Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common cancer worldwide, with high recurrence rate and
metastasis, and remains the leading cause of cancer-associated
mortality in the world (1,2). Despite advances in diagnosis and
treatment, the 5-year survival rate has not improved significantly,
and the prognosis of patients with metastatic disease has remained
poor over the past decade (3,4).
Currently, surgery and adjuvant therapy remain the most popular
means of treatment for patients with OSCC (5,6). Hence,
more exact molecular biomarkers should be identified for prognosis
prediction and for the development of novel therapeutic strategies
for OSCC.
Long non-coding RNAs (lncRNAs) are broadly defined
as non-protein coding transcripts >200 nt in length that lack
protein-coding ability (7–9). An increasing number of studies have
reported that lncRNA is a critical regulator that is involved in
multiple cellular biological processes and is associated with
tumorigenesis, progression and metastasis (10), such as lncRNA P21-associated
non-coding RNA (ncRNA) DNA damage-activated in hepatocellular
carcinoma and lncRNA HOX transcript antisense intergenic RNA in 26
human tumor types (11,12). However, limited studies have
indicated lncRNA signatures as independent biomarkers can predict
OSCC prognosis with high efficiency (13–15).
The aim of the present study was to detect a lncRNA
signature for the identification of OSCC prognostic biomarkers, by
mining lncRNA expression profiles and clinical data in a large
cohort of patients with OSCC in The Cancer Genome Atlas (TCGA)
database. Multiple bioinformatics analyses were performed, such as
Kaplan-Meier (K-M) curve survival analysis, univariate and
multivariate Cox analyses and robust likelihood-based survival
analysis. Seven lncRNAs (LINC01629, AC083967.1, AC067863.1,
AC022092.1, AC005532.1, BX323046.1 and PRR29-AS1) were identified
as potential novel independent prognostic biomarkers for the
prediction of survival in patients with OSCC.
Materials and methods
Clinical information and lncRNA
expression profile collection
OSCC lncRNA data and corresponding clinical
information were downloaded from the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/) using
TCGAbiolinks (R version 3.6) (16).
The OSCC datasets samples from the oral cavity were selected,
including buccal mucosa, tongue, lip, hard palate, alveolar ridge,
floor of the mouth and oral cavity. The latest genome annotation
files were downloaded from GENECODE database (https://www.gencodegenes.org/). Moreover, patients
were excluded from the present study for the following reasons: i)
OSCC plus other malignancies; ii) tissue samples without complete
RNA sequencing data; iii) patients receiving radiotherapy and
chemotherapy prior to surgery; and iv) missing clinical information
values. As a result, a total of 268 OSCC patients and 44 controls
were enrolled in the study.
Differential expression analysis
Differentially expressed lncRNAs (DElncRNAs)
screened from TCGA were analyzed using the ‘edgeR’ package
(17). In order to improve the
screening accuracy and simplify the screening process, the
DElncRNAs were selected with the false discovery rate set at 0.05
and the |fold change value| was >2-fold.
Identification and selection of
prognostic DElncRNAs
The DElncRNA expression profile and clinical
features were incorporated into the complete dataset and further
randomly divided into training datasets and test datasets, using
the ‘caret’ R package (version 6.0–84) (http://caret.r-forge.r-project.org/). The association
between DElncRNAs and patients' OS was analyzed in the training
dataset. Univariate Cox regression analysis was utilized to
identify significant DElncRNAs with a P<0.05 in the R
environment using ‘survival’ packages (version 3.1–8; http://cran.r-project.org/web/packages/survival/). In
order to ensure the reliability of these lncRNAs, a robust
likelihood-based survival analysis was conducted using the R
packages ‘Rbsurv’ (http://bioconductor.org/packages/release/bioc/html/rbsurv.html),
following the procedures described previously by Mao et al
(18). After selecting the DElncRNAs
with the lowest Akaike information criterion (AICs) value,
multivariate Cox regression analysis was performed on these
DElncRNAs, with a P<0.05 to screen for prognosis-associated
DElncRNAs.
Construction of a risk score system
for the key DElncRNAs
According to the results of multivariate Cox
regression analysis, the regression coefficients of prognostic
DElncRNAs were calculated and a risk scoring system was
constructed. The risk score of each patient was determined based on
the following risk formula: Risk score = ∑CoefDElncRNAs
× ExpDElncRNAs (19). In
the formula, CoefDElncRNAs represents the coefficient of
each DElncRNA and ExpDElncRNAs is the expression of each
DElncRNA.
Validation of the risk score
formula
Based on this formula, the risk scores were
calculated in the training and test datasets, and the patients were
categorized into high-risk and low-risk groups using the median
risk score as the cut-off point. To further evaluate the
specificity and sensitivity of the seven-lncRNA signature, receiver
operating characteristic (ROC) curve analysis was performed and the
ROC curve was obtained using the ‘survivalROC’ R package
(https://cran.r-project.org/web/packages/survivalROC).
Once the sensitivity and specificity reached a maximum, an optimal
cut-off point was selected. Based on the cut-off point, the risk
levels were used to classify patients into high-risk and low-risk
groups in the training datasets, the test dataset and the complete
dataset. The differences in survival were further assessed by K-M
curve analyses and log-rank test analyses, using the ‘survminer’ R
package (version, 0.4.6; http://cran.r-project.org/web/packages/survminer/index.html).
