Introduction
Cancer, as a complex disease, is the result of
long-term interaction of various exogenous and endogenous
carcinogenic factors (1–3). Normal cell division has a certain
maximum number of divisions, and it is precisely regulated by a
series of internal factors such as genes, enzymes and proteins.
Tumor cells often divide uncontrollably without following the
principles of normal self-limited cell division, and can invade or
spread to other healthy parts of the body, leading to the formation
of tumors (4). Cancer is the second
leading cause of death worldwide (5). With nearly 60% of the world's
population in Asia, 48.4% of new cancer cases and more than half of
cancer-associated deaths (57.3%) occur in this region, where cancer
mortality is higher compared with that in other regions, such as
Europe, Africa and America (5). Lung
cancer has the highest morbidity and mortality rates of all cancer
types in men in China (6). In China,
the most common cancer types among men and women are lung and
breast cancer, respectively (6).
Currently, the US Food and Drug Administration has approved ~150
anticancer drugs, which are classified into either cytotoxic or
targeted drugs. Cytotoxic drugs can kill cancer cells by targeting
mitotic and/or DNA replication pathways, whereas targeted drugs
block the growth and spread of cancer by inhibiting molecular
targets associated with cancer progression and migration (7). However, targeted drugs are often more
expensive, and cytotoxic drugs often have various side effects and
toxicity levels. Therefore, identification and isolation of natural
compounds from medicinal plants has received increasing attention
for the development of novel anticancer drugs, with the aim
overcome drug resistance and long-term survival.
Natural products as antitumor drugs
Medicinal plants have a long history of being used
to treat various types of cancer. For example, numerous Asian
countries, such as China, Japan and Thailand, have used traditional
medicinal plants to treat cancer for thousands of years (8–10).
Several of the antineoplastic drugs that have been used in a
clinical setting originate from plants, some of which have
significantly prolonged the survival time of patients. For example,
vincristine is used to treat leukemia (11), lymphoma (12), breast cancer (13), lung cancer (14) and pediatric solid cancers (15); paclitaxel is used to treat ovarian,
breast, lung, bladder and head and neck cancer (16); docetaxel is used to treat breast
(17) and lung (18) cancer; and irinotecan is used to treat
colorectal and lung cancer (19). In
addition, a number of natural products (including evodiamine,
peimine, isorhynchophylline, Coptis chinensis, ephedrine,
oridonin and matrine) improve the drug resistance of cancer cells
(breast cancer resistance to paclitaxel and epirubicin; gastric
cancer resistance to fluorouracil; lung adenocarcinoma cell
resistance to cisplatin and docetaxel; and hepatocellular carcinoma
resistance to cisplatin), which is the primary cause of cancer
chemotherapy failure (20).
Recently, an increasing number of studies have focused on the
anticancer mechanism of natural products. Wu et al (21) have reported that icariin induces
apoptosis in human lung adenocarcinoma cells by activating the
mitochondrial apoptotic pathway. Furthermore, lycorine has notable
antitumor effects in various types of cancer, such as breast,
esophageal, ovarian, prostate, melanoma and liver cancer (22). Acridone alkaloids are another class
of natural products primarily obtained from Swinglea
glutinosa, which has selective cytotoxicity against human
prostate, lung, breast and liver carcinoma cell lines (23). In the Allium cepa assay and the yeast
proliferation model, Kanchnar guggulu was evaluated for its
cytotoxicity by inhibiting mitosis and anti-proliferation effects,
confirming its potential in cancer treatment, and current studies
have shown that it has antitumor effect (24,25). In
addition, it has been confirmed that the use of a standardized
Chinese herbal formula (including Radix and Rizoma Ginseng, Rhizoma
Atractylodis, Poriae; Radix Glycyrrhiza Preparata, Rehmannia
glutinosa, Radix Paeoniae alba, Radix Angelica sinensis, Rhizoma
Chuang xiong, Ramulus Cinnamomum, Semen Armeniaca amarum, Radix
Platycodonois, Radix Saposhnikoviae, Fructus Jujubae, Massa Medica
Fermentata, Cordyceps, RhizomaDioscoreae, Radix Ophiopogonis, Radix
Bupleuri, Colla Corii asini, Semen Lablab album, Rhizoma
Zingiberis, Ganoderma and Rhodiolae crenulatae) in patients with
advanced lung cancer is acceptable and safe (26), therefore natural products have a
broad application in the development and application of antitumor
drugs.
Ailanthone (AIL) as an anti-tumor drug
Ailanthus altissima is a plant of the genus
Ailanthus in the family Simaroubaceae (27). As a traditional Chinese medicine, it
has a long history in China; for example, its bark, root bark and
fruit have been used for the treatment of ascariasis, diarrhea,
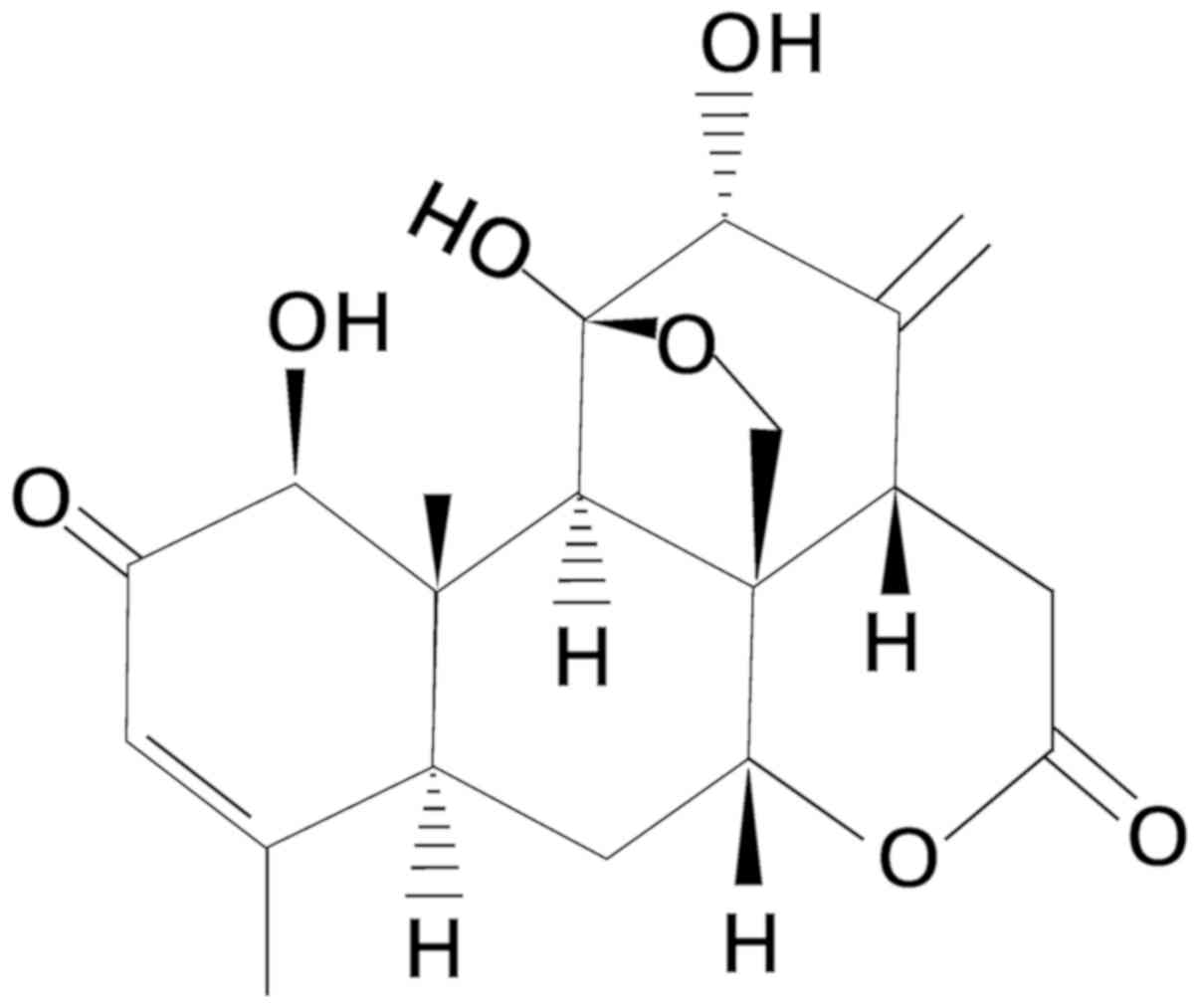
spermatorrhea, bleeding and gastrointestinal diseases (28). AIL, extracted from Ailanthus
altissima, is a pentacyclic diterpene lactone compound
(Fig. 1). It has notable clinical
benefits in the treatment of inflammation (29), malaria (30), allergies (31), tuberculosis (32), ulcer s (33), amoeba-associated disease (34) and HIV (35) and has antitumor effects (36). Numerous in vitro studies have
revealed that AIL has inhibitory effects on sever types of cancer
cells, such as melanoma (37), acute
myeloid leukemia (38,39), bladder (40), lung cancer (41,42),
gastric (43), liver (44) and breast (27,45)
cancer, vestibular schwannomas (VS) (46), osteosarcoma (47) and prostate cancer (48). The specific antitumor mechanism of
AIL is summarized in Fig 2. In
addition, AIL was found to improve the resistance of prostate
cancer cells and leukemia cells to MDV3100 and doxorubicin (DOX),
respectively (48,49). An overview of the antitumor mechanism
of AIL in various types of cancer cells will be discussed in the
present review.
