Introduction
Colorectal cancer (CRC) is the third most common
cancer worldwide, with 1.8 million new cases and 881,000 deaths
recorded in 2018 (1). CRC is also
the second leading cause of cancer-related deaths (1). The overall 5-year survival is only
65.2%, and the stage-specific 5-year survival is 93.2 for stage I,
82.5 for stage II, 59.5 for stage III and 8.1% for stage IV in USA
(2).
Recurrence following resection is a major problem in
CRC, which results in poor prognosis, particularly in patients with
stage III and high-risk stage II CRC. Currently, cancer prognosis
depends mainly on the pathological stage at the time of diagnosis.
However, patients with the same Tumor-Node-Metastasis (TNM) stage
still have different prognoses (3).
In addition to staging prognosis, assessment of
clinical biomarkers is an optimal strategy to identify patients
with higher risks of recurrence and low survival (4). This distinction is helpful for patient
management, optimization of current treatment options, and early
detection of recurrence (5). Studies
have reported that the age, sex, tumor location, grade,
inflammatory markers and number of involved lymph nodes are
prognostic factors of CRC (6).
Blood lipid markers are also associated with
survival outcomes in patients with certain malignant tumors,
including prostate, breast, gastrointestinal, cervical and lung
cancers (7–11). However, the association between blood
lipid levels and the prognosis of CRC has not been well studied,
and the results of the studies conducted thus far are inconsistent.
One study demonstrated that dyslipidemia, including high
triglyceride (TG) levels and low high-density lipoprotein (HDL)
cholesterol levels, was independently associated with improved
overall and recurrence-free survival of patients with colon cancer
(12). By contrast, a recent
prospective study by Peng et al (13) that involved 1,318 patients with CRC
reported that patients with dyslipidemia exhibited a significantly
shorter median survival duration compared with those without
dyslipidemia. Although patients with stage III and high-risk stage
II CRC primarily have a poor prognosis after radical surgery,
previous studies did not stratify patients by stage (2,12–16).
Furthermore, there are limited studies that have investigated the
role of apolipoprotein levels in CRC prognosis. Thus, the current
study aimed to investigate the association between lipid and
lipoprotein levels and the prognosis of patients with stage III and
high-risk stage II CRC.
Materials and methods
Patient selection
This retrospective study included patients diagnosed
with CRC between June 2008 and September 2011 in the Department of
Colorectal Surgery of the First Affiliated Hospital of Fujian
Medical University in Fujian (Fujian, China). A total of 246
patients (134 male and 112 female) were enrolled in this study. The
mean age of the patients was 60.56±13.23 (range, 21–84) years. The
patients exhibited no distant metastases or local recurrence and
underwent surgical resection. The TNM stages (17) of stage III and high-risk stage II CRC
were confirmed by pathological examination. Among these patients,
56.8% of patients with right-sided colon cancer and 69.2% of
patients with left-sided colon cancer received chemotherapy after
surgical resection. Neo-adjuvant chemoradiation was administered to
83.2% of patients with tumors located in the rectal area.
The inclusion criteria were as follows: i) Stage III
or high-risk stage II CRC; and ii) complete clinical data,
including age, sex, height, weight, history of diabetes mellitus
(DM), history of hypertension, smoking status, alcohol intake,
cancer site, tumor stage, histological class, differentiation and
the levels of carcinoembryonic antigen (CEA), total cholesterol
(TC), triglyceride (TG), high-density lipoprotein cholesterol
(HDL), low-density lipoprotein cholesterol (LDL), very-low-density
lipoprotein cholesterol (VLDL), apolipoprotein-A1 (APO-A1) and
apolipoprotein B (APO-B). The exclusion criteria were as follows:
i) Treatment with lipid-lowering agents or metformin; ii) presence
of infections, serious liver and kidney dysfunction, severe heart
disease or other serious illnesses; iii) history of other
malignancies; iv) incomplete preoperative laboratory data; or v)
use of anti-inflammatory medications before surgery.
Patients with high-risk stage II CRC were defined as
those who had poor prognosis with the following characteristics: T4
(stage IIB, IIC), poor histological differentiation (grades 3/4,
excluding high microsatellite instability) (18), vascular and nerve infiltration,
intestinal obstruction, tumor site perforation, positive or unclear
margins, inadequate margins and <12 lymph nodes sent for
examination.
The present study was reviewed and approved by the
Ethics Committee of The First Affiliated Hospital of Fujian Medical
University (Fujian, China), and written informed consent was
obtained from all subjects.
Data collection
Blood levels of CEA, TC, TG, HDL, LDL, VLDL, APO-A1
and APO-B were measured up to 7 days before the surgery using
standard methods. Patients were not allowed to consume meals with
high-fat content before blood tests to reflect the true level of
blood lipids. Data on clinical parameters, including age at CRC
diagnosis, sex, height, weight, body mass index (BMI), history of
DM, history of hypertension, smoking status, alcohol intake, cancer
site, TNM classification, histological type, differentiation
status, perineural invasion, vascular tumor thrombus and intestinal
obstruction were collected from the medical records. Tumor staging
for CRC in this study was based on the 7th edition of the American
Joint Committee on Cancer TNM classification (17). BMI was classified as underweight
(<18.5 kg/m2), normal weight (18.5–23.9
kg/m2), overweight (24.0–27.9 kg/m2) and
obese (≥28 kg/m2) according to the diagnostic criteria
in China (19). Smoking status was
defined as smoking ≥10 cigarettes per day at the time of CRC
diagnosis. Alcohol intake was defined as the consumption of at
least one alcoholic drink per week at the time of CRC
diagnosis.
Follow-up
Follow-up was conducted every 3–6 months for the
first 2 years after resection, every 6 months for the next 3 years
and annually thereafter. Follow-up procedures included colonoscopy,
computed tomography and CEA tests. Local recurrence and distant
metastasis from CRC were identified by endoscopy, tissue
pathological examination or imaging analysis. The last follow-up
was performed on May 1, 2018.
Statistical analysis
The primary endpoint was disease-free survival
(DFS), and the secondary endpoint was overall survival (OS). DFS
was defined as the time from the date of surgery to the date of
local tumor recurrence and/or distant metastases or the date of the
last follow-up. OS was defined as the time from the date of surgery
to the date of death or the date of the last follow-up. The optimal
cut-off values of TC, TG, HDL, LDL, VLDL, LDL/HDL ratio, APO-A1 and
APO-B were determined using the maximal x2 method to
best classify patients into two prognostic groups: Poor and good.
