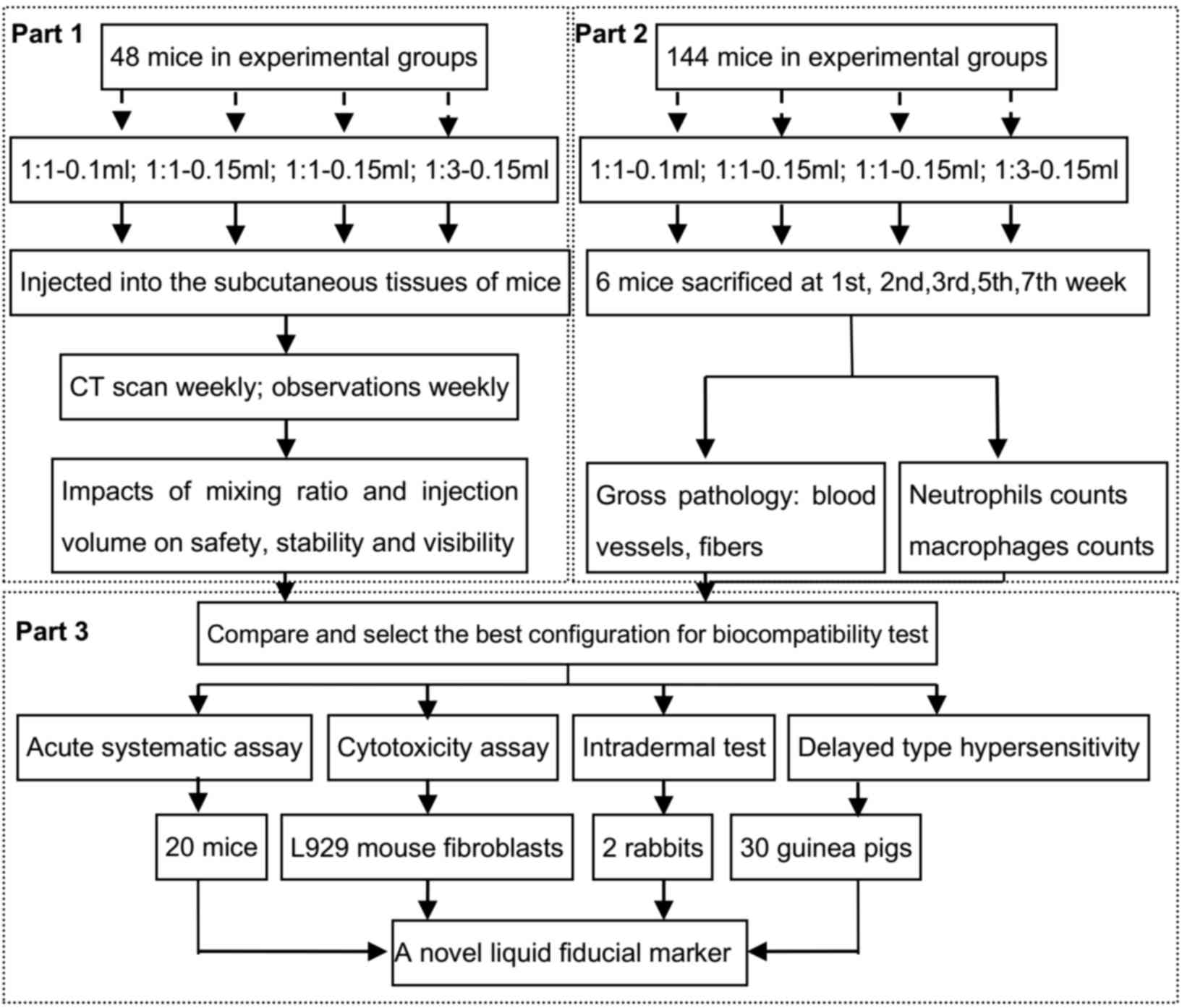
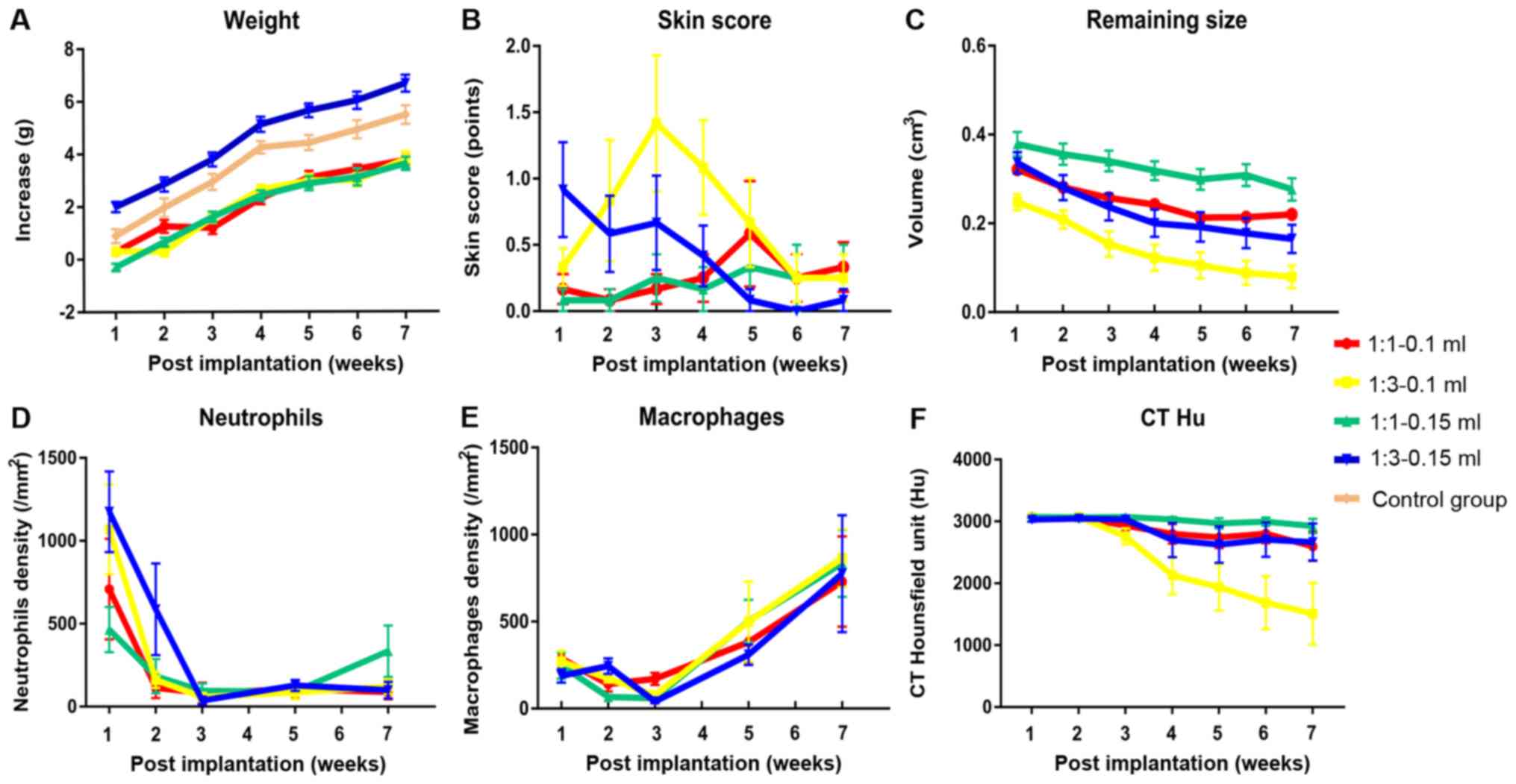
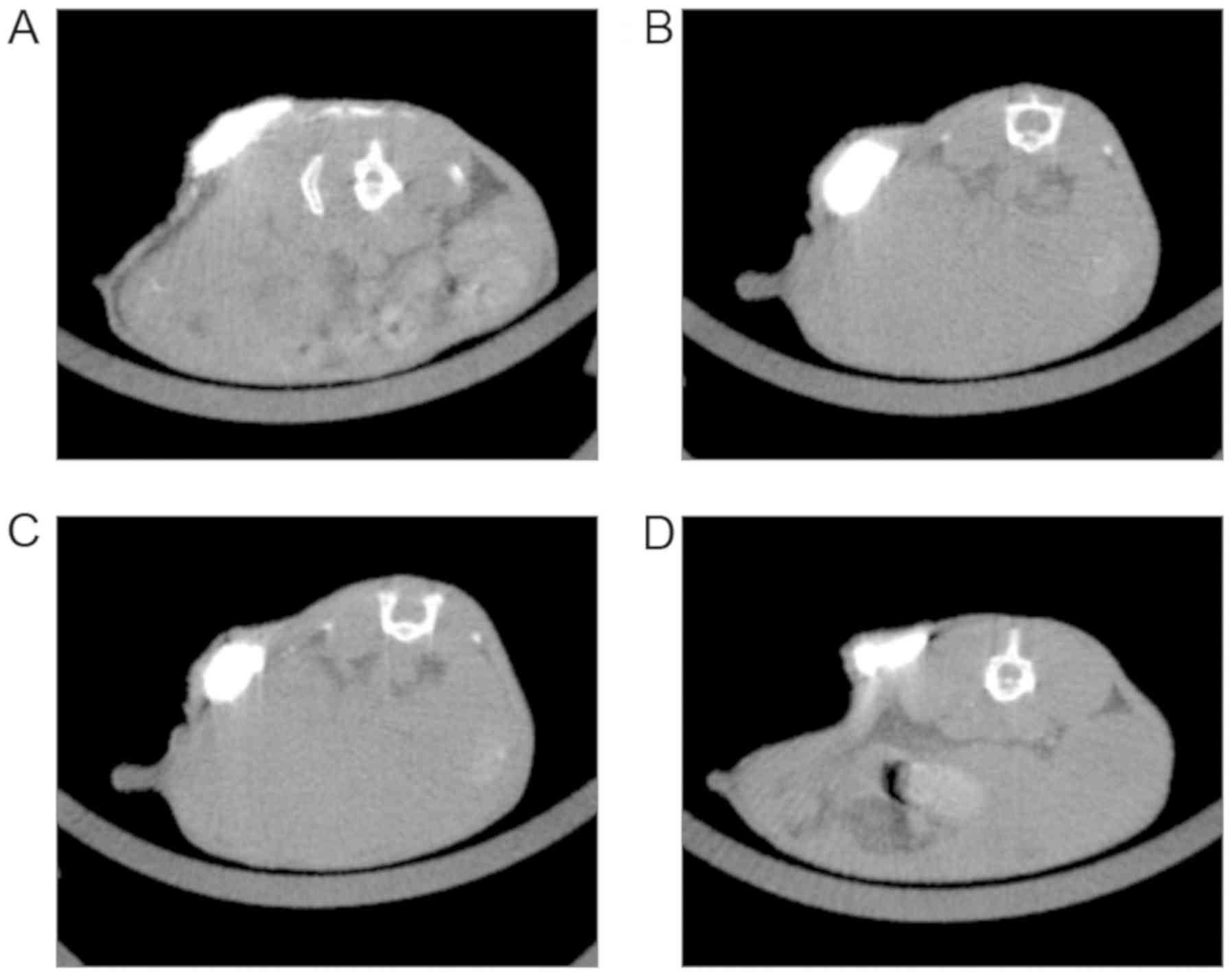
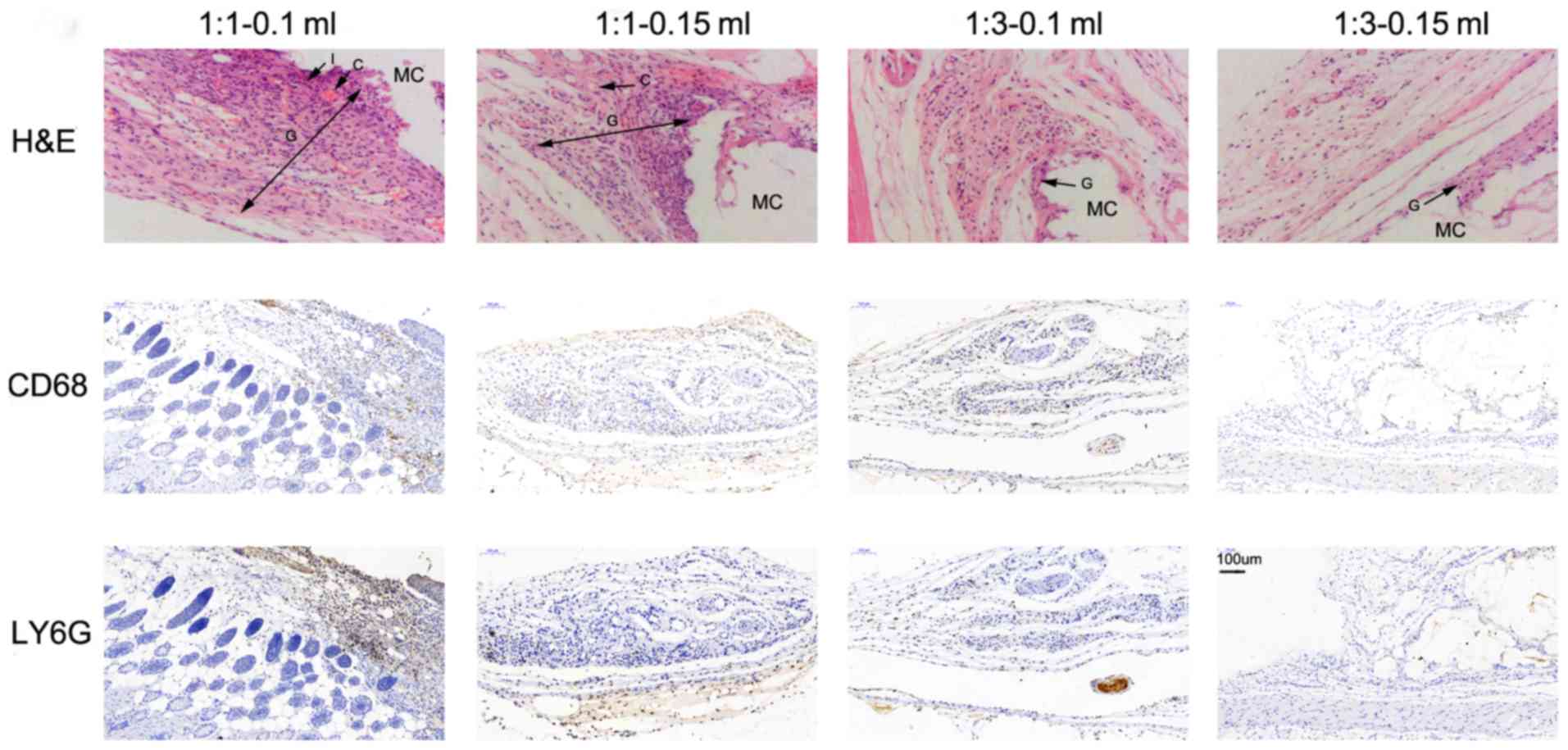
|
1
|
Welsh J, Settle SH, Amini A, Xiao L,
Suzuki A, Hayashi Y, Hofstetter W, Komaki R, Liao Z and Ajani JA:
Failure patterns in patients with esophageal cancer treated with
definitive chemoradiation. Cancer. 118:2632–2640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin P, Van Der Horst A, De Jong R, van
Hooft JE, Kamphuis M, van Wieringen N, Machiels M, Bel A, Hulshof
MC and Alderliesten T: Marker-based quantification of
interfractional tumor position variation and the use of markers for
setup verification in radiation therapy for esophageal cancer.
Radiother Oncol. 117:412–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burmeister BH, Beukema J, Guidi R, Harvey
JA, Gotley D and Smithers BM: Localization of small esophageal
cancers for radiation planning using endoscopic contrast injection:
Report on a series of eight cases. Dis Esophagus. 14:28–31. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chavalitdhamrong D, Dimaio CJ, Siersema PD
and Wagh MS: Technical advances in endoscopic ultrasound-guided
fiducial placement for the treatment of pancreatic cancer. Endosc
Int Open. 3:E373–E377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Machiels M, Van Hooft J, Jin P, van Berge
Henegouwen MI, van Laarhoven HM, Alderliesten T and Hulshof MC:
Endoscopy/EUS-guided fiducial marker placement in patients with
esophageal cancer: A comparative analysis of 3 types of markers.
