Introduction
Salvianolic acid B (Sal-B) is a water-soluble
component of Salvia miltiorrhiza Bunge with a wide spectrum
of effects, including anti-inflammatory effects, inhibition of new
vessel formation and atherogenesis, and relief of chronic hepatitis
and liver fibrosis, as well as antioxidant and tumor-modulating
effects (1–4). A previous study demonstrated that this
compound inhibits cell proliferation in head and neck squamous cell
carcinoma (5). In addition, it has
been reported to decrease viability of U87 cells in a dose- and
time-dependent manner (6).
S. miltiorrhiza Bge is the main source of
Sal-B (7–10) and it also possesses another active
ingredient, tanshinone, that is widely used clinically. Tanshinone
has been reported to inhibit oxidation of low-density lipoproteins,
improve lipid metabolism, protect endothelial cells and prevent
myocardial ischemia (11–13). In addition, this compound displays
preventive effects on cardiovascular diseases, such as
atherosclerosis, and reduces the area of myocardial infarction and
the oxygen consumption of the myocardium (14–17).
Unfortunately, the current extraction methods of Sal-B result in
the loss of fat-soluble tanshinone. Following tanshinone
extraction, the residue can be used for Sal-B extraction by process
modification; however, the yield is low and the process is
time-consuming and laborious (18).
Therefore, S. miltiorrhiza Bge is mainly used in the
extraction of tanshinone rather than Sal-B. However, S.
miltiorrhiza Bge is very expensive, and its accessibility is
limited due to its regional distribution. It is therefore
imperative to look for alternative sources of Sal-B to replace
S. miltiorrhiza Bge. In this regard, Salvia bowleyana
Dunn has been suggested as an alternative source since it is often
used as a surrogate of S. miltiorrhiza Bge. This species is
abundant in areas that exhibit high incidences of gastric cancer in
China, such as Fujian (19).
In the present study, the water-soluble components
of S. miltiorrhiza Bge and S. bowleyana Dunn were
extracted and assayed for antitumor effects on gastric cancer cell
lines. Since there have only been a few studies on Sal-B for the
prevention and treatment of gastric cancer (2,20–23), the
present study aimed to explore the potential of these species in
treating gastric cancer.
Materials and methods
Determination of Sal-B in S. bowleyana
Dunn roots
S. bowleyana Dunn plants were collected from
Lianjiang (Fuzhou, China) in July 2014 (E, 119°20′; N, 20°11′; Alt,
57 m), while S. miltiorrhiza Bge (produced in Anhui, China,
in 2015) was purchased from Hui Chun Pharmacy (Fuzhou, China). The
roots of S. bowleyana Dunn and S. miltiorrhiza Bge
were washed, dried, ground to a fine powder and passed through a
425-µm sieve respectively. Sal-B in the roots of the two plants was
purified by the following experimental steps. A total of 1 g of the
resulting powder was placed in an Erlenmeyer flask with 20 ml 60%
ethanol and left at room temperature. The rest of the powder was
stored at ≤-20°C for subsequent use. After 6–8 h, the solution was
exposed to ultrasound at 40 kHz for ~35 min and centrifuged for 15
min at 8,000 × g, and the supernatant was collected at room
temperature. Subsequently, 20 ml 60% ethanol was added to the
Erlenmeyer flask, and the solution was exposed to ultrasound at 40
kHz for 35 min and centrifuged at 8,000 × g for 15 min at room
temperature. The resultant supernatant was collected and mixed with
the supernatant collected in the first phase. Finally, the
supernatant was topped up to 1,000 ml using ultra-pure water and
stored at 4°C for use in subsequent experiments.
The extract was analyzed using Waters 2695 Alliance
HPLC high-performance liquid chromatography (Waters Corporation). A
Sal-B standard sample (≥98.3%) was purchased from Nanjing Chunqiu
Biological Engineering Co., Ltd., and was used for analysis on
ZORBAX Eclipse XDB-C18 (4.6×250 mm, 5 µm) chromatography columns
(Agilent Technologies, Inc.). The (4.6×250 mm) are the diameter and
length of the column, respectively. The particle size of the
particles is 5 µm in the column, which is the composition of the
solid phase. Results were detected using the Ultrospec™ 2100 pro
UV–Vis variable wavelength detector (Amersham; Cytiva) at a
wavelength of 286 nm. The analysis involved a mobile phase with
0.5% carboxylic acid, 99.9% acetonitrile and 99.9% methanol in the
ratio 48:7:45 (V:V:V). Other conditions included a 0.8-ml/min flow
rate and a column temperature of 28°C for a sample volume of 10 µl.
The analyzed Sal-B contents were imaged and quantified using
Empower System Suitability (24–26).
Sal-B in S. miltiorrhiza Bge root was not used in the
remaining experiments
Purification and verification of
Sal-B
A 20-g sample of powder from S. bowleyana
Dunn roots was used in the extraction of plant components as
aforementioned. The obtained supernatant was concentrated under
reduced pressure at 45°C for ethanol removal, and the solution was
subsequently diluted to 500 ml with ultra-pure water and divided
into 10 bottles (500 ml/bottle). A total of 4 g X-5 resin (Nankai
University Chemical Plant) was added into each bottle, followed by
12 h of shaking at 110 r min−1 in a rotary incubator at
room temperature to allow full absorption of the compound by the
resin. Subsequently, any resin impurities on the surface were
washed off by running water, and the rest of the solution in the
bottle was discarded. The mixture absorbed by the resin was eluted
using 60% ethanol and shaken for 1–2 h at 110 r min−1 in
a rotary incubator at room temperature until the resin became
colorless. The ethanol solution was concentrated under reduced
pressure and freeze-dried to obtain a powder. The powder was
dissolved in 10% ethanol solution and filtered using a 0.45 µm
microporous membrane. Subsequently, the filtered solution was
purified via column chromatography. Sephadex LH-20 (Amersham;
Cytiva) was used for chromatographic media in the columns
(Φ1.6×70 cm) and ethanol solutions of different volume
percentages (10, 30 and 50%) were used for eluting the columns in
sequence. Finally, the solution was concentrated to dryness in a
rotary evaporator under reduced pressure conditions, and the yield
was calculated. The purified compound powder was stored at −20°C
before HPLC and nuclear magnetic resonance (NMR) analyses.
A total of 2 mg of the powder was dissolved in 2 ml
methanol, and the filtered sterile solution was placed into a
sample bottle. Components of this purified powder were identified
by liquid chromatography-mass spectrometry (LC-MS) and liquid
chromatography-tandem mass spectrometry (LC-MS2) using
the aforementioned HPLC chromatographic conditions. Parameters for
mass spectrometry (G6520B; Agilent Technologies, Inc.) were as
follows: A negative ionization mode, fragmentor set at 100 V, mass
spectra scanning range of 200–1,200 m/z, a two-stage mass
spectrometry scanning range of 50–800 m/z, nebulizer pressure 40
psi (10 l/min), nitrogen gas temperature of 350°C and the energy of
collision chamber of 10, 20 and 40 eV (27,28). A
total of 8 mg powder was obtained and dissolved in 0.7 ml
D2O by ultrasound in a 1-ml clean centrifugal tube for
1H NMR test using the UNITY-400 NMR spectrometer
(Varian).
