Introduction
Hepatocellular carcinoma (HCC), the most common type
of liver cancer, is the fifth most frequently diagnosed cancer and
the third leading cause of cancer-associated mortality worldwide
(1). Aside from liver
transplantation, hepatic resection and chemotherapy are the main
effective treatment strategies used for HCC. However, despite
recent advances in surgery and chemotherapy, the prognosis of HCC
remains poor, with a postoperative 5-year recurrence rate of
approximately 70% (2). Therefore,
understanding the mechanisms underlying liver cancer and
identifying novel therapeutic targets for the disease is
important.
Inflammasomes are a group of intracellular
multiprotein complexes that mediate host immune responses to
pathogen infection and cellular damage (3). Chronic inflammation in the tumor
microenvironment serves a crucial role during tumor development;
however, the roles of the inflammasome during tumor progression are
complex (4). The inflammasome acts
as a double-edged sword in various types of cancer, serving as a
tumor promoter or suppressor (5).
The NLR pyrin domain containing 3 (NLRP3) inflammasome, the most
highly characterized inflammasome, consists of the scaffold protein
NLRP3, the adaptor apoptosis-associated speck-like protein
containing a CARD and caspase-1 (6).
Upon activation, NLRP3 recruits and cleaves pro-caspase-1 into its
active form, which subsequently cleaves pro-interleukin (IL)-1β
into mature biologically active IL-1β, ultimately resulting in
inflammation (7). Activated
caspase-1 also induces pyroptosis, a proinflammatory programmed
cell death (6). It has been reported
that the components of the NLRP3 inflammasome are significantly
downregulated in HCC cells (8).
Consistently, another study demonstrated that pyroptosis is
inhibited in HCC tissues and cells (9), suggesting that NLRP3-dependent
pyroptosis may serve crucial roles in liver cancer. Therefore,
investigating the mechanism underlying NLRP3-dependent pyroptosis
in liver cancer is of interest.
Long non-coding RNAs (lncRNAs) are a class of
non-coding RNAs that are >200 base pairs in length. lncRNAs are
novel regulators of various cellular processes during tumorigenesis
and tumor progression, including cell proliferation, apoptosis,
pyroptosis and cell cycle progression (10,11). A
genome-wide analysis of lncRNA profiles identified small nucleolar
RNA host gene 7 (SNHG7) as a metastasis-associated lncRNA during
HCC. It has also been reported that SNHG7 is upregulated during
HCC, indicating its critical role during the development of the
disease (11). Furthermore, The
Cancer Genome Atlas database indicated a strong correlation between
a high expression of SNHG7 and poor prognosis of patients with HCC.
Patients with HCC with a higher SNHG7 expression had a poorer
overall survival compared with patients with lower SNHG7
expression, indicating that SNHG7 may serve as a potential
indicator of HCC clinical outcomes (12). Consistently, HCC tissues with an
advanced TNM stage, high tumor grade or vascular invasion displayed
higher expression levels of SNHG7 compared with corresponding
controls (13). A recent study
demonstrated that SNHG7 acts as an oncogenic lncRNA by competing
with microRNA (miR)-34a and polypeptide
N-acetylgalactosaminyltransferase 7 (GALNT7) to regulate the
PI3K/Akt/mTOR signaling pathway during colorectal cancer (14). However, the biological functions and
regulatory mechanisms underlying SNHG7 during liver cancer
progression are not completely understood.
To the best of our knowledge, the aim of the present
study was to demonstrate for the first time that SNHG7 regulated
sirtuin 1 (SIRT1) expression by acting as a competing endogenous
RNA (ceRNA) for miR-34a, resulting in inhibition of NLRP3-dependent
pyroptosis during liver cancer.
Materials and methods
Collection of normal and HCC human
tissue samples
A total of 25 paired HCC tumor and adjacent normal
tissues (≥3 cm from the tumor margin) were collected
post-operatively from patients with HCC (mean age, 63 years; age
range, 43–72 years; 13 males and 12 females) at the People's
Hospital of Deyang City between January 2016 and December 2018. HCC
diagnosis was confirmed by two independent pathologists. The
present study was approved by the Ethics Committee of the People's
Hospital of Deyang City. Written informed consent was obtained from
all patients.
Cell culture and transfection
Normal liver epithelial THLE-3 cells, human
embryonic kidney 293 cells, and human hepatocellular carcinoma
HepG2 and SK-hep-1 cells were purchased from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences. The cell
lines were authenticated by short tandem repeat DNA profiling
analysis prior to purchase by The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences. Cells were cultured
in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin (all Gibco; Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2.
The small interfering (si)RNA targeted against
SNHG7, miR-34a mimic, miR-34a inhibitor and corresponding controls
were obtained from Guangzhou RiboBio Co., Ltd. SNHG7 was cloned
into the pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific,
Inc.), as previously described (13). Cells were seeded at a density of
2×105 cells/well in 12-well plates and were transfected
using Lipofectamine® 3000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. For siRNA and miRNA transfection, 50 nM
siRNA and/or 50 nM miRNA was transfected into cells. For
overexpression experiments, 0.5 µg plasmid DNA was transfected into
cells. pcDNA3.1 alone served as a negative control. The sequences
of the siRNAs and miRNAs used in the present study are as follows:
si-SNHG7-1, 5′-GUGCAGCAAUUACUCUUAAUU-3′; si-SNHG7-2,
5′-UUAGCAGAGUAAUUUGCACUU-3′; miR-34a mimic NC forward,
5′-UUCUCCGAACGUGUCAGGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′; miR-34a mimic forward,
5′-UGGCAGUGUCUUAGCUGGUUGU-3′ and reverse,
5′-AACCAGCUAAGACACUGCAAUU-3′; miR-34a inhibitor NC,
5′-CAGUACUUUUGUGUAGUACAA-3′; and miR-34a inhibitor,
5′-ACAACCAGCUAAGACACUGCCA-3′. Cells were harvested and subjected to
subsequent analysis 48 h post-transfection.
RNA isolation and reverse
transcription-quantitative PCR (qRT-PCR)
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the
Prime-Script RT Reagent kit (Takara Biotechnology Co., Ltd.).