Gastrointest Endosc. 82:641–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirato H, Harada T, Harabayashi T, Hida
K, Endo H, Kitamura K, Onimaru R, Yamazaki K, Kurauchi N, Shimizu
T, et al: Feasibility of insertion/implantation of 2.0-mm-diameter
gold internal fiducial markers for precise setup and real-time
tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys.
56:240–247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Habermehl D, Henkner K, Ecker S, Jakel O,
Debus J and Combs SE: Evaluation of different fiducial markers for
image-guided radiotherapy and particle therapy. J Radiat Res. 54
(Suppl 1):i61–i68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukada J, Hanada T, Kawaguchi O, Ohashi T,
Takeuchi H, Kitagawa Y, Seki S, Shiraishi Y, Ogata H and Shigematsu
N: Detection of esophageal fiducial marker displacement during
radiation therapy with a 2-dimensional on-board imager: Analysis of
internal margin for esophageal cancer. Int J Radiat Oncol Biol
Phys. 85:991–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernandez DC, Hoffe SE, Barthel JS,
Vignesh S, Klapman JB, Harris C, Almhanna K, Biagioli MC, Meredith
KL, Feygelman V, et al: Stability of endoscopic ultrasound-guided
fiducial marker placement for esophageal cancer target delineation
and image-guided radiation therapy. Pract Radiat Oncol. 3:32–39.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scherman Rydhog J, Perrin R, Jolck RI,
Gagnon-Moisan F, Larsen KR, Clementsen P, Riisgaard de Blanck S,
Fredberg Persson G, Weber DC, Lomax T, et al: Liquid fiducial
marker applicability in proton therapy of locally advanced lung
cancer. Radiother Oncol. 122:393–399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Picardi C, Rouzaud M, Kountouri M,
Lestrade L, Vallée JP, Caparrotti F, Dubouloz A, Miralbell R and
Zilli T: Impact of hydrogel spacer injections on interfraction
prostate motion during prostate cancer radiotherapy. Acta Oncol.
55:834–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dobiasch S, Kampfer S, Burkhardt R,
Schilling D, Schmid TE, Wilkens JJ and Combs SE: BioXmark for
high-precision radiotherapy in an orthotopic pancreatic tumor mouse
model: Experiences with a liquid fiducial marker. Strahlenther
Onkol. 193:1039–1047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sondergaard J, Olsen KO, Muren LP, Elstrom
UV, Grau C and Hoyer M: A study of image-guided radiotherapy of
bladder cancer based on lipiodol injection in the bladder wall.
Acta Oncol. 49:1109–1115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeuchi Y, Morishita H, Sato Y, Hamaguchi
S, Sakamoto N, Tokue H, Yonemitsu T, Murakami K, Fujiwara H, Sofue
K, et al: Guidelines for the use of NBCA in vascular embolization
devised by the Committee of Practice Guidelines of the Japanese
Society of Interventional Radiology (CGJSIR), 2012 edition. Jpn J
Radiol. 32:500–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takasawa C, Seiji K, Matsunaga K,
Matsuhashi T, Ohta M, Shida S, Takase K and Takahashi S: Properties
of N-butyl cyanoacrylate-iodized oil mixtures for arterial
embolization: In vitro and in vivo experiments. J Vasc Interv
Radiol. 23:1215–1221.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
International Organization for
Standardization, ISO 10993-10:2002, . Biological evaluation of
medical devices-Part 10: Tests for irritation and delayed-type
hypersensitivity. 2002.
|
|
17
|
International Organization for
Standardization, ISO 10993-11:1993 Biological evaluation of medical
devices-Part 11, . Tests for systemic toxicity. 1993.
|
|
18
|
International Organization for
Standardization, ISO 10993-5:1999 biological evaluation of medical
devices-Part 5, . Test for in vitro cytotoxicity. 1999.
|
|
19
|
Flecknell P, Lofgren JLS, Dysson MC,
Marini RR, Swindle MM and Wilson RP: Chapter 24 - Preanesthesia,
Anesthesia, Analgesia, and Euthanasia. Laboratory Animal Medicine
(3rd). American College of Laboratory Animal Medicine. 1135–1200.