Establishment of cell culture
The human gastric cancer HGC-27 and AGS cell lines
were obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences for use in the present study. All cells
were tested by short tandem repeat genotyping and confirmed to be
mycoplasma negative. HGC-27 cells were grown in RPMI-1640 medium,
whereas AGS cells were maintained in DMEM F12 (both Biological
Industries). The cultures were supplemented with 10% FBS
(Biological Industries), 100 U/ml penicillin G and 100 µg/ml
streptomycin (BBI Life Sciences Corporation). All cells were grown
at 37°C in a humidified incubator containing 5% CO2.
Sulforhodamine B (SRB) assay
Cells (100 µl) were seeded into 96-well plates at
concentrations of 10,000 and 8,000 cells/well for AGS and HGC-27,
respectively. After 24 h of incubation, the gastric cancer cells
were treated with different concentrations of Sal-B (100, 200, 400,
600 and 800 µM) for 48 h at 37°C. The culture media was removed,
cells were fixed with 3% trichloroacetic acid and stained with
0.057% SRB (both Sigma-Aldrich; Merck KGaA) (29). SRB was solubilized in 10 mM Tris base
solution, and its fluorescence was quantified using the Synergy HT
Multi-Mode Microplate Reader (Agilent Technologies, Inc.) at a
wavelength of 510 nm. The wells treated with Sal-B were compared
with control wells (30,31). At least three biological replicates
were performed for each assay.
Cell Counting Kit-8 (CCK-8) assay
Cells (100 µl) were seeded into 96-well plates at
concentrations of 10,000 and 8,000 cells/well for AGS and HGC-27,
respectively. After 24 h, the gastric cancer cells were treated
with different concentrations of Sal-B as described for the SRB
assay for 48 h at 37°C. After treatment, 10 µl CCK-8 solution
(TransGen Biotech Co., Ltd.) was added to each well, according to
the manufacturer's protocol, and the cells were incubated at 37°C
for 2 h. Subsequently, optical density values were measured at 450
nm using the Synergy HT Multi-Mode Microplate Reader. Drug-treated
wells were compared with solvent-controlled wells using results
obtained from three independent experiments.
Colony formation assay
The ability of cells to form colonies was analyzed
for HGC-27 and AGS cells in the exponential phase. Each cell line
was divided into 3 groups with 3 replicate wells in each group and
seeded into 6-well plates (1×107/well), and were
subsequently treated with 0, 400 or 800 µM Sal-B when the monolayer
cell density reached 70% confluency. After 48 h, adhered cells were
disassociated with trypsin and cells from each well were seeded
into 6-well plates. Additionally, 500 cells/well of AGS and HGC-27
were seeded to continue culture in complete medium without Sal-B
treatment. The cells were grown for 7 to 14 days to allow colony
formation from viable clonogenic cells. After culture, the medium
was removed, and 70% methanol was added to fix the cells for 15
min, then methanol was discarded and stained with 5% Giemsa
(Sigma-Aldrich; Merck KGaA) for 10 min after air drying at room
temperature. Subsequently, the plates were washed with PBS and
captured to calculate colony numbers, using a Canon EOS 70D
(https://www.canon.com.cn/product/70d). Statistical
results were obtained from three independent experiments.
Protection from oxidative DNA damage
induced by 2,2′-azobis (2-methylpropionamidine) dihydrochloride
(AAPH)
AAPH (Sigma-Aldrich; Merck KGaA) is an oxidizing
agent, and its formation of cationic free radicals can occur at the
first level. The ability of the extracted Sal-B to protect the
supercoiled pUC18 plasmid from AAPH was measured according to the
method previously described by Zhang and Omaye (32), with some modifications. Briefly, 2 µl
of intact pUC18 plasmid (0.1 µg/µl) was mixed with various
concentrations (0.017 mmol/l, 0.03, 0.06 and 0.13 mmol/l) of Sal-B
samples (7 µl) and 12.5 mM AAPH (6 µl) in PBS (pH 7.4), and then
the mixture was incubated at 37°C. After 1 h of incubation, the
samples were electrophoresed on a 0.8% agarose gel for 30 min and
subsequently stained for 3–5 min with 0.5 µg/ml ethidium bromide.
Analysis of DNA damage was performed on images taken using a GelDoc
EZ gel image analysis system (Bio-Rad Laboratories, Inc.) (33).
Western blot analysis
Proteins were extracted from cells using RIPA buffer
composed of 50 mM Tris HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5%
sodium deoxycholate and 0.1% SDS. Protein concentration was
measured using the Pierce BCA Protein assay kit (Thermo Fisher
Scientific, Inc.), 30 µg protein/lane separated via SDS-PAGE on a
10% gel and transferred to a nitrocellulose membrane (GE Healthcare
Life Sciences). After blocking with 5% skimmed milk powder for 1 h
at room temperature, the nitrocellulose membrane was incubated with
Caspase3 mouse mAb (1:1,000; cat. no. 9668) and GAPDH rabbit mAb
(1: 1,000; cat. no. 2118; both from Cell Signaling Technology,
Inc.) overnight at 4°C. Subsequently, the blots were stained with
the IRDye® 680RD goat-anti-rabbit IgG (cat. no.
926-68071) and IRDye® 800CW goat anti-mouse IgG (cat.
no. 926-32210; both 1:5,000 and from LI-COR Biosciences) secondary
antibodies labeled with fluorescence in a cassette at room
temperature for 1 h. Subsequently, the blots were directly imaged
and semi-quantified using the Odyssey Infrared Imaging system
(model no. 9140; LI-COR Biosciences) (34).
Flow cytometry
Cells were seeded in 6-well plates at a density of
2×105 cells/well. After 24 h, the cells were treated
with Sal-B (0, 400 and 800 µM) at 37°C for 48 h and assessed for
cellular apoptosis using the AnnexinV-FITC/PI Apoptosis Detection
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Apoptotic cells were analyzed using a flow
cytometer and FlowJo software (v10; FlowJo LLC).
Statistical analysis
Data were analyzed using one-way ANOVA with a
Dunnett's post hoc test. Analyses were performed in GraphPad Prism
v7 (GraphPad Software, Inc.). Data are presented as the mean ± SEM.
P<0.05 was considered to indicate a statistically
significant difference. The IC50 value was calculated by
Dr Fit 1.042 (35).
