Introduction
Transforming growth factor-β (TGF-β) has been shown
to possess a contradictory dual-faceted nature; it plays both as a
tumor suppressor during the initial stages of tumorigenesis as well
as an activator in tumor progression. In early-stage cancer cells,
TGF-β inhibits cell proliferation, while promoting apoptosis;
however, in the late stage of cancer, TGF-β induces invasion and
metastasis of cancer through epithelial-mesenchymal transition
(EMT), escape from immune system and facilitating angiogenesis
(1). TGF-β binds to TGF-β receptor
type I (TβR-I) and type II (TβR-II), which are transmembrane
serine/threonine kinases. Smad2 and Smad3 when phosphorylated by
TGF-β receptor, TβR-I/TβR-II hetero-tetramer, bind to Smad4 and
translocate to the nucleus. The transcription of several target
genes is regulated by the Smad2/3/4 complex in cooperation with
other cofactors (2,3). Recent preclinical and clinical trials
in tumor carcinogenesis have focused on testing inhibitors, such as
small-molecule tyrosine kinase inhibitors, antibodies and antisense
molecules, which block the TGF-β signaling pathway and TGF-β
synthesis by small compounds, antibodies and antisense molecules
(4).
Squamous cell carcinoma (SCC) is the most frequent
cancer in the oral cavity (5). EMT
is known to play an important role in cancer metastasis (6). In addition, bone morphogenetic protein
(BMP), which belongs to TGF-β superfamily, seems to be involved in
mesenchymal-epithelial transition (MET) after metastasis, but the
mechanisms have not yet been clarified (7).
EMT induces the loss of characteristics of epithelia
and the gain of characteristics of mesenchyme in differentiated
epithelial cells, which leads to increased cell migration and
invasion (8). EMT is not only an
important process in development, adult tissue maintenance and
reproduction (9,10), but also in cancer and desmoplasia in
disease (11). In general, TGF-β is
a crucial inducer of EMT (12,13).
Cadherin switch (expression changes from E-cadherin to N-cadherin)
is known to play an important role in the malignant transformation
of cancer cells in the EMT process (14). The mechanism underlying regulation of
the cadherin switch in human oral squamous cell carcinoma (hOSCC)
cells remains to be elucidated, whereas previous studies have
reported changes to the expression of various genes related to the
cadherin switch in many kinds of SCC cells other than hOSCC cells
(15,16).
In our previous study, we demonstrated that TGF-β1
induced EMT in hOSCC cell line HSC-4. We also showed that the
migratory activity of HSC-4 cells was promoted through
TGF-β1-induced integrin α3β1/FAK activation (16). In addition, we found that the
TGF-β1-induced upregulation of Slug expression, which positively
regulated the migratory activity of HSC-4 cells. TGF-β1 also
stimulates the invasion ability of HSC-4 cells through the
Slug/Wnt-5b/MMP-10 signaling axis (17). These results suggested that Slug
might be an important EMT-related transcription factor which
promotes metastasis of hOSCC cells. However, we also demonstrated
that Slug did not participate in the upregulation of N-cadherin
expression (16), suggesting that
EMT-related transcription factors other than Slug played an
important role in the process.
Sox9, also known as sex-determining region Y (SRY)
protein, is a transcription factor that regulates chondrocyte
differentiation and cartilage formation (18). Sox9 positively regulates cell
stemness (19), in conjunction with
intracellular signaling pathways, such as Wnt signaling (20). Further, it promotes N-cadherin gene
transcription in chondrocytic CFK2 cells (21). Sox9 also induces EMT, which in turn
results in neural crest formation (22) and nephrolithiasis in primary renal
tubular epithelial cells (23). In
lung adenocarcinoma, Sox9 mediates Notch-1-induced mesenchymal
phenotypes (24), and coexpression
of Sox9 and collagen type X alpha 1 in presence of TGF-β1 is
associated with tumor progression in gastric cancer (25). In contrast, knockdown of Sox9
inhibits EMT in thyroid cancer cells (26). However, it is unclear whether Sox9 is
involved in TGF-β-induced EMT and N-cadherin expression in
hOSCC.
In this study, we aimed to identify whether the
EMT-related transcription factor Sox9 upregulates N-cadherin
expression in hOSCC cells. In addition, we also aimed to elucidate
the TGF-β1-induced signals that affect the function of Sox9 in
HSC-4 cells at a molecular level.
Materials and methods
Materials
Cultured cell lines were obtained from the Japanese
Collection of Research Bioresources Cell Bank. Recombinant human
TGF-β1 was purchased from PeproTech. Protease inhibitor cocktail,
for use with mammalian cell and tissue extracts, and phosphatase
inhibitor cocktails 1 and 2 were purchased from Sigma-Aldrich. The
Protein kinase inhibitor (PKA), H-89 was obtained from Santa Cruz
Biotechnology Inc. and okadaic acid (OA) was procured from Merck
(Calbiochem, KGaA). All other purchased reagents were of analytical
grade.
Cell culture
All cell lines were grown at 37°C and 5%
CO2. Human HSC-4 SCC cells (JCRB0624) were cultured in
Eagle's minimum essential medium (MEM; Sigma-Aldrich) supplemented
with 10% fetal bovine serum (FBS; Gibco BRL). SAS cells (JCRB0260)
were cultured in PRIM1640 medium (Gibco BRL) supplemented with 10%
FBS. HO-1-N1 cells (JCRB0831) were cultured in Dulbecco's modified
Eagle's medium (DMEM) and Ham's F-12 medium (1:1; Gibco BRL) with
10% FBS. The culture medium was removed and replaced with
serum-free medium 24 h prior to the TGF-β1-stimulated experiments.
For time-course experiments, 2.0×105 hOSCC cells were
cultured in 500 µl of medium without serum containing 10 ng/ml
TGF-β1, for 1 to 48 h in 12 or 24-well tissue culture plates.
Reverse transcription-quantitative PCR
(RT-qPCR)
For total RNA preparation, 2.0×105 cells
were cultured in 24-well tissue culture plates. Total RNA was
isolated using the ISOGEN reagent (Nippon Gene), according to
manufacturer's instructions. RNA was reverse transcribed into
first-strand cDNA using a RT-PCR System kit (Takara Bio Inc.). qPCR
was performed on a Thermal Cycler Dice Real Time System (Takara
Bio) using SYBR Premix Ex Taq II (Takara Bio) with human
gene-specific primers (Table I).
