Introduction
Bladder cancer (BC) is the 7th most common cancer
affecting men in the world and the 11th most common cancer in the
total population (1). BC affects
~3.4 million people worldwide, with 430,000 new cases diagnosed in
2015 (2). In the United States,
80,470 new cases of BC and 17,670 BC-associated mortality cases
were expected to occur in 2019 (3).
Furthermore, BC incidence and mortality rates vary across countries
due to the differences in risk factors, detection and diagnostic
practices and treatments availability (4). The most common type of BC is bladder
urothelial carcinoma (BLCA), which accounts for ~90% of all cases
(5). In addition, BLCA can be low
grade or high grade (6). Low grade
BLCA rarely results in cancer invasion in the bladder muscular wall
or metastasis to other parts of the body, and patients rarely
succumb to low grade BLCA; however, the majority of BLCA-associated
mortality cases result from the high-grade disease (6). BC can also be stratified into muscle
invasive bladder cancer (MIBC) and non-muscle invasive bladder
cancer (NMIBC), according to invasion of the muscularis propria
(6). In particular, ~75% of newly
diagnosed BC cases are non-invasive, including Stages Ta, Tis or
T1, based according to the Union for International Cancer
Control/American Joint Committee on Cancer (UICC/AJCC) staging
system (8th edition) (4). NMIBC
exhibits a high prevalence due to the long-term survival rates and
the lower risk of cancer-specific mortality compared with patients
with MIBC (6). Furthermore,
improvements in the early detection and treatment of BC have
increased patient survival status; however, BC-associated mortality
remains high. It is therefore crucial to identify novel biomarkers
and potential therapeutic targets to improve the clinical treatment
of patients with BLCA.
ST3 β-galactoside α-2,3-sialyltransferase 5
(ST3GAL5) is a protein coding gene, which catalyzes the formation
of ganglioside monosialodihexosylganglioside (GM3) (7). Ganglioside GM3 is known to participate
in the induction of cell differentiation, modulation of cell
proliferation, maintenance of fibroblast morphology, signal
transduction and integrin-mediated cell adhesion (8). Furthermore, ganglioside GM3 is
associated with numerous types of tumor, including lung cancer,
brain cancer and melanomas, and was reported to significantly
influence cancer development and progression (9–12). GM3
is also upregulated in several types of cancer, such as lung and
brain cancer, and melanoma, and can be used as a tumor-associated
carbohydrate antigen in immunotherapy (9,10). In
addition, GM3 inhibits tumor cell proliferation through
angiogenesis inhibition or decrease in cell motility (9,11,13).
However, the expression profile and functional role of ST3GAL5 in
BLCA remain unclear. Therefore, to the best of our knowledge, the
present study is the first data mining study to predict the
potential role of ST3GAL5 in BLCA, based on publicly available gene
expression and clinical outcome databases.
In the present study, the expression of ST3GAL5 and
its clinical outcomes were investigated in patients with BLCA using
various public gene expression and survival datasets. In addition,
the DNA methylation and gene expression patterns of ST3GAL5 in BLCA
were analyzed. Furthermore, enrichment analyses were performed on
genes that were positively co-expressed with ST3GAL5 in BLCA, and
gene set enrichment analysis (GSEA) was also used. The findings
from the present study hypothesized that ST3GAL5 downregulation may
influence BLCA carcinogenesis, suggesting that ST3GAL5 may
represent a novel therapeutic target in BLCA.
Materials and methods
Data set acquisition and
processing
All data were acquired and processed from the public
bioinformatics databases Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo) (14), Oncomine (www.oncomine.org) (15,16),
Tumor IMmune Estimation Resource (TIMER;
cistrome.shinyapps.io/timer) (17,18),
Gene Expression across Normal and Tumor tissue (GENT;
medical-genome.kribb.re.kr/GENT) (19,20),
University of California, Santa Cruz (UCSC) Xena (xenabrowser.net)
(21), Gene expression Profiling
Interactive Analysis 2 (GEPIA2; gepia2.cancer-pku.cn) (22) and Kaplan-Meier plotter (kmplot.com/analysis) (23). The BLCA microarray datasets GSE13507
(24), GSE120736 (25) and GSE31684 (25,26) were
downloaded from the GEO database to analyze the expression of
ST3GAL5. The Lee Bladder (27),
Blaveri Bladder 2 (28),
Sanchez-Carbayo Bladder 2 (29) and
Stransky Bladder (30) datasets from
the Oncomine database were extracted and processed using the R
package ‘ROncomine’ v0.0.0.9 (github.com/yikeshu0611/ROncomine). The datasets from
Genomic Data Commons (GDC; gdc.cancer.gov), The Cancer Genome Atlas (TCGA;
cancergenome.nih.gov) and
Genotype-Tissue Expression (GTEx; commonfund.nih.gov/GTEx) databases were downloaded
using UCSC Xena browser tool (xenabrowser.net/). In the Oncomine database, the
default settings were used and the threshold parameters were as
follows: P<1×10−4, |fold change|>2 and gene rank
in the top 10%. In the GENT database, data were analyzed using the
Human Genome U133 Plus 2.0 Array platform (http://www.affymetrix.com/support/technical/byproduct.affx?product=hg-u133-plus).
Enrichment analysis
The Gene Ontology (GO) terms and Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathway enrichment analysis were
determined using the R package ‘clusterProfiler’ v3.14.3 (31), and the Reactome pathway enrichment
analysis was performed using the R package ‘ReactomePA’ v1.30.0
(32). Subsequently, the
gene-concept network analysis were performed using the R package
‘clusterProfiler’ and ‘ReactomePA’. Microarray datasets of
accession number GSE83586 (33) were
downloaded from the GEO database in order to investigate the
relevant signaling pathways using GSEA. According to the mean
expression value of ST3GAL5 in the GSE83586 dataset, the matrix
file was divided into high- and low-expression groups, and GSEA was
performed using GSEA 4.02 software (34) in order to determine the KEGG pathways
(c2.cp.kegg.v7.0.symbols) associated with high and low expression
of ST3GAL5. Gene set permutations were performed 1,000 times for
each analysis. The false discovery rate <0.25, |normalized
enrichment score| >1 and nominal P<0.05 were considered to
indicate a statistically significant difference. Subsequently,
replotting of the output from the GSEA report folder was conducted
using the R package ‘Rtoolbox’ v1.4 (github.com/PeeperLab/Rtoolbox).
Data management and statistical
analysis
Cancer staging was assessed using the 8th edition of
the UICC/AJCC cancer staging system. The gene expression profile
and survival data were downloaded, converted, constructed and
managed using Microsoft Office Excel 2016 (Microsoft Corporation).
All statistical analyses were performed using R software
(www.r-project.org; v3.6.1). The box plot was
constructed using the R package ‘ggplot2’ v3.2.1 (35). The Cnetplot was constructed using the
R package ‘clusterProfiler’ and ‘ReactomePA’. Student's t-test was
used to compare the means of two independent samples, and one-way
ANOVA was used to compare the means of multiple independent samples
followed by Bonferroni post hoc test for multiple comparisons.