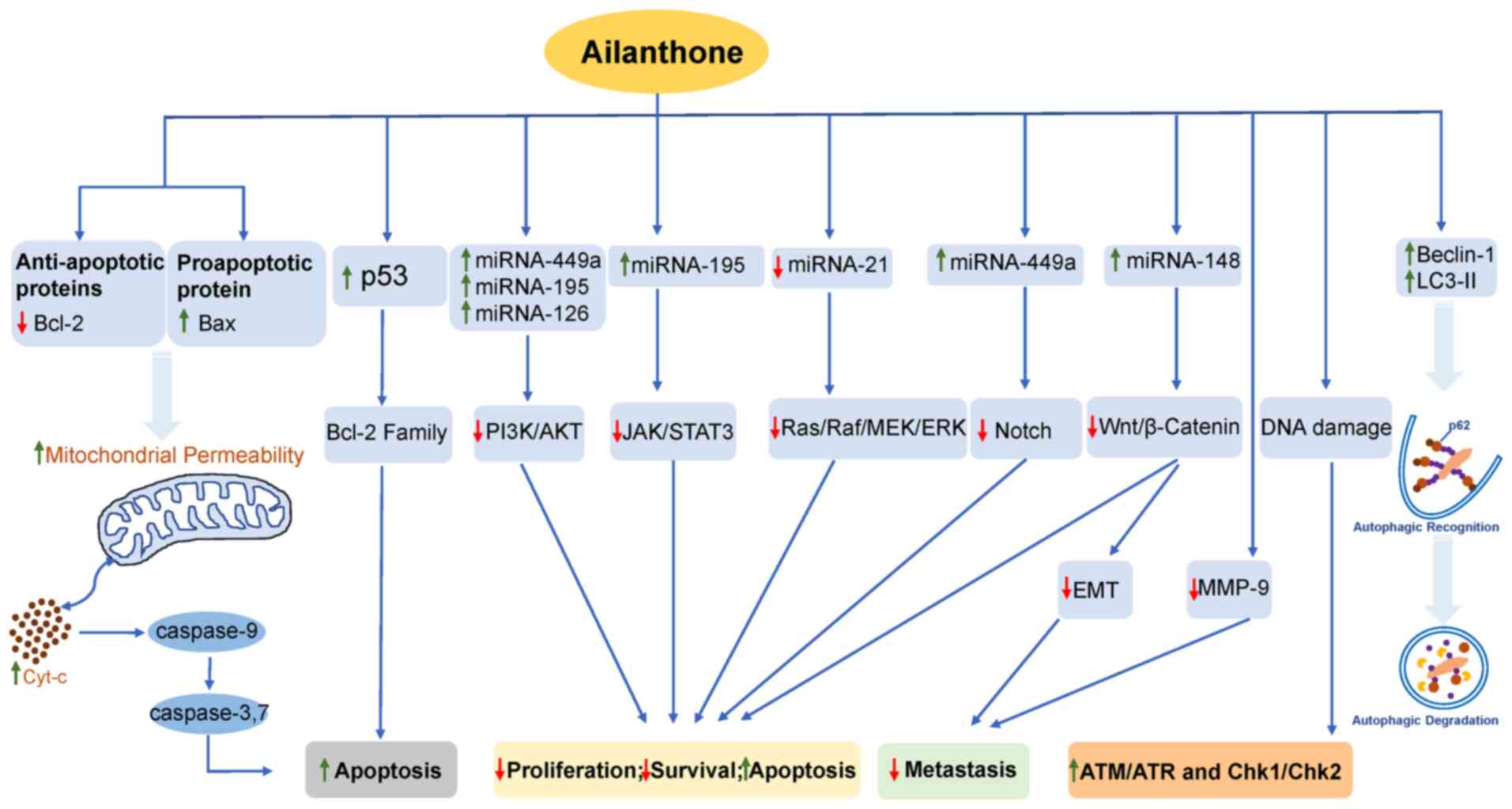 | Figure 2.Antitumor mechanism of ailanthone.
Green arrows indicates upregulation, and red arrows indicate
downregulation of molecular targets. Bcl-2, B cell lymphoma-2; Bax,
Bcl-2-associated X; mi, micro; PI3K, phosphoinositide 3-kinase;
AKT, protein kinase B; JAK, Janus kinase; STAT3, signal transducer
and activator of transcription 3; RAF, RAF proto-oncogene
serine/threonine-protein kinase; MEK, mitogen-activated protein
kinase; ERK, extracellular signal-regulated kinases; MMP-9, matrix
metalloproteinase-9; EMT, epithelial mesenchymal transition; LC3,
light chain 3; Cyt-c, cytochrome C. |
Antitumor activity of AIL against
melanoma
In 2011, a total of 9,128 melanoma deaths occurred
in the United States. The overall age-adjusted melanoma death rate
was 2.7 per 100,000 and the mortality rate of malignant melanin is
even higher (50). It has become one
of the most serious malignant tumors threatening human health
(51). Liu et al (37) demonstrated that AIL inhibited cell
proliferation and promoted apoptosis of B16 and A375 melanoma cells
in a dose-dependent manner by downregulating the phosphoinositide
3-kinase (PI3K)/protein kinase B (AKT) signaling pathway and
inducing the activation of apoptotic initiating factors. The
results of their study also revealed that the number of viable
cells significantly decreased with increasing AIL concentrations
24-h following incubation (37).
Subsequently, the potential mechanisms were explored; AIL induced
G0/G1 phase arrest in B16 cells and
G2/M phase arrest in A375 cells, significantly
increasing the apoptotic rate in a dose-dependent manner (37).
Distinct apoptotic characteristics, such as nuclear
condensation, irregular contraction of chromatin and apoptotic
bodies were observed in the AIL-treated B16 cells, whereas the
protein expression levels of p21 were increased and the levels of
cyclins E and B were decreased. These results suggested that AIL
inhibited cell proliferation by regulating cell cycle-associated
protein expression to block the cell cycle of melanoma cells; the
expression levels of PI3K, phosphorylated (p)-PI3K and p-AKT were
also decreased, indicating that AIL inhibited the activity of the
PI3K/AKT signaling pathway (37). In
addition, the decrease of the mitochondrial membrane potential,
increase of cytochrome c and Apaf-1 expression and activation of
caspase-9 and −3 indicated that AIL mediated apoptosis through the
mitochondrial pathway (37).
Overall, these results suggested that AIL may be a potential
antitumor agent to treat melanoma.
Antitumor activity of AIL against
acute myeloid leukemia (AML)
Acute myeloid leukemia (AML) is an aggressive,
heterogeneous, myeloid malignancy. It is the most common adult
acute leukemia and accounts for ~80% of cases (52). microRNAs (miRNAs/miRs) are short RNA
molecules that negatively regulate gene expression; miRNAs are
downregulated in numerous types of solid tumors, such as gastric
cancer (53), lung cancer (54) and osteosarcoma (55). Moreover, abnormal expression levels
of miRNAs (miR-128a, miR-92a, miR-143 and miR-342) are associated
with the development of AML (56,57).
miRNA-449a is downregulated in several types of solid tumors
(including gastric and liver cancer) and can regulate the
expression of tumorigenesis-associated genes, such as Flotillin 2
(58) and SOX4 (59). Based on these findings, Zhang et
al (38) explored the potential
role of miR-449a in AML and demonstrated that AIL inhibited the
activity of AML cells and induced apoptosis by targeting miR-449a.
AIL inhibited cell viability in a dose-dependent manner and exerted
significant inhibitory effects on tumor cell migration and
invasion, possibly via the downregulation of matrix
metalloproteinase (MMP)-9 and vimentin (38). In addition, AIL also notably
increased the levels of cleaved-caspase-7, −3 and −9, leading to
increased tumor cell apoptosis in the AIL-treatment group
(P<0.05) (38). Further
investigation reported that AIL significantly (P<0.01 or
P<0.001) increased the mRNA expression levels of miR-449a in AML
cells. Subsequently, following transfection of a miR-449a inhibitor
into AML cells, the levels of cleaved-caspase-7, −3 and −9 and the
apoptotic rate in the AIL-treatment group were decreased, with the
latter being significant (P<0.05). In addition, the protein
expression levels of Notch1 and 2, p-PI3K and p-AKT in the
AIL-treatment group were also lower compared with those in the
control group, and the inhibitor of miR-449a reversed this. These
results suggested that AIL upregulated the expression of miR-449a
in the Notch and PI3K/AKT signaling pathways (38). Wei et al (39) reported that AIL promoted apoptosis by
inducing autophagy in HL-60 promyelocytic leukemia cells. Their
results demonstrated that acidic vesicular organelles, which are
one of the characteristics of autophagy, were observed in the
experimental group. The protein expression levels of the
autophagy-associated proteins were determined, and the results
revealed that levels of Beclin-1 and light chain (LC)3-II were
upregulated, while the levels of p62 and LC3-I were downregulated
in a dose-dependent manner (all P<0.05) (39). Therefore, these results indicated
that AIL induced autophagy and that AIL may be a potential
treatment for AML.
Antitumor activity of AIL against
bladder cancer
Bladder cancer is one of the most common malignant
tumors in the urinary system. Global cancer statistics in 2018
found that bladder cancer is more common in men, in whom it is the
sixth most common cancer and ninth leading cause of
cancer-associated death (5).
Currently, the combination of surgery and cisplatin-based
chemotherapy is the standard treatment (60); however, cisplatin-based chemotherapy
is often accompanied by secondary drug resistance, which can reduce
the long-term therapeutic effect and is particularly evident in
invasive urothelial cancer (61).
Cisplatin resistance in patients with bladder cancer is associated
with overexpression of NF-E2-related factor (Nrf2), and increased
Nrf2 in resistant cells has been recognized as an important factor
in maintaining drug resistance (62). Nrf2 also promotes the
epithelial-mesenchymal transition by downregulating E-cadherin, and
knockdown of Nrf2 impairs tumor cell migration and invasion
(63). Yes-associated protein (YAP)
is the primary effector of the Hippo pathway, which also
participates in chemotherapy resistance of bladder cancer (64). When the Hippo pathway is inhibited,
YAP is transported into the nucleus and binds to transcription
factors, such as transcriptional enhanced associate domains, to
promote the expression of target genes (c-Myc, Cyr61 and
survivin) that regulate cell proliferation, migration and survival
(64). Conversely, knockdown of YAP
and silencing of Nrf2 can enhance the sensitivity of bladder cancer
cells to cisplatin and reduce the migration of tumor cells
(62). Daga et al (40) demonstrated that AIL inhibited the
proliferation and migration of bladder cancer cells by reducing the
expression of Nrf2, YAP and c-Myc. Moreover, a similar effect was
identified in cisplatin-resistant bladder cancer cells. The results
of MTT and colony formation assays demonstrated that AIL was more
effective compared with cisplatin in inhibiting the growth of 253J
B-V and 253J bladder cancer cell lines (40). Of note, the growth inhibition rate of
the cisplatin-resistant cell lines, 253J B-V C-r and 253J C-r was
the same as that of the sensitive cells, confirming the
cytotoxicity and antiproliferative effect of AIL. Furthermore, AIL
exhibited low cytotoxicity in normal adult HK-2 renal cortex cells,
indicating that the toxicity of AIL to normal cells was lower
compared with that of cancer cells (40). Further flow cytometry analysis
demonstrated that AIL primarily arrested cells in the
G0/G1 phase of the cell cycle and inhibited
migration and invasion; however, this did not induce apoptosis. In
addition, protein expression levels of Nrf2, YAP and c-Myc were
decreased in the AIL-treatment group. Overall, Daga et al
(40) demonstrated that AIL overcame
cisplatin resistance in bladder cancer cells by downregulating the
expression of Nrf2 and YAP, suggesting that AIL may be an effective
drug for patients with bladder cancer that are resistant to
chemotherapy.
Antitumor activity of AIL against lung
cancer
Lung cancer is one of the most common malignancies
in the world and is the leading cause of cancer-associated death,
accounting for 18.4% of deaths among patients with cancer (5). Ni et al (42) screened 3,000 herbal monomers using an
ATP luminescent high-throughput assay and demonstrated that AIL had
the potential to inhibit the proliferation of non-small cell lung
cancer (NSCLC) cells. AIL inhibited the proliferation and colony
formation of NSCLC A549, H1299 and H1975 cells in a dose- and
time-dependent manner, and the growth inhibition ability of AIL was
greater compared with that of cisplatin, which is a first-line
chemotherapeutic drug for lung cancer (42). Orthotopic lung tumor models revealed
that the volume and weight of tumors were smaller in the
AIL-treated group. In addition, flow cytometry analysis
demonstrated that AIL induced G1 or G2/M
arrest of tumor cells in a dose-independent manner and induced
apoptosis in H1975 cells, but did not induce apoptosis in A549 and
H1299 cells (42). Western blotting
showed that caspase-3 and poly-ADP-ribose polymerase (PARP) were
activated in H1975 cells, but not in A549 and H1299 cells;
similarly, following DAPI staining, AIL was observed to induce DNA
damage in H1299 and H1975 cells, but not in A549 cells (42). This indicated that AIL-mediated
growth inhibition was dependent on the induction of apoptosis and
DNA damage. The authors further elucidated its mechanism of action
using cDNA microarray analysis and reported that 1,222 genes were
significantly differentially expressed in A549, H1299 and H1975
cells; among them, four genes, namely proliferating cell nuclear
antigen (PCNA), replication protein A 1 (RPA1),
acyl-CoA desaturase and DNA ligase 1, were involved in both
nucleotide excision repair and DNA replication signaling pathways
(42). Subsequent experiments
revealed that the mRNA levels of PCNA and RPA1 were
significantly decreased in all tested cell lines, while the protein
expression levels of RPA1 were significantly decreased in a
dose-dependent manner, and PCNA levels were not altered. Lastly,
using animal experiments, it was confirmed that AIL inhibited
subcutaneous xenograft and orthotopic lung tumor growth and
prolonged the survival time of tumor-bearing mice (42). This indicated that AIL inhibited RPA1
expression in a dose-dependent manner, thus inhibiting DNA
replication and tumor cell growth.
