Introduction
Gastric cancer (GC) is the second most common cause
of cancer-associated mortality worldwide (1). In East Asia, based on GLOBOCAN 2018
data, the average incidence of gastric cancer is 32.1 per 100,000
among men and 13.2 among women (2).
For the majority of cases, patients with GC are diagnosed at the
advanced stage of disease and thus, successful therapeutic
strategies are limited, and the prognosis is poor (3–5).
Metastasis is a common trait of advanced GC progression (6); thus, in order to improve the survival
rate of patients with GC, investigations that focus on the
molecular mechanisms underlying pathogenesis of GC are
required.
MicroRNAs (miRNAs) are small conserved non-coding
RNAs, typically 20–25 nucleotides in length (7). miRNAs bind to complementary sequences
that are frequently present in the 3′-untranslated region (UTR) of
the target mRNA, thereby suppressing the translation of the target
mRNAs (7). A number of studies have
demonstrated that miRNAs play multiple roles in the pathogenesis of
GC (8–14).
The function of miR-876-5p has been investigated in
multiple types of cancer, including hepatocellular carcinoma (HCC)
(15,16) and lung cancer (17). miR-876-5p has been demonstrated to
inhibit cell proliferation of HCC and metastasis by targeting the
DNMT3A gene (16), and also inhibit
epithelial-to-mesenchymal transition (EMT) and metastasis by
targeting the BCL6 corepressor-like 1 gene (15). With regard to lung cancer, miR-876-5p
has been demonstrated to suppress EMT by targeting bone
morphogenetic protein 4 (17).
However, the role of miR-876-5p in GC is not yet fully understood.
Thus, the present study investigated the functions of miR-876-5p in
GC, with the aim of identifying potential, novel therapeutic
targets for further development in the future.
Materials and methods
Patients and tissue samples
A total of 16 GC tissues and corresponding adjacent
normal tissues were collected from 16 patients with GC between June
2015 and September 2016 who underwent surgery at the Department of
Gastroenterology, West China Hospital (Chengdu, China). The age and
sex distributions of the 16 patients with GC were listed as
follows: Age, 46–72 years; mean age, 56; male/female, 11:5. The
inclusion criteria were: i) Age ≥18 years; ii) the absence of
radiotherapy, chemotherapy or any adjuvant therapy before
hospitalization; iii) the absence of other malignant tumors; and
iv) the absence of a family history of genetic diseases. Exclusion
criteria were: i) The presence of antibiotics taking within three
months before blood collection; ii) the presence of liver
insufficiency and, iii) the presence of autoimmune system
deficiency. The distance between the tumor tissues and adjacent
non-tumor tissues was >5 cm. The histopathological diagnoses of
all patients were confirmed by a senior pathologist at West China
Hospital. The present study was approved by the Ethics Committee of
Sichuan University (Chengdu, China) and all patients provided
written informed consent.
Cell culture
The human GC cell line AGS-1 cell line and human
gastric epithelial mucosa cell line GES-1 were purchased from the
China Infrastructure of Cell Line Resources, Institute of Basic
Medical Sciences, Chinese Academy of Medical Sciences. The two cell
lines were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C and 5%
CO2. The cells were cultured until they reached 80%
confluence. Cisplatin was purchased from Hansoh Pharma. A final
concentration of 25 µM of cisplatin was added to the cultures as
previously described (18).
miR-876-5p mimics and oligonucleotide
transfection
miR-876-5p mimics, miR-876-5p antisense
oligonucleotides (ASO) and negative controls (miR-NC mimics and
miR-NC ASO) were constructed by Sangon Biotech Co., Ltd. The primer
sequences were as follows: miR-876-5p mimics, sense
5′-UGGAUUUCUUUGUGAAUCACCA-3′ and antisense
5′-GUGAUUCACAAAGAAAUCCAUU-3′; mimic control, sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′; miR-876-5p inhibitors
5′-UGGUGAUUCACAAAGAAAUCCA-3′; and inhibitor control
5′-CAGUACUUUUGUGUAGUACAA-3. A total of 2×105 AGS-1 cells
were seeded into 6-well plates overnight and transfected with 50 nM
miR-876-5p mimics or miR-NC mimics using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Similarly, AGS-1 cells were seeded and
transfected with miR-876-5p ASO (50 nM); miR-NC ASO (50 nM) was
used as control. The cell growth and apoptosis assays were
performed 24 h later.
Cell proliferation assay
Cell proliferation was assessed using an MTT assay.
The AGS-1 cells were seeded into 96-well plates at a density of
5×105 cells/plate. MTT was added to the medium at a
final concentration of 0.1 mg/ml. The purple formazan crystals were
dissolved using 100 µl of dimethyl sulfoxide and optical density
was measured using a microplate reader (Multiskan Sky Thermo Fisher
Scientific, Inc.) at a wavelength of 570 nm (19–21).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from GC tissues and AGS-1
cell lines using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The RNA was quantified by NanoDrop™ (ThermoFisher Scientific Inc.).
