Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related death (1),
in spite of recent improvements in risk factor regulation and
surveillance (2). Surgical resection
is the first choice to treat early-stage HCC. However, the 5-year
recurrence rate after hepatectomy reaches approximately 70%
(3). Therefore, most cases of
recurrent HCC develop to advanced stages (4). The prognosis of early-stage HCC has
improved thanks to clinical management, but that of advanced-stage
HCC remains extremely poor (5). The
targeted agent sorafenib, an oral multikinase inhibitor, was
introduced as first-line systemic therapy for HCC in 2007 (6). Sorafenib therapy provided an additional
treatment option for patients with advanced HCC who had local
vascular invasion or distant metastasis (6). Sorafenib prolonged the median survival
and time to radiologic progression in patients with advanced HCC
for 3 months over that of placebo (7). Recently, regorafenib, a multiple
receptor tyrosine kinase inhibitor, was approved for the treatment
of advanced HCC that has progressed after sorafenib therapy
(8). Therefore, predictive
biomarkers of the outcomes of sorafenib therapy that indicate the
best time to switch to second-line therapy should be assessed
before starting treatment. However, no biological serum marker has
been discovered for the prediction of refractory HCC under
sorafenib therapy.
MicroRNAs (miRNAs) are 18–22 nucleotide-long
endogenous noncoding RNAs (9,10) that
regulate over 200 genes (11).
Previous reports have shown altered expression of several miRNAs in
HCC tissues when compared to normal tissues (12–15).
Recently, miRNAs have been evaluated as tissue biomarkers in the
context of sorafenib response in HCC (16,17).
In this study, we evaluated whether specific
circulating miRNAs were involved with refractory HCC under
sorafenib therapy to identify miRNAs that may serve as new
predictive biomarkers for the efficacy of sorafenib therapy in this
disease.
Materials and methods
Cell culture
The human liver cancer cell lines Li-7, Hep3B,
HepG2, HLE, HLF, Alex and Huh7 were obtained from the Japanese
Cancer Research Resources Bank and transported to our laboratory.
The cell lines were authenticated by the cell bank using short
tandem repeat PCR. Mycoplasma testing has been done for cell lines
used in our experiments. Cells were grown in minimum essential
medium (MEM; Gibco-Invitrogen) supplemented with 10% fetal bovine
serum (FBS, 533–69545; Wako) and penicillin-streptomycin (100 mg/l;
Invitrogen) at 37°C in a humidified atmosphere containing 5%
CO2.
Cell proliferation assay
The cell proliferation assays were conducted using
CCK-8 according to the manufacturer's instructions. Each cell line
(0.5×104) was seeded on 96-well plates and cultured in
100 µl MEM supplemented with 10% FBS. After 24 h, seeded cells were
treated with 0, 1, 3, 5, or 10 µM sorafenib that was added to the
culture medium. At the indicated time points, the medium was
exchanged for 100 µl of MEM with CCK-8 reagent (10 µl CCK-8 and 90
µl MEM). The absorbance of each well was measured at a wavelength
of 450 nm using an auto-microplate reader.
Patients and serum samples
We obtained serum samples from 11 patients with
advanced HCC who underwent sorafenib therapy at the Kagawa
University hospital from 2012 to 2015. All samples were drawn
before sorafenib administration (Table
I). This study was conducted in accordance with the ethical
principles of the Declaration of Helsinki and approved by The
Institutional Review Board (IRB) of Kagawa University, Faculty of
Medicine (Heisei-22-063). Informed consent to use the clinical data
and samples for the present study was obtained from the patients or
their relatives. For patients who died and had no relatives listed
in their clinical records, we provided opt-out methods for the
relatives of the dead participants by publishing a summary of this
study on the university website. Ethics approval was obtained from
The Ethics Committee of Kagawa University Faculty of Medicine.
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
|
Characteristics | Effective
(n=6) | Non-effective
(n=5) | P-values |
|---|
| Age (median) | 69 (53–79) | 68 (55–76) | NS |
| Sex,
male/female | 5/1 | 4/1 | NS |
| Etiology,
B/C/NBNC | 1/3/2 | 1/3/1 | NS |
| Stage,
III/IVa/IVb | 2/3/1 | 2/2/1 | NS |
| Child-Pugh,
A/B/C | 5/1/0 | 5/1/0 | NS |
| Vessel invasion,
none/portal vein | 4/2 | 4/1 | NS |
| Reason of sorafenib
administration, TACE refractory/vascular invasion | 4/2 | 4/1 | NS |
| Initial dose,
200/400/800 | 1/4/1 | 1/3/1 | NS |
|
Primary/recurrent | 3/3 | 2/3 | NS |
miRNA microarray for liver cancer cell
lines and serum samples from advanced HCC patients
Total miRNA was extracted from liver cancer cell
lines using the Qiagen miRNeasy kit (Qiagen K.K.) according to the
instructions provided by the manufacturer. Each serum sample was
processed for total RNA extraction with the miRNeasy Mini Kit
(Qiagen) according to the manufacturer's instructions. The RNA
sample from both sets typically showed A260/280
ratios between 1.9 and 2.1 measured with an Agilent 2100
Bioanalyzer (Agilent Technologies).