Kaplan-Meier analysis and Cox proportional hazard models were used
for survival analysis by using R package ‘survival’ v3.1.8 and
‘survminer’ v0.4.6. A multivariate Cox proportional hazards
regression model was performed to adjust for covariate effects, and
stratification analysis was used to reduce the potential
confounding effect on the estimation of hazard ratio (HR). Missing
data were coded and excluded from the analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of ST3GAL5 in different
types of cancer
The differences in ST3GAL5 expression between
various types of cancer and paired healthy tissues were compared
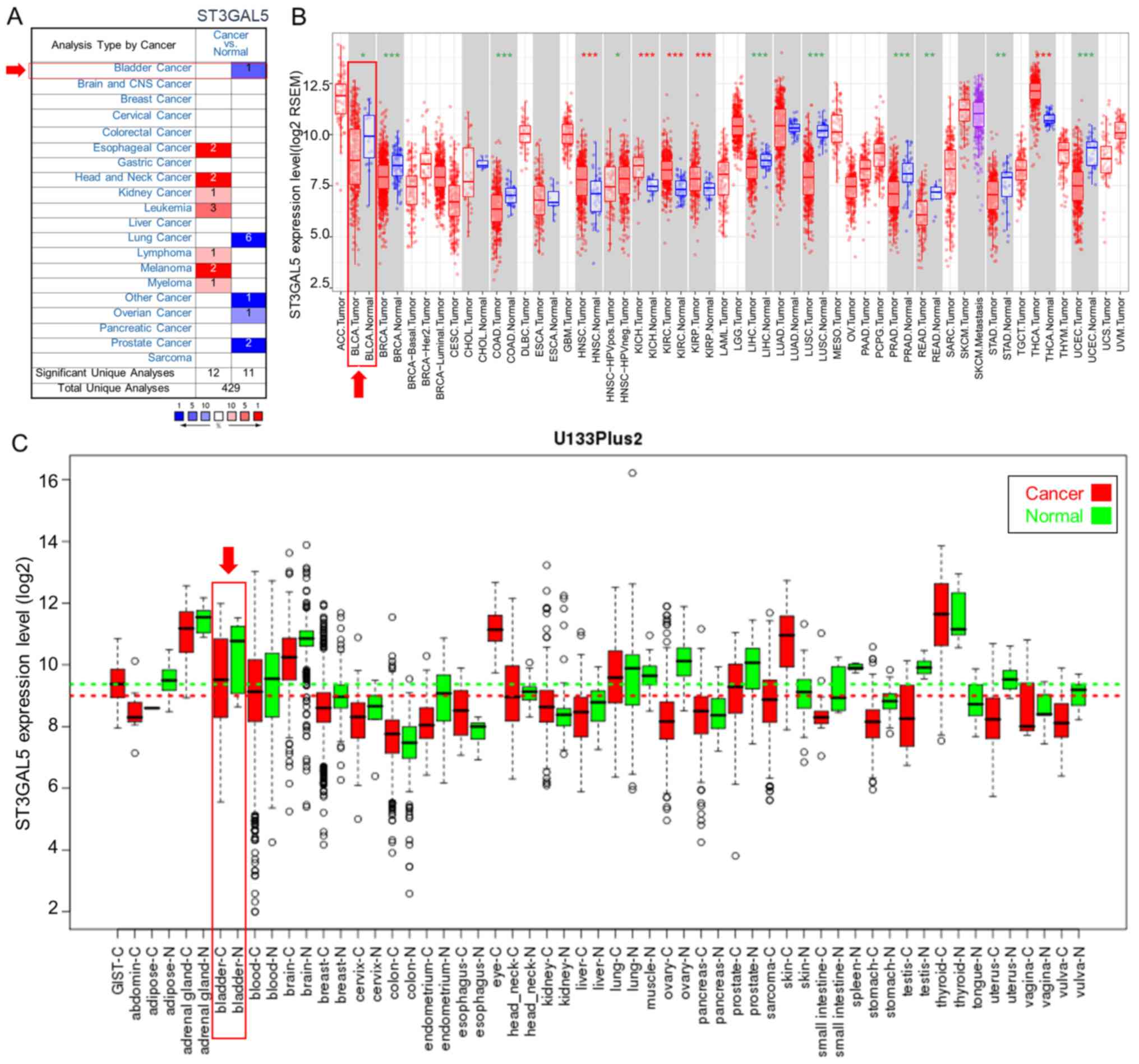
from three independent bioinformatics databases. In the Oncomine
database, the comparison between each type of cancer and healthy
tissues identified the downregulation of ST3GAL5 expression in
bladder, lung, ovarian, prostate and ‘other’ cancers, and the
upregulation of ST3GAL5 expression in esophageal cancer, head and
neck cancer, kidney cancer, leukemia, lymphoma, melanoma and
myeloma (Fig. 1A). ST3GAL5
expression was also analyzed in tumor and healthy tissues using
TCGA data and the TIMER tool (Fig.
1B). Among the different types of cancer, 10 presented
significantly lower ST3GAL5 expression, and five had significantly
higher ST3GAL5 expression compared with paired healthy tissues
(Fig. 1B). Furthermore, data from
the GENT database indicated that ST3GAL5 expression was
downregulated in certain cancer types, including bladder, blood,
brain, breast, liver, ovary, prostate, stomach and testicular
cancers (Fig. 1C). The three
databases demonstrated the downregulation of ST3GAL5 in different
cancer types. Furthermore, ST3GAL5 expression in BLCA tissues was
significantly decreased in the three databases compared with paired
healthy tissues.
Expression of ST3GAL5 in BLCA and
healthy bladder tissues
To observe the expression of ST3GAL5 in BLCA, three
independent datasets from TGCA + GTEx, Oncomine and GEO databases
were analyzed. Data from TCGA + GTEx database were acquired using
the USCS Xena browser tool. Moreover, data from the Oncomine Lee
Bladder dataset were extracted and processed using the R package
‘ROncomine’. The GEO datasets were acquired from the accession
number GSE13507. The results demonstrated a significant
downregulation of ST3GAL5 in BLCA tissues compared with healthy
bladder tissues (Fig. 2A-C).
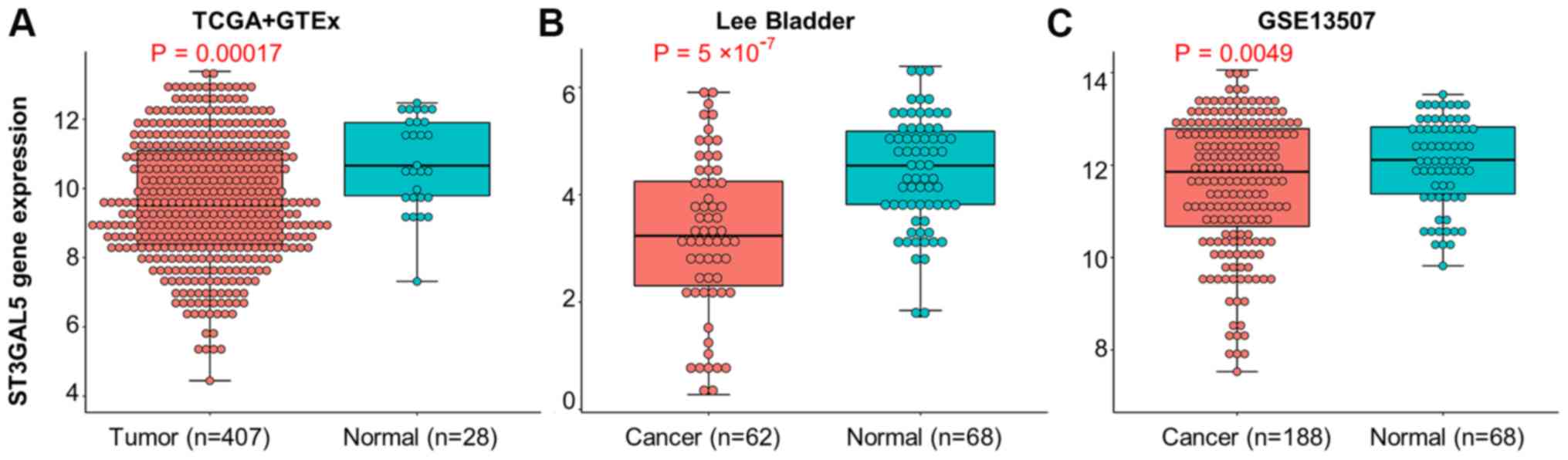 | Figure 2.Expression of ST3GAL5 between BLCA
and healthy bladder tissues. (A) Expression of ST3GAL5 in BLCA from
TCGA + GTEx databases, the data was downloaded from UCSC Xena
browser tool. (B) Expression of ST3GAL5 in BLCA from the Oncomine
database, the threshold was designed using default settings
parameters: P<1×10−4, |fold-change|>2, and gene
rank in top 10%. (C) The expressions of ST3GAL5 in BLCA from the
GEO database under accession numbers GSE13507. TCGA, The Cancer
Genome Atlas; GTEx, Genotype-Tissue Expression; UCSC, University of
California, Santa Cruz; BLCA, bladder urothelial carcinoma;
ST3GAL5, ST3 β-galactoside α-2,3-sialyltransferase 5. |
Expression of ST3GAL5 in MIBC and
high-grade BLCA tissues
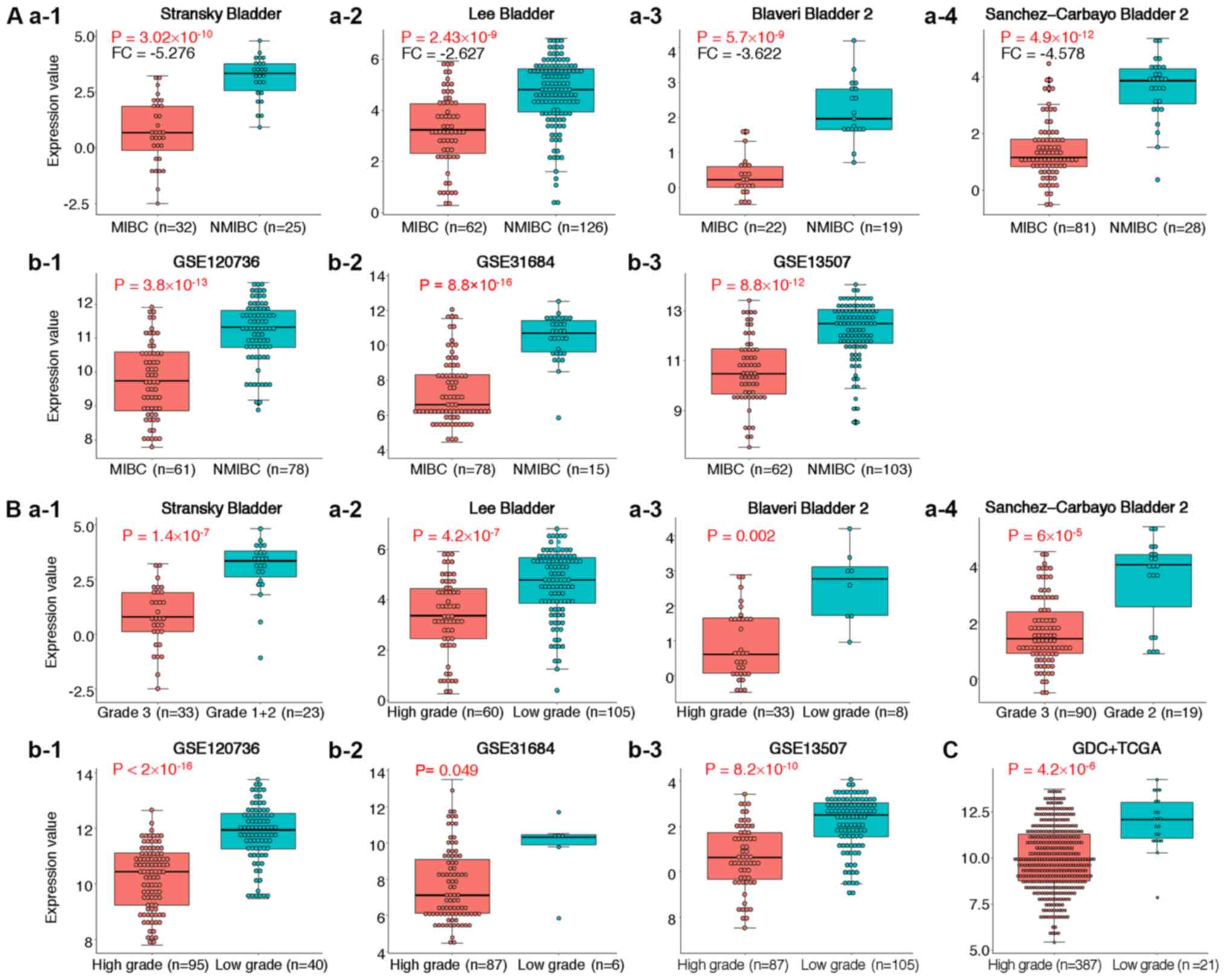
To further determine of ST3GAL5 expression in MIBC
and high-grade BLCA, four individual datasets from the Oncomine
database were analyzed (Table I).