Results
S. bowleyana Dunn roots contain high
Sal-B content
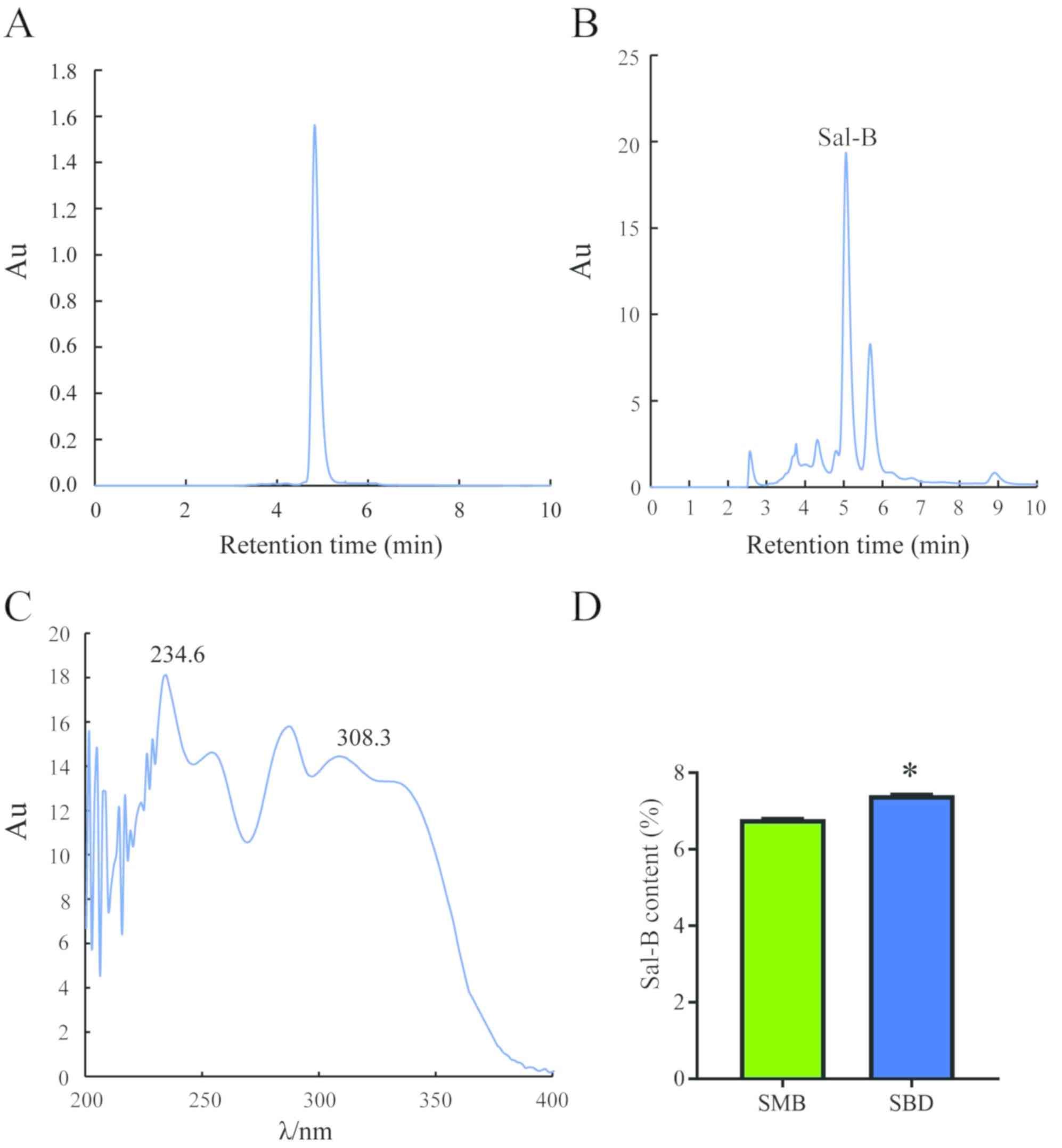
Analysis of the peak area of the standard sample
resulting from HPLC as well as linear regression [y (peak
area)=9480.2× (mg/ml)-181.39 (R2=0.9998)] allowed
detection of Sal-B in the extract (Fig.
1A). The content of Sal-B was imaged and quantified using
Empower System Suitability (Fig. 1B and
C). Quantification revealed a significantly higher Sal-B
content in S. bowleyana Dunn roots (7.42%) than in S.
miltiorrhiza Bge root (6.79%; P<0.05; Fig. 1D). After purification, the yield of
Sal-B was 4.26% and the purity of Sal-B was 93.26% by HPLC
analysis.
Sal-B in S. bowleyana Dunn roots is
verified by LC-MS and LC-MS2
The purified component extracted from S.
bowleyana Dunn roots was verified by LC-MS and
LC-MS2. Separation and purification procedures allowed
the identification of a substance with molecular formula
C36H30O16 and molecular mass of
718. The compound can lose one proton to be negatively charged
under the condition of negative ion full-wave scanning mass
spectrometry. Thus, its base peak is the molecular ion peak and the
m/z value was 717.14 (Fig. 2A). Ion
fragments of the compound from different collision energies were
collected and detected, including those at m/z 519, 339, 321 and
295 (Fig. 2B-D). These fragments
were consistent with the standard sample of Sal-B and with a
previous report (36). The current
results indicated that Sal-B was formed by condensation of
lithospermic acid and danshensu, and that its two ester bonds were
the most prone to breaking. Therefore, Sal-B is liable to lose two
danshensu units and form two different m/z 519 substances (paths a
and b; Fig. 2E). Subsequently, two
substances, m/z 339 (paths a1 and b2) and m/z
321 (paths a2 and b1), are formed, with m/z
339 having two possible structures (Fig.
2E). The ion fragments of m/z 321 are formed by removing
monomolecular danshensu. The ion fragments of the two m/z 339
structures can additionally form two different m/z 321 structures
(paths a3 and b3; Fig. 2E). The chemical structure of Sal-B
was identified according to its 1H NMR data. Compared
with the data in the literature (9),
the data of the compound was the following: 1H NMR (400
MHz, D2O), δ/ppm: 6.78 (m, 7 H; H 1), 6.63 (m, 2 H; H
2), 6.27 (d, J=8 Hz, 1 H; H3), 6.08 (d, J=2 Hz, 1 H; H 4), 5.92
(dd, 1 H; H 5), 5.80 (d, J=6 Hz, 1 H; H 6), 5.68 (d, J=16 Hz, 1 H;
H 7), 4.85 (dd, 2 H; H 8), 4.75 (dd, 9 H; H 9), 4.09 (d, J=6 Hz,
1H; H10), 2.97 (dd, 1 H; H 11), 2.80 (m, 2 H; H 12) and 2.41 (dd, 1
H; H 13) (the H number corresponds to the structure shown in
Fig. 2F). The current results
indicated that the purified product corresponded to Sal-B.