After RNA quantification with an RNA 6000 Nano kit
(Agilent Technologies), the samples were labeled using a miRCURY
Hy3/Hy5 Power labeling kit and hybridized on a human miRNA Oligo
chip10, version 14.0 (Toray Industries). Scanning was conducted
with the 3D-Gene Scanner 3000 (Toray Industries). The 3D-Gene
extraction version 1.2 software (Toray Industries) was used to read
the raw intensity of the image. The raw data were analyzed with
GeneSpringGX v 10.0 (Agilent Technologies) to determine the change
in miRNA expression. The samples were frozen at −80°C within 4 h of
collection and thawed just before analysis.
Reverse transcription (RT)-PCR for
quantifying miRNA in liver cancer cells and circulating miRNA in
serum samples
Total miRNA was extracted from liver cancer cells
using the QIAGEN miRNeasy kit (Qiagen K.K.) for miRNA
quantification according to the instructions provided by the
manufacturer. Total RNA was reverse-transcribed using TaqMan
MicroRNA Reverse Transcription kit (Life Technologies Japan). RNU6B
was used as an internal control for relative quantification of
hsa-miR-30d.
Circulating miRNA was extracted from 200 µl of serum
samples using the Qiagen miRNeasy serum-plasma kit (Qiagen K.K.)
according to the instructions provided by the manufacturer. RNA was
reverse-transcribed using the TaqMan MicroRNA Reverse Transcription
kit (Life Technologies Japan) following the manufacturer's
instructions. Caenorhabditis elegans miR-39 (cel-miR-39) was
spiked in each sample as a control for the extraction and
amplification steps. Serum miRNA was amplified using primers and
probes provided by Applied Biosystems by the TaqMan MicroRNA assay,
according to the instructions provided by the manufacturer. The
relative expression of serum miRNA was calculated using the
comparative cycle quantification (Cq) method (2−ΔΔCq)
(18) with spiked cel-miR-39 as a
normalized internal control.
Statistical analysis
Replicate data were consolidated for each sample
group as follows; differences between the effective and
non-effective groups i) in vivo and ii) in vitro. The
data were organized using the hierarchical clustering and ANOVA
functions in the GeneSpring software. Hierarchical clustering was
performed using the clustering function (condition tree) and
Euclidean correlation as a distance metric. Two-way ANOVA with
Bonferroni's correction and asymptotic P-value (<0.05)
computation without any error correction on the samples were
conducted to search for the miRNAs with the most prominent
variation across the different groups. All analyzed data were
scaled by global normalization. The statistical significance of
differentially-expressed miRNAs was analyzed by Mann-Whitney U
test. All analyses were conducted using computer-assisted JMP8.0
(SAS Institute). Paired analysis between the groups was conducted
using the Student's t-test and Fisher's exact test. P<0.01
(Cluster analysis in vitro) and P<0.05 (Cluster analysis
in vivo) were considered to indicate a significant
difference between groups.
