Introduction
Cervical carcinoma is the second most common
malignancy among females worldwide. It is preceded by a long
precursor phase, characterized by cervical intraepithelial
neoplasia (CIN), which may persist for 10–20 years. High-grade CIN
is the precursor of cervical cancer. Certain evidence indicated
that spontaneous regression occurs in ~40% of cases of CIN2
(1), suggesting that these lesions
probably have characteristics of being less aggressive compared to
CIN3 or more severe lesions. The regression rate of CIN2 is similar
to that of CIN1 in a 2-year follow-up period (2). At present, histopathological assessment
is unable to differentiate high-grade CIN lesions from others and
predict whether they may regress spontaneously. Certain prognostic
biomarkers may be helpful in this differentiation (3).
Important progress in the etiological study of
cervical cancer has been made in the last decade, and it is now
widely accepted that high-risk human papillomavirus (HR-HPV) is the
central cause of cervical cancer and precursor lesions (4). HPV E6/E7 mRNA, which is translated into
E6 and E7 proteins, is able to directly reflect the target of
malignant transformation of host cells. Testing HPV E6/E7 mRNA
transcription is considered as the gold standard for verifying the
presence of HPV. As a novel RNA in situ detection method,
the RNAscope in situ hybridization (ISH) method is able to
directly detect HPV, specifically in the process of E6/E7 mRNA
transcription, thus providing evidence of HPV transcription
activity in cells. This method may avoid errors of other indirect
methods (5). It has been reported
that the sensitivity and specificity of HPV E6/E7 mRNA detected by
the RNAscope ISH method is similar to that of p16 and Ki-67
detected by immunohistochemistry (IHC) in head and neck tumors
(6). The 2 combination algorithms
for the RNAscope HPV test and p16-IHC were reported to be superior
to p16-IHC alone in predicting the prognosis of oropharyngeal
cancers (7).
In cervical cancer detection, HPV E6/E7 mRNA is
useful for the differential diagnosis of challenging low-grade
squamous intraepithelial lesion (LSIL) vs. benign reactive change
(8). While the role of single
factors, e.g. p16 and HPVE6/E7 mRNA, has been demonstrated in
previous studies, it remains elusive whether HPV E6/E7 mRNA has a
role in the progression of CIN2 lesions. The objective of the
present study was to evaluate the usefulness of HPV E6/E7 mRNA and
p16 in predicting the evolution of CIN2 in patients without prior
treatment and to provide an objective basis for guiding clinical
treatment.
Materials and methods
Patient selection and study
design
A prospective study was performed from the files of
the Department of Pathology at the Peking University People's
Hospital (Beijing, China) between March 2013 and March 2016. In
total, 108 cases that were histologically confirmed to have CIN2 as
the most severe lesion at the time of the initial diagnosis and
those who preferred cautious waiting rather than immediate
treatment were included in the study. Patients who remained
untreated for 6 months or longer after the diagnosis of CIN2 were
also included in the study. The exclusion criteria were as follows:
Pregnancy at the time of diagnosis, a previous diagnosis of CIN3 or
more severe lesions, as well as treatments, including cervical
conization, loop electrosurgical excision procedure or
hysterectomy, prior to diagnosis (9). This study was approved by the Ethics
Committee of Peking University People's Hospital (Beijing, China)
and informed consent was waived.
In situ hybridization
HR-HPV RNAscope ISH was performed by using
RNAscope® 2.5 HD assay-brown to detect HPV E6/E7 mRNA,
including HPV type 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56,
58, 59, 66, 68, 73 and 82 (‘HR RNA18’ ISH, cocktail probe).
RNAscope assays were performed following the manufacturer's
instructions. Ubiquitin controls were performed using HeLa control
slides provided by Advanced Cell Diagnostics. After unstained
slides were cut, directed punches from formalin-fixed,
paraffin-embedded tissues were taken from areas of morphologic
correlation with H&E slides. Positive signals were recorded for
dark-brown, dot-like cytoplasmic and/or nuclear staining, while a
slide with no staining was considered as ‘negative’ (8). Each case was examined alongside a
positive control (cervical squamous cell carcinoma) and a negative
control (normal cervical squamous epithelium).