Total RNA were reverse transcribed into cDNA using the All-in-One™
miRNA First-Strand cDNA Synthesis kit (Invitrogen, Thermo Fisher
Scientific, Inc.). The PCR was performed by the SuperScript™ III
Platinum™ SYBR™ Green One-Step qPCR kit (ThermoFisher Scientific
Inc.). The primers were synthesized by Sangon Biotech Co., Ltd. The
RNA sequences for the primers mentioned above were as follows:
miR-876-5p sense 5′-AGGACUUCUCCCUCCUCCCAG-3′ and anti-sense,
5′-UCCUCUUCUCCCUCCAGGGAG-3′; U6 snRNA sense
5′-CTCGCTTCGGCAGCACATATACT-3′; and anti-sense
5′-ACGCTTCACGAATTTGCGTGTC-3′. miR-876-5p expression was normalized
to the internal reference gene U6 (18,22). PCR
conditions were as follows: Denaturation for 10 min at 95°C,
followed by 40 cycles of annealing for 1 min at 95°C and extension
for 30 sec at 60°C. The dissolution procedure was designed as
follows: 15 sec at 95°C, 30 sec at 60°C and 15 sec at 95°C.
Quantification was performed using the 2−∆∆Cq method
(23).
Mutated site and target gene
prediction
The mutated sites were generated by Gene
Site-directed Mutagenesis System (Thermo Fisher Scientific, Inc.).
The potential targets of miR-876-5p were predicted by TargetScan
software (24–29).
Apoptosis analysis
The apoptotic ability of AGS-1 cells was assessed
via flow cytometry using Annexin V-propidium iodide (PI) staining.
AGS-1 cells were suspended in Annexin V-fluorescein isothiocyanate
(FITC) (1 µg/ml) binding buffer (Abcam) at a density of
5×105 cells/ml and incubated at room temperature for 20
min. Subsequently, PI (0.1 µg/ml) (Abcam) was added to the samples,
incubated for 5 min at room temperature and analyzed using a BD
FACSVerse™ flow cytometer (BD Biosciences) using the 488 nm
excitation line (argon ion or solid-state laser), and emission was
detected at 530 nm (green for Annexin V-FITC) and 575–610 nm
(orange for PI). The data were analyzed using the BD FACSuite™
version 1.01 (BD Biosciences).
Western blot analysis
Total protein was extracted from AGS-1 cells using
lysis buffer (150 mM NaCl, 50 mM Tris-HCI, 1% Triton X-100 and 0.1%
SDS) with Protease Inhibitor Cocktail and Phosphatase Inhibitor
Cocktail (Sigma-Aldrich; Merck KGaA). Proteins (10 mg) were
separated via SDS-PAGE on a 10% gel and subsequently transferred
onto a polyvinylidene difluoride membrane. The membranes were
blocked in 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for
1 h at room temperature, prior to transforming growth factor
β-receptor 1 (TGFBR1) analysis. The membranes were incubated with
anti-TGFBR1 (1:1,000; cat. no. ab31013; Abcam) and β-actin
(1:1,000; cat. no. ab8226; Abcam) overnight at 4°C. Membranes were
washed three times with PBS and subsequently incubated with a
horseradish peroxidase-conjugated rabbit IgG antibody (1:2,000;
cat. no. ab218695; Abcam) for 2 h at room temperature. Protein
bands were visualized using the ECL Western Blotting Detection
reagents (GE Healthcare) and images were analyzed using ImageJ
software (National Institutes of Health).
Luciferase reporter plasmid
transfection
AGS-1 cells were seeded at 1×105 per well
and were serum-starved for 6 h pre-transfection. The 3′
untranslated region (UTR) of TGFBR1 was cloned into the reporter
plasmid (500 ng) and the pGL3-control (100 ng; Promega
Corporation). miR-876-5p (30 nM) were transfected into AGS-1 cells
containing the wild-type or mutant 3′-UTR plasmids using
Lipofectamine® 2000 (Invitrogen, Thermo Fisher
Scientific Inc.) for 5 min at room temperature. Mutants of the 3′
UTR of TGFBR1 were generated using the Site-directed Mutagenesis
kit (cat. no. A13282, Thermo Fisher Scientific Inc.). Cells were
harvested 24 h later, and the luciferase activity was measured
using the Dual-Luciferase Reporter Assay System (Promega
Corporation). The firefly luciferase enzyme activity was normalized
to the Renilla luciferase enzyme activity.
