Introduction
According to cancer statistics reported in the
United States in 2016, ovarian cancer is the leading cause of
gynecological malignancy-associated mortality and ~70% of patients
are diagnosed at an advanced stage (1). Therefore, it is important to
distinguish benign ovarian masses from early-stage malignant
ovarian tumors to decide whether to operate, select the method of
operation and to prevent cancer progression.
Cancer antigen (CA)125 is currently used as a
conventional marker for epithelial ovarian cancer (EOC) (2). However, the serum CA125 expression
levels are frequently increased and false-positive results may be
obtained for certain benign uterine tumors and medical conditions,
including leiomyomas, adenomyosis, infection, liver cirrhosis,
pelvic endometriosis and pregnancy (3,4). Serum
CA125 expression levels are frequently in the normal range in
early-stage invasive ovarian cancer (5). Therefore, CA125 is not recommended for
the discrimination of ovarian tumors due to low specificity.
Previously, it was demonstrated that human epididymis protein 4
(HE4) was involved in the neoplastic processes of EOC and HE4 has
been used to overcome the aforementioned limitations in the
discrimination of ovarian tumors (6,7). HE4
expression levels are increased in certain ovarian cancers and it
has the advantage of not being affected by physiological
conditions, as is the case with CA125 (8,9). Serum
HE4 expression levels are not affected by the menstrual cycle,
hormonal treatment and endometriosis, but expression levels may
increase with age and smoking (10,11).
Therefore, the Risk of Ovarian Malignancy Algorithm (ROMA) test was
developed using CA125, HE4 and menopausal status. The algorithm
test is currently used for discriminating between benign and
early-stage malignant ovarian tumors (12,13).
Studies on the efficacy of the ROMA test have
provided inconsistent results due to the different distribution of
tumor subtypes in the patient population in each study. The
expression levels of HE4 in tumors depend on the histological
subtype. Drapkin et al (14)
demonstrated that 100% of endometrioid and 93% of serous EOCs
expressed HE4; however, only 50% of clear-cell carcinomas and no
mucinous tumors were HE4-positive (14). However, no previous study has
evaluated the efficacy of the ROMA test by tumor subtype and the
majority of previous studies have only focused on the fact that
ROMA is more useful in identifying endometriosis compared with
CA125 (15–18). In addition, these studies have
included several incidences of hydrosalpinx, paratubal cysts,
inclusion cysts and advanced ovarian cancer that may be
distinguished from each other using ultrasonography, as well as
functional cysts that spontaneously disappeared in the follow-up
period.
The present study investigated the efficacy of the
ROMA test in comparison with CA125 as a tool for discriminating
between benign and early-stage ovarian cancer according to imaging
tumor subtypes associated with post-operative histopathological
findings.
Materials and methods
Patients
After obtaining approval from The Institutional
Review Board at the Asan Medical Center (Seoul, Republic of Korea;
approval no. 2019-0616), the medical records of patients who
underwent the ROMA test due to suspicion of early-stage ovarian
cancer and were subjected to surgery at Asan Medical Center (Seoul,
Republic of Korea) between September 2014 and March 2018 were
retrospectively reviewed. The clinicopathological data were
collected, including age, menopausal status, pre-operative results
regarding CA125 and the ROMA test, results of imaging analysis
(tumor size and volume), histological subtype and International
Federation of Gynecology and Obstetrics stage (19) in malignant cases.
Only patients with histologically-confirmed
diagnosis after surgery were included in the analysis. The patients
were pathologically diagnosed by topography of the ovarian
structure as the major differentiation point and additional
immunostaining was performed when detailed discrimination or origin
confirmation was required. Patients with advanced ovarian cancer
with ascites and peritoneal carcinomatosis that were sufficiently
predictable by sonography or abdominopelvic computed tomography
(APCT) prior to surgery were excluded. Patients with only
hydrosalpinx or paratubal cyst, inclusion cysts by pelvic adhesion
and inflammatory lesions were excluded from the analysis, as these
cases should have been excluded from suspicion of ovarian cancer by
pre-operative evaluation with imaging or inflammatory tests. When
ovarian masses were bilateral, they were included in the analysis
if they were of the same subtype and excluded if they were
different subtypes, as the subtype that affected the discrimination
was not known in the present study and this may serve as a
confounder. The presence of two or more tumor subtypes in one ovary
were also excluded from the analysis, as it was not known which
tumor subtype affected the blood test. Patients were excluded if
there were >3 months between the operation and the blood test.
Patients diagnosed with ovarian masses during pregnancy were
excluded due to changes in the CA125 level following the gestation
period. Patients with end-stage renal disease, diabetic
nephropathy, nephrotic syndrome, renal cancer or urosepsis were
excluded due to the possibility of serum HE4 levels being
abnormally high due to decreased elimination or increased
production from the damaged renal tubules (17). Patients who were diagnosed with other
malignancies within 5 years were excluded as it was not possible to
estimate the degree of the effect from primary site carcinoma.
Patients with transient cell carcinoma, undifferentiated carcinoma,
squamous cell carcinoma and mixed Müllerian malignant tumor were
excluded from the analysis due to the rarity of such cases.
