Introduction
Breast cancer is one of the leading causes of
cancer-related death in women (1).
Although most breast cancer patients respond to traditional
treatments, such as tumor removal surgery, chemotherapy and
radiation, the disease becomes difficult to treat and causes death
with recurrence and metastasis to distant organs. Metastasis is the
process by which cancer cells migrate from a site of origin and
develop in neighboring locations, and rather than the primary tumor
itself, metastasis is responsible for the majority of
cancer-related deaths (2–4). Recently, it has been proposed that
cancer stem cells (CSCs) exist in tumors and contribute to
tumorigenesis, aggressiveness, metastasis to distant organs,
resistance to different types of anticancer therapeutic strategies,
including radiation therapy and chemotherapy, and disease
recurrence. CSCs are known to be able to self-renew and regenerate
heterogeneous populations that consist of tumor cells after
treatment (5). Some or all of these
cells, which are present in a tumor in specific microenvironments
or niches, render tumor cells more resistant to radiation therapy
and chemotherapy, ultimately leading to tumor regrowth and distant
metastasis (6–10).
The P2Y2 receptor (P2Y2R)
belongs to the family of G protein-coupled P2Y receptors and is
most consistently expressed (or overexpressed) by tumor cells;
P2Y2R mediates various responses, including
proliferation, in many tumors (11–14) upon
activation by ATP, which is released in the tumor microenvironment
(15). In our previous study, we
reported that MDA-MB-231 breast cancer cells, which show highly
metastatic properties, release higher levels of ATP and show
greater P2Y2R activity than lowly metastatic breast
cancer cells, such as MCF-7 cells, and that P2Y2R
activation by ATP plays an important role in tumor progression and
metastasis by regulating various responses in tumor cells and by
modulating crosstalk between cancer cells and endothelial cells
(ECs) (16). In addition,
ATP-mediated activation of P2Y2R in monocytes induces
their recruitment toward a tumor and promotes inflammatory
conditions around primary tumors by secreting matrix
metalloproteinase. In breast cancer cells, P2Y2R
activation by ATP induces hypoxia-inducible factor (HIF)-1α
expression, lysyl oxidase (LOX) secretion, and collagen
crosslinking, which results in premetastatic niche formation
(17). Moreover, we found that
radiotherapy-resistant (RT-R) breast cancer cells, particularly
RT-R-MDA-MB-231 cells derived from the highly metastatic breast
cancer cell line MDA-MB-231, release high levels of ATP, promoting
invasion and tumor growth through activation of P2Y2R
(18). Furthermore, more CSCs
developed among RT-R-MDA-MB-231 cells, contributing to the
acquisition of tolerance to other anticancer therapies in addition
to radiation therapy (19). Thus, we
hypothesized that P2Y2R may have a relationship with
CSCs in breast cancer, and we aimed to evaluate CSC marker
expression in human breast cancer and to determine their
relationship with P2Y2R in human breast cancer. In this
study, we investigated the expression level of 5 CSC markers (CD24,
CD44, Oct3/4, Notch-4 and ALDH1A1) and P2Y2R in human
breast cancer patients and examined the correlation of CSC markers
with P2Y2R.
Materials and methods
Case selection
This retrospective study was approved by the
Institutional Review Board of Gyeongsang National University
Hospital with a waiver of informed consent (GNUH-2018-05-010).
Specimens from 180 breast cancer patients who underwent surgery
with wide excision or mastectomy between January 2010 and December
2012 at Gyeongsang National University Hospital, Jinju, Korea, were
selected. For each sample, formalin-fixed, paraffin-embedded and
hematoxylin and eosin-stained sections were prepared on glass
slides and assessed by two pathologists. Data from electronic
medical records, including sex, age, menstrual status, tumor size,
lymph node status, distant metastasis, and tumor stage, were
reviewed. Cancer stages were determined according to the eighth
edition of the American Joint Committee on Cancer (AJCC).
Histological type and grade were determined per the fifth edition
of the World Health Organization (WHO) classification. As shown in
Table I, all patients except one
were female (179), with a mean age of 51.5 years old (range,
25~81). Patients <50 years old accounted for 50.6; 56.1% of the
patients were premenopausal; 91.7% of patients had a tumor diameter
classified as T1/T2, whereas 8.3% had a tumor diameter classified
as T3/T4; 85.0% had an N0/N1 lymph node grade, whereas 15.0% had an
N2/N3 lymph node grade; 80.6% were at stage I/II, whereas 19.4%
were at stage III/IV. Cases were divided into three groups
according to ER, PR and HER-2 expression: i) triple-negative breast
cancer (TNBC), ER, PR and HER-2 negative (n=20; 11.1%); ii)
HER-2-breast cancer, ER and PR negative (n=7; 3.9%; iii) luminal
breast cancer, ER and/or PR positive, HER-2 negative or positive
(n=153; 85.0%). The criteria for ER- and PR-positive staining was a
score >3 by the Allred scoring system. HER-2 was considered
positive if >10% of the cancer cells presented with strong and
complete brown cell membrane staining (Table II).
 | Table I.Association between cancer stem cell
markers and clinicopathological characteristics in patients with
breast cancer. |
Table I.
