Introduction
Multiple myeloma (MM) is a malignancy of B cells
characterized by the aberrant clonal proliferation and expansion of
plasma cells producing monoclonal immunoglobulin (Ig) antibodies
(1). MM is the second most common
hematological malignancy in the world and accounts for ~13% of all
hematological malignancies (2).
Although the complete remission rate and progression-free survival
time has improved as a result of novel treatment strategies and
advances in transplantation based therapies, it remains an
incurable disease. Despite the central role of genomic changes,
epigenetic modifications, including DNA methylation, regulation by
non-coding RNAs and histone modifications are crucial in the
development and progression of MM (3–5).
MicroRNAs (miRNAs/miRs) are a class of small
non-coding RNAs measuring 17–25 nucleotides in length (6). Mature miRNAs regulate a large variety
of cellular functions including proliferation, differentiation,
angiogenesis, apoptosis, stress response and fat metabolism
(7–9).
A number of previous studies have examined the miRNA
expression profile in MM, with the aim of identifying novel
biomarkers for diagnosis or prognosis prediction, therapeutic
targets as well as improving the understanding of the underlying
mechanisms regarding miRNA regulation networks (3,10,11).
Among the numerous aberrantly expressed miRNA that have been
documented in MM, the effects of miR-181a, which is upregulated in
MM, have not been extensively examined. miR-181a was the first
miRNA reported to serve a regulatory role in B cell differentiation
in the bone marrow (BM), and ectopic expression in hematopoietic
stem/progenitor cells promoted a substantial increase in the
generation of B cells, both in vitro and in vivo
(12). Previous data have revealed
that miR-181a is a biomarker of MM and regulates the progression of
MM (13,14). In a number of different types of
tumor, miR-181a was involved in the regulation of tumor
proliferative, migratory and invasive abilities, thereby
highlighting its potential as a prognostic factor and therapeutic
target (15). In the present study,
the expression of miR-181a in patients with MM was measured, and
its effect on proliferation and invasion of MM cells was assessed.
Additionally, the underlying molecular mechanism of its regulation
functions were determined.
Materials and methods
Patient samples
A group of 31 newly diagnosed and previously
untreated patients with MM (13 females and 18 males with a median
age of 62 years and range 46–77 years) were enrolled in the present
study between December 2015 and July 2018 in Ningbo First Hospital.
Patients were diagnosed and categorized according to the
International Staging System (16).
Of the enrolled patients, 5 were classified as stage I, 10 as stage
II and 16 as stage III. The class of monoclonal proteins present
were: IgG for 13 patients; IgA for 8 patients; and light chain
disease and others for 10 patients. As a control, 13 healthy
individuals (6 females and 7 males; median age, 59 years; age
range, 39–74 years) were recruited. Healthy volunteers had not been
exposed to any known cytotoxic treatment prior to aspiration of the
BM. BM aspirates were collected from patients with MM and normal
controls into heparinized syringes in a single aspiration. All BM
aspirates were obtained from the posterior iliac crest. Written
informed consent was obtained from all patients and all experiments
were performed in compliance with the 6th revision of The
Declaration of Helsinki (2008) and approved by the Institutional
Review Board of Ningbo First Hospital. Briefly, BM samples were
diluted in PBS (Sigma-Aldrich; Merck KGaA) and separated using a
Ficoll-Paque gradient centrifugation at 1,500 × g for 15 min at 4°C
(GE Healthcare Life Sciences) according to the manufacturer's
protocol.
Cell culture
The human MM RPMI8226 cell line was obtained from
American Type Culture Collection. Cells were grown in RPMI 1640
medium (HyClone; GE Healthcare Life Sciences) supplemented with 10%
fetal calf serum (HyClone; GE Healthcare Life Sciences) and
antibiotics (100 units/ml penicillin and 100 µg/ml streptomycin) in
5% CO2 at 37°C.
Cell transfection
miR-181a inhibitor and negative control (NC)
inhibitor were purchased from Shanghai GenePharma Co., Ltd.
RPMI8226 cells in the logarithmic growth stage were seeded into
6-well plates with the density of 1×105 cells/well and
transfected with 50 nM either the miR-181a inhibitor or NC
inhibitors using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
Following transfection for 6 h, the medium was removed from the
plate, and serum-free RPMI-1640 was used to wash the cells. Cells
were incubated at 37°C in 5% CO2 for 24 h for further
use. The sequences were as follows: miR-181a inhibitor,
5′-ACUCACCGACAGCGUUGAAUGUU-3′; NC inhibitor,
5′-CAGUACUUUUGUGUAGUACAA-3′.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from freshly isolated
RPMI8226 cells using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. cDNA
was reverse transcribed from the mRNA using the One Step
PrimeScript miRNA cDNA Synthesis kit (Takara Biotechnology Co.,
Ltd.). RT-PCR was conducted using One Step TB Green®
PrimeScript™ RT-PCR kit (RR066A; Takara Biotechnology
Co., Ltd). The PCR conditions were as follows: Initial denaturation
at 72°C for 5 min, denaturation at 95°C for 10 sec, annealing at
72°C for 10 sec and final extension at 65°C for 20 sec for 35
cycles. All primer sequences used in RT-qPCR were purchased from
Invitrogen; Thermo Fisher Scientific and the primer sequences were
as follows: miR-181a forward, 5′-GCGGTAACATTCAACGCTGTCG-3′;
miR-181a reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′; and U6 reverse,
5′-AACGCTTCACGAATTTGCGT-3′. Small nuclear RNA U6 was used as an
internal reference. The RT-qPCR was performed on an ABI Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The comparative Cq method (2−ΔΔCq) was used to calculate
the relative expression level of each sample (17).