Results
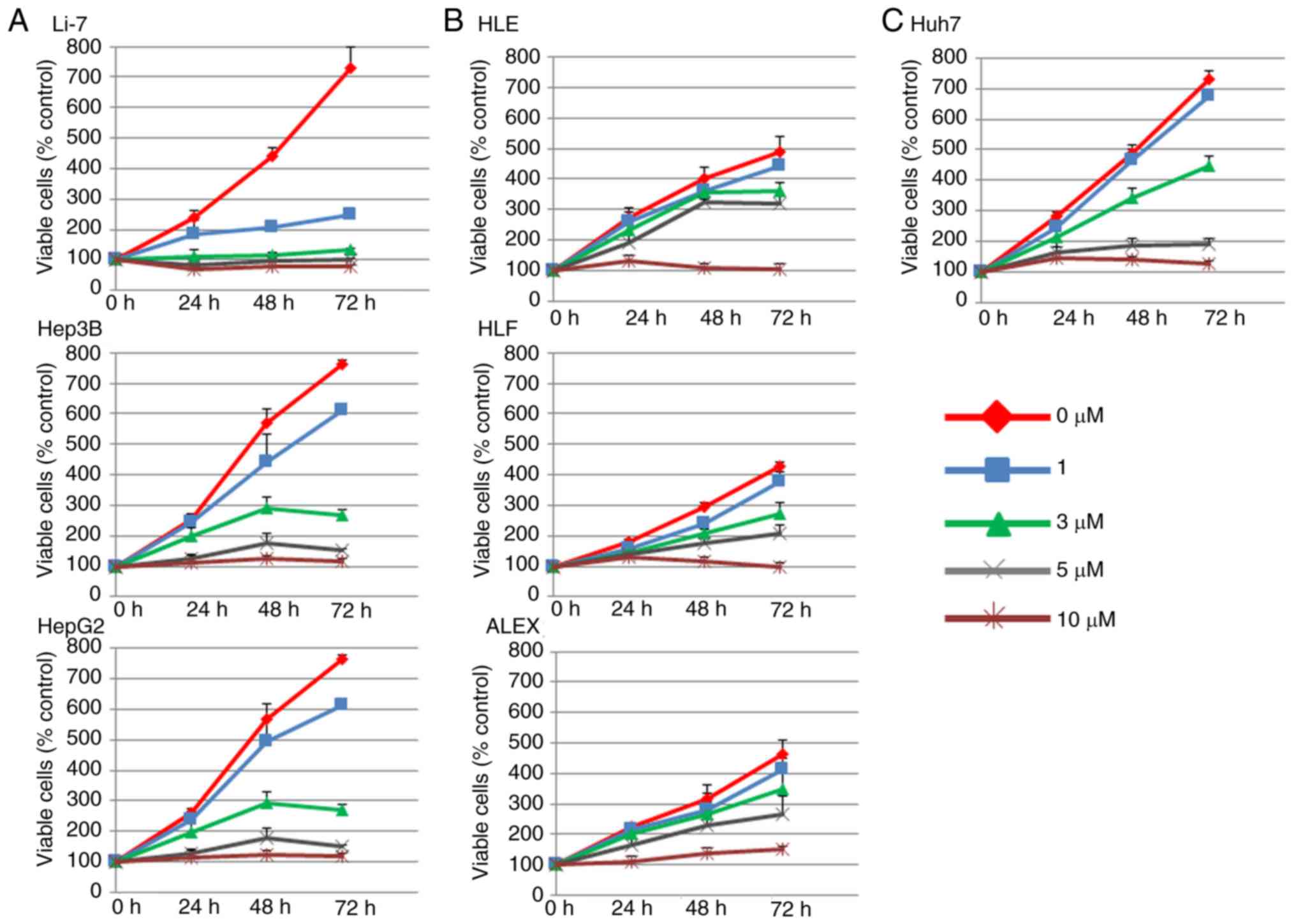
Sorafenib inhibits cell proliferation
of Li-7, Hep3B, HepG2, and Huh7, but not of HLE, HLF, and ALEX
cells
We examined the in vitro effects of sorafenib
on seven human liver cancer cell lines, Li-7, Hep3B, HepG2, HLE,
HLF, Alex and Huh7 by following the course of proliferation of each
of them for three days after sorafenib addition and evaluating the
direct relationship between the decrease of cell viability and the
inhibition of cell proliferation. Cells were cultured and with 0,
1, 3 or 10 µM sorafenib was added to the medium after 24 h. As
shown in Fig. 1, sorafenib strongly
inhibited the proliferation of Li-7, Hep3B, and HepG2 cells in a
dose- and time-dependent manner (sorafenib-effective group), but
not that of HLE, HLF, and ALEX cells (sorafenib non-effective
group). Huh7 cell growth was partially inhibited by sorafenib
(Fig. 1), so it was not included in
either group. These results demonstrated that sorafenib inhibits
the proliferation of certain human liver cancer cell lines.
Comparison of miRNA expression in
cancer cell lines between sorafenib-effective and non-effective
groups
A miRNA array was performed in all liver cancer cell
lines before sorafenib administration, and the results of the
sorafenib-effective group were compared to those of the
non-effective group. We examined the expression patterns of 2555
miRNAs extracted from the cell lines. The unsupervised hierarchical
clustering analysis, using Pearson's correlation, showed that the
sorafenib-effective group formed a cluster separated from that of
the non-effective group (Fig. 2).