Laboratory methods
The present study used the ARCHITECT CA125 II assay
(cat. no. 2P45) and the ARCHITECT HE4 assay (cat. no. 2P54; Abbott
Pharmaceutical Co. Ltd.) for the quantitative determination of
CA125 and HE4 expression levels (20). These were two-step immunoassays that
used the chemiluminescent microparticle immunoassay technology
(Abbott Pharmaceutical Co., Ltd.), which were performed according
to the manufacturer's protocol. The cut-off value was 35.0 U/ml for
CA125 and 70 pmol/ml for HE4. The expression levels of these
markers were defined as normal when lower than the cut-off value.
Patients were then stratified into low- or high-risk groups based
on laboratory methods for calculating the ROMA score and menopausal
status. Predictive index (PI) and predicted probability were
calculated using the following algorithms proposed by Moore et
al (11): Pre-menopausal PI =
−12.0 +2.38× LN (natural log) (HE4) +0.0626× LN(CA125);
Post-menopausal PI = −8.09 +1.04× LN(HE4) +0.7320× LN(CA125);
Predicted probability (ROMA, %) = exp (PI)/[1 + exp (PI)] ×100.
The cut-off value for ROMA was 7.4 for
pre-menopausal females and 25.3 for post-menopausal females.
Regarding the menopausal status, post-menopausal was defined as 1
year or more after cessation of menstrual bleeding. For patients
who underwent hysterectomy prior to menopause, an age of 50 years
or above was defined as post-menopausal. The result of the blood
tests (CA125 and ROMA test) were defined as true if it was within
the normal range (below cut-off value) for benign tumors or above
the normal range for malignant tumors.
Imaging study
For the imaging study, sonography or APCT were
routinely performed as hospital practice. There were no definite
criteria for the size or number of leiomyomas affecting the CA125
level. Therefore, one or more leiomyoma >3 cm was considered
positive for the presence of leiomyoma. All images were blindly
interpreted by two radiologists at the Asan Medical Center (Seoul,
Republic of Korea) and the total ovarian tumor volume was
calculated.
Imaging subtypes of tumors
This classification system was specifically used for
the present study. In this system, diseases that appear similar in
image and are difficult to distinguish by image alone are grouped
together. This group was subsequently used to compare and analyze
the efficacy of CA125 and ROMA tests to identify malignant tumors.
Accordingly, imaging subtypes of tumors were classified for
analysis as associated with post-operative histological findings as
follows: Serous cystadenoma, corpus luteal cyst, simple cyst,
serous borderline tumor, serous adenocarcinoma were classified as
‘serous type’; mucinous cystadenoma, mucinous borderline tumor and
mucinous adenocarcinoma were classified as ‘mucinous type’;
endometriotic cyst, seromucinous borderline tumor, endometrioid
adenocarcinoma and clear cell carcinoma were classified as
‘endometriotic type’; and mature cystic teratoma, fibroma, Brenner
tumor, germ cell tumor and sex-cord stromal cell tumor were
classified as ‘solid type’.
Statistical analysis
Associations between variable characteristics and
ovarian tumors were assessed by Mann Whitney-U tests followed by
Bonferroni's correction. Patient characteristics are presented as
the median and interquartile range. Diagnostic performance of CA125
and ROMA was compared in patients with suspected ovarian cancer
stratified by menopausal status, leiomyomas and adenomyosis. The
sensitivity, specificity, positive predictive value (PPV), negative
predictive value (NPV) and accuracy of the two methods were
compared. CA125 and the ROMA test were analyzed using imaging of
tumor subtype. For the analysis of discrepancies in the two
prediction methods, McNemar's test was used to assess how the two
measurements differed without considering the outcome data. The net
reclassification index (NRI) indicated the extent to which the ROMA
test improved prediction compared with CA125 using outcome data.
Due to the fact that the ROMA test had different scales for
pre-menopausal and post-menopausal patients, receiver operating
characteristic (ROC) analysis was also performed for these groups.
The P-values for comparing the two ROC curves were calculated using
Delong's test for two associated ROC curves. For P-values subjected
to Bonferroni correction, P>0.0167 (0.05/3) was considered to
indicate a statistically significant difference. For all other
data, P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software version 21.0 (IBM Corp.).
Results
Patient characteristics
Among the 1,207 patients identified in the initial
search, 981 met the inclusion criteria and were included in the
retrospective analysis. Of these patients, 816 (83.2%) were
diagnosed with benign disease, 75 (7.6%) with borderline disease
and 90 (9.2%) had cancer. All serous adenocarcinoma included in the
study were of high grade. The characteristics of the patients are
summarized in Table I. Patients who
were diagnosed with ovarian cancer were older and more likely to be
post-menopausal (P<0.001). Of the patients diagnosed with
ovarian cancer, 63.3% were judged as being high-risk according to
the ROMA test and 67.8% had CA125 levels of >35 U/ml. This
sensitivity was further reduced by including patients with
borderline tumors: A total of 47.3% (78/165) were judged as being
high-risk using the ROMA test and 58.2% (96/165) had CA125 levels
of >35 U/ml. In the same patient group, a total of 65.5% were
judged as being high-risk using the ROMA test and/or had CA125
levels of >35 U/ml (Table I). The
tumor volume was by far the largest among borderline tumors
(P<0.001). Histology, stage, and degree of differentiation of
ovarian tumors are summarized in Table
II. One or more leiomyomas sized >3 cm were detected in 119
patients (12.1%), and adenomyosis was suspected in 118 patients
(12.0%) using imaging (data not shown).
 | Table I.Characteristics of patients with
ovarian tumors. |
Table I.