Correlation analysis and function
enrichment
In order to explore the potential function for the
prognostic lncRNA, correlation analysis was performed between gene
expression and prognostic lncRNA expression. The correlated genes
were screened based on the following criteria: P<0.05 and
|Pearson coefficient| >0.3. The selected genes were further
applied to conduct pathway enrichment analysis using the online
tool ‘metascape’ (http://metascape.org/gp/index.html). The significant
pathways were screened according to the criterion: q value
<0.05
Results
Preprocessing clinical features and
screening for DElncRNAs
The RNA sequencing (RNAseq) expression profile data
and detailed clinical information on patients with head and neck
cell squamous carcinoma were downloaded from the TCGA database. To
obtain the OSCC datasets, samples from the oral cavity were
selected, including buccal mucosa, tongue, lip, hard palate,
alveolar ridge, floor of the mouth and oral cavity. After excluding
unclear clinical information, a final sample of 268 patients was
considered (Table I). The RNAseq
expression datasets consisted of coding and non-coding genes. To
obtain the lncRNA datasets, the latest genome annotation files
(https://www.gencodegenes.org/) were
downloaded. Based on the annotation file, expression data of a
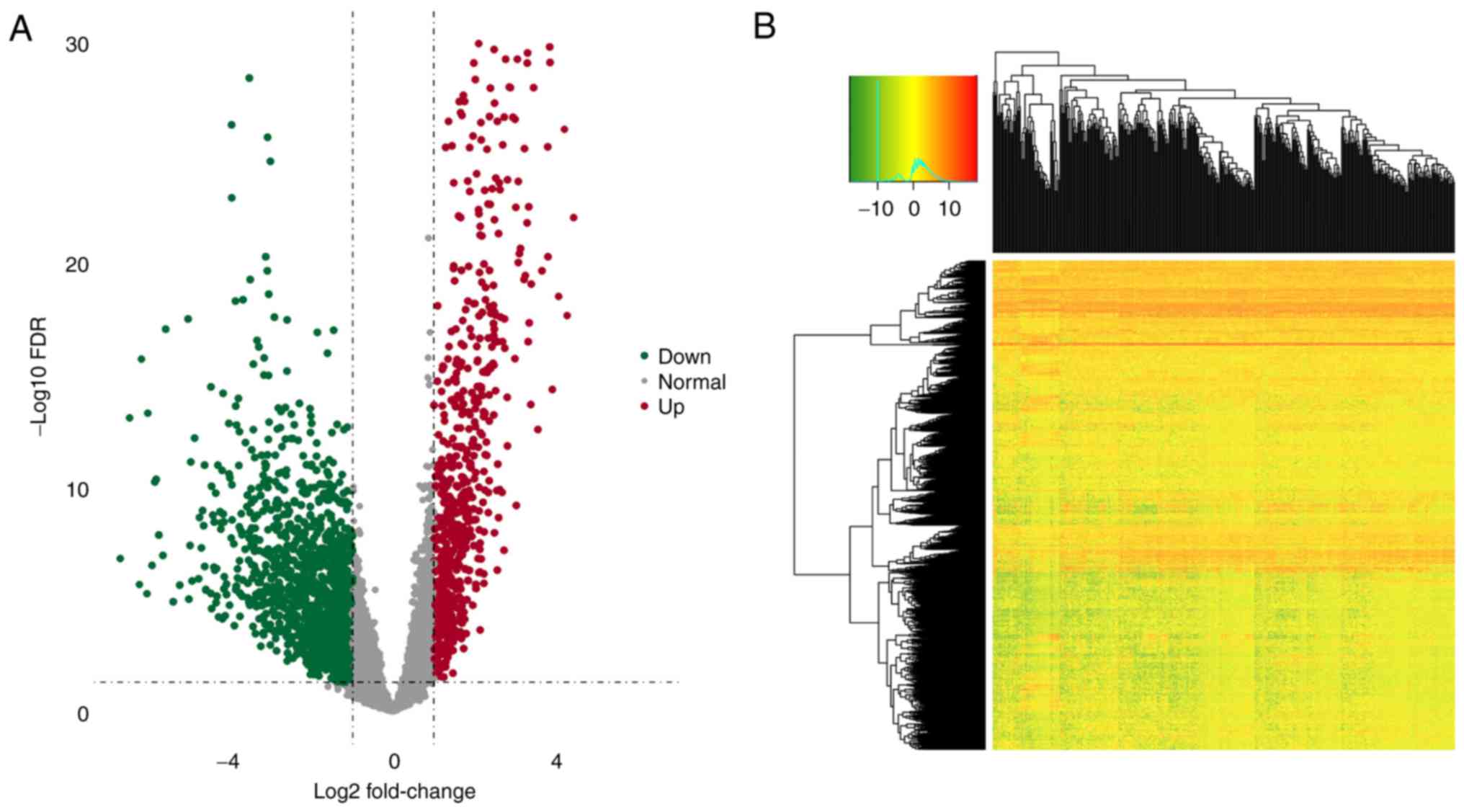
total of 15,183 lncRNAs were extracted. In addition, 2,157
significant DElncRNAs (including 1,454 upregulated and 703
downregulated DElncRNAs) were identified between OSCC tumor and
normal tissues (fold change >2; P<0.05; Fig. 1A and B). The expression data and
clinical features of 2,157 lncRNAs were further integrated into a
complete dataset and patient samples were randomly divided into
training (n=134) and test (n=134) datasets.
 | Table I.Clinical information of patients and
pre-screening of the clinical factors associated with the survival
of OSCC based on the Cox regression analysis. |
Table I.
Clinical information of patients and
pre-screening of the clinical factors associated with the survival
of OSCC based on the Cox regression analysis.
