Introduction
Radiotherapy serves an increasingly important role
in the multimodality treatment of esophageal cancer (1,2).
However, esophageal cancer presents difficulties for precision
radiotherapy, such as uncertainties with target delineation and
intra-/interfractional tumor position variations (2). One important factor is that the true
extent of a tumor is difficult to visualize using computed
tomographic (CT) images, which is crucial for the planning of
radiotherapy despite the assistance of other current medical
examinations, such as esophageal barium meal, MRI(Magnetic
Resonance Imaging) and PET-CT(Positron Emission Tomography-Computed
Tomography) (3). Endoscopy is used
as the standard for determining mucosal tumor extensions in the
craniocaudal direction and the endoscopic ultrasonography
(EUS)-guided placement of fiducial markers is a novel method prior
to targeted radiation therapy, wherein fiducial markers are
implanted into the target lesion margin to target and track the
location of the tumor in real time. This may provide higher
delineation accuracy and the possibility of daily image-guided
treatment set-up verification for image-guided radiotherapy (IGRT)
(4,5). Previous studies have focused on the use
of metal clips, including silver, gold and titanics; however, a
high rate of hemorrhage and perforation, risk of chyme rub,
esophageal peristalsis and a short residence time limit further
application of such solid markers in clinical use (2,5–9). Liquid radiopaque materials have several
advantages over metal clips for IGRT, including strong attachment
to the subcutaneous tissue, controllable density, smaller and
controllable size and that these materials can be metabolized
(10–12). The use of lipiodol (Lip) has been
studied in bladder cancer and lung cancer, wherein Lip is readily
identifiable using radiographic and CT imaging without major
adverse side effects. However, the disadvantages of using lipiodol
alone include extravasations and the difficulty in producing a
consistent marker size (13).
The combination of N-butyl cyanoacrylate (NBCA) and
Lip as an embolization treatment has been widely used (14). The use of NBCA as a fiducial marker
in IGRT has the potential to overcome the aforementioned
shortcomings of IGRT. Dilution of NBCA using lipiodol for
intravascular embolization treatment has been shown to affect the
duration of vascular occlusion, radiopacity and the polymerization
speed of the fiducial marker, which could lead to the catheter
becoming sticky or delays embolization and activates cell
infiltration (14,15). However, the appropriate mixing ratio
and injection dosage of NBCA/Lip as fiducial markers is unknown and
the safety and feasibility of this combination remains unclear,
highlighting the need for further research prior to its use in
clinical practice.
In the present study, in order to identify the most
appropriate application of NBCA/Lip as fiducial markers, four
different mixing ratios and injection volumes were injected into
subcutaneous mouse tissue, followed by quantitative macroscopic and
histopathological comparisons to determine the safety of these
combinations. Finally, the most appropriate combination was
selected and the biocompatibility was further assessed based on the
International Organization for Standardization (ISO) (16–18).
Materials and methods
Reagents and animals
The sterile ready-to-inject NBCA liquid (Histoacryl;
B. Braun Melsungen AG) was diluted using lipiodol, using pipettes
and 22-gauge needles. A total of 224 BALB/c 6-week-old male mice
(Hua Fukang Bioscience, Ltd.), weighing 16–22 g; 30 3-month-old
male Hartley Albino guinea pigs (Hua Fukang Bioscience, Ltd.),
weighing 300–400 g; and two two-month-old male New Zealand white
rabbits (Hua Fukang Bioscience, Ltd.), weighing 2–2.5 kg were
allowed free access to food and water throughout the experiments.
The housing conditions were as follows: 24±2°C, 50±10% relative
humidity, and 12-h light/dark cycle. The acclimatization period was
5 days prior to the experiments. The present study was approved by
The Scientific Review and Ethics Committee of Shandong Cancer
Hospital and Institute (Jinan, China). The animals were shaved and
the skin around the implantation sites was disinfected with 3%
iodine and 75% alcohol prior to injection (19). Injection of the mixture was performed
according to previously described procedures, with some
modifications; increased injection depth in case of skin ischemia
and injections were slow but withdrawn quickly to cause a
hemispherical hump (4,20–23). The
mice were anesthetized using a 80 mg/kg ketamine hydrochloride and
5 mg/kg xylazine hydrochloride intraperitoneal injection. The
euthanasia of guinea pigs was conducted by intraperitoneal
injection with sodium pentobarbital, 150 mg/kg. The rabbits were
euthanized with intravenous administered overdose of sodium
pentobarbital (100 mg/kg). Total anesthetic depth was defined as
loss of response to the irritation of toes, cornea and skin and the
loss of righting reflex. The experimental dilutions were injected
into the subcutaneous tissues of dorsal skin between the upper
limbs forming a round bulge at a 45° angle using 1 ml syringes. The
mice were euthanized when the humane endpoints were observed or
markers were no longer detected by CT imaging or palpation, using
an overdose of ketamine (240 mg/kg) and xylazine (20 mg/kg)
injected into the lateral tail vein. Death confirmation depended on
a combination of criteria, including lack of pulse, breathing,
corneal reflex and response to firm toe pinch, inability to hear
respiratory and touch of heartbeat for more than 30 sec with a
stethoscope (24). Animals with any
observable acute signs of pain and distress, such as increased
scratching, poorer coat condition, weight loss, and a growing
abdomen, which indicate a continuing and possibly irreversible
deterioration in an animal's condition should be removed from the
procedure and receive the appropriate treatment.
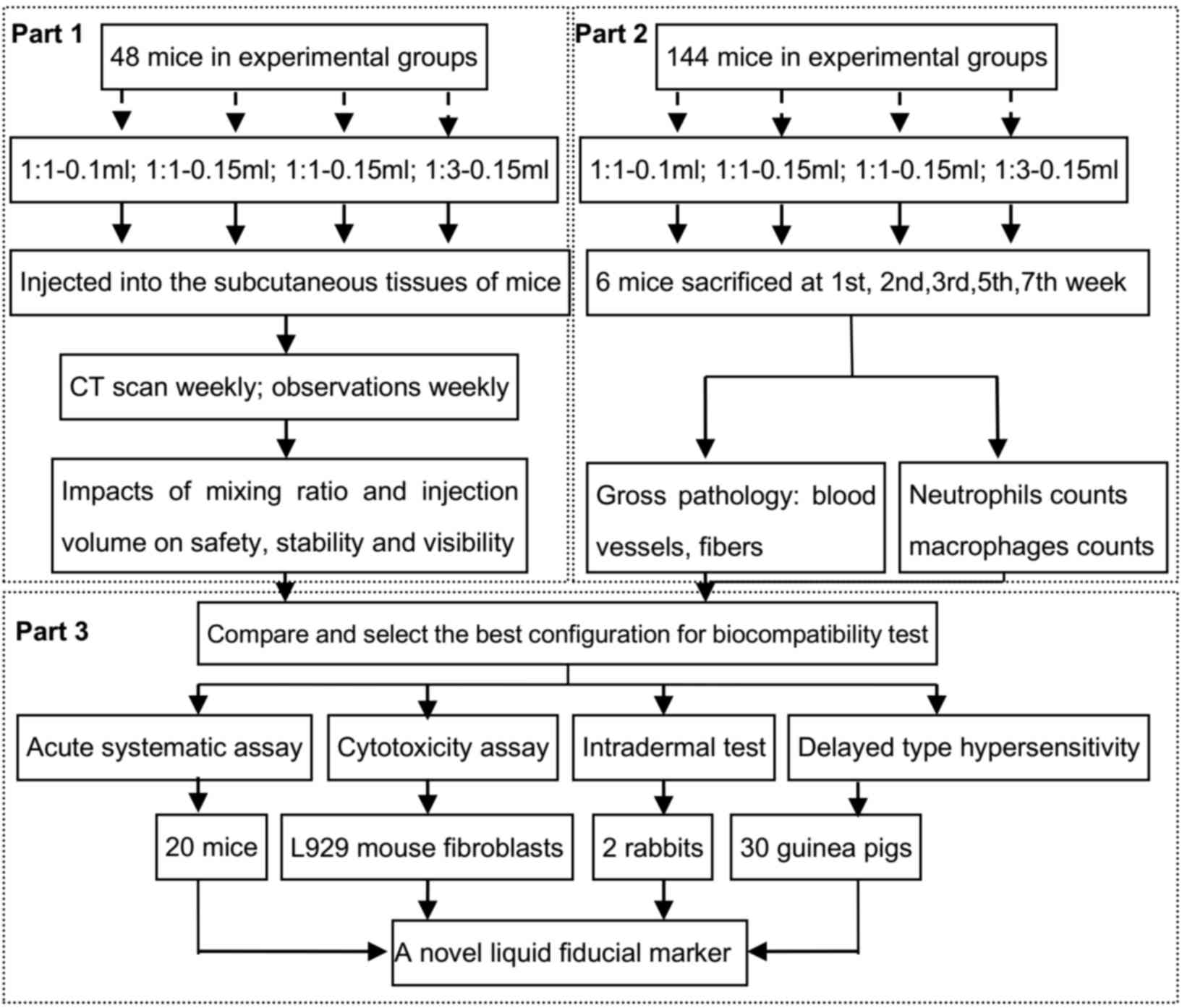
Experimental design
The study was designed in 3 parts: Macroscopic
evaluation (part 1), microscopic evaluation (part 2) and
biocompatibility evaluation (part 3) (Fig. 1). The macroscopic evaluations
included analysis of systematic and local toxicity of materials and
operation. The skin status and individual weight were routinely
monitored weekly at certain points over a period of 7 weeks. Part 2
was conducted to examine the histopathological changes using
hematoxylin and eosin (H&E) staining and immunohistochemical
analysis. All the measurements (parts 1: systemic side reactions,
skin status scores, body weights, permanence of marker, quality of
imaging; part 2: histopathological changes, inflammatory cells)
were calculated and compared to determine the optimal NBCA/Lip
combination. Then the biocompatibility of the chosen combination
was investigated in part 3 using L929 mouse fibroblasts (Kunming
Cell Bank of Type Culture Collection).