Characteristics of patients with
ovarian tumors.
| Parameter | Benign disease
(n=816) | Borderline disease
(n=75) | Ovarian cancer
(n=90) | P-value |
|---|
| Age (years) | 41.00
(31.00–49.00)a | 41.00
(30.50–54.00)b | 49.50
(42.00–55.00) | <0.001 |
| Menopausal
status |
|
|
|
|
| Pre- | 628
(77.0)a | 51 (68.0) | 50 (55.6) | <0.001 |
|
Post- | 188 (23.0) | 24 (32.0) | 40 (44.4) | <0.001 |
| CA125 (U/ml) | 24.00
(14.00–57.10)a | 33.50
(18.50–62.20)c | 61.50
(25.60–238.82) | <0.001 |
| CA125 >35
U/ml | 301
(36.9)a | 35 (46.7) | 61 (67.8) | <0.001 |
| HE4 level
(pmol/l) | 39.80
(33.50–48.60)a | 45.00
(35.15–52.90)a | 64.45
(43.58–93.78) | <0.001 |
| ROMA high risk | 135
(16.5)a,d | 21
(28.0)a | 57 (63.3) | <0.001 |
| Tumor volume
(cm3) | 153.50
(60.00–371.25)a,e | 1,300.00
(227.50–3,154.50) | 548.00
(214.75–1,554.00) | <0.001 |
 | Table II.Histology, stage and degree of
differentiation of ovarian tumors. |
Table II.
Histology, stage and degree of
differentiation of ovarian tumors.
| Parameter | Benign disease
(n=816) | Borderline disease
(n=75) | Ovarian cancer
(n=90) |
|---|
| Pathology |
|
|
|
| Serous
cystadenoma | 67 (8.2) | 0 (0.0) | 0 (0.0) |
| Mucinous
cystadenoma | 128 (15.7) | 0 (0.0) | 0 (0.0) |
|
Endometriotic cyst | 304 (37.3) | 0 (0.0) | 0 (0.0) |
| Mature
cystic teratoma | 177 (21.7) | 0 (0.0) | 0 (0.0) |
| Corpus
luteal cyst | 37 (4.5) | 0 (0.0) | 0 (0.0) |
|
Fibroma | 54 (6.6) | 0 (0.0) | 0 (0.0) |
| Simple
cyst | 45 (5.5) | 0 (0.0) | 0 (0.0) |
| Brenner
tumor | 4 (0.5) | 0 (0.0) | 0 (0.0) |
| Serous
borderline tumor | 0 (0.0) | 17 (22.7) | 0 (0.0) |
| Mucinous
borderline tumor | 0 (0.0) | 48 (64.0) | 0 (0.0) |
|
Seromucinous borderline
tumor | 0 (0.0) | 10 (13.3) | 0 (0.0) |
|
High-grade serous
adenocarcinoma | 0 (0.0) | 0 (0.0) | 23 (25.6) |
|
Mucinous adenocarcinoma | 0 (0.0) | 0 (0.0) | 20 (22.2) |
|
Endometrioid
adenocarcinoma | 0 (0.0) | 0 (0.0) | 18 (20.0) |
| Clear
cell carcinoma | 0 (0.0) | 0 (0.0) | 14 (15.6) |
| Germ
cell tumor | 0 (0.0) | 0 (0.0) | 6 (6.7) |
|
Sex-cord stromal cell
tumor | 0 (0.0) | 0 (0.0) | 9 (10.0) |
| FIGO stage |
|
|
|
| I | 0 (0.0) | 74 (98.7) | 67 (74.4) |
|
II–III | 0 (0.0) | 1 (1.3) | 23 (25.6) |
| Degree of
differentiation |
|
|
|
|
Well-differentiated | 0 (0.0) | 0 (0.0) | 26 (28.9) |
|
Moderately differentiated | 0 (0.0) | 0 (0.0) | 11 (12.2) |
| Poorly
differentiated | 0 (0.0) | 0 (0.0) | 44 (48.9) |
|
Unknown | 0 (0.0) | 0 (0.0) | 9 (10.0) |
Influence of menopausal status,
leiomyoma and adenomyosis
Table III
summarizes comparisons of the diagnostic performance of CA125 and
ROMA in patients with suspected ovarian cancer stratified by
menopausal status, leiomyomas and adenomyosis. The sensitivity of
CA125 was higher compared with that of ROMA in pre-menopausal
(0.584 vs. 0.455) but not in post-menopausal females (0.578 vs.
0500). The specificity (0.818 vs. 0.556), PPV (0.288 vs. 0.175) and
accuracy (0.768 vs. 0.560) of ROMA were higher compared with those
of CA125 in pre-menopausal but not in post-menopausal females. The
specificity and accuracy of ROMA in all patients were higher
compared with those of CA125, regardless of leiomyomas or
adenomyosis; however, this difference was not observed in
post-menopausal females. A possible explanation for these
differences may be due to the fact that mostly endometriotic types
are present in pre-menopausal females.
 | Table III.Predictive value of CA125 and ROMA in
patients with suspected ovarian cancer stratified by menopausal
status, leiomyomas and adenomyosis. |
Table III.