Association between cancer stem cell
markers and clinicopathological characteristics in patients with
breast cancer.
|
|
| CD24 (%) | CD44 (%) | Oct3/4 (%) | Notch-4 (%) | ALDH1A1 (%) |
|---|
|
|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Total patients: 180
(%) | Negative | Positive | P-value | Negative | Positive | P-value | Low | High | P-value | Low | High | P-value | Negative | Positive | P-value |
|---|
| Age, years |
|
|
| 0.6504 |
|
| 0.2627 |
|
| 0.3424 |
|
| 0.4559 |
|
| 0.6187 |
|
<50 | 91 (50.6) | 55 (60.4) | 36 (39.6) |
| 25 (27.5) | 66 (72.5) |
| 20 (22.0) | 71 (78.0) |
| 42 (46.1) | 49 (53.9) |
| 90 (99.0) | 1 (1.0) |
|
|
≥50 | 89 (49.4) | 50 (56.2) | 39 (43.8) |
| 32 (36.0) | 57 (64.0) | | 14 (15.7) | 75 (84.3) | | 36 (40.4) | 53 (59.6) | | 87 (97.7) | 2 (2.3) | |
| Menstrual
status |
|
|
| 0.1268 |
|
| 0.075 |
|
| 0.4451 |
|
| 0.8802 |
|
| 0.2582 |
|
Premenopausal | 101 (56.1) | 54 (53.5) | 47 (46.5) |
| 38 (37.6) | 63 (62.4) |
| 17 (16.8) | 84 (83.2) |
| 44 (43.6) | 57 (56.4) |
| 98 (97.0) | 3 (3.0) |
|
|
Postmenopausal | 78 (43.3) | 51 (65.4) | 27 (34.6) | | 19 (24.4) | 59 (75.6) | | 17 (21.8) | 61 (78.2) | | 33 (42.3) | 45 (57.7) | | 78 (100.0) | 0 (0.0) | |
| None
(male) | 1 (0.6) | 1 (100.0) | 0 (0.0) | | 0 (0.0) | 1 (100.0) | | 0 (0.0) | 1 (100.0) | | 1 (100.0) | 0 (0.0) | | 1 (100.0) | 0 (0.0) | |
| ER status |
|
|
| 0.0812 |
|
| 0.5931 |
|
| 0.8332 |
|
| <0.0001 |
|
| 0.1871 |
|
Negative | 50 (27.8) | 24 (48.0) | 26 (52.0) |
| 14 (28.0) | 36 (72.0) |
| 10 (20.0) | 40 (80.0) |
| 37 (74.0) | 13 (26.0) |
| 48 (96.0) | 2 (4.0) |
|
|
Positive | 130 (72.2) | 81 (62.3) | 49 (37.7) | | 43 (33.1) | 87 (66.9) | | 24 (18.5) | 106 (81.5) | | 41 (31.5) | 89 (68.5) | | 129 (99.2) | 1 (0.8) | |
| PR status |
|
|
| 0.5021 |
|
| 0.8587 |
|
| 0.141 |
|
| 0.0185 |
|
|
|
|
Negative | 50 (27.8) | 27 (54.0) | 23 (46.0) |
| 15 (30.0) | 35 (70.0) |
| 13 (26.0) | 37 (74.0) |
| 29 (58.0) | 21 (42.0) |
| 49 (98.0) | 1 (2.0) | |
|
Positive | 130 (72.2) | 78 (60.0) | 52 (40.0) | | 42 (32.3) | 88 (67.7) | | 21 (16.2) | 109 (83.8) | | 49 (37.7) | 81 (62.3) | | 128 (98.5) | 2 (1.5) | |
| HER-2 status |
|
Negative | 151 (83.9) | 91 (60.3) | 60 (39.7) | 0.3039 | 45 (29.8) | 106 (70.2) | 0.2757 | 29 (19.2) | 122 (80.8) | 1 | 61 (40.4) | 90 (59.6) | 0.1006 | 148 (98.0) | 3 (2.0) | |
|
Positive | 29 (16.1) | 14 (48.3) | 15 (51.7) | | 12 (41.4) | 17 (58.6) | | 5 (17.2) | 24 (82.8) | | 17 (58.6) | 12 (41.4) | | 29 (100.0) | 0 (0.0) | |
| Tumor size |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T1/T2 | 165 (91.7) | 98 (59.4) | 67 (40.6) | 0.4152 | 55 (33.3) | 110 (66.7) | 0.15 | 30 (18.2) | 135 (81.8) | 0.054 | 69 (41.8) | 96 (58.2) | 0.1861 | 162 (98.2) | 3 (1.8) | |
|
T3/T4 | 15 (8.3) | 7 (46.7) | 8 (53.3) | | 2 (13.3) | 13 (86.7) | | 4 (26.7) | 11 (73.3) | | 9 (60.0) | 6 (40.0) | | 15 (100.0) | 0 (0.0) | |
| Lymph node
involvement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N0/N1 | 153 (85.0) | 88 (57.5) | 65 (42.5) | 0.6751 | 45 (29.4) | 108 (70.6) | 0.1768 | 29 (18.9) | 124 (81.1) | 1 | 63 (41.2) | 90 (58.8) | 0.207 | 150 (98.0) | 3 (2.0) | |
|
N2/N3 | 27 (15.0) | 17 (63.0) | 10 (37.0) | | 12 (44.4) | 15 (55.6) | | 5 (18.5) | 22 ((81.5) | | 15 (55.6) | 12 (44.4) | | 27 (100.0) | 0 (0.0) | |
| Distant
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 175 (97.2) | 102 (58.3) | 73 (41.7) | 0.65 | 55 (31.4) | 120 (68.6) | 0.6524 | 33 (18.9) | 142 (81.1) | 1 | 75 (42.9) | 100 (57.1) | 0.6537 | 172 (98.3) | 3 (1.7) |
|
|
Yes | 5 (2.8) | 4 (80.0) | 1 (20.0) | | 2 (40.0) | 3 (60.0) | | 1 (20.0) | 4 (80.0) | | 3 (60.0) | 2 (40.0) | | 5 (100.0) | 0 (0.0) |
|
| Tumor stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I/II | 145 (80.6) | 85 (58.6) | 60 (41.4) | 1 | 44 (30.3) | 101 (69.7) | 0.4273 | 27 (18.6) | 118 (81.4) | 0.8136 | 58 (40.0) | 87 (60.0) | 0.0868 | 142 (97.9) | 3 (2.1) | |
|
III/IV | 35 (19.4) | 21 (60.0) | 14 (40.0) | | 13 (37.1) | 22 (62.9) | | 7 (20.0) | 28 (80.0) | | 20 (57.1) | 15 (42.9) | | 35 (100.0) | 0 (0.0) |
|
 | Table II.Expression of cancer stem cell
markers in different subtypes of breast cancer. |
Table II.