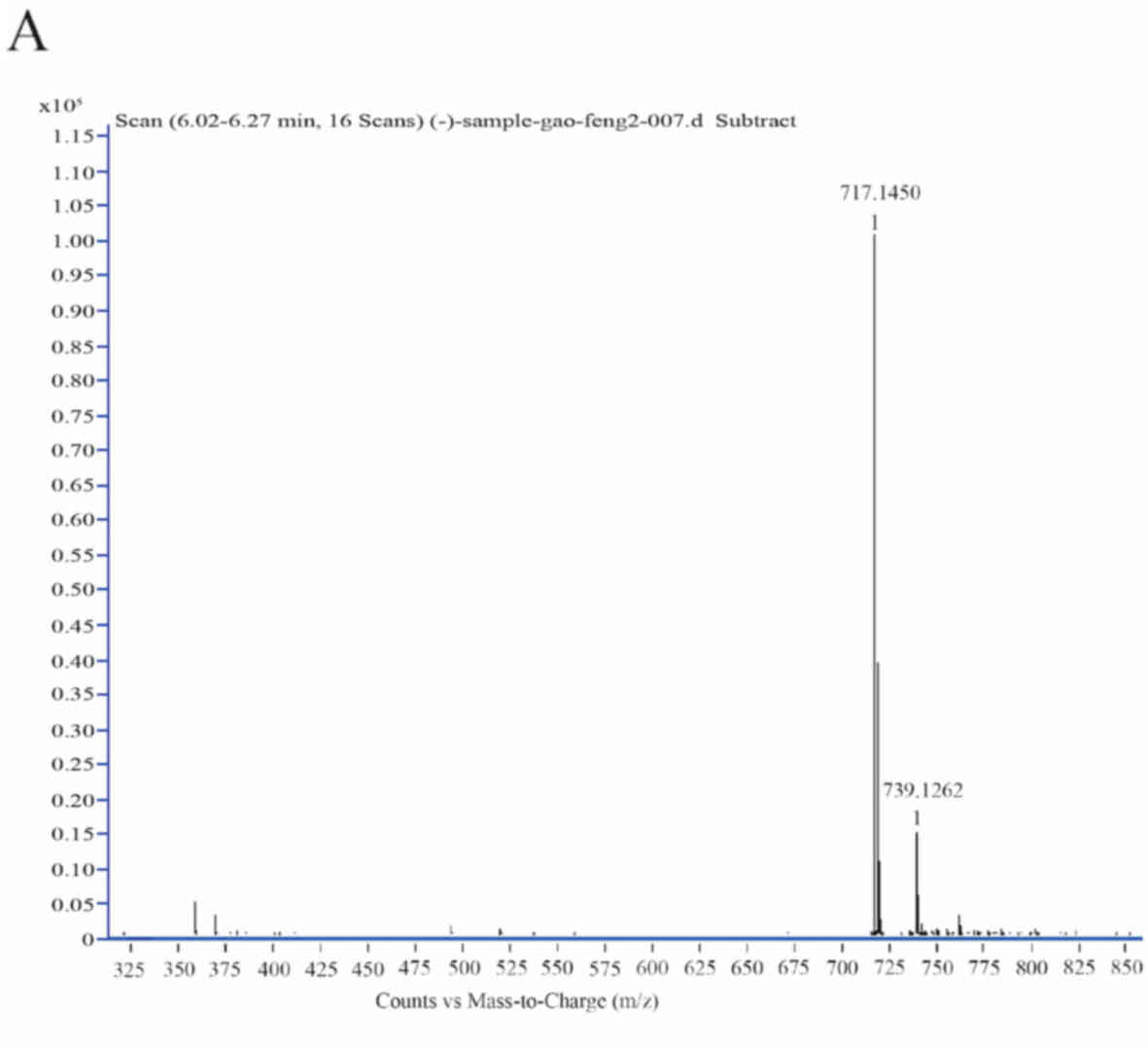 | Figure 2.Sal-B identification via liquid
chromatography-mass spectrometry, LC-MS2 and
1H NMR spectrum. (A-D) Mass spectrum via negative ESI
for Sal-B. LC-MS2, liquid chromatography-tandem mass
spectrometry; NMR, nuclear magnetic resonance; ESI, electrospray
ionization; Sal-B, salvianolic acid B. Sal-B identification via
liquid chromatography-mass spectrometry, LC-MS2 and
1H NMR spectrum. (E) Proposed ESI-MS2
fragmentation pathway of Sal-B. (F) 1H NMR spectrum of
Sal-B (D2O). LC-MS2, liquid
chromatography-tandem mass spectrometry; NMR, nuclear magnetic
resonance; ESI, electrospray ionization; Sal-B, salvianolic acid
B. |
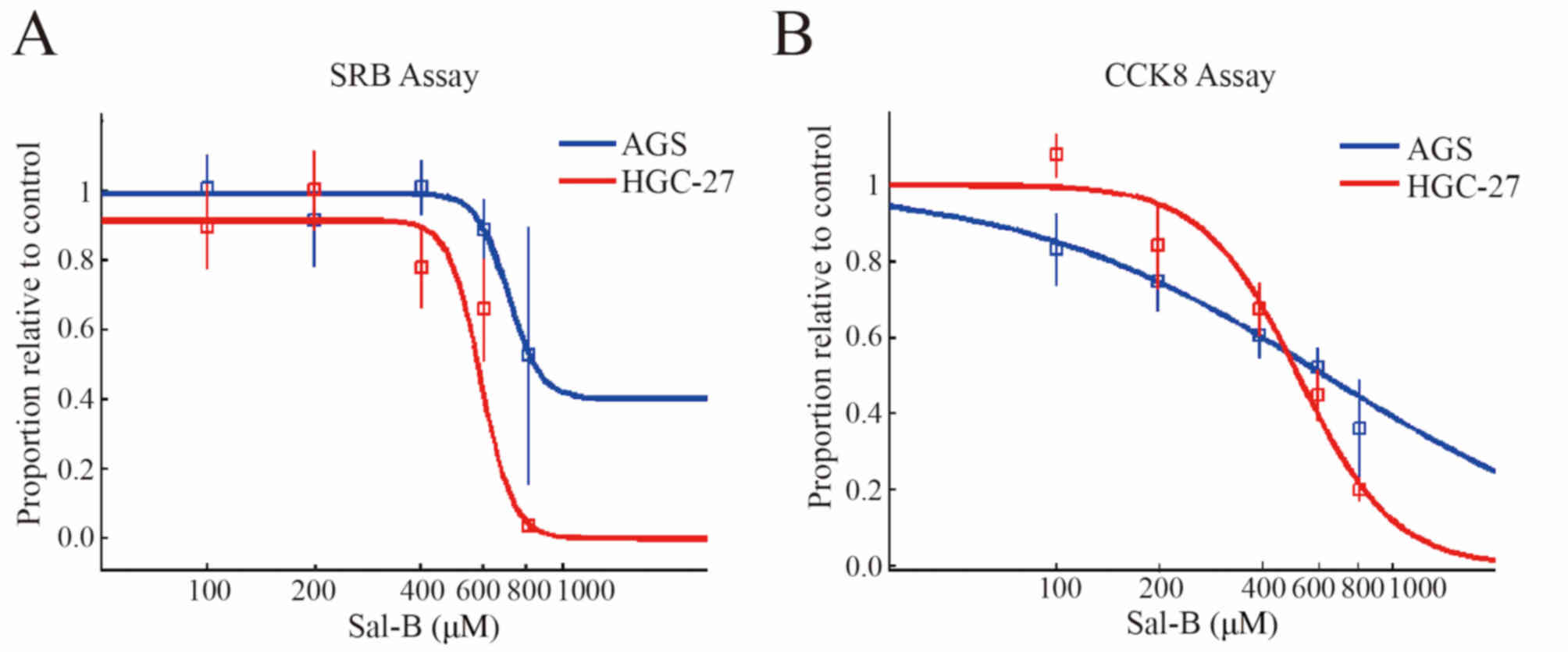
Sal-B effectively inhibits
proliferation of gastric cancer cells
A number of biological experiments were conducted to
examine the anti-gastric cancer effect of purified Sal-B extracted
from S. bowleyana Dunn. Treating AGS and HGC-27 cells with
different concentrations of purified Sal-B inhibited cell
proliferation. This is based on the different IC50
values obtained from the SRB (AGS, ~824 µM; HGC, ~576 µM; Fig. 3A) and CCK-8 assays (AGS, ~615 µM;
HGC, ~511 µM; Fig. 3B).