Subsequently, qPCR was performed using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.) and the ABI PRISM 7500 real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. qPCR reaction was
performed using the following conditions: Pre-denaturation at 95°C
for 1 min, 40 cycles of denaturation at 95°C for 30 sec, annealing
at 67°C for 30 sec and extension at 72°C for 30 sec, followed by a
final extension step at 72°C for 5 min. The following primers were
used for qPCR: SNHG7 forward, 5′-TTGCTGGCGTCTCGGTTAAT-3′ and
reverse, 5′-GGAAGTCCATCACAGGCGAA-3′; miR-34a forward,
5′-CACGGACTCGGGGCATTTGGAGATTTT-3′ and reverse,
5′-CTGTCTAGATCGCTTATCTTCCCCTTGG-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
NLRP3 forward, 5′-CACCTGTTGTGCAATCTGAAG-3′ and reverse,
5′-GCAAGATCCTGACAACATGC-3′; caspase-1 forward,
5′-CCTTAATATGCAAGACTCTCAAGGA-3′ and reverse,
5′-TAAGCTGGGTTGTCCTGCACT-3′; IL-1β forward,
5′-TACCTGTCCTGCGTGTTGAA-3′ and reverse,
5′-TCTTTGGGTAATTTTTGGGATCT-3′; SIRT1 forward,
5′-TGCTGGCCTAATAGAGTGGCA-3′ and reverse,
5′-CTCAGCGCCATGGAAAATGT-3′; and GAPDH forward,
5′-AACGTGTCAGTGGTGGACCTG-3′ and reverse,
5′-AGTGGGTGTCGCTGTTGAAGT-3′. The specificity of the fluorescent
signal was verified by melting curve and 1% agarose gel
electrophoresis. miRNA and mRNA expression levels were quantified
using the 2−ΔΔCq method (15) and normalized to the internal
reference genes U6 and GAPDH, respectively.
RNA fluorescence in situ hybridization
(FISH)
RNA FISH was performed using the Fluorescent in
Situ Hybridization kit (Guangzhou RiboBio Co., Ltd.), according
to the manufacturer's protocol. HCC and paired adjacent normal
tissues were fixed with 4% paraformaldehyde at room temperature for
8 h, embedded in paraffin and cut into 7-µm-thick sections. The
Cy3-conjugated SNHG7 probe was synthesized by Guangzhou RiboBio
Co., Ltd. as previously described (16). For Cy3 detection, fluorescence images
(magnification, ×400) were acquired using a fluorescence confocal
laser-scanning microscope with excitation at 543 nm (Olympus
Corporation). Slides were mounted with Prolong Gold Antifade
reagent with DAPI (Invitrogen; Thermo Fisher Scientific, Inc.).
Western blotting
Total protein from HepG2 and SK-hep-1 cells was
extracted using RIPA lysis buffer (Pierce; Thermo Fisher
Scientific) and quantified using the BCA protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Equal amounts of protein (40
µg/lane) were separated via 4–12% SDS-PAGE and transferred onto
PVDF membranes (EMD Millipore), which were blocked with 5% non-fat
milk at room temperature for 1 h. Subsequently, the membranes were
incubated at 4°C overnight with primary antibodies targeted
against: SIRT1 (1:500; cat. no. sc-74504; Santa Cruz Biotechnology,
Inc.), NLRP3 (1:1,000; cat. no. ab214185; Abcam), caspase-1
(1:1,000; cat. no. 4199; Cell Signaling Technology, Inc.), IL-1β
(1:1,000; cat. no. sc-52012; Santa Cruz Biotechnology, Inc.) and
GAPDH (1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.).
Following primary incubation, the membranes were incubated with
HRP-conjugated goat anti-rabbit (cat. no. 31460) or goat anti-mouse
(cat. no. 31430) (both 1:5,000; Invitrogen; Thermo Fisher
Scientific, Inc.) secondary antibodies at room temperature for 1 h.
Protein bands were visualized using SuperSignal West Pico PLUS
Chemiluminescent substrate (Pierce) and exposed to X-ray film.
Protein expression was quantified using ImageJ v1.52a software
(National Institutes of Health) with GAPDH as the loading
control.
Dual-luciferase reporter assay
The potential binding sites between SNHG7 and
miR-34a, and the putative association between miR-34a and SIRT1
were predicted using bioinformatics analysis, namely TargetScan
(www.targetscan.org) and miRanda
(www.microrna.org/microrna/searchMirnas.do). The
putative binding site between miR-34a and the 3′-untranslated
region (UTR) of SNHG7 or SIRT1 was cloned into the pmirGLO vector
(Promega Corporation). The mutant (MUT) 3′UTR of SNHG7 or SIRT1 was
synthesized using the QuickChange Lighting Multi Site-Directed
Mutagenesis kit (Agilent Technologies, Inc.) according to the
manufacturer's protocol. 293 cells (2×105 cells/well)
were co-transfected with 0.5 µg SNHG7-wild-type (WT),
SIRT1-3′UTR-WT, SNHG7-MUT or SIRT1-3′UTR-MUT vectors and 50 nM
miR-34a mimic NC or miR-34a mimic using Lipofectamine®
3000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Following
incubation at 37°C for 48 h, luciferase activities were measured
using the Dual Luciferase Reporter assay system (Promega
Corporation), according to the manufacturer's protocol. Firefly
luciferase activities were normalized to Renilla luciferase
activities.
Analysis of DNA fragmentation
A TUNEL assay was performed to detect DNA
fragmentation of individual cells. Briefly, HepG2 cells
(2×105 cells/well) were seeded onto coverslips in
12-well dishes. Following transfection, cells were fixed with 4%
paraformaldehyde at room temperature for 10 min and permeabilized
using 0.1% Triton X-100 at room temperature for 10 min.
Subsequently, cells were incubated with TUNEL reaction mixture
(Roche Diagnostics), according to the manufacturer's protocol. For
one test, 45 µl TUNEL Label and 5 µl TUNEL Enzyme were prepared
prior to use. Slides were incubated with TUNEL reaction mixture at
37°C for 1 h. After rinsing with PBS, slides were mounted with
Prolong Gold Antifade reagent with DAPI (Invitrogen; Thermo Fisher
Scientific, Inc.). Stained cells were observed using a fluorescence
confocal laser-scanning microscope (Olympus Corporation). The cells
were observed at 5–10 fields of view (magnification, ×400), and the
TUNEL-positive cells were counted per 100 cells in each field of
view.
Cytotoxicity assay
Cytotoxicity assay was performed using HepG2 cells.
Cell culture medium was collected and centrifuged at 12,000 × g at
4°C for 15 min to remove the cell debris. Pyroptotic cell death was
determined by quantifying lactate dehydrogenase (LDH) content of
the cell culture medium using the LDH Cytotoxicity Detection kit
(Clontech Laboratories, Inc.), according to the manufacturer's
protocol.
Statistical analysis
All experiments were performed at least three times.
Data are expressed as the mean ± standard deviation. Differences
among groups were analyzed using one-way ANOVA followed by
Dunnett's post hoc test. Differences between two groups were
analyzed using the paired Student's t-test. Statistical analyses
were performed using GraphPad Prism software (version 6.0; GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
SNHG7 is upregulated in HCC tissues
and cell lines, and mediates NLRP3-dependent pyroptosis during
HCC
To investigate the biological function of SNHG7
during HCC, the expression of SNHG7 in HCC and adjacent normal
tissues was assessed. SNHG7 expression levels were significantly
higher in HCC tissues compared with adjacent normal tissues
(Fig. 1A). RNA FISH confirmed that
SNHG7 expression was increased in HCC tissues compared with
adjacent normal tissues (Fig. 1B).