Expression of cancer stem cell
markers in different subtypes of breast cancer.
|
| CD24 (%) | CD44 (%) | Oct3/4 (%) | Notch-4 (%) | ALDH1A1 (%) |
|---|
|
|
|
|
|
|
|
|---|
| Breast cancer
type | Negative | Positive | P-value | Negative | Positive | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| TNBC | 11 (55.0) | 9 (45.0) | 0.3456 | 3 (15.0) | 17 (85.0) | 0.2964 | 3 (15.0) | 17 (85.0) | 0.9795 | 15 (75.0) | 5 (25.0) | 0.0013 | 19 (95.0) | 1 (5.0) | 0.4504 |
| HER2+
(ER−/PR−) | 6 (85.7) | 1 (14.3) |
| 3 (42.9) | 4 (57.1) |
| 1 (14.3) | 6 (85.7) |
| 5 (71.4) | 2 (28.6) |
| 7 (100.0) | 0 (0.0) |
|
| Luminal | 92 (60.1) | 61 (39.9) |
| 43 (28.1) | 110 (71.9) |
| 25 (16.3) | 128 (83.7) |
| 56 (36.6) | 97 (63.4) |
| 151 (98.7) | 2 (1.3) |
|
Tissue microarray and
immunohistochemistry
Representative hematoxylin and eosin-stained
sections on glass slides containing prominent intratumoral regions
from 180 breast cancer patient specimens were chosen. Two 2-mm
tissue cores were obtained from each representative paraffin block
and transferred to recipient tissue microarray (TMA) blocks, and
immunohistochemial staining was performed on the TMA blocks using
anti-P2Y2R polyclonal (1:200 dilution, #PA1-46150;
Thermo Fisher Scientific, Inc.), anti-CD24 monoclonal (1:100
dilution, #ab31622; Abcam), anti-CD44 monoclonal (1:100 dilution,
#ab51037; Abcam), anti-OCT3/4 polyclonal (1:100 dilution, #ab18976;
Abcam), anti-Notch-4 polyclonal (1:50 dilution, #ab199295; Abcam),
and anti-ALDH1A1 monoclonal (1:100 dilution, #ab52492; Abcam)
primary antibodies.
Evaluation of
immunohistochemistry
CD24 and CD44 were interpreted as positive when
staining in the cell membranes was observed in at least 10% of the
cells. ALDH1A1 was interpreted as positive when staining in the
cytoplasm was observed in at least 10% of the cells. Cytoplasmic
expression of Oct3/4 was detected and classified into two groups:
Low expression (0~50% cells) or high expression (51~100% cells).
Membrane and nuclear expression of Notch-4 was recorded. A
semiquantitative scoring system was used, evaluating both the
staining intensity (0, no stain; 1+, weak stain; 2+, moderate
stain; 3+, strong stain) and percentage of stained cells (0,
<5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; and 4, >75%). Scores for
staining intensity and the percentage of positive cells were then
multiplied to generate the immunoreactivity score (IS) for each
case. All cases were sorted into two groups according to IS. High
expression of Notch-4 was defined as detectable immunoreactivity in
the cytoplasm and nucleus with IS ≥4. Only nuclear expression of
P2Y2R was recorded, given a score from 0 to 12 according
to the intensity (0, 1+, 2+, 3+) and percentage of positive tumor
cells (0=all negative, 1+=0~10%, 2+=11~50%; 3+=51~80% and 4+=more
than 81% of cells).
Statistical analysis
All statistics were analyzed using GraphPad Prism7
software (GraphPad Software, Inc.). One-way ANOVA followed by
Newman-Keuls post hoc test was carried out to compare different
groups. Data are presented as the mean ± SEM. Coefficients of
correlation (r) were determined by the Pearson correlation method.
P<0.05 was considered to indicate a statistically significant
difference. Correlation analyses were performed using the
chi-square test and Fisher's exact test. SPSS version 25.0 (IBM
Corp.) was used for the analysis.