Cell proliferation analysis
The proliferative capacity of the RPMI8226 cells was
analyzed using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) based on the dehydrogenase activity detection
in viable cells. Briefly, cells were seeded into 96-well plates at
a density of 1×105 cells per well. After 24 h of
culturing, 10 µl CCK-8 solution was added into each well and cells
were incubated for 2 h at room temperature. The absorbance at 450
nm was measured using a microplate reader (Model 550; Bio-Rad
Laboratories, Inc.).
Cell cycle analysis
RPMI8226 cells in the logarithmic phase were
harvested and fixed in ice-cold 70% ethanol on ice for 20 min. The
cells were rinsed in PBS, and stained using 20 mg/ml propidium
iodide (PI) and 10 U/ml RNase A (Abcam) in a 37°C water bath for 30
min. Cell cycle distribution was determined using flow cytometry
(FACScan; BD Biosciences) and the data were analyzed using ModFit
LT™ version 2.0 (Verity Software House, Inc.) to
determine the percentage of cells in each phase of the cell
cycle.
Wound healing assay
Following transfection, the RPMI8226 cells were
collected and seeded in a 48-well plate at a density of
1×106 per well. Upon reaching a confluent monolayer, a
line-shaped scratch was created by carefully dragging a 200 µl
pipette tip through the cell layer. Cells were washed with PBS 3
times and incubated at room temperature at 5% CO2 for 24
h. The degree of scratch healing was observed by a light microscope
at magnification, ×200 and measured and used to determine the cell
migratory ability.
Western blot analysis
SDS-PAGE and western blot analysis were performed to
measure the protein expression levels of cyclin D1 (CCND1) protein
using β-actin as an internal reference. Briefly, RPMI8226 cell
lysates were prepared using lysis buffer containing protease
inhibitors (Beyotime Institute of Biotechnology). The
concentrations of protein were measured using a Bradford protein
assay (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. A total of 20 µg protein extracts were
resolved using 10% SDS-PAGE and transferred to a PVDF membrane
(Bio-Rad Laboratories, Inc.). The membrane was blocked using 5%
non-fat milk in TBS-Tween solution (Beijing Solarbio Science &
Technology Co., Ltd.) for 2 h at room temperature and immunoblotted
overnight at 4°C with a mouse anti-CCND1 monoclonal antibody (cat.
no., sc-450; 1:1,000; Santa Cruz Biotechnology, Inc.) or mouse