The R MaxStat package (https://CRAN.R-project.org/package=maxstat) in R
version 3.5.2 software (https://cran.r-project.org) was used for this analysis
(20). The Kaplan-Meier method was
used to establish the effect of each variable on DFS, and log-rank
tests were used to compare the survival curves. Univariate and
multivariate analyses using the Cox proportional hazards model were
also performed to identify the prognostic impact of clinical
parameters and blood lipid index on DFS and OS. Significant
variables in univariate analysis (P<0.1) were entered into
regression models with increasing complexity, and significance was
assessed using analysis of variance followed by a Bonferroni post
hoc test. Data were represented as hazard ratios (HRs) and 95%
confidence intervals (CIs). A predictive nomogram of 1-, 3- and
5-year CRC mortality was established based on clinical and
clinicopathological parameters. The variables from Cox multivariate
analysis were included in the nomogram analysis. The nomogram was
implemented by the regression modeling strategy (RMS) package
(http://biostat.mc.vanderbilt.edu/wiki/Main/RmS) in R
version 3.5.2 software (Institute for Statistics and Mathematics).
The concordance index was used to evaluate the predictive accuracy
of the nomogram. All statistical analyses were performed using SPSS
19.0 (SPSS, Inc.) and R version 3.5.2 software. P<0.05 was
considered to indicate a statistically significant difference
Results
Optimal cut-off values of lipid
indices by sex
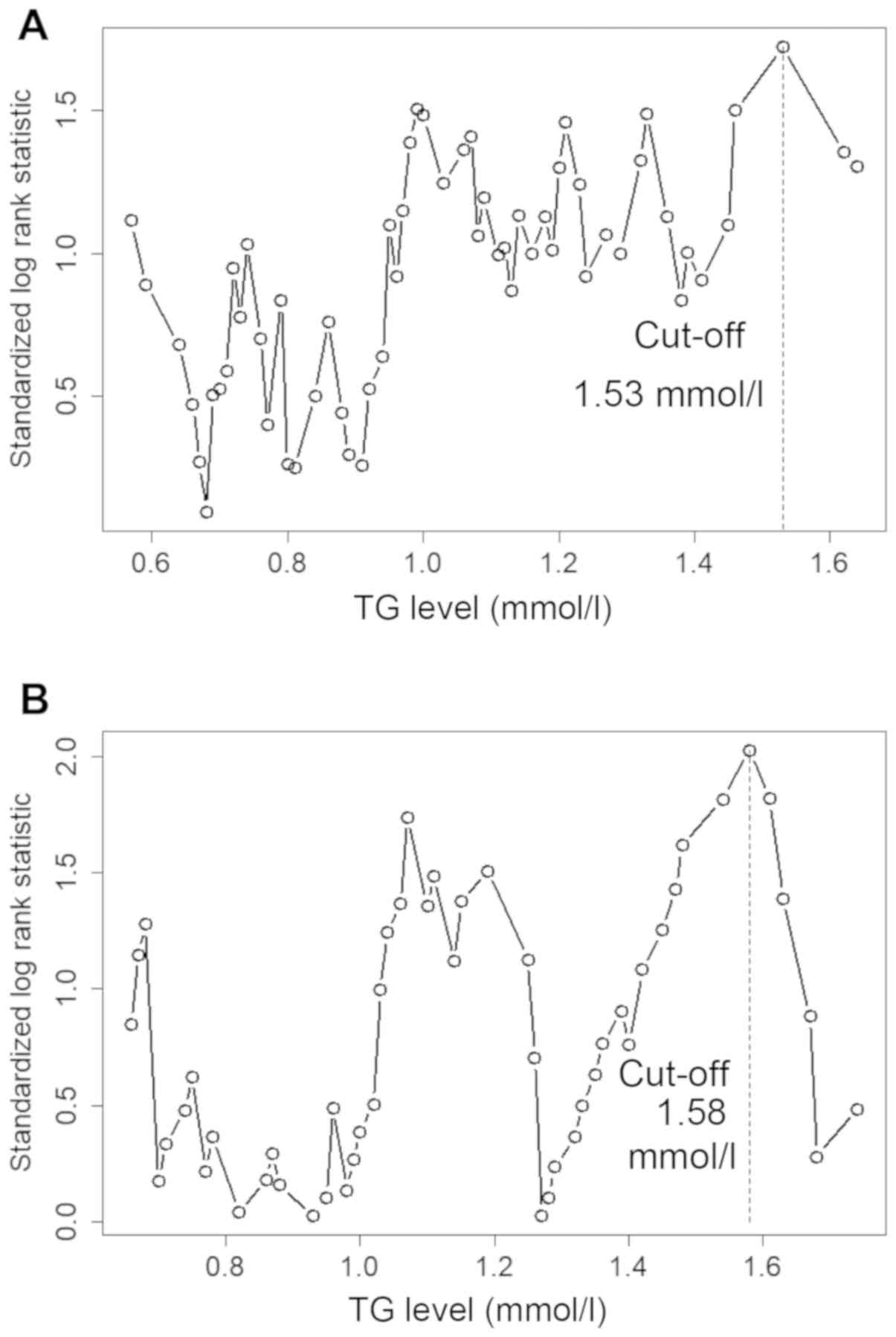
