Introduction
Melanoma originates from neural crest of epiblast
and is a malignant melanoma originating from the pigmented areas of
the skin (cutaneous melanoma elaborated in this study), mucosa, eye
and the central nervous system (1,2). The
pathogenesis of melanoma is still unclear. Several studies have
reported that melanoma is closely related to factors, such as race,
large amount of ultraviolet radiation, abuse of estrogen drugs and
immunodeficiency (3–5). Due to various carcinogenic factors,
melanoma is one of the most invasive and migratory malignant solid
tumors that spreads rapidly and may lead to the death of a great
number of patients after several months of diagnosis (6,7).
Currently, surgery is still an important treatment for melanoma in
patients insensitive to radiotherapy or chemotherapy (8,9). Recent
studies have suggested that gene therapy can provide a new
direction for the treatment of melanoma, and therefore gene therapy
has become a promising research field worldwide (10–12). The
pathogenesis of melanoma is a complex multi-step process involving
numerous genes (13). The malignant
transformation process of normal cells into tumor cells involves
various gene changes (14,15). In malignant tumors, the expression of
tumor suppressor genes is decreased or the function is absent
(16). It is worth noting that the
threat of cancer to human life is mainly attributed to malignant
growth, due to invasion and metastasis, rather than the growth in
the primary site. Melanoma is one of the most invasive and
migratory malignant solid tumors (17). Degradation of extracellular matrix
and basement membrane is a key step in the complex process of
melanoma leaving the primary site, invading the surrounding tissues
and passing through the basement membrane to enter the lymph or
blood circulation and transfer to newly diffused tissues (18,19).
Epithelial-mesenchymal transition (EMT) was proposed
by Greenburg and Hay in 1982 (20).
EMT refers to the phenomenon of epithelial cells transforming into
mesenchymal cells under specific physiological and pathological
conditions (21,22). Cells may lose their connection and
polarity, change in morphology, and their migration ability may be
enhanced, thus gaining invasion and metastasis abilities (23,24). It
has been reported that although melanocytes are not traditional
epithelial components, there is EMT in the process of melanoma
invading dermal stroma (25). EMT
has been reported in previous studies to be involved in the
invasion and metastasis of melanoma (26–28).
During EMT, there are obvious differences in the expression of
EMT-related molecules (29), whose
comparison in melanocytes and different melanoma cell lines
(30) has led to the discovery of
abnormal expression of epithelial cadherin (E-cadherin),
neurotrophic cadherin (N-cadherin) and vimentin in melanocytes
(31,32).
JAK/STAT signaling pathway is a widely used signal
transduction pathway of hematopoietic growth factor, which mediates
a number of physiological processes of cells (33). JAK/STAT signaling pathway may play
critical roles in ontogenesis, hematopoietic regulation and immune
response (34). Persistent
activation of JAK/STAT signaling pathway is related to the
occurrence of various human tumors (35,36).
Current studies have confirmed that external stimulators are
closely associated with the regulation of various cell behaviors
and the activation of signal transduction pathways in cells
(33,37,38).
JAK/STAT signal transduction pathway is one of the most commonly
studied pathways and plays an important role in the process of cell
signal transduction. JAK is a class of intracytoplasmic
non-receptor soluble tyrosine protein kinases, including the four
subfamilies of JAK1, JAK2, JAK3 and TYK2 (39). STAT is a cytoplasmic protein that can
bind to the DNA of the target gene regulatory region, which is the
downstream substrate of JAK (40).
STAT family includes seven members, i.e., STAT1-4, STAT5A, STAT5B
and STAT6 (41). After tyrosine
phosphorylation of JAK family, STATs in cytoplasm are recruited and
phosphorylated to form dimers into the nucleus (42). In the nucleus, STATs bind to the
promoter of target genes, and then activate gene expression, so as
to regulate physiological and pathological reactions of cells, and
function significantly in the process of cell growth, activation,
differentiation and apoptosis (43).
Moreover, AG490 is a selective inhibitor of JAK2 tyrosine
phosphorylation (44) which can
effectively block the downstream signal transduction and STAT3
activation, and then inhibit corresponding physiological and
pathological effects.
The role of JAK/STAT signaling pathway has been
recognized in multiple human malignancies. However, there are few
studies on the aforementioned targets in cutaneous melanoma.
Furthermore, chemokin CXCL8, also known as interleukin-8, is a
pro-inflammatory molecule that functions significantly in tumor
microenvironment and has been screened to be associated with the
development of various tumors, considering its roles in directing
neutrophils and oligodendrocytes to combat infection and tumor
cells in the progression of metastasis. The present study was
carried out to investigate the effect of CXCL8 silencing-mediated
activation of JAK-STAT signaling pathway on EMT of human cutaneous
melanoma cells. This study is expected to provide potential
reference for target gene screening during genetic therapy and
updated therapeutic approaches for inhibiting the development of
cutaneous melanoma.
Patients and methods
Reagents and equipment
Laboratory reagents: 95% medical ethanol and
absolute ethanol (Tianjin Fuyu Fine Chemical Co., Ltd.); xylene
(Shanghai YuanMu Biological Technology Co., Ltd.); polyformaldehyde
(Sigma-Aldrich; Merck KGaA); hematoxylin and eosin (H&E;
Beijing Solarbio Science & Technology Co., Ltd.); protein
denaturant (Shanghai Sibas Biotechnology Development Co., Ltd.),
primary polyclonal antibody CXCL8 (Abcam), goat anti-rabbit IgG
antibody (Wuhan Boster Biological Technology, Ltd.), ECL kit
(Amersham; GE Healthcare). DMEM (HyClone; GE Healthcare), PBS and
trypsin (both from Beijing Solarbio Science & Technology Co.,
Ltd.), BCA kit (23250; Thermo Fisher Scientific, Inc.), Annexin
V-FITC/PI apoptotic detection kit (Nanjing KeyGen Biotech Co.,
Ltd.).
Main experimental equipment: Electronic analytical
balance (Shanghai Ping Xuan Scientific Instruments, Ltd.); RM2126
paraffin slicing machine (Shanghai Leica Instruments Ltd.); Olympus
fluorescent microscope and inverted fluorescent microscope (both
from Olympus Corporation); gel imaging system (ChemiDoc MP; Bio-Rad
Laboratories, Inc.); pipette (0.5-10 µl, 20-200 µl,
Research® plus 100-1,000 µl; Beijing Gilson Science
& Technology Co., Ltd.).
Research subjects
Melanoma tissues were collected from 80 patients
with cutaneous melanoma admitted to the General Hospital of Ningxia
Medical University (Yinchuan, China) from January 2016 to January
2018. The collected melanoma tissues served as the experimental
group and the adjacent normal tissues as the control group. All
cases were confirmed by histopathology. The experimental group
consisted of 53 males and 27 females, with an average age of
61.55±9.04 years, and the age at onset ranged from 32 to 80 years.
Among the 80 cases, there were 50 cases of superficial diffusion
and 30 cases of nodular metastasis, 41 cases of non-lymph node
metastasis and 39 cases of lymph node metastasis.
Inclusion criteria of eligible cases: i) patients
who were diagnosed with cutaneous melanoma by histopathology; ii)
patients with complete medical records; iii) patients with no
previous history of radiotherapy and chemotherapy. Exclusion
criteria: i) Patients who were not treated in the aforementioned
hospital after diagnosis; ii) patients who had a history of
treatment in other hospitals; iii) patients with other malignant
tumors. Tumor tissues in the center of the lesion and non-tumor
tissues adjacent to the cancer (5 cm) were collected and frozen in
cryopreservation tubes. Next, the tumor tissues were quickly frozen
in liquid nitrogen tanks (−196°C). The samples were stored in a
refrigerator at −80°C. The study was approved by the Ethics
Committee of the General Hospital of Ningxia Medical University.
Patients who participated in this research had complete clinical
data. Signed informed consents were obtained from the patients or
their guardians.