Statistical analysis
All data were analyzed using SPSS software version
16.0 (SPSS, Inc.). All experiments were performed in triplicate and
data are presented as the mean ± standard deviation. Two-tailed
Student's t-test was used in order to analyze the mean values
between two groups; one-way ANOVA was used in order to test the
mean values among three groups or more, followed by
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
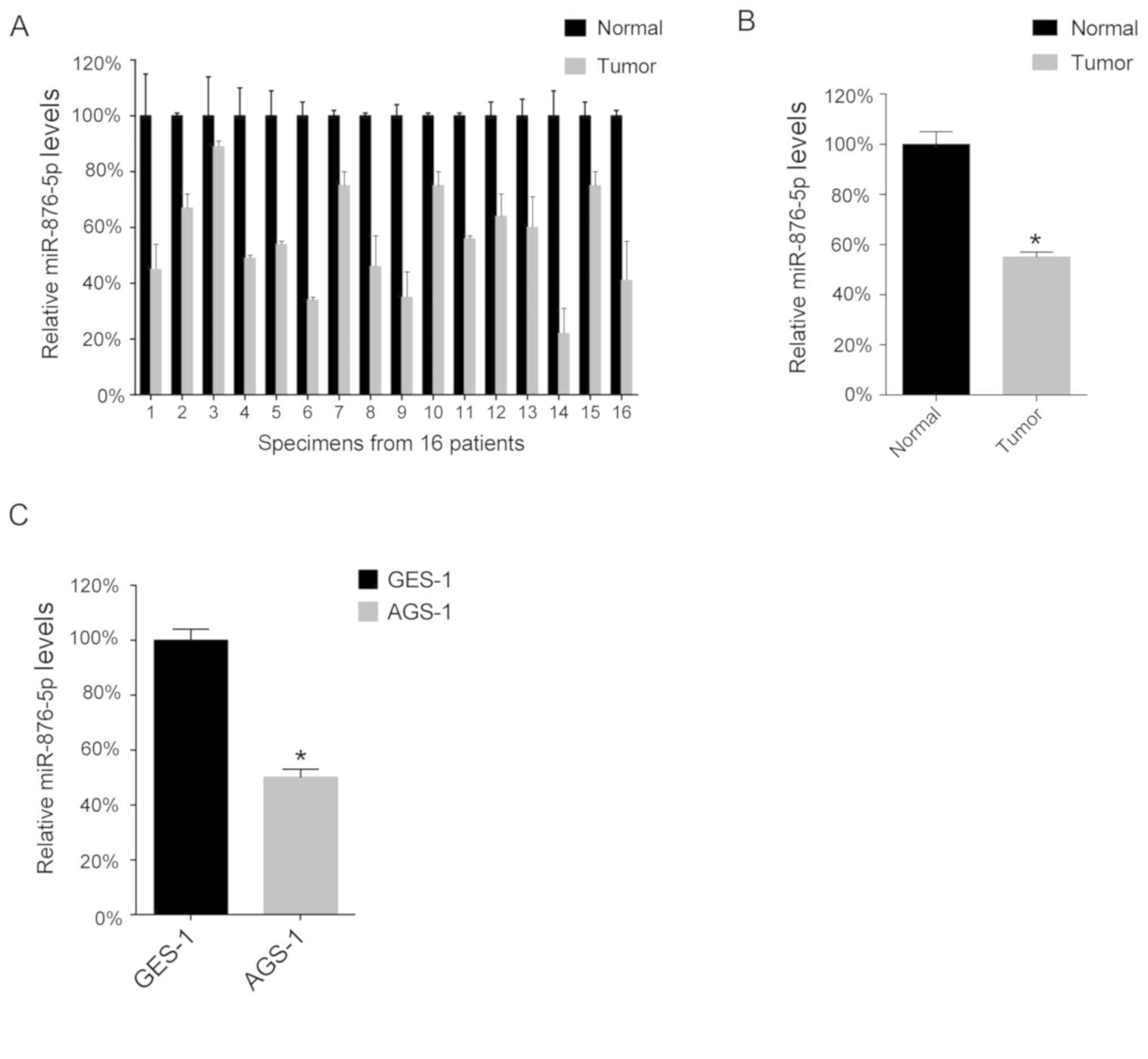
miR-876-5p levels in GC tissues
The present study performed a RT-qPCR analysis in
order to determine miR-876-5p levels in the 16 GC tissues and 16
corresponding normal adjacent tissues. The results of the present
study demonstrated that miR-876-5p levels are downregulated in GC
tissues compared with normal tissues (Fig. 1A), as the mean levels of miR-876-5p
in the 16 GC tissues was lower compared with that in the 16 normal
adjacent tissues (Fig. 1B).
Furthermore, the present study analyzed miR-876-5p levels in the GC
cell lines and demonstrated that the AGS-1 cell line expressed
lower levels of miR-876-5p compared with the GES-1 cell line
(Fig. 1C).