Target gene expression was normalized to an internal β-actin
reference and expressed in terms of fold-change relative to the
control sample (27).
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Target mRNA | Oligonucleotide
sequence, 5′-3′ | Predicted size,
bp |
|---|
| E-cadherin | (F)
TACACTGCCCAGGAGCCAGA | 103 |
|
| (R)
TGGCACCAGTGTCCGGATTA |
|
| Fibronectin | (F)
AACTTCGAATTATGAGCAGGACCAG | 151 |
|
| (R)
GCCCTCAGAAGTGCAATCAGTGTA |
|
| Laminin α3 | (F)
TCGGTCACACCAAAGCAGTCTC | 93 |
|
| (R)
TGTGTCCAGTTCCAGGTGCAG |
|
| N-cadherin | (F)
CGAATGGATGAAAGACCCATCC | 171 |
|
| (R)
GCCACTGCCTTCATAGTCAAACACT |
|
| Slug | (F)
TGTTGCAGTGAGGGCAAGAA | 158 |
|
| (R)
GACCCTGGTTGCTTCAAGGA |
|
| Sox9 | (F)
GGAGATGAAATCTGTTCTGGGAATG | 149 |
|
| (R)
TTGAAGGTTAACTGCTGGTGTTCTG |
|
|
Thrombospondin-1 | (F)
GGAGACAAAGACTGGCTTCTGGAC | 66 |
|
| (R)
GGCCACTGCAGGTGATGAGTAA |
|
| β-actin | (F)
GGAGATTACTGCCCTGGCTCCTA | 89 |
|
| (R)
GACTCATCGTACTCCTGCTTGCTG |
|
Suppression of gene expression by
small interfering RNAs (siRNA)
The sense sequences of human Slug siRNA (MISSION
siRNA, Hs_SNAIS_9785, Sigma-Aldrich), and Sox9 siRNA (siRNA, Life
Technologies) are 5′-GCAUUUGCAGACAGGUCAATT-3′ and
5′-UGAAGAAGGAGAGCGAGGAGGACAA-3′, respectively. Logarithmically
growing cells were seeded at a density of 1×105 cells in
24-well tissue culture plates and transfected with 10 nM of a
specific siRNA using Lipofectamine RNAiMAX (Life Technologies),
according to manufacturer's instructions. Forty-eight hours after
transfection, cells were stimulated using 10 ng/ml TGF-β1 and then
were used for RT-qPCR analysis to analyze vimentin gene expression
or for wound healing assay, as described below. Stealth™ RNAi
Negative Control High GC Duplex (Life Technologies), which does not
possess significant homology to vertebrate gene sequences, was used
as a negative control. Suppression of gene expression by siRNA was
evaluated by RT-qPCR and western blot analyses were performed for
targeted molecules.
Western blot analysis
For western blot experiments, 3.0×106
cells were lysed in RIPA buffer (Sigma-Aldrich) containing a
protease and phosphatase inhibitor cocktail (Sigma-Aldrich). The
protein content of the samples was measured using BCA reagent
(Thermo Fisher Scientific, Inc.). For the preparation of cell
lysates to examine marker proteins, 1.0×106 cells were
cultured in a 6-well plate in serum-free MEM with or without 10
ng/ml TGF-β1 for the indicated times. Cells were dissolved in SDS
sample buffer containing a protease and phosphatase inhibitor
cocktail (Sigma-Aldrich). Acrylamide gels of 12.5% (ATTO Co.) for
SDS-PAGE were used for protein separation, and the proteins were
subsequently transferred onto PVDF membranes (Merck). Membranes
were probed with primary antibodies, including mouse
anti-N-cadherin (1:250, H-2; Santa Cruz Biotechnology) and rabbit
anti-Sox9 (1:1,000, AB5535; Chemicon International Inc.)
antibodies, while a mouse anti-β-actin antibody (1:1,000, clone C4;
Santa Cruz) was used as a loading control in siRNA experiments. The
blots were then incubated with alkaline phosphatase-conjugated
secondary antibody, and subsequently, signals were detected using
an alkaline phosphatase substrate kit (BCIP/NBT Substrate kit;
Vector Laboratories Inc.).
Cell migration assay with a Boyden
chamber
The Boyden chamber-based cell migration assays were
performed as follows. First, the cells were transfected with Slug
siRNA as described above. Then, they were treated with 10 ng/ml
TGF-β1 under serum-free conditions for 48 h. Subsequently, the
cells were plated at a density of 1.0×105 cells in the
upper chamber of a Boyden chamber apparatus in serum-free media and
were allowed to migrate into a medium containing 10% FBS in the
lower chamber for 24 h at 37°C. Following the 24 h incubation
period, the filter was fixed in 4% paraformaldehyde and stained
with DAPI for 10 min. The cells that migrated to the underside of
the membrane were counted in nine random fields under a
fluorescence microscope. Data are the average of triplicate
experiments. The values indicate the mean number of migrating cells
compared with control. The level of significance was determined
using the Tukey's multiple comparison test.
Immunofluorescence analysis of
cultured cells
Cells plated on 8-well chamber slides were incubated
at 37°C for 24 h and then stimulated with 10 ng/ml TGF-β1 for an
additional time period of 48 h. Slides were fixed with 4%
paraformaldehyde at room temperature for 15 min. Cells were then
incubated with specific antibodies with 1:200 dilutions of mouse
anti-Slug (A-7; Santa Cruz), rabbit anti-Sox9 (H-90; Santa Cruz)
and rabbit anti-phospho-Sox9 (Ser181) (CSB-PA050120; Cusbio
Technology) antibodies for 16 h at 4°C. After rinsing with
phosphate-buffered saline (PBS), cells were incubated with
secondary antibodies Alexa Fluor® 488 goat anti-mouse or
anti-rabbit antibodies (1:1,000; Life Technologies) for 1 h at room
temperature and then stained with DAPI (1:500, Sigma-Aldrich) for
10 min. Slides were then washed and imaged using a fluorescence
microscope (IX70; Olympus).