Macroscopic evaluations
There were four treatment groups with two mixing
ratios (1:1 and 1:3) and two injection volumes (0.1 and 0.15 ml) as
follows: 1:1–0.1, 1:1–0.15, 1:3–0.1 and 1:3–0.15 ml. The ratios and
dilutions used in the present study were based on pre-experiments,
clinical experience and literature review (14,15,25–27).
Sixty male Balb/c mice were equally and randomly divided into four
treatment groups and a control group. The four treatment groups
received different dilutions of NBCA/Lip and the control group
received the same total volume (0.15 ml) of saline solution to
allow comparisons of weight increase with the treatment groups.
Sample size required in previous studies was usually 6–12 mice for
four groups with the two-sided significant level of 0.05 and a
power of 90%, however the present study was a type of multiple
factorial exploration and a high power and effect size was needed
for the credibility of the study (28–32). In
addition, in the preliminary experiment, two mice died, which may
have been caused by the blood vessel injections (14), therefore, the heterogeneity of
variance could be decreased by 12 mice/group. Baseline data were
acquired immediately following implantation and the skin scores,
body weights and CT image scans were examined weekly over a period
of 7 weeks. Follow-up CT scans were conducted monthly until 6
months or when the mixtures could no longer be detected by CT
imaging or palpation. The initial observations continued from the
time immediately following implantation until the animals
completely recovered from the anesthesia and every hour during the
subsequent 12-h period. Observable side effects included lethargy,
tremors and spasms, a staggering gait, twitches, a scruffy coat and
death. The skin scoring system includes the sum of erythema scores
(0, no reaction; 1, very mild; 2, mild with clear erythema or
minimal ulceration; 3, medium erythema or mild ulceration; 4,
severe reaction and eschar formation) and swelling scores (0, no
reaction; 1, minimal swelling; 2, mild with clear edema no more
than the local margin; 3, medium; 4, severe with 1 cm above the
skin and more than the injection sites). The higher the score, the
worse the skin status was (16).
Permanence of imaging
All injected markers were evaluated for remaining
volume using a MicroCAT II system (Siemens Healthineers) and this
was contoured using a Hounsfield Unit (HU) thresholding function
with a lower limit of 300 HU for defining the contour of the marker
and no upper limit was defined. Subsequent analysis was performed
using Imaging Biomarker Explorer Software version V1.0 β (MD
Anderson Cancer Center).
Microscopic examinations
In Part 2, 144 mice were randomly divided into 4
treatment groups (n=36) and 6 randomly selected mice were
sacrificed from each group for histopathological examinations
conducted at weeks 1, 2, 3, 5 and 7 post-injection and the
remaining mice were used for follow-up observation.
Histopathological analysis
The mice were directly fixed on the operating table
after death and a necropsy was performed within 2 min after
euthanasia. After hair removal, the mixture, together with covered
skin and subcutaneous tissue, was separated from the back of the
mice with a scalpel and scissors, and the skin tissue containing
mixtures was fixed with 10% formalin at 4°C for 24 h and embedded
in paraffin. The slices with 4-um thickness were subjected to
H&E staining, which included the interface between mixtures and
the tissue, and were evaluated by a pathologist blinded to the
group conditions, treatment and sampling time of the specimens
under a light microscope at ×200 magnification.
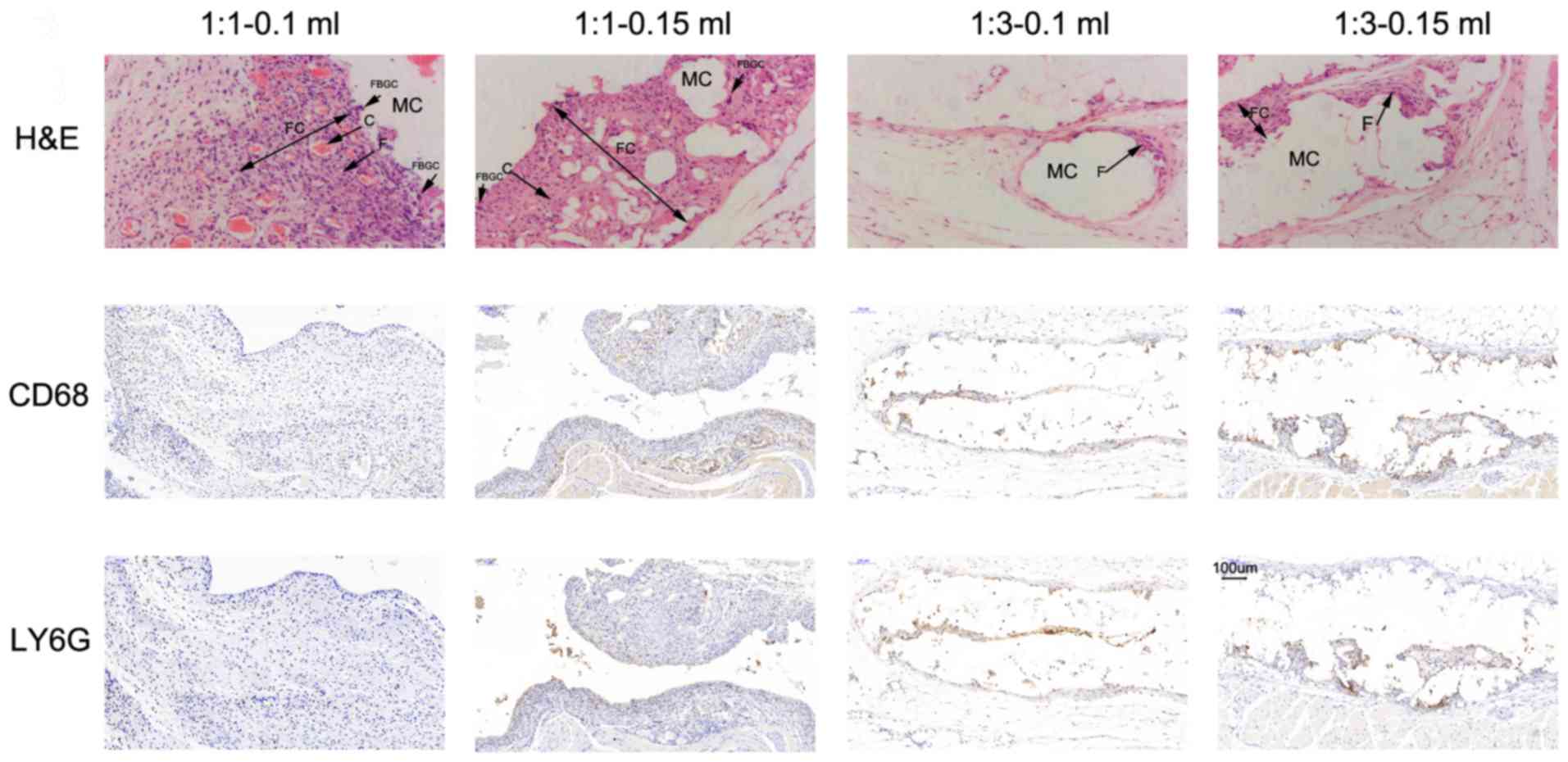
Immunohistochemistry
The presence of inflammatory cells for quantitative
comparisons of reactions to the NBCA/Lip mixture were assessed
using immunohistochemistry. Following deparaffinization in xylene
(Servicebio Technology Co., Ltd.), the sections were heated (98°C)
for antigen retrieval by microwaving for 10 min in citrate buffer
(pH 6.0). The primary antibodies, LY6G and CD68 (cat. no. GB11229;
Servicebio Technology Co., Ltd.) diluted at 1:800, were added to
detect neutrophils and macrophages, respectively. The corresponding
secondary antibody, HRP goat anti-rabbit IgG (cat. no. G23303;
Servicebio Technology Co., Ltd.) diluted at 1:200, was added and
3,3-diaminobenzidine (DAB) was added as a chromogen. Hematoxylin
(Servicebio Technology Co., Ltd.) was used as the counterstain at
15°C for 3 minutes. The sections were then dehydrated in graded
(50-100%) alcohols, followed by xylene for 5 min each,
cover-slipped and visualized under a light microscope. Positive
staining was indicated by a brown color. A 5×5 mm-square grid was
inserted into one of the ocular sections of a microscope. The
images were captured under the microscope at a magnification of
×200 and were analyzed using Image Pro Plus version 6.0 software
(Media Cybernetics Inc.). At least 5 squares were positioned for
each section.