anti-β-actin primary antibody (cat. no., BM0627; 1:1,000; Wuhan
Boster Biological Technology, Ltd.). Membranes were washed 3 times
in TBS-Tween, and subsequently incubated with a secondary antibody
IgG H&L Cy3® (cat. no., ab97035; Abcam) for 1 h at
room temperature. Enhanced chemiluminescence was used to visualize
the signal and the signal intensity was quantified using
VisionWorks LS version 7.0 Analyzing System (Analytik Jena US
LLC).
Statistical analysis
Data were analyzed using SPSS version 20.0 (IBM
Corp.) to determine statistical significance. All data are
expressed as the mean ± standard deviation. Differences between two
groups were compared using a Student's t-test. Multiple comparisons
were performed using a one-way analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-181a expression is upregulated in
patients with MM and an MM cell line
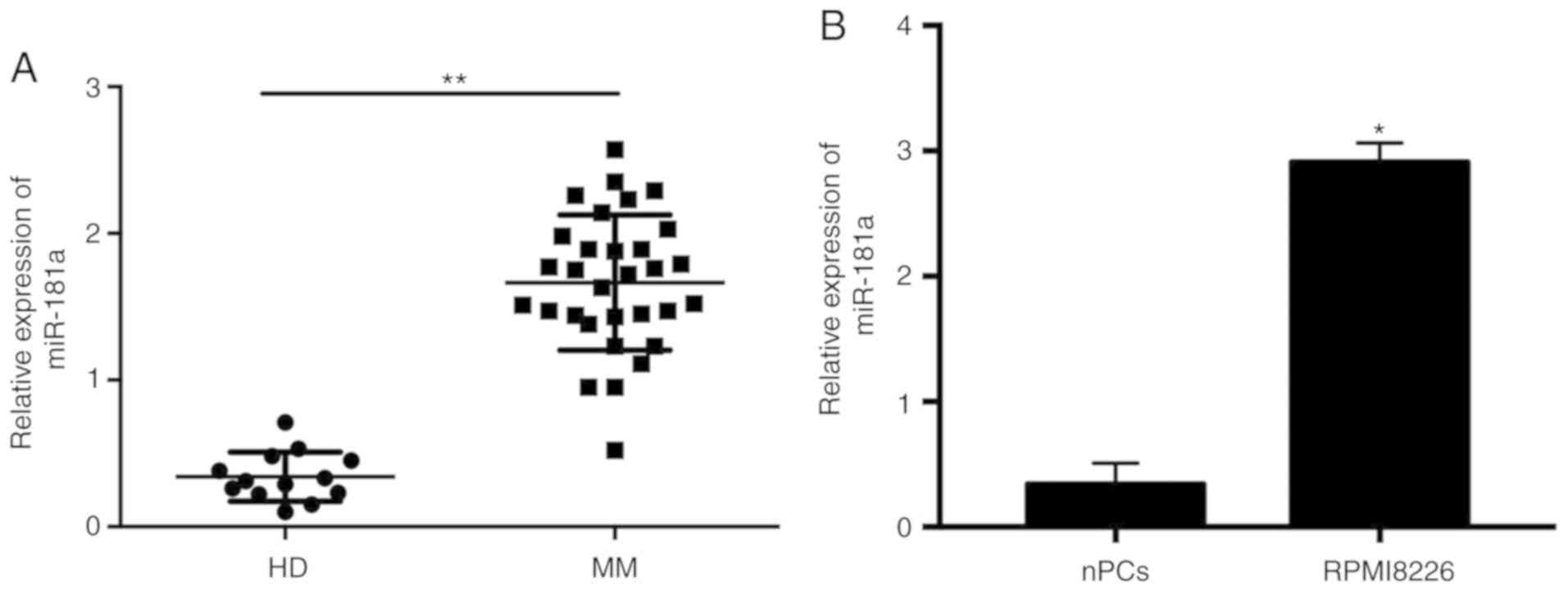
To determine the expression level of miR-181a in
patients with MM, RT-qPCR analysis was performed using the MM cells
obtained from patients with MM and the normal plasma cells (nPCs)
from the healthy controls. In the BM aspirates of the 31 patients
with MM and 13 healthy donors, miR-181a expression was
significantly increased in the patients with MM patients without
treatment compared with the nPCs (Fig.
1A). The expression level of miR-181a was significantly
increased in the RPMI8226 cell line compared with the healthy
control (Fig. 1B).
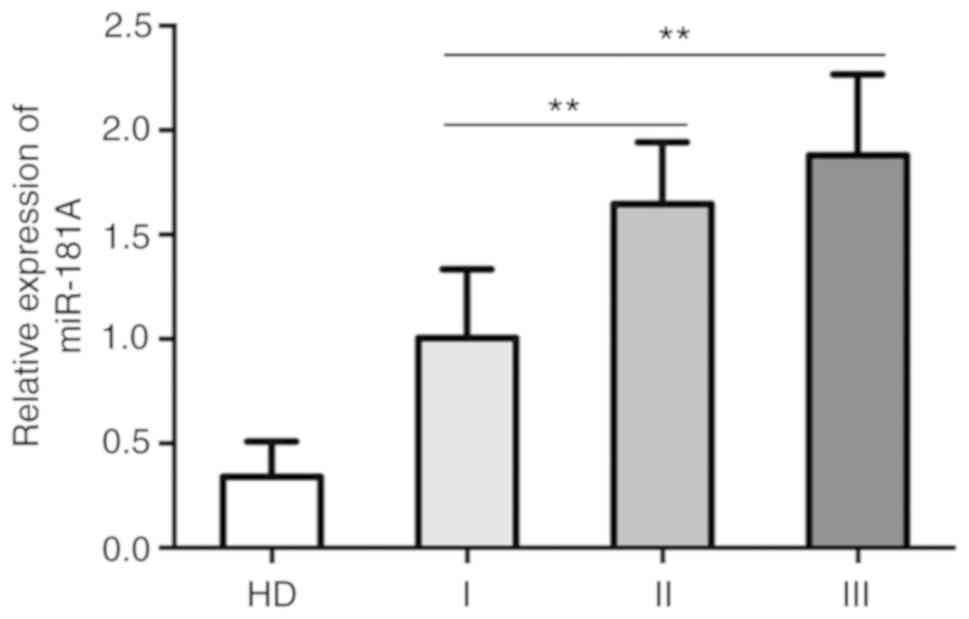
The association between the expression of miR-181a
and the clinical features of the patients was subsequently
assessed. There was no association between the expression profile
of miR-181a and age, sex and cancer isotype; however, expression
was increased in the later stages of MM compared with earlier
disease stages (Table I). Patients
with either stage II (1.65±0.30) and stage III (1.88±0.39) disease
presented with significantly increased expression levels of
miR-181a compared with patients with stage I MM (1.01±0.33).
However, there was no significant difference observed between
patients with stage II and III MM (Fig.
2). The expression levels of miR-181a were also increased in
patients with MM with renal lesions compared with patients without
lesions. These results suggested an oncogenic function for miR-181a
in the pathogenesis and progression of MM.
 | Table I.Relative expression of miR-181a in
patients with multiple myeloma with different clinical
characteristics. |
Table I.