Statistical analysis
All experiments were performed at least in
triplicate. Results are expressed as mean ± standard deviation
(SD). Differences between two groups (control and TGF-β1-treated
cells) for the time course of Sox9 expression, and the expressions
of Slug, Sox9 and N-cadherin in hOSCC cells were analyzed using
unpaired two-tailed Student's t-test. Differences among multiple
samples for the siRNA- or inhibitor-treated experiments were
compared using Tukey's multiple comparison test following ANOVA
with IBM SPSS Statistics 24 software. P<0.05 was considered to
indicate a statistically significant difference.
Results
EMT-inducible transcription factors
other than Slug is involved in the TGF-β-induced EMT in hOSCC
cells
We reported that Slug plays an important role in the
TGF-β-induced downregulation of E-cadherin expression in HSC-4
cells (16,17). We also found that Slug increased the
expression of the mesenchymal marker, vimentin, but not N-cadherin.
We previously demonstrated that the same Slug siRNA significantly
downregulated mRNA expression of Slug in HSC-4 cells (16). Therefore, we further examined whether
Slug increased the expression of mesenchymal markers other than
vimentin to verify the extent to which Slug affected the
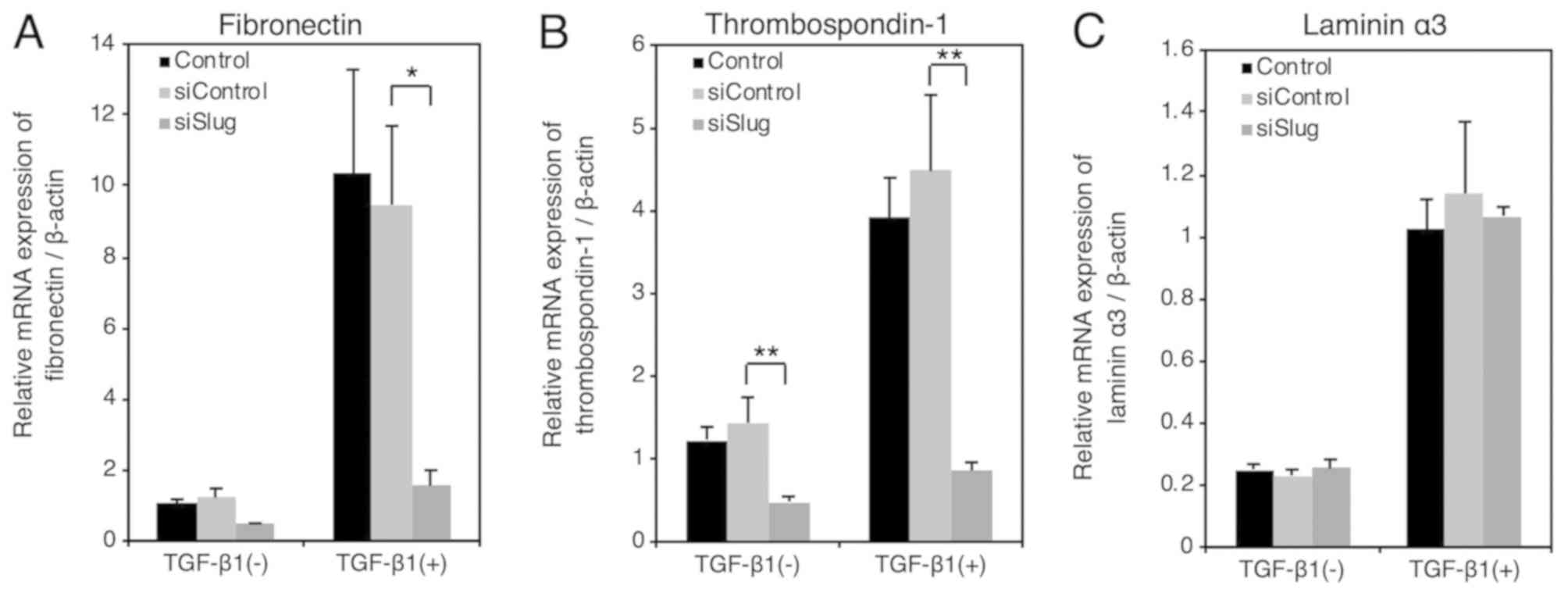
TGF-β-induced EMT in HSC-4 cells. RT-qPCR analysis revealed that
the TGF-β-induced expression of mesenchymal markers fibronectin
(Fig. 1A) and thrombospondin-1
(Fig. 1B) were significantly
downregulated following the administration of Slug siRNA. The
status of Laminin α3 was not affected by Slug siRNA (Fig. 1C), suggesting that transcription
factors other than Slug must also be involved in the TGF-β-induced
EMT in HSC-4 cells.