Results
CD44, Oct3/4 and Notch-4 CSC markers
are significantly expressed in the tumor tissue of breast cancer
patients
First, we examined the expression of the CSC markers
CD24, CD44, Oct3/4, Notch-4 and ALDH1A1 in tumor tissues (n=180)
and normal epithelial tissues (n=20) obtained from breast cancer
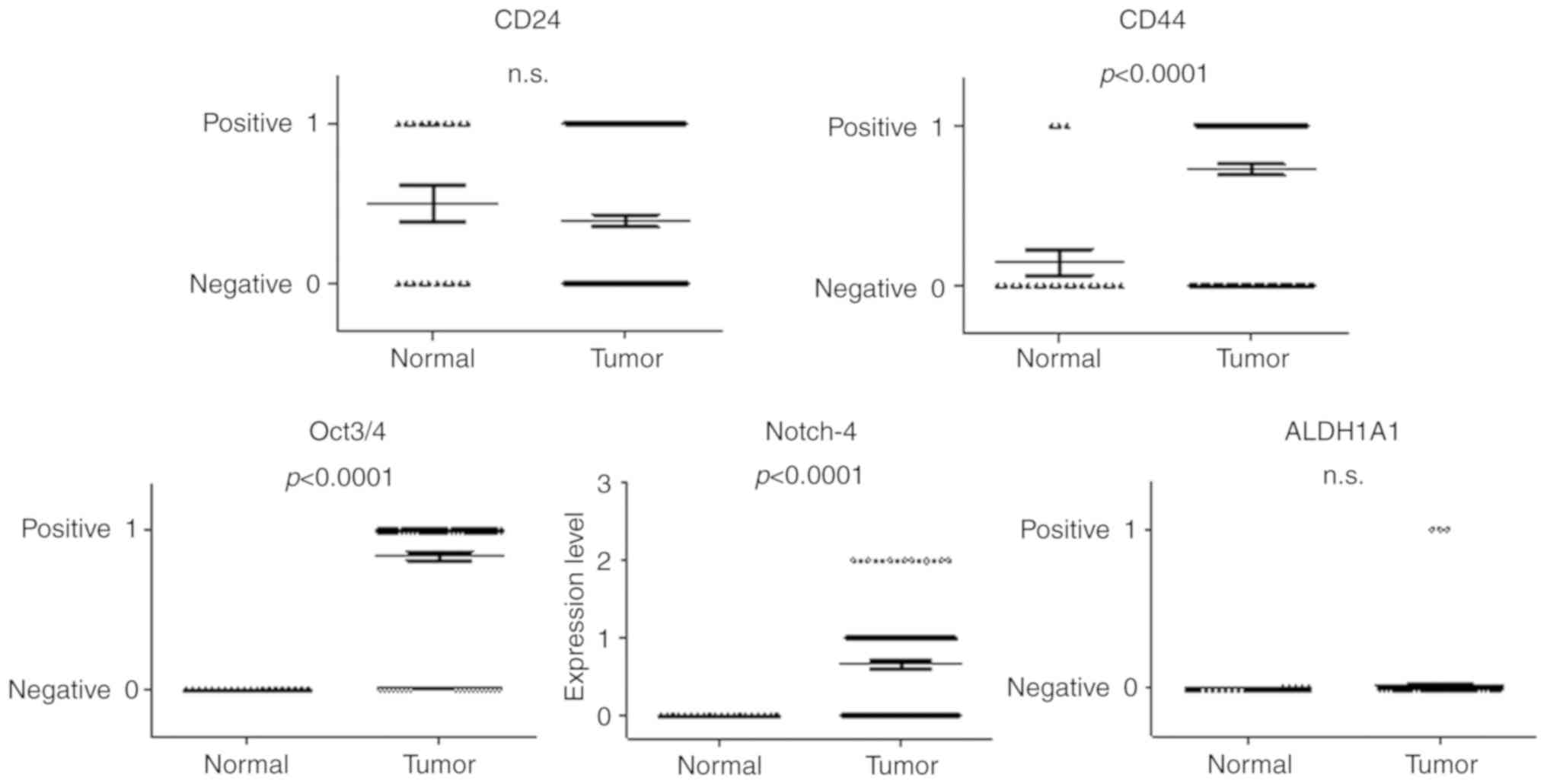
patients. As shown in Fig. 1,
expression of CD44, Oct3/4 and Notch-4, but not CD24 and ALDH1A1,
was significantly induced in the tumor tissues compared to the
normal epithelial tissues of breast cancer patients.
P2Y2R expression is also
significantly increased in tumor tissue and has a significant
correlation only with Notch-4 of the cancer stem cell markers
evaluated
In our previous study, we suggested that
P2Y2R has an important role in tumor progression and
metastasis in highly metastatic breast cancer cells, such as
MDA-MB-231 and RT-R breast cancer cells (16–19). In
addition, Ko et al (19).