Relative expression of miR-181a in
patients with multiple myeloma with different clinical
characteristics.
| Characteristics | Cases (n) | miR-181a level | P-value |
|---|
| Sex |
|
| 0.743 |
| Male | 18 | 1.64±0.46 |
|
|
Female | 13 | 1.70±0.48 |
|
| Age, years |
|
|
|
|
<65 | 16 | 1.52±0.48 | 0.080 |
| ≥65 | 15 | 1.81±0.41 |
|
| Stage |
|
| 0.000a |
| I | 5 | 1.01±0.33 |
|
| II | 10 | 1.65±0.30 |
|
|
III | 16 | 1.88±0.39 |
|
| Phenotype |
|
| 0.859 |
|
IgG | 13 | 1.64±0.41 |
|
|
IgA | 8 | 1.62±0.49 |
|
|
Other | 10 | 1.73±0.54 |
|
| Renal lesion |
|
| 0.022a |
|
Yes | 17 | 1.83±0.38 |
|
| No | 14 | 1.46±0.49 |
|
Downregulation of miR-181a inhibits
the proliferation and migration of MM cells
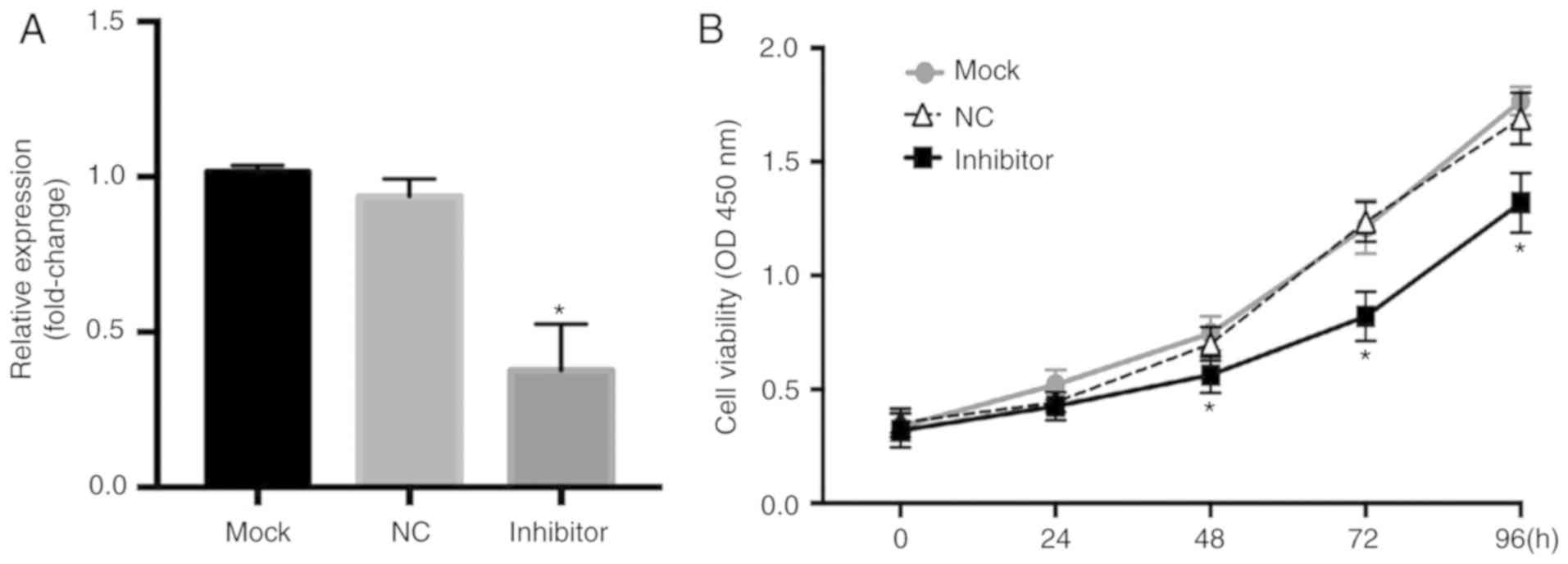
To determine the effects of miR-181a expression in
an MM cell line, miR-181a was inhibited in RPMI8226 cells by
transfection with an miR-181a inhibitor (Fig. 3A). By measuring the optical density
of the cells, it was demonstrated that the survival rate increased