The results from meta-analysis demonstrated that ST3GAL5 was
significantly downregulated in MIBC across the four datasets
(Table I). In these four datasets,
ST3GAL5 was significantly downregulated in MIBC and high-grade BLCA
(Fig. 3A-a1-4 and B-a1-4). The data
acquired from the GEO datasets GSE120736, GSE31684 and GSE13507
also presented significantly lower expression of ST3GAL5 in MIBC
and high-grade BLCA (Fig. 3A-b1-3 and
B-b1-3, respectively). In addition, decreased expression of
ST3GAL5 in high-grade BLCA tissues was reported in the GDC + TCGA
BLCA datasets, using the USCS Xena browser tool (Fig. 3C). Taken together, these results
demonstrated that ST3GAL5 downregulation was associated with MIBC
and high-grade BLCA.
 | Table I.Comparison of ST3 β-galactoside
α-2,3-sialyltransferase 5 across four datasets in the
downregulation analysis from the Oncomine database. |
Table I.
Comparison of ST3 β-galactoside
α-2,3-sialyltransferase 5 across four datasets in the
downregulation analysis from the Oncomine database.
| Dataset | FC | P-value | Gene rank | MIBC | NMIBC |
|---|
| Sanchez-Carbayo
Bladder 2 | −9.324 |
2.43×10−9 | 315 (in top
3%) | 32 | 25 |
| Blaveri Bladder
2 | −3.622 |
2.86×10−9 | 66 (in top 2%) | 62 | 126 |
| Stransky
Bladder | −5.276 |
3.02×10−10 | 3 (in top 1%) | 22 | 19 |
| Lee Bladder | 2.627 |
2.43×10−12 | 7 (in top 1%) | 81 | 28 |
Association between ST3GAL5 expression
and clinicopathological characteristics of patients with BLCA
The present study investigated the association
between ST3GAL5 mRNA expression, promoter methylation level and the
clinicopathological characteristics of patients with BLCA from the
TCGA-BLCA dataset by using the Xena web tool. Compared with healthy
bladder tissues, the expression of ST3GAL5 was downregulated in
tissues from primary tumors, Stage IV cancer, extreme weight,
smoking for >15 years, non-papillary tumors and nodal metastasis
status N1 (Table II). Furthermore,
ST3GAL5 expression was downregulated in male and female patients
with BLCA. However, the level of ST3GAL5 promoter methylation in
patients with BLCA was significantly decreased, regardless of
patient clinicopathological characteristics, including cancer
stage, ethnicity, sex, age, weight, smoking status, nodal
metastasis status and histological subtype compared with healthy
patients (Fig. 4). Therefore, it was
hypothesized that decreased ST3GAL5 promoter methylation may be
positively associated with numerous clinicopathological
characteristics of patients with BLCA.
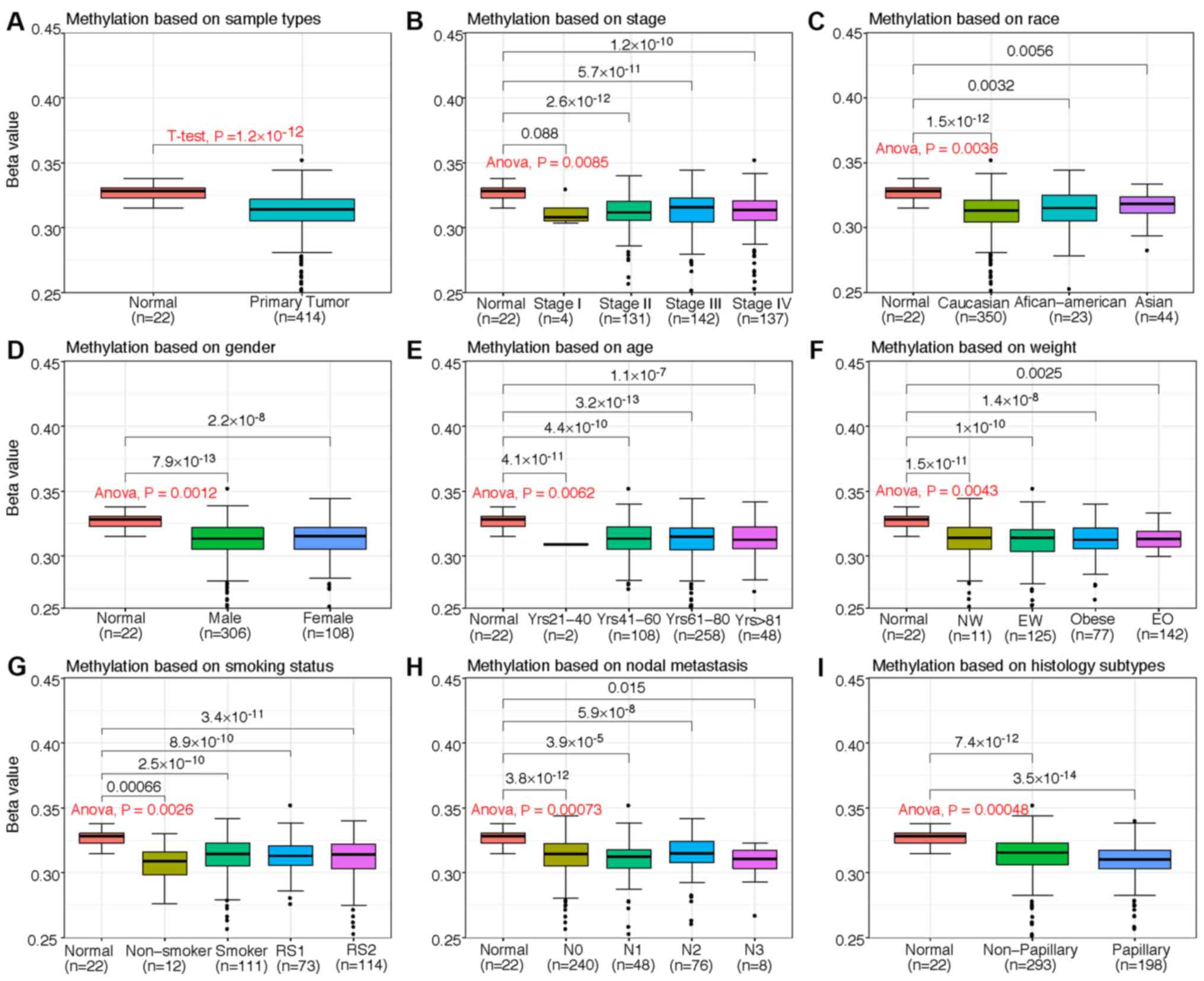 | Figure 4.Box plots from TCGA clinical data
according to categorization of the clinicopathological
characteristics of patients with BLCA and healthy patients
(normal). Promoter methylation of ST3GAL5 in BLCA (different color
plot) and healthy (red plot) tissues based on (A) sample type, (B)
individual cancer stages, (C) ethnicity, (D) sex, (E) age, (F)
weight, (G) smoking status, (H) nodal metastasis status and (I)
histological subtype. The β-value for assessment of methylation
level ranged from 0 (least methylated) to 1 (most methylated). NW,
normal weight; EW, extreme weight; EO, extreme obese; RS1, reformed
smoker (<15 years); RS2, reformed smoker (>15 years); TCGA,
The Cancer Genome Atlas; BLCA, bladder urothelial carcinoma;
ST3GAL5, ST3 β-galactoside α-2,3-sialyltransferase 5; Yrs, years;
N1, metastases in 1–3 axillary lymph nodes; N2, metastases in 4–9
axillary lymph nodes; N3, metastases in ≥10 axillary lymph
nodes. |
 | Table II.Association between ST3 β-galactoside
α-2,3-sialyltransferase 5 expression and clinicopathological
characteristics of patients with bladder urothelial carcinoma. |
Table II.