2015. View Article : Google Scholar
|
|
20
|
Pos F, Bex A, Dees-Ribbers HM, Betgen A,
Van Herk M and Remeijer P: Lipiodol injection for target volume
delineation and image guidance during radiotherapy for bladder
cancer. Radiother Oncol. 93:364–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rose M, Siva S, Ball D, Irving LB and
Steinfort DP: Bronchoscopic delivery of lipiodol as a fiducial
marker in lung tumors before radiotherapy. J Thorac Oncol.
9:1579–1583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Freilich JM, Spiess PE, Biagioli MC,
Fernandez DC, Shi EJ, Hunt DC, Gupta S and Wilder RB: Lipiodol as a
fiducial marker for image-guided radiation therapy for bladder
cancer. Int Braz J Urol. 40:190–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rydhog JS, Mortensen SR, Larsen KR,
Clementsen P, Jølck RI, Josipovic M, Aznar MC, Specht L, Andresen
TL, Rosenschöld PM and Persson GF: Liquid fiducial marker
performance during radiotherapy of locally advanced non-small cell
lung cancer. Radiother Oncol. 121:64–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leary S, Underwood W, Anthony R, et al:
AVMA Guidelines for the Euthanasia of Animals: 2013 Edition.
American Veterinary Medical Association. 2013.
|
|
25
|
Seewald S, Ang TL, Imazu H, Naga M, Omar
S, Groth S, Seitz U, Zhong Y, Thonke F and Soehendra N: A
standardized injection technique and regimen ensures success and
safety of N-butyl-2-cyanoacrylate injection for the treatment of
gastric fundal varices (with videos). Gastrointest Endosc.
68:447–454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stoesslein F, Ditscherlein G and Romaniuk
PA: Experimental studies on new liquid embolization mixtures
(histoacryl-lipiodol, histoacryl-panthopaque). Cardiovasc Intervent
Radiol. 5:264–267. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwon WJ, Kim HJ, Jeong YJ, Lee CH, Kim KI,
Kim YD and Lee JH: Direct lipiodol injection used for a
radio-opaque lung marker: stability and histopathologic effects.
Exp Lung Res. 37:310–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang Y, Qin J, Peng WD, Shi S, Fu SZ, Guo
G, Hu HZ, Zhao X, Wei YQ and Qian ZY: Acute toxicity evaluation of
biodegradable in situ gel-forming controlled drug delivery system
based on thermosensitive PEG-PCL-PEG hydrogel. J Biomed Mater Res B
Appl Biomater. 91:26–36. 2009.PubMed/NCBI
|
|
29
|
Wu Y, Wang L, Zhang K, Zhou L, Zhang X,
Jiang X and Zhu C: N-Butyl-2-cyanoacrylate-based injectable and in
situ-forming implants for efficient intratumoral chemotherapy. Drug
Deliv. 24:729–736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dalu A, Blaydes BS, Lomax LG and Delclos
KB: A comparison of the infammatory response to a
polydimethylsiloxane implant in male and female Balb/c mice.
Biomaterials. 21:1947–1957. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmidt SAJ, Lo S and Hollestein LM:
Research Techniques Made Simple: Sample size estimation and power
calculation. J Invest Dermatol. 138:1678–1682. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buch T, Moos K, Ferreira FM, Fröhlich H,
Gebhard C and Tresch A: Benefits of a factorial design focusing on
inclusion of female and male animals in one experiment. J Mol Med
(Berl). 97:871–877. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh J, Greer PB, White MA, Parker J,
Patterson J, Tang CI, Capp A, Wratten C and Denham JW:
Treatment-related morbidity in prostate cancer: A comparison of
3-dimensional conformal radiation therapy with and without image
guidance using implanted fiducial markers. Int J Radiat Oncol Biol
Phys. 85:1018–1023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kirby AN, Jena R, Harris EJ, Evans PM,
Crowley C, Gregory DL and Coles CE: Tumour bed delineation for
partial breast/breast boost radiotherapy: What is the optimal
number of implanted markers? Radiother Oncol. 106:231–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaisman B, Motiei M, Nyska A and Domb AJ:
Biocompatibility and safety evaluation of a ricinoleic acid-based
poly(ester- anhydride) copolymer after implantation in rats. J
Biomed Mater Res A. 92:419–431. 2010.PubMed/NCBI
|
|
36
|
Miura C, Shimizu Y, Imai Y, Mukai T,
Yamamoto A, Sano Y, Ikeo N, Isozaki S, Takahashi T, Oikawa M, et
al: In vivo corrosion behaviour of magnesium alloy in association
with surrounding tissue response in rats. Biomed Mater.