Predictive value of CA125 and ROMA in
patients with suspected ovarian cancer stratified by menopausal
status, leiomyomas and adenomyosis.
| Type | Sensitivity (95%
CI) | Specificity (95%
CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95%
CI) |
|---|
| Pre-menopause |
|
|
|
|
|
|
CA125 | 0.584 | 0.556 | 0.175 | 0.893 | 0.56 |
|
| (0.482–0.681) | (0.516–0.595) | (0.136–0.219) | (0.858–0.921) | (0.523–0.596) |
|
ROMA | 0.455 | 0.818 | 0.288 | 0.903 | 0.768 |
|
| (0.356–0.558) | (0.786–0.848) | (0.219–0.364) | (0.876–0.926) | (0.736–0.798) |
| Post-menopause |
|
|
|
|
|
|
CA125 | 0.578 | 0.883 | 0.627 | 0.86 | 0.806 |
|
| (0.448–0.701) | (0.828–0.925) | (0.491–0.75) | (0.803–0.906) | (0.751–0.853) |
|
ROMA | 0.5 | 0.888 | 0.604 | 0.839 | 0.79 |
|
| (0.372–0.628) | (0.834–0.93) | (0.46–0.735) | (0.781–0.887) | (0.734–0.838) |
| Leiomyoma - |
|
|
|
|
|
|
CA125 | 0.573 | 0.659 | 0.261 | 0.88 | 0.644 |
|
| (0.49–0.654) | (0.623–0.694) | (0.215–0.312) | (0.849–0.906) | (0.611–0.676) |
|
ROMA | 0.447 | 0.857 | 0.396 | 0.88 | 0.785 |
|
| (0.366–0.53) | (0.829–0.882) | (0.322–0.474) | (0.854–0.903) | (0.756–0.812) |
| Leiomyoma + |
|
|
|
|
|
|
CA125 | 0.667 | 0.442 | 0.147 | 0.902 | 0.471 |
|
| (0.384–0.882) | (0.345–0.543) | (0.073–0.254) | (0.786–0.967) | (0.378–0.564) |
|
ROMA | 0.733 | 0.683 | 0.25 | 0.947 | 0.689 |
|
| (0.449–0.922) | (0.584–0.771) | (0.132–0.403) | (0.869–0.985) | (0.598–0.771) |
| Adenomyosis - |
|
|
|
|
|
|
CA125 | 0.565 | 0.688 | 0.282 | 0.879 | 0.666 |
|
| (0.483–0.645) | (0.653–0.722) | (0.233–0.336) | (0.849–0.905) | (0.634–0.698) |
|
ROMA | 0.461 | 0.87 | 0.436 | 0.881 | 0.797 |
|
| (0.381–0.543) | (0.843–0.894) | (0.358–0.515) | (0.855–0.904) | (0.769–0.824) |
| Adenomyosis + |
|
|
|
|
|
|
CA125 | 0.818 | 0.252 | 0.101 | 0.931 | 0.305 |
|
| (0.482–0.977) | (0.173–0.346) | (0.047–0.183) | (0.772–0.992) | (0.224–0.397) |
|
ROMA | 0.636 | 0.598 | 0.14 | 0.941 | 0.602 |
|
| (0.308–0.891) | (0.499–0.692) | (0.058–0.267) | (0.856–0.984) | (0.507–0.691) |
Efficacy according to imaging tumor
subtype
Data on the efficacy of CA125 and ROMA according to
tumor subtype are presented in Table
IV. The subtypes of tumors were classified based on the
post-operative histological findings. A total of 189 cases (19.3%)
were of the serous type, 196 (20.0%) were mucinous, 346 (35.3%)
were endometriotic and 250 (25.5%) were of the solid type. In the
analysis by tumor subtype, the ROMA test had better efficacy in
terms of specificity (0.753 vs. 0.303), PPV (0.257 vs. 0.120), NPV
(0.935 vs. 0.876) and accuracy (0.737 vs. 0.350) compared with
CA125 in the endometriotic type and somewhat superior results in
terms of specificity (0.881 vs. 0.791) and accuracy (0.852 vs.
0.768) in the solid type. In the serous and mucinous type, the
efficacy of the ROMA test was not significantly different from that
of CA125, while the sensitivity of CA125 in the serous type was
higher compared with that of the ROMA test (0.950 vs. 0.750).
 | Table IV.Predictive efficacy of CA125 and ROMA
according to imaging tumor subtype. |
Table IV.