Similarly, SNHG7 expression levels were significantly upregulated
in liver cancer cell lines HepG2 and SK-hep-1 compared with the
normal liver epithelial THLE-3 cell line (Fig. 1C), indicating that SNHG7 may serve
important roles during liver cancer tumorigenesis. Furthermore, a
previous study demonstrated that pyroptosis is inhibited in HCC
tissues and cells (9); therefore, to
investigate whether SNHG7 was involved in liver cancer cell
pyroptosis, loss-of-function experiments were performed. si-SNHG7-1
and si-SNHG7-2 significantly decreased SNHG7 expression levels in
HepG2 and SK-hep-1 cells compared with si-NC. However, si-SNHG7-1
silenced SNHG7 expression more effectively compared with
si-SNHG-7-2, so si-SNHG7-1 was used for subsequent experiments
(Fig. 1D). SNHG7 knockdown
significantly increased the mRNA expression levels of
pyroptosis-related NLRP3, caspase-1 and IL-1β in HepG2 and SK-hep-1
cells compared with si-NC (Fig.
1E-G). Consistently, the western blotting results indicated
that the protein expression levels of NLRP3, caspase-1 and IL-1β
were significantly increased in SNHG7-knockdown cells compared with
si-NC-transfected cells (Fig. 1H).
The results suggested that SNHG7 may serve a crucial role during
NLRP3-dependent pyroptosis in liver cancer cells.
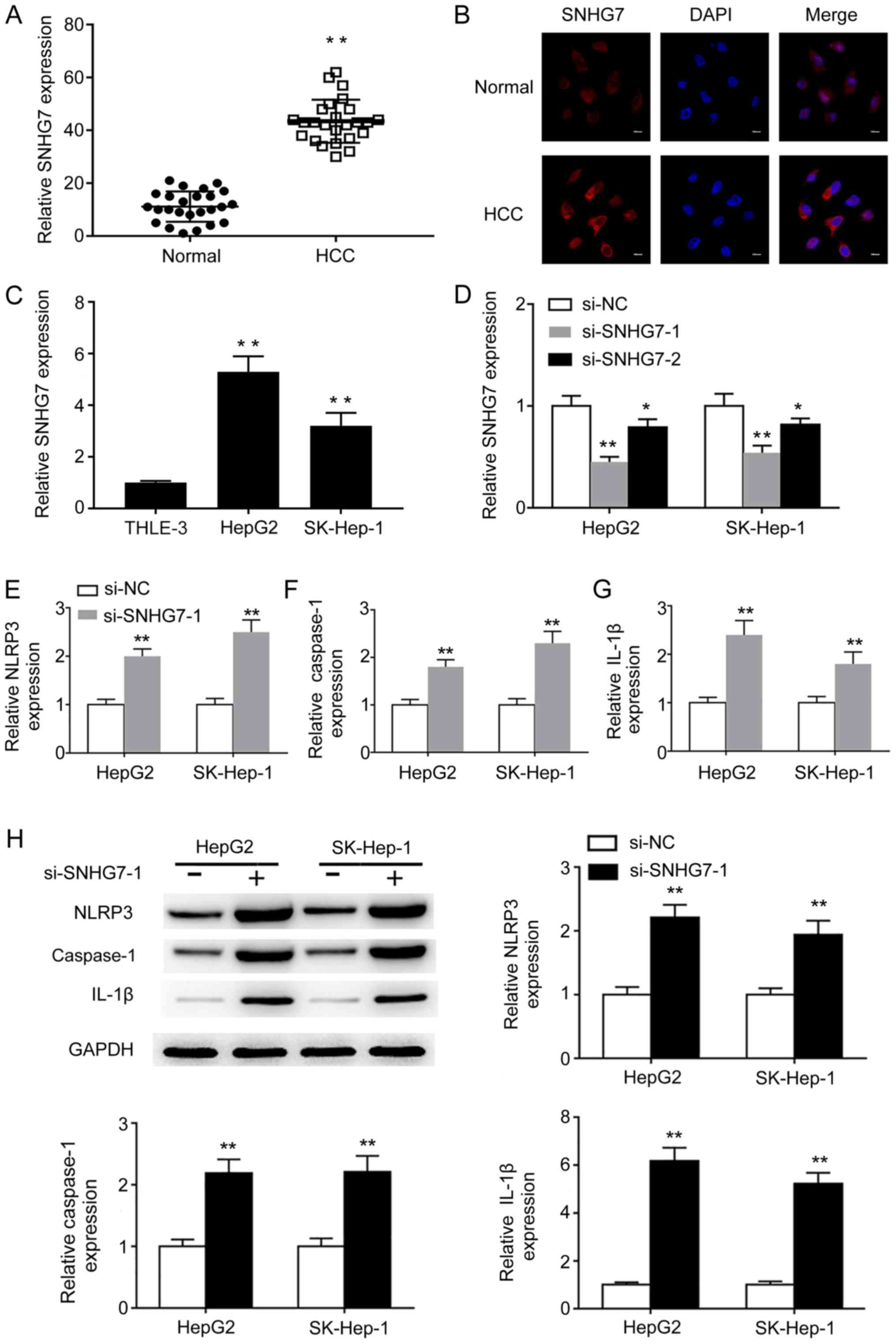 | Figure 1.SNHG7 is upregulated in HCC and
mediates NLRP3-dependent pyroptosis. (A) SNHG7 mRNA expression
levels in paired HCC and adjacent normal tissues were determined by
RT-qPCR. (B) The expression of SNHG7 in paired HCC and adjacent
normal tissues was determined by RNA FISH (scale bar, 10 µm). (C)
SNHG7 mRNA expression levels in THLE-3, HepG2 and SK-Hep-1 cells
were determined by RT-qPCR. mRNA expression levels of (D) SNHG7,
(E) NLRP3, (F) caspase-1 and (G) IL-1β were determined by RT-qPCR.
(H) Protein expression levels of NLRP3, caspase-1 and IL-1β were
determined by western blotting and semi-quantified. *P<0.05 and
**P<0.01 vs. the corresponding control. SNHG7, small nucleolar
RNA host gene 7; HCC, hepatocellular carcinoma; NLRP3, NLR pyrin
domain containing 3; RT-qPCR, reverse transcription-quantitative
PCR; FISH, fluorescence in situ hybridization; IL-1β,
interleukin-1β; si, small interfering RNA; NC, negative
control. |
SNHG7 sponges miR-34a to repress its
expression in liver cancer cells
To further understand the mechanism underlying SNHG7
during NLRP3-dependent pyroptosis, bioinformatics analysis was
performed to predict the putative interactions between SNHG7 and
miRNAs. Among the predicted miRNAs, the expression levels of
miR-34a were significantly lower in HCC tissues compared with
adjacent normal tissues (Fig. 2A).