showed that induced expression of CSC markers in RT-R-MDA-MB-231
cells. Therefore, in the present study, we examined whether
expression of P2Y2R and CSC markers is induced in breast
cancer patients and, if so, whether there is a relationship between
P2Y2R and CSC markers in these patients. As depicted in
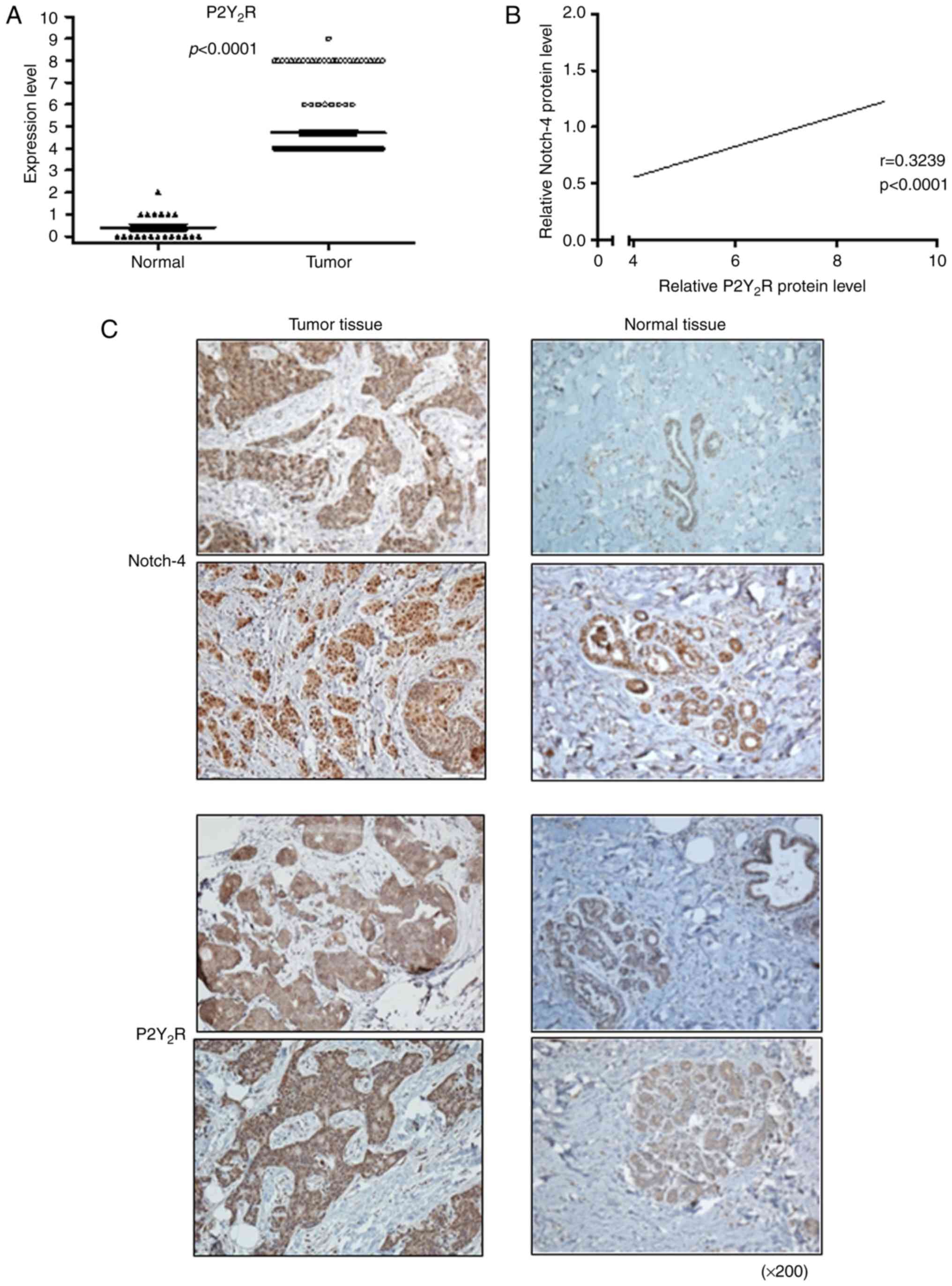
Fig. 2A, we confirmed that
P2Y2R expression was increased in the tumor tissues of
breast cancer patients compared to normal epithelial tissues. We
also found that P2Y2R expression had a significant
correlation only with Notch-4 in breast cancer patients (Fig. 2B). Immunohistochemical staining
results showed that Notch-4 and P2Y2R were highly
expressed in tumor tissues compared to normal tissues (Fig. 2C).
Discussion
Breast CSCs are characterized by high expression of
CD44 and low expression of CD24
(CD44+/CD24−/low), and Notch-4, Oct3/4 and
ALDH1 have also been suggested as CSC markers (20–24). The
present study shows that of CSC markers, expression of CD44, Oct3/4
and Notch-4, but not CD24 and ALDH1A1, was significantly induced in
tumor tissues compared to normal epithelial tissues obtained from
breast cancer patients. Recent studies have suggested that ALDH1, a
detoxifying enzyme responsible for the oxidation of retinol to
retinoic acid, may be a potent marker of breast CSCs (24–27).
However, there is controversy regarding the use of ALDH1 as a
breast CSC marker. Resetkova et al (28). reported that ALDH1-positive cells did
not significantly increase following neoadjuvant chemotherapy in
surgical specimens, whereas other researchers (25–27),
including Tanei et al (24),
reported that ALDH1 was a more significant predictive marker than
CD44+/CD24− for the identification of breast
CSCs with respect to resistance to chemotherapy. In our previous
in vitro study (19), ALDH1
levels were significantly increased in RT-R-MDA-MB-231 cells,
indicating an increased number of CSCs, compared to MDA-MB-231
cells; furthermore, ALDH1 was not expressed in MCF-7 and T47D
cells, even in RT-R-MCF-7 and RT-R-T47D cells, suggesting that
ALDH1 may be a potent marker for breast CSCs and that
ALDH1-positive breast CSCs may play an important role in
radioresistance. ALDH1 has three main isotypes, ALDH1A1, ALDH1A2,
and ALDH1A3 (28). Recent reports
suggest that ALDH1, and its isotype ALDH1A1 in particular, are
useful CSC markers that may be used to enrich tumor-initiating
subpopulations from various cell lines and primary tumors and that
they are associated with cancer progression (29–31).
Therefore, among the isotypes of ALDH1, we investigated expression
of ALDH1A1 in tumor tissues of human breast cancer patients.
However, among our 180 breast cancer patients, expression of
ALDH1A1 was not induced in tumor tissues compared to normal
epithelial tissues. Thus, more work is needed to clarify this
issue.