H&E staining
During H&E staining, human cutaneous melanoma
tissues and adjacent normal tissues were divided into melanoma
group and normal control group, respectively. The main reagent
formulations were as follows: i) Hematoxylin staining: Hematoxylin
(1 g), ethanol (10 ml), distilled water (200 ml), potassium alum
(20 g), HgO (0.5 g); ii) eosin staining: Eosin (2.5-5 g), distilled
water (500 ml); iii) hydrochloric acid alcohol differentiation
solution: 0.5 ml hydrochloric acid and 100 ml 75% alcohol to mix.
a) Tissue sample dewaxing: The tissue sample slices were placed in
xylene, soaked fully for 10 min, then replaced in xylene and
continuously soaked for 10 min. b) Hydration of tissue samples: The
tissue samples soaked in xylene were first immersed in absolute
ethanol for 5 min, and then in 95, 85 and 70% ethanol for 5 min for
complete hydration. c) Hematoxylin staining, differentiation, and
bluing: The dewaxed tissue samples were placed in hematoxylin
solution, stained for 5 min, and washed with tap water for 5 min;
the stained tissue samples were placed in alcohol hydrochloride
differentiation solution for several seconds; the slice was washed
for 10 min with tap water, and the nuclear staining was observed
under the microscope. d) Eosin staining: Tissue samples were placed
in 0.5% eosin solution and stained for 5 min. v) Dehydration: After
H&E staining, the tissue samples were put into 95% ethanol I,
95% ethanol II, 100% ethanol I and 100% ethanol II for 2 min,
respectively. e) Tissue sample slice air-drying and sealing: The
dehydrated tissue sample slice was soaked in xylene twice for 4 min
each time, and then the tissue sample slice was dried and sealed
with neutral gum. f) Finally, the slices were observed and
photographed under a microscope. With the morphological image
analysis system, different groups were analyzed (magnification,
×400), and the images were collected randomly. The experiment was
repeated 3 times.
RT-qPCR
Some melanoma tissues and adjacent normal tissues
were ground into fine powder by adding liquid nitrogen. Total RNA
was extracted using TRIzol® reagent (Sangon Biotech Co.,
Ltd.), and RNA concentration and purity were determined. Total RNA
was reverse transcribed into cDNA (total volume, 20 µl) using the
PrimeScript™ RT reagent kit (Takara Bio, Inc.), according to the
manufacturer's protocol. The reaction conditions of the reverse
transcription of RNA into cDNA were: Incubation at 25°C for 10 min,
additional incubation at 42°C for 50 min, and heating at 95°C for 5
min. cDNA was diluted with DEPC water and blended. Fluorescent
RT-qPCR was performed following the manufacturer's protocol. During
RT-qPCR double-labeled fluorescent probes (5′-end of the
fluorescent reporting group, 3′-end of the fluorescent quenching
group) were used, in which the 5′-end was
6-carboxy-fluorescene-labeled fluorescence group and the 3′-end was
6-carboxytetramethylrhodamine-labeled fluorescence group (both from
Applied Biosystems; Thermo Fisher Scientific, Inc.). The primers of
CXCL8, JAK2, STAT3, E-cadherin, N-cadherin, vimentin and β-actin
were designed and synthesized by Wuhan Boster Biological
Technology, Ltd.: CXCL8 forward (5′-3′), ATGGCT GCTGAACCAGTAGA and
reverse (5′-3′), CTAGTCTTC GTTTTGAACAG; JAK2 forward (5′-3′),
GGGTGTTCGCGT CGCCACTT and reverse (5′-3′), CAGATCGGGCGACCA GAGCG;
STAT3 forward (5′-3′), GCGGCAGTTTCTGGC CCCTT and reverse (5′-3′),
CGGGCCACAATCCGGGCAAT; E-cadherin forward primer (5′-3′),
ATGCTGATGCCCCCA ATACC and reverse (5′-3′), ATCTTGCCAGGTCCTTTGCT;
N-cadherin forward (5′-3′), CCGGAGAACAGTCTCCAACTC and reverse
(5′-3′), CCCACAAACAGCACCAGTC; vimentin forward (5′-3′),
GGTGCAATCGTGATCTGGGA and reverse (5′-3′), GTCTTTGCTCGAATGTGCGG; and
β-actin forward primer (5′-3′), CCCAGCACAATGAAGATCAAGATCAT and
reverse (5′-3′), ATCTGCTGGAAGGTGGACAGCGA. The reaction system
volume was 25 µl and was consisted of cDNA (1 µl), 10X PCR buffer
(2.5 µl), 10 mmol/l dNTPs (2 µl), PCR upstream primers (1 µl), PCR
downstream primers (1 µl), Taq DNA polymerase (1 µl), and deionized
water (16.5 µl). Fluorescent RT-qPCR was performed in ABI
PRISM® 7300 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction conditions were as follows:
Pre-denaturation at 95°C for 5 min, denaturation at 94°C for 1 min,
annealing at 54°C for 45 sec, extension at 72°C for 1 min, in a
total of 30 cycles, followed by extension at 72°C for 10 min.
β-actin was used as internal reference. All primers were designed
and synthesized by Wuhan Boster Biological Technology, Ltd. The
2−∆∆Cq method (45) was
used to express the multiplier relationship between the target gene
expression in the experimental group and the control group. The
experiment was repeated 3 times independently.
Western blot analysis
Some melanoma and adjacent normal tissues were
ground with liquid nitrogen into a homogeneous fine powder and 1 ml
of modified RIPA lysis buffer (Beyotime Institute of Biotechnology)
was added for protein extraction. Melanoma and adjacent normal
tissues were ground into homogenate in an ice bath. Protein lysate
was added to the melanoma tissues at 4°C for 30 min and shaken
every 10 min for the extraction of total protein. According to the
manufacturer's instructions of the BCA kit, the content of total
protein was determined and frozen at −80°C. Protein (50 µg) was
collected and mixed with denaturant, boiled for 10 min for
denaturation, and then separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After
electrophoresis, the protein was transferred from the SDS-PAGE gel
to a nitrocellulose membranes using the electrical transfer method.
Nitrate cellulose membranes were blocked overnight at 4°C in PBST
(PBS + Tween-20) containing 10% skimmed milk powder. The membranes
were rinsed with PBST (5 min for 3 times) and incubated overnight
with the primary antibodies: rabbit polyclonal CXCL8 antibody
(1:1,000; ab84995), rabbit monoclonal JAK2 antibody (1:1,000;
ab108596), rabbit anti-STAT3 antibody (1:100, ab76315), rabbit
monoclonal E-cadherin antibody (1:1,000; ab194982), rabbit
monoclonal vimentin antibody (1:1,000; ab92547) and rabbit
polyclonal N-cadherin antibody (1:1,000; ab76057) (all from Abcam),
respectively, followed by PBS washing 3 times at room temperature,
5 min each. Next, the membranes were incubated with goat anti-mouse
IgG antibody labeled with horseradish peroxidase (1:2,000; ab6789;
Wuhan Boster Biological Technology, Ltd.) for 120 min at room
temperature. The reference gene was mouse monoclonal β-actin
antibody (1:500; ab8226; Abcam). The membranes were washed 3 times
with TBST, and ECL kit was used for luminescence reaction. Protein
bands were visualized using Bio-Rad Image Reader imaging analyzer
(Bio-Rad Laboratories, Inc.). Quantity One software 4.6.6 software
(Bio-Rad Laboratories, Inc.) was used for the analysis of the gray
value of the bands. The experiment was repeated three times.
Screening of cell lines, cell culture
and transfection
Human melanoma cell lines A375 (cat. no. AC148),
SK-MEL-28 (cat. no. AC339802) and SK-MEL-1 (cat. no. AC100491)
(Shanghai Institute of Cell Research, Chinese Academy of Sciences)
were selected for subculture and were inoculated in culture medium.
The cells were cultured in a thermostat at 37°C and 5%
CO2. The medium consisted of 10% fetal bovine serum,
RPMI-1640 medium and penicillin-streptomycin. Every 24-48 h, the
medium was replaced, digested and passaged with 0.25% trypsin, and
the cells in logarithmic phase were selected for the experiment.
The expression of CXCL8 in A375, SK-MEL-28 and SK-MEL-1 cells was
detected by fluorescent RT-qPCR, and the optimal cell line was
screened out for subsequent experiments.