Moreover, miR-34a expression levels were significantly decreased in
HepG2 and SK-hep-1 cells compared with THLE-3 cells (Fig. 2B), suggesting that miR-34a was
negatively regulated by SNHG7 during liver cancer; therefore, SNHG7
may function as a molecular sponge of miR-34a. A dual-luciferase
reporter assay was conducted to verify the direct binding between
SNHG7 and miR-34a. The potential binding sites were predicted using
TargetScan and miRanda (Fig. 2C).
miR-34a mimic significantly reduced the luciferase activities of
the SNHG7-WT group compared with the miR-34a mimic NC. By contrast,
miR-34a mimic displayed no effect on the luciferase activities of
the SNHG7-MUT group compared with miR-34a mimic NC (Fig. 2D). Gain- and loss-of-function
experiments were conducted. SNHG7 overexpression significantly
increased the expression levels of SNHG7 in HepG2 and SK-hep-1
cells compared with the vector control (Fig. 2E). SNHG7 overexpression and knockdown
experiments suggested that miR-34a was negatively regulated by
SNHG7 in HepG2 and SK-hep-1 cells (Fig.
2F and G). Collectively, the results indicated that SNHG7 acted
as a sponge of miR-34a to repress its expression in HepG2 and
SK-hep-1 cells.
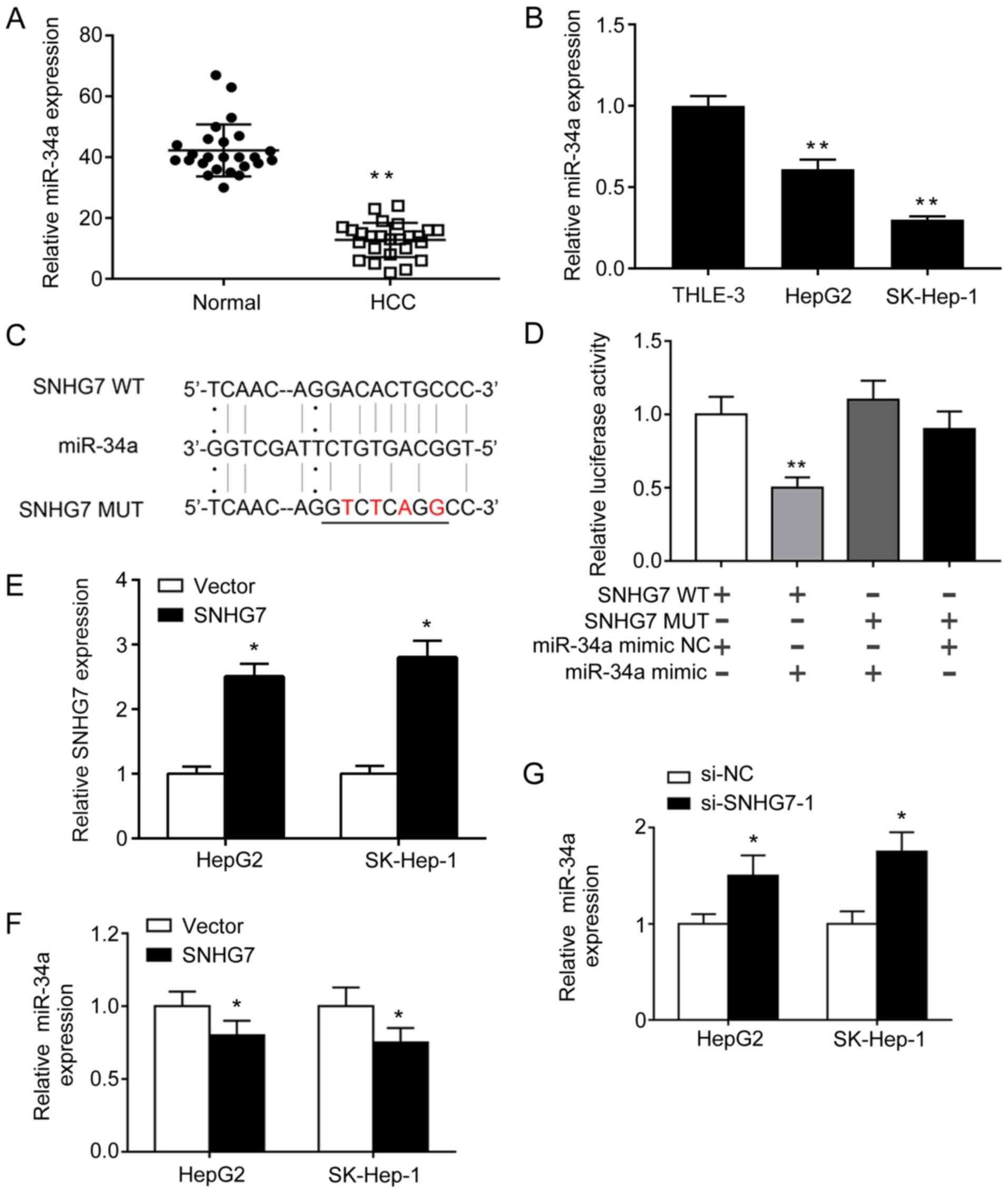 | Figure 2.SNHG7 sponges miR-34a to repress its
expression in HCC cells. (A) mRNA expression levels of miR-34a in
(A) paired HCC and adjacent normal tissues, as well as in (B)
THLE-3, HepG2 and SK-Hep-1 cells were determined by RT-qPCR. (C)
The potential binding sites between SNHG7 and miR-34a. A mutation
was generated in the miR-34a binding site of the SNHG7 sequence.
The mutations were indicated in red. (D) Luciferase activities were
determined by a dual-luciferase reporter assay. (E) SNHG7 mRNA
expression levels following SNHG7 overexpression were determined by
RT-qPCR. miR-34a expression levels following SNHG7 (F)
overexpression and (G) knockdown were determined by RT-qPCR.
*P<0.05 and **P<0.01 vs. the corresponding control. SNHG7,
small nucleolar RNA host gene 7; miR, microRNA; HCC, hepatocellular
carcinoma; RT-qPCR, reverse transcription-quantitative PCR; WT,
wild-type; MUT, mutant; si, small interfering; NC, negative
control. |
SIRT1 is a direct target of
miR-34a
Increasing evidence has suggested that lncRNAs
function as miRNA sponges and inhibit miRNA function, thus
regulating miRNA target gene expression (17,18).