11:0250012016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schneider G and Otto K: In vitro and in
vivo studies on the use of Histoacryl(®) as a soft
tissue glue. Eur Arch Otorhinolaryngol. 269:1783–1789. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vinters HV, Galil KA, Lundie MJ and
Kaufmann JC: The histotoxicity of cyanoacrylates. A selective
review. Neuroradiology. 27:279–291. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gounis MJ, Lieber BB, Wakhloo AK, Siekmann
R and Hopkins LN: Effect of glacial acetic acid and ethiodized oil
concentration on embolization with N-butyl 2-cyanoacrylate: An in
vivo investigation. AJNR Am J Neuroradiol. 23:938–944.
2002.PubMed/NCBI
|
|
40
|
Wikholm G: Occlusion of cerebral
arteriovenous malformations with N-butyl cyano-acrylate is
permanent. AJNR Am J Neuroradiol. 16:479–482. 1995.PubMed/NCBI
|
|
41
|
Sadato A, Wakhloo AK and Hopkins LN:
Effects of a mixture of a low concentration of n-butylcyanoacrylate
and ethiodol on tissue reactions and the permanence of arterial
occlusion after embolization. Neurosurgery. 47:1195–1204. 2000.
View Article : Google Scholar
|
|
42
|
Gruber A, Mazal PR, Bavinzski G, Killer M,
Budka H and Richling B: Repermeation of partially embolized
cerebral arteriovenous malformations: A clinical, radiologic, and
histologic study. AJNR Am J Neuroradiol. 17:1323–1331.
1996.PubMed/NCBI
|
|
43
|
Rafat M, Xeroudaki M, Koulikovska M,
Sherrell P, Groth F, Fagerholm P and Lagali N: Composite
core-and-skirt collagen hydrogels with differential degradation for
corneal therapeutic applications. Biomaterials. 83:142–155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wilson DJ, Chenery DH, Bowring HK, Wilson
K, Turner R, Maughan J, West PJ and Ansell CW: Physical and
biological properties of a novel siloxane adhesive for soft tissue
applications. J Biomater Sci Polym Ed. 16:449–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brothers MF, Kaufmann JC, Fox AJ and
Deveikis JP: n-Butyl2-cyanoacrylate-substitute for IBCA in
interventional neurodiology: Histopathologic and polymerization
time studies. AJNR Am J Neurodiol. 10:777–786. 1989.
|
|
46
|
Pollak JS and White RI Jr: The use of
cyanoacrylate adhesives in peripheral embolization. J Vasc Interv
Radiol. 12:907–913. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ishikawa M, Horikawa M, Yamagami T, Uchida
BT, Awai K and Kaufman JA: Embolization of Arteriovenous
Malformations: Effect of flow control and composition of n-Butyl-2
cyanoacrylate and iodized oil mixtures with and without ethanol in
an in vitro model. Radiology. 279:910–916. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lock M, Malayeri AA, Mian OY, Mayr NA,
Herman JM and Lo SS: Computed tomography imaging assessment of
postexternal beam radiation changes of the liver. Future Oncol.
12:2729–2739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Montanaro L, Arciola CR, Cenni E, Ciapetti
G, Savioli F, Filippini F and Barsanti LA: Cytotoxicity, blood
compatibility and antimicrobial activity of two cyanoacrylate glues
for surgical use. Biomaterials. 22:59–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Derks CM and Jacobovitz-Derks D: Embolic
pneumopathy induced by oleic acid. A systematic morphologic study.
Am J Pathol. 87:143–158. 1977.PubMed/NCBI
|
|
51
|
Wang YM, Cheng LF and Li N:
Histopathological study of vascular changes after intra-arterial
and intravenous injection of N-butyl-2-cyanoacrylate. Chin J Dig
Dis. 7:175–179. 2006. View Article : Google Scholar : PubMed/NCBI
|