Furthermore, 89 miRNAs were significantly differentially expressed
between the groups (Fig. 2).
Patient characteristics in the
sorafenib-effective and non-effective groups
We included the serum samples of 11 patients (9
males and 2 females with a median age of 69 years, ranging between
53 and 79 years) in the miRNA analysis. All patients received
sorafenib therapy. The parameter association between the effective
and non-effective groups is summarized in Table I. The analysis of age, sex, etiology,
tumor stage, Child-Pugh classification, vessel invasion, reason of
sorafenib administration, initial sorafenib dose, and primary or
recurrent cancer were conducted using various categories of the
Student's t-test and Fisher's exact test. No statistically
significant differences were observed between the effective and
non-effective groups regarding patient background.
Comparison of miRNA expression in
serum samples of HCC patients before sorafenib administration
between the sorafenib-effective and non-effective groups
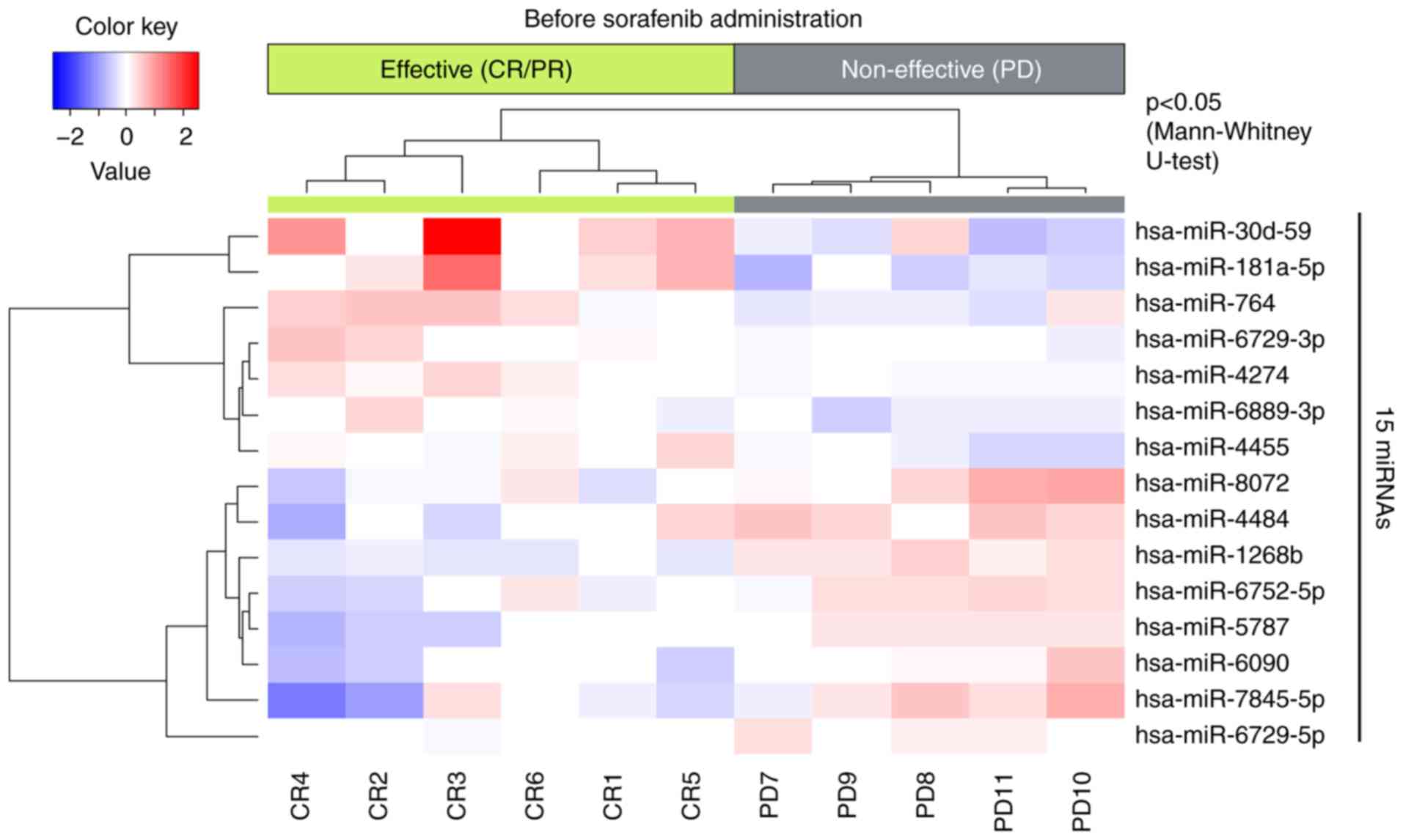
We also performed miRNA array using serum samples
from patients with HCC and compared the results of the
sorafenib-effective and non-effective groups (Fig. 3). The unsupervised hierarchical
clustering analysis, using Pearson's correlation, showed that the
effective group formed a cluster separated from that of the
non-effective group. Additionally, 10 miRNAs had significantly
different expression patterns in the sorafenib-effective group when
compared to the non-effective group.