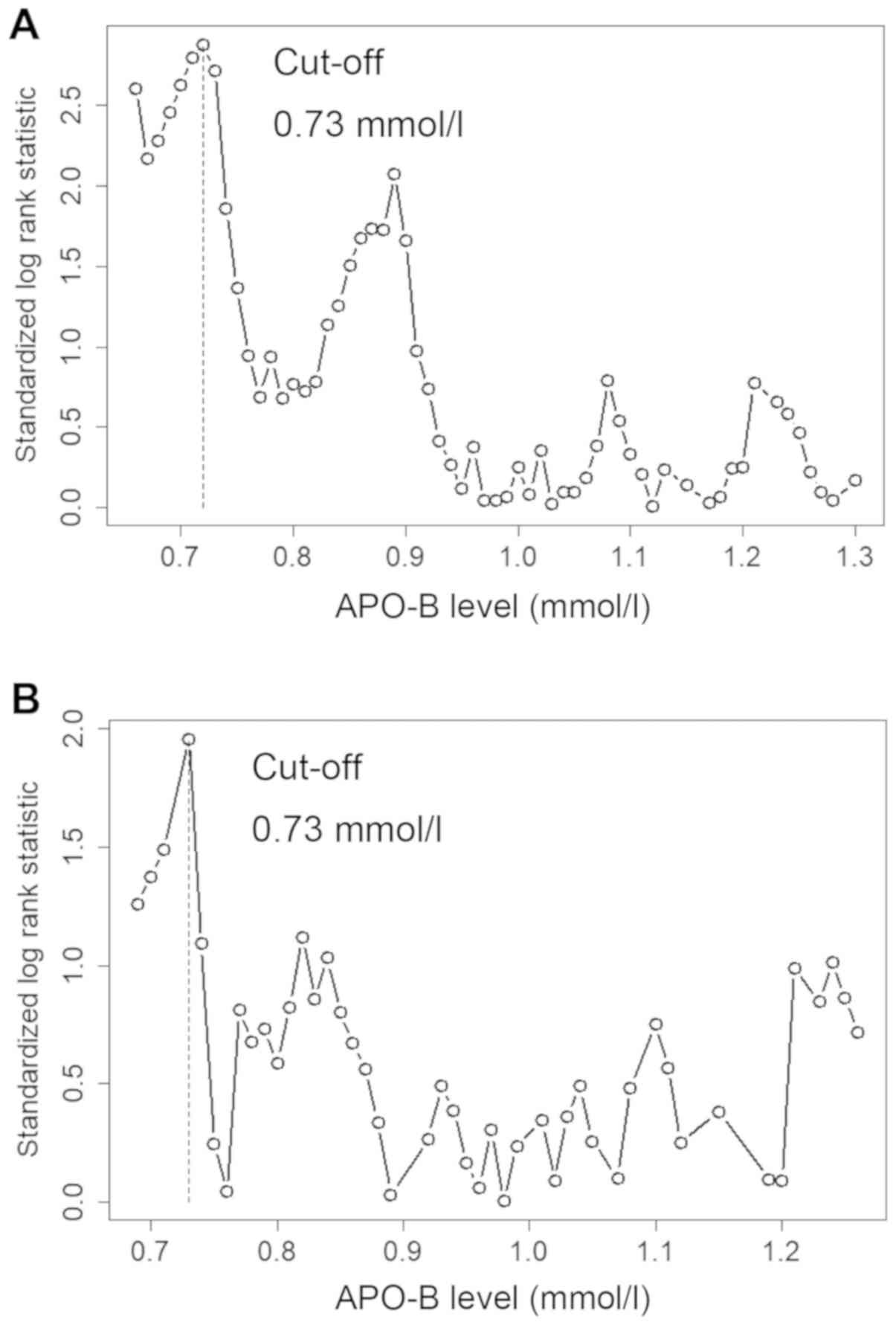
The optimal cut-off values for predicting DFS in men
were determined to be as follows: TC, 3.30 mmol/l; TG, 1.53 mmol/l;
HDL, 0.98 mmol/l; LDL, 1.91 mmol/l; VLDL, 0.20 mmol/l; LDL/HDL,
2.09; APO-A1, 1.05 g/l; and APO-B, and 0.73 g/l. The optimal
cut-off values for predicting DFS in women were as follows: TC,
5.23 mmol/l; TG, 1.58 mmol/l; HDL, 1.18 mmol/l; LDL, 1.91 mmol/l;
VLDL, 0.46 mmol/l; LDL/HDL, 1.35 mmol/l; APO-A1, 1.14 g/l; and
APO-B, 0.73 g/l (Figs. 1 and
2; Table
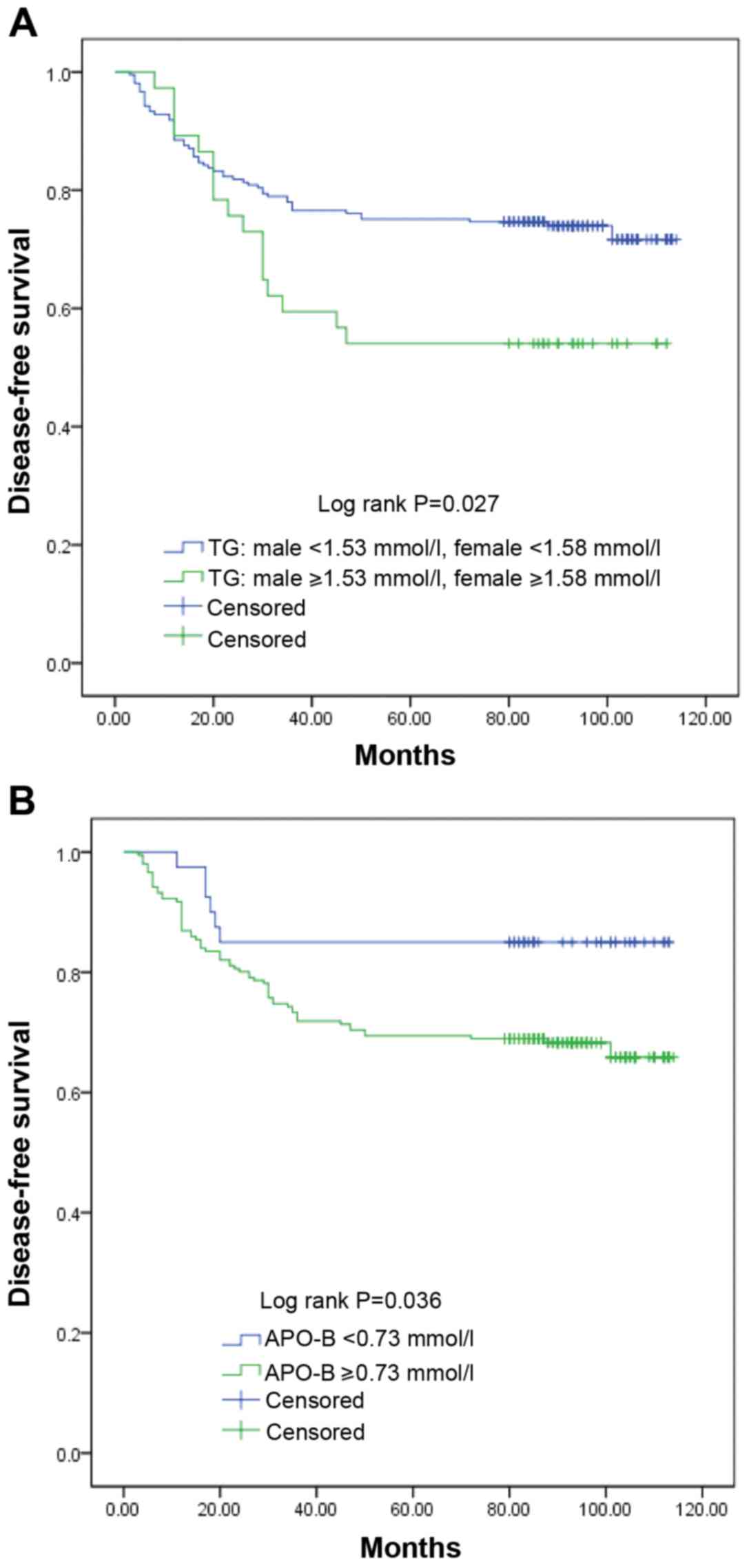
I). The Kaplan-Meier analysis demonstrated that high TG and
APO-B levels were significantly associated with poor DFS time
(Fig. 3; Table I).
 | Table I.Optimal cut-off values of the lipid
indices by sex. |
Table I.
Optimal cut-off values of the lipid
indices by sex.
|
|
|
| Cut-off |
|
|---|
|
|
|
|
|
|
|---|
| Index | Reference
range | No. of patients in
the normal range, n (%) | Male | Female | P-value |
|---|
| TC, mmol/l | 3.60–5.69 | 177 (71.95) | 3.3 | 5.23 | 0.113 |
| TG, mmol/l | 0.34–1.70 | 220 (89.43) | 1.53 | 1.58 | 0.027a |
| HDL, mmol/l | >1.04 | 160 (65.04) | 0.98 | 1.18 | 0.643 |
| LDL, mmol/l | <3.64 | 212 (86.18) | 1.91 | 1.91 | 0.123 |
| VLDL, mmol/l | <0.78 | 242 (98.37) | 0.20 | 0.46 | 0.112 |
| LDL/HDL |
|
| 2.09 | 1.35 | 0.544 |
| APOA1, g/l | 1.2–1.6 | 194 (78.86) | 1.05 | 1.14 | 0.071 |
| APO-B, g/l | 0.6–1.1 | 181(73.58) | 0.73 | 0.73 | 0.036a |
Patient characteristics
A total of 246 patients were included in this study.