Cells were divided into five groups: Blank group
(human cutaneous melanoma cells), NC group (human cutaneous
melanoma cells + blank vector plasmid transfection), CXCL8 siRNA
group (human cutaneous melanoma cells + CXCL8 silent expression
vector plasmid transfection), AG490 group (human cutaneous melanoma
cells + JAK-STAT signal pathway inhibitor transfection), CXCL8
siRNA + AG490 group (human cutaneous melanoma cells + JAK-STAT
signaling pathway inhibitor + CXCL8 silent expression vector
plasmid transfection). Transfection plasmids were purchased from
Thermo Fisher Scientific, Inc., and JAK-STAT signaling pathway
inhibitor was purchased from Beijing Baioleibo Technology Co., Ltd.
Cells were inoculated into 6-well plates 24 h before transfection.
When the cell confluency reached ~50-60%, the cells were
transfected into a human cutaneous melanoma cell line by
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were transfected for 6 h and then
cultured for 48 h, before collected for subsequent experiments.
CCK-8 assay for the detection of cell
proliferation after transfection
When the cell growth density reached ~80%, all the
cells in logarithmic growth phase were washed twice with PBS
solution and digested by 0.25% trypsin to form a single cell
suspension. After counting, the cells with adjusted density of
3×103 were inoculated into 96-well plates with a volume
of 200 µl/well, with 6 replicates made for each sample. Cells were
evenly dispersed by gentle shaking. Subsequently, the 96-well
plates were incubated at 37°C and 5% CO2 saturated
humidity. The plates were taken out at 24, 48, 72 and 96 h,
respectively, followed by the addition of 10 µl CCK-8
(Sigma-Aldrich; Merck KGaA) per well for continuous culture for 3
h. The absorbance values (A values) of each well were read at 450
nm by a microplate reader. Each experiment was repeated 3 times.
Cell viability curves were plotted with time as abscissa and A
value as ordinate.
Flow cytometry
For the apoptosis detection by flow cytometry, human
cutaneous melanoma cell lines were selected. The experimental
groups were the Blank group, NC group, CXCL8 siRNA group, AG490
group and CXCL8 siRNA + AG490 group. i) Cell collection: Adherent
cells were digested and directly collected into 5 ml centrifugal
tubes. The number of cells per sample was 1 to 5×106/ml.
After centrifugation at 800 × g for 5 min, the culture medium was
abandoned. ii) Preparation of cell suspension: Cells were washed
with PBS and centrifuged at 800 × g for 5 min, and the supernatant
was discarded. iii) Annexin V-FITC/PI double labeled staining by
using a flow cytometer (Thermo Fisher Scientific, Inc.), with the
apoptotic rate analyzed by ModFit LT 3.0 software (Verity Software
House, Inc): 500 µl cells were suspended by a binding buffer, 5 µl
PI and 5 µl Annexin V-FITC were added to each tube, and the cells
were incubated at room temperature in the dark for 15 min. iv)
Annexin V-FITC and PI fluorescence were detected by flow cytometry,
and apoptosis was detected.
Statistical analysis
Statistical data were processed and analyzed by the
SPSS 21.0 statistical software (IBM Corp.). Normal distribution and
homogeneous tests of variance were performed for all data. The
parameters of the experimental measurement data conforming to a
normal distribution were expressed as the mean ± standard
deviation. Data were analyzed between two groups using the
independent samples t-test. One-way analysis of variance (ANOVA)
was used for comparisons among groups, followed by the Bonferroni
post hoc. P<0.05 was considered to indicate a statistically
significant difference.
Results
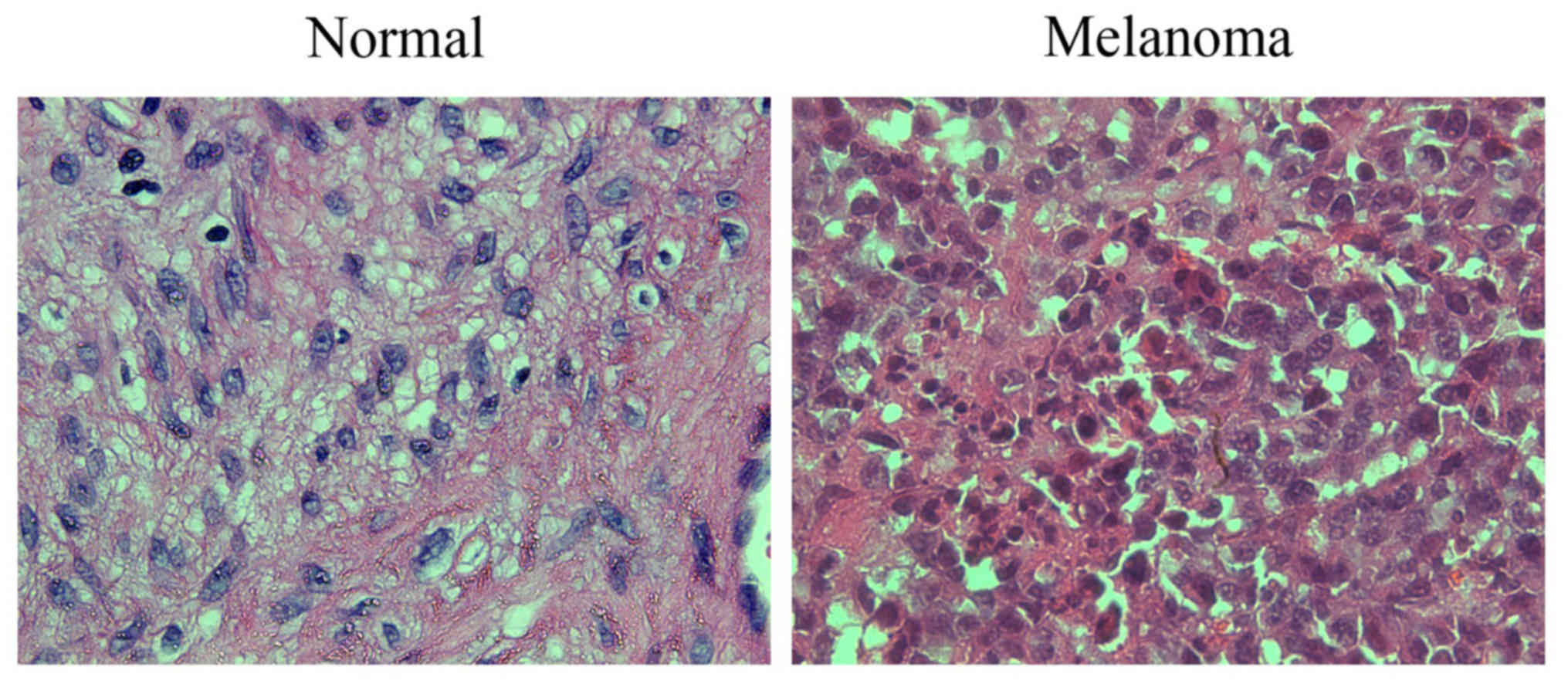
H&E staining in human cutaneous
melanoma
H&E staining revealed that compared with the
adjacent normal tissues, the cells in the melanoma tissues
presented obvious atypia, had oval appearance with different shapes
and sizes in most cases, indicating compact arrangement, obvious
nucleoli, mitotic figures, large cytoplasm, diffuse distribution,
and increased production of melanin granules between and within
cells (Fig. 1).