TGF-β1 upregulates expression of
transcription factor Sox9
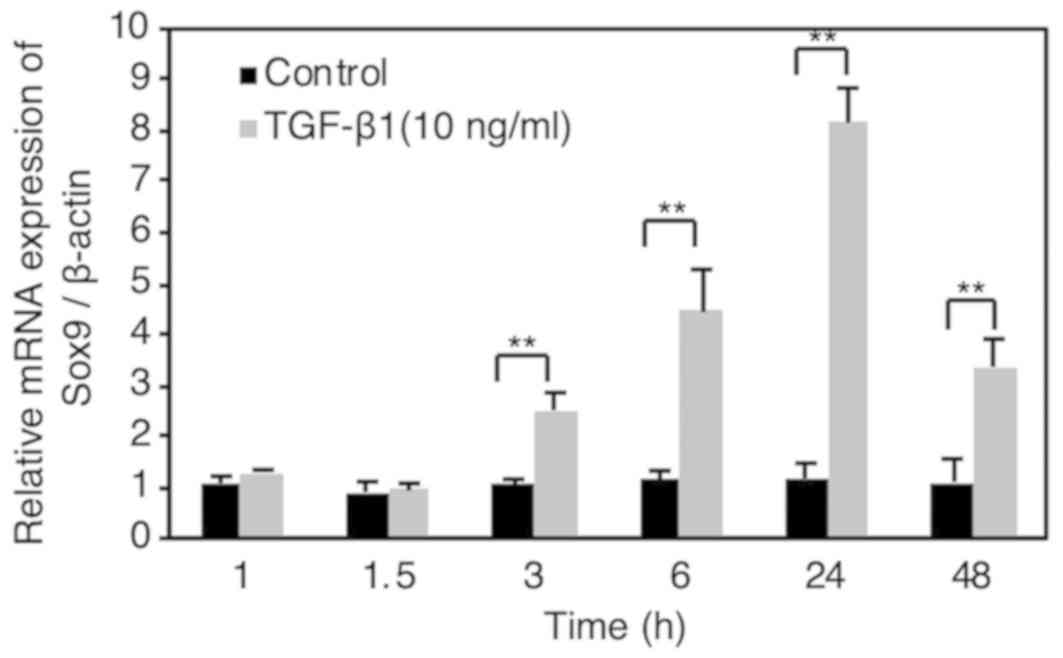
The mRNA expression of Sox9 was found to be
significantly upregulated at 3–48 h after TGF-β1 stimulation (10
ng/ml) in HSC-4 cells (Fig. 2). The
expression of Sox9 mRNA continuously increased between 3 and 24 h
following TGF-β1 stimulation, peaking at 24 h and then decreased at
48 h after stimulation. We previously reported that TGF-β1 (10
ng/ml) increased the mRNA expression of EMT-related transcription
factor, Slug, at 1.5 h following stimulation, in a Smad signal
transduction mechanism-dependent manner, in HSC-4 cells (17). These results indicate that Sox9 is
not a direct target of Smad signaling.
TGF-β1 upregulates expression of
N-cadherin, and promotes the migration of HSC-4 cells
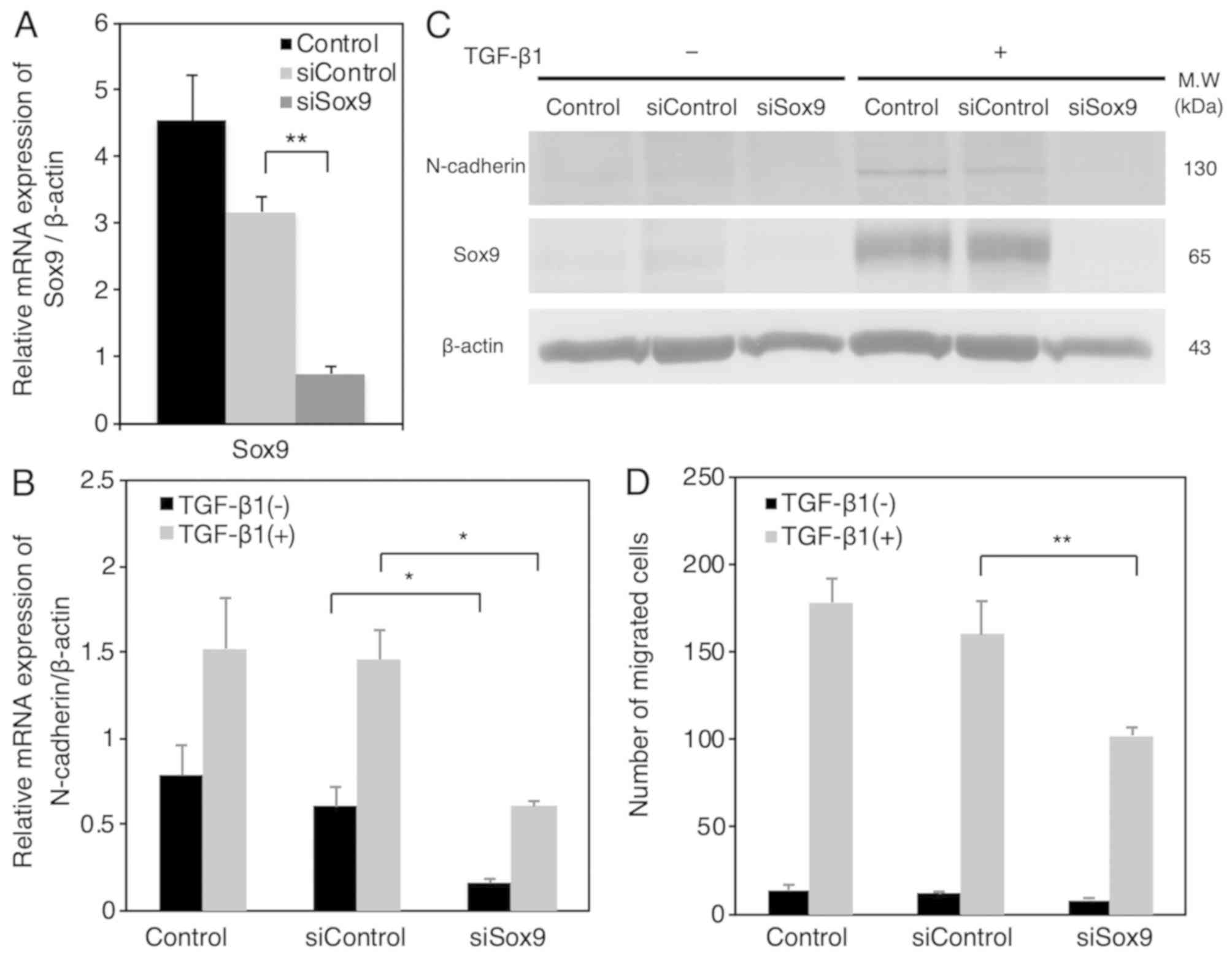
As shown in Fig. 3A,
we confirmed that control siRNA did not affect Sox9 mRNA
expression, and that Sox9 siRNA significantly suppressed Sox9 mRNA
expression in HSC-4 cells. Notably, Sox9 siRNA significantly
abrogated the TGF-β1-induced upregulation of N-cadherin mRNA
expression (Fig. 3B). In addition,
we confirmed that Sox9 siRNA suppressed the TGF-β1-induced
upregulation of N-cadherin expression at protein level (Fig. 3C). TGF-β1-induced cell migration was
significantly and incompletely decreased by Sox9 siRNA in HSC-4
cells (Fig. 3D). These results
suggest that N-cadherin promotes the migration activity of HSC-4
cells partially through Sox9-dependent pathway.