Association between ST3 β-galactoside
α-2,3-sialyltransferase 5 expression and clinicopathological
characteristics of patients with bladder urothelial carcinoma.
|
|
| Expression
value | P-value |
|
|
|
|
|
| Parameter | Sample (n) | Mean | SD | t-test or
ANOVA | Multiple
comparisons |
|---|
| Sample type |
|
|
| 0.023 |
|
|
Healthy | 21 | 9.772 | 1.979 |
|
|
| Primary
tumor | 408 | 8.855 | 1.792 |
|
|
| Cancer stage |
|
|
|
1.88×10−6 |
|
|
Healthy | 21 | 9.772 | 1.979 |
|
|
| Primary
tumor |
|
|
|
|
|
|
Stage I | 4 | 10.241 | 1.705 |
| 1.000 |
|
Stage II | 130 | 9.470 | 1.839 |
| 1.000 |
|
Stage III | 140 | 8.644 | 1.761 |
| 0.067 |
|
Stage IV | 134 | 8.432 | 1.676 |
| 0.014 |
| Ethnicity |
|
|
| 0.121 |
|
|
Healthy | 21 | 9.772 | 1.979 |
|
|
| Primary
tumor |
|
|
|
|
|
|
Caucasian | 347 | 8.852 | 1.817 |
| 0.151 |
|
African-American | 21 | 9.147 | 1.745 |
| 1.000 |
|
Asian | 40 | 8.715 | 1.829 |
| 0.192 |
| Sex |
|
|
| 0.067 |
|
|
Healthy | 21 | 9.772 | 1.979 |
|
|
| Primary
tumor |
|
|
|
|
|
|
Male | 302 | 8.821 | 1.846 |
| 0.064 |
|
Female | 106 | 8.945 | 1.718 |
| 0.174 |
| Age, years |
|
|
| 0.018 |
|
|
Healthy | 21 | 9.772 | 1.979 |
|
|
| Primary
tumor |
|
|
|
|
|
|
21-40 | 2 | 10.690 | 0.396 |
| 1.000 |
|
41-60 | 106 | 9.156 | 1.813 |
| 1.000 |
|
61-80 | 253 | 8.700 | 1.760 |
| 0.095 |
|
>80 | 47 | 8.919 | 2.024 |
| 0.737 |
| Weight |
|
|
| 0.012 |
|
|
Healthy | 21 | 9.772 | 1.979 |
|
|
| Primary
tumor |
|
|
|
|
|
|
Normal weight | 140 | 9.144 | 1.860 |
| 1.000 |
|
Extreme
weight | 124 | 8.509 | 1.828 |
| 0.035 |
|
Obese | 75 | 8.961 | 1.670 |
| 0.720 |
|
Extreme obese | 10 | 8.986 | 1.889 |
| 1.000 |
|
NA | 59 |
|
|
|
|
| Smoking habits |
|
|
| 0.028 |
|
|
Healthy | 21 | 9.772 | 1.979 |
|
|
| Primary
tumor |
|
|
|
|
|
|
Non-smoker | 12 | 9.061 | 1.967 |
| 1.000 |
|
Smoker | 109 | 9.075 | 1.797 |
| 1.000 |
|
Reformed smoker 1
(<15 years) | 72 | 8.947 | 1.716 |
| 0.700 |
|
Reformed smoker 2
(>15 years) | 113 | 8.509 | 1.888 |
| 0.039 |
|
NA | 102 |
|
|
|
|
| Nodal metastasis
status |
|
|
| 0.004 |
|
|
Healthy | 21 | 9.772 | 1.979 |
|
|
| Primary
tumor |
|
|
|
|
|
|
N0 | 237 | 9.033 | 1.857 |
| 0.721 |
|
N1 | 46 | 8.177 | 1.759 |
| 0.008 |
|
N2 | 75 | 8.635 | 1.584 |
| 0.109 |
|
N3 | 8 | 8.631 | 1.693 |
| 1.000 |
|
NA | 42 |
|
|
|
|
| Histological
subtypes |
|
|
|
1.66×10−7 |
|
|
Healthy | 21 | 9.772 | 1.979 |
|
|
| Primary
tumor |
|
|
|
|
|
|
Non-papillary | 271 | 8.544 | 1.654 |
| 0.007 |
|
Papillary | 132 | 9.525 | 1.964 |
| 1.000 |
|
NA | 5 |
|
|
|
|
Association between ST3GAL5 expression
and survival prognosis in patients with BLCA
To investigate the association between ST3GAL5
expression and survival prognosis in patients with BLCA,
Kaplan-Meier survival analysis was performed using data from the
GDC, TCGA and GEO databases via the UCSC Xena browser and
Kaplan-Meier plotter web tools. The 5-year overall survival (OS),
disease specific survival, progression free interval and relapse
free survival were all positively associated with lower ST3GAL5
expression in patients with BLCA (Fig.
5A-E).
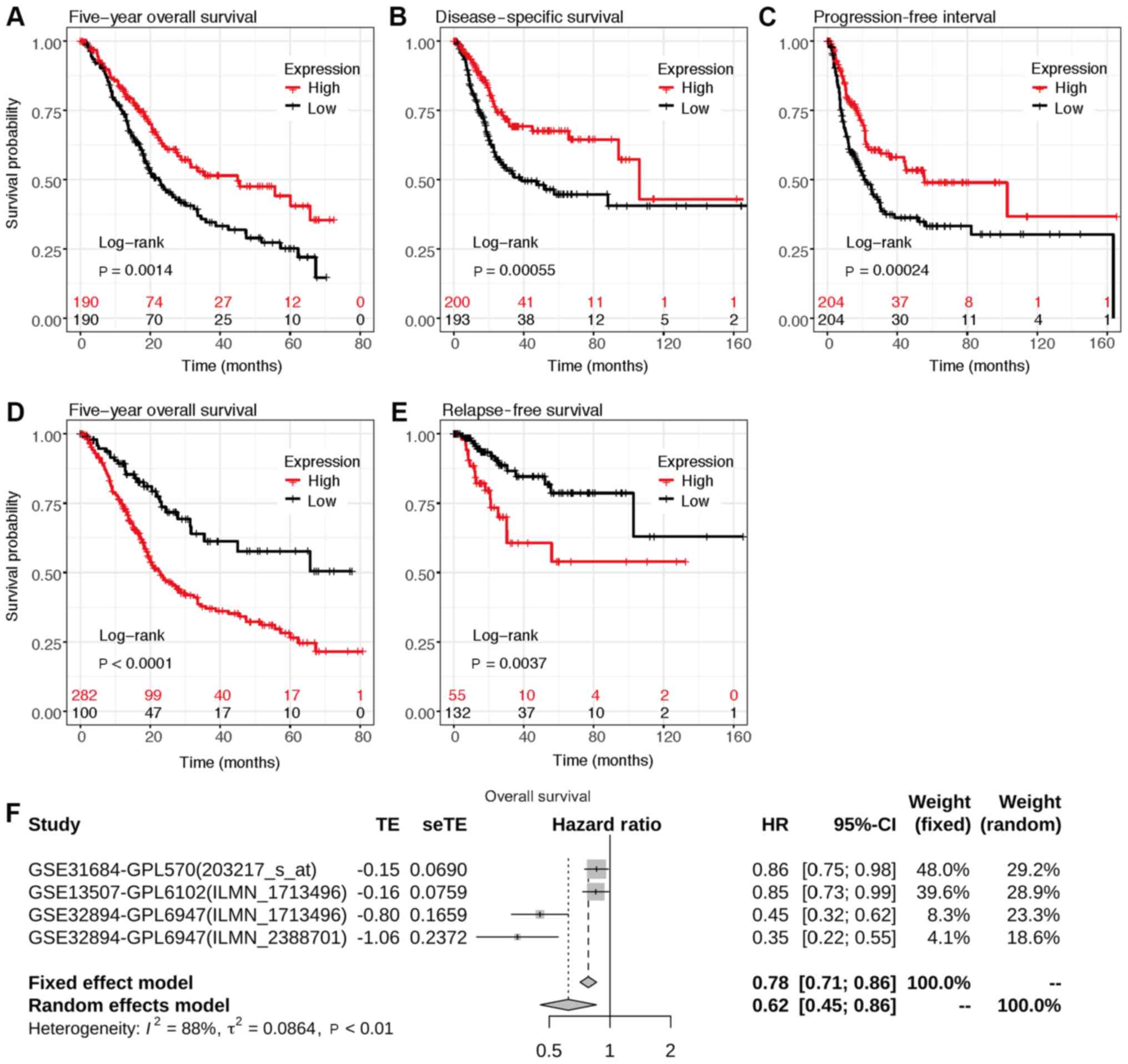 | Figure 5.Assessment of the 5-year overall
survival according to ST3GAL5 expression in patients with BLCA.