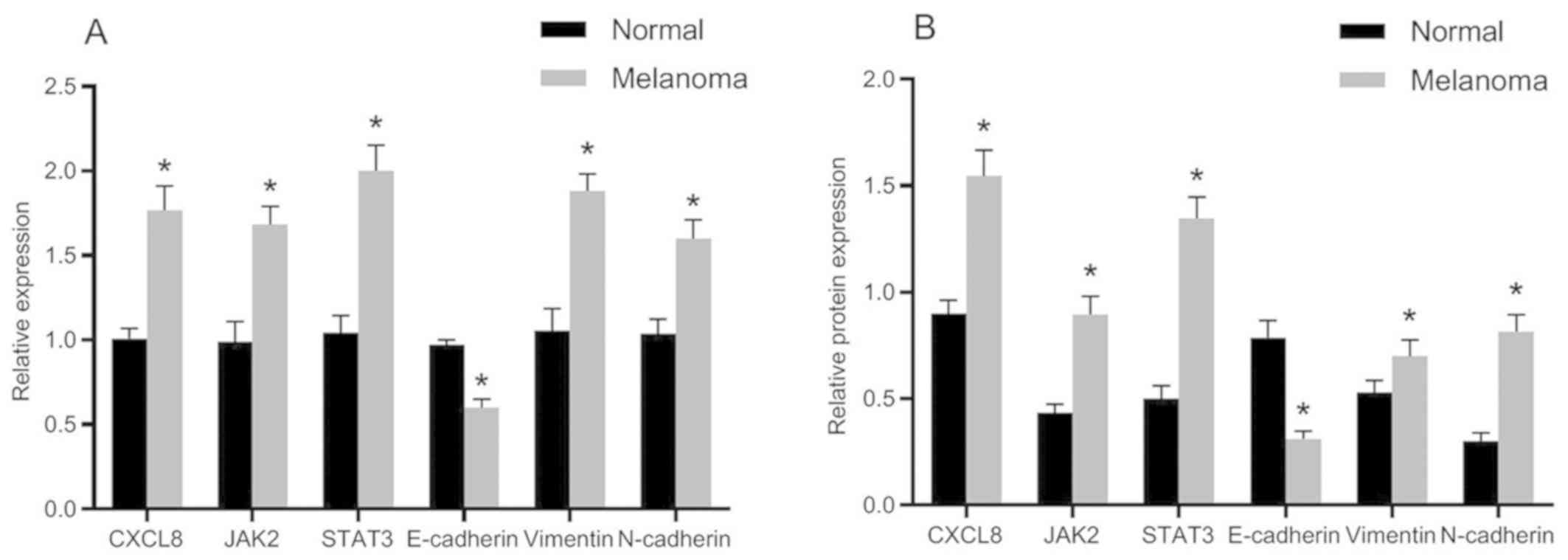
mRNA and protein expression of related
genes
The mRNA and protein expression levels of related
genes in normal and melanoma tissues are presented in Fig. 2. Compared with the adjacent normal
tissues, the mRNA and protein expression levels of E-cadherin were
significantly decreased in melanoma tissues, whereas the mRNA and
protein expression levels of CXCL8, JAK2, STAT3, vimentin and
N-cadherin were obviously increased. The differences were
statistically significant (P<0.05).
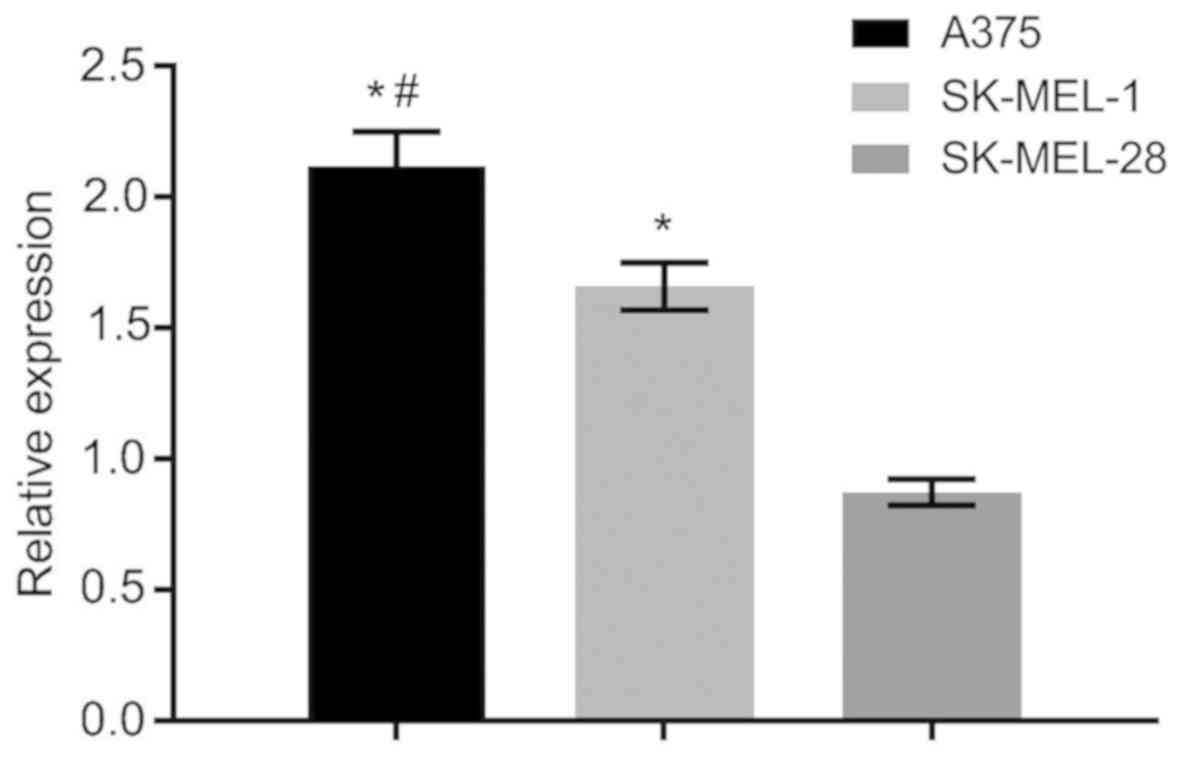
Screening of cell lines
The expression of CXCL8 gene in A375 human cutaneous
melanoma cells was the highest compared with that in SK-MEL-28 and
SK-MEL-1 human cutaneous melanoma cells, with statistically
significant differences (P<0.05) (Fig. 3). Thus, A375 human cutaneous melanoma
cells were screened out for subsequent experiments.
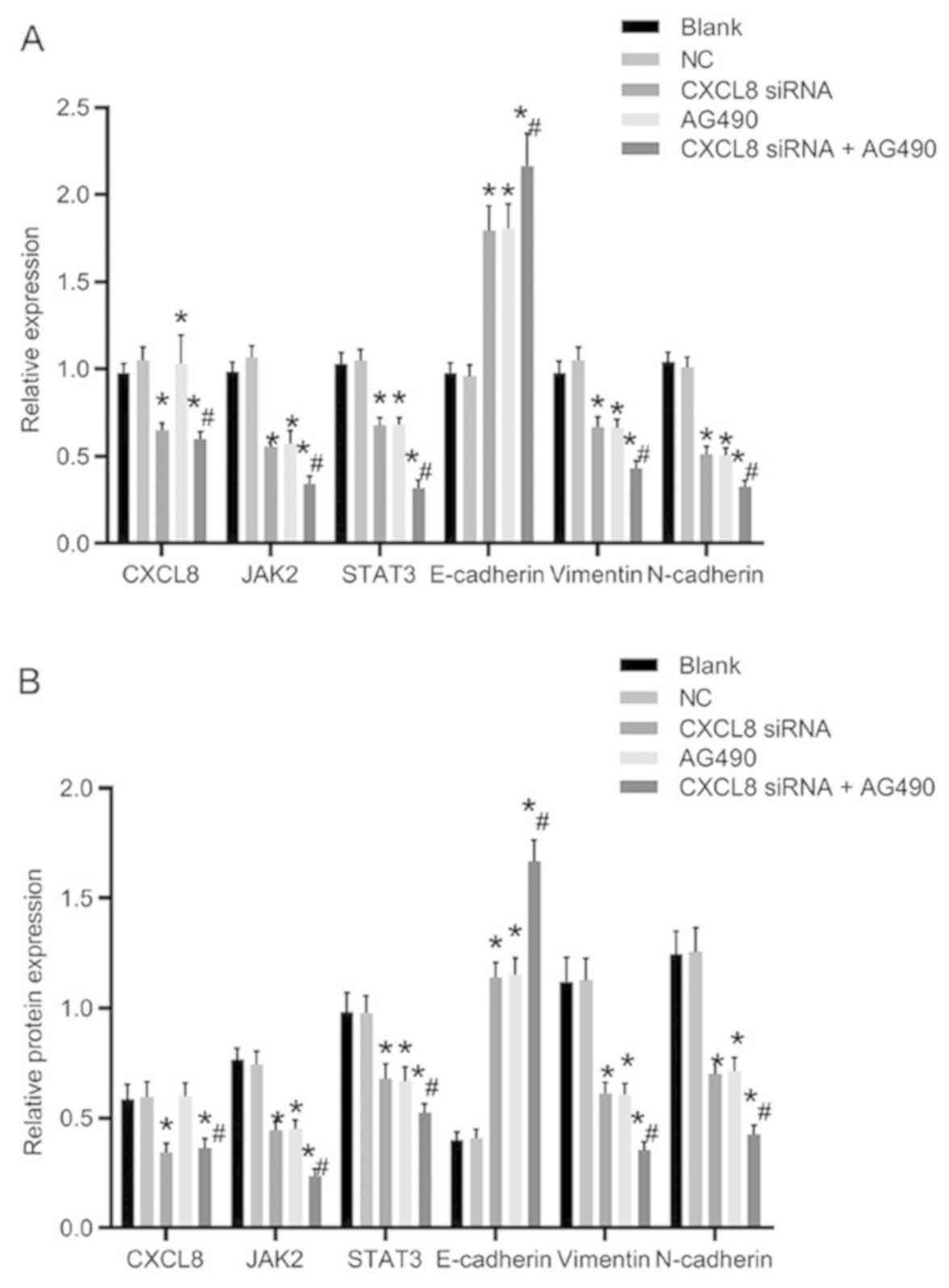
mRNA and protein expression of related
genes after cell transfection
The mRNA and protein expression levels of related
genes after transfection are presented in Fig. 4. The CXCL8 siRNA group and CXCL8
siRNA + AG490 group showed significantly decreased CXCL8 expression
compared with the blank group (P<0.05), whereas there was no
significant difference in the CXCL8 expression compared with the NC
and AG490 groups (P>0.05). Compared with the blank group, the
expression levels of JAK2, STAT3, vimentin and N-cadherin in the
CXCL8 siRNA group, AG490 group and CXCL8 siRNA + AG490 group were
significantly decreased, whereas the expression level of E-cadherin
was increased (P<0.05). There was no obvious difference in the
expression levels of the related genes compared with those in the
CXCL8 siRNA group and AG490 group (P>0.05). Compared with CXCL8
siRNA group, the expression levels of JAK2, STAT3, vimentin and
N-cadherin in CXCL8 siRNA + AG490 group were obviously decreased,
whereas the E-cadherin expression was significantly increased
(P<0.05).