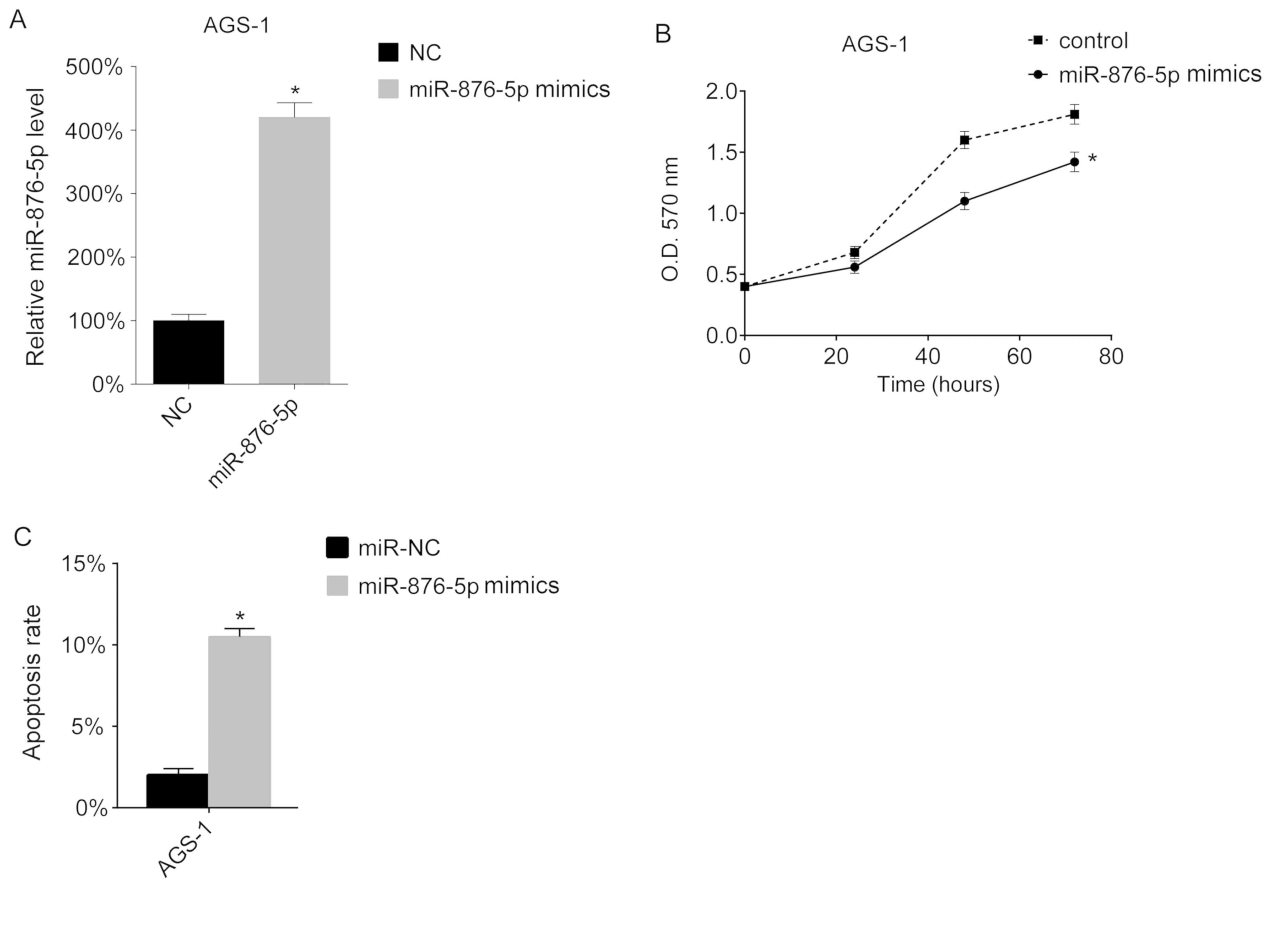
Overexpression of miR-876-5p inhibits
cellular proliferation and promotes apoptosis
The present study assessed the function of
miR-876-5p in vitro. miR-876-5p levels in AGS-1 cells were
overexpressed using miR-876-5p mimics. The results of the present
study demonstrated that transfection with miR-876-5p mimics
increased the miR-876-5p levels in AGS-1 cells (Fig. 2A). Subsequently, cell proliferation
was assessed using an MTT assay. The results of the present study
demonstrated that overexpression of miR-876-5p inhibits
proliferation of AGS-1 cells (Fig.
2B). The present study assessed the apoptotic ability of the
transfected cells and demonstrated that miR-876-5p increased the
apoptotic rate of AGS-1 cells (Fig.
2C).
Suppression of miR-876-5p promotes
cellular proliferation and inhibits apoptosis
miR-876-5p knockdown in AGS-1 cells was performed in
the present study, via miR-876-5p ASO transfection. The miR-876-5p
levels of AGS-1 cells were analyzed via RT-qPCR, and the results of
the present study demonstrated that miR-876-5p ASO transfection
downregulates miR-876-5p levels in AGS-1 (Fig. 3A). Furthermore, the results of the
MTT assay in the present study demonstrated that miR-876-5p ASO
transfection promotes the proliferation of AGS-1 cells (Fig. 3B). Subsequently, AGS-1 cells were
treated with cisplatin following miR-876-5p ASO transfection. The
results of the present study demonstrated that cisplatin increased
the apoptotic rate of AGS-1 cells, whereas the downregulation of
miR-876-5p decreased the apoptotic rate induced by cisplatin
(Fig. 3C).