Hou et al (41) demonstrated that AIL inhibited the
PI3K/AKT and Janus kinase (JAK)/signal transducer and activator of
transcription (STAT)3 signaling pathways by increasing the
expression levels of miR-195, as well as by promoting apoptosis and
autophagy in the A549 lung cancer cell line. Abnormal expression of
miR-195 is associated with the development and progression of
numerous types of tumors, such as breast (65), lung (66), liver (67) and prostate cancer (68). The viability of A549 cells treated
with different concentrations of AIL was significantly decreased
(P<0.01 or P<0.001) compared with that in the control group.
Cell proliferation and cyclin D1 expression levels were also
significantly decreased in cells treated with AIL (P<0.01),
suggesting that AIL also inhibited the proliferation of lung cancer
cells. Apoptotic analysis demonstrated that AIL significantly
increased the rate of apoptosis of tumor cells, and the protein
expression levels of cleaved-caspase-3 and −9 were increased,
further indicating that AIL pomoted apoptosis (41). However, Ni et al (42) did not report that AIL induces
apoptosis in A549 cells. Considering that a previous study
identified that miR-195 was associated with lung cancer (66), Hou et al (41) detected the expression levels of
miR-195 and the protein expression levels of autophagy-related
proteins Beclin-1 and p62 rin AIL-treated A549 cells. It was
concluded that AIL promoted apoptosis and autophagy by upregulating
miR-195, which was verified by knockdown of miR-195. Also the
upregulation of miR-195 inhibited the PI3K/AKT and JAK/STAT3
signaling pathways (41). Therefore,
AIL was hypothesized to exert its anticancer effects by
upregulating the expression of miR-195 in lung cancer.
Antitumor activity of AIL against
gastric cancer
The incidence of gastric cancer in East Asia
(including Mongolia, China, Japan and Korea) is notably higher
compared with that in other regions, such as Northern America,
Northern Europe, and Africa (5).
Gastric cancer has become the fifth most frequently diagnosed
cancer and the third leading cause of cancer-associated death in
the world (5). Chen et al
(43) explored the antitumor effect
of AIL on human SGC-7901 gastric cancer cells. The results revealed
that AIL inhibited the proliferation of SGC-7901 cells in a dose-
and time-dependent manner. The IC50 value of AIL in
SGC-7901 cells at 24 h was significantly (P<0.05) lower than
that of the taxol group, which was used as a positive control. The
apoptotic rate was also significantly (P<0.001) increased with
increasing concentrations of AIL. Furthermore, AIL significantly
increased the percentage of cells in the G2/M phase in a
dose-dependent manner. The protein expression levels of Bcl-2 and
Bax were down- and upregulated, respectively, in cells treated with
AIL. Characteristic apoptotic morphology (nuclear shrinkage and
chromatin condensation) were also observed in the AIL-treatment
group following Hoechst 33258 staining, indicating that AIL induced
apoptosis in SGC-7901 cells (43).
Antitumor activity of AIL against
liver cancer
Liver cancer was the sixth most commonly diagnosed
cancer and the fourth leading cause of cancer-associated death
worldwide in 2018 (5). The incidence
rate of liver cancer is often higher in countries with a lower
Human Development Index (5). Primary
liver cancer includes hepatocellular carcinoma (HCC), comprising
75-85% of total cases, intrahepatic cholangiocarcinoma, comprising
10-15% of cases, and other rare types (5). The 5 year survival rate of patients
with liver cancer is still low (~18%), even following systemic
treatment (69). Zhuo et al
(44) investigated the anticancer
effect of AIL in Huh7 human HCC cells. AIL reduced the viability of
Huh7 cells in a dose- and time-dependent manner. Colony formation
was also inhibited in a dose-dependent manner. Flow cytometry
analysis showed that after 48 h of exposure to different
concentrations of AIL (0, 0.2, 0.4, or 0.8 µM), the percentage of
cells in the G0/G1 phase increased notably,
confirming that AIL arrested the cell cycle. The expression levels
of proteins regulating the cell cycle were investigated, and it was
demonstrated that AIL decreased the expression of cyclins D and E,
CDK2, CDK4 and CDK6, and increased the expression of p21 and p27
(44). The expression levels of cell
division cycle 25A, which acts as an upstream regulator of the
CDK/cyclin complex, and retinoblastoma protein (Rb), which is a
positive regulator of the cell cycle, were also significantly
inhibited by AIL. In addition, AIL induced double-stranded DNA
breakage and activated ataxia telangiectasia mutated
proteins/ataxia telangiectasia, Rad3-assocaited proteins and
Chk1/Chk2 pathways in Huh7 cells, which may lead to
G0/G1 cell cycle arrest (44). Furthermore, AIL also induced
apoptosis of Huh7 cells in a dose-dependent manner, and
significantly increased the levels of cleaved caspase-9 and −3,
indicating that AIL can induce caspase-dependent apoptosis; in
addition, AIL mediated apoptosis via the mitochondrial pathway,
which was determined by detection of apoptosis-inducing factor and
endonuclease G levels in the cytoplasm of Huh7 cells and the
detection of decreased mitochondrial membrane potential (44). This study further revealed that AIL
inhibited the PI3K/AKT signaling pathway using western blotting.
Furthermore, the authors confirmed that AIL inhibited the growth
and angiogenesis of Huh7 cell xenografts in nude mice, and its low
toxicity was also verified (44).
These results suggested that AIL exerted notable antitumor activity
in Huh7 cells and may have potential as a novel drug for the
treatment of HCC.
Antitumor activity of AIL against
breast cancer
Breast cancer is the most commonly diagnosed cancer
in the world, and was leading cause of cancer-associated death in
women in 2018 (5). It has been
reported that AIL significantly inhibits the proliferation of human
MCF-7 breast cancer cells in a time- and dose-dependent manner
(27). The inhibition rates of MCF-7
cells were 7.38-35.95, 22.73-47.6 and 35.64-56.76% at 24, 48 and 72
h, respectively, following treatment with different concentrations
of AIL (0.5, 1.0, 2.0, 4.0 and 8.0 µg/ml) (27). AIL also promoted apoptosis in a
dose-dependent manner, and the apoptosis rate in the 8.0 µg/ml
group was 75.51%, which was significantly increased compared with
that of the control group (P<0.01). In addition, the percentage
of cells in G0/G1 phase were cells increased,
whereas the percentage of cells in S and G2/M phase were
notably decreased compared with that of the control group (27). In addition, the apoptosis of breast
cancer MCF-7 cells in the AIL group was increased by upregulating
the expression levels of Bax, caspase-3 and downregulating the
expression of Bcl-2 (27). This
indicated that AIL arrested the cell cycle and promoted apoptosis
in breast cancer cells.
Gao et al (45) reported that AIL significantly reduced
the viability of breast cancer cells, suppressed cell proliferation
and induced apoptosis. AIL downregulated cyclin D1 and upregulated
p53 and p21 protein levels in MDA-MB-231 cells (45). The IC50 of AIL on
MDA-MB-231 cells was 9.8 µM at 48 h, which was lower compared with
that of human non-tumorigenic breast epithelial MCF-12A cells; this
indicated that AIL had lower cytotoxic effects on normal cells
compared with that in cancer cells (45). In addition, AIL significantly
decreased the percentage of BrdU-positive cells compared with the
control group (P<0.05). An apoptosis assay revealed that AIL
significantly increased the percentage of apoptotic cells, and AIL
increased the protein levels of cleaved caspase-3 and −9
(P<0.001). Transwell assay results revealed that AIL
significantly reduced cell migration and invasion; also the levels
of migration- and invasion-associated proteins MMP-9 and vimentin
were significantly (P<0.05, P<0.001) decreased in the
AIL-treated group (45). The data
from reverse transcription-quantitative PCR indicates that
upregulation of miR-148a may mediate cell proliferation, apoptosis,
migration and invasion affected by AIL in MDA-MB-231 cells.
Furthermore, it was demonstrated that AIL inhibited AMP-activated
protein kinase (AMPK) and Wnt/β-catenin signaling pathway by
regulating miR-148a in MDA-MB-231 cells (45). Overall, these two studies suggested
that AIL had potential in the treatment of breast cancer.
Antitumor activity of AIL against
VS
VS, also known as acoustic schwannoma, originates
from the myelin-forming Schwann cells that surround the vestibular
branches of the eighth (auditory) cranial nerve (70). VS accounts for 6-7% of all
intracranial tumors, 90% of which are located in the
cerebellopontine angle (71). Yang
et al (46) demonstrated the
anticancer effect of AIL in human VS cells. AIL inhibited VS cell
proliferation at 0.4, 0.6, 0.8 and 1 µM after 48-h treatment, and
promoted apoptosis in a dose-dependent manner via downregulation of
miR-21. Administration of AIL significantly reduced the mRNA and
protein expression levels of cyclin D1, which is a critical target
of proliferative signals in the G1 phase to promote cell
cycle progression (46). AIL
significantly increased the apoptotic rate (P<0.001) and the
expression levels of cleaved caspase-9 and −3. In addition,
increased expression levels of the autophagy-associated proteins
Beclin-1 and LC3-II, and decreased levels of p62 in the AIL group
compared with those in the control cells suggested that AIL can
promote the autophagy of VS cells (46). Previous studies have demonstrated
that high levels of miR-21 expression are associated with several
types of cancer (72–74), thus it was explored whether miR-21
functioned in AIL-mediated growth inhibition of VS cells. miR-21
levels were increased in VS cells compared with those in healthy
tissue using microarray analysis techniques (46). AIL significantly decreased the levels
of miR-21 in tumor cells (P<0.01), indicating that miR-21 was
negatively regulated by AIL; overexpression of miR-21 reversed the
aforementioned results, suggesting that miR-21 participated in the
antitumor mechanism of AIL (46).
Furthermore, the possible mechanism by which AIL induced apoptosis
and autophagy in VS cells was explored, reporting that AIL blocked
the Ras/RAF proto-oncogene serine/threonine-protein kinase
(Raf)/mitogen-activated protein kinase kinase (MEK)/ERK and mTOR
pathways in a miR-21-dependent manner (46). Therefore, these results demonstrated
that AIL may be a potential antitumor agent for treating VS.