Statistical analysis of miRNA
expression between cancer cell lines and serum samples of HCC
patients
We detected 3 miRNAs that were significantly changed
between the sorafenib-effective and non-effective groups jointly in
the in vitro and in vivo experiments (Table II). The hsa-miR-296-5p was
up-regulated in the effective groups of both the liver cancer cell
lines and the serum samples of HCC patients. The hsa-miR-6729-5p
was down-regulated in the effective groups of both sets of samples.
In contrast, hsa-miR-30d was down-regulated in the effective group
of the liver cancer cell lines but up-regulated in the serum
samples (Table II). Among 3 miRNAs,
hsa-miR-30d might not be leaked, but actively secreted from the
cancer cell.
 | Table II.Statistical analysis of miR
expressions between cancer cell lines and serum samples of patients
with hepatocellular carcinoma. |
Table II.
Statistical analysis of miR
expressions between cancer cell lines and serum samples of patients
with hepatocellular carcinoma.
|
| Cell | Serum |
|---|
|
|
|
|
|---|
|
|
| Fold Change |
| Fold Change |
|---|
|
|
|
|
|
|
|---|
| Targeted miR | P-values |
Effective/Non-effective | P-values |
Effective/Non-effective |
|---|
| hsa-miR-296-5p | 0.049 | 1.175 | 0.044 | 1.402 |
| hsa-miR-30d-5p | 0.0004 | 0.513 | 0.017 | 2.540 |
|
hsa-miR-6729-5p | 0.029 | 0.888 | 0.016 | 0.860 |
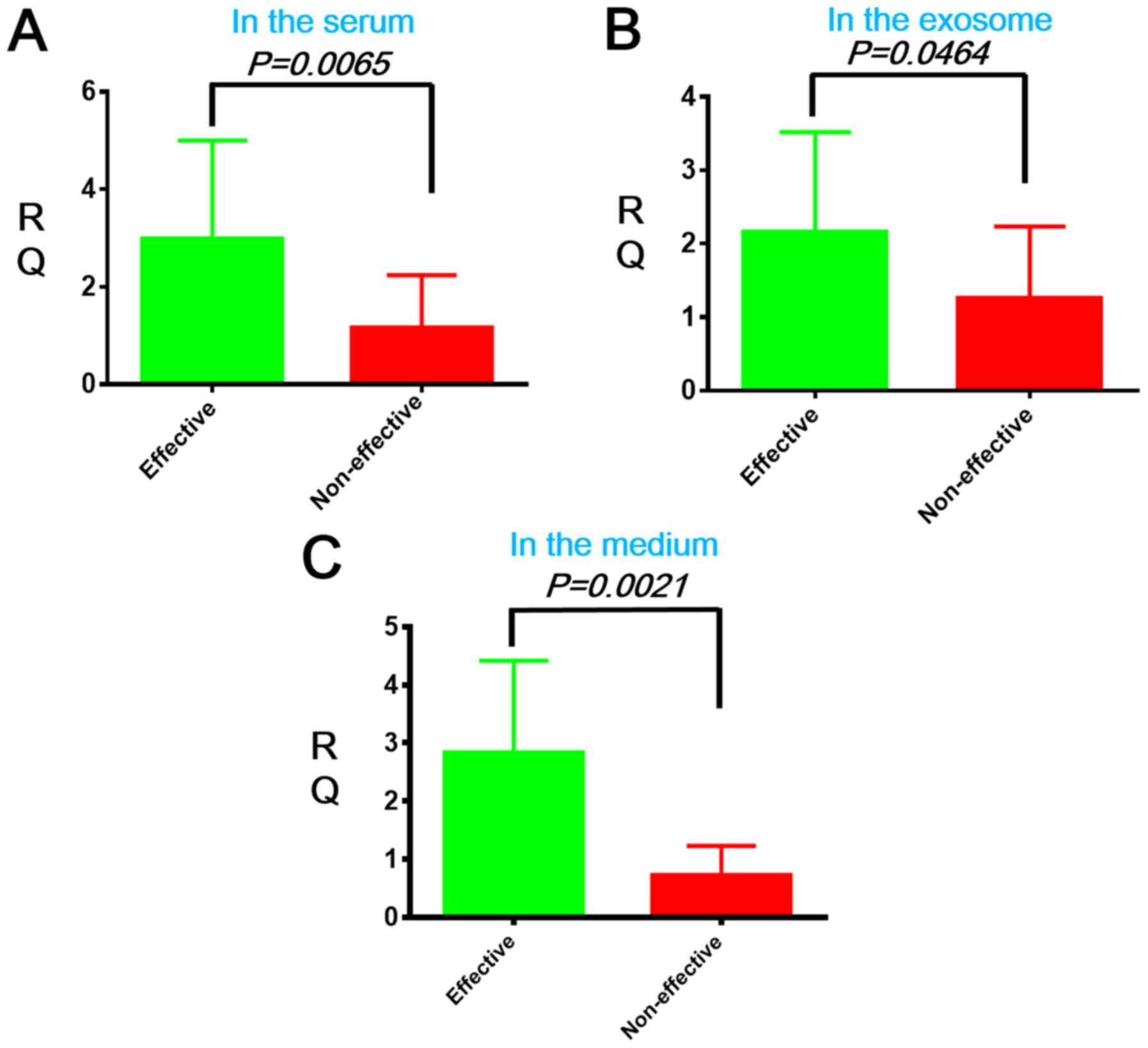
To determine if hsa-miR-30d was secreted from the
liver cancer cell to the extracellular fluid, we compared its
expression levels in the serum of patients with HCC before
sorafenib administration in the sorafenib-effective and
non-effective groups using real-time quantitative (RTq-PCR).
hsa-miR-30d expression was up-regulated in the serum and exosomes
of HCC patients of the effective group when compared to that of the
non-effective group (*P=0.0065 and #P=0.0464,
respectively, Fig. 4A and B). We
also quantified hsa-miR-30d expression in the medium of liver