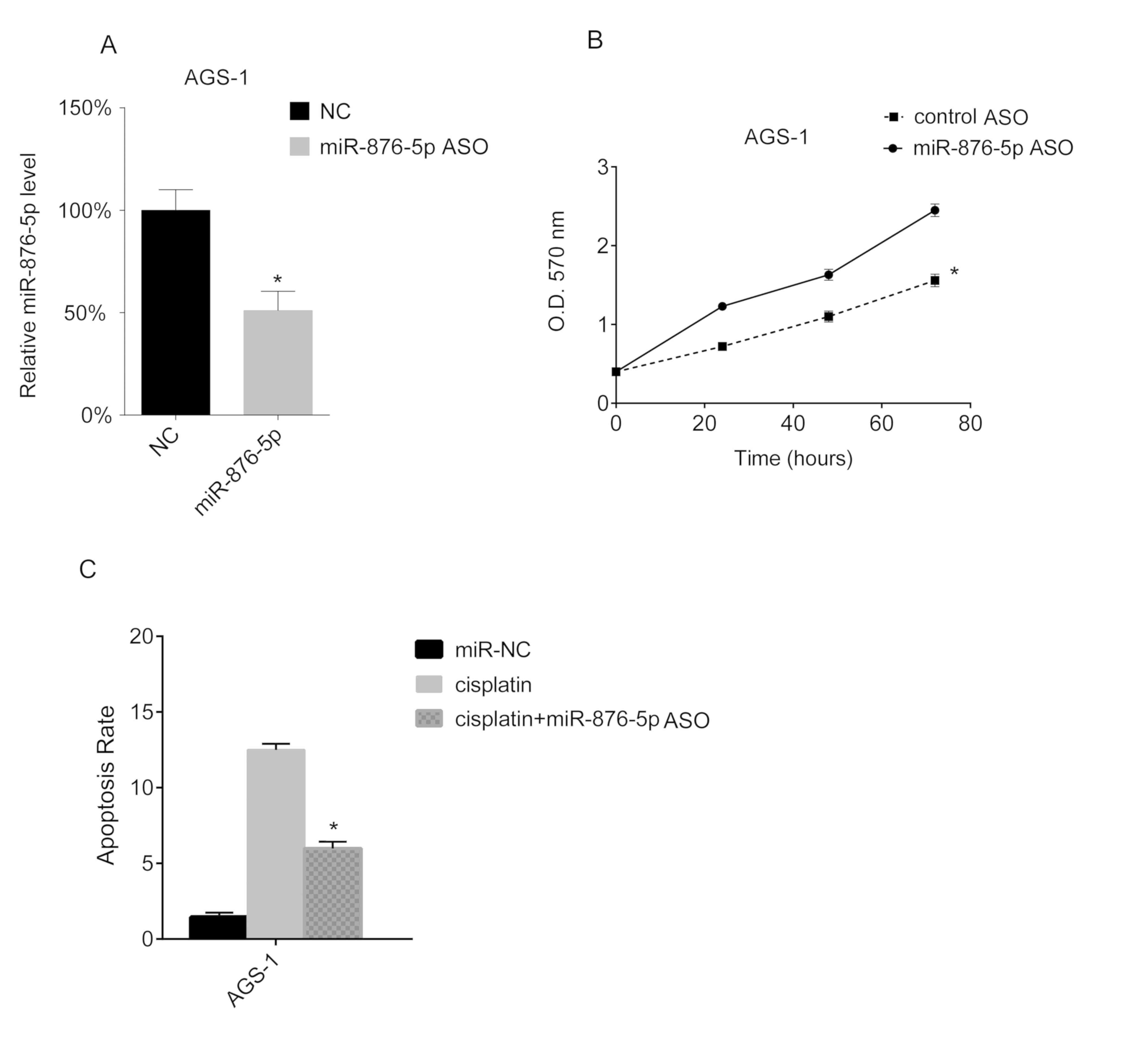 | Figure 3.miR-876-5p knockdown promotes
proliferation and inhibits apoptosis of AGS-1 cells. AGS-1 cells
were seeded into 24-well plates at a density of 1×106
cells/well overnight. miR-876-5p mimics were transfected into AGS-1
cells. (A) After 24 h, miR-876-5p levels in AGS-1 cells were
analyzed using reverse transcription-quantitative PCR. The
miR-876-5p levels in miR-NC mimic-transfected cells. The miR-876-5p
levels in the control group were arbitrarily defined as 100%. (B)
Cellular proliferation was assessed using an MTT assay 24 h after
transfection, at 24, 48 and 72 h. (C) Cell apoptosis rates were
assessed by FACS 24 h after transfection with miR-876-5p ASO.