|
| Cox regression
analysis |
|---|
|
|
|
|---|
| TCGA OSCC set
(n=268) | Hazard ratio (95%
CI) | P-value |
|---|
| Age | 1.364
(1.064–1.748) | 0.014a |
| Clinical_M | 1.923
(0.476–7.771) | 0.359 |
| Clinical_N | 1.128
(0.879–1.447) | 0.346 |
| Tumor_stage | 1.178
(0.888–1.563) | 0.257 |
| Sex | 0.957
(0.736–1.243) | 0.742 |
Identification of prognostic lncRNAs
associated with OSCC
In total, 2,157 DElncRNAs in the training dataset
were first used for univariate Cox regression analysis, with
P<0.05, and 81 significant DElncRNAs were identified and further
fitted to a robust likelihood-based survival analysis. As Table
Shown in Table II, 12 feasible and
reliable DElncRNAs were selected (Table
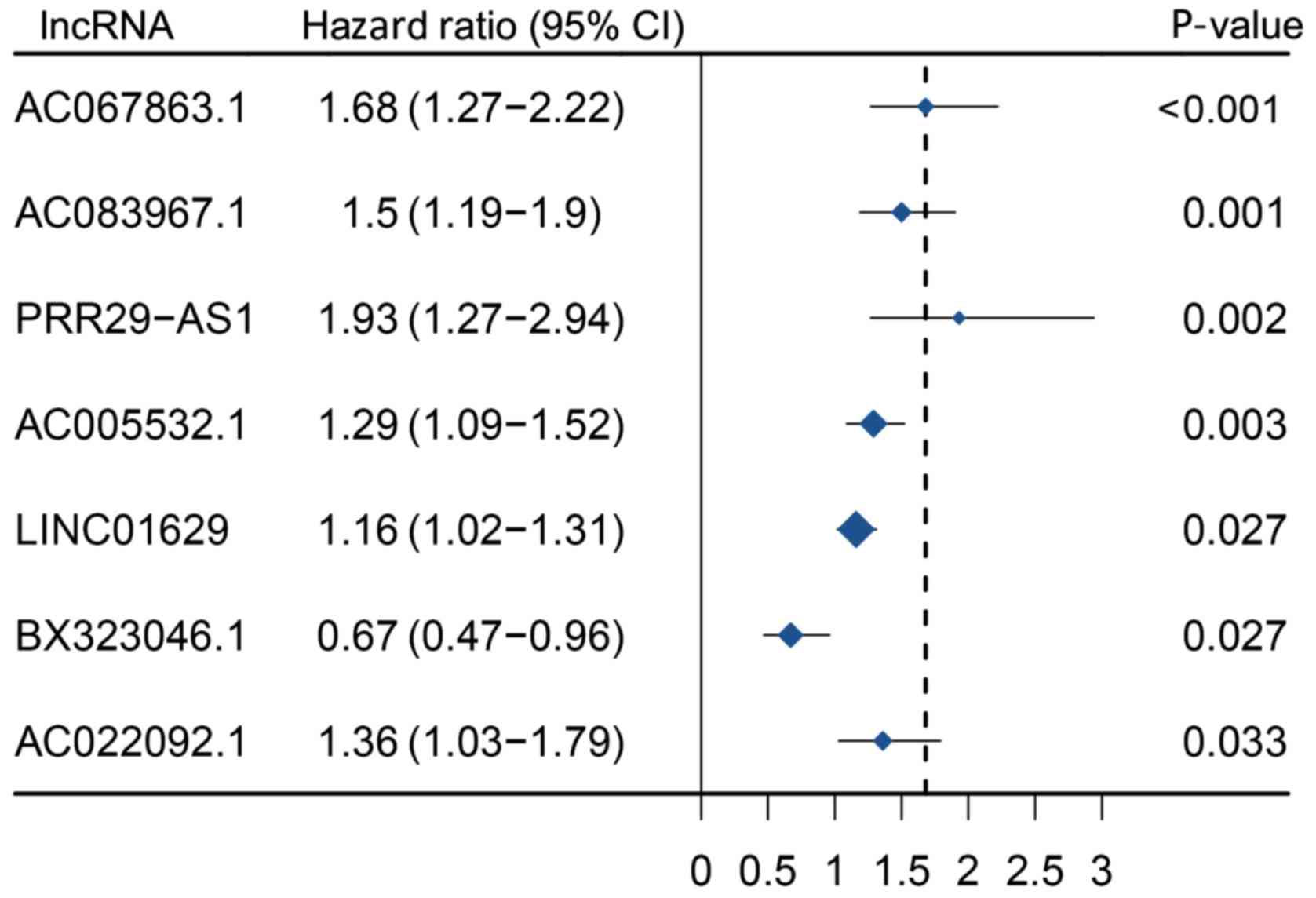
II). Furthermore, multivariate Cox regression analysis yielded
seven prognosis-associated lncRNAs, including LINC01629,
AC083967.1, AC067863.1, AC022092.1, AC005532.1, BX323046.1 and
PRR29-AS1 (Fig. 2). To
comprehensively understand the association between the
seven-DElncRNA signature and the prognosis of OSCC, a
seven-DElncRNA risk scoring system was constructed, based on its
Cox regression coefficient. Risk score = (0.1448) ×
ExpLINC01629 + (0.4073) × ExpAC083967.1 +
(0.5187) × ExpAC067863.1 + (0.3046) ×
ExpAC022092.1 + (0.2533) × ExpAC005532.1 +
(−0.4013) × ExpBX323046.1 + (0.6586) ×
ExpPRR29-AS1. The risk score of the seven-DElncRNA
signature was calculated for each sample in the training and test
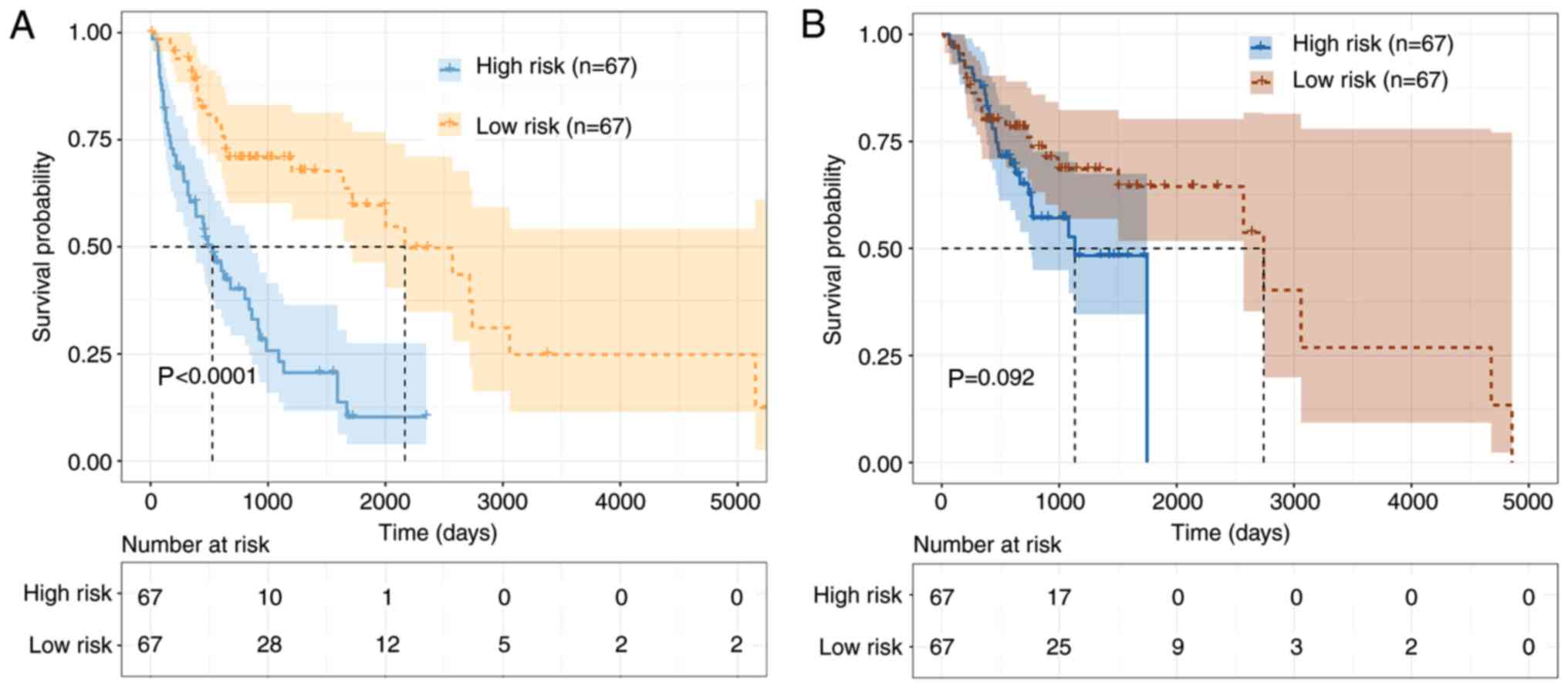
datasets separately. Patients were categorized into the high-risk
group (N=67) and low-risk group (N=67), based on the median risk