TGF-β1 upregulates expression levels
of Sox9 and/or N-cadherin in hOSCC cells other than HSC-4
cells
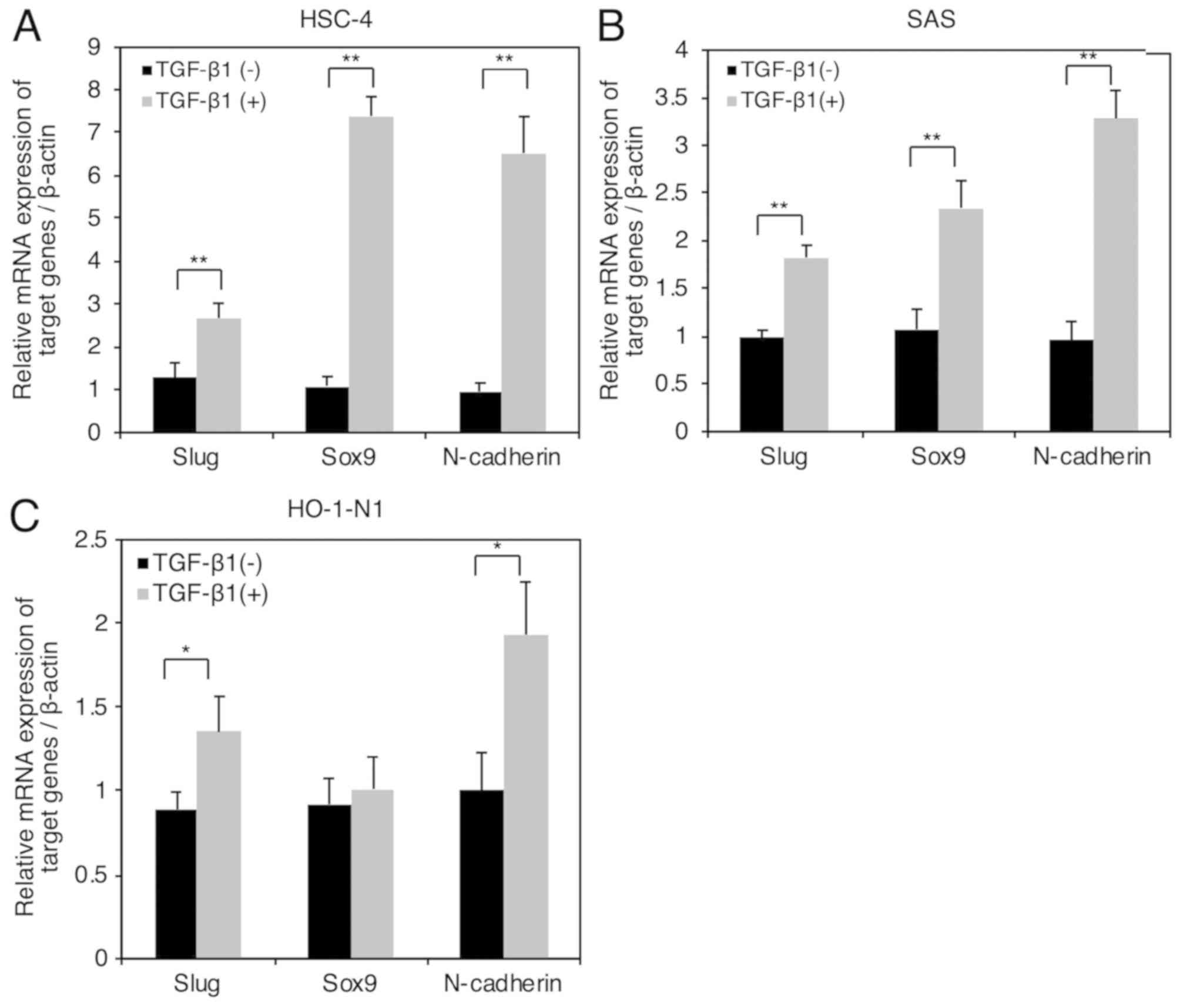
We previously reported that SAS and HO-1-N1 were
TGF-β1-responsive hOSCC cells: TGF-β1 upregulated expression of
fibronectin and plasminogen activator inhibitor-1 (16). Here, we examined whether TGF-β1
upregulated Slug, Sox9, and N-cadherin in SAS cells and HO-1-N1
cells as in HSC-4 cells. We found that TGF-β1 (10 ng/ml)
significantly upregulated the mRNA expressions of Slug, and
N-cadherin at 24 h following stimulation with TGF-β1 (Fig. 4A, B and C). In contrast, TGF-β1 (10
ng/ml) significantly upregulated the mRNA expression of Sox9 in
both HSC-4 cells and SAS cells, but not in HO-1-N1 cells (Fig. 4A, B and C), suggesting that the
TGF-β1-induced upregulation of N-cadherin expression is not always
dependent on the upregulation of Sox9 expression in hOSCC
cells.