Of these, 44 (17.9%) had right-sided colon cancer, 65 (26.4%) had
left-sided colon cancer, and 137 (55.7%) had rectal cancer. The
baseline clinical and laboratory characteristics are listed in
Table II. The median follow-up
duration was 74 (range, 6–114) months. The median follow-up
duration for patients who were alive at the end of this study was
95 months (range, 79–114). At the end of the study, 60 patients
(24.4%) had distant metastasis that mainly involved the lungs
(43.3%) and the liver (33.3%). Local recurrence occurred in 13
(5.3%) cases.
 | Table II.Demographic and clinical
characteristics of the study population (n=246). |
Table II.
Demographic and clinical
characteristics of the study population (n=246).
|
Characteristics | Value | % | Median (min,
max) |
|---|
| Number of
patients | 246 |
|
|
| Age (≥60
years) | 139 | 56.5 |
|
| Sex (male) | 134 | 54.5 |
|
| History of DM | 40 | 16.3 |
|
| History of HP | 61 | 24.8 |
|
| Smoking | 33 | 13.4 |
|
| Alcohol intake | 18 | 7.32 |
|
| BMI,
kg/m2 |
|
| 22.32 (15.78,
46.08) |
|
<18.5 | 27 | 11.0 |
|
|
18.5–23.9 | 138 | 56.1 |
|
|
24.0–27.9 | 70 | 28.5 |
|
|
≥28 | 11 | 4.47 |
|
| Gross
classification |
|
|
|
|
Elevated | 73 | 29.7 |
|
|
Ulcerative | 146 | 59.3 |
|
|
Infiltrative | 27 | 11.0 |
|
| TNM Stage |
|
|
|
|
High-risk II | 73 | 29.7 |
|
|
III | 173 | 70.3 |
|
| Vascular tumor
thrombus | 58 | 23.6 |
|
| Perineural
invasion | 28 | 11.4 |
|
| Differentiation
status |
|
|
|
|
Poor | 51 | 20.7 |
|
|
High/moderate | 195 | 79.3 |
|
| No. of retrieved
lymph nodes ≥12 | 90 | 36.6 |
|
| Intestinal
obstruction | 18 | 7.3 |
|
| TG, mmol/l |
|
| 1.03 (0.39,
4.77) |
| Men
≥1.53, women ≥1.58 | 36 | 14.6 |
|
| APO-B, mmol/l |
|
| 0.92 (0.4,
1.86) |
|
APO-B≥0.73 | 206 | 83.7 |
|
| CEA, ng/ml |
|
| 3.92 (0.203,
266.1) |
| CEA
≥5.0 | 102 | 41.5 |
|
| Tumor location |
|
|
|
|
Right-sided colon | 44 | 17.89 |
|
|
Left-sided colon | 65 | 26.42 |
|
|
Rectum | 137 | 55.69 |
|
Univariate and multivariate survival
analysis
Univariate survival analysis was used to study the
associations of sex, age at diagnosis, history of DM, history of
hypertension, smoking, alcohol intake, BMI, histological type, TNM
stage, TG, APO-B, CEA, differentiation, perineural invasion,
vascular tumor thrombus and intestinal obstruction with DFS and OS.
The results of the univariate analysis demonstrated that TG (men
≥1.53 mmol/l, women ≥1.58 mmol/l), APO-B (≥0.73 mmol/l), TNM stage,
tumor location, perineural invasion and poor differentiation were
identified as significant prognostic factors for DFS (Table III). TG, APO-B, TNM stage, tumor
location and perineural invasion were identified as significant
prognostic factors for OS (Table
III). Variables with P<0.1 in the univariate Cox regression
analysis were used in the multivariate analysis based on forward
stepwise selection. CEA and BMI were also analyzed in multivariate
analysis (Table III).
 | Table III.Univariate and multivariate analyses
for DFS and OS among patients with colorectal cancer. |
Table III.
Univariate and multivariate analyses
for DFS and OS among patients with colorectal cancer.
|
| DFS | OS |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.772 | 0.483–1.233 | 0.279 |
|
|
| 0.838 | 0.536–1.311 | 0.438 |
|
|
|
| Age (≥60 vs.
<60) | 0.698 | 0.441–1.105 | 0.125 |
|
|
| 0.814 | 0.523–1.267 | 0.362 |
|
|
|
| DM | 1.250 | 0.698–2.239 | 0.453 |
|
|
| 1.122 | 0.630–2.000 | 0.695 |
|
|
|
| Hypertension | 0.867 | 0.704–1.067 | 0.179 |
|
|
| 0.876 | 0.724–1.060 | 0.173 |
|
|
|
| Smoking | 1.222 | 0.658–2.269 | 0.526 |
|
|
| 1.159 | 0.626–2.146 | 0.638 |
|
|
|
| Alcohol | 0.868 | 0.350–2.152 | 0.760 |
|
|
| 0.829 | 0.335–2.053 | 0.686 |
|
|
|
| BMI (≥28.0 vs.
24.0–27.9 vs. 18.5–23.9 vs. <18.5) | 0.997 | 0.716–1.387 | 0.985 | 0.845 | 0.590–1.210 | 0.359 | 0.965 | 0.700–1.329 | 0.825 | 0.791 | 0.556–1.125 | 0.192 |
| TG (male ≥1.53,
female ≥1.58 vs. male <1.53, female <1.58) | 1.825 | 1.059–3.143 | 0.030a | 1.987 | 1.057–3.737 | 0.033a | 2.229 | 1.351–3.678 | 0.002 | 2.374 | 1.318–4.275 | 0.004a |
| APO-B (≥0.73 vs.