Predictive efficacy of CA125 and ROMA
according to imaging tumor subtype.
| Type | Sensitivity (95%
CI) | Specificity (95%
CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95%
CI) |
|---|
| Serous (n=189) |
|
|
|
|
|
|
CA125 | 0.95 | 0.846 | 0.623 | 0.984 | 0.868 |
|
| (0.831–0.994) | (0.777–0.9) | (0.49–0.744) | (0.945–0.998) | (0.811–0.913) |
|
ROMA | 0.75 | 0.893 | 0.652 | 0.93 | 0.862 |
|
| (0.588–0.873) | (0.831–0.937) | (0.498–0.786) | (0.875–0.966) | (0.805–0.908) |
| Mucinous
(n=196) |
|
|
|
|
|
|
CA125 | 0.338 | 0.867 | 0.575 | 0.712 | 0.684 |
|
| (0.228–0.463) | (0.796–0.921) | (0.409–0.73) | (0.634–0.781) | (0.614–0.748) |
|
ROMA | 0.235 | 0.875 | 0.5 | 0.683 | 0.653 |
|
| (0.141–0.354) | (0.805–0.927) | (0.319–0.681) | (0.606–0.753) | (0.582–0.719) |
| Endometriotic
(n=346) |
|
|
|
|
|
|
CA125 | 0.69 | 0.303 | 0.12 | 0.876 | 0.35 |
|
| (0.529–0.824) | (0.251–0.358) | (0.082–0.168) | (0.798–0.932) | (0.299–0.403) |
|
ROMA | 0.619 | 0.753 | 0.257 | 0.935 | 0.737 |
|
| (0.456–0.764) | (0.701–0.801) | (0.176–0.354) | (0.896–0.962) | (0.687–0.783) |
| Solid (n=250) |
|
|
|
|
|
|
CA125 | 0.4 | 0.791 | 0.109 | 0.954 | 0.768 |
|
| (0.163–0.677) | (0.734–0.842) | (0.041–0.222) | (0.914–0.979) | (0.711–0.819) |
|
ROMA | 0.4 | 0.881 | 0.176 | 0.958 | 0.852 |
|
| (0.163–0.677) | (0.832–0.919) | (0.068–0.345) | (0.922–0.981) | (0.802–0.894) |
Analysis of discrepancies
Table V presents an
analysis of discrepancies between the two prediction methods.
McNemar's test was used to assess how the two measurements
differed, without considering the outcome data. And the NRI
indicated the extent to which the ROMA test improved prediction
compared with CA125, using outcome data. A total of 276 patients
(28.1%) had discrepancies between the results of the two predictive
methods. In a total of 151 cases of the endometriotic type,
accounting for >50% of these patients, CA125 levels were
elevated but they were classified as low-risk by the ROMA test
(McNemar's test P<0.001). These results demonstrated that the
ROMA test provided superior prediction in the endometriotic type
compared with CA125 (NRI=0.379). However, there was no significant
difference between the results of the two predictive methods in the
mucinous type. Furthermore, the NRI values compared with CA125
suggested that ROMA did not provide any improved prediction in the
mucinous type (NRI=0.095) and solid type (NRI=0.089), and had a
worse predictive ability in the serous type (NRI=−0.153).
 | Table V.Analysis of discrepancies between the
CA125 and ROMA prediction methods. |
Table V.
Analysis of discrepancies between the
CA125 and ROMA prediction methods.
| Type | CA125 and ROMA
low | CA125 high and ROMA
low | CA125 low and ROMA
high | CA125 and ROMA
high | McNemar's test
P-value | NRI |
|---|
| Serous (n=189) | 116 (61.4) | 27 (14.3) | 12 (6.3) | 34 (18.0) | 0.025 | −0.153 |
| Mucinous
(n=196) | 143 (73.0) | 21 (10.7) | 13 (6.6) | 19 (9.7) | 0.230 | −0.095 |
| Endometriotic
(n=346) | 94 (27.2) | 151 (43.6) | 11 (3.2) | 90 (26.0) | <0.001 | 0.379 |
| Solid (n=250) | 185 (74.0) | 31 (12.4) | 10 (4.0) | 24 (9.6) | 0.002 | 0.089 |