As mentioned above, P2Y2R has, according
to an in vitro study, an important role in tumor progression
and metastasis. We also wondered whether P2Y2R is highly
expressed in the tumor tissues of breast cancer patients. As
expected, P2Y2R expression was significantly induced in
tumor tissues compared to normal epithelial tissues. More
interestingly, we found that P2Y2R expression has a
significant correlation with Notch-4 in breast cancer patients.
Many studies have confirmed increased expression of the Notch
receptor and its ligands in breast cancer tissue compared with
normal breast tissue (32–34). The Notch receptor family comprises
four transmembrane proteins. Rizzo et al (34) reported that Notch-1 and Notch-4
expression was low in normal breast tissue but that invasive ductal
carcinoma and invasive lobular carcinoma exhibited 81 and 93%
Notch-4 positivity, respectively. In particular, the role of
Notch-4 in epithelial tumors was established by insertional
mutagenesis in mice infected with mouse mammary tumor virus
(35,36). Notch-4 is expressed in stem cells of
the mammary gland terminal duct and is involved in the formation of
branching structures that precede poorly differentiated
adenocarcinoma and the incorporation of TAC-2 cells into duct
branches. Notch-4 has also been implicated in growth factor β
function, aggressive tumor phenotypes, and the transformation from
normal mouse mammary epithelial cells to heterotypic cells
(35–38). These results establish that the
Notch-4 signaling pathway has an important role in regulating the
growth and development of the mammary gland. Abnormal expression of
Notch-4 may inhibit mammary stem cell differentiation, and Notch-4
gene mutations may enhance mammary epithelial cell proliferation,
thus leading to the occurrence of breast cancer. According to Wang
et al (39), Notch-4
expression was significantly higher in patients with TNBC and
HER-2-overexpressing breast cancer than in those with luminal
breast cancer. They also suggested that Notch-4 expression is
associated with aggressive clinicopathological and biological
phenotypes and may predict poor prognosis in luminal breast cancer
patients. Nonetheless, in the present study, Notch-4 was not
significantly associated with tumor size, lymph node involvement,
or clinical TNM stage (Table I).
Moreover, as shown in Table II,
Notch-4 levels were increased in non-TNBC and luminal breast cancer
types compared with TNBC (Table
II). In survival analysis, Notch-4 expression did not show any
significance in the breast cancer patients enrolled in this study
(data not shown). Interestingly, Notch-4 expression was
significantly associated with P2Y2R expression in breast
cancer cells, even though expression of P2Y2R was also
not associated with tumor size, lymph node involvement, clinical
TNM stage, TNBC (Table SI) or the
overall survival rate (P=0.245; data not shown). As this study was
performed with specimens from breast cancer patients who underwent
surgery with wide excision or mastectomy, the patients enrolled
were in the early phase rather than in the late phase. This fact is
a possible reason why no significant relationship between several
CSC markers and survival rate or recurrence was found. Although
Notch-4 does not appear to be associated with the presence of
hormone receptors, tumor size, and clinical TNM stage in human
breast cancer patients, it is very meaningful that Notch-4 showed a
notable correlation with P2Y2R, which has important
roles in tumor progression and metastasis, as noted in a previous
study. As we mentioned in the Introduction, in in vitro
study and in vivo mice model, P2Y2R was closely
related with the tumor progression, metastasis and acquisition of
tolerance to other anticancer therapies (16~19). Accordingly, we
surmise that we might obtain more impressive results regarding the
relationship between Notch-4 and P2Y2R in the prognosis
of breast cancer patients, including the survival rate, by studying
the tumor tissues of breast cancer patients in a later phase.
The above limitation might also be applied to the
fact that expression of other CSCs, namely, CD44 and Oct3/4, in
tumor tissues displayed no significant correlation with
P2Y2R expression in breast cancer patients. As we stated
above, RT-R-MDA-MB-231 cells derived from the highly metastatic
breast cancer cell line MDA-MB-231 exhibited increased expression
of CSC markers, including CD44 and Oct3/4, with promotion of
invasion and tumor growth through activation of P2Y2R.
Thus, it is also expected that expression of CD44 and Oct3/4 is
related to P2Y2R expression as well as Notch-4.
Regardless, we found no significant relationship between CD44 or
Oct3/4 and P2Y2R. CD44 is a transmembrane receptor that
is associated with cancer-initiating cell development and tumor
metastasis (40–42). CD44 binds to hyaluronic acid (HA),
resulting in effective activation of STAT-3 pathways, which play
important roles in the regulation of the growth and maintenance of
CSCs (43). In addition, STAT-3
activation has been associated with the resistance of tumor cells
to chemotherapeutic agents and γ radiation (44,45).
Oct3/4, also known as Oct3, Oct4 or Pit-Oct0Unc class 5 homeobox 1
(POU5F1), is a transcription factor (46), and overexpression of Oct3/4 has been
shown to enhance the stemness of CSCs and to induce the
pathogenesis of several human cancers (47–49).
Based on these reports, it is possible to expect that data based on
tumor tissues of breast cancer patients in a later phase may reveal
a significant correlation between CD44 or Oct3/4 and
P2Y2R.