Antitumor activity of AIL against
osteosarcoma
Statistics from 1973 to 2004 show that osteosarcoma
is the most common primary malignant tumor of bone in children and
adolescents in the United States (75). At present, complete surgical
resection combined with chemotherapy is the primary method of
treatment for osteosarcoma (76). A
number of studies have demonstrated that abnormal expression of
miRNA (miR-27a, 95-3p, 195 and 133b) was associated with
osteosarcoma growth, metastasis and prognosis (77–79). For
example, Kong et al (47)
revealed that different concentrations of AIL inhibited MG63
osteosarcoma cell viability (P<0.01 or P<0.001) and
proliferation (P<0.01) compared with those in the control group
by increasing the levels of miR-126. Furthermore, cell migration
and invasion were also inhibited by AIL, whereas the rate of
apoptosis was increased. Protein expression levels of cyclin D1 and
Bcl-2 were decreased, while Bax, cleaved PARP and cleaved caspase-3
were increased following treatment of MG63 cells with AIL compared
with untreated cells (47). In
addition, PTEN protein expression levels were increased; however,
PI3K and AKT phosphorylation levels were decreased (47). This indicated that the activation of
the PI3K/AKT pathway was suppressed by AIL in MG63 cells. miR-126
was expressed at low levels in osteosarcoma cell lines MG63, U2OS,
HOS and Saos-2 compared with those in normal osteoblast hFOB1 cells
(47). These effects of AIL inducing
MG63 cell proliferation, migration and invasion were all reversed
when miR-126 was knocked down, indicating that AIL exerts its
antiosteosarcoma effect by upregulating miR-126.
Antitumor activity of AIL against
prostate cancer
In 2018, prostate cancer is the second most common
type of cancer in the world and the fifth leading cause of
cancer-associated death among men (5). Androgen deprivation therapy is the
primary treatment for metastatic prostate cancer and includes three
methods: Surgery, radiotherapy and castration drugs, such as
Goserelin (80). Drug castration
therapy primarily targets androgen receptors (ARs), which serve an
important role in the development of prostate cancer. When ARs are
phosphorylated following activation via endogenous androgen ligands
(testosterone and dihydrotestosterone), the ligand-receptor
complex, in association with coregulatory factors (for example,
c-Fos, c-Jun, NFκB and sex-determining region Y gene translocates
into the nucleus and binds to specific genomic DNA regions to
regulate target gene expression (81). Androgen binding is the most important
stimulator of androgen receptor activation (81); therefore, drug castration therapy is
primarily aimed at eliminating this stimulus. A previous study has
reported that >80% of patients respond to castration therapy in
the early stages of treatment; however, almost all patients
eventually progress to the terminal stage of castration-resistant
prostate cancer (CRPC) (82). The
drugs currently used in CRPC are docetaxel (83), cabazitaxel (84), abiraterone (85), radium-223 (86) and enzalutamide (87). In addition, the AR antagonist MDV3100
has also been reported to be effective against CRPC (88). Most of the AR antagonists used in
clinic target the ligand-binding domain of the receptor. Therefore,
AR shear variants (AR-Vs) that lack the ligand binding domain are
resistant to antiandrogen therapy, including MDV3100 and
abiraterone (89). He et al
(48) demonstrated that AIL targeted
p23 to overcome MDV3100 resistance in prostate cancer cell lines.
The group used dihydrotestosterone to stimulate 22RV1 prostate
cancer cells to activate the ligand-dependent receptor full-length
AR (AR-FL), and transfected with AR1-651 (this segment of AR lacks
a ligand-binding domain (LBD), but can be continuously activated to
introduce AR-Vs. After 12-h incubation with various natural
compounds, a dual luciferase assay was used to detect AR
transcriptional activity, and it was observed that AIL effectively
reduced the transcriptional activities of AR-FL and AR-Vs. The same
results were also demonstrated in LNCaP and c4-2b prostate cancer
cell lines (48). A sulforhodamine B
assay confirmed that AIL inhibited the proliferation of several
AR-positive prostate cancer cell lines, including LNCaP, c4-2b,
22RV1 and LAPC4. However, its proliferation inhibitory effect was
weaker in AR-negative tumor cell lines and normal prostate cells
(48). Similarly, AIL was more
effective at inhibiting AR-positive cell migration compared with
that of AR-negative prostate cancer cells. After combining AIL (0.1
µM) with the AR antagonists bicalutamide (BIC) and MDV3100, c4-2b
androgen-insensitive and 22RV1 castration-resistant cells
proliferation was inhibited, indicating that AIL overcame drug
resistance (48). AIL was also
demonstrated to significantly inhibit the increase in tumor volume
in 22RV1 ×enografts in animal experiments with BALB/c nude mice. In
addition, the oral bioavailability of AIL was 25.7% and did not
exhibit significant hepatotoxicity; however, there was some damage
to the gastric mucosa (48). Of
note, VCaP xenografts were more sensitive to AIL compared with
MDV3100. CRPC 22RV1 ×enografts were resistant to BIC and MDV3100
treatment; however, AIL markedly inhibited tumor growth and reduced
the tumor volume by 82% (95% confidence intervals, 70-95%)
(48). AIL also significantly
reduced the expression levels of AR proteins in LNCaP, 22RV1,
LNCaP-MDV3100-R and VCaP prostate cancer cells in a dose-dependent
manner, and these reductions were not associated with the presence
or absence of androgen stimulation (48). An immunoprecipitation assay showed
that AIL induced AR protein degradation and ubiquitination by
preventing the interaction between AR and its molecular chaperones,
heat shock protein HSP90 and HSP70; in addition, AIL downregulated
AKT and CDK4 expression, which may be associated with inhibition of
proliferation (48).
In the absence of a ligand, the AR resides in the
cytosol bound to a complex of HS, chaperone and co-chaperone
proteins (90). This protein complex
is also termed foldosome and component proteins, including HSP90,
HSP70 and p23, in which p23 serves an important role in maintaining
the stability of the foldosome (90). Using the ProteOn XPR36 system, it was
demonstrated that AIL could bind to p23 and inhibit its interaction
with HSP90 in the foldosome, thus destabilizing the complex
(90). In summary, targeting p23 is
the main mechanism by which AIL induces the degradation of AR, and
destabilizes the folding complex. Therefore, AIL is a potential
candidate for the treatment of prostate cancer, which requires
further investigation.
Preclinical safety evaluation of AIL
Unfortunately, most of the aforementioned studies
did not evaluate the effect of AIL on normal cell lines (27,37–40,43,46,47).
Only a few studies have evaluated the cytotoxicity of AIL in normal
cells or the toxicity and the side effects in animal models
(42,44,45,48). Gao
et al (45) and He et
al (48) have reported that
cancer cells are more sensitive to AIL compared with normal cells.
Traditional chemotherapeutic drugs have various side effects, such
as myelosuppression, hepatotoxicity, nephrotoxicity, digestive
tract reaction, neurotoxicity and pulmonary fibrosis, which
markedly reduce the quality of life of patients with cancer and
hinder the progress of treatment (91). Antitumor drugs extracted from
traditional Chinese medicine exhibit less toxicity and fewer side
effects compared with traditional chemotherapeutics. For example,
Ni et al (42) demonstrated
that AIL significantly inhibited NSCLC cell proliferation in
vitro and tumor growth in vivo with low toxicity, and no
damage was observed in the liver and kidney of SCID-Bg mice
following AIL treatment. He et al (48) found that AIL does not induce
significant hepatotoxicity in BALB/c nude mice; however, it can
cause some damage to the stomach. To evaluate the in vivo
cytotoxic effects of AIL, Zhuo et al (44) observed hematoxylin and eosin-stained
sections from the heart, lung, liver, kidney and spleen and
revealed no notable morphological changes were found in the
AIL-treated animals.
In a recent study, Tang et al (92) used Kunming mice to evaluate the
toxicity and safety of AIL. The acute toxicity experiments
indicated that the main organs affected by AIL were the liver,
spleen, intestine, colon and stomach. According to the toxicity of
the classification standard, Globally Harmonized System of
Classification and Labeling of Chemicals (93), the median lethal dose of AIL is 27.3
mg/kg, which is level 2 (severe). The primary causes of death from
AIL included gastrointestinal hemorrhage and liver steatosis
(92). In addition, the toxicity of
AIL in the blood system was also investigated; AIL significantly
reduced the numbers of red blood cells (RBC), hemoglobin,
hematocrit and mean corpuscular hemoglobin concentration and
increase the MCN, RDWCV and platelet counts in mice; however, this
process was reversed following drug withdrawal, indicating that AIL
may have hematologic toxicity, but it was not the primary cause of
death in mice (92). In the autopsy
report, in addition to gastrointestinal hemorrhage and liver
steatosis in the high concentration group, hepatic steatosis,
cholestasis, splenomegaly and chronic gastritis were also observed
in the low concentration treatment group. In addition, the study
showed that AIL had reproductive toxicity, as the testis and
epididymis of male mice had marked atrophy and pathological damage,
while ovarian follicle development was hindered, with corpus luteum
necrosis in female mice (92).
Antitumor mechanism of AIL
Effects on apoptosis
One of the antitumor effects of AIL is the
activation of the apoptosis pathway. Apoptosis is a form of
programmed cell death regulated by genes, through which abnormal
cells in the body can be removed and to maintain homeostasis.
Defects within apoptosis are associated with numerous diseases,
such as autoimmunity, degenerative diseases and cancer (94). Dysregulation of apoptosis may lead to
the formation of tumor cells (95).
Therefore, molecular pathways that promote apoptosis have become
effective targets against tumor growth. AIL has been demonstrated
to induce apoptosis in numerous types of cancer cells via the
intrinsic and extrinsic pathways by regulating multiple molecular
targets (37,43), including the caspase and Bcl-2 family
proteins (43), transcription
factors (such as β-catenin) (45),
tumor suppressor genes (such as TP53) (45) and signaling pathways, such as
PI3K/AKT (37) and JAK/STAT3
(41).
Members of the Bcl-2 family
The Bcl-2 family of proteins are key regulators of
apoptosis via the mitochondrial apoptosis pathway and by promoting
the caspase cascade activation (96,97). The
balanced ratio of various Bcl-2 proteins determines whether the
cell undergoes apoptosis or survives (98). AIL treatment downregulates Bcl-2 and
upregulates Bax proteins in melanoma (37), gastric cancer (43), breast cancer (27) and osteosarcoma (47) cells. Mitochondrial membrane potential
changes have been observed in melanoma (37) and hepatocellular carcinoma (44) cells treated with AIL compared with
untreated cells, suggesting that the mitochondrial apoptosis
pathway is involved.
TP53
The tumor suppressor protein p53, encoded by the
TP53 gene in humans, serves an important role in preventing
cancer development (99).
TP53 is the most frequently mutated gene in human cancer
(100). p53 can also promote
apoptosis, relying on the induction of pro-apoptotic Bcl-2 proteins
(99). In addition, p53 is an
important mutual regulator of AMPK (101). AIL notably upregulated p53 protein
levels in MDA-MB-231breast cancer cells, which promoted apoptosis
(45).
Effects on signaling pathways
PI3K/AKT/mTOR signaling pathway
The PI3K/AKT/mTOR signaling pathway is one of the
most important intracellular pathways that is frequently activated
in a wide range of cancer types, including melanoma (102), breast cancer (103), lung and colorectal cancer (104). The PI3K/AKT/mTOR signaling pathway
regulates cell proliferation, differentiation and metabolism,
leading to anti-apoptosis and cancer cell survival. In addition,
activation of the PI3K/AKT/mTOR pathway is also associated with
tumor pathogenesis (including breast cancer, melanoma, gastric,
lung, pancreatic and thyroid cancer and acute myeloid leukemia) and
drug resistance, such as etoposide, DOX, cytarabine (105–107).