*P<0.05, cisplatin vs. cisplatin + miR-876-5p ASO. miR-876-5p,
microRNA-876-5p; miR-NC, microRNA-control; FACS,
fluorescence-activated cell sorting; NC, control; miR-876-5p ASO,
microRNA-876-5p antisense oligonucleotides; OD, optical
density. |
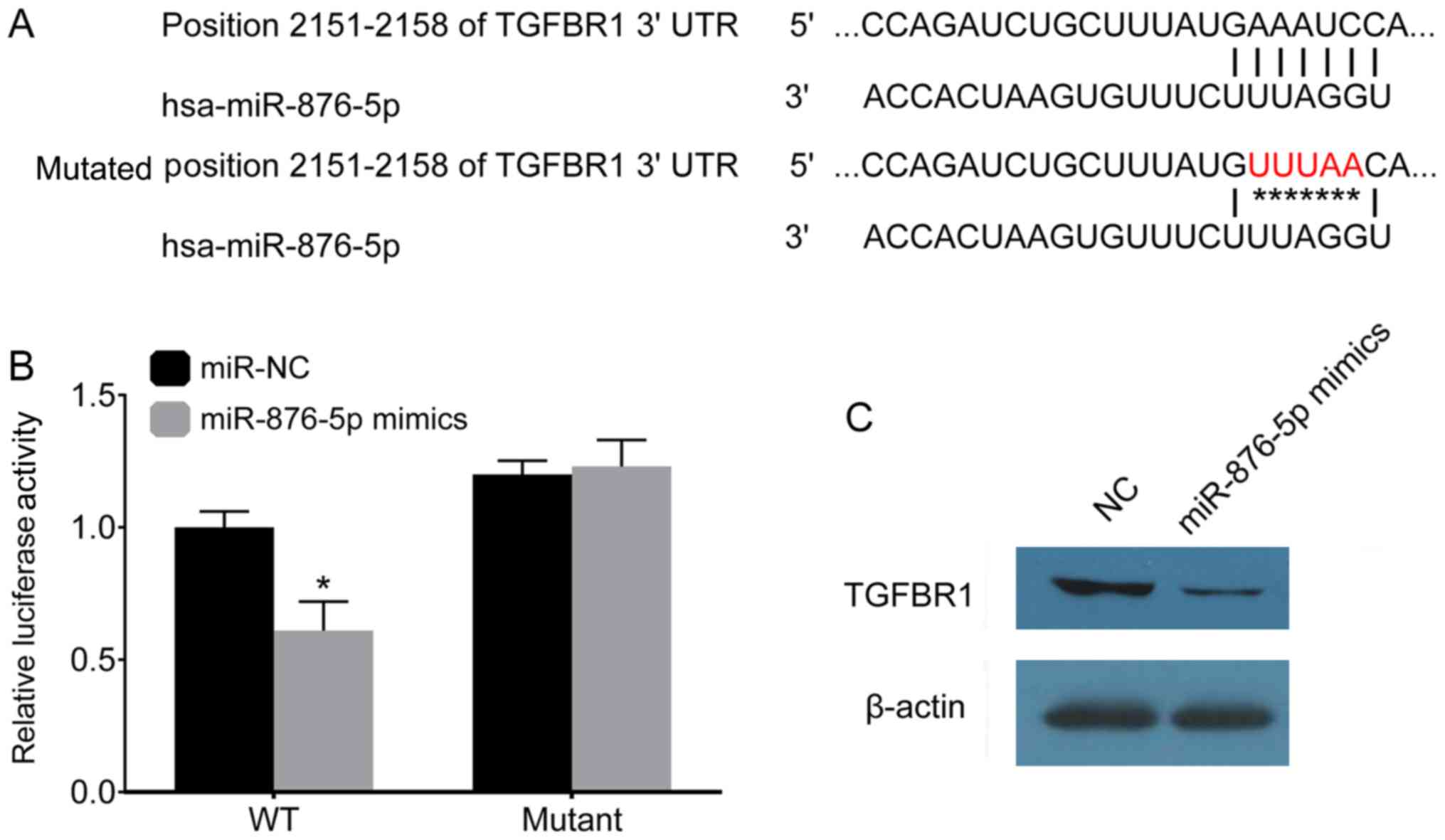
miR-876-5p targets TGFBR1
Physiologically, miRNAs play a role in targeting
genes. The present study used the TargetScan software in order to
predict the potential target genes of miR-876-5p. The results of
the present study demonstrated that TGFBR1 may be targeted by
miR-876-5p. The present study mutated the 2,151-2,158 position of
TGFBR1 (Fig. 4A), in order to
investigate the association between miR-876-5p and TGFBR1. The
3′-UTR TGFBR1 mutant was generated and subsequently transfected
into a luciferase reporter plasmid. miR-876-5p and the 3′-UTR
TGFBR1 mutant were co-transfected into AGS-1 cells. At 24 h
post-transfection, miR-876-5p was demonstrated to decrease the
luciferase activity of the 3′-UTR of wild-type TGFBR1, but not that
of the 3′-UTR of mutated TGFBR1 (Fig.
4B). After 48 h, TGFBR1 protein levels were determined using
western blot analysis, and the results of the present study
demonstrated that miR-876-5p mimics inhibited the expression of
TGFBR1 in AGS-1 cells (Fig. 4D).