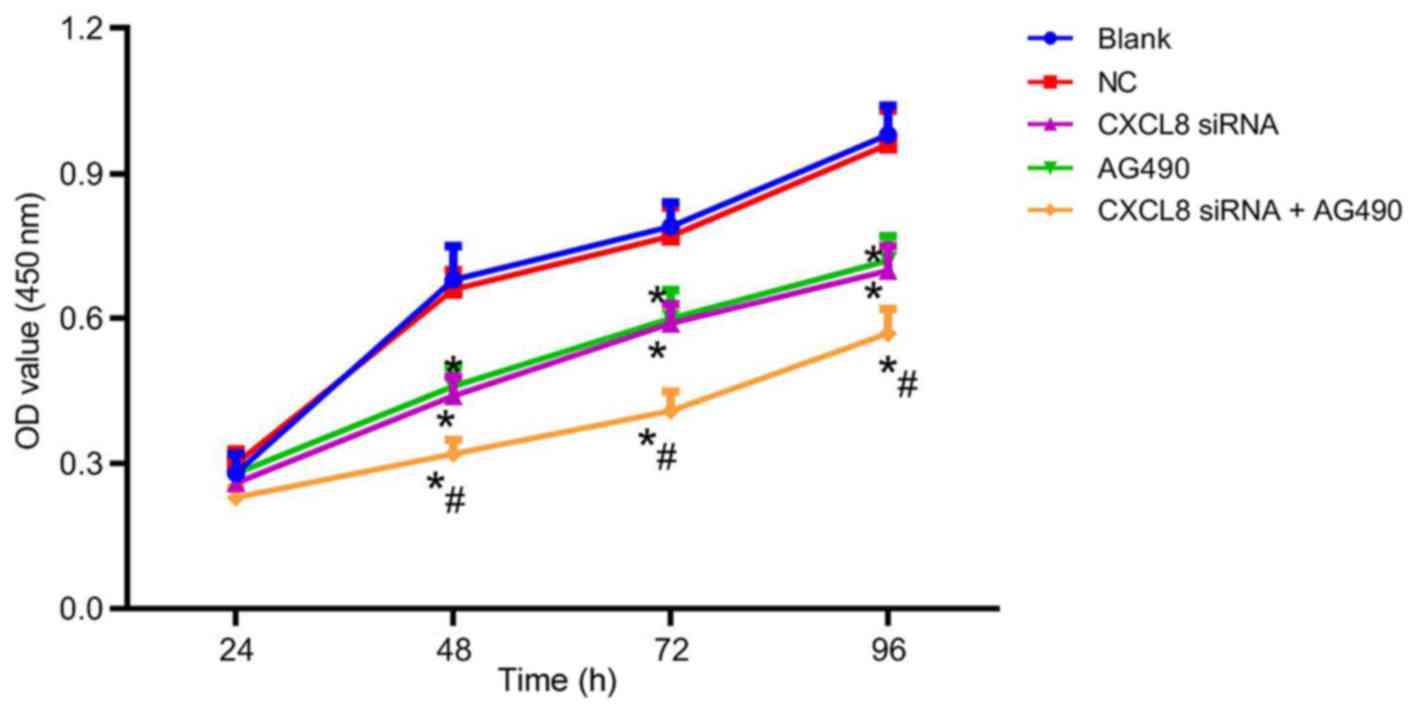
Changes of cell proliferation ability
after cell transfection
After cell transfection, the cell proliferation
ability of each group is presented in Fig. 5. There was no obvious difference in
cell proliferation among groups at 24 h (P>0.05). At 48, 72 and
96 h, the cell proliferation ability in the CXCL8 siRNA group,
AG490 group and CXCL8 siRNA + AG490 group was decreased compared
with that in the blank group (P<0.05). There was no significant
difference in the cell proliferation of the NC group at the
different time-points (P>0.05); and no significant difference
was presented between the cell proliferation ability of the CXCL8
siRNA group and AG490 group at the different time-points
(P>0.05). Compared with CXCL8 siRNA group, CXCL8 siRNA + AG490
group showed a significant decrease in cell proliferation
(P<0.05).
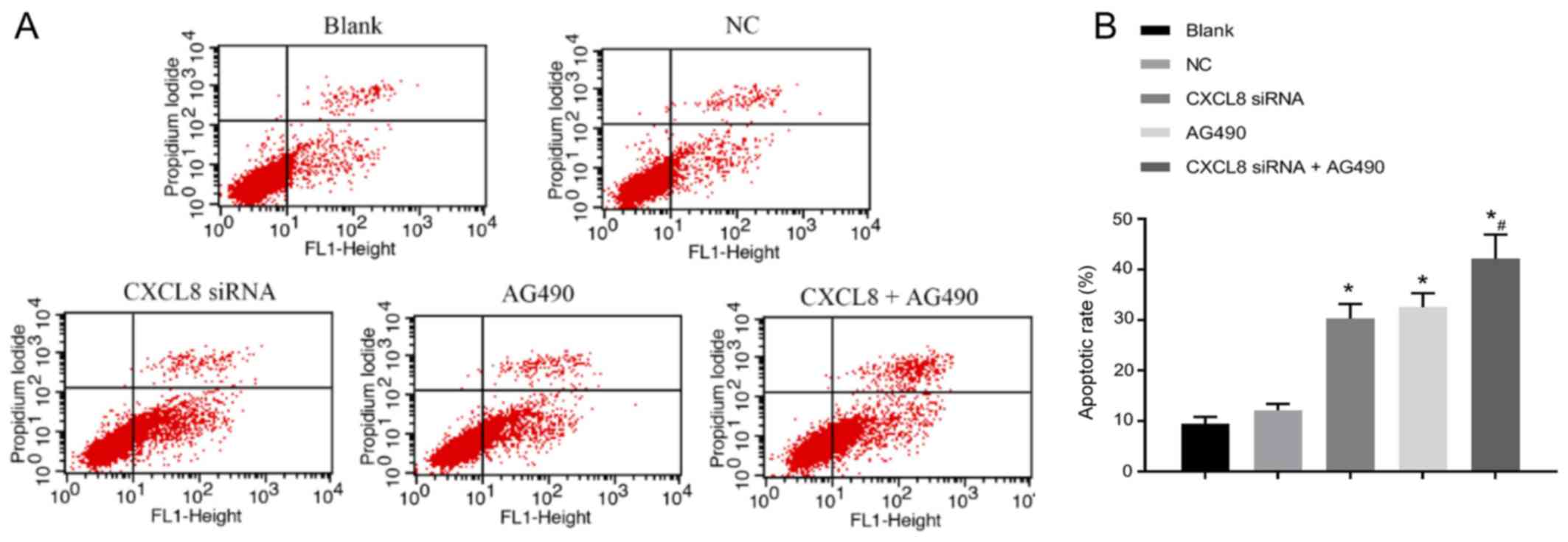
Changes of cell apoptosis ability
after cell transfection
The apoptotic status is shown in Fig. 6. Compared with the blank group, the
apoptotic rate of cells in the CXCL8 siRNA group, AG490 group and
CXCL8 + AG490 group was increased (P<0.05). There was no
significant difference in cell apoptosis when compared with NC
group (P>0.05). In addition, no significant difference was
detected in cell apoptosis between CXCL8 siRNA group and AG490
group (P>0.05). Compared with CXCL8 siRNA group, CXCL8 siRNA +
AG490 group presented obvious increase in cell apoptosis
(P<0.05).