score. As shown in Fig. 3, the K-M
curves revealed a significant difference between the high-risk and
low-risk groups in the training dataset, which were not
significantly associated with OSCC in the test dataset
(P=0.092).
 | Table II.Prognostic value of lncRNAs was
screened by performing forward selection in the training dataset
(n=134). |
Table II.
Prognostic value of lncRNAs was
screened by performing forward selection in the training dataset
(n=134).
| lncRNA | nloglik | AIC |
|---|
| LINC01629 | 606.51 |
1215.01a |
| AP002989.1 | 603.15 |
1210.29a |
| AC073578.1 | 597.29 |
1200.58a |
| AC083967.1 | 592.62 |
1193.23a |
| AC067863.1 | 588.19 |
1186.39a |
| AC069503.1 | 586.91 |
1185.82a |
| HCG14 | 586.51 |
1187.02a |
| AC022092.1 | 585.00 |
1186.00a |
| AC005532.1 | 579.36 |
1176.71a |
| BX323046.1 | 577.73 |
1175.45a |
| PRR29-AS1 | 573.64 |
1169.29a |
| AC130456.2 | 572.02 |
1168.05a |
| BLACE | 571.05 | 1168.10 |
| LINC00628 | 570.71 | 1169.43 |
| SMARCA5-AS1 | 569.99 | 1169.98 |
| KLHL30-AS1 | 569.56 | 1171.12 |
| AL391001.1 | 569.55 | 1173.11 |
| AC008011.2 | 569.10 | 1174.20 |
| AL035661.1 | 569.03 | 1176.06 |
| AC007786.1 | 567.94 | 1175.88 |
| LINC02453 | 567.09 | 1176.18 |
| AL021026.1 | 567.08 | 1178.15 |
| AC026471.6 | 566.29 | 1178.59 |
| AC093510.2 | 565.64 | 1179.29 |
| BDNF-AS | 565.43 | 1180.85 |
| MIR503HG | 562.80 | 1177.61 |
| AC097634.1 | 561.28 | 1176.56 |
| AC090337.1 | 559.59 | 1175.19 |
| CBR3-AS1 | 559.16 | 1176.32 |
Evaluation and validation of the
seven-DElncRNAs signature
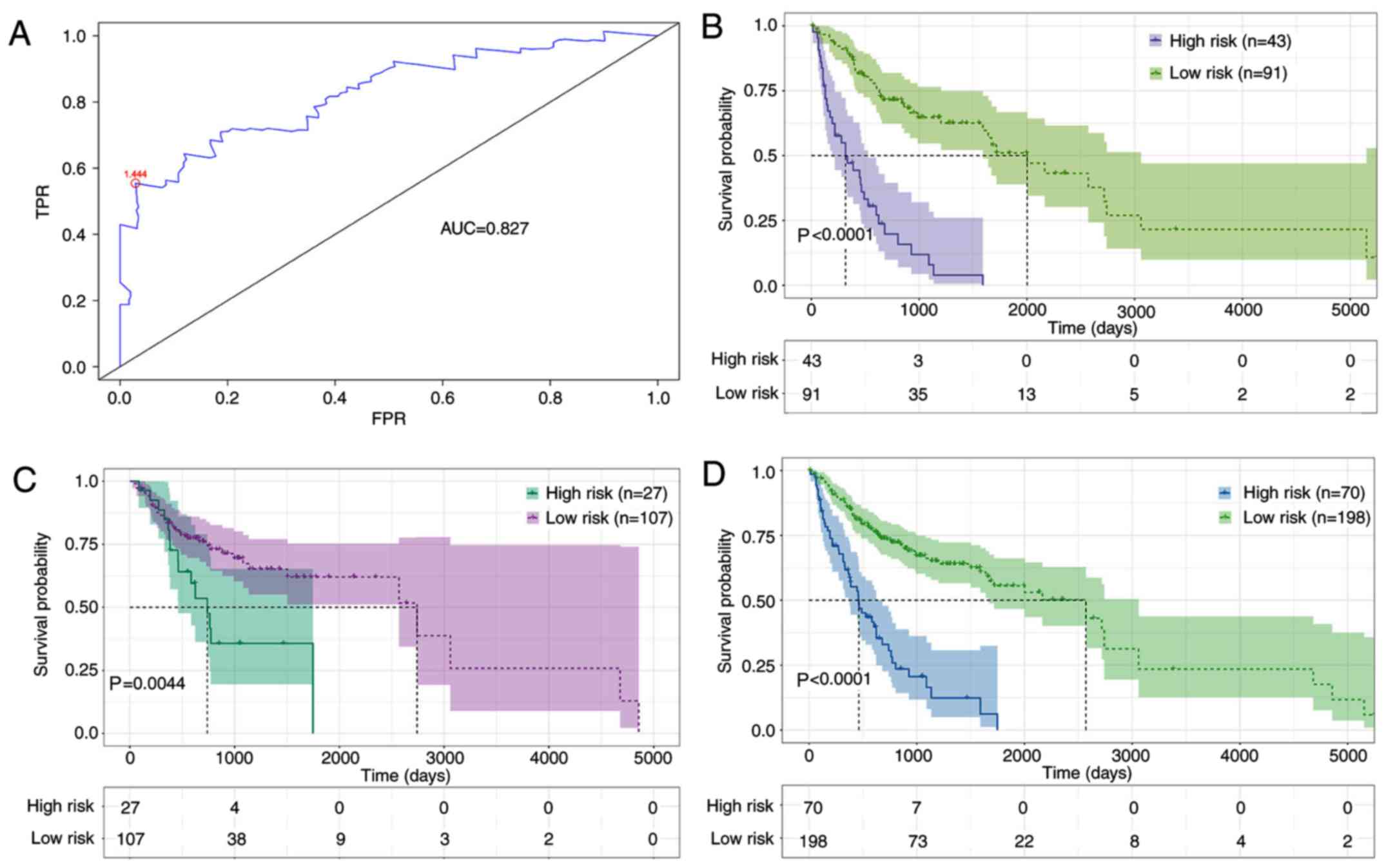
In order to evaluate the sensitivity and specificity