Therefore, the present study aimed to identify the direct target of
miR-34a by bioinformatics analysis. Among the putative theoretical
targets, the present study focused on SIRT1 due to its reported
role in NLRP3-dependent pyroptosis (19). The mRNA expression levels of SIRT1
were examined in HCC tissues and cells. SIRT1 was significantly
upregulated in HCC tissues compared with adjacent normal tissues
(Fig. 3A). Similarly, the mRNA
expression levels of SIRT1 were significantly higher in HepG2 and
SK-hep-1 cells compared with THLE-3 cells (Fig. 3B). The complementary binding site
between miR-34a and the 3′UTR of SIRT1 was predicted (Fig. 3C). miR-34a mimic significantly
reduced the luciferase activities of the SIRT1-3′UTR-WT group
compared with miR-34a NC, indicating that SIRT1 was a direct target
of miR-34a (Fig. 3D). To further
investigate the effect of miR-34a on SIRT1 expression, HepG2 and
SK-hep-1 cells were transfected with miR-34a inhibitor, miR-34a
mimic and corresponding miRNA controls. miR-34a inhibitor and
miR-34a mimic significantly decreased and increased miR-34a
expression levels in HepG2 cells, respectively, compared with the
corresponding controls (Fig. 3E).
The protein expression levels of SIRT1 were significantly increased
by miR-34a inhibitor, but significantly decreased by miR-34a mimic
compared with the corresponding controls in liver cancer cells
(Fig. 3F). In HepG2 cells, the
expression levels of pyroptosis-related NLRP3, caspase-1 and IL-1β
were significantly decreased by miR-34a inhibitor and significantly
increased by miR-34a mimic compared with the corresponding controls
(Fig. 3F). Similar results were
observed in SK-hep-1 cells (data not shown). Collectively, the
results indicated that SIRT1 was a direct target of miR-34a;
therefore, miR-34a/SIRT1 may be associated with NLRP3-dependent
pyroptosis in liver cancer cells.
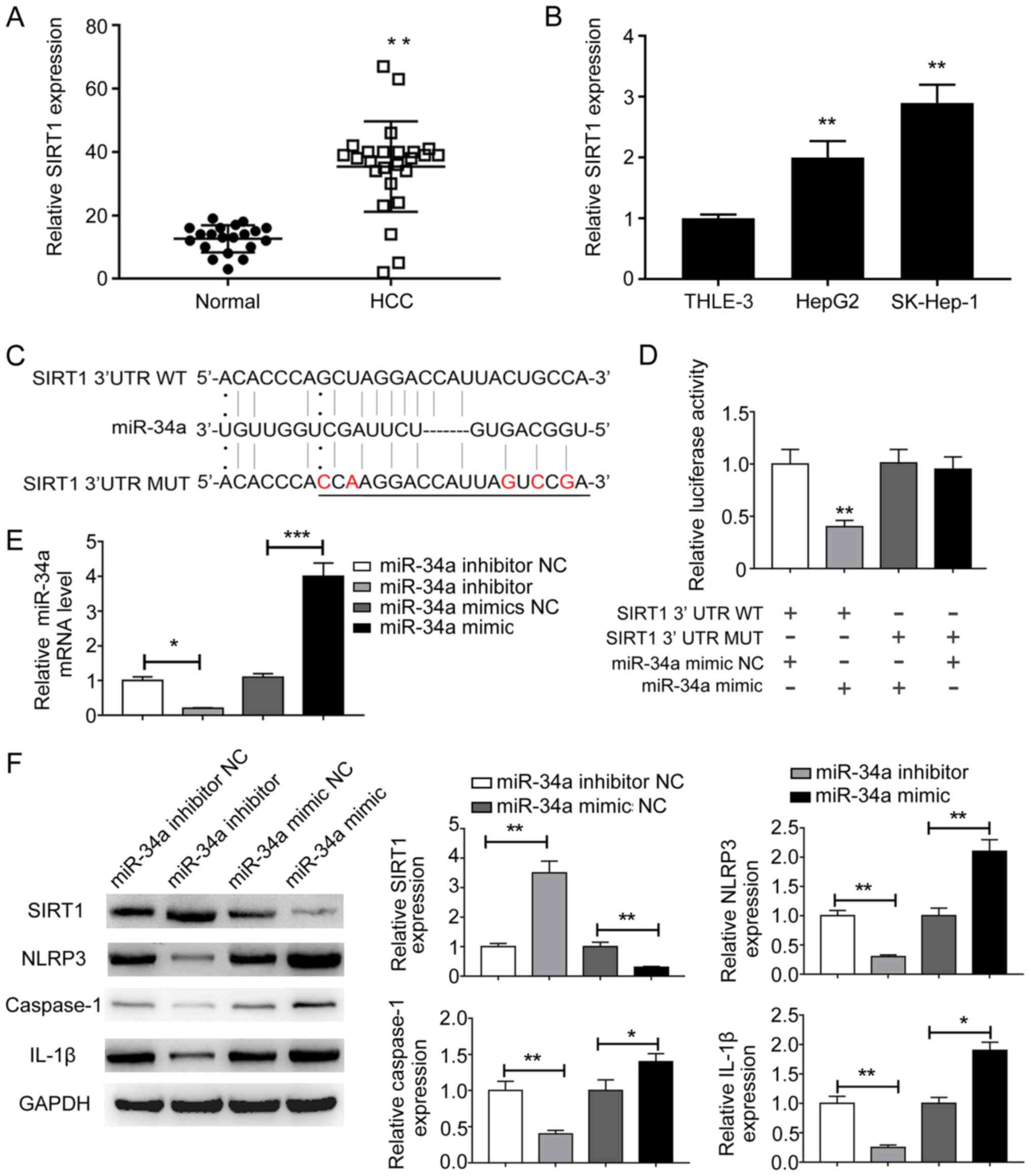 | Figure 3.SIRT1 is a direct target of miR-34a.
(A) mRNA expression levels of SIRT1 in (A) paired HCC and adjacent
normal tissues, as well as in (B) THLE-3, HepG2 and SK-Hep-1 cells
were determined by RT-qPCR. (C) The potential binding sites between
miR-34a and the 3′UTR of SIRT1. A mutation was generated in the
miR-34a binding site of the 3′UTR sequence of SIRT1. The mutations
were indicated in red. (D) Luciferase activities were determined by
a dual-luciferase reporter assay. (E) miR-34a expression levels
following miR-34a overexpression or knockdown were determined by
RT-qPCR. (F) Protein expression levels of SIRT1, NLRP3, caspase-1
and IL-1β following miR-34a overexpression or knockdown were
determined by western blotting. *P<0.05 and **P<0.01 vs. the
corresponding control. SIRT1, sirtuin 1; miR, microRNA; HCC,
hepatocellular carcinoma; RT-qPCR, reverse
transcription-quantitative PCR; 3′UTR, 3′-untranslated region;
NLRP3, NLR pyrin domain containing 3; IL-1β, interleukin-1β; WT,
wild-type; MUT, mutant; NC, negative control. |
NLRP3-dependent pyroptosis is
regulated by the SNHG7/miR-34a/SIRT1 axis during liver cancer
To further determine the effect of the
SNHG7/miR-34a/SIRT1 axis on pyroptosis in liver cancer cells,
si-SNHG7, miR-34a mimic and corresponding controls were
co-transfected into liver cancer cells. Liver cancer cells
transfected with si-SNHG7 or miR-34a mimic displayed significantly
reduced mRNA expression levels of SIRT1 compared with the negative
control group; co-transfection of si-SNHG7 and miR-34a mimic also
significantly reduced SIRT1 expression levels in HepG2 cells
compared with the negative control group (Fig. 4A). By contrast, the mRNA expression
levels of NLRP3, caspase-1 and IL-1β were significantly increased
in cells transfected with si-SNHG7 or miR-34a mimic compared with
the negative control group; additionally, the co-transfection group
displayed higher expression levels compared with the si-SNHG7 or
miR-34a mimics group (Fig. 4B-D).