cancer cells by RTq-PCR and found that HCC cells of the effective
group up-regulated hsa-miR-30d when compared to the non-effective
group ($P=0.0021, Fig.
4C).
Discussion
HCC is one of the most common forms of cancer
worldwide (19), and the prognosis
of patients in advanced stages remains very poor (5). Sorafenib, an oral multikinase inhibitor
that suppresses angiogenesis in advanced-stage HCC, was the first
drug approved to treat unresectable HCC (20). A recent clinical trial demonstrated
that sorafenib is an effective first-line systemic therapy for
patients with advanced HCC, but that only a subset of patients
responds to the treatment (20).
Regorafenib was recently approved as second-line systemic therapy
for the treatment of advanced HCC that has progressed after
sorafenib therapy (8). Therefore,
biomarkers that predict the efficacy of sorafenib therapy and allow
to switch smoothly from sorafenib to regorafenib are needed.
In the present study, we determined that circulating
hsa-miR-30d is a potential predictive biomarker for sorafenib
response when measured in the serum samples of HCC patients
(Figs. 3 and 4; Table
II). Recently, miRNAs such as miR-181a-5p and miR-339-5p, have
been shown to predict early response to sorafenib treatment
(21). Pretreatment-circulating
miRNAs such as miR-21, miR-18a, miR-221, miR-139-5p, miR-224, and
miR-10b-3p, were related to positive radiological responses in
sorafenib-treated HCC patients (22). Additionally, hsa-miR-30d is
associated with cell cycle arrest-related and apoptosis-induced
molecules (23). Therefore, the
down-regulation of hsa-miR-30d in HCC tissues might inhibit tumor
growth through cell cycle arrest and apoptosis. On the other hand,
secreted hsa-miR-30d might inhibit cell cycle arrest-related
molecules in adjacent HCC tissues and induce non-tumor cell
proliferation. These results, along with our data, suggest that
miRNAs, including hsa-miR-30d, can serve as pivotal predictive
biomarkers of the effectiveness of sorafenib therapy.