Biocompatibility tests
The combination of 1:1–0.15 ml NBCA/Lip, which was
chosen based on the comparison of the safety and effectiveness of
different concentrations and proportions of the mixture in the
first part and the second part, was subjected to biocompatibility
evaluations, including four tests according to the ISO criteria.
The conditioned media was extracted using 20 ml 9% sodium chloride
(SC) and 20 ml cottonseed oil (CSO), respectively. An acute
systematic assay was evaluated using 20 mice by intraperitoneal
injection of 1:1–0.15 ml NBCA/Lip to examine the acute systematic
toxicity according to ISO 10993.11 (17). The cytotoxic evaluation of the
mixture was determined by performing an MTT assay using L929 mouse
fibroblast according to ISO 10993.5 (18). L929 cells were cultured for 24 h at
37°C in 96-well culture plates at an initial cell count of 10,000
cells/well and were subsequently exposed to the conditioned media
(as aforementioned). Extraction solution from polyethylene was used
as the negative control and the positive control was 10% DMSO. The
optical density of the supernatant was measured using a microplate
reader (CrymeiBio, Ltd.) at 570 nm. The delayed type
hypersensitivity evaluation was ranked according to the Magnusson
and Kligman scoring system in 30 Hartley Albino guinea pigs (Hua
Fukang Bioscience, Ltd.), according to ISO 10993.10 (16). An intradermal test was administered
with the samples injected in every 20 dorsal sites of two New
Zealand white rabbits (Hua Fukang Bioscience, Ltd.), including 5
sites each for the SC treatment group, SC control group, CSO
treatment group and the CSO control group. Skin scores were
observed at 24, 48 and 72 h post implantation, according to ISO
10993.10 (16).
Statistical analysis
All statistical analyses were performed using SPSS
version 22.0 (IBM Corp) and the data are expressed as mean ±
standard error of the mean (unless otherwise shown). The two-way
and three-way mixed ANOVAs were performed to examine the effects of
factors of mixing ratio, injection volume and time and the
differences between the four settings. The variables of mixing
ratio and injection volume were treated as between-subject factors.
Tests of within-subjects post-hoc simple effects were performed
using Bonferroni's correction. P<0.05 was considered to indicate
a statistically significant difference.
Results
Macroscopic evaluations
Within-subject effects and between-subject effects
are listed in Tables SI and
SII. The results of simple effects
and the main effect between mixing ratio and injection volume are
listed in Table SIII and those
involving the factor of time are listed in Table SIV.
No limitation of movement or change in behavior was
observed. Two mice may have died of accidental intravenous
injection of mixtures in the preliminary experiment after autopsy.
Based on this knowledge, in the experiment, no death occurred and
none of the mice had a severe infection or operation-related
complications in the immediate and follow-up observations.
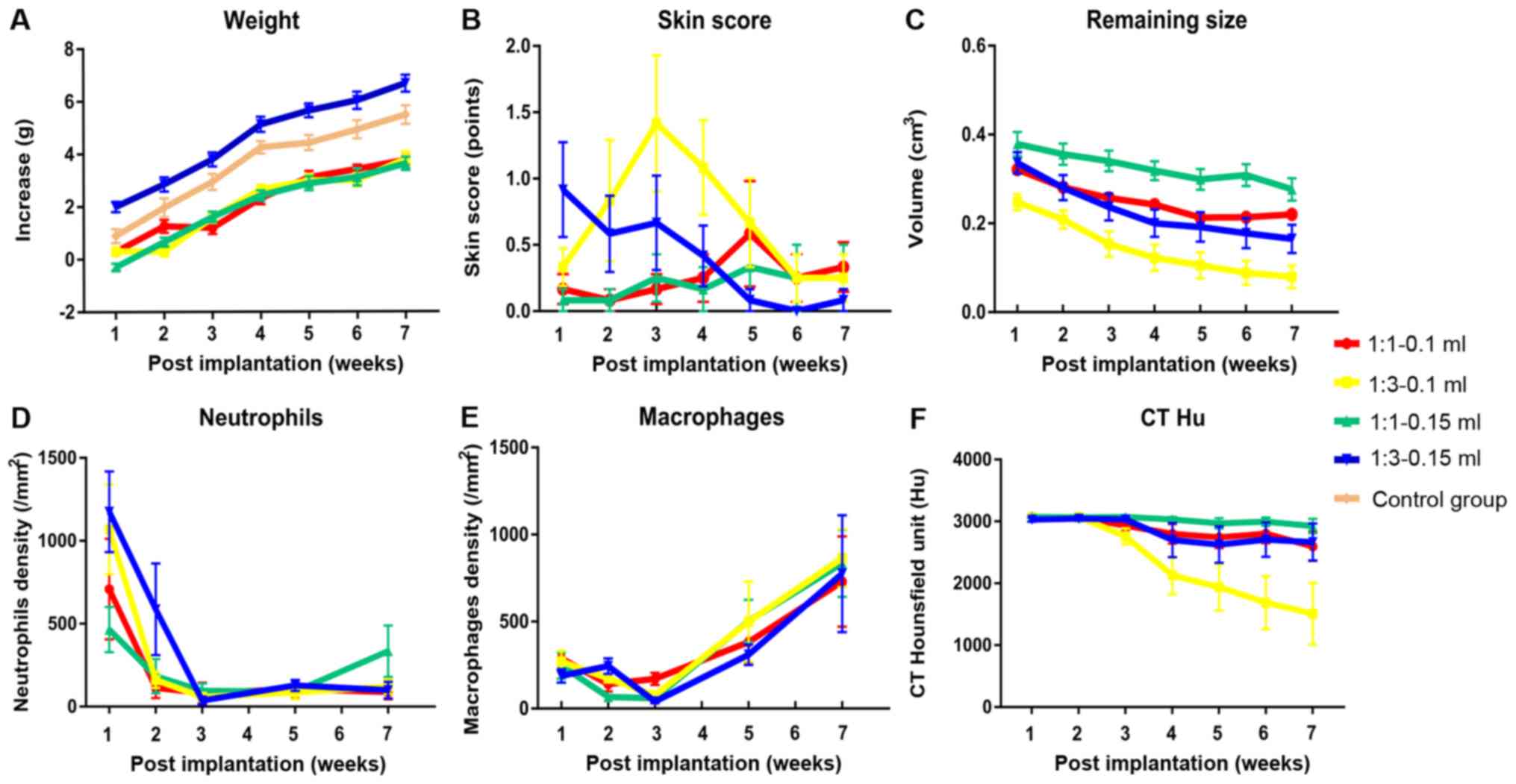
Weight changes of mice in part 1
Statistical analysis revealed that when the mixing
ratio was 1:1, the injection volume had no significant effect on
the weight; however, when the ratio was 1:3, the injection volume
of 0.1 ml induced a significant reduction in weight of 1.976 g
compared with the volume of 0.15 ml (P<0.001; Table SIII). Further analyses also revealed
that when the injection volume was 0.1 ml, the mixing ratio had no
significant effect; however, the mixing ratio of 1:1 was associated
with a significant 2.084 g weight loss compared with the mixing
ratio of 1:3 at the injection volume of 0.15 ml (P<0.001;
Table SIII). At each time point,
the injection volume of 0.1 ml significantly decreased weight
compared with the volume of 0.15 ml (P<0.001), excluding the 2nd
week (P=0.104) (Table SIV). In
multiple comparisons, apart from the 1:3–0.15 ml group which was
significantly larger compared with the other three settings, the
average weight of the other three settings was significantly lower
compared with the control group (1:1–0.1 ml, 22.09±0.19 g; 1:1–0.15
ml, 22.00±0.21 g; 1:3–0.1 ml, 22.10±0.21 g; 1:3–0.15 ml, 24.17±0.26
g; control group, 23.37±0.27 g; Table
I and Fig. 2A).
 | Table I.Changes in mice measured over time.