PI3K, AKT or mTOR kinase inhibitors are already in clinical
development (102,104). It has been demonstrated that AIL
treatment suppresses the PI3K/AKT/mTOR pathway by decreasing the
phosphorylation of PI3K and AKT, thus inducing apoptosis. In
melanoma (37), acute myeloid
leukemia (38), lung cancer
(41), liver cancer (44), VS (46) and osteosarcoma (47), AIL exerts its antitumor activity
mainly by inhibiting the PI3K/AKT pathway.
JAK/STAT3 signaling pathway
The JAK/STAT3 signaling pathway serves a key role
in cell survival and apoptosis; it is activated and its components
are abnormally expressed in a variety of tumors, including leukemia
(108), prostate cancer (109), renal cell carcinoma (110), lung (111), colon (112) and pancreatic cancer (113). In recent years, the JAK/STAT3
signaling pathway has been considered as a potential target for
antitumor therapy (114). The
phosphorylation of STAT3 increased in various types of cancer
(109); therefore, analysis of
p-STAT3 protein expression levels can be used to determine whether
AIL acts on the JAK/STAT3 signaling pathway. A previous study has
demonstrated that AIL exerts its antitumor effects on lung cancer
by upregulating miR-195, which inhibits the JAK/STAT3 signaling
pathway (41).
Ras/Raf/MEK/ERK signaling pathway
The Ras/Raf/MEK/ERK signaling pathway also serves a
pivotal role in tumor cell survival. Activation of the Ras protein
is observed in ~30% of human cancer types, including pancreatic,
lung, endometrium, ovary, prostate, stomach, liver and breast
cancer (115). ERK promotes
survival, metastasis and cell proliferation, primarily by
activating the epidermal growth factor receptor and Ras small
guanosine triphosphatases; p-ERK translocates into the nucleus and
regulates various transcription factors, such as the Ets family of
transcription factors (116). In
VS, AIL acts on this signaling pathway to exert its antitumor
effects (46).
Wnt/β-catenin signaling pathway
Dysregulation in the Wnt/β-catenin pathway has been
observed in numerous types of human cancer, such as colon cancer,
melanoma, pancreas cancer and adrenocortical carcinoma (117). Wnt/β-catenin is an important signal
transduction pathway for the regulation of cell proliferation,
apoptosis and metastasis (118).
The expression levels of β-catenin are associated with poor
prognosis in patients with breast cancer (119). Gao et al (45) reported that AIL inhibited the
activation of the AMPK and Wnt/β-catenin signaling pathways by
regulating miR-148a in MDA-MB-231 cells.
Notch signaling pathway
Notch can function as a proto-oncogene in tumors,
including breast cancer and lymphoid malignancies (for example T
cell acute lymphoblastic leukemia, B cell chronic lymphocytic
leukemia and splenic marginal zone lymphoma), and can also serve as
a tumor suppressor gene (120).
Notch signaling pathway is regulated at the transcriptional or
post-transcriptional levels. The ubiquitination pathway, miRNA
(including miR-1, −34, −146, −199 and −200) and Cyclin/Cdk complex
can all affect the Notch signaling pathway (121). Notch is particularly important in
the hematopoietic system (122).
Notch mediates the proliferation, self-renewal and differentiation
of stem and progenitor cells to generate mature cells in the blood
(122). The activation of Notch
signaling is associated with poor prognosis of patients with AML
(123), and targeting Nocth1 has
been considered as a novel strategy for AML treatment (124). AIL has been demonstrated to
deactivate the Notch and PI3K/AKT signaling pathways by
upregulating miR-449a expression (38).
Effects on cell proliferation and cell cycle
Cell proliferation is highly regulated in normal
cells, and dysregulation of the cell cycle may lead to excessive or
uncontrolled proliferation and promote metastasis (4). A number of studies have documented that
AIL mediates its antiproliferative effect in cancer cells via
modulation of various molecular targets, such as cyclin, CDKs, CDK
inhibitors (CKIs) and the Rb gene (125). Cyclin binds to specific CDKs to
form cyclin/CDK complexes that are important in regulating
transcription, DNA repair, differentiation and apoptosis. The
synthesis and destruction of cyclins is one of the primary means of
regulating the cell cycle in vivo (125). CKI, as a negative regulator of the
cell cycle, can be divided into two classes: The Ink4 family and
the Cip/Kip family (126). AIL
exerts its effects by regulating cyclin, CDK and CKI expression.
Cyclins E and B were downregulated and p21 was upregulated in
melanoma (37) and breast cancer
(45), whereas cyclin D1 was
downregulated in lung (41) and
breast (45) cancer, VS (46) and osteosarcoma (47). In hepatocellular carcinoma, the
expression levels of cyclins D and E were inhibited and CDK2, 4 and
6 were decreased, while the expression levels of p21 and p27 was
increased (44). The expression of
CDK4 was downregulated in prostate cancer (48). In addition, the Rb gene, one
of the most important antioncogenes, can be phosphorylated by
cyclin D/CDK4 or cyclin E/CDK2, releasing the transcription factor
E2F. Transcription factor E2F regulates the expression of numerous
genes, including cyclins E and A, cdk1, B-myb, dihydrofolate
reductase, thymidine kinase and DNA polymerase (127). These genes serve an important role
in the cell cycle and DNA synthesis (127,128).
AIL can also act on the retinoblastoma (Rb) gene in HCC,
reducing the expression of Rb protein, which is a positive
regulator of the cell cycle (44).
Deregulation of cell metabolism and proliferation
are major characteristics of tumor cells. AMPKs are activated when
cells are metabolically stressed. AMPK activation regulates various
cellular processes, such as cell proliferation, polarity, autophagy
and apoptosis (129). Gao et
al (45) demonstrated that AIL
inhibited breast cancer cell proliferation by inhibiting AMPK.
Effects on autophagy
Autophagy serves a pivotal role in the cellular
homeostasis of specific tissues, including liver tissue and
skeletal muscle. Its functions include cell survival regulation
(such as the response to metabolic alterations, recycling damaged
macromolecules and organelles) and various forms of programmed cell
death (130). Autophagy can be
considered as a tumor-suppressing process in specific tissues
(131). The detection of
autophagy-associated proteins Beclin-1, p62 and LC3-I/II can be
used to analyze the role of AIL in promoting autophagy (130). Beclin-1 serves an important role in
the formation of autophagosomes, which can initiate autophagy by
binding to type III phosphatidylinositol and triphosphate kinase,
and is considered to be a marker of autophagy initiation (132). p62 is an adaptor molecule involved
in the activation of autophagy, targeting polyubiquitinated protein
aggregates to autophagic lysosomes and degrading autophagic
lysosomes (133). Therefore, p62
expression levels are a reliable indicator of autophagy (133). LC3 protein is sheared at the
carboxyl end by cysteine protease ATg4 with endonuclease activity,
which exposes glycine residues and produces LC3-I localized in the
cytoplasm (134). After being
modified and processed by a ubiquitin-like system, including Atg7
and Atg3, LC3 is covalently bound to phosphatidylethanolamine to
form LC3-II, which is localized to the autophagosome membrane
(135). The LC3-I/II ratio can be
used to assess the rate of autophagy (134). By detecting the aforementioned
proteins, it was demonstrated that AIL induced autophagy in
promyelocytic leukemia (39), lung
cancer (41) and VS (46) cells.
Effects on cell invasion and metastasis
Invasion and metastasis occur in the moderate and
advanced stages of various types of cancer (136,137).
As aforementioned, it has been shown that AIL inhibits metastasis
in cancer cells by modulating molecular targets, including matrix
metalloproteinases (MMPs). MMPs can degrade various protein
components in the extracellular matrix, destroy the histological
barrier of tumor cell invasion and serve a key role in tumor
invasion and metastasis (138).
Overexpression of MMPs, particularly MMP-2 (gelatinase A) and MMP-9
(gelatinase B) has been associated with tumor progression,
metastasis and poor prognosis in breast, lung, colon, gastric,
pancreatic, and prostate cancer (139). Zhang et al (38) and Gao et al (45) demonstrated that the inhibitory effect
of AIL on invasion and migration of breast cancer cells and acute
myeloid leukemia cells was associated with decreased MMP-9
expression.
Effects on drug resistance
Tumor chemotherapy is often accompanied by drug
resistance difficulties. It has been demonstrated that traditional
Chinese medicine plant extracts, such as curcumin (140), matrine (141) and resveratrol (142), can improve drug resistance and
reduce the use of chemotherapy drugs. He et al (48) also confirmed that AIL improved
resistance to MDV3100 in prostate cancer cell lines.
Conclusions
The antitumor effect of AIL involves numerous
mechanisms (Table I); however, the
current research on the underlying mechanisms of AIL function is
still relatively superficial. Based on existing studies, it has
been observed in few studies in this review that the advantages of
AIL as an antitumor agent are its relatively low toxicity and fewer
side effects compared with existing chemotherapeutic drugs
(42,44,48).
However, it cannot be ignored that the median lethal dose in mice
observed by Tang et al (92)
was rated as level 2 (severe). Considering that the research
investigating AIL is still in its infancy, there are few
comparative studies on the efficacy of AIL and existing
chemotherapy drugs. Therefore, this conclusion remains to be
verified. Furthermore, the majority of studies lack in vivo
experiments and clinical trials, and there are no further studies
on the bioavailability and side effects of AIL. Therefore, in
subsequent studies, researchers should focus on the efficacy of AIL
compared with existing chemotherapy drugs, as well as in
vivo and clinical trials. It is hypothesized that in the
future, when its efficacy is demonstrated to be favorable compared
with existing chemotherapy, AIL may be used as an effective novel
anticancer treatment.
 | Table I.Mechanism and biological effects of
ailanthone in cancer cells. |
Table I.