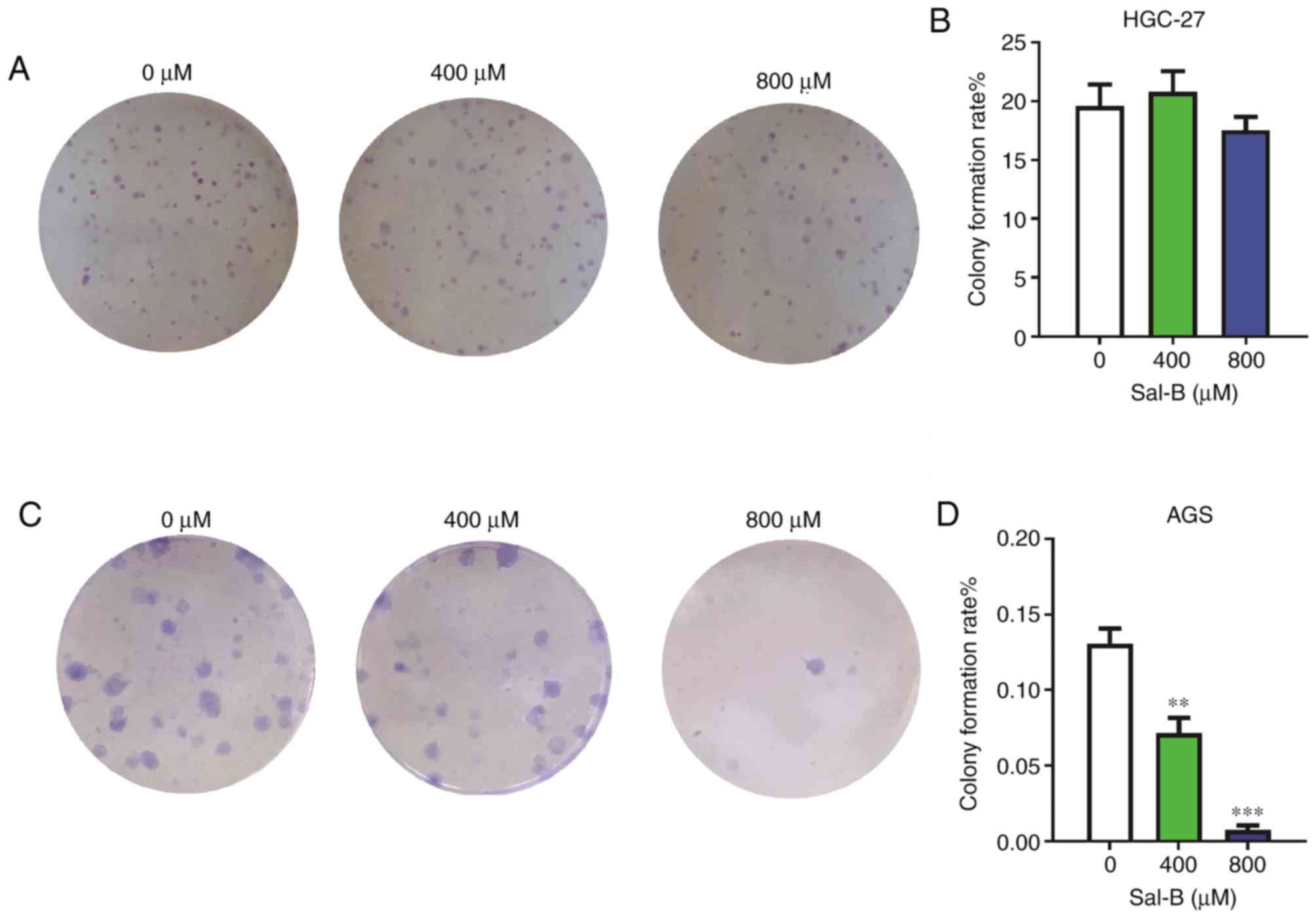
A colony formation assay comparing Sal-B-treated
HGC-27 cells with control cells did not reveal significant
differences (Fig. 4A and B).
However, colony formation in AGS cells was strongly suppressed at
400 and 800 µM (Fig. 4C and D).