All data are presented as the means ± standard deviation. |
Table I.
Changes in mice measured over time.
All data are presented as the means ± standard deviation.
| A, weight, g |
|---|
|
|---|
|
|
| Post-implantation
week |
|
|---|
|
|
|
|
|
|---|
| Ratio | Volume, ml | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall mean |
|---|
| 1:1 | 0.1 | 20.36±.81 | 21.31±1.00 | 21.25±1.00 | 22.38±1.13 | 23.16±1.12 | 23.47±.91 | 23.81±1.02 | 22.09±.19 |
| 1:1 | 0.15 | 20.28±.51 | 21.23±.57 | 22.18±.65 | 22.98±.80 | 23.44±.79 | 23.68±.89 | 24.19±.73 | 22.00±.21 |
| 1:3 | 0.1 | 20.98±1.01 | 20.95±1.07 | 22.26±1.30 | 23.33±1.39 | 23.65±1.36 | 23.68±1.22 | 24.46±1.16 | 22.10±.21 |
| 1:3 | 0.15 |
20.30±1.05a–c |
21.58±1.28a–c |
22.09±1.19a–c |
23.42±1.20a–c |
23.95±1.13a–c |
24.33±1.16a–c |
24.97±1.23a–c |
24.17±.26a–c |
| 0 | 0 |
20.49±.95d |
21.58±1.28c |
22.54±1.18a,b |
23.85±1.09a–c |
24.02±1.29b |
24.53±1.51a–c |
25.08±1.48a–c |
23.37±.27a–c |
|
| B, skin
score |
|
|
|
|
Post-implantation week |
|
|
|
|
|
|
| Ratio | Volume,
ml | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall
mean |
|
| 1:1 | 0.1 | 0.17±.39 | 0.08±.29 | 0.17±.39 | 0.25±.62 | 0.58±1.38 | 0.25±.62 | 0.33±.65 | 0.27±.71 |
| 1:1 | 0.15 | 0.08±.21 | 0.08±.28 | 0.25±.62 | 0.17±.58 | 0.33±.31 | 0.25±.87 | 0.25±.87 | 0.70±.20 |
| 1:3 | 0.1 | 0.33±.50 | 0.83±1.56 |
1.42±1.79a,b | 1.08±1.24 | 0.67±1.16 | 0.25±.62 | 0.25±.62 | 0.69±1.19 |
| 1:3 | 0.15 | 0.92±1.24 | 0.58±1.00 | 0.67±1.23 | 0.42±.79 | 0.08±.29 | 0.00±.00 | 0.08±.29 | 0.39±.86 |
|
| C, remaining
size, cm3 |
|
|
|
|
Post-implantation week |
|
|
|
|
|
|
| Ratio | Volume,
ml | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall
mean |
|
| 1:1 | 0.1 | 0.32±.06 | 0.28±.05 | 0.26±.05 | 0.24±.06 | 0.21±.06 | 0.21±.08 | 0.22±.08 | 0.25±.07 |
| 1:1 | 0.15 | 0.37±.09 | 0.36±.08 | 0.34±.08 | 0.32±.07 | 0.30±.08 |
0.31±.08a | 0.28±.09 |
0.33±.09a |
| 1:3 | 0.1 |
0.24±.06b |
0.21±.07b |
0.15±.10a,b |
0.12±.10b,c |
0.11±.10a,b |
0.08±.09a,b |
0.08±.09a,b |
0.14±.10b |
|
| D, CT,
HU |
|
|
|
|
Post-implantation week |
|
|
|
|
|
|
| Ratio | Volume,
ml | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall
mean |
|
| 1:3 | 0.15 | 0.34±.08 | 28±.10 |
0.24±.10b |
0.20±.11c |
0.19±.12b |
0.18±.12b |
0.16±.12b |
0.23±.12c |
| 1:1 | 0.1 | 3,071.00±.00 | 3,060.33±36.96 |
2,854.17±1,029.89 |
2,564.50±946.95 |
2,566.83±939.97 |
2,566.83±939.97 |
2,379.92±1055.00 |
2,715.63±101.60 |
| 1:1 | 0.15 | 3,071.00±.00 | 3,063.83±24.83 | 3,071.00±.00 |
3,030.92±138.85 |
2,925.75±404.22a |
2,994.83±226.28a |
2,925.75±404.22a | 2,313.60±43.03 |
| 1:3 | 0.1 | 3,071.00±.00 | 3,071.00±.00 |
2,757.33±450.279 |
2,128.0±1,057.97b |
1,906.58±1,195.10b |
1,709.0±1,342.24b |
1,552.25±1,425.80b |
3,017.86±157.00b |
| 1:3 | 0.15 |
3,027.75±111.82 | 3,043.42±95.55 |
3,000.33±139.58 |
2,680.17±815.70 |
2,550.08±930.57 |
2,554.50±928.50 |
2,400.58±1,065.80c |
2,750.98±190.50 |
|
| E, macrophages
count |
|
|
|
Post-implantation week |
|
|
|
|
|
|
| Ratio | Volume,
ml | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall
mean |
|
| 1:1 | 0.1 | 288±103 | 141±104 | 171±85 | – | 382±282 | – | 730±636 | 342±361 |
| 1:1 | 0.15 | 271±151 | 173±47 | 77±39 | – | 500±564 | – | 864±408 | 342±380 |
| 1:3 | 0.1 | 247±183 | 67±28 | 59±38 | – | 503±298 | – | 834±471 | 377±405 |
| 1:3 | 0.15 | 188±93 | 244±109 | 41±27 | – | 309±144 | – | 775±823 | 311±426 |
|
| F, neutrophil
count |
|
|
|
Post-implantation week |
|
|
|
|
|
|
| Ratio | Volume,
ml | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall
mean |
|
| 1:1 | 0.1 | 708±743 | 113±148 | 81±154 | – | 107±123 | – | 87±104 | 219±405 |
| 1:1 | 0.15 | 465±334 | 184±252 | 95±108 | – | 90±42 | – | 334±380 | 234±277 |
| 1:3 | 0.1 |
1,069±661b | 161±116 | 59±61 | – | 80±101 | – | 128±109 | 299±478 |
| 1:3 | 0.15 |
1,175±596b | 586±677 | 37±58 | – | 128±79 | – | 52±45 | 405±571 |
Skin status score of mice in part
1
For skin status, the effect of different ratios on
skin score was dependent on time. It was observed that before the
4th week, the ratio of 1:1 was significantly associated with a more
favorable skin status and after that the ratio of 1:1 was
associated with a worse skin status, although this difference was
not significant (Table SIV).
Injection volume had no significant effect on skin status (Table SIII). The overall worst skin score
was observed in the 1:1–0.15 ml group and the best skin score was
associated with the 1:1–0.1 ml group; however, multiple comparisons
revealed that this difference was not significant (1:1–0.1 ml,
0.27.71; 1:1–0.15 ml, 0.70±.20; 1:3–0.1 ml, 0.69±1.19; 1:3–0.15 ml,
0.39±.86; Table I and Fig. 2B).