Survival plot data from the UCSC Xena browse tool, gene expression
data and (A) overall survival, (B) disease specific survival and
(C) progression free interval information were downloaded from
TCGA-BLCA datasets. Survival plots from the KM plotter, gene
expression data and (D) overall survival and (E) relapse free
survival information were downloaded from the GEO, EGA and TCGA
datasets. Analyses focused on ST3GAL5 expression in patients with
BLCA. (F) Forest plots of the GEO datasets evaluating association
of ST3GAL5 with the overall survival in patients with BLCA using
GENT2 web tool. TCGA, The Cancer Genome Atlas; BLCA, bladder
urothelial carcinoma; ST3GAL5, ST3 β-galactoside
α-2,3-sialyltransferase 5; GEO, Gene Expression Omnibus; EGA,
European Genome-phenome Archive; GENT, Gene Expression across
Normal and Tumor tissue; UCSC, University of California, Santa
Cruz; HR, hazard ratio. |
Meta-survival analysis of OS was performed using
data from GENT2 web (gent2.appex.kr/gent2) tools and depicted as
forest plots (Fig. 5F). The results
demonstrated that low ST3GAL5 expression was associated with poor
OS [P<0.001; HR, 2.934; 95% CI (1.916–4.493);
τ2, 0.086; I2, 0.884]. These results
indicated the prognostic relevance of ST3GAL5 expression in
patients with BLCA.
Enrichment analysis genes co-expressed
with ST3GAL5 in BLCA samples
The top 250 genes that were positively co-expressed
with ST3GAL5 in BLCA samples were identified using Oncomine
Stransky Bladder dataset and GEPIA2 and UALCAN web tools. The genes
common to these three databases were selected for further analysis.
In total, 33 genes were identified as positively co-expressed with
ST3GAL5 in BLCA samples. The names of the genes were as follows:
[methyltransferase like 7A, SMAD6, ATPase phospholipid transporting
8B1, sortilin related receptor 1, trafficking kinesin protein 1,
pterin-4 α-carbinolamine dehydratase 1, cytochrome b5 type A,
isocitrate dehydrogenase (NADP(+)) 1, fructose-bisphosphatase 1,
GATA binding protein 3, cathepsin H, dual specificity phosphatase
2, TP53 target 1, inhibitor of DNA binding (ID) 1, phospholipase C
eta 2, solute carrier family (SLC) 14 member 1 (Kidd blood group),
arachidonate 5-lipoxygenase, PPFIA binding protein 2, transmembrane
protein 63A, 4-aminobutyrate aminotransferase, intraflagellar
transport 140, SLC23A2, zinc finger protein 211, keratin associated
protein 5–9, oviductal glycoprotein 1, family with sequence
similarity 13 member A, ID2, carbonyl reductase 4,
glycerol-3-phosphate dehydrogenase 1 like, carnitine
O-octanoyltransferase, tubulin tyrosine ligase like 3, aldehyde
dehydrogenase 4 family member A1 and malic enzyme 3].
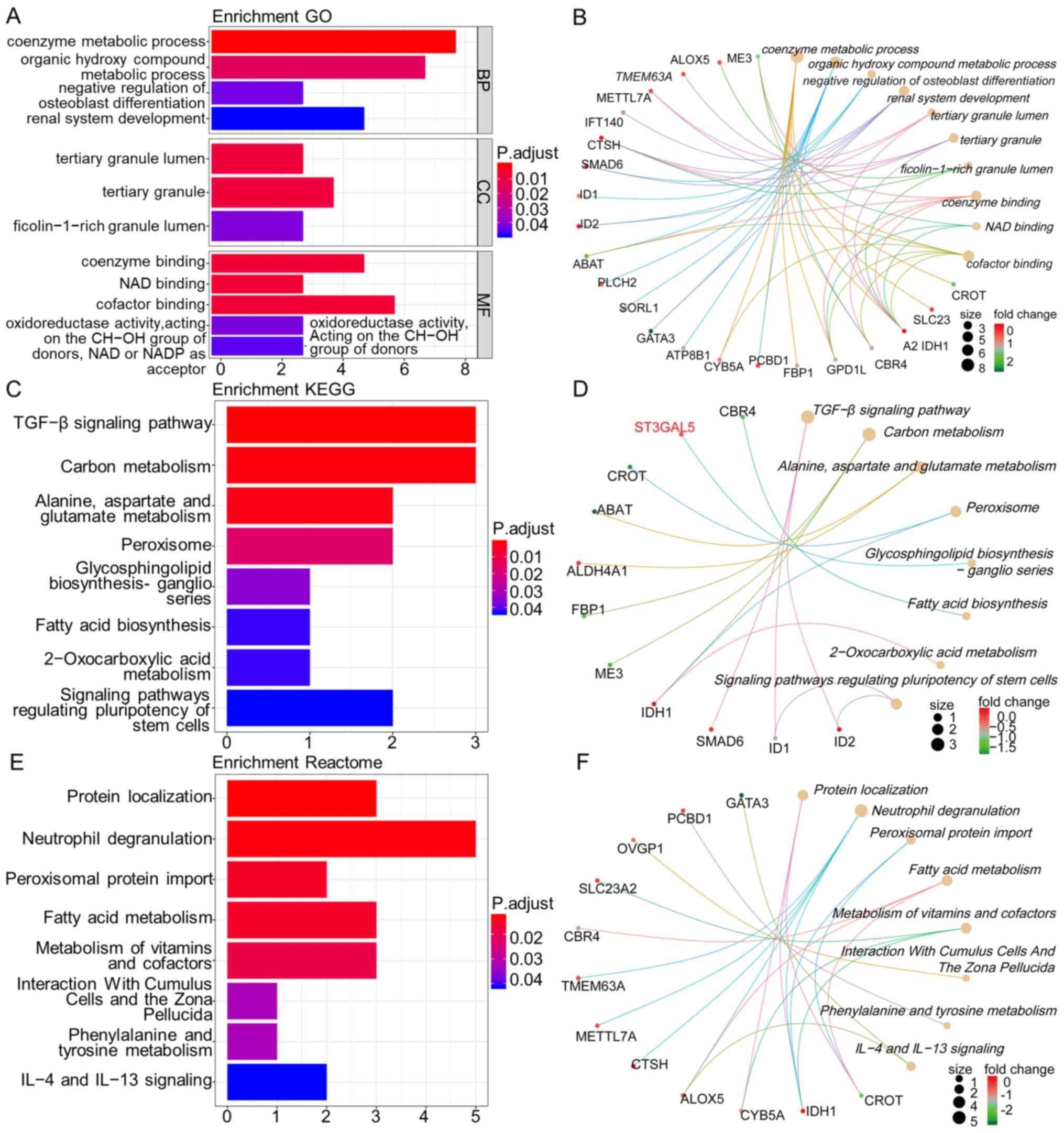
Subsequently, GO, KEGG and Reactome pathway
enrichment analyses, and gene-concept network analysis were
performed with ST3GAL5 and the 33 positively co-expressed genes by
using the R packages ‘clusterProfiler’ and ‘ReactomePA’. GO terms
functional enrichment analysis was performed with ST3GAL5 and its
associated genes to determine the functions associated with
biological processes (BP), molecular functions (MF) and cellular
components (CC). ST3GAL5 and its co-expressed genes were
predominantly associated with ‘coenzyme metabolic process’,
‘organic hydroxy compound metabolic process’, ‘negative regulation
of osteoblast differentiation’, ‘renal system development’,
‘tertiary granule lumen’, ‘tertiary granule’, ‘ficolin-1-rich
granule lumen’, ‘coenzyme binding’, ‘NAD binding’ and ‘cofactor
binding’ (Fig. 6A and B).
Furthermore, the KEGG pathways analysis for ST3GAL5
and its co-expressed genes demonstrated their association with
‘transforming growth factor (TGF)-β signaling pathway’, ‘carbon
metabolism’, ‘alanine, aspartate and glutamate metabolism’,
‘peroxisome’, ‘glycosphingolipid biosynthesis-ganglio series’,
‘fatty acid biosynthesis’, ‘2-oxocarboxylic acid metabolism’ and
‘signaling pathways regulating pluripotency of stem cells’
(Fig. 6C and D).