Taken together, there is no report to date on the
correlation between Notch-4 and P2Y2R in tumor
progression or tumorigenesis. Thus, it might be valuable to
investigate how P2Y2R correlates with Notch-4, and
further study may uncover a novel strategy for developing targeted
therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education, Science and Technology (grant
no. 2018R1A2B6001786).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DCK performed the experiments, analyzed the data and
drafted the initial manuscript. HJ performed the experiments,
analyzed and interpreted the data. JSL collected patients' samples,
performed experiments and analyzed the data. ES performed
experiments and analyzed the data. GWL and HJK designed the study,
interpreted the data and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the Good
Clinical Practice guidelines and the Declaration of Helsinki. This
retrospective study was approved by the Institutional Review Board
of Gyeongsang National University Hospital with a waiver of
informed consent (GNUH-2018-05-010).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALDH
|
aldehyde dehydrogenase
|
|
AM
|
adhesion molecule
|
|
CSC
|
cancer stem cell
|
|
EC
|
endothelial cell
|
|
EMT
|
epithelial mesenchymal transition
|
|
HIF
|
hypoxia-inducible factor
|
|
LOX
|
lysyl oxidase
|
|
MMP
|
matrix metalloproteinase
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steeg PS: Cancer: Micromanagement of
metastasis. Nature. 449:671–673. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eccles SA and Welch DR: Metastasis: Recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bertolini G, Roz L, Perego P, Tortoreto M,
Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S,
et al: Highly tumorigenic lung cancer CD133+ cells display
stem-like features and are spared by cisplatin treatment. Proc Natl
Acad Sci USA. 106:16281–16286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Creighton CJ, Li X, Landis M, Dixon JM,
Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A,
Herschkowitz JI, et al: Residual breast cancers after conventional
therapy display mesenchymal as well as tumor-initiating features.
Proc Natl Acad Sci USA. 106:13820–13825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Lewis MT, Huang J, Gutierrez C,
Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC,
et al: Intrinsic resistance of tumorigenic breast cancer cells to
chemotherapy. J Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst. 98:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abraham BK, Fritz P, McClellan M,
Hauptvogel P, Athelogou M and Brauch H: Prevalence of
CD44+/CD24-/low cells in breast cancer may not be associated with
clinical outcome but may favor distant metastasis. Clin Cancer Res.
11:1154–1159. 2005.PubMed/NCBI
|
|
11
|
White N, Butler PE and Burnstock G: Human
melanomas express functional P2X(7) receptors. Cell Tissue Res.
321:411–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schafer R, Sedehizade F, Welte T and
Reiser G: ATP-and UTP-activated P2Y receptors differently regulate
proliferation of human lung epithelial tumor cells. Am J Physiol
Lung Cell Mol Physiol. 285:L376–L385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shabbir M, Ryten M, Thompson C,
Mikhailidis D and Burnstock G: Purinergic receptor-mediated effects
of ATP in high-grade bladder cancer. BJU Int. 101:106–112.
2008.PubMed/NCBI
|
|
14
|
Janssens R and Boeynaems JM: Effects of
extracellular nucleotides and nucleosides on prostate carcinoma
cells. Br J Pharmacol. 132:536–546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pellegatti P, Raffaghello L, Bianchi G,
Piccardi F, Pistoia V and Di Virgilio F: Increased level of
extracellular ATP at tumor sites: In vivo imaging with plasma
membrane luciferase. PLoS One. 3:e25992008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin H, Eun SY, Lee JS, Park SW, Lee JH,
Chang KC and Kim HJ: P2Y2 receptor activation by nucleotides
released from highly metastatic breast cancer cells increases tumor
growth and invasion via crosstalk with endothelial cells. Breast
Cancer Res. 16:R772014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joo YN, Jin H, Eun SY, Park SW, Chang KC
and Kim HJ: P2Y2R activation by nucleotides released from the
highly metastatic breast cancer cell MDA-MB-231 contributes to
pre-metastatic niche formation by mediating lysyl oxidase
secretion, collagen crosslinking, and monocyte recruitment.
Oncotarget. 5:9322–9334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin H, Ko YS and Kim HJ: P2Y2R-mediated
inflammasome activation is involved in tumor progression in breast
cancer cells and in radiotherapy-resistant breast cancer. Int J
Oncol. 53:1953–1966. 2018.PubMed/NCBI
|
|
19
|
Ko YS, Jin H, Lee JS, Park SW, Chang KC,
Kang KM, Jeong BK and Kim HJ: Radioresistant breast cancer cells
exhibit increased resistance to chemotherapy and enhanced invasive
properties due to cancer stem cells. Oncol Rep. 40:3752–3762.
2018.PubMed/NCBI
|
|
20
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in propagation of tumorigenic breast cancer cells
with stem/progenitor cell properties. Cancer Res. 65:5506–5511.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koo BS, Lee SH, Kim JM, Huang S, Kim SH,
Rho YS, Bae WJ, Kang HJ, Kim YS, Moon JH and Lim YC: Oct4 is a
critical regulator of stemness in head and neck squamous carcinoma
cells. Oncogene. 34:2317–2324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai YH, VanDussen KL, Sawey ET, Wade AW,
Kasper C, Rakshit S, Bhatt RG, Stoeck A, Maillard I, Crawford HC,
et al: ADAM10 regulates Notch function in intestinal stem cells of
mice. Gastroenterology. 147:822–834.e13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanei T, Morimoto K, Shimazu K, Kim SJ,
Tanji Y, Taguchi T, Tamaki Y and Noguchi S: Association of breast
cancer stem cells identified by aldehyde dehydrogenase 1 expression
with resistance to sequential Paclitaxel and epirubicin-based
chemotherapy for breast cancers. Clin Cancer Res. 15:4234–4241.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morimoto K, Kim SJ, Tanei T, Shimazu K,
Tanji Y, Taguchi T, Tamaki Y, Terada N and Noguchi S: Stem cell
marker aldehyde dehydrogenase 1-positive breast cancers are
characterized by negative estrogen receptor, positive human
epidermal growth factor receptor type 2, and high Ki67 expression.