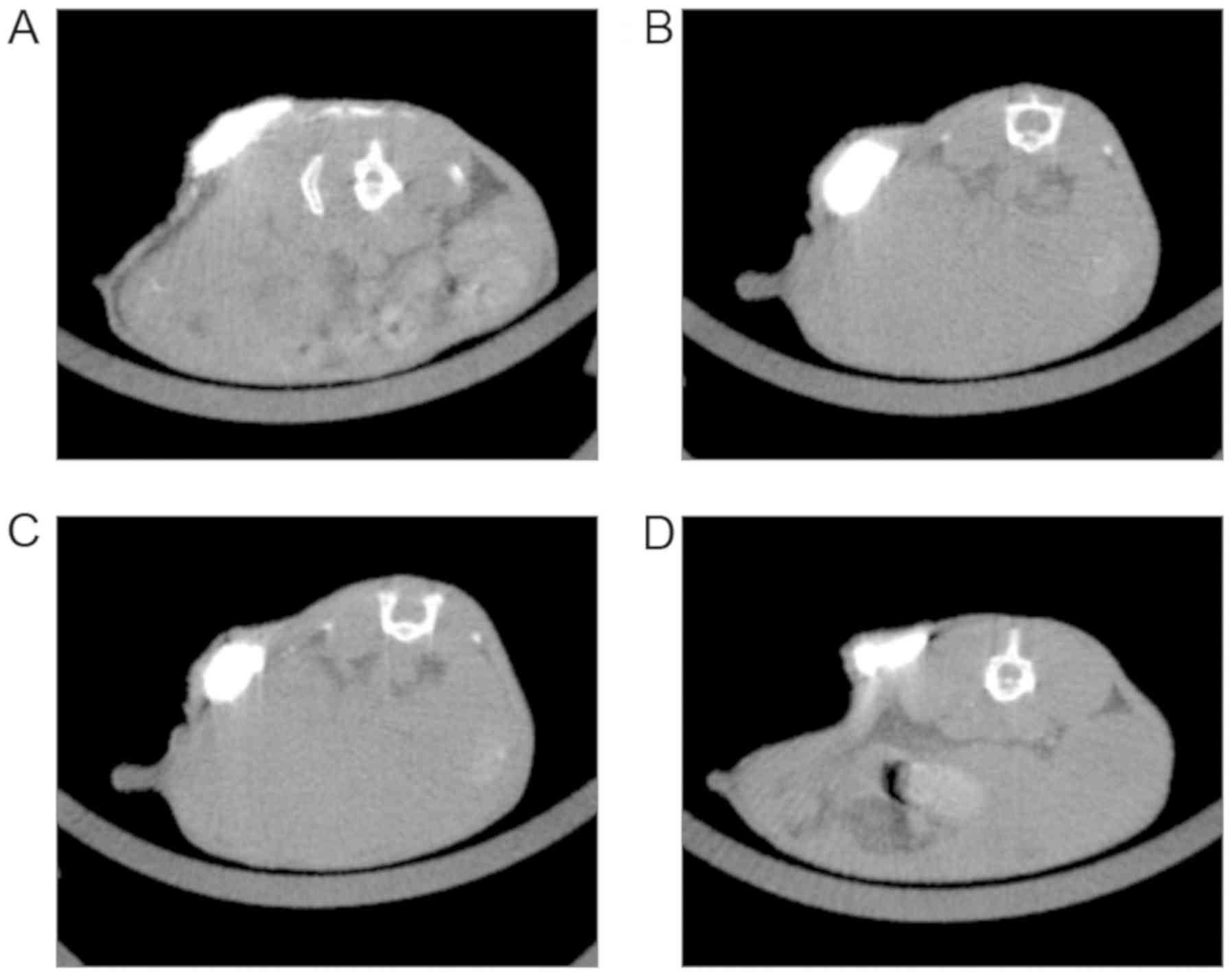
Permanence of imaging effects of
markers in part 1
After 7 weeks of follow-up, NBCA/Lip injection
nodules were observed in a total of 42 mice using CT images between
the four treatment groups (1:1–0.1 ml, 11/12; 1:1–0.15 ml, 12/12;
1:3–0.1 ml, 8/12; 1:3–0.15 ml, 11/12). No obvious artifacts were
observed using CT imaging. For the 1:3–0.1 ml combination, 33.3% of
nodules could not be analyzed, indicating the most rapid
degradation rate of the treatment mixture. The 1:3–0.15 ml group
maintained a stable nodule for 7 weeks and fig.3 shows a series of images of a nodule
(Arrows in Fig. 3) in this treatment
at 1, 3, 5, 7 weeks post implantation, which was not observed on
the follow-up images at 3 months. Further analysis on the
association between time and the mixing ratio showed that over the
7 weeks, the mixing ratio of 1:1 created a larger nodule compared
with 1:3 (P<0.001, Table SIV)
and the injection volume 0.1 ml was 0.079 cm3 larger
than the injection volume of 0.15 ml (P=0.001, Table SIII). Multiple comparisons revealed
significant differences between the 1:1–0.1 ml group vs. 1:3–0.1 ml
group, 1:1–0.15 ml group vs. 1:3–0.1 ml group, 1:1–0.15 ml group
vs. 1:3–0.15 ml group (Table I and
Fig. 2C).
Dynamic changes of CT Hounsfield Units
(HU)
CT values are expressed in HU and were obtained by
drawing the volumetric region of interest. The contrasted results
between the different groups varied according to the post-treatment
weeks. From the 4th week, the difference between the 1:1–0.15 and
1:3–0.1 ml groups became significant until the 7th week.
Significant differences were also observed between the 1:1–0.1 and
1:1–0.15 ml groups at the 6th and 7th week. For the 1:3–0.1 and
1:3–0.15 ml groups, the significant time point was the 7th week.
Generally, at 7 weeks, the CTHU in the 1:3–0.1 ml group declined
the most rapidly and the CTHU in 1:1–0.15 ml group kept stable
change (Fig. 2F).
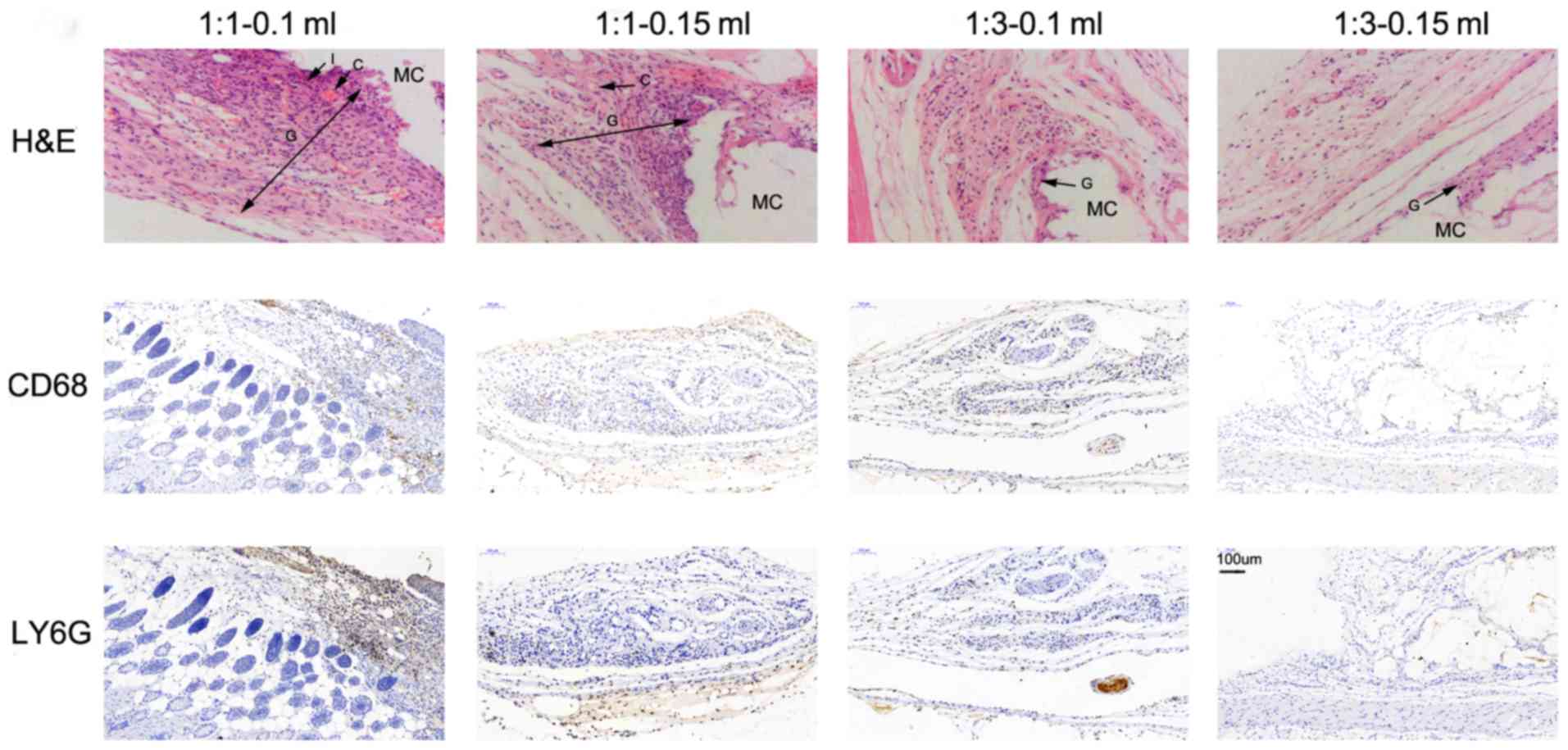
Histopathological analysis of markers
in mice in part 2
Overall, the histopathology observed as a result of
the different NBCA/Lip mixtures included inflammation, tissue
granulation, minimal foreign body reactions and varying levels of
fibrous capsule formation (Figs. 4
and 5). In the acute stage at 2
weeks, granulation was observed alongside focal infiltration of
neutrophils as well as mild edema and dilated capillaries in all
four treatment groups (Fig. 4A-D).