Mechanism and biological effects of
ailanthone in cancer cells.
| A, Melanoma |
|---|
| Author, year | Mechanism | Biological
effects | Model | Ref. |
|---|
| Liu et al,
2019 | G0/S
arrest, G2/M arrest; Apoptotic body formation; ↑p21; ↓
Cyclin E and B; ↓ PI3K, p-PI3K, p-AKT; ↑ Caspase-9,
cleaved-caspase-3, −9; ↓Mitochondrial membrane potential; ↓Bcl-2;
↑Bax; ↑ Cytochrome c; ↑Apaf-1, | ↓ Proliferation;
↑Apoptosis | Human melanoma
cells B16, mouse melanoma cells A375 | (37) |
|
| B, AML |
|
| Author,
year |
Mechanism | Biological
effects | Model | Ref. |
|
| Zhang et al,
2019 | ↑ miR-449a; ↓
Notch1, Notch2, p-PI3K, p-AKT; ↓MMP-9, vimentin;
↑cleaved-caspase-7, −3, −9 | ↑ Apoptosis; ↓
Migration and invasion | Human AML cells
(KG1, HL60, U-937, THP-1 and OCI-AML2) | (38) |
| Wei et al,
2018 |
G0/G1 arrest; ↑
Autophagy; Acidic vesicular organelles (AVOs); ↑ Beclin-1, LC3-II;
↓ p62, LC3-I | ↓ Proliferation;
↑Apoptosis | Human AML cells
HL60 | (39) |
|
| C, Bladder
cancer |
|
| Author,
year |
Mechanism | Biological
effects | Model | Ref. |
|
| Daga et al,
2019 |
G0/G1 arrest; ↓
Nrf2, YAP, c-Myc | ↓ Proliferation; ↓
Migration and invasion | Human Bladder tumor
cells (T24,253J B-V, 253J B-V and, 253J C-r) | (40) |
|
| D, Lung
cancer |
|
| Author,
year |
Mechanism | Biological
effects | Model | Ref. |
|
| Ni et al,
2017 | DNA damage (H1299
and H1975 cells); G1 or G2/M arrest; ↓RPA1; ↑
Cleaved- caspase-3(H1975); ↓DNA replication | ↑ Apoptosis
(H1975); ↓H1975 subcutaneously xenograft and orthotopic lung tumor
growth; ↑Survival of tumor-bearing mice; ↓Viability | Human NSCLC cells
(A549, H1299 and H1975) | (42) |
| Hou et al,
2019 | ↑ miR-195; ↑
Cleaved-caspase-3, −9; ↑ Beclin-1; ↓ p62; ↓ p-PI3K, p-AKT, p-JAK,
p-STAT3 | ↓ Proliferation;
↑Apoptosis; ↑ Autophagy | Human NSCLC cells
A549 | (41) |
|
| E, Gastric
cancer |
|
| Author,
year |
Mechanism | Biological
effects | Model | Ref. |
|
| Chen et al,
2017 | G2/M
arrest; ↓ Bcl-2; ↑ Bax | ↓ Proliferation;
↑Apoptosis; ↓ Viability | Human gastric
cancer cells SGC-7901 | (43) |
|
| F, Liver
cancer |
|
| Author,
year |
Mechanism | Biological
effects | Model | Ref. |
|
| Zhuo et al,
2015 |
G0/G1 arrest;
↓Cyclin D, cyclin E; ↓CDK2, CDK4, CDK6; ↑ p21, p27; ↓ CDC25A; ↓Rb;
↑p-H2AX; DNA damage; ↑ p-ATM, p-ATR; ↑p-Chk1, p-Chk2;
↑Cleaved-caspase-3, −9;↑Cleaved PARP; ↓Mitochondrial membrane
potential; ↓p-PI3K, p-AKT | ↓ Proliferation; ↓
Viability; ↓ Huh7 subcutaneously xenograft | Human hepatic cell
lines (HepG2, Hep3B and Huh7) | (44) |
|
| G, Breast
cancer |
|
| Author,
year |
Mechanism | Biological
effects | Model | Ref. |
|
| Wang et al,
2018 |
G0/G1 arrest;
↓Bcl-2; ↑Bax; ↑Caspase-3; | ↓ Proliferation; ↓
Viability; ↑ Apoptosis | Breast cancer cells
MCF-7 | (27) |
| Gao et al,
2019 | ↑
Cleaved-caspase-3, −9; ↓ MMP-9, vimentin; ↓ CyclinD1; ↑ p53, p21; ↑
miR-148a; ↓ p-AMPK, β-catenin | ↓ Migration and
invasion; ↓ Viability; ↓ Proliferation; ↑ Apoptosis | Breast cancer cells
MDA-MB-231, human non-tumorigenic breast epithelial cells
MCF-12A | (45) |
|
| H, Vestibular
schwannoma |
|
| Author,
year |
Mechanism | Biological
effects | Model | Ref. |
|
| Yang et al,
2018 | ↓ miR-21;
↑Beclin-1; ↓p62, LC3-I; ↑Cleaved-caspase-3, −9; ↓ Viability;
↓Cyclin D1; ↓Ras, Raf, p-MEK, p-ERK, p-mTOR and p-p70S6K | ↓ Proliferation; ↓
Viability; ↑ Apoptosis; ↑ Autophagy | Human vestibular
schwannoma cells | (46) |
|
| I,
Osteosarcoma |
|
| Author,
year |
Mechanism | Biological
effects | Model | Ref. |
|
| Kong et al,
2019 | ↑
Cleaved-caspase-3; ↑ Cleaved-PARP; ↓ CyclinD1; ↓Bcl-2; ↑Bax; ↑
PTEN; ↓ p-PI3K, p-AKT; ↑miR-126 | ↓ Viability; ↓
Proliferation; ↑ Apoptosis; ↓ Migration and invasion | Osteosarcoma cell
lines (MG63, U2OS, HOS and Saos-2), normal osteoblast hFOB1
cells | (47) |
|
| J, Prostate
cancer |
|
| Author,
year |
Mechanism | Biological
effects | Model | Ref. |
|
| He et al,
2016 | ↓ AR protein; ↑ AR
degradation; ↓AKT; ↓CDK4 | ↓ Proliferation;
↓Migration; ↑Drug(MDV3100) sensitivity; ↓Tumor growth and
metastasis (in vivo) | Prostate cancer
cell lines (LNCaP, c4-2b, 22RV1 and LAPC4) | (48) |
Acknowledgements
Not applicable.
Funding
This study was funded by the Traditional Chinese
Medicine Science and Technology Project of Zhejiang Province (grant
no. 2018ZA109), Medical and Health Science and Technology Project
of Zhejiang Province (grant no. 2018ZH026), Natural Science
Foundation of Ningbo (grant nos. 2016A610157 and 2018A610371) and
the Science and Technology Projects of Zhejiang Province (grant no.
LGF19H030007).
Availability of data and materials
Not applicable.
Authors' contributions
HD and ZY conceived and designed the study and
prepared the manuscript. XY, CH, KG and XL were responsible for the
literature search, data visualization and analysis. XL and YJ
searched for the relevant literature and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AIL
|
ailanthone
|
|
AML
|
acute myeloid leukemia
|
|
MMP
|
matrix metalloproteinase
|
|
Nrf2
|
NF-E2-related factor
|
|
YAP
|
Yes-associated protein
|
|
JAK
|
Janus kinase
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
RAF
|
RAF proto-oncogene
serine/threonine-protein kinase
|
|
MEK
|
mitogen-activated protein kinase
kinase
|
|
ERK
|
extracellular signal-regulated
kinases
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
AKT
|
protein kinase B
|
|
PARP
|
poly-ADP-ribose polymerase
|
|
mTOR
|
mammalian target of rapamycin
|
|
Bcl-2
|
B cell lymphoma-2
|
|
Bax
|
Bcl-2-associated X
|
|
AR
|
androgen receptors
|
|
CRPC
|
castration-resistant prostate
cancer
|
|
AMPK
|
AMP-activated protein kinase
|
|
CKI
|
CDK inhibitor
|
References
|
1
|
Blattner WA: Human retroviruses: Their
role in cancer. Proc Assoc Am Physicians. 111:563–572. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomlinson IP, Novelli MR and Bodmer WF:
The mutation rate and cancer. Proc Natl Acad Sci USA.
93:14800–14803. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wogan GN, Hecht SS, Felton JS, Conney AH
and Loeb LA: Environmental and chemical carcinogenesis. Semin
Cancer Biol. 14:473–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Zheng R, Zhang S, Zhang S, Zeng H,
Xia C, Zuo T, Yang Z, Zou X and He J: Cancer incidence and
mortality in China, 2013. Cancer Lett. 401:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tseng HH and He B: Molecular markers as
therapeutic targets in lung cancer. Chin J Cancer. 32:59–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura K, Shinozuka K and Yoshikawa N:
Anticancer and antimetastatic effects of cordycepin, an active
component of cordyceps sinensis. J Pharmacol Sci. 127:53–56. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lumlerdkij N, Tantiwongse J,
Booranasubkajorn S, Boonrak R, Akarasereenont P, Laohapand T and
Heinrich M: Understanding cancer and its treatment in thai
traditional medicine: An ethnopharmacological-anthropological
investigation. J Ethnopharmacol. 216:259–273. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bezwoda WR, MacDonald DF, Gear JS, Derman
DP, Bothwell TH, Sqi S, Hurwitz S and Lewis D: Combination
chemotherapy including bleomycin in the treatment of advanced
hodgkin's disease. S Afr Med J. 53:369–373. 1978.PubMed/NCBI
|
|
12
|
Durant JR, Gams RA, Bartolucci AA and
Dorfman RF: BCNU with and without cyclophosphamide, vincristine,
and prednisone (COP) and cycle-active therapy in non-hodgkin's
lymphoma. Cancer Treat Rep. 61:1085–1096. 1977.PubMed/NCBI
|
|
13
|
Wong MY and Chiu GN: Liposome formulation
of co-encapsulated vincristine and quercetin enhanced antitumor
activity in a trastuzumab-insensitive breast tumor xenograft model.
Nanomedicine. 7:834–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Munker S, Vogelhuber M, Bornschein J,
Stroszczynski C, Evert M, Schlitt H, Herr W and Teufel A: EpiCO
(epirubicin, cyclophosphamide and vincristine) as treatment for
extrapulmonary high-grade neuroendocrine neoplasms. Z
Gastroenterol. 58:133–136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Büyükkapu Bay S, Kebudi R, Görgün O,
Zülfikar B, Darendeliler E and Çakır FB: Vincristine, irinotecan,
and temozolomide treatment for refractory/relapsed pediatric solid
tumors: A single center experience. J Oncol Pharm Pract.
25:1343–1348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weaver BA: How taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caparica R, Bruzzone M, Poggio F, Ceppi M,
de Azambuja E and Lambertini M: Anthracycline and taxane-based
chemotherapy versus docetaxel and cyclophosphamide in the adjuvant
treatment of HER2-negative breast cancer patients: A systematic
review and meta-analysis of randomized controlled trials. Breast
Cancer Res Treat. 174:27–37. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reck M, Brahmer J, Bennett B, Taylor F,
Penrod JR, DeRosa M, Dastani H, Spigel DR and Gralla RJ: Evaluation
of health-related quality of life and symptoms in patients with
advanced non-squamous non-small cell lung cancer treated with
nivolumab or docetaxel in checkmate 057. Eur J Cancer. 102:23–30.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
da Rocha AB, Lopes RM and Schwartsmann G:
Natural products in anticancer therapy. Curr Opin Pharmacol.
1:364–369. 2001. View Article : Google Scholar
|
|
20
|
Wang P, Yang HL, Yang YJ, Wang L and Lee
SC: Overcome cancer cell drug resistance using natural products.
Evid Based Complement Alternat Med. 2015:7671362015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Kong W, Qi X, Wang S, Chen Y, Zhao
Z, Wang W, Lin X, Lai J, Yu Z and Lai G: Icariin induces apoptosis
of human lung adenocarcinoma cells by activating the mitochondrial
apoptotic pathway. Life Sci. 15:1168792019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang P, Zhang M, Yu D, Liu W, Hu L, Zhang
B, Zhou Q and Cao Z: Lycorine inhibits melanoma cell migration and
metastasis mainly through reducing intracellular levels of
beta-catenin and matrix metallopeptidase 9. J Cell Physiol.
234:10566–10575. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Segun PA, Ismail FMD, Ogbole OO, Nahar L,
Evans AR, Ajaiyeoba O and Sarker SD: Acridone alkaloids from the
stem bark of citrus aurantium display selective cytotoxicity
against breast, liver, lung and prostate human carcinoma cells. J
Ethnopharmacol. 227:131–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomar P, Dey YN, Sharma D, Wanjari MM,
Gaidhani S and Jadhav A: Cytotoxic and antiproliferative activity
of kanchnar guggulu, an ayurvedic formulation. J Integr Med.