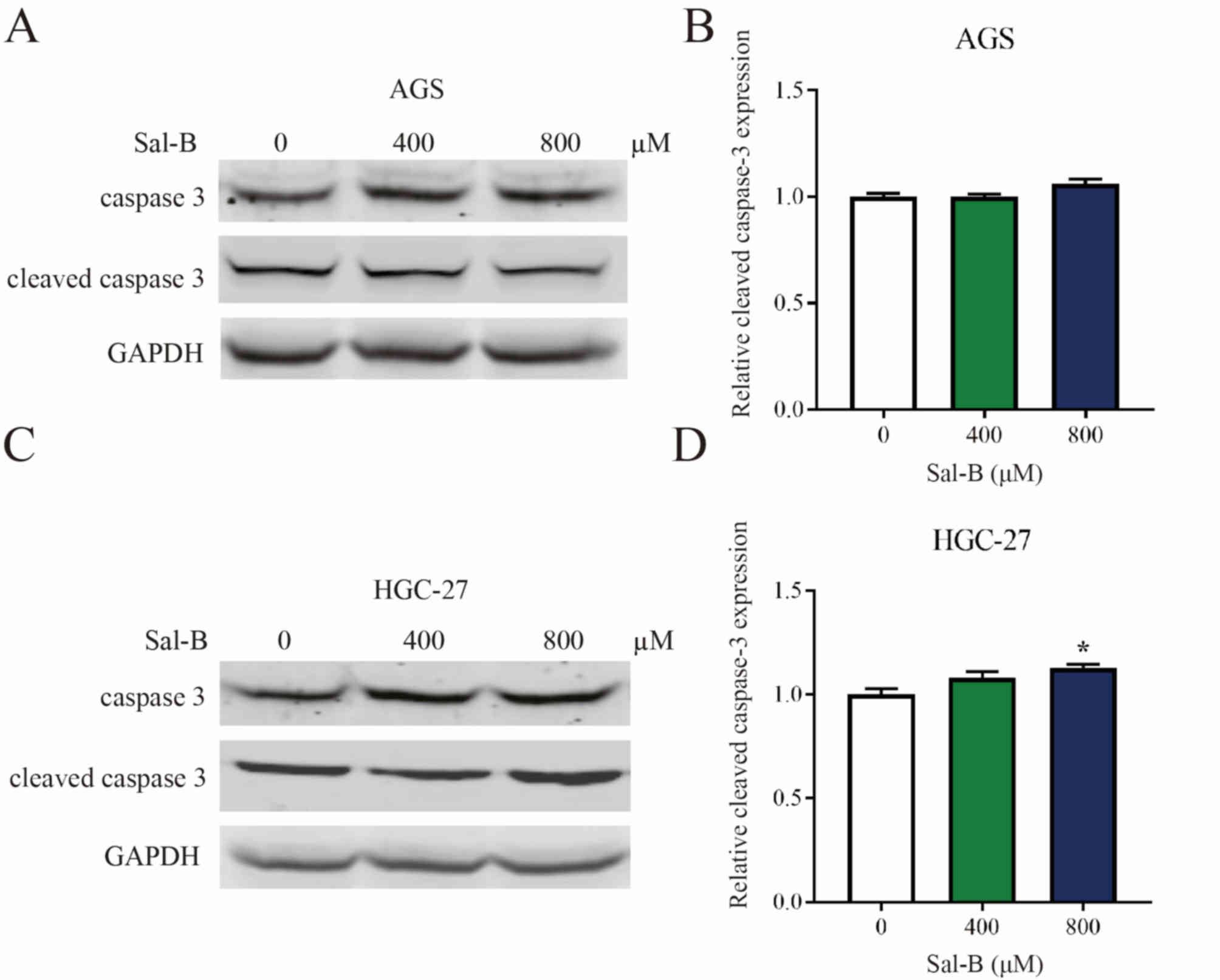
Western blot analysis of AGS and HGC-27 cells treated with varying
concentrations of Sal-B revealed that the compound had a
significant effect on the expression levels of cleaved caspase-3
only when treating HGC-27 cells with 800 µM Sal-B (Fig. 5A-D). Alternative assessment of
apoptosis using flow cytometry revealed similar results in AGS and
HGC-27 cells (Fig. 5E-H).
Finally, the ability of Sal-B from S.
bowleyana Dunn to prevent oxidative DNA damage was examined
(Fig. 6). It was observed that
oxidative DNA damage was gradually diminished with increasing Sal-B
concentration. Oxidative DNA damage was rarely observed at 0.06
mmol/l Sal-B; this concentration may effectively prevent DNA from
being sheared. To some extent, the low level of apoptosis may be
associated with the protective effect of Sal-B on DNA.
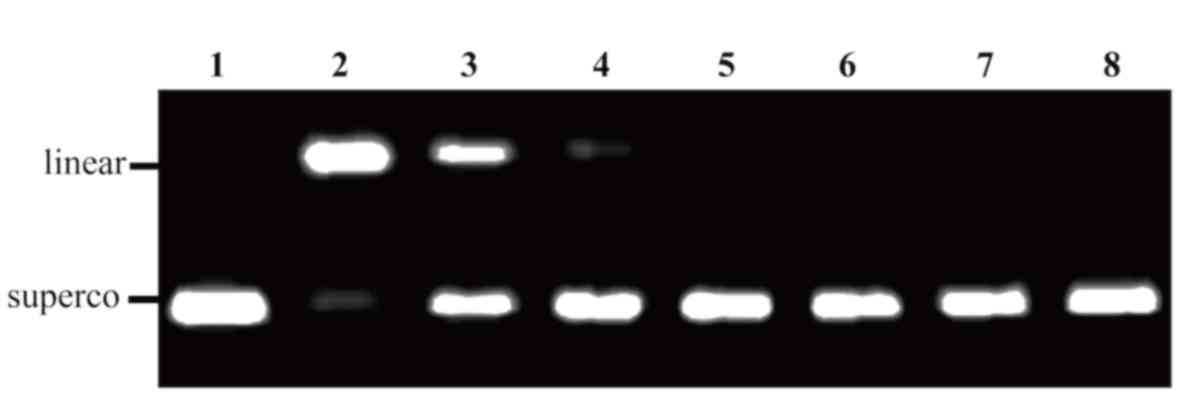 | Figure 6.Protective effects of Sal-B from
Salvia bowleyana Dunn on oxidative DNA damage induced by
AAPH. Lane 1, native DNA; lane 2, DNA treated with AAPH and
solvent; lane 3, DNA treated with AAPH and 0.004 mmol/l Sal-B; lane
4–7, DNA treated with AAPH and 0.017, 0.03, 0.06 and 0.13 mmol/l
Sal-B, respectively; lane 8, DNA treated with AAPH and 0.08 mmol/l
rutin. A total of 100 ng pUC18 DNA and 12.5 mmol/l AAPH were used
in every appropriate lane. Sal-B, salvianolic acid B; AAPH,
2,2′-azobis (2-methylpropionamidine) dihydrochloride; superco,
supercoiled. |
Discussion
The molecular weight of purified monomeric compound
extracted from S. bowleyana Dunn was successfully detected
using HPLC-MS, while ion fragments were analyzed via
HPLC-MS2. Results from ion fragments were consistent
with the decomposition product of Sal-B, and Sal-B content from
S. bowleyana Dunn roots was significantly higher than that
from S. miltiorrhiza Bge roots. The current extraction
method was therefore improved and relatively simplified compared
with previous methods (37,38).
S. bowleyana Dunn is widely distributed in
the Fujian province, where there is a high incidence of gastric
cancer (39). The extraction of
compounds from S. bowleyana Dunn and the potential
identification of anti-gastric cancer activities may result in a
beneficial use of the plant in this region. The findings of the
present study add substantial knowledge to the few reports
regarding the anti-gastric cancer effects of Sal-B (40–43). In
the current study, Sal-B extracted from S. bowleyana Dunn
had an inhibitory effect on the proliferation of AGS and HGC cells.