Next, Reactome pathway analysis of ST3GAL5 and its
co-expressed genes identified highlighted their association with
‘protein localization’, ‘neutrophil degranulation’, ‘peroxisomal
protein import’, ‘fatty acid metabolism’, ‘metabolism of vitamins
and cofactors’, ‘interaction with cumulus cells and the zona
pellucida’, ‘phenylalanine and tyrosine metabolism’ and
‘interleukin (IL)-4 and IL-13 signaling’ (Fig. 6E and F). All these pathways may
therefore be associated with BLCA tumor progression and
tumorigenesis.
GSEA analysis between high and low
ST3GAL5 expression in BLCA
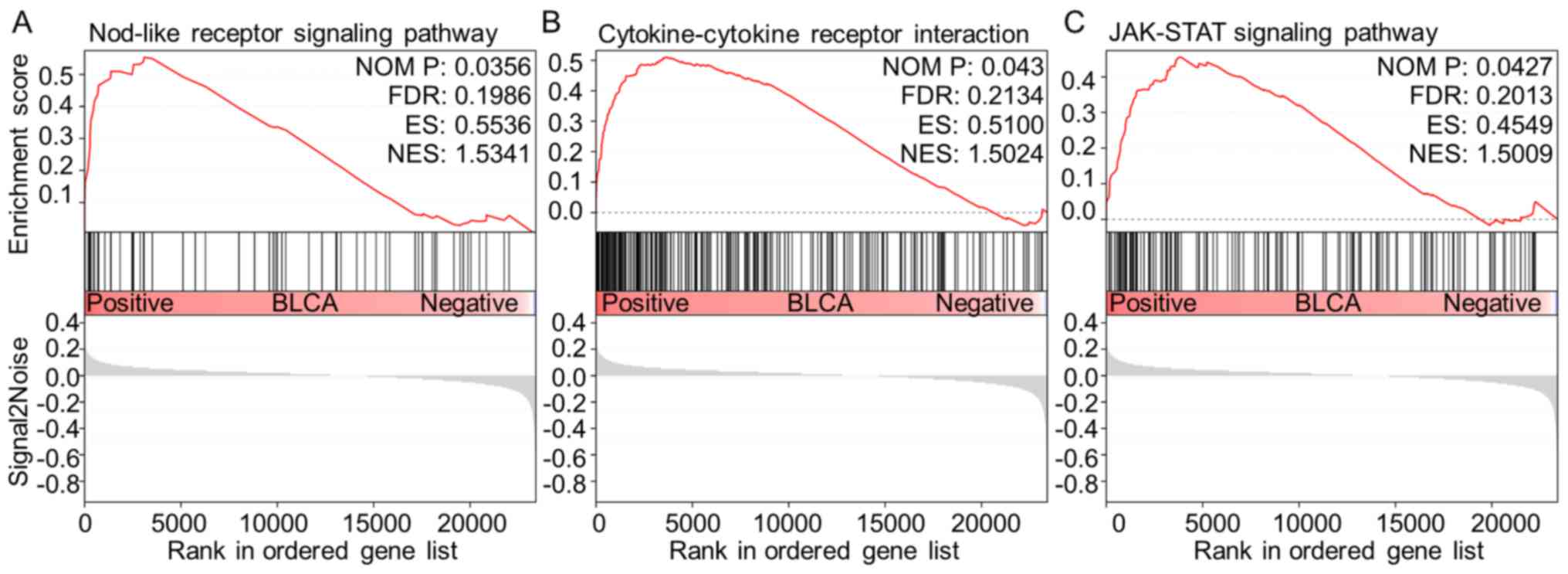
To further identify the signaling pathways that are
differentially activated in BLCA, GSEA was performed to investigate
the difference between high- (n=124) and low-ST3GAL5 (n=183)
expression groups by using the GEO dataset GSE83586. Three
tumor-associated pathways were identified as significantly
associated with the downregulation of ST3GAL5 expression in BLCA
tissues, including ‘NOD-like receptor (NLR) signaling pathway’,
‘cytokine-cytokine receptor interaction’ and ‘Janus kinase
(JAK)-STAT signaling pathway’ (Table
III; Fig. 7).
 | Table III.Gene set enrichment analysis in the
group with low expression levels of ST3 β-galactoside
α-2,3-sialyltransferase 5 in bladder urothelial carcinoma. |
Table III.
Gene set enrichment analysis in the
group with low expression levels of ST3 β-galactoside
α-2,3-sialyltransferase 5 in bladder urothelial carcinoma.
| Gene set name | NES | NOM P-value | FDR |
|---|
|
KEGG_SYSTEMIC_LUPUS_ERYTHEMATOSUS | 1.788003 | 0.00396 | 0.212486 |
|
KEGG_AUTOIMMUNE_THYROID_DISEASE | 1.596979 | 0.027237 | 0.242572 |
|
KEGG_FC_GAMMA_R_MEDIATED_PHAGOCYTOSIS | 1.593668 | 0.028689 | 0.212411 |
|
KEGG_ALLOGRAFT_REJECTION | 1.579419 | 0.044747 | 0.208527 |
|
KEGG_GLYCOSAMINOGLYCAN_BIOSYNTHESIS_CHONDROITIN_SULFATE | 1.577063 | 0.026923 | 0.189083 |
|
KEGG_NOD_LIKE_RECEPTOR_SIGNALING_PATHWAY | 1.534106 | 0.035644 | 0.198596 |
|
KEGG_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION | 1.502448 | 0.042969 | 0.213385 |
|
KEGG_JAK_STAT_SIGNALING_PATHWAY | 1.500872 | 0.042718 | 0.201288 |
Discussion
BC is the most common malignancy of the urinary
system, and ~90% of BC cases are urothelial carcinoma (5). Furthermore, BC can be low grade or high
grade and can also be divided into MIBC and NMIBC; low grade BC
rarely invades the muscular wall of the bladder and patients rarely
succumb to low grade BC, while high grade BC is more likely to
result in mortality (6).
Furthermore, patients with NMIBC exhibit a favorable outcome
(5-year overall survival of 95 vs. 69% in MIBC) (36). However, 70% of patients with BC will
experience recurrence following initial treatment (surgery,
radiotherapy or chemotherapy), including 30% out of the 70% of
patients presenting with muscle invasive disease (37). In addition, cancer recurrence and
progression lead to a higher disease stage, ending therefore in a
less favorable outcome (38).
Currently, the etiology of muscle invasion and high-grade
progression in BC remains unknown. It is therefore crucial to
identify an effective biomarker that could predict muscle invasion,
high grade and prognosis in patients with BC.
To the best of our knowledge, ST3GAL5 expression and
its effect on muscle invasion, cancer grade and prognosis in
patients with BLCA have not yet been investigated. The present
study investigated therefore the potential role of ST3GAL5 in BLCA.
In this study, bioinformatics analysis of multiple independent
public databases was performed. The results demonstrated that
ST3GAL5 was downregulated in various types of cancer, including BC,
and that its expression in BLCA tissues was lower compared with
healthy bladder tissues. In addition, ST3GAL5 downregulation was
positively associated with muscle invasion, high grade and a poor
prognosis in patients with BLCA. Collectively, these findings
indicated that ST3GAL5 may be considered as a tumor suppressor gene
in BLCA, and may therefore inhibit the progression of BC to MIBC
and high grade BLCA. These results also highlighted the potential
role of ST3GAL5 as a therapeutic target in BC. However, further
investigation is required to determine the underlying mechanisms of
ST3GAL5 in BC progression and in the prognosis of patients with
BC.
The association between ST3GAL5 expression, promoter
methylation level and the clinicopathological characteristics of
patients with BLCA was examined using TCGA data from the Xena
browser. The results demonstrated that ST3GAL5 expression was
downregulated in high stages and moderate nodal metastasis status
compared with healthy bladder tissues. However, the level of
ST3GAL5 promoter methylation was significantly decreased in BCLA
tissues compared with healthy bladder tissues regardless of the
patients' clinicopathological characteristics, including cancer
stage, ethnicity, sex, age, weight, smoking status, nodal
metastasis status and histological subtype. Furthermore, analysis
of ST3GAL5 expression and DNA methylation status indicated that
ST3GAL5 gene expression may be associated with certain CpG island
sites. CpG islands are CG-rich stretches in the genome concentrated
near transcription start sites; in normal cells they are protected
and therefore are in a non-methylated state, but in tumors they are
specifically methylated. These findings suggested therefore that
ST3GAL5 promoter methylation may be associated with the
clinicopathological characteristics of patients with BC.
ST3GAL5 is a protein that catalyzes the formation of
ganglioside GM3 (7). ST3GAL5 is
upregulated in several types of cancer, such as lung and brain
cancer, and melanoma, and can serve as a tumor-associated
carbohydrate antigen in immunotherapy for cancer (9,10).