16:411–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dhiman K: Ayurvedic intervention in the
management of uterine fibroids: A case series. Ayu. 35:303–308.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kasymjanova G, Tran AT, Cohen V, Pepe C,
Sakr L, Small D, Agulnik JS and Jagoe RT: The use of a standardized
Chinese herbal formula in patients with advanced lung cancer: A
feasibility study. J Integr Med. 16:390–395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang R, Lu Y, Li H, Sun L, Yang N, Zhao M,
Zhang M and Shi Q: Antitumor activity of the ailanthus altissima
bark phytochemical ailanthone against breast cancer MCF-7 cells.
Oncol Lett. 15:6022–6028. 2018.PubMed/NCBI
|
|
28
|
Rahman S, Fukamiya N, Ohno N, Tokuda H,
Nishino H, Tagahara, Lee KH and Okano M: Inhibitory effects of
quassinoid derivatives on epstein-barr virus early antigen
activation. Chem Pharm Bull (Tokyo). 45:675–677. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho SK, Jeong M, Jang DS and Choi JH:
Anti-Inflammatory effects of canthin-6-one alkaloids from ailanthus
altissima. Planta Med. 84:527–535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okunade AL, Bikoff RE, Casper SJ, Oksman
A, Goldberg DE and Lewis WH: Antiplasmodial activity of extracts
and quassinoids isolated from seedlings of ailanthus altissima
(Simaroubaceae). Phytother Res. 17:675–677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin M, Yang JH, Lee E, Lu Y, Kwon S, Son
KH, Son KH, Son JK and Chang HW: Antiasthmatic activity of
luteolin-7-O-glucoside from Ailanthus altissima through the
downregulation of T helper 2 cytokine expression and inhibition of
prostaglandin E2 production in an ovalbumin-induced asthma model.
Biol Pharm Bull. 32:1500–1503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rahman S, Fukamiya N, Okano M, Tagahara K
and Lee KH: Anti-Tuberculosis activity of quassinoids. Chem Pharm
Bull (Tokyo). 45:1527–1529. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Melanchauski LS, Broto AP, Moraes TM,
Nasser ALM, Said A, Hawas UW, Rashed K, Vilegas W and Hiruma-Lima
CA: Gastroprotective and antisecretory effects of ailanthus excelsa
(Roxb). J Nat Med. 64:109–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wright CW, O'Neill MJ, Phillipson JD and
Warhurst DC: Use of microdilution to assess in vitro antiamoebic
activities of brucea javanica fruits, simarouba amara stem, and a
number of quassinoids. Antimicrob Agents Chemother. 32:1725–1729.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kundu P and Laskar S: A brief resume on
the genus Ailanthus: Chemical and pharmacological aspects.
Phytochem Rev. 9:379–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kato T, Suzumura Y, Fukushima M, Honda T,
Nakanishi T and Noguchi T: Antitumor activity of novel ailanthone
derivatives in vitro and in vivo. Anticancer Res. 8:573–579.
1988.PubMed/NCBI
|
|
37
|
Liu W, Liu X, Pan Z, Wang D, Li M, Chen X,
Zhou L, Xu M, Li D and Zheng Q: Ailanthone induces cell cycle
arrest and apoptosis in melanoma B16 and A375 cells. Biomolecules.
9:2752019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Zhang C and Min D: Ailanthone
up-regulates miR-449a to restrain acute myeloid leukemia cells
growth, migration and invasion. Exp Mol Pathol. 108:114–120. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei C, Chen C, Cheng Y, Zhu L, Wang Y, Luo
C, He Y, Yang Z and Ji Z: Ailanthone induces autophagic and
apoptotic cell death in human promyelocytic leukemia HL-60 cells.
Oncol Lett. 16:3569–3576. 2018.PubMed/NCBI
|
|
40
|
Daga M, Pizzimenti S, Dianzani C, Cucci
MA, Cavalli R, Grattarola M, Ferrara B, Scariot V, Trotta F and
Barrera G: Ailanthone inhibits cell growth and migration of
cisplatin resistant bladder cancer cells through down-regulation of
Nrf2, YAP, and c-Myc expression. Phytomedicine. 56:156–164. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hou S, Cheng Z, Wang W, Wang X and Wu Y:
Ailanthone exerts an antitumor function on the development of human
lung cancer by upregulating microRNA-195. J Cell Biochem.
120:10444–10451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ni Z, Yao C, Zhu X, Gong C, Xu Z, Wang L,
Li S, Zou C and Zhu S: Ailanthone inhibits non-small cell lung
cancer cell growth through repressing DNA replication via
downregulating RPA1. Br J Cancer. 117:1621–1630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Y, Zhu L, Yang X, Wei C, Chen C, He Y
and Ji Z: Ailanthone induces G2/M cell cycle arrest and apoptosis
of SGC7901 human gastric cancer cells. Mol Med Rep. 16:6821–6827.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhuo Z, Hu J, Yang X, Chen M, Lei X, Deng
L, Yao N, Peng Q, Chen Z, Ye W and Zhang D: Ailanthone inhibits
huh7 cancer cell growth via cell cycle arrest and apoptosis in
vitro and in vivo. Sci Rep. 5:161852015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao W, Ge S and Sun J: Ailanthone exerts
anticancer effect by up-regulating miR-148a expression in
MDA-MB-231 breast cancer cells and inhibiting proliferation,
migration and invasion. Biomed Pharmacother. 109:1062–1069. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang P, Sun D and Jiang F: Ailanthone
promotes human vestibular schwannoma cell apoptosis and autophagy
by downregulation of miR-21. Oncol Res. 26:941–948. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kong D, Ying B, Zhang J and Ying H: The
anti-osteosarcoma property of ailanthone through regulation of
miR-126/VEGF-A axis. Artif Cells Nanomed Biotechnol. 47:3913–3919.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
He Y, Peng S, Wang J, Chen H, Cong X, Chen
A, Hu M, Qin M, Wu H, Gao S, et al: Ailanthone targets p23 to
overcome MDV3100 resistance in castration-resistant prostate
cancer. Nat Commun. 7:131222016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Han F, Liu G, Sun C and Wei J: Ailanthone
reverses multidrug resistance by inhibiting the
P-glycoprotein-mediated efflux in resistant K562/A02 cells. Cell
Mol Biol (Noisy-le-grand). 64:55–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guy GP Jr, Thomas CC, Thompson T, Watson
M, Massetti GM and Richardson LC; Centers for Disease Control and
Prevention (CDC), : Vital signs: Melanoma incidence and mortality
trends and projections - United States, 1982-2030. MMWR Morb Mortal
Wkly Rep. 64:591–596. 2015.PubMed/NCBI
|
|
51
|
Lens MB and Dawes M: Global perspectives
of contemporary epidemiological trends of cutaneous malignant
melanoma. Br J Dermatol. 150:179–185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yamamoto JF and Goodman MT: Patterns of
leukemia incidence in the United States by subtype and demographic
characteristics, 1997-2002. Cancer Causes Control. 19:379–390.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shin VY and Chu KM: miRNA as potential
biomarkers and therapeutic targets for gastric cancer. World J
Gastroenterol. 20:10432–10439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen YJ, Guo YN, Shi K, Huang HM, Huang
SP, Xu WQ, Li ZY, Wei KL, Gan TQ and Chen G: Down-Regulation of
microRNA-144-3p and its clinical value in non-small cell lung
cancer: A comprehensive analysis based on microarray,
miRNA-sequencing, and quantitative real-time PCR data. Respir Res.
20:482019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yuan G, Zhao Y, Wu D, Gao C and Jiao Z:
miRNA-20a upregulates TAK1 and increases proliferation in
osteosarcoma cells. Future Oncol. 14:461–469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
De Luca L, Trino S, Laurenzana I,
Tagliaferri D, Falco G, Grieco V, Bianchino G, Nozza F, Campia V,
D'Alessio F, et al: Knockdown of miR-128a induces Lin28a expression
and reverts myeloid differentiation blockage in acute myeloid
leukemia. Cell Death Dis. 8:e28492017. View Article : Google Scholar
|
|
57
|
Elhamamsy AR, El Sharkawy MS, Zanaty AF,
Mahrous MA, Mohamed AE and Abushaaban EA: Circulating miR-92a,
miR-143 and miR-342 in plasma are novel potential biomarkers for
acute myeloid leukemia. Int J Mol Cell Med. 6:77–86.
2017.PubMed/NCBI
|
|
58
|
Li Q, Peng J, Li X, Leng A and Liu T:
MiR-449a targets Flot2 and inhibits gastric cancer invasion by
inhibiting TGF-beta-mediated EMT. Diagn Pathol. 10:2022015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sandbothe M, Buurman R, Reich N, Greiwe L,
Vajen B, Gürlevik E, Schäffer V, Eilers M, Kühnel F, Vaquero A, et
al: The microRNA-449 family inhibits TGF-beta-mediated liver cancer
cell migration by targeting SOX4. J Hepatol. 66:1012–1021. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
DeGeorge KC, Holt HR and Hodges SC:
Bladder cancer: Diagnosis and treatment. Am Fam Physician.
96:507–514. 2017.PubMed/NCBI
|
|
61
|
Nadal R and Bellmunt J: Management of
metastatic bladder cancer. Cancer Treat Rev. 76:10–21. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ciamporcero E, Daga M, Pizzimenti S,
Roetto A, Dianzani C, Compagnone A, Palmieri A, Ullio C, Cangemi L,
Pili R and Barrera G: Crosstalk between Nrf2 and YAP contributes to
maintaining the antioxidant potential and chemoresistance in
bladder cancer. Free Radic Biol Med. 115:447–457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rojo de la Vega M, Chapman E and Zhang DD:
NRF2 and the hallmarks of cancer. Cancer Cell. 34:21–43. 2018.
View Article : Google Scholar
|
|
64
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang Y, Zhang X, Zou C, Kung HF, Lin MC,
Dress A, Wardle F, Jiang BH and Lai L: miR-195 inhibits tumor
growth and angiogenesis through modulating IRS1 in breast cancer.
Biomed Pharmacother. 80:95–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu B, Qu J, Xu F, Guo Y, Wang Y, Yu H and
Qian B: miR-195 suppresses non-small cell lung cancer by targeting
CHEK1. Oncotarget. 6:9445–9456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yu S, Jing L, Yin XR, Wang MC, Chen YM,
Guo Y, Nan KJ and Han LL: miR-195 suppresses the metastasis and
epithelial-mesenchymal transition of hepatocellular carcinoma by
inhibiting YAP. Oncotarget. 8:99757–99771. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang X, Tao T, Liu C, Guan H, Huang Y, Xu
B and Chen M: Downregulation of miR-195 promotes prostate cancer
progression by targeting HMGA1. Oncol Rep. 36:376–382. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Brackmann DE: Vestibular schwannoma
(acoustic neuroma). Otolaryngol Clin North Am. 45:xiii–xv. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rosahl S, Bohr C, Lell M, Hamm K and Iro
H: Diagnosis and management of vestibular schwannomas - an
interdisciplinary challenge. Laryngorhinootologie. 96:S152–S182.
2017.(In German). PubMed/NCBI
|
|
72
|
Feng YH, Wu CL, Tsao CJ, Chang JG, Lu PJ,
Yeh KT, Uen YH, Lee JC and Shiau AL: Deregulated expression of
sprouty2 and microRNA-21 in human colon cancer: Correlation with
the clinical stage of the disease. Cancer Biol Ther. 11:111–121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu X, Zhuo S, Zheng C and Gao G:
MicroRNA-21 correlates TGF-beta1 pathway of pancreatic ductal
adenocarcinoma. Zhong Nan Da Xue Xue Bao. 44:749–756. 2019.(In
Chinese). PubMed/NCBI
|
|
74
|
Bharali D, Banerjee BD, Bharadwaj M,
Husain SA and Kar P: Expression analysis of microrna-21 and
microrna-122 in hepatocellular carcinoma. J Clin Exp Hepatol.