<0.73) | 2.369 | 1.027–5.461 | 0.043a | 2.920 | 1.031–8.276 | 0.044a | 2.282 | 1.098–4.741 | 0.027 | 2.425 | 1.019–5.775 | 0.045a |
| CEA (≥5.0 vs
<5.0) | 1.170 | 0.738–1.855 | 0.505 | 1.138 | 0.686–1.889 | 0.617 | 1.153 | 0.740–1.799 | 0.529 | 1.117 | 0.686–1.819 | 0.655 |
| Gross
classification | 1.055 | 0.781–1.426 | 0.726 |
|
|
| 0.824 | 0.575–1.180 | 0.291 |
|
|
|
| TNM Stage (III
stage vs. high-risk II) | 3.253 | 1.668–6.342 | 0.001a | 3.258 | 1.606–6.608 | 0.001a | 2.454 | 1.376–4.376 | 0.002a | 2.772 | 1.485–5.177 | 0.001a |
| Tumor location
(rectum vs. left colon vs. right colon) | 1.578 | 1.120–2.223 | 0.009a | 1.329 | 0.911–1.940 | 0.140 | 1.537 | 1.112–2.125 | 0.009a | 1.319 | 0.924–1.882 | 0.127 |
| Vascular tumor
thrombus | 1.243 | 0.743–2.079 | 0.408 |
|
|
| 1.151 | 0.697–1.898 | 0.583 |
|
|
|
| Perineural
invasion | 2.693 | 1.543–4.699 | 0.001a | 2.891 | 1.573–5.312 | 0.001a | 2.811 | 1.638–4.823 | 0.001a | 2.963 | 1.641–5.353 | 0.001a |
| Differentiation
status (poor vs. high/moderate) | 1.687 | 1.016–2.801 | 0.043a | 0.825 | 0.624–1.091 | 0.177 | 0.877 | 0.681–1.130 | 0.310 | 0.801 | 0.612–1.047 | 0.105 |
| No. of retrieved
lymph nodes (≥12 vs. <12) | 1.202 | 0.752–1.922 | 0.441 |
|
|
| 1.049 | 0.664–1.659 | 0.837 |
|
|
|
| Intestinal
obstruction | 1.377 | 0.631–3.003 | 0.421 |
|
|
| 1.497 | 0.720–3.112 | 0.280 |
|
|
|
In the multivariate Cox analysis, high TG levels
(HR, 1.987; 95% CI, 1.057–3.737), high APO-B levels (HR, 2.920; 95%
CI, 1.031–8.276), advanced TNM stage (HR, 3.258; 95% CI,
1.606–6.608) and perineural invasion (HR, 2.891; 95% CI,
1.573–5.312) were identified as independent prognostic factors for
DFS in patients with CRC (Table
III). High TG levels (HR, 2.374; 95% CI, 1.318–4.275), high
APO-B levels (HR, 2.425; 95% CI, 1.019–5.775), advanced TNM stage
(HR, 2.772; 95% CI, 1.485–5.177) and perineural invasion (HR,
2.963; 95% CI, 1.641–5.353) were identified as independent
prognostic factors for OS in patients with CRC (Table III).
Nomogram
To assess the prognostic values of TG and APO-B for
DFS and OS in patients with CRC, nomograms were constructed
(Fig. 4). The variables from Cox
multivariate analysis, including TG, APO-B, TNM stage, tumor
location, perineural invasion, differentiation, CEA and BMI, were
incorporated into the nomograms. The concordance index of the
nomogram that included TG and APO-B values were 0.754 for DFS and
0.768 for OS, whereas the concordance index of the nomogram without
TG and APO-B values were 0.732 for DFS and 0.726 for OS. These
outcomes indicated that the concordance index of the nomogram
involving TG and APO-B values may be improved compared with that of
the nomogram without these values in predicting the clinical
outcomes of CRC patients.
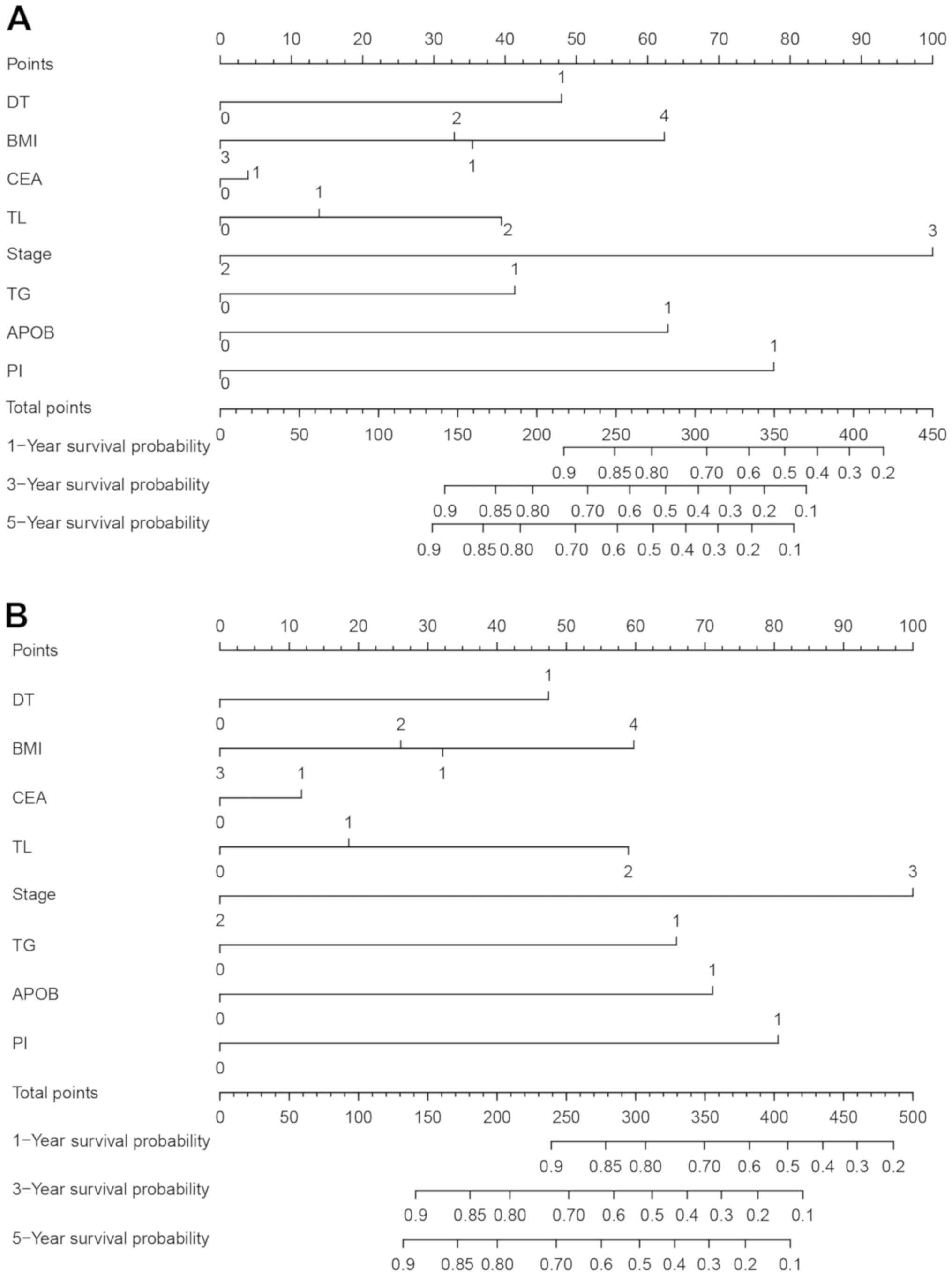 | Figure 4.Prognostic nomograms for predicting
(A) OS and (B) DFS in patients with stage III and high-risk stage
II CRC. DT: 0, well/moderate; 1, poor. BMI: 1, <18.5
kg/m2; 2, 18.5–23.9 kg/m2; 3, 24.0–27.9
kg/m2; 4, ≥28.0 kg/m2. CEA: 0, <5.0 ng/ml
1, ≥5.0 ng/ml. TL: 0, right colon; 1, left colon; 2, rectum. Stage:
2, high-risk TNM stage II; 3, TNM stage III. TG: 0, male <1.53
mmol/l, female <1.58 mmol/l; 1, male ≥1.53 mmol/l, female ≥1.58
mmol/l. APO-B: 0, <0.73 mmol/l; 1, ≥ 0.73 mmol/l. PI: 0, no
perineural invasion; 1, perineural invasion. DFS, disease-free
status; OS, overall survival; DT, differentiation status; BMI, body
mass index; CEA, carcinoembryonic antigen; TL, tumor location; TG,
triglyceride; APO-B, apolipoprotein B; PI, perineural invasion. |
Discussion
In the present study, the effects of blood lipid and
apolipoprotein on the prognosis of radical CRC were investigated.