| Total (n=981) | 538 (54.8) | 230 (23.4) | 46 (4.7) | 167 (17.0) | <0.001 | 0.094 |
ROC curves for ROMA test and
CA125
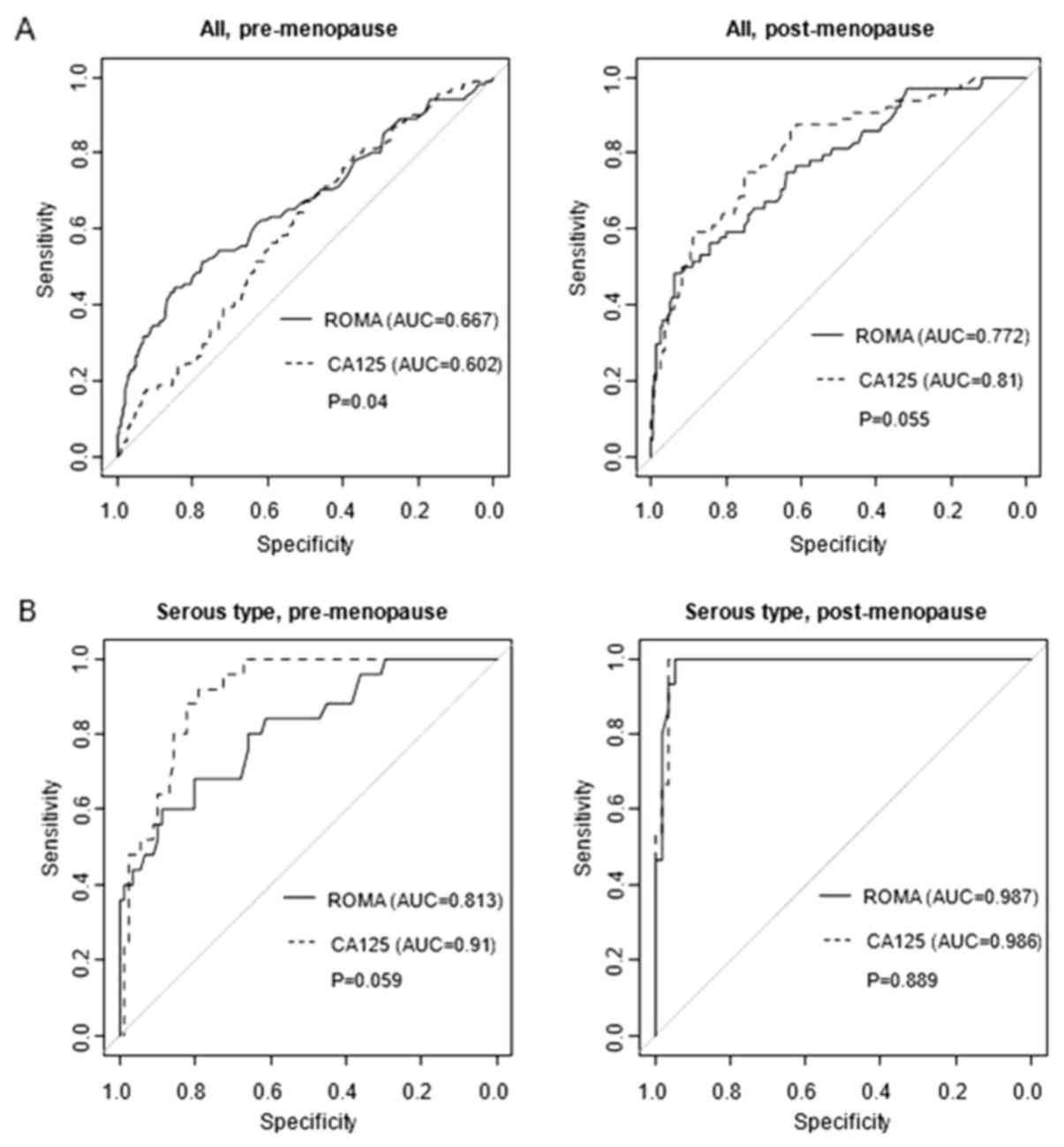
Fig. 1 provides the
ROC curves for each tumor type in pre-menopausal and
post-menopausal females. The ROMA test had better predictive
accuracy compared with CA125 in the overall ROC curve based on the
analysis of pre-menopausal patients. However, in the analysis by
tumor subtype, these comparative advantages were only observed in
pre-menopausal cases of the endometriotic type, but not in the
other tumor subtypes or post-menopausal females.
Discussion
All previous studies evaluating the ROMA test
demonstrated and emphasized its superior specificity compared with
that of the CA125 test. However, these studies did not separately
analyze the histological subtypes of ovarian tumors (21–23).
Although Kim et al (15)
investigated the diagnostic performance of ROMA for ovarian cancer,
two-thirds of the patients did not have ovarian tumors but had
adenomyosis, leiomyomas, an endometrial pathology or ovarian tumors
without biopsy. As mentioned above, the expression levels of HE4 in
tumors vary depending on the histological subtype. Therefore, it is
necessary to assess the expected degree of prediction accuracy of
the ROMA test compared with that of CA125 in discriminating ovarian
tumors for each tumor subtype. According to the results of the
present study, the superiority of the ROMA test in the
identification of malignant ovarian tumors compared with CA125 was
observed only in pre-menopausal cases of the endometriotic type and
comparative advantages were not observed in other tumor
subtypes.
The specificity and accuracy of ROMA were higher
compared with those of the CA125 test regardless of leiomyomas or
adenomyosis in pre-menopausal females but not in post-menopausal
females. This difference may be due to most cases of endometriosis
being pre-menopausal females and ROMA being superior to CA125 in
discriminating endometriotic type ovarian tumors. In the presence
of leiomyomas and adenomyosis, the specificity and accuracy of
CA125 and ROMA was decreased; however, the specificity and accuracy
of CA125 were decreased compared with those of the ROMA test. This
may be due to the inclusion of HE4, which is not affected by
leiomyomas or adenomyosis.
According to a previous meta-analysis of five
studies, ROMA had a sensitivity of 0.873 (95% CI, 0.752–0.940) and
a specificity of 0.855 (95% CI, 0.719–0.932) (24). In the present study, the sensitivity
of ROMA (0.455 in pre-menopausal vs. 0.500 in post-menopausal
females) and CA125 (0.584 in pre-menopausal vs. 0.578 in
post-menopausal females) were lower compared with the previously
reported results. This may be due to the present study including
only histologically-confirmed patients after surgery, while
advanced ovarian cancer or hydrosalpinx that were sufficiently
predictable using imaging were excluded from the analysis. The
inclusion of a number of patients with borderline tumors may also
be the cause of the comparatively lower sensitivities obtained in
the present study.