Furthermore, ST3GAL5, which encodes GM3, inhibits tumor cell
proliferation through angiogenesis inhibition or decrease in cell
motility (9). Previous studies
reported that ST3GAL5 exerts some anti-proliferative effects in
colon cancer (39), breast cancer
(40,41), liver cancer (42) and other types of tumor (9,10).
Although some studies demonstrated that ST3GAL5 has anti-tumor
effects in human bladder cancer (11,14,39,43), the
underlying mechanisms remain unknown. Furthermore, it was reported
that ST3GAL5 effects could be associated with tumor cell apoptosis
and angiogenesis inhibition (9,12,44).
However, the expression profile and functional role of ST3GAL5 in
BLCA remain unknown.
In the present study, the biological effect of
ST3GAL5 in BLCA was investigated by using bioinformatics analysis
of multiple public databases. Co-expressed genes that were
positively associated with ST3GAL5 expression were identified in
three public databases, and intersecting genes from all databases
were considered to be significantly co-expressed genes. R packages
were then used to identify the signaling pathways associated with
the genes that were positively co-expressed with ST3GAL5 in BLCA
samples. Furthermore, from the perspective of functional
classification, GO enrichment analysis of BP, CC and MF was
performed on ST3GAL5 and its co-expressed genes. The results from
KEGG pathway analysis revealed that the ‘TGF-β signaling pathway’
was significantly associated with ST3GAL5 expression. The
deregulation of this pathway has been reported to result in tumor
progression (45). In healthy and
early-stage cancer, such as breast and prostate cancer cells, the
TGF-β pathway exerts tumor-suppressive properties; however, its
activation in late-stage cancer can promote tumor progression, via
metastasis and chemoresistance (45,46).
Furthermore, the dual function and pleiotropic nature of TGF-β
signaling makes of it a challenging target; therefore, careful
therapeutic dosage of TGF-β drugs and careful patient selection are
required (46). In the present
study, although ST3GAL5 expression was downregulated in BLCA
tissues compared with healthy bladder tissues, ST3GAL5 expression
was significantly downregulated in high grade and advanced stage
BLCA in multiple databases. The significant downregulation in high
grade and advanced stage BLCA may due to the associated activation
of ST3GAL5 and its co-expressed genes following the increase in
TGF-β signaling transduction. Another pathway associated with
ST3GAL5 expression was ‘carbon metabolism’. Cells require
one-carbon units for nucleotide synthesis, methylation and
reductive metabolism, which support the high proliferative rate of
cancer cells (47). A previous study
reported that polymorphisms in one-carbon metabolism and
susceptibility to BC suggested that variation in glutathione
synthesis may contribute to the risk of BC (48). In the present study, Reactome pathway
analysis demonstrated that the main pathway associated with ST3GAL5
expression was ‘protein localization’, and previous studies
reported that changes in subcellular localization of
tumor-associated proteins can influence protein structure and
biological function, which are associated with tumorigenesis, tumor
progression and patient prognosis (49–52).
Another pathway associated with ST3GAL5 expression was ‘neutrophil
degranulation’. Neutrophils have been shown to be the first
responders to inflammation and infection (53). The role of neutrophils in cancer is
multifactorial, but is not fully understood. Furthermore,
neutrophils reflect a state of host inflammation, which is a
hallmark of cancer (54), and can
participate in different stages of the oncogenic process including
tumor initiation, growth, proliferation and metastasis (55,56).
Neutrophil granule proteins released upon cell activation have also
been associated with tumor progression, and this differential
granule mobilization may allow neutrophils and possibly associated
cancer cells to exit the bloodstream and enter inflamed and
infected tissues (53). Since
neutrophils are immune cells, tumor immunity must also be
considered in order to predict the prognosis of patients with BC.
Takeuchi et al (55),
reported that the Tumour-associated macrophage polarized M2
phenotype influences microangiogenesis, pathological outcome, tumor
grade and tumor invasiveness in BC. In the present study, GO
analysis of the BP and MF domains identified co-enzyme involvement
in BP and MF, suggesting that co-enzymes may serve an important
role in the tumorigenesis and tumor progression of BC. However,
further in vitro and in vivo studies are required to
elucidate the biological role of ST3GAL5 in BC. Taken together,
these findings highlighted the important role of ST3GAL5 and its
co-expressed genes in various carcinogenic processes.
A large BLCA dataset from the GEO database was
investigated in the present study. According to the mean value of
ST3GAL5 expression, the dataset was divided into low- and
high-expression groups, and GSEA was used to compare the two
groups. The results identified three tumor-associated pathways that
were associated with ST3GAL5 in BLCA, the ‘NLR signaling pathway’,
‘cytokine-cytokine receptor interaction’ and ‘JAK-STAT signaling
pathway’. NLR signaling pathway is an immunology-signaling pathway,
in which cytosolic NLRs are associated with certain diseases,
including infections, cancer and autoimmune and inflammatory
disorders (57). Furthermore, NLRs
and their downstream signaling components can engage in an
intricate crosstalk with cell death and autophagy pathways, which
are critical processes for cancer progression (58). Kent and Blander (57) reported that chronic inflammation
resulting from the activated NLRs signaling pathway is a powerful
driver of carcinogenesis, which promotes gene mutation, tumor
growth and progression. In the present study, results from KEGG
analysis suggested that lower expression of ST3GAL5 were enriched
in the ‘NLR signaling pathway’. Ozaki and Leonard (59) demonstrated that cytokines are crucial
intercellular regulators and mobilizers of cells that are involved
in innate and adaptive inflammatory host defenses, cell
proliferation, cell differentiation, cell death, angiogenesis, and
development and repair processes associated with the restoration of
homeostasis. In the current study, the ‘cytokine-cytokine receptor
interaction’ was positively associated with lower ST3GAL5
expression, suggesting that ST3GAL5 downregulation may promote
oncogenesis by affecting the immune status of patients with BC. The
JAK-STAT pathway is an essential signaling pathway involved with
numerous cytokines and proliferation factors in mammals (60). Previous studies (60,61)
assessing the JAK-STAT signaling pathway reported highly conserved
programs linking cytokine signaling to the regulation of essential
cellular mechanisms, including cell proliferation, cell invasion,
cell survival, inflammation and immunity. Furthermore, aberrant
active regulation of JAK-STAT signaling pathway has been reported
to contribute to cancer progression and development of metastasis
(60,61). In the present study, results from
pathway analysis suggested that the low expression of ST3GAL5 may
positively affect the progression of BLCA via tumor immunity,
cytokine interaction and cytokine transduction. The results from
this study suggested that immunotherapy may be used in the
treatment of BLCA, and that ST3GAL5 may be considered as a novel
target and potential biomarker in BLCA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key
Scientific and Technological Projects of Xinjiang Production and
Construction Corps (grant no. 2018AB023).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available in the GEO (www.ncbi.nlm.nih.gov/geo), Oncomine (www.oncomine.org) and TGCA (cancergenome.nih.gov) repositories.
Authors' contributions
JL and QW participated in the design of the present
study, performed the statistical analysis and drafted the
manuscript. SO, ZN and GD performed the study and collected
background information and data. SO was a major contributor in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BC
|
bladder cancer
|
|
BLCA
|
bladder urothelial carcinoma
|
|
MIBC
|
muscle invasive bladder cancer
|
|
NMIBC
|
non-muscle invasive bladder cancer
|
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in Europe: Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2015 Disease and Injury Incidence and
Prevalence Collaborators, . Global, regional, and national
incidence, prevalence, and years lived with disability for 310
diseases and injuries, 1990–2015: A systematic analysis for the
Global Burden of Disease Study 2015. Lancet Lond Engl.
388:1545–1602. 2016. View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, Vecchia CL,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyazaki J and Nishiyama H: Epidemiology
of urothelial carcinoma. Int J Urol. 24:730–734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Witjes JA, Lebret T, Compérat EM, Cowan
NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J,
Rouanne M, et al: Updated 2016 EAU Guidelines on Muscle-invasive
and Metastatic Bladder Cancer. Eur Urol. 71:462–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berselli P, Zava S, Sottocornola E, Milani
S, Berra B and Colombo I: Human GM3 synthase: A new mRNA variant
encodes an NH2-terminal extended form of the protein. Biochimica
Biophysica Acta. 1759:348–358. 2006. View Article : Google Scholar
|
|
8
|
Inokuchi JI, Inamori KI, Kabayama K,
Nagafuku M, Uemura S, Go S, Suzuki A, Ohno I, Kanoh H and Shishido
F: Biology of GM3 Ganglioside. Prog Mol Biol Transl. 156:151–195.
2018. View Article : Google Scholar
|
|
9
|
Zheng C, Terreni M, Sollogoub M and Zhang
Y: Ganglioside GM3 and its role in cancer. Curr Med Chem.
26:2933–2947. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hakomori SI and Handa K: GM3 and cancer.
Glycoconjugate J. 32:1–8. 2015. View Article : Google Scholar
|
|
11
|
Satoh M, Ito A, Nojiri H, Handa K,
Numahata K, Ohyama C, Saito S, Hoshi S and Hakomori SI: Enhanced
GM3 expression, associated with decreased invasiveness, is induced
by brefeldin A in bladder cancer cells. Int J Oncol. 19:723–731.