The results of the present study demonstrated that high TG and
APO-B levels were independent prognostic factors for poor DFS and
OS in patients with stage III and high-risk stage II CRC.
To the best of our knowledge, this is the first
study to describe the relationship between APO-B levels and CRC
prognosis. The patients were into two groups using a cut-off value
of 0.73 mmol/l based on the R MaxStat method. Multivariate analysis
and the nomogram demonstrated that a baseline high serum APO-B
level was a predictor of poor DFS and OS in patients with CRC.
APO-B is the main apolipoprotein of chylomicrons, VLDL,
intermediate-density lipoprotein and LDL particles (21). Thus, it is responsible for
transporting fat molecules to all peripheral tissues. APO-B is also
the major structural protein for atherogenic APO-B-containing
lipoproteins (22). The
epidemiological evidence for the association between APO-B levels
and CRC prognosis has not been confirmed, and the conclusions
concerning this association are controversial. Borgquist et
al (23) conducted a prospective
cohort study that included 28,098 individuals with an average
follow-up of 14.3 years and reported that the levels of APO-B were
positively associated with the risk of CRC and lung cancer. Another
study with an average follow-up of 15.6 years conducted by Katzke
et al (24) revealed that
high levels of circulating APO-B were positively associated with
the risk of breast cancer. By contrast, in a study on tumor
prognosis, Chen et al (25)
reported that high levels of APO-B were beneficial for the OS of
patients with lung cancer. In a retrospective study involving 1,201
patients with gastric cancer, those with high APO-B/APO-A1 ratios
had a shorter OS (26).
The present study demonstrated that patients with
high TG levels (men, ≥1.53 mmol/l; women, ≥1.58 mmol/l) had worse
DFS and OS. TGs constitute a part of the lipid profile in the human
body and are the major component of chylomicrons and VLDL (27). TGs are formed from fatty acids and
stored in the adipose tissue, and they are the main form of energy
storage and circulation (28). TGs
are also involved in protein transport and serve as energy sources
obtained from dietary fat (27).
Patients with obesity or DM often present with high TG levels
(29). In addition, a high TG level
is one of the diagnostic criteria for the metabolic syndrome
(27). Due to the known associations
of obesity, DM and the metabolic syndrome with CRC progression, a
growing number of studies have focused on the relationship between
TG levels and CRC (30,31). A meta-analysis of 16 studies
indicated that high levels of TG increased the overall risk of
cancer by 20% (32). The results of
another meta-analysis of 17 studies, which assessed the association
between CRC risk and TG levels, demonstrated that high TG levels
increased the overall CRC risk by 6%; however, this increase was
not statistically significant (33).
A large prospective study that included 514,097 participants with
an average follow-up of 13.4 years indicated that high levels of
serum TG were associated with a significant two-fold increase in
the risk of colon cancer in men but not in women (34).
Epidemiological studies on the association between
serum TG levels and cancer prognosis are sparse. Although some
studies have assessed the relationship between serum TG levels and
prognosis in tumors such as CRC, breast and prostate cancer, the
results are inconsistent. In breast cancer, high preoperative serum
TG levels were identified as a significant independent predictor of
improved DFS (35). However, in
prostate cancer, patients with high TG levels may be at a higher
risk of developing aggressive prostate cancer compared with those
with low TG levels (36). The
association between TG levels and CRC prognosis remains unclear. In
a large retrospective study, high TG levels were independently
associated with improved OS and recurrence-free survival rates in
patients with colon cancer (12). In
another study, TG levels were not associated with progression-free
survival in patients with colon cancer (37).
Differences in the study population, follow-up time,
endpoints and statistical adjustment for confounding factors may
have resulted in the conflicting results in the aforementioned
studies. In addition, the blood lipid cut-off points were different
among previous studies, and these studies did not exclude patients
who were using lipid-lowering drugs. To minimize the impact of
confounding factors on outcomes, the present study selected
patients with stage III and high-risk stage II CRC who had a higher
risk of recurrence compared with other patients with non-metastatic
CRC who were followed up for a longer period of time. In addition,
the present study eliminated the possible bias that could have been
introduced by lipid-lowering drugs by excluding patients receiving
these medications. The measurements of blood lipid levels were
performed under conditions where patients were not allowed to eat a
high-fat diet following hospitalization, which ensured the accuracy
of these measurements. Considering that blood lipid composition and
metabolism in patients with CRC are different from those in healthy
individuals (38), the prognostic
significance of the established cut-off values was evaluated using
a maximal x2 method instead of using conventional
cut-off values. The maximal x2 method can define a
subset with the greatest survival difference (39). In the present study, the cut-off
values of TG and APO-B were lower compared with the conventional
cut-off values, which may be due to the influence of cancer on the
metabolic indicators and nutritional status of patients. To the
best of our knowledge, this is the first time such an approach has
been used in a study to determine the prognostic impact of lipid
cut-off points in CRC. After controlling for confounding factors,
the present study demonstrated that high TG levels were associated
with a 9.87% greater fully adjusted risk for cancer recurrence.
However, Kaplan-Meier survival curves demonstrated that mortality
in the low TG group was high in the first 20 months after surgery.
This increase in mortality may be attributable to cardiovascular
events. Mortality caused by cardiovascular events tends to increase
with the increase in TG levels (40), which reduces the proportion of
cancer-related deaths in the high TG group. The impaired exercise
capacity after surgery has adverse effects on heart function in
patients (41); therefore, the risk
of cardiovascular complications may be higher during the early
postoperative period.
The potential mechanisms for the association between
hypertriglyceridemia and cancer development are as follows: i)
Increased chronic inflammatory response in patients with
hypertriglyceridemia can stimulate cancer cell growth (42); ii) TG levels are positively
associated with fecal bile acids, which have been demonstrated to
be cytotoxic; this may cause DNA damage and promote CRC progression
(43); iii) the results of
nutritional studies have demonstrated that a high-fat diet was
associated with accelerated tumor growth and metastasis (44); and iv) there is experimental evidence
that fatty acid synthase, an enzyme that synthesizes fatty acids,
affects tumorigenesis (45,46).