TGF-β1 promotes phosphorylation and
nuclear translocation of Sox9 in HSC-4 cells
The transcription factor Sox9 is known to
translocate from cytoplasm into the nucleus in response to TGF-β1
stimulation in interstitial cells (28). In addition, cyclic AMP-dependent
protein kinase (PKA)-mediated phosphorylation of Sox9 plays an
important role in its transcriptional activity (29). We confirmed that TGF-β1 (10 ng/ml)
induced nuclear translocation of Slug, as previously reported
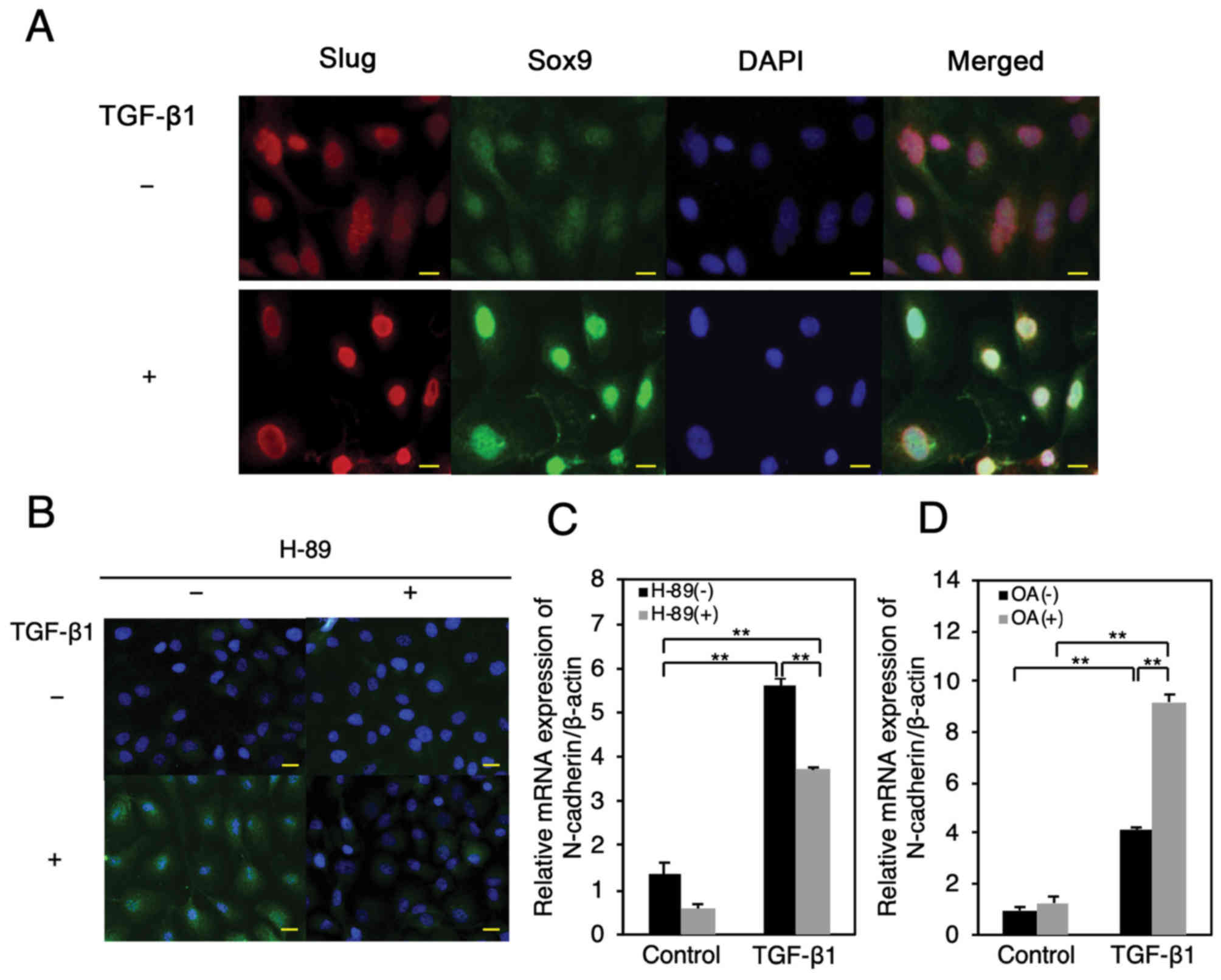
(30) (Fig. 5A). Similarly, we found that TGF-β1
(10 ng/ml) promoted nuclear translocation of total Sox9 and
Ser-181-phosphorylated, which is known to activate the
transcriptional activity of Sox9-target genes in chondrocytes
(31) (Fig. 5A and B). Furthermore, we found that
TGF-β1 stimulation resulted in an increase in total Sox9 and pSox9
levels (Fig. 5A and B).
Interestingly, the pretreatment of HSC-4 cells with the PKA
inhibitor, H-89 (15 µM), before TGF-β1 stimulation inhibited the
TGF-β1-induced phosphorylation and nuclear translocation of pSox9
(Fig. 5B). Further, we observed that
the PKA inhibitor significantly abrogated the TGF-β1 (10
ng/ml)-induced upregulation of N-cadherin mRNA expression (Fig. 5C). It was previously reported that
protein phosphatase A2 (PP2A) negatively regulates PKA activity
(32,33). We confirmed that the PP2A inhibitor
okadaic acid (50 nM) clearly and significantly enhanced TGF-β1 (10
ng/ml)-induced upregulation of N-cadherin mRNA expression (Fig. 5D). These results suggest that the
TGF-β1 promoted phosphorylation and nuclear translocation of Sox9
occurs in a PKA-dependent manner, possibly resulting in the
upregulation of N-cadherin expression in HSC-4 cells.
Discussion
We demonstrate that TGF-β1 increased N-cadherin
expression, and migratory activity in HSC-4 cells through
upregulation of Sox9 expression, and promotion of Sox9 nuclear
translocation. Interestingly, Zhang et al reported that
TGF-β, secreted from tumor-associated macrophages, induces EMT in
non-small lung cancer through activation of Sox9-mediated signals
(34). In contrast, Wnt and/or Hippo
pathways are known to play important roles in TGF-β1-induced
expression of Sox9 (20,35). In addition, Dyer et al
reported that BMP-2-induced Smad1/5/8-mediated signal increased
Sox9 protein levels in the atrioventricular cushions during EMT
(36). However, we confirmed that
BMP-2 (10 ng/ml) did not increase Sox9 mRNA levels in HSC-4 cells
(data not shown).
We previously reported that Slug is an EMT-related
transcription factor that upregulates expression of vimentin,
Wnt-5B, and MMP-10 (16,17). Similarly, in this study, transfection
of HSC-4 cells with Slug siRNA demonstrated that Slug promotes gene
expressions of fibronectin and thrombospondin-1. Notably, the
expression levels of thrombospondin-1 were found to be
significantly downregulated by siSlug in the absence of TGF-β1
stimulation. Collectively, these findings suggest two
possibilities; that Slug mediated the fundamental machinery of
transcription of fibronectin and thrombospondin-1 genes, or that
HSC-4 cells autonomously secreted TGF-β1.