significantly over time in the normal control RPMI8226 cells, and a
significant decrease in the viability of cells was observed in the
cells transfected with the miR-181a inhibitor compared with the
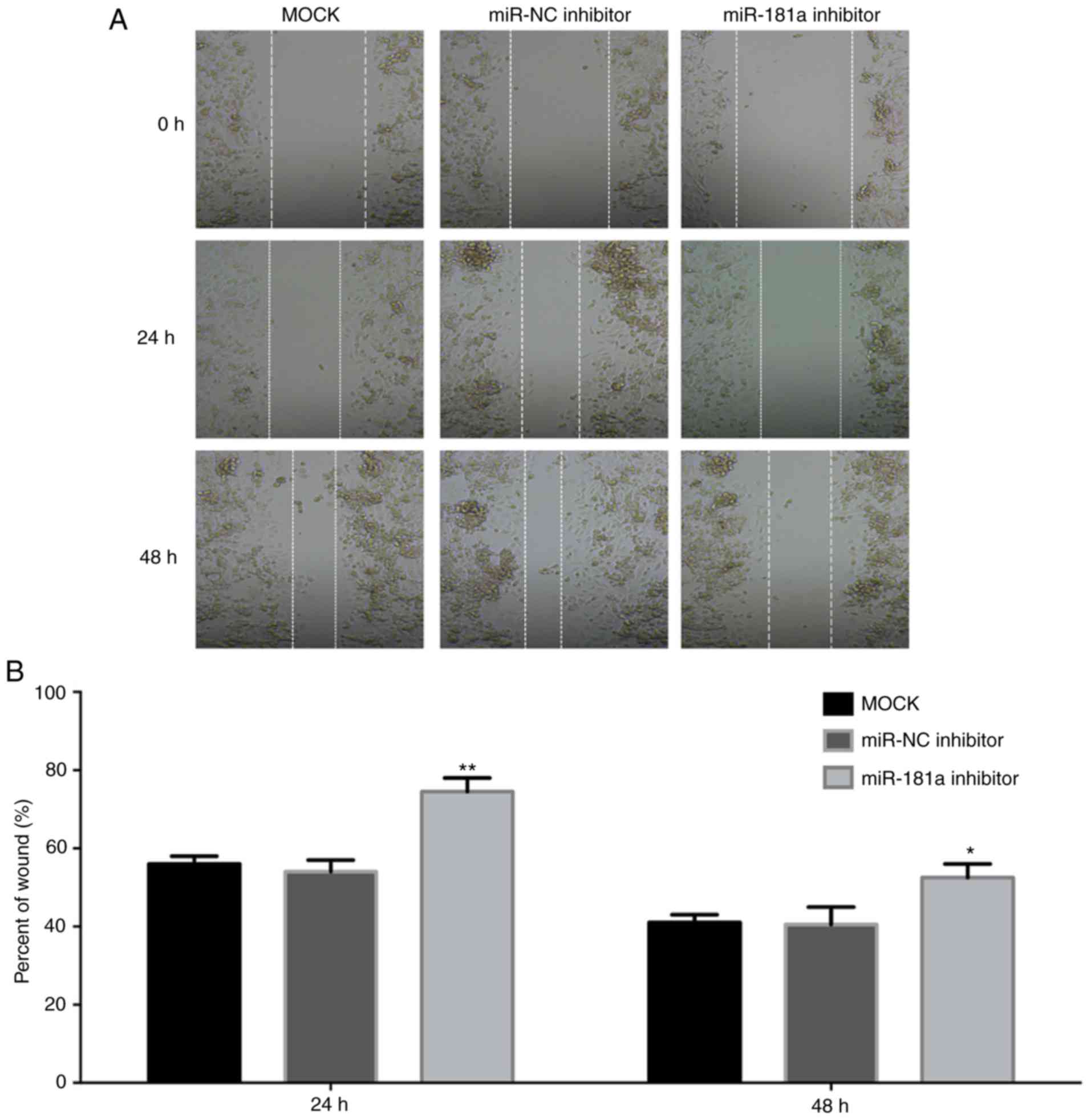
miR-NC group (Fig. 3B). A wound
healing assay was used to determine the effect of miR-181a on the
mobility of MM cells (Fig. 4). The
edge of the wound was neat following scratching. After 24 h
incubation, the cells migrated into the wound. Compared with the
non-transfected cells and the cells transfected with the NC
inhibitor, the number of cells transfected with the miR-181a
inhibitor migrating into the wound was significantly decreased
(P<0.05). Together, these data suggest that miR-181a serves an
important role in promoting proliferation and the migratory ability
of MM cells.
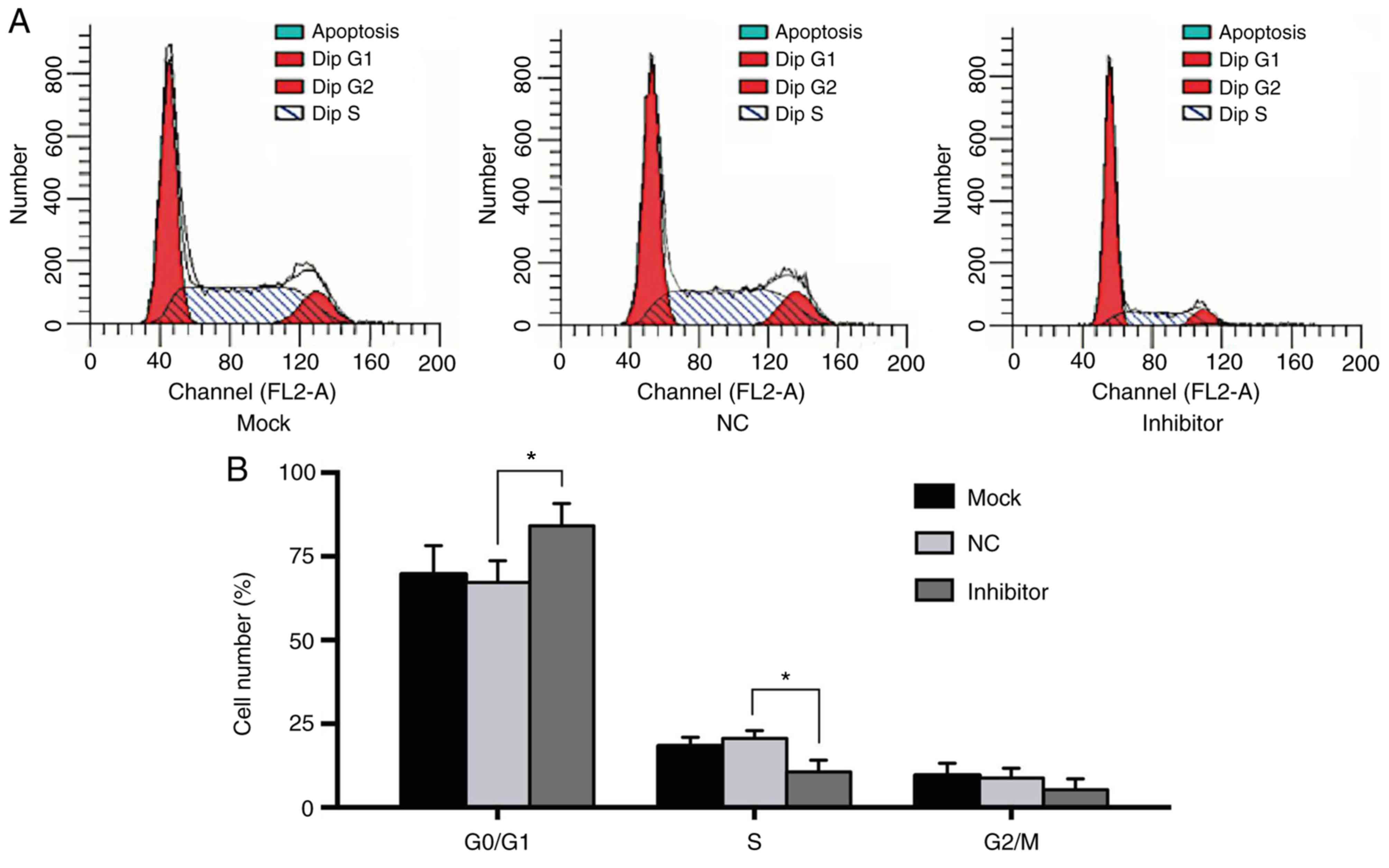
Knockdown of miR-181a results in cell
cycle arrest in MM cells