However, a more intense reaction could be distinguished at the
ratio of 1:1 (Fig. 4A and B)
compared with the ratio of 1:3 (Fig. 4C
and D). Chronic granulomatous vasculitis and fibrosis of the
nodules followed in the chronic stage (Fig. 5). At the 3rd week, chronic
inflammation initiated an increase of fibroblast infiltration into
the granulated tissue with local vascularization in all 4 groups,
which reached a peak at the 5th week. By the 7th week, a fibrous
capsule was marked with fibroblasts and multinuclear giant cells
embracing particles in groups treated with the ratio of 1:1
(Fig. 5A and B). However, the
premature tissue granulation with less intense fibroblast
proliferation and fewer foreign giant cells were primarily observed
in the groups treated with the ratio of 1:3 (Fig. 5C and D). Regardless of the different
treatment combinations, the general appearance of the nodules
ressemble that of a hard nodule until the 5th week; subsequently,
the nodules treated with the 1:3 ratio were palpated softer
compared with those treated with the ratio of 1:1. At the 3rd
month, no nodule was palpated and visible on the CT images in the
groups treated with the ratio of 1:3 and at 6 months for groups
treated with 1:1.
Neutrophil counts of tissue in mice in
part 2
LY6G-positve cells were quantified. For interactive
effects, a significant difference was only observed between time
and mixing ratio, (Df=1.741; F=3.626; P=0.043; Table SI). Simple effects analysis revealed
that the ratio of 1:1 was associated with fewer neutrophils
compared with the ratio of 1:3 at the 1st week and no differences
were observed at other time points (Table SIV). When the mixing ratio was 1:3,
compared with the 1st week, the number of neutrophils
significantly decreased gradually over time (P=0.042; Table SIV). Injection volume did not have a
significant effect on the neutrophils counts (Table SIII). Finally, the differences
between the four groups were examined and no significant
differences were observed (Table I
and Fig. 2D).
Macrophage counts of tissue in mice in
part 2
Statistical tests of model effects on macrophage
counts revealed no significant associations between cell counts and
injection volume and injection ratio (Tables SI and SII). The analysis of the main effects
demonstrated that compared with the 1st week, macrophage
counts decreased significantly before the 3rd week and subsequently
increased significantly in all groups (Table SIV). As for the four settings, no
significant difference in macrophage counts was observed between
post-implantation week 1 and 7 (Table
I and Fig. 2E).
Biocompatibility tests in part 3
Based on the results of part 1 and part 2, the
combination of 1:3–0.15 ml was selected to further analyze
biocompatibility, following the ISO 10993 criteria. The results of
acute systematic assay showed that within the 72-h evaluations, no
death occurred in the treatment or control group and the weight of
mice increased gradually, irrespective of the extraction liquid
(Fig. SI). The results of the MTT
assay reported that the relative proliferation rate of the
treatment group was 95% at the grade I level of cytotoxicity
(Table SV). According to the
Magnusson and Kligman classification criteria (16), the absence of distinct erythema and
edema on guinea pigs' skin demonstrated a reaction grade <1 and
all the guinea pigs exhibited no skin allergies in the treatment
group. No difference was greater than 1 at 24, 48 and 72 h
post-injection, which meant that either the SC or CSO extraction
for the intradermal test were safe on rabbits within the 72-h
evaluation (Table SVI).
Discussion
Fiducial placement is well established in breast and
prostate cancer (33,34) and the previous literature has
suggested its value in radiation therapy for upper gastrointestinal
tumors (2,5,8,9). In the present study, the effects of
mixing ratio and injection volume were evaluated and it was
demonstrated that the higher concentration of NBCA (1:1) was
associated with a more intense tissue response, resulting in a
larger remaining volume and a longer degradation time compared with
the lower mixing ratio (1:3). Nodules formed by a larger injection
volume (0.15 ml) were associated with a larger remaining volume as
observed using CT imaging. The combination of 1:3–0.15 ml injection
into subcutaneous tissue was associated with improved fusion with
local tissue and this combination maintained a more stable imaging
nodule in CT images during the initial 7 weeks. The final
biocompatibility test demonstrated the safety of 1:3–0.15 ml
NBCA/Lip according to the ISO criteria. Therefore, this ratio
should be further investigated in experiments involving larger
animals.
The two ratios and two volumes used in the present
study were selected based on pre-experimental data. A high
viscosity and long polymerization time of fiducial markers, which
increases proportionately with decreased NBCA volume, will impede
the operation and limit the precision of marking the site (14,15,25). In
a study conducted by Stoesslein et al (26), when the NBCA concentration increased
from 20 to 25%, this yielded an increase in polymerization time
from 7.5±0.8 to 11.8±1.5 sec (26).
In addition, in the preliminary experiment on BALB/c mice, NBCA was
diluted over 1:3 resulted in various flat shapes easily squeezed by
surrounding tissues, and when injecting volumes was 0.1–0.2 ml, a
nodule ~1 cm in diameter on the CT images was created without
significant leakage, which was in accordance with a previous study
(27).
The present evaluations of the reactions to
different mixture compositions of NBCA/Lip demonstrated that the
1:3–0.15 ml combination was the safest. Body weight and skin status
at the injection site were individually monitored as these were
expected to be sensitive indicators of adverse reactions associated
with the implantation (35,36). Although the results did not indicate
growth retardation as a result of implantation, it was observed
that the combination of 1:3–0.15 ml had the least impact on animal
weight compared with the 3 other mixtures. Notably, with the
configurations of 1:1–0.15 and 1:3–0.1 ml, significantly greater
extents of erythema and edema were observed. Schineider and Otto
(37) reported that the reaction
degree of soft tissue to Histoacryl® was associated with
the mixture volumes and ratios in vitro and in vivo,
as further supported by the present study. The present study
reported that with greater volumes of NBCA, the extent of the
reaction was greater and the degradation rate was considerably
attenuated. Inflammatory reactions can occur in chemically or
physically injured tissues (2,9,38). However, the minimal dosage and
appropriate configuration of NBCA/Lip limited the toxicity observed
in the present study.
The biodegradation of an implant in vivo
depends on a number of factors, including the microscopic
appearance of the implantation, the interaction of tissue-interface
and the histopathological process (2,39). When
NBCA/Lip is treated as permanent embolic material, the microscopic
appearance of the solidified mass after blocking blood vessels has
been reported to be a honeycombed lattice containing blood clots in
the channel, which may become organized and recanalized (40–42).
NBCA adheres to the tissue, but lipiodol reduces this capacity and
causes peripheral solidification, which notably affects the
microscopic tissue interface. Generally, the low concentration and
a small volume of NBCA/Lip may raise concerns regarding the
remaining time of the radio-opaque mass on radiographs. For
example, in the present study the 1:3–0.1 ml combination resulted
in the smallest number of markers visible on CT images at the
7th week. In addition, the skin score of 1:3–0.1 ml
increased after injection, reached its peak during the 3rd week,
and subsequently decreased gradually, returning to the mean value
at 6th week. One explanation for this could be that this
combination degraded faster, in accordance with the present
examinations of imaging volume that the setting of 1:3–0.1 ml had
only 8 nodules on images at 7 weeks and the lowest CT HU among four
configurations. Low density of NBCA and low injection volume may
create a looser structure, resulting in a faster degradation and
affect the skin reaction at early stage. Moreover, it was
hypothesized that when the density of NBCA is low, the
polymerization time is longer, which results in lower catheter
occlusion and more distal penetration into small vessels, further
irritating the skin. This hypothesis is supported by previous
studies investigating embolization treatment (43–47). In
the present study, increasing the injection volume or the
concentration of NBCA created a larger remaining size of the nodule
over time. The worse skin status in treatment of 1:3–0.1 ml may be
partially explained by the low concentration of NBCA in NBCA/Lip.
Rydhog et al (23) estimated
the relative volume change of a liquid fiducial marker for
radiotherapy in patients with lung cancer and at end of
radiotherapy treatment was −23% for tumor injections and −5% for
lymph node injected markers. Thick fibrosis and decreased blood
flow, as a result of the increased extracellular matrix and reduced
blood circulation, are consequently considered to be involved in
the persistence of the CT imaging effect.
Another factor in the measurement of the structural
stability of fiducial markers is HU variation, which can impact the
ability to consistently measure the marker volume (23). As for the radio-opacity (HU), Rydhog
et al (23) reported notably
lower radio-opacity of markers injected into the lymph node
compared with those injected into the tumors. The authors
attributed this to the degree of tissue condensing following
injection, wherein tissue volumetric integrity was better preserved
and an improved representation of the tumor border was observed.