Discussion
Invasion and migration of malignant tumor cells is a
complex multi-step and multi-factor process (46,47).
Melanoma is highly malignant and prone to hematogenous and
lymphatic metastasis with poor prognosis (1). Early detection and diagnosis are
particularly important for the prediction of melanoma prognosis
(4). The preferred treatment for
malignant melanoma is early surgical excision (8). Chemotherapy and radiotherapy have been
used to prevent the recurrence of lesions, remove small foci and
metastases, and prevent metastasis after surgical resection
(9). However, melanoma is
insensitive to current radiotherapy and chemotherapy, and most of
the anti-neoplastic drugs currently used in clinic have strong
toxicity and side-effects, which seriously affect the quality of
life of cancer patients (9).
Therefore, searching and screening for safe, effective, less toxic
side-effects of anticancer strategies are highly valued.
JAK-STAT signal transduction pathway is a classic
signal transduction pathway, which participates in cell
proliferation, survival, transformation, migration and other
processes (33,34). Among the six family members of STATs,
STAT1, STAT3 and STAT5 are the most common (41). It has been reported for numerous
tumors that blocking the functions of STAT3 and STAT5 alone can
inhibit the growth of tumor cells and induce apoptosis (48). Previous studies have shown that STAT3
is expressed at high levels and is activated in cervical and
endometrial cancer tissues (49,50).
Blocking the persistent signal pathway of STAT3 is considered to be
able to inhibit the growth of cancer cells, which may become a new
therapeutic target (51). Pan et
al (52) have reported that high
mRNA expression levels of STAT3 in colon cancer, breast cancer and
glioblastoma are related significantly to shorter survival times.
In addition, cell proliferation and apoptosis maintain homeostasis
under normal conditions, and the mechanism regulating the balance
maintains the normal physiological function of the body (53). Once this balance is destroyed, it
leads to numerous serious pathological changes, such as tumors,
degenerative diseases and autoimmune diseases. Development of
tumors are not only related to abnormal proliferation and
differentiation of tumor cells, but also to the changes of cell
death (54). It has been proven that
the reduction of apoptosis can cause tumorigenesis and promote
malignant transformation and evolution of cancer cells by escaping
(55). Therefore, the strategy of
inducing apoptosis of cancer cells has become the focus of cancer
treatment in recent decades.
In the present study, H&E staining showed that
compared with the adjacent normal tissues, melanoma cells in
tissues present obvious atypia, oval appearance with different
shapes and sizes in most cases, compact arrangement, obvious
nucleoli, mitotic figures, large cytoplasm, diffuse distribution,
and increased production of melanin granules between and within
cells, showing the biological features of melanoma cells.
Subsequent gene expression detection showed that the expression of
E-cadherin in melanoma tissues was significantly decreased compared
with that of the adjacent normal tissues, whereas the expression
levels of CXCL8, JAK2, STAT3, vimentin, and N-cadherin were
significantly increased. These results suggest that there may be
high CXCL8 expression and activation of JAK-STAT signaling pathway,
and presence of EMT in cutaneous melanoma. In the malignant
evolution of 90% of epithelial malignant tumors, there may be
active downregulation of epithelial cells, decreased polarity of
inter-cellular homogeneous adhesion system, disengagement from
constraint of inherent organizational structure, acquisition of
phenotypes and related gene changes, resulting in cell invasion and
migration, with EMT (56,57). During EMT, strong phenotypic
characteristics of epithelial cells, such as intercellular adhesion
and polar distribution, are replaced by mesenchymal phenotype
(decreased adhesion, fibroblast phenotype, increased mobility),
which is an important link in malignant evolution and metastasis of
cancer cells (58,59). It has been reported that human cancer
cells can secrete CXCL8 through autocrine and/or paracrine pathways
and interact with chemokine receptors to promote the proliferation
and migration of tumor cells (60).
Furthermore, important markers of EMT are loss of homogeneous
adhesion between epithelial cells (decrease in E-cadherin
expression, increase in N-cadherin and vimentin expression)
(61,62), resulting in cell adhesion complex
disintegration, cell adhesion and cell proliferation disorder,
morphological and structural disorders of epithelial cells, loss of
cell polarity and contact inhibition, cell growth disorder and
infiltration into surrounding tissues (63).
Cell line experiment was carried out, and the
highest expression level of CXCL8 gene was found in A375 human
cutaneous melanoma cells, when compared with that in SK-MEL-28 and
SK-MEL-1 human cutaneous melanoma cell lines. Thus, A375 cells were
screened out for subsequent experiments. The results for the mRNA
and protein expression levels of related genes after transfection
were: Compared with the blank group, CXCL8 siRNA group and CXCL8
siRNA + AG490 group showed evidently decreased CXCL8 expression.
The expression levels of JAK2, STAT3, N-cadherin and vimentin in
CXCL8 siRNA group, AG490 group and CXCL8 siRNA + AG490 group were
decreased, whereas the expression of E-cadherin was increased. The
aforementioned trends are especially significant in CXCL8 siRNA +
AG490 group. In subsequent research of biological characteristics,
cell proliferation test results showed that at 48, 72 and 96 h,
CXCL8 siRNA group, AG490 group and CXCL8 siRNA + AG490 group had
reduced cell proliferation, with more evident decrease of cell
proliferation in CXCL8 siRNA + AG490 group. The results indicated
that the silenced expression of CXCL8 can inhibit the proliferation
of cancer cells, while JAK-STAT signaling pathway activation may
promote the proliferation of cancer cells. Apoptotic status of each
group further revealed that compared with the blank group, the
apoptotic rate in the CXCL8 siRNA group, AG490 group and CXCL8 +
AG490 group was increased, and it was in particular significantly
increased in the CXCL8 siRNA + AG490 group. These results suggest
that suppressed expression of CXCL8 can promote apoptosis of cancer
cells, whereas activation of JAK-STAT signaling pathway can inhibit
apoptosis of cancer cells.
In conclusion, silencing of CXCL8 gene expression
may inhibit EMT and cell proliferation while promoting cell
apoptosis of human cutaneous melanoma cells by inhibiting JAK-STAT
signaling pathway activation. In clinical practice, the study of
the association of CXCL8 and JAK-STAT signaling pathway with EMT
and apoptosis of cutaneous melanoma will have essential impact on
the diagnosis, treatment and drug screening of cutaneous melanoma,
especially with the discovery of more signal transduction
substrates, activators and inhibitors.