On the contrary, we found that TGF-β1-induced
expression of mesenchymal marker, Laminin α3, was not abrogated by
Slug siRNA, indicating that Slug does not participate in the
TGF-β1-induced expression of Laminin α3. However, RT-qPCR analysis
revealed that the TGF-β1-induced expression of Laminin α3 was
significantly downregulated by Sox9 siRNA (data not shown),
suggesting that TGF-β1-induced expression of Laminin α3 was
mediated by Sox9 and not by Slug. Interestingly, a cooperative
interplay of Slug and Sox9 in EMT was observed in early neural
crest development (22) and in
mammary stem cells (19). Moreover,
Slug and Sox9 were found to cooperatively and positively regulate
the expressions of tenascin-C and periostin, which are
tumor-initiating niche factors in breast cancer cells (37). Slug also regulates Sox9 stability in
lung carcinoma cells (38). Whether
the signal crosstalk between Slug- and Sox9-mediated signals played
an important role in the TGF-β1-induced EMT in hOSCC cells remains
under investigation.
The phosphorylation sites of Sox9 have been reported
as serine (S) residues 64 and 181 (29,31).
Particularly, the phosphorylation of S181 played a crucial role in
the nuclear translocation of Sox9 (31). We observed that Sox9 gets
translocated into nuclei in response to TGF-β1-stimulation. In
addition, we demonstrated that the nuclear-translocated Sox9 is
phosphorylated at S181 by TGF-β1-stimulation. It was reported that
Sox9 is phosphorylated by cyclic AMP-dependent protein kinase A
(PKA), resulting in enhancement of transcriptional activity of Sox9
(29). This led us to examine
whether PKA was involved in the TGF-β1-induced upregulation of
N-cadherin expression. The results of our study showed that the PKA
inhibitor, H-89, partially, but significantly suppressed the
TGF-β1-induced upregulation of N-cadherin expression, suggesting
that TGF-β1-induced upregulation of N-cadherin expression was only
partly mediated by a PKA-dependent signal. In addition, these
results further implicated that the TGF-β1-induced phosphorylation
of Sox9 (S181) could be possibly mediated by PKA. In contrast, it
was demonstrated that TGF-β1-stimulated Smad3/4 directly activated
PKA through an interaction between Smad4 and a regulatory subunit
of PKA (39,40). In addition, Chowdhury et al
also reported TGF-β activated PKA in colon cancer cells (33). Corroborating these findings, we
previously showed that TGF-β1 induced activation of Smad2/3 in
HSC-4 cells (16), suggesting the
possible involvement of Smad2/3 in activation of PKA in
TGF-β1-stimulated HSC-4 cells.
In summary, we have demonstrated that TGF-β1 induces
N-cadherin expression through upregulated expression and promotion
of nuclear translocation of Sox9, thus resulting in the progression
of EMT in hOSCC cells.
Acknowledgements
The authors would like to thank Dr Takahiro Chiba
(Division of Oral and Maxillofacial Surgery, Department of
Reconstructive Oral and Maxillofacial Surgery, Iwate Medical
University School of Dentistry) for assistance with the cell
cultures of hOSCC cell lines and RT-qPCR analysis.
Funding
The present study was supported in part by JSPS
KAKENHI (grant nos. JP18K17237, JP16H05534 and JP17K11851) from The
Ministry of Education, Culture, Sports, Science and Technology of
Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH, DS and MK performed western blotting and reverse
transcription-quantitative PCR analyses, fluorescence
immunostaining and cell migration assays. TH, HY, AI and MK
designed the present study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BMP
|
bone morphogenetic protein
|
|
EMT
|
epithelial-mesenchymal transition
|
|
hOSCC
|
human oral squamous cell carcinoma
|
|
TGF-β
|
transforming growth factor-β
|
|
RT-qPCR
|
reverse transcription-qPCR
|
|
TβR-I
|
TGF-β receptor type I
|
|
TβR-II
|
TGF-β receptor type II
|
References
|
1
|
Principe DR, Doll JA, Bauer J, Jung B,
Munshi HG, Bartholin L, Pasche B, Lee C and Grippo PJ: TGF-β:
Duality of function between tumor prevention and carcinogenesis. J
Natl Cancer Inst. 106:djt3692014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mu Y, Gudey SK and Landström M: Non-Smad
signaling pathways. Cell Tissue Res. 347:11–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyazawa K, Shinozaki M, Hara T, Furuya T
and Miyazono K: Two major Smad pathways in TGF-beta superfamily
signalling. Genes Cells. 7:1191–1204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Y, Di C, Zhang X, Wang J, Wang F, Yan
JF, Xu C, Zhang J, Zhang Q, Li H, et al: Transforming growth factor
β signaling pathway: A promising therapeutic target for cancer. J
Cell Physiol. 235:1903–1914. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lambert R, Sauvaget C, de Camargo Cancela
M and Sankaranarayanan R: Epidemiology of cancer from the oral
cavity and oropharynx. Eur J Gastroenterol Hepatol. 23:633–641.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Graves CA, Abboodi FF, Tomar S, Wells J
and Pirisi L: The translational significance of
epithelial-mesenchymal transition in head and neck cancer. Clin
Transl Med. 3:602014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YL, Ju HZ, Liu SF, Lee TC, Shih YW,
Chuang LY, Guh JY, Yang YY, Liao TN, Hung TJ and Hung MY: BMP-2
suppresses renal interstitial fibrosis by regulating
epithelial-mesenchymal transition. J Cell Biochem. 112:2558–2565.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith A, Teknos TN and Pan Q: Epithelial
to mesenchymal transition in head and neck squamous cell carcinoma.
Oral Oncol. 49:287–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weber CE, Li NY, Wai PY and Kuo PC:
Epithelial-mesenchymal transition, TGF-β, and osteopontin in wound
healing and tissue remodeling after injury. J Burn Care Res.