of the seven-DElncRNA signature, ROC analysis was performed on the
training dataset. Fig. 4A shows a
value of the area under the curve of 0.827. It has been
demonstrated that the cut-off points with the maximal sensitivity
and specificity could achieve a good classification (15). The optimal cut-off point reached
1.444, and the optimal cut-off value was used to further divide
patient samples into the high-risk group (N=43) and the low-risk
group (N=91) in the training dataset (Fig. 4B). K-M curves and log-rank test
results suggested that a significant difference existed in survival
time between the high-risk and the low-risk group (P<0.0001). In
addition, K-M curves and log-rank tests were also performed in the
test and complete datasets. The patients were separated into the
high-risk and the low-risk group in the test dataset (P=0.0044) and
in the complete dataset (P<0.0001), using the same risk formula
(Fig. 4C and D). Additionally,
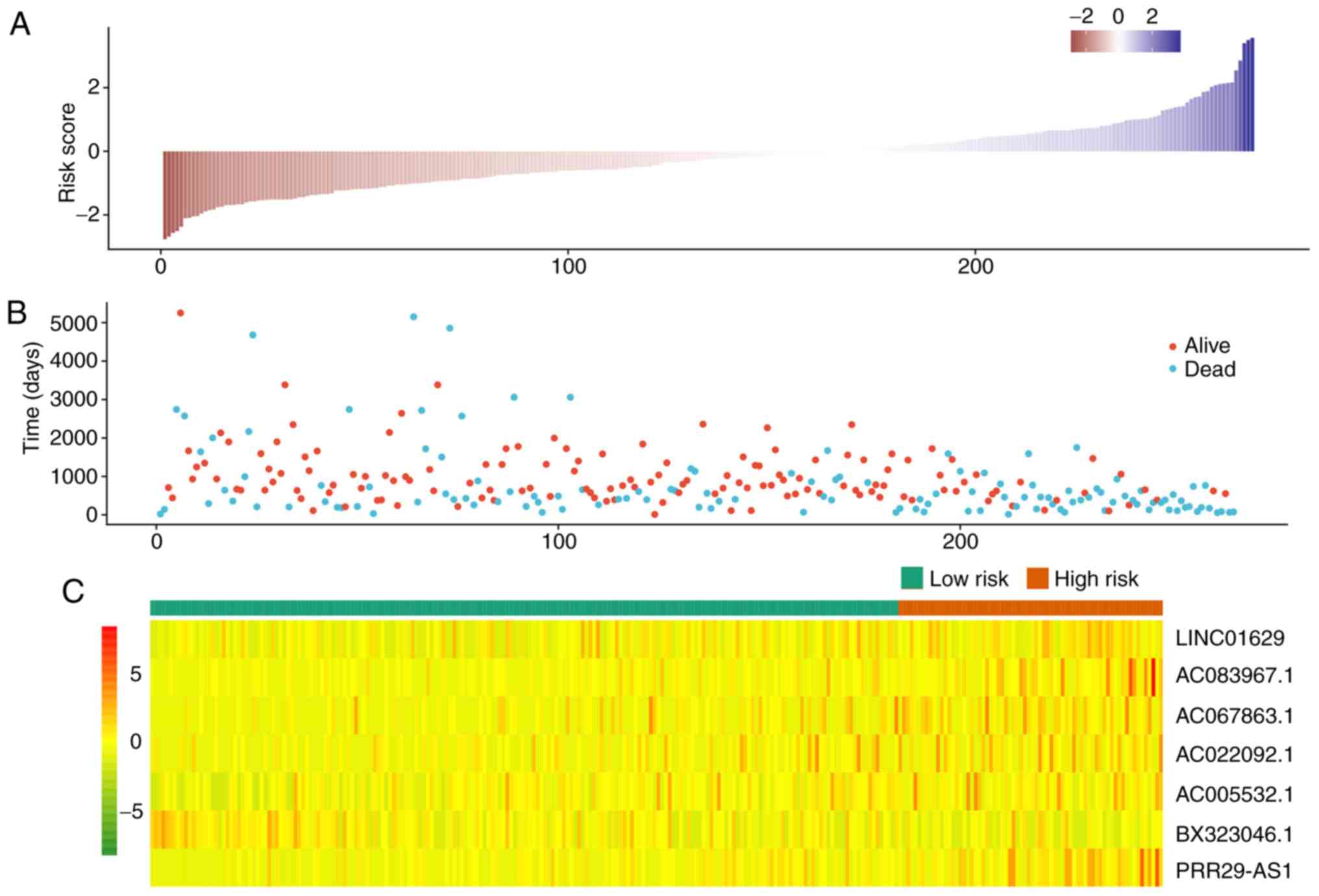
compared to the expression level of BX323046.1, the other lncRNAs
were at a high expression level in the high-risk group. Most cases
of mortality were observed in the high-risk group, and patients
with extended survival time were observed in the low-risk group in
the complete dataset (Fig. 5). Based
on the aforementioned results, age factors were found to be
associated with the survival time of OSCC. Therefore, the datasets
were regrouped based on the median age (62 years) to investigate
the applicability of the seven-DElncRNAs signature. Patients were
divided into a younger group (N=141) and an older group (N=127).