2001.PubMed/NCBI
|
|
12
|
Kawashima N, Nishimiya Y, Takahata S and
Nakayama KI: Induction of Glycosphingolipid GM3 expression by
valproic acid suppresses cancer cell growth. J Biol Chem.
291:21424–21433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohyama C: Glycosylation in bladder cancer.
Int J Clin Oncol. 13:308–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edgar R, Domrachev M and Lash AE: Gene
Expression Omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pander A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li B, Severson E, Pignon JC, Zhao H, Li T,
Novak J, Jiang P, Shen H, Aster JC, Rodig S, et al: Comprehensive
analyses of tumor immunity: Implications for cancer immunotherapy.
Genome Biol. 17:1742016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park SJ, Yoon BH, Kim SK and Kim SY:
GENT2: An updated gene expression database for normal and tumor
tissues. BMC Med Genomics. 12 (Suppl 5):1012019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin G, Kang TW, Yang S, Baek SJ, Jeong YS
and Kim SY: GENT: Gene expression database of normal and tumor
tissues. Cancer Informatics. 10:149–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldman M, Craft B, Hastie M, Repečka K,
McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, et al: The UCSC
Xena platform for public and private cancer genomics data
visualization and interpretation. Biorxiv 326470. 2019.
|
|
22
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anaya J, Reon B, Chen WM, Bekiranov S and
Dutta A: A pan-cancer analysis of prognostic genes. Peer J.
3:e14992016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS,
Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, et al: Predictive value
of progression-related gene classifier in primary non-muscle
invasive bladder cancer. Mol Cancer. 9:32010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riester M, Taylor JM, Feifer A, Koppie T,
Rosenberg JE, Downey RJ, Bochner BH and Michor F: Combination of a
novel gene expression signature with a clinical nomogram improves
the prediction of survival in high-risk bladder cancer. Clin Cancer
Res. 18:1323–1333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riester M, Werner L, Bellmunt J,
Selvarajah S, Guancial EA, Weir BA, Stack EC, Park RS, O'Brien R,
Schutz FA, et al: Integrative analysis of 1q23.3 copy-number gain
in metastatic urothelial carcinoma. Clin Cancer Res. 20:1873–1883.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JS, Leem SH, Lee SY, Kim SC, Park ES,
Kim SB, Kim SK, Kim YJ, Kim WJ and Chu IS: Expression signature of
E2F1 and its associated genes predict superficial to invasive
progression of bladder tumors. J Clin Oncol. 28:2660–2667. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blaveri E, Simko JP, Korkola JE, Brewer
JL, Baehner F, Mehta K, Devries S, Koppie T, Pejavar S, Pejavar S,
et al: Bladder cancer outcome and subtype classification by gene
expression. Clin Cancer Res. 11:4044–4055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanchez-Carbayo M, Socci ND, Lozano J,
Saint F and Cordon-Cardo C: Defining molecular profiles of poor
outcome in patients with invasive bladder cancer using
oligonucleotide microarrays. J Clin Oncol. 24:778–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stransky N, Vallot C, Reyal F,
Bernard-Pierrot I, de Medina SG, Segraves R, de Rycke Y, Elvin P,
Cassidy A, Spraggon C, et al: Regional copy number-independent
deregulation of transcription in cancer. Nat Genet. 38:1386–1396.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu G and He QY: ReactomePA: An
R/Bioconductor package for reactome pathway analysis and
visualization. Mol Biosyst. 12:477–479. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sjödahl G, Eriksson P, Liedberg F and
Höglund M: Molecular classification of urothelial carcinoma: Global
mRNA classification versus tumour-cell phenotype classification. J
Pathology. 242:113–125. 2017. View Article : Google Scholar
|
|
34
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc National Acad Sci. 102:15545–15550. 2005. View Article : Google Scholar
|
|
35
|
Villanueva RAM and Chen ZJ: ggplot2:
Elegant graphics for data analysis. (2nd). Meas Interdiscip Res
Perspectives. 17:160–167. 2019. View Article : Google Scholar
|
|
36
|
Nykopp TK, Batista da Costa J, Mannas M
and Black PC: Current clinical trials in non-muscle invasive
bladder cancer. Curr Urol Rep. 19:1012018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heijden AGvd and Witjes JA: Recurrence,
progression, and follow-up in non-muscle-invasive bladder cancer.
Eur Urol Suppl. 8:556–562. 2009. View Article : Google Scholar
|
|
39
|
Wang H, Isaji T, Satoh M, Li D, Arai Y and
Gu J: Antitumor effects of exogenous ganglioside GM3 on bladder
cancer in an orthotopic cancer model. Urology. 81:210.e211–215.
2012.
|
|
40
|
Li Q, Sun M, Yu M, Fu Q, Jiang H, Yu G and
Li G: Gangliosides profiling in serum of breast cancer patient: GM3
as a potential diagnostic biomarker. Glycoconjugate J. 36:419–428.
2019. View Article : Google Scholar
|
|
41
|
Guthmann MD, Castro MA, Cinat G, Venier C,
Koliren L, Bitton RJ, Vázquez AM and Fainboim L: Cellular and
humoral immune response to N-Glycolyl-GM3 elicited by prolonged
immunotherapy with an anti-idiotypic vaccine in High-Risk and
metastatic breast cancer patients. J Immunother. 29:215–223. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cai H, Zhou H, Miao Y, Li N, Zhao L and
Jia L: MiRNA expression profiles reveal the involvement of miR-26a,
miR-548l and miR-34a in hepatocellular carcinoma progression
through regulation of ST3GAL5. Lab Invest. 97:530–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawamura S, Ohyama C, Watanabe R, Satoh M,
Saito S, Hoshi S, Gasa S and Orikasa S: Glycolipid composition in
bladder tumor: A crucial role of GM3 ganglioside in tumor invasion.
Int J Cancer. 94:343–347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chung TW, Choi HJ, Kim SJ, Kwak CH, Song
KH, Jin UH, Chang YC, Chang HW, Lee YC, Ha KT and Kim CH: The
ganglioside GM3 is associated with cisplatin-induced apoptosis in
human colon cancer cells. PLoS One. 9:e927862014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Massagué J: TGFβ in Cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Colak S and Ten Dijke P: Targeting TGF-β
signaling in cancer. Trends Cancer. 3:56–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Newman AC and Maddocks ODK: One-carbon
metabolism in cancer. Brit J Cancer. 116:1499–1504. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moore LE, Malats N, Rothman N, Real FX,
Kogevinas M, Karami S, García-Closas R, Silverman D, Chanock S,
Welch R, et al: Polymorphisms in one-carbon metabolism and
trans-sulfuration pathway genes and susceptibility to bladder
cancer. Int J Cancer. 120:2452–2458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li X, Che K, Wang L, Zhang T, Wang G, Pang
Z, Shen H and Du J: Subcellular localization of β-arrestin1 and its
prognostic value in lung adenocarcinoma. Medicine. 96:e84502017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu J, Wang H, Huang C and Qian H:
Subcellular localization of MTA proteins in normal and cancer
cells. Cancer Metastasis Rev. 33:843–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim HJ, Lee SY, Kim CY, Kim YH, Ju W and
Kim SC: Subcellular localization of FOXO3a as a potential biomarker
of response to combined treatment with inhibitors of PI3K and
autophagy in PIK3CA-mutant cancer cells. Oncotarget. 8:6608–6622.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Boudhraa Z, Bouchon B, Viallard C, D'Incan
M and Degoul F: Annexin A1 localization and its relevance to
cancer. Clin Sci (Lond). 130:205–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mollinedo F: Neutrophil degranulation,
plasticity, and cancer metastasis. Trends Immunol. 40:228–242.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hanahan D and Weinberg Robert A: Hallmarks
of cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takeuchi H, Tanaka M, Tanaka A, Tsunemi A
and Yamamoto H: Predominance of M2-polarized macrophages in bladder
cancer affects angiogenesis, tumor grade and invasiveness. Oncol
Lett. 11:3403–3408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Coffelt SB, Wellenstein MD and de Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:431–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kent A and Blander JM: Nod-like receptors:
Key molecular switches in the conundrum of cancer. Front Immunol.
5:1852014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Saxena M and Yeretssian G: NOD-like
receptors: Master regulators of inflammation and cancer. Front
Immunol. 5:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ozaki K and Leonard WJ: Cytokine and
cytokine receptor pleiotropy and redundancy. J Biol Chem.
277:29355–29358. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pencik J, Pham HTT, Schmoellerl J,
Javaheri T, Schlederer M, Culig Z, Merkel O, Moriggl R, Grebien F
and Kenner L: JAK-STAT signaling in cancer: From cytokines to
non-coding genome. Cytokine. 87:26–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Trivedi S and Starz-Gaiano M: Drosophila
Jak/STAT signaling: Regulation and relevance in human cancer and
metastasis. Int J Mol Sci. 19:40562018. View Article : Google Scholar
|