Cancer Sci. 100:1062–1068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Charafe-Jauffret E, Ginestier C, Iovino F,
Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci
F, Jacquemier J, et al: Aldehyde dehydrogenase 1-positive cancer
stem cells mediate metastasis and poor clinical outcome in
inflammatory breast cancer. Clin Cancer Res. 16:45–55. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Resetkova E, Reis-Filho JS, Jain RK, Mehta
R, Thorat MA, Nakshatri H and Badve S: Prognostic impact of ALDH1
in breast cancer: A story of stem cells and tumor microenvironment.
Breast Cancer Res Treat. 123:97–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang EH, Hynes MJ, Zhang T, Ginestier C,
Dontu G, Appelman H, Fields JZ, Wicha MS and Boman BM: Aldehyde
dehydrogenase 1 is a marker for normal and malignant human colonic
stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng S, Yang X, Lassus H, Liang S, Kaur S,
Ye Q, Li C, Wang LP, Roby KF, Orsulic S, et al: Distinct expression
levels and patterns of stem cell marker, aldehyde dehydrogenase
isoform 1 (ALDH1), in human epithelial cancers. PLoS One.
5:e102772010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ciccone V, Terzuoli E, Donnini S,
Giachetti A, Morbidelli L and Ziche M: Stemness marker ALDH1A1
promotes tumor angiogenesis via retinoic acid/HIF-1α/VEGF
signalling in MCF-7 breast cancer cells. J Exp Clin Cancer Res.
37:3112018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parr C, Watkins G and Jiang WG: The
possible correlation of Notch-1 and Notch-2 with clinical outcome
and tumour clinicopathological parameters in human breast cancer.
Int J Mol Med. 14:779–786. 2004.PubMed/NCBI
|
|
33
|
Zardawi SJ, Zardawi I, McNeil CM, Millar
EK, McLeod D, Morey AL, Crea P, Murphy NC, Pinese M, Lopez-Knowles
E, et al: High Notch1 protein expression is an early event in
breast cancer development and is associated with the HER-2
molecular subtype. Histopathology. 56:286–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rizzo P, Miao H, D'Souza G, Osipo C, Song
LL, Yun J, Zhao H, Mascarenhas J, Wyatt D, Antico G, et al:
Cross-talk between notch and the estrogen receptor in breast cancer
suggests novel therapeutic approaches. Cancer Res. 68:5226–5235.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gallahan D and Callahan R: Mammary
tumorigenesis in feral mice: Identification of a new int locus in
mouse mammary tumor virus (Czech II)-induced mammary tumors. J
Virol. 61:66–74. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith GH, Gallahan D, Diella F, Jhappan C,
Merlino G and Callahan R: Constitutive expression of a truncated
INT3 gene in mouse mammary epithelium impairs differentiation and
functional development. Cell Growth Differ. 6:563–577.
1995.PubMed/NCBI
|
|
37
|
Uyttendaele H, Soriano JV, Montesano R and
Kitajewski J: Notch4 and Wnt-1 proteins function to regulate
branching morphogenesis of mammary epithelial cells in an opposing
fashion. Dev Biol. 196:204–217. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weijzen S, Rizzo P, Braid M, Vaishnav R,
Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC,
et al: Activation of Notch-1 signaling maintains the neoplastic
phenotype in human Ras-transformed cells. Nat Med. 8:979–986. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang JW, Wei XL, Dou XW, Huang WH, Du CW
and Zhang GJ: The association between Notch4 expression, and
clinicopathological characteristics and clinical outcomes in
patients with breast cancer. Oncol Lett. 15:8749–8755.
2018.PubMed/NCBI
|
|
40
|
Marhaba R and Zöller M: CD44 in cancer
progression: Adhesion, migration and growth regulation. J Mol
Histol. 35:211–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Orian-Rousseau V: CD44, a therapeutic
target for metastasising tumours. Eur J Cancer. 46:1271–1277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marotta LL, Almendro V, Marusyk A,
Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ,
Choudhury SA, Maruyama R, et al: The JAK2/STAT3 signaling pathway
is required for growth of CD44+CD24− stem
cell-like breast cancer cells in human tumors. J Clin Invest.
121:2723–2735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tan Q, Wang H, Hu Y, Hu M, Li X,
Aodengqimuge, Ma Y, Wei C and Song L: Src/STAT3-dependent heme
oxygenase-1 induction mediates chemoresistance of breast cancer
cells to doxorubicin by promoting autophagy. Cancer Sci.
106:1023–1032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bonner JA, Trummell HQ, Willey CD, Plants
BA and Raisch KP: Inhibition of STAT-3 results in
radiosensitization of human squamous cell carcinoma. Radiother
Oncol. 92:339–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ling GQ, Chen DB, Wang BQ and Zhang LS:
Expression of the pluripotency markers Oct3/4, Nanog and Sox2 in
human breast cancer cell lines. Oncol Lett. 4:1264–1268. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y,
Cao X, Ling EA and Hao A: Oct4 is expressed in human gliomas and
promotes colony formation in glioma cells. Glia. 57:724–733. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matsuoka J, Yashiro M, Sakurai K, Kubo N,
Tanaka H, Muguruma K, Sawada T, Ohira M and Hirakawa K: Role of the
stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J
Surg Res. 174:130–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tai MH, Chang CC, Kiupel M, Webster JD,
Olson LK and Trosko JE: Oct4 expression in adult human stem cells:
Evidence in support of the stem cell theory of carcinogenesis.
Carcinogenesis. 26:495–502. 2005. View Article : Google Scholar : PubMed/NCBI
|