In addition, our study demonstrated that hsa-miR-30d
was down-regulated in liver cancer cells, but some cell lines such
as Li-7 and Hep3B, showed cell cycle inhibitory effects of
sorafenib. The relationship between hsa-miR-30d down-regulation and
direct inhibitory effects of sorafenib in liver cancer cell lines
remains unclear. In our present study, although low hsa-miR-30d
expression in the cells revealed inhibitory effects for Li-7 and
Hep3B cells, active secretion of hsa-miR-30d from liver cancer
cells was detected in the serum of HCC patients and medium of liver
cancer cell lines. In order to confirm if hsa-miR-30d is involved
in sorafenib therapy, we examined the hsa-miR-30d expression in the
medium of liver cancer cell lines 48 h after sorafenib
administration using real-time RT-PCR (Fig. S1). In Fig. S1, the hsa-miR-30d expression was
significantly increased in the mediums of effective group as
compared to those of non-effective group. This suggests our
hypothesis that hsa-miR-30d does not induce direct inhibitory
effect in the cell, but hsa-miR-30d secreted from liver cancer
cells might be involved in the sensitivity of sorafenib for liver
cancer cells.
The highlight of our study is that the expression
levels of hsa-miR-30d diverged between cancer cell lines and serum
samples in HCC patients. Miquelestorena-Standley et al
demonstrated that miR-152 was down-regulated in the serum of HCC
patients when compared to that of non-HCC patients, while miR-152
expression in tissue did not change between HCC and non-HCC
(24). In our study, hsa-miR-30d
secretion was actively regulated by liver cancer cells
independently from miRNA leakage due to liver damage, such as
inflammation and tumor necrosis (Fig.
4; Table II). The results of
Coenen-Stass et al (25)
support our data by showing that some circulating miRNAs might be
actively secreted and involved with the clinicopathological
features of patients with HCC (26).
It has also been demonstrated that miRNA leakage from hepatocytes,
as in the case of miR-122, can easily be influenced by liver
damage, including inflammation, steatosis, and fibrosis (27). Therefore, circulating miRNAs, such as
miR-122, which are involved in hepatocyte damage, are not useful
biomarkers to evaluate HCC treatment. hsa-miR-30d-5p could serve as
a better and more specific biomarker of HCC because it is
independent of inflammation and tumor necrosis.
The weakness of this study is the small number of
HCC patients and lack of functional analysis of hsa-miR-30d. We
could target several miRNAs for predictive markers for the
sorafenib therapy, but the relationship between those miRNAs and
clinical parameters, such as overall survival, progression free
survival, and disease control rate, remains still unknown. In
addition, direct inhibitory effect by hsa-miR-30d for liver cancer
cells also remains elusive. Future study enrolling larger number of
HCC patients and functional analysis of hsa-miR-30d will reveal
more new evidences for sorafenib therapy.
In conclusion, hsa-miR-30d is up-regulated in the
sera of HCC patients before sorafenib therapy and down-regulated in
liver cancer cells. The secretion of hsa-miR-30d is actively
controlled by HCC cells independent of miRNA leakage due to liver
damage. Therefore, hsa-30d-5p might serve as a new predictive
biomarker of sorafenib therapy outcomes in patients with HCC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Kayo Hirose, Ms.
Fuyuko Kokado and Ms. Keiko Fujikawa (Department of
Gastroenterology and Neurology, Kagawa University Faculty of
Medicine) for technical assistance during the microRNA array.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AM designed the concept of the present study. TK,
AM, HI, KF, JT, KT, MN, KO, TT, TN, HY, KK, KO, YS, TH and TM
performed outpatient service, obtained the informed consents,
preserved the samples, and performed analysis and interpretation of
data. AM, TK, KF, HI, AN and TM analyzed the serum microRNAs. TK
and AM performed the in vitro experiments. TK and AM wrote
the draft of the manuscript, and TM reviewed it. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the ethical principles of the Declaration of Helsinki and approved
by The Institutional Review Board of Kagawa University, Faculty of
Medicine (Heisei-22-063). Informed consent to use the clinical data
and samples for the present study was obtained from the patients or
their relatives. For patients who died and had no relatives listed
in their clinical records, opt-out methods were provided for the
relatives of the dead participants by publishing a summary of this
study on the university website.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNA
|
microRNA
|
|
HCC
|
hepatocellular carcinoma
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
References
|
1
|
Morishita A and Masaki T: miRNA in
hepatocellular carcinoma. Hepatol Res. 45:128–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lacaze L and Scotté M: Surgical treatment
of intra hepatic recurrence of hepatocellular carcinoma. World J
Hepatol. 7:1755–1760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Imamura H, Matsuyama Y, Tanaka E, Ohkubo
T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T,
Kawasaki S and Makuuchi M: Risk factors contributing to early and
late phase intrahepatic recurrence of hepatocellular carcinoma
after hepatectomy. J Hepatol. 38:200–207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: A global and regional perspective. Oncologist. 15 (Suppl
4):S5–S13. 2010. View Article : Google Scholar
|
|
6
|
Kudo M: Systemic therapy for
hepatocellular carcinoma: 2017 update. Oncology. 93 (Suppl
1):S135–S146. 2017. View Article : Google Scholar
|
|
7
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pelosof L, Lemery S, Casak S, Jiang X,
Rodriguez L, Pierre V, Bi Y, Liu J, Zirkelbach JF, Patel A, et al:
Benefit-risk summary of regorafenib for the treatment of patients
with advanced hepatocellular carcinoma that has progressed on
Sorafenib. Oncologist. 23:496–500. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Belghiti J and Kianmanesh R: Surgical
treatment of hepatocellular carcinoma. HPB (Oxford). 7:42–49. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masaki T: MicroRNA and hepatocellular
carcinoma. Hepatol Res. 39:751–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M
and Rajewsky N: Combinatorial microRNA target predictions. Nat
Genet. 37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gramantieri L, Ferracin M, Fornari F,
Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E,
Grazi GL, et al: Cyclin G1 is a target of miR-122a, a microRNA
frequently down-regulated in human hepatocellular carcinoma. Cancer
Res. 67:6092–6099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong QW, Lung RW, Law PT, Lai PB, Chan KY,
To KF and Wong N: MicroRNA-223 is commonly repressed in
hepatocellular carcinoma and potentiates expression of Stathmin1.