In clinical practice, gynecologists establish the
prediction marker after determining the approximate tumor subtype
and degree of doubt regarding malignancy using preoperative
imaging. When determining the approximate tumor subtype through
sonography or APCT, knowledge of the accuracy of the prediction
marker for each tumor subtype makes it possible to select the test
according to the expectation of each test. The present study
compared the predictive value of CA125 and the ROMA test for
identifying ovarian tumors according to tumor subtypes by imaging
associated with post-operative histological findings. The imaging
test performed in all patients to confirm the suspicion of
leiomyomas or adenomyosis, which was reaffirmed to determine
whether these factors affected the accuracy of the CA125 and ROMA
tests. Previous studies on ROMA only focused on epithelial ovarian
tumors and did not assess malignant germ cell tumors or epithelial
borderline tumors (15–17). The present study evaluated the
utility of prediction markers in more diverse tumors, including 75
borderline tumors and 15 germ cell or sex-cord stromal cell
tumors.
The present study had certain limitations. First, it
had a retrospective design and thus, it was not possible to
identify any physical conditions that may have affected CA125 or
HE4, including inflammation or smoking at the time of examination.
Furthermore, the present study had the precondition that the type
of ovarian tumor was able to be clearly distinguished through
sonography or APCT. Most ovarian tumors can be accurately
distinguished as specific tumor types via imaging tests prior to
surgery. However, this is difficult in some tumors. It may be
challenging to differentiate hemorrhagic corpus luteal cysts from
endometriotic cysts. Finally, adenomyosis was a suspicious result
during imaging; however, this was not histologically confirmed.
However, if adenomyosis was sufficiently severe to affect CA125
levels, it should be detectable using ultrasound.
In conclusion, the present study compared the
predictive value of CA125 and the ROMA test for differentiating
ovarian tumors according to imaging tumor subtypes associated with
post-operative histopathological findings. To the best of our
knowledge, the present study was the first to analyze the
discrimination performance of ROMA for ovarian tumors in each tumor
subtype for patients undergoing surgery. In the endometriotic type
ovarian tumor, the superiority of the ROMA test compared with CA125
was confirmed in the triage of ovarian tumor. However, these
comparative advantages were not observed in the other tumor
subtypes and post-menopausal females. Therefore, it was
demonstrated that the ROMA test was more beneficial compared with
the conventional CA125 as a triage biomarker, but only for
endometriotic-type ovarian tumors as determined using imaging. Most
benign ovarian tumors occur in women of childbearing age in their
20 to 40s with periodic ovulation; however, the frequency of
malignancies is higher in ovarian tumors that develop after
menopause (25). The present study
reported that the ROMA test is not superior to CA125 in
post-menopausal females. This highlights the requirement to develop
better tumor-specific biomarkers, including circulating tumor DNA.
In the future, it will be important to improve the accuracy and
convenience of relevant tests to facilitate their clinical
application.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJL and YMK conceptualized and designed the study
and drafted the initial manuscript. JSK, SHN and YJL collected the
data, performed initial analyses and reviewed and revised the
manuscript. DYK and YTK performed statistical analysis, interpreted
the data, reviewed and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki. This study was approved by The
Institutional Review Board of the Asan Medical Center of Ulsan
University (approval no. 2019-0616).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ROMA
|
Risk of Ovarian Malignancy
Algorithm
|
|
CA125
|
cancer antigen 125
|
|
EOC
|
epithelial ovarian cancer
|
|
HE4
|
human epididymis protein 4
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bast RC, Feeney M, Lazarus H, Nadler LM,
Colvin RB and Knapp RC: Reactivity of a monoclonal antibody with
human ovarian carcinoma. J Clin Invest. 68:1331–1337. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sevinc A, Adli M, Kalender ME and Camci C:
Benign causes of increased serum CA-125 concentration. Lancet
Oncol. 8:1054–1055. 2019. View Article : Google Scholar
|
|
4
|
Medeiros LR, Rosa DD, da Rosa MI and
Bozzetti MC: Accuracy of CA 125 in the diagnosis of ovarian tumors:
A quantitative systematic review. Eur J Obstet Gynecol Reprod Biol.
142:99–105. 2019. View Article : Google Scholar
|
|
5
|
Engelen MJ, de Bruijn HW, Hollema H, ten
Hoor KA, Willemse PH, Aalders JG and van der Zee AG: Serum CA 125,
carcinoembryonic antigen, and CA 19-9 as tumor markers in
borderline ovarian tumors. Gynecol Oncol. 78:16–20. 2019.
View Article : Google Scholar
|
|
6
|
Simmons AR, Baggerly K and Bast RC: The
emerging role of HE4 in the evaluation of epithelial ovarian and
endometrial carcinomas. Oncology (Williston Park). 27:548–556.
2019.
|
|
7
|
Lu R, Sun X, Xiao R, Zhou L, Gao X and Guo
L: Human epididymis protein 4 (HE4) plays a key role in ovarian
cancer cell adhesion and motility. Biochem Biophys Res Commun.