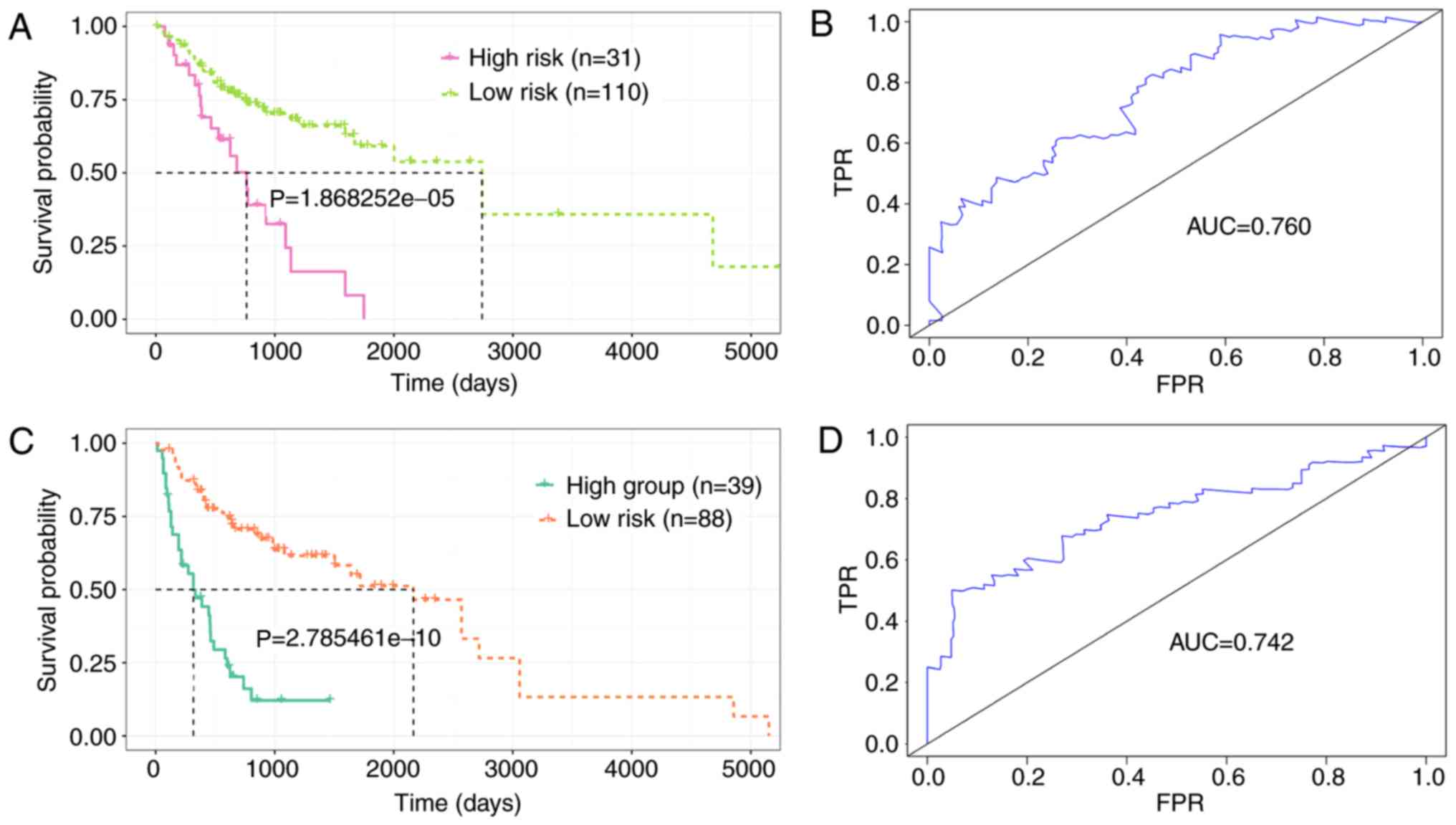
Using the same formula, the younger group was further categorized
into the high-risk (N=31) and the low-risk group (N=110), based on
the optimal cut-off (Fig. 6A).
Similarly, the older group was divided into the high-risk (N=39)
and the low risk-group (N=88; Fig.
6C). The K-M curves demonstrated that the patients in the
high-risk group had shorter OS (P<0.0001), and the area under
the curve (AUC) values were 0.760 and 0.742 for the younger and
older group, respectively, indicating that the seven-DElncRNA
signature was independent to age (Fig.
6B and D). Additionally, the association between the
seven-DElncRNA signature and clinical phenotypes was investigated.
The results demonstrated that the seven-DElncRNA signature can
serve as an independent predictor among the clinical phenotypes
(Fig. S1). In order to explore the
potential functions of the seven DElncRNAs, a correlation analysis
between genes and the seven lncRNAs was performed. As a result, a
total of 287 genes were screened from the correlation analysis by
setting the following criteria: P<0.001 and absolute value of
Pearson correlation coefficient >0.3. Further pathway enrichment
analysis for the corresponding genes revealed that these lncRNA may
be involved in the ‘PPAR signaling pathway’ and ‘cell cycle’
(P<0.05; Fig. S2).
Discussion
OSCC is an aggressive malignancy of the head and
neck, with a 5-year survival rate of 40–50% worldwide (20,21).