Gastroenterology. 135:257–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varnholt H, Drebber U, Schulze F,
Wedemeyer I, Schirmacher P, Dienes HP and Odenthal M: MicroRNA gene
expression profile of hepatitis C virus-associated hepatocellular
carcinoma. Hepatology. 47:1223–1232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaira V, Roncalli M, Carnaghi C, Faversani
A, Maggioni M, Augello C, Rimassa L, Pressiani T, Spagnuolo G, Di
Tommaso L, et al: MicroRNA-425-3p predicts response to sorafenib
therapy in patients with hepatocellular carcinoma. Liver Int.
35:1077–1086. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gyöngyösi B, Végh É, Járay B, Székely E,
Fassan M, Bodoky G, Schaff Z and Kiss A: Pretreatment MicroRNA
level and outcome in Sorafenib-treated hepatocellular carcinoma. J
Histochem Cytochem. 62:547–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kane RC, Farrell AT, Madabushi R, Booth B,
Chattopadhyay S, Sridhara R, Justice R and Pazdur R: Sorafenib for
the treatment of unresectable hepatocellular carcinoma. Oncologist.
14:95–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishida N, Arizumi T, Hagiwara S, Ida H,
Sakurai T and Kudo M: MicroRNAs for the prediction of early
response to sorafenib treatment in human hepatocellular carcinoma.
Liver Cancer. 6:113–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoon EL, Yeon JE, Ko E, Lee HJ, Je JH, Yoo
YJ, Kang SH, Suh SJ, Kim JH, Seo YS, et al: An explorative analysis
for the role of serum miR-10b-3p levels in predicting response to
sorafenib in patients with advanced hepatocellular carcinoma. J
Korean Med Sci. 32:212–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Díez-Planelles C, Sánchez-Lozano P, Crespo
MC, Gil-Zamorano J, Ribacoba R, González N, Suárez E,
Martínez-Descals A, Martínez-Camblor P, Álvarez V, et al:
Circulating microRNAs in Huntington's disease: Emerging mediators
in metabolic impairment. Pharmacol Res. 108:102–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miquelestorena-Standley E, Tallet A,
Collin C, Piver E, De Muret A, Salamé E, Bourlier P, Kervarrec T,
Guyétant S and Pagès JC: Interest of variations in microRNA-152 and
−122 in a series of hepatocellular carcinomas related to hepatitis
C virus infection. Hepatol Res. 48:566–573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coenen-Stass AM, Betts CA, Lee YF, Mäger
I, Turunen MP, El Andaloussi S, Morgan JE, Wood MJ and Roberts TC:
Selective release of muscle-specific, extracellular microRNAs
during myogenic differentiation. Hum Mol Genet. 25:3960–3974. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu AM, Yao TJ, Wang W, Wong KF, Lee NP,
Fan ST, Poon RT, Gao C and Luk JM: Circulating miR-15b and miR-130b
in serum as potential markers for detecting hepatocellular
carcinoma: A retrospective cohort study. BMJ Open. 2:e0008252012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakao K, Miyaaki H and Ichikawa T:
Antitumor function of microRNA-122 against hepatocellular
carcinoma. J Gastroenterol. 49:589–593. 2014. View Article : Google Scholar : PubMed/NCBI
|