Acknowledgements
We would like to thank our team members for their
work and our colleagues for their valuable scientific advice and
helpful suggestions.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH wrote the manuscript, analyzed and interpreted
the patients' data. LY and TM performed H&E staining, PCR,
western blot analysis, CCK-8 assay and flow cytometry, and were
responsible for the statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the General Hospital of Ningxia Medical University (Yinchuan,
China). Patients who participated in this research had complete
clinical data. Signed informed consents were obtained from the
patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aggarwal R, Dhawan S and Chopra P: Primary
gastric melanoma: A diagnostic challenge. J Gastrointest Cancer. 45
(Suppl 1):33–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varamo C, Occelli M, Vivenza D, Merlano M
and Lo Nigro C: MicroRNAs role as potential biomarkers and key
regulators in melanoma. Genes Chromosomes Cancer. 56:3–10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu-Smith F and Ziogas A: An age-dependent
interaction between sex and geographical UV index in melanoma risk.
J Am Acad Dermatol. Dec 2–2017.(Epub ahead of print).
|
|
4
|
Nissen LHC, Pierik M, Derikx LAAP, de Jong
E, Kievit W, van den Heuvel TRA, van Rosendael AR, Plasmeijer EI,
Dewint P, Verhoeven RHA, et al: Risk factors and clinical outcomes
in patients with IBD with melanoma. Inflamm Bowel Dis.
23:2018–2026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hübner J, Waldmann A, Eisemann N, Noftz M,
Geller AC, Weinstock MA, Volkmer B, Greinert R, Breitbart EW and
Katalinic A: Association between risk factors and detection of
cutaneous melanoma in the setting of a population-based skin cancer
screening. Eur J Cancer Prev. 27:563–569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fratangelo F, Camerlingo R, Carriero MV,
Pirozzi G, Palmieri G, Gentilcore G, Ragone C, Minopoli M, Ascierto
PA and Motti ML: Effect of ABT-888 on the apoptosis, motility and
invasiveness of BRAFi-resistant melanoma cells. Int J Oncol.
53:1149–1159. 2018.PubMed/NCBI
|
|
7
|
Ramgolam K, Lauriol J, Lalou C, Lauden L,
Michel L, de la Grange P, Khatib AM, Aoudjit F, Charron D, Alcaide-
Loridan C, et al: Melanoma spheroids grown under neural crest cell
conditions are highly plastic migratory/invasive tumor cells
endowed with immunomodulator function. PLoS One. 6:e187842011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tchernev G: One step melanoma surgery for
patient with thick primary melanomas: ‘To break the rules, you must
first master them!’. Open Access Maced J Med Sci. 6:367–371. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marinova L, Yordanov K and Sapundgiev N:
Primary mucosal sinonasal melanoma - case report and review of the
literature. The role of complex treatment-surgery and adjuvant
radiotherapy. Rep Pract Oncol Radiother. 16:40–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tesic N, Kamensek U, Sersa G, Kranjc S,
Stimac M, Lampreht U, Preat V, Vandermeulen G, Butinar M, Turk B,
et al: Endoglin (CD105) silencing mediated by shRNA under the
control of endothelin-1 promoter for targeted gene therapy of
melanoma. Mol Ther Nucleic Acids. 4:e2392015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Menezes ME, Talukdar S, Wechman SL, Das
SK, Emdad L, Sarkar D and Fisher PB: Prospects of gene therapy to
treat melanoma. Adv Cancer Res. 138:213–237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohammed-Saeid W, Chitanda J, Al-Dulaymi
M, Verrall R and Badea I: Design and evaluation of RGD-modified
gemini surfactant-based lipoplexes for targeted gene therapy in
melanoma model. Pharm Res. 34:1886–1896. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piccinin S, Doglioni C, Maestro R,
Vukosavljevic T, Gasparotto D, D'Orazi C and Boiocchi M: p16/CDKN2
and CDK4 gene mutations in sporadic melanoma development and
progression. Int J Cancer. 74:26–30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singer M, Wang C, Cong L, Marjanovic ND,
Kowalczyk MS, Zhang H, Nyman J, Sakuishi K, Kurtulus S, Gennert D,
et al: A distinct gene module for dysfunction uncoupled from
activation in tumor-infiltrating T cells. Cell. 166:1500–1511.e9.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Han N, Zhou S, Zhou R, Yuan X, Xu
H, Zhang C, Yin T and Wu K: The DACH/EYA/SIX gene network and its
role in tumor initiation and progression. Int J Cancer.
138:1067–1075. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Volodko N, Gordon M, Salla M, Ghazaleh HA
and Baksh S: RASSF tumor suppressor gene family: Biological
functions and regulation. FEBS Lett. 588:2671–2684. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel M, Boo H, Kandasamy S, Patel D and
Iorio A: The advantages of dermoscopy in the diagnosis of acral
melanoma from other podiatric lesions: A literature review. J Am
Podiatr Med Assoc. 106:32016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benton G, Kleinman HK, George J and
Arnaoutova I: Multiple uses of basement membrane-like matrix
(BME/Matrigel) in vitro and in vivo with cancer cells. Int J
Cancer. 128:1751–1757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Sun X, Nan N, Cao KX, Ma C, Yang
GW, Yu MW, Yang L, Li JP, Wang XM, et al: Elemene inhibits the
migration and invasion of 4T1 murine breast cancer cells via
heparanase. Mol Med Rep. 16:794–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greenburg G and Hay ED: Cytoskeleton and
thyroglobulin expression change during transformation of thyroid
epithelium to mesenchyme-like cells. Development. 102:605–622.
1988.PubMed/NCBI
|
|
21
|
Han ZH, Wang F, Wang FL, Liu Q and Zhou J:
Regulation of transforming growth factor β-mediated
epithelial-mesenchymal transition of lens epithelial cells by c-Src
kinase under high glucose conditions. Exp Ther Med. 16:1520–1528.
2018.PubMed/NCBI
|
|
22
|
Ning X, Zhang H, Wang C and Song X:
Exosomes released by gastric cancer cells induce transition of
pericytes into cancer-associated fibroblasts. Med Sci Monit.
24:2350–2359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dettman RW and Simon HG: Rebooting the
collagen gel: artificial hydrogels for the study of epithelial
mesenchymal transformation. Dev Dyn. 247:332–339. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang P, Dai H and Peng L: AGEs induce
epithelial to mesenchymal transformation of human peritoneal
mesothelial cells via upregulation of STAT3. Glycoconj J.
36:155–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou L, Yang K, Randall Wickett R and
Zhang Y: Dermal fibroblasts induce cell cycle arrest and block
epithelial-mesenchymal transition to inhibit the early stage
melanoma development. Cancer Med. 5:1566–1579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li CY, Wang Q, Shen S, Wei XL and Li GX:
Oridonin inhibits migration, invasion, adhesion and TGF-β1-induced
epithelial-mesenchymal transition of melanoma cells by inhibiting
the activity of PI3K/Akt/GSK-3β signaling pathway. Oncol Lett.
15:1362–1372. 2018.PubMed/NCBI
|
|
27
|
Tai KF and Wang CH: Using adenovirus armed
short hairpin RNA targeting transforming growth factor β1 inhibits
melanoma growth and metastasis in an ex vivo animal model. Ann
Plast Surg. 71 (Suppl 1):S75–S81. 2013.PubMed/NCBI
|
|
28
|
Sinnberg T, Levesque MP, Krochmann J,
Cheng PF, Ikenberg K, Meraz-Torres F, Niessner H, Garbe C and Busch
C: Wnt-signaling enhances neural crest migration of melanoma cells
and induces an invasive phenotype. Mol Cancer. 17:592018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tu Z, Xie S, Xiong M, Liu Y, Yang X, Tembo
KM, Huang J, Hu W, Huang X, Pan S, et al: CXCR4 is involved in
CD133-induced EMT in non-small cell lung cancer. Int J Oncol.
50:505–514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crosson WP, Berlinberg E and Nazarian R:
Identification of targetable EMT markers in cancer stem-like cells
derived from therapeutic resistant melanoma. Cancer Res. 77:Abst
3882. 2017.PubMed/NCBI
|
|
31
|
Ding Y, Li X, Hong D, Jiang L, He Y and
Fang H: Silence of MACC1 decreases cell migration and invasion in
human malignant melanoma through inhibiting the EMT. Biosci Trends.