BMI is another important metabolic index; the
present study reported that patients with an overweight BMI
classification had a favorable prognosis compared with that of
patients with a normal or obese BMI classification, which was
consistent with the results demonstrated in other studies (47,48).
The present study had some limitations, including
its retrospective design. In addition, dietary fat intake (as the
percentage of total calories) and metabolic changes during
follow-up could not be assessed; therefore, the impact of these
factors on prognosis were not analyzed in this study. Since the
lipid-specific genetic risk score was not analyzed, it was not
possible to evaluate the role of genetic factors in the association
between lipid levels and CRC prognosis. Lastly, since this was a
single-center study with a small sample size, the possibility of
selection bias could not be completely eliminated.
In conclusion, TG and APO-B levels at diagnosis may
be independent prognostic factors for stage III and high-risk stage
II CRC. Adjusting these parameters at diagnosis to appropriate
levels may be beneficial to patients with CRC and may also enable
physicians to choose appropriate treatment regimens for these
patients. The present study makes a significant contribution to the
identification of biomarkers that may be used to accurately predict
CRC prognosis, and both TG and APO-B levels can be detected through
a low-cost, convenient method. Further prospective, multi-center
studies are needed to investigate the exact mechanisms of the
association between these biomarkers and CRC prognosis and their
biological significance.
Acknowledgements
The abstract of the present study has been
previously published as part of a poster presentation at the 23rd
Scientific Meeting of the Chinese Diabetes Society held in Xiamen,
Fujian Province, China on November 20–23, 2019.
Funding
This study was supported by Grants from The National
Natural Science Foundation of China (grant no. 81870572); The
Medical Innovation Fund Project of Fujian Province (grant no.
2018-CX-23) and The Education and Scientific Research Projects of
Young and Middle-aged Teachers in Fujian Province (grant no.
JAT190207).
Availability of data and materials
All datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
LYY, XMS and SJY conceived and designed the study.
XQC and PWW obtained and analyzed the data and edited the drafts.
PWW and DHL reviewed the results and participated in the discussion
of the data. DHL performed the statistical analysis. XQC and XMS
wrote the manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Fujian Medical
University (approval no. MRCTA, ECFAH of FMU [2019] 200). Written
informed consent for data collection and analysis was obtained from
the respective patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APO-A1
|
apolipoprotein-A1
|
|
APO-B
|
apolipoprotein B
|
|
BMI
|
body mass index
|
|
CI
|
confidence interval
|
|
CEA
|
carcinoembryonic antigen
|
|
CRC
|
colorectal cancer
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
DM
|
diabetes mellitus
|
|
DT
|
differentiation status
|
|
HDL
|
high-density lipoprotein
|
|
HR
|
hazard ratio
|
|
LDL
|
low-density lipoprotein
|
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
TNM
|
Tumor-Node-Metastasis
|
|
VLDL
|
very low-density lipoprotein
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new American joint committee on
cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hari DM, Leung AM, Lee JH, Sim MS, Vuong
B, Chiu CG and Bilchik AJ: AJCC cancer staging manual 7th edition
criteria for colon cancer: Do the complex modifications improve
prognostic assessment? J Am Coll Surg. 217:181–190. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cerottini JP, Caplin S, Pampallona S and
Givel JC: Prognostic factors in colorectal cancer. Oncol Rep.
6:409–414. 1999.PubMed/NCBI
|
|
5
|
Micu BV, Vesa SC, Pop TR and Micu CM:
Evaluation of prognostic factors for 5 year-survival after surgery
for colorectal cancer. Ann Ital Chir. 91:41–48. 2020.PubMed/NCBI
|
|
6
|
Rashtak S, Ruan X, Druliner BR, Liu H,
Therneau T, Mouchli M and Boardman LA: Peripheral neutrophil to
lymphocyte ratio improves prognostication in colon cancer. Clin
Colorectal Cancer. 16:115–123 e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma C, Xie J, Luo C, Yin H, Li R, Wang X,
Xiong W, Zhang T, Jiang P, Qi W, et al: OxLDL promotes
lymphangiogenesis and lymphatic metastasis in gastric cancer by
upregulating VEGFC expression and secretion. Int J Oncol.
54:572–584. 2019.PubMed/NCBI
|
|
8
|
Nowak C and Ärnlöv J: A Mendelian
randomization study of the effects of blood lipids on breast cancer
risk. Nat Commun. 9:39572018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murtola TJ, Kasurinen TVJ, Talala K, Taari
K, Tammela TLJ and Auvinen A: Serum cholesterol and prostate cancer
risk in the Finnish randomized study of screening for prostate
cancer. Prostate Cancer Prostatic Dis. 22:66–76. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raju K, Punnayanapalya SS, Mariyappa N,
Eshwarappa SM, Anjaneya C and Kai LJ: Significance of the plasma
lipid profile in cases of carcinoma of cervix: A tertiary hospital
based study. Asian Pac J Cancer Prev. 15:3779–3784. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan TWM, Zhang X, Wang C, Yang Y, Kang WY,
Arnold S, Higashi RM, Liu J and Lane AN: Exosomal lipids for
classifying early and late stage non-small cell lung cancer. Anal
Chim Acta. 1037:256–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Mauldin PD, Ebeling M, Hulsey TC,
Liu B, Thomas MB, Camp ER and Esnaola NF: Effect of metabolic
syndrome and its components on recurrence and survival in colon
cancer patients. Cancer. 119:1512–1520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng F, Hu D, Lin X, Chen G, Liang B, Chen
Y, Li C, Zhang H, Xia Y, Lin J, et al: An in-depth prognostic
analysis of baseline blood lipids in predicting postoperative
colorectal cancer mortality: The FIESTA study. Cancer Epidemiol.
52:148–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manfredi S, Bouvier AM, Lepage C, Hatem C,
Dancourt V and Faivre J: Incidence and patterns of recurrence after
resection for cure of colonic cancer in a well defined population.
Br J Surg. 93:1115–1122. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benson AB III, Schrag D, Somerfield MR,
Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J,
McAllister P, Van Cutsem E, et al: American society of clinical
oncology recommendations on adjuvant chemotherapy for stage II
colon cancer. J Clin Oncol. 22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Connor ES, Greenblatt DY, LoConte NK,
Gangnon RE, Liou JI, Heise CP and Smith MA: Adjuvant chemotherapy
for stage II colon cancer with poor prognostic features. J Clin
Oncol. 29:3381–3388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual, 7th ed.
Springer. (New York, NY). 2010.
|
|
18
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumours of the digestic system.