419:274–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schummer M, Ng WV, Bumgarner RE, Nelson
PS, Schummer B, Bednarski DW, Hassell L, Baldwin RL, Karlan BY and
Hood L: Comparative hybridization of an array of 21,500 ovarian
cDNAs for the discovery of genes overexpressed in ovarian
carcinomas. Gene. 238:375–385. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Granato T, Porpora MG, Longo F, Angeloni
A, Manganaro L and Anastasi E: HE4 in the differential diagnosis of
ovarian masses. Clin Chim Acta. 446:147–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hallamaa M, Suvitie P, Huhtinen K,
Matomäki J, Poutanen M and Perheentupa A: Serum HE4 concentration
is not dependent on menstrual cycle or hormonal treatment among
endometriosis patients and healthy premenopausal women. Gynecol
Oncol. 125:667–672. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moore RG, Miller MC, Eklund EE, Lu H, Bast
RC and Lambert-Messerlian G: Serum levels of the ovarian cancer
biomarker HE4 are decreased in pregnancy and increase with age. Am
J Obstet Gynecol. 206:349.e1–349.e7. 2012. View Article : Google Scholar
|
|
12
|
Moore RG, McMeekin DS, Brown AK,
DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC
Jr and Skates SJ: A novel multiple marker bioassay utilizing HE4
and CA125 for the prediction of ovarian cancer in patients with a
pelvic mass. Gynecol Oncol. 112:40–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore RG, Miller MC, Disilvestro P,
Landrum LM, Gajewski W, Ball JJ and Skates SJ: Evaluation of the
diagnostic accuracy of the risk of ovarian malignancy algorithm in
women with a pelvic mass. Obstet Gynecol. 118:280–288. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drapkin R, von Horsten HH, Lin Y, Mok SC,
Crum CP, Welch WR and Hecht JL: Human epididymis protein 4 (HE4) is
a secreted glycoprotein that is overexpressed by serous and
endometrioid ovarian carcinomas. Cancer Res. 65:2162–2169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim B, Park Y, Kim B, Ahn HJ, Lee KA,
Chung JE and Han SW: Diagnostic performance of CA 125, HE4, and
risk of Ovarian Malignancy Algorithm for ovarian cancer. J Clin Lab
Anal. 33:e226242019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Molina R, Escudero JM, Augé JM, Filella X,
Foj L, Torné A, Lejarcegui J and Pahisa J: HE4 a novel tumour
marker for ovarian cancer: Comparison with CA 125 and ROMA
algorithm in patients with gynaecological diseases. Tumor Biol.
32:1087–1095. 2011. View Article : Google Scholar
|
|
17
|
Romeo V, Framarino Dei Malatesta M, Nudo
F, Simonelli L, Derme M and Berloco PB: Is HE4 serum level a valid
screening test in women candidates for kidney transplant? A case
report and a review of literature. Clin Ter. 165:e162–e165.
2014.PubMed/NCBI
|
|
18
|
Anton C, Carvalho FM, Oliveira EI, Maciel
GAR, Baracat EC and Carvalho JP: A comparison of CA125, HE4, risk
ovarian malignancy algorithm (ROMA), and risk malignancy index
(RMI) for the classification of ovarian masses. Clinics (Sao
Paulo). 67:437–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mutch DG and Prat J: 2014 FIGO staging for
ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol.
133:401–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruggeri G, Bandiera E, Zanotti L, Belloli
S, Ravaggi A, Romani C, Bignotti E, Tassi RA, Tognon G, Galli C, et
al: HE4 and epithelial ovarian cancer: Comparison and clinical
evaluation of two immunoassays and a combination algorithm. Clin
Chim Acta. 412:1447–1453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lenhard M, Stieber P, Hertlein L,
Kirschenhofer A, Fürst S, Mayr D, Nagel D, Hofmann K, Krocker K and
Burges A: The diagnostic accuracy of two human epididymis protein 4
(HE4) testing systems in combination with CA125 in the differential
diagnosis of ovarian masses. Clin Chem Lab Med. 49:2081–2088. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan KK, Chen CA, Nam JH, Ochiai K,
Wilailak S, Choon AT, Sabaratnam S, Hebbar S, Sickan J, Schodin BA
and Sumpaico WW: The use of HE4 in the prediction of ovarian cancer
in Asian women with a pelvic mass. Gynecol Oncol. 128:239–244.
2019. View Article : Google Scholar
|
|
23
|
Karlsen MA, Høgdall EV, Christensen IJ,
Borgfeldt C, Kalapotharakos G, Zdrazilova-Dubska L, Chovanec J, Lok
CA, Stiekema A, Mutz-Dehbalaie I, et al: A novel diagnostic index
combining HE4, CA125 and age may improve triage of women with
suspected ovarian cancer-An international multicenter study in
women with an ovarian mass. Gynecol Oncol. 138:640–646. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dayyani F, Uhlig S, Colson B, Simon K,
Rolny V, Morgenstern D and Schlumbrecht M: Diagnostic performance
of risk of ovarian malignancy algorithm against CA125 and HE4 in
connection with ovarian cancer: A Meta-analysis. Int J Gynecol
Cancer. 26:1586–1593. 2019. View Article : Google Scholar
|
|
25
|
Wolman I: Berek and Novak's Gynecology
15th Edition. J Obstet Gynecol India. 2014.
|