The western blotting results indicated that protein expression
levels displayed a similar pattern (Fig.
4E). In addition, the present study aimed to determine the
effect of the SNHG7/miR-34a/SIRT1 axis on cell death. A TUNEL assay
was performed to detect DNA fragmentation in liver cancer cells.
Liver cancer cells transfected with si-SNHG7 or miR-34a mimic
displayed an increased proportion of TUNEL-positive cells compared
with the negative control group, and co-transfection of si-SNHG7
and miR-34a mimic further increased the proportion of
TUNEL-positive HepG2 cells (Fig.
4F). Furthermore, pyroptosis is generally assessed by
quantifying cytosolic LDH release (20). In HepG2 cells, LDH release was
significantly increased in the cell culture media of cells
transfected with si-SNHG7 or miR-34a mimic compared with the
negative control group (Fig. 4G).
LDH release was even higher in the si-SNHG7+miR-34a mimics group
compared with that in the si-SNHG7 or miR-34a mimics groups
(Fig. 4G). Similar results were
observed in SK-hep-1 cells (data not shown). Collectively, the
results indicated that the SNHG7/miR-34a/SIRT1 ceRNA network
regulated NLRP3-dependent pyroptosis in liver cancer cells.
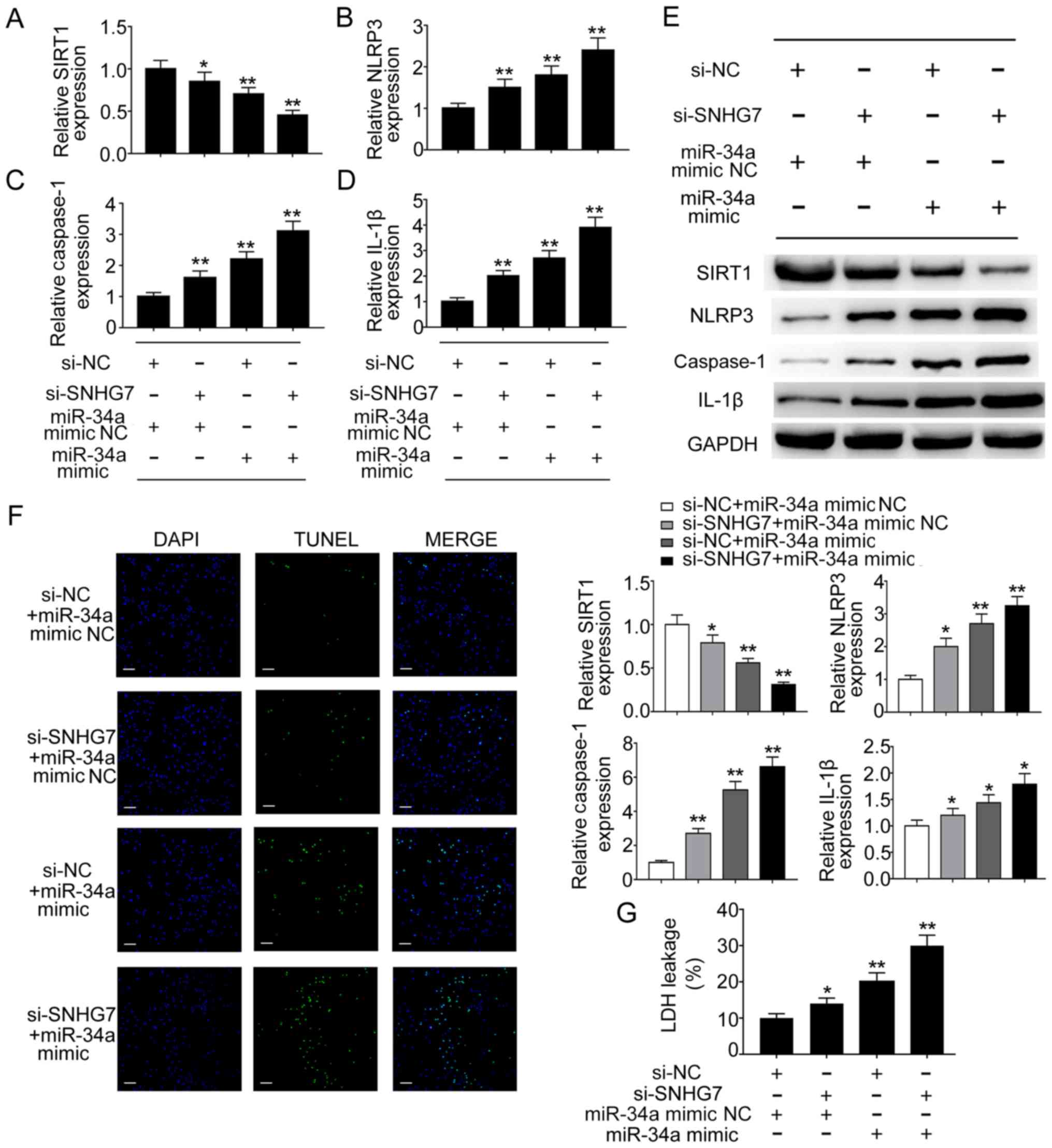 | Figure 4.NLRP3-dependent pyroptosis is
regulated by the SNHG7/miR-34a/SIRT1 axis in HCC. mRNA expression
levels of (A) SIRT1, (B) NLRP3, (C) caspase-1 and (D) IL-1β were
determined by RT-qPCR. (E) Protein expression levels of SIRT1,
NLRP3, caspase-1 and IL-1β were determined by western blotting. (F)
DNA fragmentation was assessed using the TUNEL assay. Blue
indicates DAPI nuclear staining and green indicates TUNEL staining
(scale bar, 50 µm). (G) Pyroptotic cell death was determined by
quantifying LDH release in the cell culture media. *P<0.05 and
**P<0.01 vs. the corresponding control. NLRP3, NLR pyrin domain
containing 3; SNHG7, small nucleolar RNA host gene 7; miR,
microRNA; SIRT1, sirtuin 1; HCC, hepatocellular carcinoma; IL-1β,
interleukin-1β; RT-qPCR, reverse transcription-quantitative PCR;
LDH, lactate dehydrogenase; si, small interfering RNA; NC, negative
control. |
Discussion
The present study demonstrated that SNHG7 and SIRT1
were upregulated, and miR-34a was downregulated in liver cancer
tissues and cell lines compared with adjacent normal tissues and
cell lines. The results indicated that SNHG7 sponged miR-34a to
suppress its expression, and SIRT1 was identified as a direct
target of miR-34a in liver cancer cells. Gain- and loss-of-function
experiments demonstrated that SNHG7 repressed miR-34a by acting as
a ceRNA, thus enhancing the expression of SIRT1 and inhibiting
NLRP3-dependent pyroptosis. Collectively, the results indicated
that the SNHG7/miR-34a/SIRT1 axis may serve as a potential
therapeutic target for liver cancer.