To investigate the effect of miR-181a on cell cycle
progression in MM cells, RPMI8226 cells were transfected with the
miR-181a inhibitor or NC inhibitor, or mock-transfected (Fig. 5). The cytometry results demonstrated
that the cells transfected with the miR-181a-inhibitor exhibited a
larger proportion of cells in the G0/G1 phase, and smaller
proportion of cells in the S and G2/M phases, suggesting that the
inhibition of miR-181a induced G0/G1 cell cycle arrest in RPMI8226
cells. The results suggested that miR-181a contributed to tumor
progression of MM by promoting cell cycle progression from G0/G1 to
S.
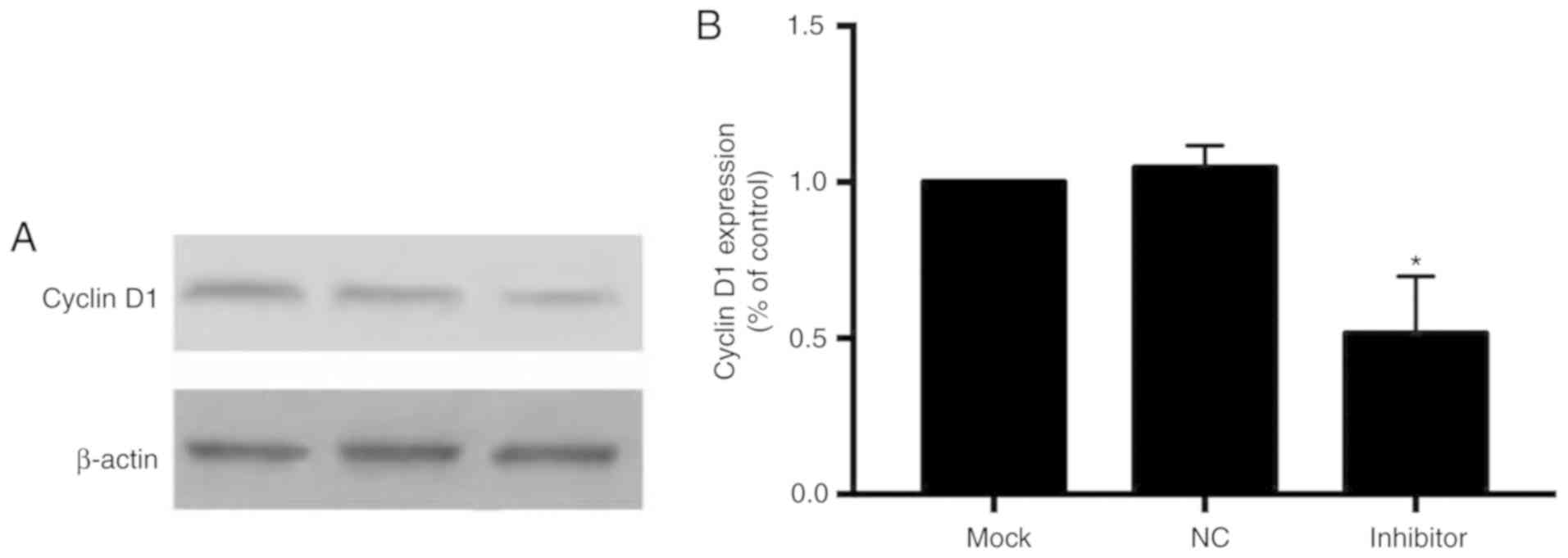
Inhibition of miR-181a suppresses
expression of CCND1
To determine the underlying molecular mechanism of
action of miR-181a, western blot analysis was performed to
determine the expression of the cell cycle regulator CCND1. In
cells transfected with the miR-181a inhibitor, CCND1 expression was
downregulated compared with the miR-NC group (Fig. 6).