Other studies have demonstrated that a large volume of liquid
material leads to hardened artifacts and results in decreased
imaging quality, rendering the radiotherapy unprecise (12,48). In
the present study, the injection volume of 0.15 ml was not shown to
reduce the CT imaging quality. Overall, it is valuable that the
application of 1:3–0.15 ml created clear visible nodules for 7
weeks, which then rapidly diminished, meeting the four criteria of
fiducial markers as outlined by Habermehl et al (7), including the visibility, the absence of
artifacts, easy application and sufficient immovability.
Following an evaluation of safety and stability of
different combinations of NBCA/Lip, the 1:3–0.15 ml setting is
recommended for the use of this fiducial mixture in further
research. Given that no previous data have illustrated the
biocompatibility of this specified setting and biocompatibility is
a prerequisite for use in human patients, the present toxicity
evaluation was conducted following the ISO criteria and the results
revealed that this material has a good biocompatibility.
Cyanoacrylates are hypothesized to be cytotoxic
primarily at the initial dehydration stage of the tissues before
full polymerization and are then removed slowly by macrophages
(15,21,37,38,49,50). The
release of formaldehyde, a compound produced in the process of the
polymerization of cyanoacrylates, which releases fewer residues
during cell culture and, therefore, should be less cytotoxic, was
demonstrated to be less cytotoxic compared with cyanoacrylates in a
previous study (49). Data regarding
the biocompatibility of NBCA and Lip are rare; however, the
histopathological examinations of embolization in veins and
arteries of different parts of the body are within the acceptable
range (26,39,41,51).
Furthermore, the dosage used in the present study was less than
that used in interventional embolization, contributing to the good
biocompatibility of NBCA and Lip as fiducial markers.
Clinically, fiducial markers should be injected
close to the tumor margins, which would likely influence the
histopathology and degradation rate (12,21–23).
Therefore, it is difficult to determine whether the histological
changes and the permanent effects are due to the underlying
disease. Moreover, the tissue reactions and degradation rates
reported in the present study demonstrate that the extrapolation of
the different mixing ratios and volumes to a wider range should be
approached with caution. Accordingly, these results highlight the
need for further assessment of the NBCA/Lip mixture quantities
prior to the use of this mixture in human patients.
In conclusion, the combination of 1:3–0.15 ml
NBCA/Lip is a good fiducial marker in vivo which may have
valuable applications in IGRT, as these mixture quantities were
associated with limited granulomatous reactions, creating a
well-balanced integration with tissue environment and, importantly,
maintaining a stable nodule size and density for 7 weeks post
implantation. Evaluations following ISO also showed the good
biocompatibility of this setting. Overall, NBCA/Lip with the
appropriate combination is a promising fiducial marker for IGRT and
should be further investigated in other preclinical models.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science
Foundation of China (grant no. 81972864), Science and Technology
Support Plan for Youth Innovation Teams of Universities in Shandong
Province (grant no. 2019KJL001) and Science and Technology Plan of
Jinan (grant no. 201907113).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS, YS, CL, LW, XS and WL conducted the experiments
and LS wrote the manuscript. XD and DX conducted the acquisition of
data, data analysis and interpretation of data. JY and XM designed
the study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal protocols were conducted in compliance with
the regulations of the Shandong Cancer Hospital and the present
study as approved by The Scientific Review and Ethics Committee of
Shandong Cancer Hospital and Institute (Jinan, China; approval no.
SDTHEC-2019030915).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Welsh J, Settle SH, Amini A, Xiao L,
Suzuki A, Hayashi Y, Hofstetter W, Komaki R, Liao Z and Ajani JA:
Failure patterns in patients with esophageal cancer treated with
definitive chemoradiation. Cancer. 118:2632–2640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin P, Van Der Horst A, De Jong R, van
Hooft JE, Kamphuis M, van Wieringen N, Machiels M, Bel A, Hulshof
MC and Alderliesten T: Marker-based quantification of
interfractional tumor position variation and the use of markers for
setup verification in radiation therapy for esophageal cancer.
Radiother Oncol. 117:412–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burmeister BH, Beukema J, Guidi R, Harvey
JA, Gotley D and Smithers BM: Localization of small esophageal
cancers for radiation planning using endoscopic contrast injection:
Report on a series of eight cases. Dis Esophagus. 14:28–31. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chavalitdhamrong D, Dimaio CJ, Siersema PD
and Wagh MS: Technical advances in endoscopic ultrasound-guided
fiducial placement for the treatment of pancreatic cancer. Endosc
Int Open. 3:E373–E377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Machiels M, Van Hooft J, Jin P, van Berge
Henegouwen MI, van Laarhoven HM, Alderliesten T and Hulshof MC:
Endoscopy/EUS-guided fiducial marker placement in patients with
esophageal cancer: A comparative analysis of 3 types of markers.
Gastrointest Endosc. 82:641–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirato H, Harada T, Harabayashi T, Hida
K, Endo H, Kitamura K, Onimaru R, Yamazaki K, Kurauchi N, Shimizu
T, et al: Feasibility of insertion/implantation of 2.0-mm-diameter
gold internal fiducial markers for precise setup and real-time
tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys.
56:240–247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Habermehl D, Henkner K, Ecker S, Jakel O,
Debus J and Combs SE: Evaluation of different fiducial markers for
image-guided radiotherapy and particle therapy. J Radiat Res. 54
(Suppl 1):i61–i68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukada J, Hanada T, Kawaguchi O, Ohashi T,
Takeuchi H, Kitagawa Y, Seki S, Shiraishi Y, Ogata H and Shigematsu
N: Detection of esophageal fiducial marker displacement during
radiation therapy with a 2-dimensional on-board imager: Analysis of
internal margin for esophageal cancer. Int J Radiat Oncol Biol
Phys. 85:991–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernandez DC, Hoffe SE, Barthel JS,
Vignesh S, Klapman JB, Harris C, Almhanna K, Biagioli MC, Meredith
KL, Feygelman V, et al: Stability of endoscopic ultrasound-guided
fiducial marker placement for esophageal cancer target delineation
and image-guided radiation therapy. Pract Radiat Oncol. 3:32–39.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scherman Rydhog J, Perrin R, Jolck RI,
Gagnon-Moisan F, Larsen KR, Clementsen P, Riisgaard de Blanck S,
Fredberg Persson G, Weber DC, Lomax T, et al: Liquid fiducial
marker applicability in proton therapy of locally advanced lung
cancer. Radiother Oncol. 122:393–399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Picardi C, Rouzaud M, Kountouri M,
Lestrade L, Vallée JP, Caparrotti F, Dubouloz A, Miralbell R and
Zilli T: Impact of hydrogel spacer injections on interfraction
prostate motion during prostate cancer radiotherapy. Acta Oncol.
55:834–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dobiasch S, Kampfer S, Burkhardt R,
Schilling D, Schmid TE, Wilkens JJ and Combs SE: BioXmark for
high-precision radiotherapy in an orthotopic pancreatic tumor mouse
model: Experiences with a liquid fiducial marker. Strahlenther
Onkol. 193:1039–1047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sondergaard J, Olsen KO, Muren LP, Elstrom
UV, Grau C and Hoyer M: A study of image-guided radiotherapy of
bladder cancer based on lipiodol injection in the bladder wall.
Acta Oncol. 49:1109–1115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeuchi Y, Morishita H, Sato Y, Hamaguchi
S, Sakamoto N, Tokue H, Yonemitsu T, Murakami K, Fujiwara H, Sofue
K, et al: Guidelines for the use of NBCA in vascular embolization
devised by the Committee of Practice Guidelines of the Japanese
Society of Interventional Radiology (CGJSIR), 2012 edition. Jpn J
Radiol. 32:500–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takasawa C, Seiji K, Matsunaga K,
Matsuhashi T, Ohta M, Shida S, Takase K and Takahashi S: Properties
of N-butyl cyanoacrylate-iodized oil mixtures for arterial
embolization: In vitro and in vivo experiments. J Vasc Interv
Radiol. 23:1215–1221.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
International Organization for
Standardization, ISO 10993-10:2002, . Biological evaluation of
medical devices-Part 10: Tests for irritation and delayed-type
hypersensitivity. 2002.
|
|
17
|
International Organization for
Standardization, ISO 10993-11:1993 Biological evaluation of medical
devices-Part 11, . Tests for systemic toxicity. 1993.
|
|
18
|
International Organization for
Standardization, ISO 10993-5:1999 biological evaluation of medical
devices-Part 5, . Test for in vitro cytotoxicity. 1999.
|
|
19
|
Flecknell P, Lofgren JLS, Dysson MC,
Marini RR, Swindle MM and Wilson RP: Chapter 24 - Preanesthesia,
Anesthesia, Analgesia, and Euthanasia. Laboratory Animal Medicine
(3rd). American College of Laboratory Animal Medicine. 1135–1200.