Discussion
The results of the present study demonstrated that
miR-876-5p levels were downregulated in GC tumor tissues compared
with normal adjacent tissues. Furthermore, overexpression of
miR-876-5p inhibited cellular proliferation and promoted apoptosis,
whereas miR-876-5p knockdown promoted cellular proliferation and
inhibited apoptosis. The results of the present study suggest that
TGFBR1 is targeted by miR-876-5p. To the best of our knowledge, the
results of the present study are the first to report miR-876-5p
involvement in human GC. As miR-876-5p may be associated with the
survival time of patients with GC, future studies are required with
larger patient sample sizes, in order to accurately assess survival
time analysis in mice models. Furthermore, future studies are
critical in order to assess the association between miR-876-5p
levels and the function in drug-treatment pressure.
A previous study demonstrated the involvement of
miR-876-5p in viral infections. Wang et al (30) demonstrated that severe human
enterovirus led to elevated expression of circulating miR-876-5p.
Furthermore, miR-876-5p has been reported to participate in the
miRNA-based antiviral defense mechanism against influenza virus in
humans, by targeting the matrix protein of human influenza A H1N1
virus (31).
Previous studies have demonstrated that miR-876-5p
inhibits cell proliferation, metastasis and EMT in hepatocellular
carcinoma and lung cancer (15–17). The
results of the present study further confirmed the inhibitory role
of miR-876-5p and proposed TGFBR1 as a target gene. TGFBR1 is the
receptor of TGF-β, which is known to induce EMT (32). Thus, miR-876-5p may have the
potential to regulate EMT through TGFBR1 targeting.
TGF-β has a dual role in cancer development, acting
as a tumor suppressor and a tumor promoter; it promotes
tumorigenesis indirectly by acting on the tumor microenvironment
(33). Thus, there is potential that
TGFBR1 may play a similar dual role in the pathogenesis of GC. It
is predicted that the inhibition of TGFBR1 by miR-876-5p may result
in inactivation of the TGF-β signaling pathway. The results of the
present study demonstrated that miR-876-5p targeted TGFBR1, but the
role of TGFBR1 is not yet fully understood. Furthermore, the
results of the present study suggest that miR-876-5p may be
involved in the complex TGF-β signaling pathway by targeting
TGFBR1. Another study demonstrated that variants exist in the whole
TGFBR1 gene, represented by the single nucleotide polymorphisms,
and that genetic variants in TGFBR1 may play a role in the
development of GC (34). The results
of the present study indicate that miR-876-5p could regulate TGFBR1
by binding to its 3′-UTR. Whether the variants of TGFBR1 have an
effect on the regulation function of miR-876-5p warrants further
investigations. Overall, the results of the present study confirm
the inhibitory role of miR-876-5p in GC.
Acknowledgements
The authors would like to thank Dr Ke Lei
(Department of Oncology, West China Hospital, Sichuan University)
for discussions regarding the present study.
Funding
This study was supported by a grant from the Science
Supporting Funding of Sichuan University (grant no.20180110).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HZ, YZ, JY and JX collected patient data and
performed cell experiments. JX, SY and LX performed PCR, western
blotting and other molecular experiments. YY and XS contributed to
the study design and manuscript writing. ZJ and CZ contributed to
funding support, data analysis and revising the manuscript for
important intellectual content. All the authors have read and
approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Sichuan University (Chengdu, China) and the patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sitarz R, Skierucha M, Mielko J, Offerhaus
GJA, Maciejewski R and Polkowski WP: Gastric cancer: Epidemiology,
prevention, classification, and treatment. Cancer Manag Res.
10:239–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention. Prz
Gastroenterol. 14:26–38. 2019.PubMed/NCBI
|
|
3
|
Mocellin S, Verdi D, Pooley KA and Nitti
D: Genetic variation and gastric cancer risk: A field synopsis and
meta-analysis. Gut. 64:1209–1219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riquelme I, Saavedra K, Espinoza JA, Weber
H, García P, Nervi B, Garrido M, Corvalán AH, Roa JC and Bizama C:
Molecular classification of gastric cancer: Towards a
pathway-driven targeted therapy. Oncotarget. 6:24750–24779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coccolini F, Montori G, Ceresoli M, Cima
S, Valli MC, Nita GE, Heyer A, Catena F and Ansaloni L: Advanced
gastric cancer: What we know and what we still have to learn. World
J Gastroenterol. 22:11392016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giordano S and Columbano A: MicroRNAs: New
tools for diagnosis, prognosis, and therapy in hepatocellular
carcinoma? Hepatology. 57:840–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao F, Zhang C, Zhou C, Sun W, Liu X,
Zhang P, Han J, Xian L, Bai D, Liu H, et al: A critical role of
toll-like receptor 2 (TLR2) and its' in vivo ligands in
radio-resistance. Sci Rep. 5:130042015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng B, Liang L, Wang C, Huang S, Cao X,
Zha R, Liu L, Jia D, Tian Q, Wu J, et al: MicroRNA-148a suppresses
tumor cell invasion and metastasis by downregulating ROCK1 in
gastric cancer. Clin Cancer Res. 17:7574–7583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Dong G, Wang B, Gao W and Yang Q:
miR-543 promotes gastric cancer cell proliferation by targeting
SIRT1. Biochem Biophys Res Commun. 469:15–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou X, Zhu W, Li H, Wen W, Cheng W, Wang
F, Wu Y, Qi L, Fan Y, Chen Y, et al: Diagnostic value of a plasma
microRNA signature in gastric cancer: A microRNA expression
analysis. Sci Rep. 5:112512015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ibarrola-Villava M, Llorca-Cardeñosa MJ,
Tarazona N, Mongort C, Fleitas T, Perez-Fidalgo JA, Roselló S,
Navarro S, Ribas G and Cervantes A: Deregulation of ARID1A, CDH1,
cMET and PIK3CA and target-related microRNA expression in gastric
cancer. Oncotarget. 6:269352015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge X, Liu X, Lin F, Li P, Liu K, Geng R,
Dai C, Lin Y, Tang W, Wu Z, et al: MicroRNA-421 regulated by HIF-1α
promotes metastasis, inhibits apoptosis, and induces cisplatin
resistance by targeting E-cadherin and caspase-3 in gastric cancer.