9:294–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kim YH, Goh TS, Lee CS, Oh SO, Kim JI,
Jeung SH and Pak K: Prognostic value of microRNAs in osteosarcoma:
A meta-analysis. Oncotarget. 8:8726–8737. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hesse E and Taipaleenmaki H: MicroRNAs in
bone metastasis. Curr Osteoporos Rep. 17:122–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sasaki R, Osaki M and Okada F:
MicroRNA-Based diagnosis and treatment of metastatic human
osteosarcoma. Cancers. 11:5532019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Huggins C and Hodges CV: Studies on
prostatic cancer. I. The effect of castration, of estrogen and
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate. CA Cancer J Clin. 22:232–240. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Koochekpour S: Androgen receptor signaling
and mutations in prostate cancer. Asian J Androl. 12:639–657. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Harris WP, Mostaghel EA, Nelson PS and
Montgomery B: Androgen deprivation therapy: Progress in
understanding mechanisms of resistance and optimizing androgen
depletion. Nat Clin Pract Urol. 6:76–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ryan CJ, Smith MR, Fizazi K, Saad F,
Mulders PFA, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small
EJ, et al: Abiraterone acetate plus prednisone versus placebo plus
prednisone in chemotherapy-naive men with metastatic
castration-resistant prostate cancer (COU-AA-302): final overall
survival analysis of a randomised, double-blind, placebo-controlled
phase 3 study. Lancet Oncol. 16:152–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al: Alpha emitter radium-223 and survival in metastatic prostate
cancer. N Engl J Med. 369:213–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Beer TM, Armstrong AJ, Rathkopf DE, Loriot
Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J,
Chowdhury S, et al: Enzalutamide in metastatic prostate cancer
before chemotherapy. N Engl J Med. 371:424–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-V7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cano LQ, Lavery DN and Bevan CL:
Mini-Review: Foldosome regulation of androgen receptor action in
prostate cancer. Mol Cell Endocrinol. 369:52–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tao WW, Jiang H, Tao XM, Jiang P, Sha LY
and Sun XC: Effects of acupuncture, tuina, tai chi, qigong, and
traditional Chinese medicine five-element music therapy on symptom
management and quality of life for cancer patients: A
meta-analysis. J Pain Symptom Manage. 51:728–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tang S, Ma X, Lu J, Zhang Y, Liu M and
Wang X: Preclinical toxicology and toxicokinetic evaluation of
ailanthone, a natural product against castration-resistant prostate
cancer, in mice. Fitoterapia. 136:1041612019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Winder C, Azzi R and Wagner D: The
development of the globally harmonized system (GHS) of
classification and labelling of hazardous chemicals. J Hazard
Mater. 125:29–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. BioMed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cory S, Roberts AW, Colman PM and Adams
JM: Targeting BCL-2-like proteins to kill cancer cells. Trends
Cancer. 2:443–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lowe SW, Schmitt EM, Smith SW, Osborne BA
and Jacks T: P53 is required for radiation-induced apoptosis in
mouse thymocytes. Nature. 362:847–849. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kastenhuber ER and Lowe SW: Putting p53 in
context. Cell. 170:1062–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang Z, Wang N, Liu P and Xie X: AMPK and
cancer. Exp Suppl. 107:203–226. 2016.PubMed/NCBI
|
|
102
|
Aziz SA, Jilaveanu LB, Zito C, Camp RL,
Rimm DL, Conrad P and Kluger HM: Vertical targeting of the
phosphatidylinositol-3 kinase pathway as a strategy for treating
melanoma. Clin Cancer Res. 16:6029–6039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Brachmann SM, Hofmann I, Schnell C,
Fritsch C, Wee S, Lane H, Wang S, Echeverria CG and Maira SM:
Specific apoptosis induction by the dual PI3K/mTor inhibitor
NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells.
Proc Natl Acad Sci USA. 106:22299–22304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Martinelli E, Troiani T, D'Aiuto E,
Morgillo F, Vitagliano D, Capasso A, Costantino S, Ciuffreda LP,
Merolla F, Vecchione L, et al: Antitumor activity of pimasertib, a
selective MEK 1/2 inhibitor, in combination with PI3K/mTOR
inhibitors or with multi-targeted kinase inhibitors in
pimasertib-resistant human lung and colorectal cancer cells. Int J
Cancer. 133:2089–2101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lim HJ, Crowe P and Yang JL: Current
clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment
of human cancer. J Cancer Res Clin Oncol. 141:671–689. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hassan B, Akcakanat A, Holder AM and
Meric-Bernstam F: Targeting the PI3-kinase/Akt/mTOR signaling
pathway. Surg Oncol Clin N Am. 22:641–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Panda M and Biswal BK: Cell signaling and
cancer: A mechanistic insight into drug resistance. Mol Biol Rep.
46:5645–5659. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Koskela HL, Eldfors S, Ellonen P, van
Adrichem AJ, Kuusanmäki H, Andersson EI, Lagström S, Clemente MJ,
Olson T, Jalkanen SE, et al: Somatic STAT3 mutations in large
granular lymphocytic leukemia. N Engl J Med. 366:1905–1913. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Cocchiola R, Rubini E, Altieri F,
Chichiarelli S, Paglia G, Romaniello D, Carissimi S, Giorgi A,
Giamogante F, Macone A, et al: STAT3 post-translational
modifications drive cellular signaling pathways in prostate cancer
cells. Int J Mol Sci. 20:8152019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Horiguchi A, Oya M, Shimada T, Uchida A,
Marumo K and Murai M: Activation of signal transducer and activator
of transcription 3 in renal cell carcinoma: A study of incidence
and its association with pathological features and clinical
outcome. J Urol. 168:762–765. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhu H, Chang LL, Yan FJ, Hu Y, Zeng CM,
Zhou TY, Yuan T, Ying MD, Cao J, He QJ and Yang B: AKR1C1 activates
STAT3 to promote the metastasis of non-small cell lung cancer.
Theranostics. 8:676–692. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Nishimoto A, Kugimiya N, Hosoyama T, Enoki
T, Li TS and Hamano K: JAB1 regulates unphosphorylated STAT3
DNA-binding activity through protein-protein interaction in human
colon cancer cells. Biochem Biophy Res Commun. 438:513–518. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao
AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL and Xie K: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wu M, Song D, Li H, Yang Y, Ma X, Deng S,
Ren C and Shu X: Negative regulators of STAT3 signaling pathway in
cancers. Cancer Manag Res. 11:4957–4969. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Stirewalt DL, Kopecky KJ, Meshinchi S,
Appelbaum FR, Slovak ML, Willman CL and Radich JP: FLT3, RAS, and
TP53 mutations in elderly patients with acute myeloid leukemia.
Blood. 97:3589–3595. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol. 4:a0080522012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tan SH and Barker N: Wnt signaling in
adult epithelial stem cells and cancer. Prog Mol Biol Transl Sci.
153:21–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Taketo MM: Shutting down wnt
signal-activated cancer. Nat Genet. 36:320–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Radtke F and Raj K: The role of Notch in
tumorigenesis: Oncogene or tumour suppressor? Nat Rev Cancer.
3:756–767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Huang T, Zhou Y, Cheng AS, Yu J, To KF and
Kang W: NOTCH receptors in gastric and other gastrointestinal
cancers: Oncogenes or tumor suppressors? Mol Cancer. 15:802016.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lobry C, Oh P, Mansour MR, Look AT and
Aifantis I: Notch signaling: Switching an oncogene to a tumor
suppressor. Blood. 123:2451–2459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xu X, Zhao Y, Xu M, Dai Q, Meng W, Yang J
and Qin R: Activation of Notch signal pathway is associated with a
poorer prognosis in acute myeloid leukemia. Med Oncol.
28:S483–S489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ono M, Takimoto R, Osuga T, Okagawa Y,
Hirakawa M, Yoshida M, Arihara Y, Uemura N, Hayasaka N, Miura S, et
al: Targeting notch-1 positive acute leukemia cells by novel
fucose-bound liposomes carrying daunorubicin. Oncotarget.
7:38586–38597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Johnson DG and Walker CL: Cyclins and cell
cycle checkpoints. Ann Rev Pharmacol Toxicol. 39:295–312. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Geng Y, Eaton EN, Picon M, Roberts JM,
Lundberg AS, Gifford A, Sardet C and Weinberg RA: Regulation of
cyclin E transcription by E2Fs and retinoblastoma protein.
Oncogene. 12:1173–1180. 1996.PubMed/NCBI
|
|
128
|
Ohtani K, DeGregori J and Nevins JR:
Regulation of the cyclin E gene by transcription factor E2F1. Proc
Natl Acad Sci USA. 92:12146–12150. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Luo Z, Zang M and Guo W: AMPK as a
metabolic tumor suppressor: Control of metabolism and cell growth.
Future Oncol. 6:457–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Galluzzi L, Bravo-San Pedro JM, Levine B,
Green DR and Kroemer G: Pharmacological modulation of autophagy:
Therapeutic potential and persisting obstacles. Nat Rev Drug
Discov. 16:487–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Choi KS: Autophagy and cancer. Exp Mol
Med. 44:109–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Komatsu M, Waguri S, Koike M, Sou YS, Ueno
T, Hara T, Mizushima N, Iwata JI, Ezaki J, Murata S, et al:
Homeostatic levels of p62 control cytoplasmic inclusion body
formation in autophagy-deficient mice. Cell. 131:1149–1163. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Tanida I, Minematsu-Ikeguchi N, Ueno T and
Kominami E: Lysosomal turnover, but not a cellular level, of
endogenous LC3 is a marker for autophagy. Autophagy. 1:84–91. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tanida I, Ueno T and Kominami E: LC3 and
Autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Mundade R, Imperiale TF, Prabhu L, Loehrer
PJ and Lu T: Genetic pathways, prevention, and treatment of
sporadic colorectal cancer. Oncoscience. 1:400–406. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Coleman MP: Cancer survival: Global
surveillance will stimulate health policy and improve equity.
Lancet. 383:564–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Hung WC, Tseng WL, Shiea J and Chang HC:
Skp2 overexpression increases the expression of MMP-2 and MMP-9 and
invasion of lung cancer cells. Cancer Lett. 288:156–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Karthika C and Sureshkumar R: Can curcumin
along with chemotherapeutic drug and lipid provide an effective
treatment of metastatic colon cancer and alter multidrug
resistance? Med Hypotheses. 132:1093252019. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
An Q, Han C, Zhou Y, Li F, Li D, Zhang X,
Yu Z, Duan Z and Kan Q: Matrine induces cell cycle arrest and
apoptosis with recovery of the expression of miR-126 in the A549
non-small cell lung cancer cell line. Mol Med Rep. 14:4042–4048.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Bjorklund M, Roos J, Gogvadze V and
Shoshan M: Resveratrol induces SIRT1- and energy-stress-independent
inhibition of tumor cell regrowth after low-dose platinum
treatment. Cancer Chemother Pharmacol. 68:1459–1467. 2011.
View Article : Google Scholar : PubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBI
|