2015. View Article : Google Scholar
|
|
20
|
Pos F, Bex A, Dees-Ribbers HM, Betgen A,
Van Herk M and Remeijer P: Lipiodol injection for target volume
delineation and image guidance during radiotherapy for bladder
cancer. Radiother Oncol. 93:364–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rose M, Siva S, Ball D, Irving LB and
Steinfort DP: Bronchoscopic delivery of lipiodol as a fiducial
marker in lung tumors before radiotherapy. J Thorac Oncol.
9:1579–1583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Freilich JM, Spiess PE, Biagioli MC,
Fernandez DC, Shi EJ, Hunt DC, Gupta S and Wilder RB: Lipiodol as a
fiducial marker for image-guided radiation therapy for bladder
cancer. Int Braz J Urol. 40:190–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rydhog JS, Mortensen SR, Larsen KR,
Clementsen P, Jølck RI, Josipovic M, Aznar MC, Specht L, Andresen
TL, Rosenschöld PM and Persson GF: Liquid fiducial marker
performance during radiotherapy of locally advanced non-small cell
lung cancer. Radiother Oncol. 121:64–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leary S, Underwood W, Anthony R, et al:
AVMA Guidelines for the Euthanasia of Animals: 2013 Edition.
American Veterinary Medical Association. 2013.
|
|
25
|
Seewald S, Ang TL, Imazu H, Naga M, Omar
S, Groth S, Seitz U, Zhong Y, Thonke F and Soehendra N: A
standardized injection technique and regimen ensures success and
safety of N-butyl-2-cyanoacrylate injection for the treatment of
gastric fundal varices (with videos). Gastrointest Endosc.
68:447–454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stoesslein F, Ditscherlein G and Romaniuk
PA: Experimental studies on new liquid embolization mixtures
(histoacryl-lipiodol, histoacryl-panthopaque). Cardiovasc Intervent
Radiol. 5:264–267. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwon WJ, Kim HJ, Jeong YJ, Lee CH, Kim KI,
Kim YD and Lee JH: Direct lipiodol injection used for a
radio-opaque lung marker: stability and histopathologic effects.
Exp Lung Res. 37:310–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang Y, Qin J, Peng WD, Shi S, Fu SZ, Guo
G, Hu HZ, Zhao X, Wei YQ and Qian ZY: Acute toxicity evaluation of
biodegradable in situ gel-forming controlled drug delivery system
based on thermosensitive PEG-PCL-PEG hydrogel. J Biomed Mater Res B
Appl Biomater. 91:26–36. 2009.PubMed/NCBI
|
|
29
|
Wu Y, Wang L, Zhang K, Zhou L, Zhang X,
Jiang X and Zhu C: N-Butyl-2-cyanoacrylate-based injectable and in
situ-forming implants for efficient intratumoral chemotherapy. Drug
Deliv. 24:729–736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dalu A, Blaydes BS, Lomax LG and Delclos
KB: A comparison of the infammatory response to a
polydimethylsiloxane implant in male and female Balb/c mice.
Biomaterials. 21:1947–1957. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmidt SAJ, Lo S and Hollestein LM:
Research Techniques Made Simple: Sample size estimation and power
calculation. J Invest Dermatol. 138:1678–1682. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buch T, Moos K, Ferreira FM, Fröhlich H,
Gebhard C and Tresch A: Benefits of a factorial design focusing on
inclusion of female and male animals in one experiment. J Mol Med
(Berl). 97:871–877. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh J, Greer PB, White MA, Parker J,
Patterson J, Tang CI, Capp A, Wratten C and Denham JW:
Treatment-related morbidity in prostate cancer: A comparison of
3-dimensional conformal radiation therapy with and without image
guidance using implanted fiducial markers. Int J Radiat Oncol Biol
Phys. 85:1018–1023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kirby AN, Jena R, Harris EJ, Evans PM,
Crowley C, Gregory DL and Coles CE: Tumour bed delineation for
partial breast/breast boost radiotherapy: What is the optimal
number of implanted markers? Radiother Oncol. 106:231–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaisman B, Motiei M, Nyska A and Domb AJ:
Biocompatibility and safety evaluation of a ricinoleic acid-based
poly(ester- anhydride) copolymer after implantation in rats. J
Biomed Mater Res A. 92:419–431. 2010.PubMed/NCBI
|
|
36
|
Miura C, Shimizu Y, Imai Y, Mukai T,
Yamamoto A, Sano Y, Ikeo N, Isozaki S, Takahashi T, Oikawa M, et
al: In vivo corrosion behaviour of magnesium alloy in association
with surrounding tissue response in rats. Biomed Mater.
11:0250012016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schneider G and Otto K: In vitro and in
vivo studies on the use of Histoacryl(®) as a soft
tissue glue. Eur Arch Otorhinolaryngol. 269:1783–1789. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vinters HV, Galil KA, Lundie MJ and
Kaufmann JC: The histotoxicity of cyanoacrylates. A selective
review. Neuroradiology. 27:279–291. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gounis MJ, Lieber BB, Wakhloo AK, Siekmann
R and Hopkins LN: Effect of glacial acetic acid and ethiodized oil
concentration on embolization with N-butyl 2-cyanoacrylate: An in
vivo investigation. AJNR Am J Neuroradiol. 23:938–944.
2002.PubMed/NCBI
|
|
40
|
Wikholm G: Occlusion of cerebral
arteriovenous malformations with N-butyl cyano-acrylate is
permanent. AJNR Am J Neuroradiol. 16:479–482. 1995.PubMed/NCBI
|
|
41
|
Sadato A, Wakhloo AK and Hopkins LN:
Effects of a mixture of a low concentration of n-butylcyanoacrylate
and ethiodol on tissue reactions and the permanence of arterial
occlusion after embolization. Neurosurgery. 47:1195–1204. 2000.
View Article : Google Scholar
|
|
42
|
Gruber A, Mazal PR, Bavinzski G, Killer M,
Budka H and Richling B: Repermeation of partially embolized
cerebral arteriovenous malformations: A clinical, radiologic, and
histologic study. AJNR Am J Neuroradiol. 17:1323–1331.
1996.PubMed/NCBI
|
|
43
|
Rafat M, Xeroudaki M, Koulikovska M,
Sherrell P, Groth F, Fagerholm P and Lagali N: Composite
core-and-skirt collagen hydrogels with differential degradation for
corneal therapeutic applications. Biomaterials. 83:142–155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wilson DJ, Chenery DH, Bowring HK, Wilson
K, Turner R, Maughan J, West PJ and Ansell CW: Physical and
biological properties of a novel siloxane adhesive for soft tissue
applications. J Biomater Sci Polym Ed. 16:449–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brothers MF, Kaufmann JC, Fox AJ and
Deveikis JP: n-Butyl2-cyanoacrylate-substitute for IBCA in
interventional neurodiology: Histopathologic and polymerization
time studies. AJNR Am J Neurodiol. 10:777–786. 1989.
|
|
46
|
Pollak JS and White RI Jr: The use of
cyanoacrylate adhesives in peripheral embolization. J Vasc Interv
Radiol. 12:907–913. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ishikawa M, Horikawa M, Yamagami T, Uchida
BT, Awai K and Kaufman JA: Embolization of Arteriovenous
Malformations: Effect of flow control and composition of n-Butyl-2
cyanoacrylate and iodized oil mixtures with and without ethanol in
an in vitro model. Radiology. 279:910–916. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lock M, Malayeri AA, Mian OY, Mayr NA,
Herman JM and Lo SS: Computed tomography imaging assessment of
postexternal beam radiation changes of the liver. Future Oncol.
12:2729–2739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Montanaro L, Arciola CR, Cenni E, Ciapetti
G, Savioli F, Filippini F and Barsanti LA: Cytotoxicity, blood
compatibility and antimicrobial activity of two cyanoacrylate glues
for surgical use. Biomaterials. 22:59–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Derks CM and Jacobovitz-Derks D: Embolic
pneumopathy induced by oleic acid. A systematic morphologic study.
Am J Pathol. 87:143–158. 1977.PubMed/NCBI
|
|
51
|
Wang YM, Cheng LF and Li N:
Histopathological study of vascular changes after intra-arterial
and intravenous injection of N-butyl-2-cyanoacrylate. Chin J Dig
Dis. 7:175–179. 2006. View Article : Google Scholar : PubMed/NCBI
|