The ability of AGS cells to form colonies was strongly inhibited
when cells were treated with high concentrations of Sal-B, while no
significant inhibition was observed in HGC-27 cells. In addition,
no significant differences were observed in the apoptotic marker
cleaved caspase-3 when AGS and HGC-27 cells were treated with
different concentrations of Sal-B. Although there was a significant
difference in apoptosis between the control group and HGC-27 cells
treated with 800 µM Sal-B, the number of apoptotic cells was low.
Sal-B may inhibit apoptosis by decreasing oxidative damage to
mitochondrial DNA and protecting mitochondrial function.
Additionally, Sal-B has been demonstrated to decrease the release
of cytochrome c from mitochondrial cells into the cytosol,
thus inhibiting activated caspase-3 (44). However, in the present study Sal-B
inhibited the proliferation of AGS and HGC-27 cells, suggesting
that they may be directly killed. Sal-B is easily degraded within a
few hours of exposure to an alkaline solution, which may be one of
the causes of this phenomenon (45–47).
Furthermore, different tumor cells have different levels of
resistance to the same reagent; therefore, the development and
assessment of stable Sal-B (alone or in combination with other
drugs) may open more frontiers on its application. Further analyses
to unravel the underlying mechanism of action for the inhibition of
proliferation should be performed in future research.
Overall, the findings of the present study revealed
that S. bowleyana Dunn contains a higher Sal-B content than
S. miltiorrhiza Bge, and that it may be a novel source of
this potentially anti-gastric cancer compound.
Acknowledgements
Not applicable.
Funding
The present study was supported by the International
S&T Cooperation Program of China (grant no. 2016YFE0121900),
the Scientific Research Innovation Team Construction Program of
Fujian Normal University (grant no. IRTL1702), the United Fujian
Provincial Health and Education Project for Tackling the Key
Research (grant no. WKJ2016-2-27), the Natural Science Foundation
of Fujian Province (grant no. 2016Y0029) and the National Special
Fund for Chinese medicine resources Research in the Public Interest
of China (grant no. 2018-43).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
BC designed and performed the experiments, and
prepared the figures. CH designed and performed the experiments,
drafted the initial manuscript, and prepared the figures. YZ
extracted Sal-B and performed partial verification experiments. XT,
SL and QW performed the experiments and analyzed the data. YL
conceived and designed the experiments and drafted the initial
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee HJ, Seo M and Lee EJ: Salvianolic acid
B inhibits atherogenesis of vascular cells through induction of
Nrf2-dependent heme oxygenase-1. Curr Med Chem. 21:3095–3106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guan Y, Zhu JP, Shen J, Jia YL, Jin YC,
Dong XW and Xie QM: Salvianolic acid B improves airway
hyperresponsiveness by inhibiting MUC5AC overproduction associated
with Erk1/2/P38 signaling. Eur J Pharmacol. 824:30–39. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan ZK, Lv G, Wang YF, Li G, Yu DS, Wang
YS, Zhang YQ, Mei XF and Cao Y: The protective effect of
salvianolic acid B on blood-spinal cord barrier after compression
spinal cord injury in rats. J Mol Neurosci. 51:986–993. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Z, Li JN, Bai ZQ and Lin X: Antagonism
by salvianolic acid B of lipopolysaccharide-induced disseminated
intravascular coagulation in rabbits. Clin Exp Pharmacol Physiol.
41:502–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao Y, Xie T, Korotcov A, Zhou Y, Pang X,
Shan L, Ji H, Sridhar R, Wang P, Califano J and Gu X: Salvianolic
acid B inhibits growth of head and neck squamous cell carcinoma in
vitro and in vivo via cyclooxygenase-2 and apoptotic pathways. Int
J Cancer. 124:2200–2209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ZS, Luo P, Dai SH, Liu ZB, Zheng XR
and Chen T: Salvianolic acid B induces apoptosis in human glioma
U87 cells through p38-mediated ROS generation. Cell Mol Neurobiol.
33:921–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li HB, Lai JP, Jiang Y and Chen F:
Preparative isolation and purification of salvianolic acid B from
the Chinese medicinal plant Salvia miltiorrhiza by
high-speed counter-current chromatography. J Chromatogr A.
943:235–239. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhi W and Deng Q: Purification of
salvianolic acid B from the crude extract of Salvia
miltiorrhiza with hydrophilic organic/salt-containing aqueous
two-phase system by counter-current chromatography. J Chromatogr A.
1116:149–152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun Y, Zhu H, Wang J, Liu Z and Bi J:
Isolation and purification of salvianolic acid A and salvianolic
acid B from Salvia miltiorrhiza by high-speed
counter-current chromatography and comparison of their antioxidant
activity. J Chromatogr B Analyt Technol Biomed Life Sci.
877:733–737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuang H, Wang Y, Hu J, Wang C, Lu S and Mo
X: A method for preparation of an internal layer of artificial
vascular graft co-modified with Salvianolic acid B and heparin. Acs
Appl Mater Interfaces. 10:19365–19372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shang Q, Xu H and Huang L: Tanshinone IIA:
A promising natural cardioprotective agent. Evid Based Complement
Alternat Med. 2012:7164592012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu DM, Wang YJ, Han XR, Wen X, Li L, Xu L,
Lu J and Zheng YL: Tanshinone IIA prevents left ventricular
remodelling via the TLR4/MyD88/NF-κB signalling pathway in rats
with myocardial infarction. J Cell Mol Med. 22:3058–3072. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XX, Yang JX, Pan YY and Zhang YF:
Protective effects of tanshinone IIA on endothelial progenitor
cells injured by tumor necrosis factor-α. Mol Med Rep.
12:4055–4062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue Y, Yan J and Feng J: Treatment with
tanshinone IIA suppresses disruption of the blood-brain barrier and
reduces expression of adhesion molecules and chemokines in
experimental autoimmune encephalomyelitis. Eur J Pharmacol.
771:18–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao H, Liu X, Sun W, Kang N, Liu Y, Yang
S, Xu QM, Wang C and Chen X: Total tanshinones exhibits
anti-inflammatory effects through blocking TLR4 dimerization via
the MyD88 pathway. Cell Death Dis. 8:e30042017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao S, Liu Z, Li H, Little PJ, Liu P and
Xu S: Cardiovascular actions and therapeutic potential of
tanshinone IIA. Atherosclerosis. 220:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang J, Little PJ and Xu S:
Atheroprotective effects and molecular targets of tanshinones
derived from herbal medicine danshen. Med Res Rev. 38:201–228.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou J, He J, Jin X, Hu T and Zhang Y:
Study on optimisation of extraction process of tanshinone IIA and
its mechanism of induction of gastric cancer SGC7901 cell
apoptosis. Afr J Tradit Complement Altern Med. 10:456–458. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JB, Wang ZW, Li Y, Huang CQ, Zheng
CH, Li P, Xie JW, Lin JX, Lu J, Chen QY, et al: CDK5RAP3 acts as a
tumor suppressor in gastric cancer through inhibition of β-catenin
signaling. Cancer Lett. 385:188–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tao L, Wang S, Zhao Y, Sheng X, Wang A,
Zheng S and Lu Y: Phenolcarboxylic acids from medicinal herbs exert
anticancer effects through disruption of COX-2 activity.