Oncotarget. 26:24466–24682. 2016. View Article : Google Scholar
|
|
14
|
Du Y, Wang L, Wu H, Zhang Y, Wang K and Wu
D: MicroRNA-141 inhibits migration of gastric cancer by targeting
zinc finger E-box-binding homeobox 2. Mol Med Rep. 12:3416–3422.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Q, Zhu Q, Zhou Z, Wang Y, Liu X, Yin G,
Tong X and Tu K: MicroRNA-876-5p inhibits epithelial-mesenchymal
transition and metastasis of hepatocellular carcinoma by targeting
BCL6 corepressor like 1. Biomed Pharmacother. 103:645–652. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Xie Y, Li X, Lin J, Zhang S, Li Z,
Huo L and Gong R: miR-876-5p acts as an inhibitor in hepatocellular
carcinoma progression by targeting DNMT3A. Pathol Res Pract.
214:1024–1030. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao L, Lv L, Feng J, Chen Y, Wang X, Han S
and Zhao H: MiR-876-5p suppresses epithelial-mesenchymal transition
of lung cancer by directly down-regulating bone morphogenetic
protein 4. J Biosci. 42:671–681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song B, Ji W, Guo S, Liu A, Jing W, Shao
C, Li G and Jin G: miR-545 inhibited pancreatic ductal
adenocarcinoma growth by targeting RIG-I. FEBS Lett. 588:4375–4381.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hansen MB, Nielsen SE and Berg K:
Re-examination and further development of a precise and rapid dye
method for measuring cell growth/cell kill. J Immunol Methods.
119:203–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Freimoser FM, Jakob CA, Aebi M and Tuor U:
The MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium
bromide] assay is a fast and reliable method for colorimetric
determination of fungal cell densities. Appl Environ Microbiol.
65:3727–3729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meletiadis J, Meis JF, Mouton JW, Donnelly
JP and Verweij PE: Comparison of NCCLS and 3-(4,
5-dimethyl-2-thiazyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT)
methods of in vitro susceptibility testing of filamentous fungi and
development of a new simplified method. J Clin Microbiol.
38:2949–2954. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song B, Zhang C, Li G, Jin G and Liu C:
MiR-940 inhibited pancreatic ductal adenocarcinoma growth by
targeting MyD88. Cell Physiol Biochem. 35:1167–1177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garcia DM, Baek D, Shin C, Bell GW,
Grimson A and Bartel DP: Weak seed-pairing stability and high
target-site abundance decrease the proficiency of lsy-6 and other
microRNAs. Nat Struct Mol Biol. 18:1139–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Friedman RC, Farh KK-H, Burge CB and
Bartel DP: Most mammalian mRNAs are conserved targets of microRNAs.
Genome research. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee S, Paulson KG, Murchison EP, Afanasiev
OK, Alkan C, Leonard JH, Byrd DR, Hannon GJ and Nghiem P:
Identification and validation of a novel mature microRNA encoded by
the Merkel cell polyomavirus in human Merkel cell carcinomas. J
Clin Virol. 52:272–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang RY, Weng KF, Huang YC and Chen CJ:
Elevated expression of circulating miR876-5p is a specific response
to severe EV71 infections. Sci Rep. 6:241492016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang H, Li Z, Li Y, Liu J, Li X, Shen T,
Duan Y, Hu M and Xu D: A computational method for predicting
regulation of human microRNAs on the influenza virus genome. BMC
Syst Biol. 7 (Suppl 2):S32013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katz LH, Li Y, Chen JS, Muñoz NM, Majumdar
A, Chen J and Mishra L: Targeting TGF-β signaling in cancer. Exp
Opin Therapeutic Targets. 17:743–760. 2013. View Article : Google Scholar
|
|
34
|
Chen J, Miao L, Jin G, Ren C, Ke Q, Qian
Y, Dong M, Li H, Zhang Q, Ding Y, et al: TGFBR1 tagging SNPs and
gastric cancer susceptibility: A two-stage case-control study in
Chinese population. Mol Carcinog. 53:109–116. 2014. View Article : Google Scholar : PubMed/NCBI
|