With advancements to high-throughput sequencing
techniques, a large number of dysregulated lncRNAs have been
identified in HCC. Functional studies have revealed that
dysregulated lncRNAs serve critical roles during the tumorigenesis
and progression of HCC (17,18). lncRNA SNHG7, which is located on
chromosome 9q34.3, has been identified as a novel oncogenic gene
and has recently received increasing attention in the field of
cancer research (21). Increasing
evidence has indicated that SNHG7 regulates cell proliferation,
migration, invasion and apoptosis in a variety of different types
of cancer, including non-small cell lung, gastric, prostate and
colorectal cancer, as well as in glioblastoma (14,22–25). For
example, SNHG7 promotes lung cancer cell proliferation, migration
and invasion, and inhibits apoptosis by inducing Fas apoptotic
inhibitory molecule 2 expression (22). Furthermore, by comparing lncRNA
profiles between early recurrence HCC tissues with metastasis and
late recurrence HCC tissues without metastasis, SNHG7 was
identified as a metastasis-related lncRNA (12). Moreover, Cancer Cell Line
Encyclopedia RNA sequencing results demonstrated that SNHG7 is
highly expressed in HCC cell lines, and SNHG7 knockdown inhibits
HCC cell invasion (12). However,
the role of SNHG7 in liver cancer is still not completely
understood. The present study indicated that SNHG7 was
significantly upregulated in liver cancer tissues and cell lines
compared with adjacent normal tissues and cell lines. Consistently,
recent studies have also demonstrated that SNHG7 is significantly
upregulated in HCC tissues and cells (13,26).
Another recent study reported that lncRNA growth arrest specific 5
serves as an ovarian tumor suppressor by inducing inflammasome
formation and pyroptosis (27). By
contrast, the present study indicated that SNHG7 knockdown
upregulated the expression of pyroptosis-related NLRP3, caspase-1
and IL-1β compared with the negative control group, which indicated
that SNHG7 may display an oncogenic role by inhibiting pyroptosis
in liver cancer.
Novel lncRNA/miRNA/mRNA networks based on ceRNA
hypotheses have been identified in a variety of different types of
cancer. For example, SNHG7 promotes glioblastoma cell
proliferation, migration and invasion by inhibiting miR-5095
(25). SNHG7 also acts as a ceRNA
against miR-503 to promote prostate cancer tumorigenesis (24). Recent studies have reported that
SNHG7 accelerates the progression and metastasis of HCC by
regulating miR-122-5p/ribosomal protein L4 or RNA binding motif
protein 5 (13,26). However, the ceRNA network that
regulates pyroptosis in liver cancer is not completely understood.
The present study indicated that miR-34a was negatively regulated
by SNHG7 in liver cancer. Previous studies have suggested that
miR-34a expression is lost or downregulated in numerous types of
cancer, including HCC (28,29). miR-34a inhibits HCC glycolysis by
targeting lactate dehydrogenase A, leading to reduced cell
proliferation and invasion (28).
However, aside from glucose metabolism, the role of miR-34a during
HCC has not been previously reported. The present study further
indicated that SNHG7 directly bound to and negatively regulated
miR-34a in HepG2 and SK-Hep-1 cells, which was consistent with a
recent study that reported that SNHG7 acts as a ceRNA to regulate
GALNT7 and the PI3K/Akt/mTOR signaling pathway by sponging miR-34a
in colorectal cancer (14).
SIRT1, a well-studied member of the sirtuin family,
serves crucial roles in various cellular events, including aging,
metabolism, inflammation and tumorigenesis (30,31).
SIRT1 serves as a tumor promoter or suppressor dependent on the
tumor type, as well as the temporal or spatial distribution of
upstream and downstream factors (32). SIRT1 is frequently upregulated in HCC
tissues and cell lines; however, SIRT1 knockdown and inhibition of
SIRT1 activity by the small molecule inhibitor cambinol impairs
cell proliferation, increases cell differentiation and inhibits
tumor growth in vivo (33).
The results of the present study support the aforementioned
finding, demonstrating that SIRT1 was highly expressed in HCC
tissues and cell lines. Mechanistic studies suggested that miR-34a
inhibited SIRT1 expression by directly binding to the 3′UTR of
SIRT1 in liver cancer cells. miR-34a-mediated inhibition of SIRT1
increased the expression of NLRP3, caspase-1 and IL-1β. To the best
of our knowledge, the present study was the first to indicate that
miR-34a-mediated suppression of SIRT1 regulated pyroptosis in liver
cancer cells. TUNEL and LDH release assays following
co-transfection of si-SNHG7 and miR-34a mimic further demonstrated
that SNHG7 knockdown reduced SIRT1 expression to promote
inflammasome formation and pyroptosis via sponging miR-34a. The
results of the present study were consistent with a previous study,
which reported that lack of SIRT1 expression enhances NLRP3
inflammasome activation and caspase-1 cleavage in vascular
endothelial cells (19).
The present study further demonstrated the
antipyroptotic properties of SNHG7 in liver cancer. The results
suggested that SNHG7 may act as an endogenous sponge of miR-34a,
thereby regulating the expression of SIRT1. Moreover, the results
indicated that SIRT1 induction may inhibit NLRP3
inflammasome-induced caspase-1 cleavage and IL-1β release to
inhibit pyroptosis in liver cancer cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC and QZ designed the study. MH performed the
western blotting experiments. JC collected the tissue samples. CL
performed the RT-qPCR and cell culture experiments. All authors
participated in analyzing the data and drafting the manuscript. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethic
Committee of People's Hospital of Deyang City. Written informed
consent was obtained from all patients prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNA
|
long non-coding RNA
|
|
SNHG7
|
small nucleolar RNA host gene 7
|
|
HCC
|
hepatocellular carcinoma
|
|
SIRT1
|
sirtuin 1
|
|
NLRP3
|
NLR pyrin domain containing 3
|
|
ceRNA
|
competing endogenous RNA
|
|
LDH
|
lactate dehydrogenase
|
References
|
1
|
Gerbes A, Zoulim F, Tilg H, Dufour JF,
Bruix J, Paradis V, Salem R, Peck-Radosavljevic M, Galle PR, Greten
TF, et al: Gut roundtable meeting paper: Selected recent advances
in hepatocellular carcinoma. Gut. 67:380–388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dhir M, Melin AA, Douaiher J, Lin C, Zhen
WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK and Are C: A
review and update of treatment options and controversies in the
management of hepatocellular carcinoma. Ann Surg. 263:1112–1125.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Franchi L, Eigenbrod T, Muñoz-Planillo R
and Nuñez G: The inflammasome: A caspase-1-activation platform that
regulates immune responses and disease pathogenesis. Nat Immunol.