Although great efforts have been made over the past few decades to
develop signatures for prognostic predictions, the lack of
specificity and sensitivity to predict survival remains due to the
complex molecular and cellular heterogeneity of OSCC. Therefore,
the identification of an effective and independent molecular
biomarker of OSCC is required.
The development of high-throughput technologies has
promoted the discovery and study of ncRNAs, including lncRNAs,
which account for only a small proportion (2%) of transcribed genes
in eukaryotic species (22). A
number of studies have reported that lncRNAs serve a pivotal role
in complex biological processes (23–25),
including tumor promotion and tumor suppression. For example, Liu
et al (26) reported that
DiGeorge syndrome critical region gene 5 is involved in cervical
cancer progression by modulating the Wnt pathway. Guo et al
(27) found that the lncRNA
CEBPA-AS1 is associated with poor prognosis and promotes
tumorigenesis via CEBPA/Bcl2 in OSCC, which may contribute to
improving the effects of clinical treatment in OSCC. However, to
the best of our knowledge, the prognostic values of lncRNAs in OSCC
have not been comprehensively examined. Therefore, it is of great
significance to explore the lncRNAs associated with prognosis,
which will provide a potential mechanism and help to identify
effective therapeutic targets for patients with OSCC.
The association between dysregulated lncRNAs and the
prognosis of OSCC has been studied using a single biomarker or in
small-scale studies (28,29). However, compared with single clinical
biomarkers, integrating multiple biomarkers in a large clinical
cohort can improve predictive accuracy (30). At present, the TCGA database has
introduced a novel approach to this genomic analysis (31). TCGA is a comprehensive expression
database of a variety of cancer types, which collects
high-throughput methods at various genomic and proteomic levels as
well as clinical information, including stage and grade of tumor,
survival time, age, sex and ethnicity (32). Based on large datasets provided by
the public TCGA database, a number of studies have investigated the
diagnostic and prognostic value of lncRNAs in various types of
cancer (33–35).
In the present study, RNAseq and relevant clinical
data of the TCGA OSCC cohort were downloaded, resulting in the
identification of seven DElncRNAs associated with OSCC survival in
the training dataset, including LINC01629, AC083967.1, AC067863.1,
AC022092.1, AC005532.1, BX323046.1 and PRR29-AS1. Based on
regression coefficients from multivariate Cox regression analysis,
a seven-DElncRNAs risk scoring system was built and used to
calculate the risk score for each patient. According to the median
risk score, patients were classified into high-risk and low-risk
groups in the training and test datasets. The K-M curve analysis
results demonstrated that the low-risk group had a longer survival
time compared with the high-risk group. Furthermore, ROC analysis
was performed to estimate the sensitivity and specificity of the
seven-DElncRNAs signature. In addition, an optimal cut-off point
was identified from the ROC analysis and the patients were further
divided into the high-risk and the low-risk group. Additionally,
the risk score formula and the optimal cut-off points were
validated in the test and complete datasets. According to K-M curve
and log-rank test analysis, the patients in the low-risk group had
a significantly longer OS and fewer cases of mortality compared
with patients in the high-risk group. Furthermore, the associations
of the seven DElncRNAs were also identified, and pathway enrichment
analysis for these genes revealed that they may be involved in
‘PPAR signaling pathway’ and ‘cell cycle’.
To the best of our knowledge, the present study was
the first to identify seven DElncRNAs associated with the prognosis
of OSCC survival and their role in the prognosis of cancer, which
will provide useful information for further studies on OSCC.
Additionally, the optimal cut-off point for the risk level
classification was more effective and accurate compared with the
median risk cut-off point. For the median risk score, the high-risk
group and the low-risk group were not significant in the test
dataset (P=0.092), whereas a significant difference was observed
based on the optimal cut-off (P=0.0044). To the best of our
knowledge, this was the first attempt to determine an optimal
cut-off in OSCC. Additionally, age is an important factor that is
associated with survival in OSCC, and therefore, the seven-DElncRNA
signature was employed to analyze the clinical effect in different
age groups. The results indicated that the seven-DElncRNA signature
was a good classification system and further categorized patients
into the high-risk and low-risk groups with significant P-values,
according to the median age (62 years), demonstrating that the
seven-DElncRNA signature was an independent predictor for the
prognosis of OSCC according to age.
However, several limitations of the present study
should be taken into consideration. Firstly, only the seven-lncRNAs
signature was analyzed and validated in the TCGA datasets, and
therefore it is necessary to gain more expression profiles of
OSCC-associated lncRNAs for further validation. Additionally, in
vivo and in vitro experiments should be conducted to
verify the molecular function and mechanisms of the seven lncRNAs
in OSCC. In addition, in the risk formula, the combination of
differential multiples of the lncRNAs expression was not
considered, since the risk formula was not only established on the
lncRNA expression, and the regression coefficient for each lncRNA
was also important.
In conclusion, the present study explored the
aberrantly expressed lncRNAs in OSCC profiles from the large-scale
TCGA database. Furthermore, a seven-DElncRNA signature was
identified, which was associated with OS in OSCC and could act as a
potential independent biomarker for the prediction of prognosis in
patients with OSCC. Nevertheless, future studies are required to
evaluate and validate the molecular mechanisms of these DElncRNAs
in prospective clinical trials.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used/and or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XA designed the study. YW, RF and JW collected the
clinical information, lncRNA expression data, and revised the
figures and manuscript. TM and QS analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chan GG, Tai BC, Liang S, Lim DT and Soo
KC: Squamous cell carcinoma of the head and neck
(HNSCC)-multi-modality treatment and impact on survival. Asian J
Surg. 25:35–40. 2002.PubMed/NCBI
|
|
2
|
Friemel J, Foraita R, Günther K, Heibeck
H, Günther G, Pflueger M, Pohlabeln H, Behrens T, Bullerdiek J,
Nimzyk R and Ahrens W: Pretreatment oral hygiene habits and
survival of head and neck squamous cell carcinoma (HNSCC) patients.
BMC Oral Health. 16:332016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koch FP, Kunkel M, Biesterfeld S and
Wagner W: Diagnostic efficiency of differentiating small cancerous
and precancerous lesions using mucosal brush smears of the oral
cavity-a prospective and blinded study. Clin Oral Investig.