Phytomedicine. 21:1473–1482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen GY, Shu YC, Chuang DY and Wang YC:
Inflammatory and apoptotic regulatory activity of tanshinone IIA in
helicobacter pylori-infected cells. Am J Chin Med. 44:1187–1206.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen CY, Li H, Yuan YN, Dai HQ and Yang B:
Antioxidant activity and components of a traditional Chinese
medicine formula consisting of Crataegus pinnatifida and Salvia
miltiorrhiza. BMC Complement Altern Med. 13:992013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang K, Yang Q, Ma Q, Wang B, Wan Z, Chen
M and Wu L: Protective effects of salvianolic acid a against
dextran sodium sulfate-induced acute colitis in rats. Nutrients.
10:E7912018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pasakova I, Klimes J, Sochor J and
Hrabalek A: Optimization of HPLC chromatographic conditions for
determination of Transkarbam 12 and its degradation products. J
Pharm Biomed Anal. 42:136–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo Q, Wang D, Wei Z and Wang Z: Optimized
chromatographic conditions for separation of halogenated acetic
acids by ultra-performance liquid chromatography-electrospray
ionization-mass spectrometry. J Chromatogr A. 1277:26–34. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Demiralay EC: An experimental design
approach to optimization of the liquid chromatographic separation
conditions for the determination of metformin and glibenclamide in
pharmaceutical formulation. Acta Chim Slov. 59:307–314.
2012.PubMed/NCBI
|
|
27
|
Jones-Lepp TL and Momplaisir GM: New
applications of LC-MS and LC-MS2 toward understanding
the environmental fate of organometallics. Trac-Trend Anal Chem.
24:590–595. 2005. View Article : Google Scholar
|
|
28
|
Wang Z, Cao B, Yu A, Zhang H and Qiu F:
Ultrasound-assisted ionic liquid-based homogeneous liquid-liquid
microextraction high-performance liquid chromatography for
determination of tanshinones in Salvia miltiorrhiza Bge
root. J Pharm Biomed Anal. 104:97–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Skehan P, Storeng R, Scudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woolston C and Martin S: Analysis of tumor
and endothelial cell viability and survival using sulforhodamine B
and clonogenic assays. Methods Mol Biol. 740:45–56. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fricker SP: The application of
sulforhodamine B as a colorimetric endpoint in a cytotoxicity
assay. Toxicol In Vitro. 8:821–822. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang P and Omaye ST: DNA strand breakage
and oxygen tension: Effects of beta-carotene, alpha-tocopherol and
ascorbic acid. Food Chem Toxicol. 39:239–246. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu QP, Cao XM, Hao DL and Zhang LL:
Chemical composition, antioxidant, DNA damage protective, cytotoxic
and antibacterial activities of cyperus rotundus rhizomes essential
oil against foodborne pathogens. Sci Rep. 7:452312017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin Y, Richards FM, Krippendorff BF,
Bramhall JL, Harrington JA, Bapiro TE, Robertson A, Zheleva D and
Jodrell DI: Paclitaxel and CYC3, an aurora kinase A inhibitor,
synergise in pancreatic cancer cells but not bone marrow precursor
cells. Br J Cancer. 107:1692–1701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Di Veroli GY, Fornari C, Goldlust I, Mills
G, Koh SB, Bramhall JL, Richards FM and Jodrell DI: An automated
fitting procedure and software for dose-response curves with
multiphasic features. Sci Rep. 5:147012015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng G, Xiao H, Liu J and Liang X:
Identification of phenolic constituents in Radix Salvia
miltiorrhizae by liquid chromatography/electrospray ionization
mass spectrometry. Rapid Commun Mass Spectrom. 20:499–506. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Xiao S, Sun L, Ge Z, Fang F,
Zhang W, Wang Y and Cheng Y: Rapid screening of bioactive compounds
from natural products by integrating 5-channel parallel
chromatography coupled with on-line mass spectrometry and
microplate based assays. Anal Chim Acta. 777:49–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, Morris-Natschke SL and Lee KH: New
developments in the chemistry and biology of the bioactive
constituents of Tanshen. Med Res Rev. 27:133–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wong BC, Lam SK, Wong WM, Chen JS, Zheng
TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al: Helicobacter
pylori eradication to prevent gastric cancer in a high-risk region
of China: A randomized controlled trial. JAMA. 291:187–194. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zheng X, Chen S, Yang Q, Cai J, Zhang W,
You H, Xing J and Dong Y: Salvianolic acid A reverses the
paclitaxel resistance and inhibits the migration and invasion
abilities of human breast cancer cells by inactivating transgelin
2. Cancer Biol Ther. 16:1407–1414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang QL, Wu Q, Tao YY, Liu CH and
El-Nezami H: Salvianolic acid B modulates the expression of
drug-metabolizing enzymes in HepG2 cells. Hepatobiliary Pancreat
Dis Int. 10:502–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li H, Shi L, Wei J, Zhang C, Zhou Z, Wu L
and Liu W: Cellular uptake and anticancer activity of salvianolic
acid B phospholipid complex loaded nanoparticles in head and neck
cancer and precancer cells. Colloids Surf B Biointerfaces.
147:65–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hao Y, Zhou Y, Ji H, Fang Y, Pang X,
Southerland W, Califano J and Gu X: Salvianolic acid B specifically
inhibits cyclooxygenase-2 in human carcinoma cell lines in vitro
and in vivo. Cancer Res. 68:2008.
|
|
44
|
Yan X, Zhou T, Tao Y, Wang Q, Liu P and
Liu C: Salvianolic acid B attenuates hepatocyte apoptosis by
regulating mediators in death receptor and mitochondrial pathways.
Exp Biol Med (Maywood). 235:623–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li H, Wang S, Xie Y, Zhang B, Wang J, Yang
Q and Cao W: Simultaneous determination of danshensu, salvianolic
acid B, and paeonol in ShuangDan oral liquid by HPLC. J AOAC Int.
96:20–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xintian Z and Haibin Q: Characterisation
of the degradation of salvianolic acid B using an on-line
spectroscopic analysis system and multivariate curve resolution.
Phytochem Anal. 23:103–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Luo L, Yang B, Zhang G, Zhu W, Liu Y, Wei
X, Kang X and Qu Z: Degradation kinetics of chlorogenic acid in
honeysuckle during modified atmosphere heat pump drying. Int J
Agric & Biol Eng. 9:159–168. 2016.
|