(4th). (Lyon). IARC press. 2010.
|
|
19
|
Zhou BF and Cooperative Meta-Analysis
Group of the Working Group on Obesity in China: Predictive values
of body mass index and waist circumference for risk factors of
certain related diseases in Chinese adults-study on optimal cut-off
points of body mass index and waist circumference in Chinese
adults. Biomed Environ Sci. 15:83–96. 2002.PubMed/NCBI
|
|
20
|
Torsten H and Berthold L: On the exact
distribution of maximally selected rank statistics. Comput Stat
Data Anal. 43:121–137. 2003. View Article : Google Scholar
|
|
21
|
Brown MS and Goldstein JL: A
receptor-mediated pathway for cholesterol homeostasis. Science.
232:34–47. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kane JP: Apolipoprotein B: Structural and
metabolic heterogeneity. Annu Rev Physiol. 45:637–650. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borgquist S, Butt T, Almgren P, Shiffman
D, Stocks T, Orho-Melander M, Manjer J and Melander O:
Apolipoproteins, lipids and risk of cancer. Int J Cancer.
138:2648–2656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katzke VA, Sookthai D, Johnson T, Kühn T
and Kaaks R: Blood lipids and lipoproteins in relation to incidence
and mortality risks for CVD and cancer in the prospective
EPIC-Heidelberg cohort. BMC Med. 15:2182017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen S, Lai Y, He Z, Li J, He X, Shen R,
Ding Q, Chen H, Peng S and Liu W: Establishment and validation of a
predictive nomogram model for non-small cell lung cancer patients
with chronic hepatitis B viral infection. J Transl Med. 16:1162018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma MZ, Yuan SQ, Chen YM and Zhou ZW:
Preoperative apolipoprotein B/apolipoprotein A1 ratio: A novel
prognostic factor for gastric cancer. Onco Targets Ther.
11:2169–2176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Cholesterol Education Program
(NCEP) Expert Panel on Detection, Evaluation, and Treatment of High
Blood Cholesterol in Adults (Adult Treatment Panel III), . Third
report of the national cholesterol education program (NCEP) expert
panel on detection, evaluation, and treatment of high blood
cholesterol in adults (adult treatment panel III) final report.
Circulation. 106:3143–3421. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Angelin B, Eriksson M and Rudling M: Bile
acids and lipoprotein metabolism: A renaissance for bile acids in
the post-statin era? Curr Opin Lipidol. 10:269–274. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fasshauer M and Paschke R: Regulation of
adipocytokines and insulin resistance. Diabetologia. 46:1594–1603.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Croft B, Reed M, Patrick C, Kovacevich N
and Voutsadakis IA: Diabetes, obesity, and the metabolic syndrome
as prognostic factors in stages I to III colorectal cancer
patients. J Gastrointest Cancer. 50:221–229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen Z, Ye Y, Bin L, Yin M, Yang X, Jiang
K and Wang S: Metabolic syndrome is an important factor for the
evolution of prognosis of colorectal cancer: Survival, recurrence,
and liver metastasis. Am J Surg. 200:59–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Melvin JC, Holmberg L, Rohrmann S, Loda M
and Van Hemelrijck M: Serum lipid profiles and cancer risk in the
context of obesity: Four meta-analyses. J Cancer Epidemiol.
2013:8238492013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Esposito K, Chiodini P, Capuano A,
Bellastella G, Maiorino MI, Rafaniello C, Panagiotakos DB and
Giugliano D: Colorectal cancer association with metabolic syndrome
and its components: A systematic review with meta-analysis.
Endocrine. 44:634–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borena W, Stocks T, Jonsson H, Strohmaier
S, Nagel G, Bjørge T, Manjer J, Hallmans G, Selmer R, Almquist M,
et al: Serum triglycerides and cancer risk in the metabolic
syndrome and cancer (Me-Can) collaborative study. Cancer Causes
Control. 22:291–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Tang H, Wang J and Xie X, Liu P,
Kong Y, Ye F, Shuang Z, Xie Z and Xie X: The effect of preoperative
serum triglycerides and high-density lipoprotein-cholesterol levels
on the prognosis of breast cancer. Breast. 32:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hayashi N, Matsushima M, Yamamoto T,
Sasaki H, Takahashi H and Egawa S: The impact of
hypertriglyceridemia on prostate cancer development in patients
aged ≥60 years. BJU Int. 109:515–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stocks T, Lukanova A, Bjørge T, Ulmer H,
Manjer J, Almquist M, Concin H, Engeland A, Hallmans G, Nagel G, et
al: Metabolic factors and the risk of colorectal cancer in 580,000
men and women in the metabolic syndrome and cancer project
(Me-Can). Cancer. 117:2398–2407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pakiet A, Kobiela J, Stepnowski P,
Sledzinski T and Mika A: Changes in lipids composition and
metabolism in colorectal cancer: A review. Lipids Health Dis.
18:292019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lausen B, Hothorn T, Bretz F and
Schumacher M: Assessment of optimal selected prognostic factors.
Biom J. 46:364–374. 2004. View Article : Google Scholar
|
|
40
|
Ridker PM: Fasting versus nonfasting
triglycerides and the prediction of cardiovascular risk: Do we need
to revisit the oral triglyceride tolerance test? Clin Chem.
54:11–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cramer L, Hildebrandt B, Kung T, Wichmann
K, Springer J, Doehner W, Sandek A, Valentova M, Stojakovic T,
Scharnagl H, et al: Cardiovascular function and predictors of
exercise capacity in patients with colorectal cancer. J Am Coll
Cardiol. 64:1310–1319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim MH, Kim HN and Choi WS: The
association between subclinical inflammation and abnormal glucose
and lipid metabolisms in normal-weight Korean individuals. Nutr
Metab Cardiovasc Dis. 28:1106–1113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McKeown-Eyssen G: Epidemiology of
colorectal cancer revisited: Are serum triglycerides and/or plasma
glucose associated with risk? Cancer Epidemiol Biomarkers Prev.
3:687–695. 1994.PubMed/NCBI
|
|
44
|
Rossi S, Graner E, Febbo P, Weinstein L,
Bhattacharya N, Onody T, Bubley G, Balk S and Loda M: Fatty acid
synthase expression defines distinct molecular signatures in
prostate cancer. Mol Cancer Res. 1:707–715. 2003.PubMed/NCBI
|
|
45
|
Flavin R, Zadra G and Loda M: Metabolic
alterations and targeted therapies in prostate cancer. J Pathol.
223:283–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Migita T, Ruiz S, Fornari A, Fiorentino M,
Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescandolo E, Shin E,
et al: Fatty acid synthase: A metabolic enzyme and candidate
oncogene in prostate cancer. J Natl Cancer Inst. 101:519–532. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aparicio T, Ducreux M, Faroux R, Barbier
E, Manfredi S, Lecomte T, Etienne PL, Bedenne L, Bennouna J, Phelip
JM, et al: Overweight is associated to a better prognosis in
metastatic colorectal cancer: A pooled analysis of FFCD trials. Eur
J Cancer. 98:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee J, Meyerhardt JA, Giovannucci E and
Jeon JY: Association between body mass index and prognosis of
colorectal cancer: A meta-analysis of prospective cohort studies.
PLoS One. 10:e01207062015. View Article : Google Scholar : PubMed/NCBI
|