Discussion
miRNAs have been demonstrated to serve a number of
essential and diverse physiological and pathological roles in cells
(7) and the abnormal expression of
numerous miRNAs are implicated in the development and progression
of several different types of cancer (18). Furthermore, several miRNAs have been
identified as oncogenes or tumor suppressors in the carcinogenesis
of MM (3,10,11),
highlighting the varying effects of different miRNAs in the
development of MM (11,19).
miR-181a was recently determined to be an important
noncoding RNA with a wide range of regulatory functions in normal
hematopoiesis, organ development and pathological processes of a
number of different types of cancer and autoimmune disorders
(20–23). In MM, it was one of the most highly
upregulated miRNAs in both patient samples and cell lines, and was
also demonstrated to exhibit similar expression patterns in
peripheral blood and the BM (24).
However, its potential role in the pathogenesis of MM remains
poorly understood.
In the present study, the expression levels of
miR-181a were significantly increased in BM samples from patients
recently diagnosed with MM and in the MM RPMI8226 cell line
compared with normal plasma cells, which was consistent with the
results from previous studies (10,13,25). In
the present study, upregulation of miR-181a was associated with
disease stage, suggesting its potential contribution to the
progression of MM. Aberrant expression of miR-181a has been
observed in cases of monoclonal gammopathy of undetermined
significance, a probable early pathogenic event of MM (10). Notably, in plasma cell leukemia,
which is a rare and aggressive form of MM, characterized by poor
prognosis and the presence of malignant plasma cells circulating in
the peripheral blood, miR-181a expression levels were considerably
increased compared with MM. Additionally, compared with patients
who responded to treatment, patients who did not respond to
treatment exhibited increased levels of miR-181a (26). Taken together, it may be hypothesized
that miR-181a serves a potential role during the entire process of
the MM development and progression.
In vitro assays were performed to determine
the biological effects of miR-181a in the MM RPMI8226 cell line.
Decreasing expression of miR-181a exerted an inhibitory effect on
the proliferative and migratory capabilities of the RPMI8226 cells.
Furthermore, cell cycle analysis revealed G0/G1 arrest when
miR-181a was inhibited in MM cells. These results suggest that
miR-181a may function as a novel oncogene in MM and serve a
regulatory role in the tumorigenesis of MM.
To identify the regulatory network underlying the
effects of miR-181a, the present study focused on CCND1. CCND1 is a
cell cycle-regulating protein encoded by the CCND1 gene and is
required for the progression through the G1 phase to the S phase in
both normal cells and cancerous cells (27,28).
CCND1 is activated in MM as an oncogene, and regulates the cell
cycle and promotes proliferation (29). The results demonstrated that CCND1
expression was decreased in the miR-181a-inhibited MM cells
compared with normal MM cells, and the results consistent with
previous studies using co-immunoprecipitation analysis (30–32). The
downregulation of CCND1 as a result of miR-181a inhibition in the
MM cells may partially explain the effects of miR-181a on the cell
cycle distribution results.
However, there are several limitations of the
present study. Firstly, only one MM cell line, RPMI8226, was used
for the in vitro experiments. Secondly, the in vitro
results were not confirmed in vivo.
In conclusion, miR-181a was significantly
upregulated in clinical BM samples from patients with MM and an MM
cell line compared with the normal controls. Furthermore, the
overexpression of miR-181a promoted cell proliferation, migration
and cell cycle progression, promoting the development of MM.
miR-181a regulation of CCND1 was identified as a potential
molecular mechanism underlying the promotion of cell cycle
progression, and this interaction was abrogated using an miR-1881a
inhibitor. The results of the present study highlight the potential
of miR-181a as a novel therapeutic target and the use of synthetic
miR-181a inhibitors as novel therapeutic strategies for treating
patients with MM.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Ningbo, China (grant nos. 2017A610177 and
2017A610210) and The Medical and Health Technology Program of
Zhejiang Province (grant no. 2018KY678).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY, MG, PZ, GO, QM and KX conceived the study. XY,
MG and PZ designed the study and performed data analysis. GO, QM
and KX conducted the experiments and interpreted the data. XY and
MG wrote the manuscript. PZ, GO, QM and KX critically revised the
manuscript for important content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and all experiments were performed in compliance with the
Declaration of Helsinki (2008) and approved by the Institutional
Review Board of Ningbo First Hospital (approval no.
2015087533).
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kyle R and Rajkumar SV: Multiple myeloma.
Blood. 111:2962–2972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA. Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar
|
|
3
|
Rastgoo N, Abdi J, Hou J and Chang H: Role
of epigenetics-microRNA axis in drug resistance of multiple
myeloma. J Hematol Oncol. 10:1212017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dupéré-Richer D and Licht JD: Epigenetic
regulatory mutations and epigenetic therapy for multiple myeloma.