15:763–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Snow GB, Annyas AA, Slooten EA Van,
Bartelink H and Hart AA: Prognostic factors of neck node
metastasis. Clin Otolaryngol Allied Sci. 7:185–192. 2010.
View Article : Google Scholar
|
|
5
|
Mcguire S: Geneva, Switzerland: World
Health Organization, International Agency for Research on cancer,
WHO Press, 2015. Adv Nutr. 7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kokemueller H, Rana M, Rublack J, Eckardt
A, Tavassol F, Schumann P, Lindhorst D, Ruecker M and Gellrich NC:
The Hannover experience: Surgical treatment of tongue cancer-A
clinical retrospective evaluation over a 30 years period. Head Neck
Oncol. 3:272011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stein LD: Human genome: End of the
beginning. Nature. 431:915–916. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gibb EA, Brown CJ and Wan Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tano K and Akimitsu N: Long non-coding
RNAs in cancer progression. Front Genet. 3:2192012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng C, Hu W, Weng X, Tong R, Cheng S,
Ding C, Xiao H, Lv Z, Xie H, Zhou L, et al: Over expression of long
Non-coding RNA PANDA promotes hepatocellular carcinoma by
inhibiting senescence Associated inflammatory factor IL8. Sci Rep.
7:41862017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu X, Liu Z, Ning X, Huang L and Jiang B:
The long noncoding RNA HOTAIR promotes colorectal cancer
progression by sponging miR-197. Oncol Res. 26:473–481. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao C, Zou H, Wang J, Shen J and Liu H: A
three long noncoding RNA-Based signature for oral squamous cell
carcinoma prognosis prediction. DNA Cell Biol. 37:888–895. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diao P, Song Y, Ge H, Wu Y, Li J, Zhang W,
Wang Y and Cheng J: Identification of 4-lncRNA prognostic signature
in head and neck squamous cell carcinoma. J Cell Biochem.
120:10010–10020. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Y, Pan Y and Liu J: Functional
analysis of lncRNAs based on competitive endogenous RNA in tongue
squamous cell carcinoma. PeerJ. 7:e69912019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Colaprico A, Silva TC, Olsen C, Garofano
L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM,
Castiglioni I, et al: TCGAbiolinks: An R/Bioconductor package for
integrative analysis of TCGA data. Nucleic Acids Res. 44:e712016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robinson MD, Mccarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao X, Qin X, Li L, Zhou J, Zhou M, Li X,
Xu Y, Yuan L, Liu QN and Xing H: A 15-long non-coding RNA signature
to improve prognosis prediction of cervical squamous cell
carcinoma. Gynecol Oncol. 149:181–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong J and Xu M: A 19-miRNA Support Vector
Machine classifier and a 6-miRNA risk score system designed for
ovarian cancer patients. Oncol Rep. 41:3233–3243. 2019.PubMed/NCBI
|
|
20
|
Qiu Z, Sun W, Gao S, Zhou H, Tan W, Cao M
and Huang W: A 16-gene signature predicting prognosis of patients
with oral tongue squamous cell carcinoma. Peerj. 5:e40622017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Kolokythas A, Schwartz JL, Epstein
JB and Adami GR: microRNA from brush biopsy to characterize oral
squamous cell carcinoma epithelium. Cancer Med. 6:67–78. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li CH and Chen Y: Targeting long
non-coding RNAs in cancers: Progress and prospects. Int J Biochem
Cell Biol. 45:1895–1910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Ann Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Chang Y, Lu S and Xiang YY:
Downregulation of long noncoding RNA DGCR5 contributes to the
proliferation, migration, and invasion of cervical cancer by
activating Wnt signaling pathway. J Cell Physiol. 234:11662–11669.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo Y, Ma Y, Hu X, Song R, Zhu L and Zhong
M: Long non-coding RNA CEBPA-AS1 correlates with poor prognosis and
promotes tumorigenesis via CEBPA/Bcl2 in oral squamous cell
carcinoma. Cancer Biol Therapy. 19:205–213. 2017. View Article : Google Scholar
|
|
28
|
Fang Z, Zhao J, Xie W, Sun Q, Wang H and
Qiao B: LncRNA UCA1 promotes proliferation and cisplatin resistance
of oral squamous cell carcinoma by sunppressing miR-184 expression.
Cancer Med. 6:2897–2908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang S, Zhang S, Wang P, Yang C, Shang C,
Yang J and Wang J: LncRNA, TUG1 regulates the oral squamous cell
carcinoma progression possibly via interacting with
Wnt/beta-catenin signaling. Gene. 608:49–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li S, Chen X, Liu X, Yu Y, Pan H, Haak R,
Schmidt J, Ziebolz D and Schmalz G: Complex integrated analysis of
lncRNAs-miRNAs-mRNAs in oral squamous cell carcinoma. Oral Oncol.
73:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tomczak K, Czerwinska P and Wiznerowicz M:
The cancer genome atlas (TCGA): An immeasurable source of
knowledge. Contemp Oncol (Pozn). 19:A68–A77. 2015.PubMed/NCBI
|
|
32
|
Cancer Genome Atlas Research Network, .
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The Cancer Genome
Atlas Pan-Cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin H, Wang X, Zhang X, Wang Y, Zeng Y,
Xiong Y, Li T, Lin R, Zhou Q, Ling H, et al: Integrated analysis of
long noncoding RNA associated-competing endogenous RNA as
prognostic biomarkers in clear cell renal carcinoma. Cancer Sci.
109:3336–3349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou M, Zhang Z, Zhao H, Bao S, Cheng L
and Sun J: An immune-related six-lncrna signature to improve
prognosis prediction of glioblastoma multiforme. Mol Neurobiol.
55:3684–3697. 2018.PubMed/NCBI
|
|
35
|
Wang R, Du L, Yang X, Jiang X, Duan W, Yan
S, Xie Y, Zhu Y, Wang Q, Wang L, et al: Identification of long
noncoding RNAs as potential novel diagnosis and prognosis
biomarkers in colorectal cancer. J Cancer Res Clin Oncol.
142:2291–2301. 2018. View Article : Google Scholar
|