33:311–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He J, Xu Y, Koya D and Kanasaki K: Role of
the endothelial-to-mesenchymal transition in renal fibrosis of
chronic kidney disease. Clin Exp Nephrol. 17:488–497. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCormack N and O'Dea S: Regulation of
epithelial to mesenchymal transition by bone morphogenetic
proteins. Cell Signal. 25:2856–2862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121:727–735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saito D, Kyakumoto S, Chosa N, Ibi M,
Takahashi N, Okubo N, Sawada S, Ishisaki A and Kamo M: Transforming
growth factor-β1 induces epithelial-mesenchymal transition and
integrin α3β1-mediated cell migration of HSC-4 human squamous cell
carcinoma cells through Slug. J Biochem. 153:303–315. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hino M, Kamo M, Saito D, Kyakumoto S,
Shibata T, Mizuki H and Ishisaki A: Transforming growth factor-β1
induces invasion ability of HSC-4 human oral squamous cell
carcinoma cells through the Slug/Wnt-5b/MMP-10 signalling axis. J
Biochem. 159:631–640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bi W, Deng JM, Zhang Z, Behringer RR and
de Crombrugghe B: Sox9 is required for cartilage formation. Nat
Genet. 22:85–89. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo W, Keckesova Z, Donaher JL, Shibue T,
Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zürrer-Härdi U, Bell
G, et al: Slug and Sox9 cooperatively determine the mammary stem
cell state. Cell. 148:1015–1028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma F, Ye H, He HH, Gerrin SJ, Chen S,
Tanenbaum BA, Cai C, Sowalsky AG, He L, Wang H, et al: SOX9 drives
WNT pathway activation in prostate cancer. J Clin Invest.
126:1745–1758. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Panda DK, Miao D, Lefebvre V, Hendy GN and
Goltzman D: The transcription factor SOX9 regulates cell cycle and
differentiation genes in chondrocytic CFK2 cells. J Biol Chem.
276:41229–41236. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakai D, Suzuki T, Osumi N and Wakamatsu
Y: Cooperative action of Sox9, Snail2 and PKA signaling in early
neural crest development. Development. 133:1323–1333. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He D, Lu Y, Hu H, Zhang J, Qin B, Wang Y,
Xing S, Xi Q and Wang S: The Wnt11 signaling pathway in potential
cellular EMT and osteochondral differentiation progression in
nephrolithiasis formation. Int J Mol Sci. 16:16313–16329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Capaccione KM, Hong X, Morgan KM, Liu W,
Bishop JM, Liu L, Markert E, Deen M, Minerowicz C, Bertino JR, et
al: Sox9 mediates Notch1-induced mesenchymal features in lung
adenocarcinoma. Oncotarget. 5:3636–3650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li T, Huang H, Shi G, Zhao L, Li T, Zhang
Z, Liu R, Hu Y, Liu H, Yu J and Li G: TGF-β1-SOX9 axis-inducible
COL10A1 promotes invasion and metastasis in gastric cancer via
epithelial-to-mesenchymal transition. Cell Death Dis. 9:8492018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang J and Guo L: Knockdown of SOX9
inhibits the proliferation, invasion, and EMT in thyroid cancer
cells. Oncol Res. 25:167–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huk DJ, Austin BF, Horne TE, Hinton RB,
Ray WC, Heistad DD and Lincoln J: Valve endothelial cell-derived
Tgfβ1 signaling promotes nuclear localization of Sox9 in
interstitial cells associated with attenuated calcification.
Arterioscler Thromb Vasc Biol. 36:328–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang W, Zhou X, Lefebvre V and de
Crombrugghe B: Phosphorylation of SOX9 by cyclic AMP-dependent
protein kinase A enhances SOX9's ability to transactivate a Col2a1
chondrocyte-specific enhancer. Mol Cell Biol. 20:4149–4158. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Serrano I, McDonald PC, Lock FE and Dedhar
S: Role of the integrin-linked kinase (ILK)/Rictor complex in
TGFβ-1-induced epithelial-mesenchymal transition (EMT). Oncogene.
32:50–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haudenschild DR, Chen J, Pang N, Lotz MK
and D'Lima DD: Rho kinase-dependent activation of SOX9 in
chondrocytes. Arthritis Rheum. 62:191–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hong K, Lou L, Gupta S, Ribeiro-Neto F and
Altschuler DL: A novel Epac-Rap-PP2A signaling module controls
cAMP-dependent Akt regulation. J Biol Chem. 283:23129–23138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chowdhury S, Howell GM, Rajput A, Teggart
CA, Brattain LE, Weber HR, Chowdhury A and Brattain MG:
Identification of a novel TGFβ/PKA signaling transduceome in
mediating control of cell survival and metastasis in colon cancer.
PLoS One. 6:e193352011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Che D, Yang F, Chi C, Meng H,
Shen J, Qi L, Liu F, Lv L, Li Y, et al: Tumor-associated
macrophages promote tumor metastasis via the TGF-β/SOX9 axis in
non-small cell lung cancer. Oncotarget. 8:99801–99815.
2017.PubMed/NCBI
|
|
35
|
Zhou H, Li G, Huang S, Feng Y and Zhou A:
SOX9 promotes epithelial-mesenchymal transition via the Hippo-YAP
signaling pathway in gastric carcinoma cells. Oncol Lett.
18:599–608. 2019.PubMed/NCBI
|
|
36
|
Dyer L, Lockyer P, Wu Y, Saha A, Cyr C,
Moser M, Pi X and Patterson C: BMPER promotes
epithelial-mesenchymal transition in the developing cardiac
cushions. PLoS One. 10:e01392092015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fazilaty H, Gardaneh M, Akbari P, Zekri A
and Behnam B: SLUG and SOX9 cooperatively regulate tumor initiating
niche factors in breast cancer. Cancer Microenviron. 9:71–74. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luanpitpong S, Li J, Manke A, Brundage K,
Ellis E, McLaughlin SL, Angsutararux P, Chanthra N, Voronkova M,
Chen YC, et al: SLUG is required for SOX9 stabilization and
functions to promote cancer stem cells and metastasis in human lung
carcinoma. Oncogene. 35:2824–2833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang L, Duan CJ, Binkley C, Li G, Uhler
MD, Logsdon CD and Simeone DM: A transforming growth factor
beta-induced Smad3/Smad4 complex directly activates protein kinase
A. Mol Cell Biol. 24:2169–2180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang H, Li G, Wu JJ, Wang L, Uhler M and
Simeone DM: Protein kinase A modulates transforming growth factor-β
signaling through a direct interaction with Smad4 protein. J Biol
Chem. 288:8737–8749. 2013. View Article : Google Scholar : PubMed/NCBI
|