Curr Opin Hematol. 24:336–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dimopoulos K, Gimsing P and Grønbæk K: The
role of epigenetics in the biology of multiple myeloma. Blood
Cancer J. 4:e2072014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He L and Hannon GJ: Erratum: MicroRNAs:
Small RNAs with a big role in gene regulation. Nat Rev Genet.
5:522–531. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garzon R, Fabbri M, Cimmino A, Calin GA
and Croce CM: MicroRNA expression and function in cancer. Trends
Mol Med. 12:580–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuehbacher A, Urbich C and Dimmeler S:
Targeting microRNA expression to regulate angiogenesis. Trends
Pharmacol Sci. 29:12–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pichiorri F, Suh SS, Ladetto M, Kuehl M,
Palumbo T, Drandi D, Taccioli C, Zanesi N, Alder H, et al:
MicroRNAs regulate critical genes associated with multiple myeloma
pathogenesis. Proc Natl Acad Sci USA. 105:12885–12890. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abdi J, Jian H and Chang H: Role of
micro-RNAs in drug resistance of multiple myeloma. Oncotarget.
7:60723–60735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Yébenes VG, Bartolomé-Izquierdo N and
Ramiro AR: Regulation of B cell development and function by
microRNAs. Immunol Rev. 253:25–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng J, Thakur A, Zhang S, Dong Y, Wang X,
Yuan R, Zhang K and Guo X: Expressions of miR-181a and miR-20a in
RPMI8226 cell line and their potential as biomarkers for multiple
myeloma. Tumour Biol. 36:8545–8552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu N, Yang J, Yuan R, Peng J, Liu L and
Guo X: Effects of miR-181a on the biological function of multiple
myeloma. Oncol Rep. 42:291–300. 2019.PubMed/NCBI
|
|
15
|
Cao Y, Zhao D, Li P, Wang L, Qiao B, Qin
X, Li L and Wang Y: MicroRNA-181a-5p impedes IL-17-induced
non-small cell lung cancer proliferation and migration through
targeting VCAM-1. Cell Physiol Biochem. 42:346–356. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bi C and Chng WJ: MicroRNA: Important
player in the pathobiology of multiple myeloma. Biomed Res Int.
2014:5215862014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang S, Wu S, Ding J, Lin J, Wei L, Gu J
and He X: MicroRNA-181a modulates gene expression of zinc finger
family members by directly targeting their coding regions. Nucleic
Acids Res. 38:7211–7218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Xu QH, Dong YH, Li GX, Yang L, Wang
LW and Li HY: MiR-181a upregulation is associated with
epithelial-to-mesenchymal transition (EMT) and multidrug resistance
(MDR) of ovarian cancer cells. Eur Rev Med Pharmacol Sci.
20:2004–2010. 2016.PubMed/NCBI
|
|
22
|
Ghorbani S, Talebi F, Chan WF, Masoumi F,
Vojgani M, Power C and Noorbakhsh F: MicroRNA-181 variants regulate
T cell phenotype in the context of autoimmune neuroinflammation.
Front. Immunol. 8:7582017.
|
|
23
|
Verduci L, Azzalin G, Gioiosa S, Carissimi
C, Laudadio I, Fulci V and Macino G: microRNA-181a enhances cell
proliferation in acute lymphoblastic leukemia by targeting EGR1.
Leuk Res. 39:479–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yyusnita, Norsiah, Zakiah I, Chang KM,
Purushotaman VS, Zubaidah Z and Jamal R: MicroRNA (miRNA)
expression profiling of peripheral blood samples in multiple
myeloma patients using microarray. Malaysian J Pathol. 34:133–143.
2012.
|
|
25
|
Li Y, Li D, Yan Z, Qi K, Chen L, Zjang Z,
Fan G, Li H, Xu K and Li Z: Potential relationship and clinical
significance of miRNAs and Th17 cytokines in patients with multiple
myeloma. Leuk Res. 38:1130–1135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lionetti M, Musto P, Di Martino MT, Fabris
S, Agnelli L, Todoerti K, Tuana G, Mosca L, Gallo Cantafio ME,
Grieco V, et al: Biological and clinical relevance of miRNA
expression signatures in primary plasma cell leukemia. Clin Cancer
Res. 19:3130–3142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lesage D, Troussard X and Sola B: The
enigmatic role of cyclin D1 in multiple myeloma. Int J Cancer.
115:171–176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hafner M, Landthaler M, Burger L, Khorshid
M, Hausser J, Berninger P, Rothballer A, Ascano M Jr, Jungkamp AC,
Munschauer M, et al: Transcriptome-wide identification of
RNA-binding protein and microRNA target sites by PAR-CLIP. Cell.
141:129–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balakrishnan I, Yang X, Brown J,
Ramakrishnan A, Torok-Storb B, Kabos P, Hesselberth JR and Pillai
MM: Genome-wide analysis of miRNA-mRNA interactions in marrow
stromal cells. Stem Cells. 32:662–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Farazi TA, Ten Hoeve JJ, Brown M,
Mihailovic A, Horlings HM, van de Vijver MJ, Tuschl T and Wessels
LF: Identification of distinct miRNA target regulation between
breast cancer molecular subtypes using AGO2-PAR-CLIP and patient
datasets. Genome Biol. 15:R92014. View Article : Google Scholar : PubMed/NCBI
|