10:258–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wels C, Joshi S, Koefinger P, Bergler H
and Schaider H: Transcriptional activation of ZEB1 by Slug leads to
cooperative regulation of the epithelial-mesenchymal
transition-like phenotype in melanoma. J Invest Dermatol.
131:1877–1885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang JS, Guh JY, Chen HC, Hung WC, Lai YH
and Chuang LY: Role of receptor for advanced glycation end-product
(RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen
production in NRK-49F cells. J Cell Biochem. 81:102–113. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang R, Huang S, Fu X, Huang G, Yan X, Yue
Z, Chen S, Li Y and Xu A: The conserved ancient role of chordate
PIAS as a multilevel repressor of the NF-κB pathway. Sci Rep.
7:170632017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Erdman VV, Nasibullin TR, Tuktarova IA,
Somova RS and Mustafina OE: Association analysis of polymorphic
gene variants in the JAK/STAT signaling pathway with aging and
longevity. Russian Journal of Genetics. Russ J Genet. 55:728–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mullen M and Gonzalez-Perez RR:
Leptin-induced JAK/STAT signaling and cancer growth. Vaccines
(Basel). 4:262016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mi C, Ma J, Wang KS, Wang Z, Li MY, Li JB,
Li X, Piao LX, Xu GH and Jin X: Amorfrutin A inhibits TNF-α induced
JAK/STAT signaling, cell survival and proliferation of human cancer
cells. Immunopharmacol Immunotoxicol. 39:338–347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yeh CM, Chang LY, Lin SH, Chou JL, Hsieh
HY, Zeng LH, Chuang SY, Wang HW, Dittner C, Lin CY, et al:
Epigenetic silencing of the NR4A3 tumor suppressor, by aberrant
JAK/STAT signaling, predicts prognosis in gastric cancer. Sci Rep.
6:316902016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abramovich C, Yakobson B, Chebath J and
Revel M: A protein-arginine methyltransferase binds to the
intracytoplasmic domain of the IFNAR1 chain in the type I
interferon receptor. EMBO J. 16:260–266. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khanna P, Chua PJ, Wong BSE, Yin C, Thike
AA, Wan WK, Tan PH and Baeg GH: GRAM domain-containing protein 1B
(GRAMD1B), a novel component of the JAK/STAT signaling pathway,
functions in gastric carcinogenesis. Oncotarget. 8:115370–115383.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Slattery ML, Lundgreen A, Kadlubar SA,
Bondurant KL and Wolff RK: JAK/STAT/SOCS-signaling pathway and
colon and rectal cancer. Mol Carcinog. 52:155–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma JF, Sanchez BJ, Hall DT, Tremblay AK,
Di Marco S and Gallouzi IE: STAT3 promotes IFNγ/TNFα-induced muscle
wasting in an NF-κB-dependent and IL-6-independent manner. EMBO Mol
Med. 9:622–637. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nosaka T, Kawashima T, Misawa K, Ikuta K,
Mui AL and Kitamura T: STAT5 as a molecular regulator of
proliferation, differentiation and apoptosis in hematopoietic
cells. EMBO J. 18:4754–4765. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gurbuz V, Konac E, Varol N, Yilmaz A,
Gurocak S, Menevse S and Sozen S: Effects of AG490 and S3I-201 on
regulation of the JAK/STAT3 signaling pathway in relation to
angiogenesis in TRAIL-resistant prostate cancer cells in
vitro. Oncol Lett. 7:755–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hwang TL, Changchien TT, Wang CC and Wu
CM: Claudin-4 expression in gastric cancer cells enhances the
invasion and is associated with the increased level of matrix
metalloproteinase-2 and −9 expression. Oncol Lett. 8:1367–1371.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Porther N and Barbieri MA: The role of
endocytic Rab GTPases in regulation of growth factor signaling and
the migration and invasion of tumor cells. Small GTPases.
6:135–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dong B, Liang Z, Chen Z, Li B, Zheng L,
Yang J, Zhou H and Qu L: Cryptotanshinone suppresses key
onco-proliferative and drug-resistant pathways of chronic myeloid
leukemia by targeting STAT5 and STAT3 phosphorylation. Sci China
Life Sci. 61:999–1009. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen W, He W, Cai H, Hu B, Zheng C, Ke X,
Xie L, Zheng Z, Wu X and Wang H: A-to-I RNA editing of BLCAP lost
the inhibition to STAT3 activation in cervical cancer. Oncotarget.
8:39417–39429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wallbillich JJ, Josyula S, Saini U,
Zingarelli RA, Dorayappan KD, Riley MK, Wanner RA, Cohn DE and
Selvendiran K: High glucose-mediated STAT3 activation in
endometrial cancer is inhibited by metformin: Therapeutic
implications for endometrial cancer. PLoS One. 12:e01703182017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bao X, Ren T, Huang Y, Sun K, Wang S, Liu
K, Zheng B and Guo W: Knockdown of long non-coding RNA HOTAIR
increases miR-454-3p by targeting Stat3 and Atg12 to inhibit
chondrosarcoma growth. Cell Death Dis. 8:e26052017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pan Y, Wang S, Su B, Zhou F, Zhang R, Xu
T, Zhang R, Leventaki V, Drakos E, Liu W, et al: Stat3 contributes
to cancer progression by regulating Jab1/Csn5 expression. Oncogene.
36:1069–1079. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jullien N, Roche C, Brue T,
Figarella-Branger D, Graillon T, Barlier A and Herman JP:
Dose-dependent dual role of PIT-1 (POU1F1) in somatolactotroph cell
proliferation and apoptosis. PLoS One. 10:e01200102015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Horton BL, Williams JB, Cabanov A,
Spranger S and Gajewski TF: Intratumoral CD8+ T-cell
apoptosis is a major component of T-cell dysfunction and impedes
antitumor immunity. Cancer Immunol Res. 6:14–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang Y, Sheng H, Meng L, Yue H, Li B,
Zhang A, Dong Y and Liu Y: RBM5 inhibits tumorigenesis of gliomas
through inhibition of Wnt/β-catenin signaling and induction of
apoptosis. World J Surg Oncol. 15:92017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Feng X, Zhao L, Shen H, Liu X, Yang Y, Lv
S and Niu Y: Expression of EMT markers and mode of surgery are
prognostic in phyllodes tumors of the breast. Oncotarget.
8:33365–33374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun B, Zhang D, Zhao N and Zhao X:
Epithelial-to-endothelial transition and cancer stem cells: Two
cornerstones of vasculogenic mimicry in malignant tumors.
Oncotarget. 8:30502–30510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bo H, Zhang S, Gao L, Chen Y, Zhang J,
Chang X and Zhu M: Upregulation of Wnt5a promotes
epithelial-to-mesenchymal transition and metastasis of pancreatic
cancer cells. BMC Cancer. 13:4962013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Q, Qu C, Xie F, Chen L, Liu L, Liang
X, Wu X, Wang P and Meng Z: Curcumin suppresses
epithelial-to-mesenchymal transition and metastasis of pancreatic
cancer cells by inhibiting cancer-associated fibroblasts. Am J
Cancer Res. 7:125–133. 2017.PubMed/NCBI
|
|
60
|
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T,
Chen Y, Han X and Wu K: The CXCL8-CXCR1/2 pathways in cancer.
Cytokine Growth Factor Rev. 31:61–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kai Y, Chen QW, Sun YF, Lin JA and Xu JH:
Loss of BMI-1 dampens migration and EMT of colorectal cancer in
inflammatory microenvironment through TLR4/MD-2/MyD88-mediated
NF-κB signaling. J Cell Biochem. 119:1922–1930. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang W, Wang JG, Xu J, Zhou D, Ren K, Hou
C, Chen L and Liu X: HCRP1 inhibits TGF-β induced
epithelial-mesenchymal transition in hepatocellular carcinoma. Int
J Oncol. 50:1233–1240. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xiong S and Xiao GW: Reverting doxorubicin
resistance in colon cancer by targeting a key signaling protein,
steroid receptor coactivator. Exp Ther Med. 15:3751–3758.
2018.PubMed/NCBI
|