10:241–247. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greten FR and Grivennikov SI: Inflammation
and cancer: Triggers, mechanisms, and consequences. Immunity.
51:27–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kantono M and Guo B: Inflammasomes and
cancer: The dynamic role of the inflammasome in tumor development.
Front Immunol. 8:11322017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X,
Zhao W, Huai W, Guo P and Han L: Deregulation of the NLRP3
inflammasome in hepatic parenchymal cells during liver cancer
progression. Lab Invest. 94:52–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu Q, Jiang Y, Zhang W, Xu C, Du W,
Tuguzbaeva G, Qin Y, Li A, Zhang L, Sun G, et al: Pyroptosis is
involved in the pathogenesis of human hepatocellular carcinoma.
Oncotarget. 7:84658–84665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheetham SW, Gruhl F, Mattick JS and
Dinger ME: Long noncoding RNAs and the genetics of cancer. Br J
Cancer. 108:2419–2425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:R17–R29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui H, Zhang Y, Zhang Q, Chen W, Zhao H
and Liang J: A comprehensive genome-wide analysis of long noncoding
RNA expression profile in hepatocellular carcinoma. Cancer Med.
6:2932–2941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang X, Sun L, Wang L, Yao B, Mo H and
Yang W: lncRNA SNHG7 accelerates the proliferation, migration and
invasion of hepatocellular carcinoma cells via regulating
miR-122-5p and RPL4. Biomed Pharmacother. 118:1093862019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Zeng C, Hu J, Pan Y, Shan Y, Liu B
and Jia L: Long non-coding RNA-SNHG7 acts as a target of miR-34a to
increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in
colorectal cancer progression. J Hematol Oncol. 11:892018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu F, Dong P, Mao Y, Zhao B, Huang Z and
Zheng J: Loss of lncRNA-SNHG7 promotes the suppression of hepatic
stellate cell activation via miR-378a-3p and DVL2. Mol Ther Nucleic
Acids. 17:235–244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu S, Xu J, Xu X, Hu S, Li B and Li W: The
expression of astrocyte elevated gene-1 in human non-small-cell
lung cancer and its relationship with postoperative chemotherapy
and radiotherapy. Histopathology. 67:817–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huo X, Han S, Wu G, Latchoumanin O, Zhou
G, Hebbard L, George J and Qiao L: Dysregulated long noncoding RNAs
(lncRNAs) in hepatocellular carcinoma: Implications for
tumorigenesis, disease progression, and liver cancer stem cells.
Mol Cancer. 16:1652017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Wang P, Yang X, Wang W, Zhang J, He
Y, Zhang W, Jing T, Wang B and Lin R: SIRT1 inhibits inflammatory
response partly through regulation of NLRP3 inflammasome in
vascular endothelial cells. Mol Immunol. 77:148–156. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rayamajhi M, Zhang Y and Miao EA:
Detection of pyroptosis by measuring released lactate dehydrogenase
activity. Methods Mol Biol. 1040:85–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shan Y, Ma J, Pan Y, Hu J, Liu B and Jia
L: lncRNA SNHG7 sponges miR-216b to promote proliferation and liver
metastasis of colorectal cancer through upregulating GALNT1. Cell
Death Dis. 9:7222018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
She K, Huang J, Zhou H, Huang T, Chen G
and He J: lncRNA-SNHG7 promotes the proliferation, migration and
invasion and inhibits apoptosis of lung cancer cells by enhancing
the FAIM2 expression. Oncol Rep. 36:2673–2680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang MW, Liu J, Liu Q, Xu QH, Li TF, Jin S
and Xia TS: LncRNA SNHG7 promotes the proliferation and inhibits
apoptosis of gastric cancer cells by repressing the P15 and P16
expression. Eur Rev Med Pharmacol Sci. 21:4613–4622.
2017.PubMed/NCBI
|
|
24
|
Qi H, Wen B, Wu Q, Cheng W, Lou J, Wei J,
Huang J, Yao X and Weng G: Long noncoding RNA SNHG7 accelerates
prostate cancer proliferation and cycle progression through cyclin
D1 by sponging miR-503. Biomed Pharmacother. 102:326–332. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren J, Yang Y, Xue J, Xi Z, Hu L, Pan SJ
and Sun Q: Long noncoding RNA SNHG7 promotes the progression and
growth of glioblastoma via inhibition of miR-5095. Biochem Biophys
Res Commun. 496:712–718. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun BZ, Ji DG, Feng ZX and Wang Y: Long
noncoding RNA SNHG7 represses the expression of RBM5 to strengthen
metastasis of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci.
23:5699–5704. 2019.PubMed/NCBI
|
|
27
|
Li J, Yang C, Li Y, Chen A, Li L and You
Z: lncRNA GAS5 suppresses ovarian cancer by inducing inflammasome
formation. Biosci Rep. 38(pii): BSR201711502018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang HF, Wang YC and Han YD: MicroRNA-34a
inhibits liver cancer cell growth by reprogramming glucose
metabolism. Mol Med Rep. 17:4483–4489. 2018.PubMed/NCBI
|
|
29
|
Slabáková E, Culig Z, Remšík J and Souček
K: Alternative mechanisms of miR-34a regulation in cancer. Cell
Death Dis. 8:e31002017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X and Kazgan N: Mammalian sirtuins and
energy metabolism. Int J Biol Sci. 7:575–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng T, Wang J, Jiang H and Liu L: Hippo
signaling in oval cells and hepatocarcinogenesis. Cancer Lett.
302:91–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang Y and Nicholl MB: Sirtuin 1 in
malignant transformation: Friend or foe? Cancer Lett. 306:10–14.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Portmann S, Fahrner R, Lechleiter A, Keogh
A, Overney S, Laemmle A, Mikami K, Montani M, Tschan MP, Candinas D
and Stroka D: Antitumor effect of SIRT1 inhibition in human HCC
tumor models in vitro and in vivo. Mol Cancer Ther. 12:499–508.
2013. View Article : Google Scholar : PubMed/NCBI
|


