IHC
For p16INK4a detection, the CINtec
Histology Kit (clone E6H4; Roche Diagnostics) was used following
the manufacturer's protocol. IHC was performed with the Ventana
Benchmark XT-automatic staining machine. ‘Positivity’ referred to
nuclear and/or cytoplasm staining and was defined as a continuous
staining of cells in the basal and para-basal layers, with or
without staining of superficial squamous cell layers. A ‘negative’
slide was defined as having no staining, staining of only isolated
cells or small cell clusters, or non-continuous staining (i.e.
usually patchy or focal cell pattern) (10,11).
Ki-67 rat anti-human monoclonal antibody (cat. no.
EP5; 1:200) was obtained from Beijing Zhongshan Jinqiao
Biotechnology Co. Positive Ki-67 scores of 1, 2 and 3 were assigned
to samples in which nuclear staining was detectable in <5, 6–25
and >25%, respectively, of the epithelial region (excluding
basal cells), the IHC of Ki-67 were performed following the
manufacturer's instructions (12).
Thin-prep cytologic test (TCT)
TCTs were performed by using the Thin-Prep T2000
slide processor (Hologic) and stained using the Papanicolaou
method. Cytological diagnoses were made according to the Bethesda
System (13).
Pathological evaluation
All of the H&E-stained slides, p16, Ki-67
immunostaining and HPV E6/E7 mRNA were independently reviewed by 2
pathologists, XZ and DS blinded to the previous results.
H&E-stained slides and the results for p16, Ki-67 and HPVE6/E7
mRNA were reviewed.
Follow-up
A follow-up visit was performed at least 6 months
after the initial CIN2 diagnosis. At the follow-up visit, cervical
samples were processed for Pap tests and HR-HPV testing. Certain
patients underwent colposcopy and cervical biopsy. The progression
of cervical lesions was defined as follows (14,15).
Progression: High-grade squamous intraepithelial lesion (HSIL)/CIN3
or squamous cell carcinoma by histological diagnosis, except for
adenocarcinoma in situ or cervical adenocarcinoma.
Regression: A lesion with a histological diagnosis lower than CIN2,
including negative cytologic or biopsy findings, LSIL or atypical
squamous cells of undetermined significance as the most severe
diagnosis after follow-up. Persistence: Histological CIN2 was
diagnosed.
Statistical analysis
The data were analyzed by SPSS statistics v25.0
software (IBM Corp.). The positive and negative proportions of p16
and HPV E6/E7 mRNA were determined among the cases. The association
of positive HPV E6/E7 mRNA and positive p16 with subsequent CIN3
were analyzed using Fisher's exact tests. Receiver operating
characteristic (ROC) curves were plotted with GraphPad Prism 8.0
software (GraphPad Software, Inc.) and areas under the curve (AUCs)
were determined for further assessment of the prognostic value.
P<0.05 was considered to indicate statistical significance and
predict prognosis of CIN2.
Results
Clinicopathological
characteristics
In total, 108 cases with biopsy specimens diagnosed
as CIN2 and available follow-up data were included. The patients'
age ranged from 19 to 66 years, with a mean age of 46.3 years.
Follow-up intervals ranged from 6 to 36 months, with a mean of 26.3
months and a median of 20 months.
Of the 108 cases with CIN2 status, 20 cases
progressed to HSIL/CIN3. Patients who progressed to CIN3 were
immediately treated by cone resection. In none of the cases,
progression to invasive squamous cell carcinoma was observed. There
were 36 cases with persistence of CIN2 after follow-up and 52 cases
exhibited regression to grades ≤LSIL/CIN1. This was at the
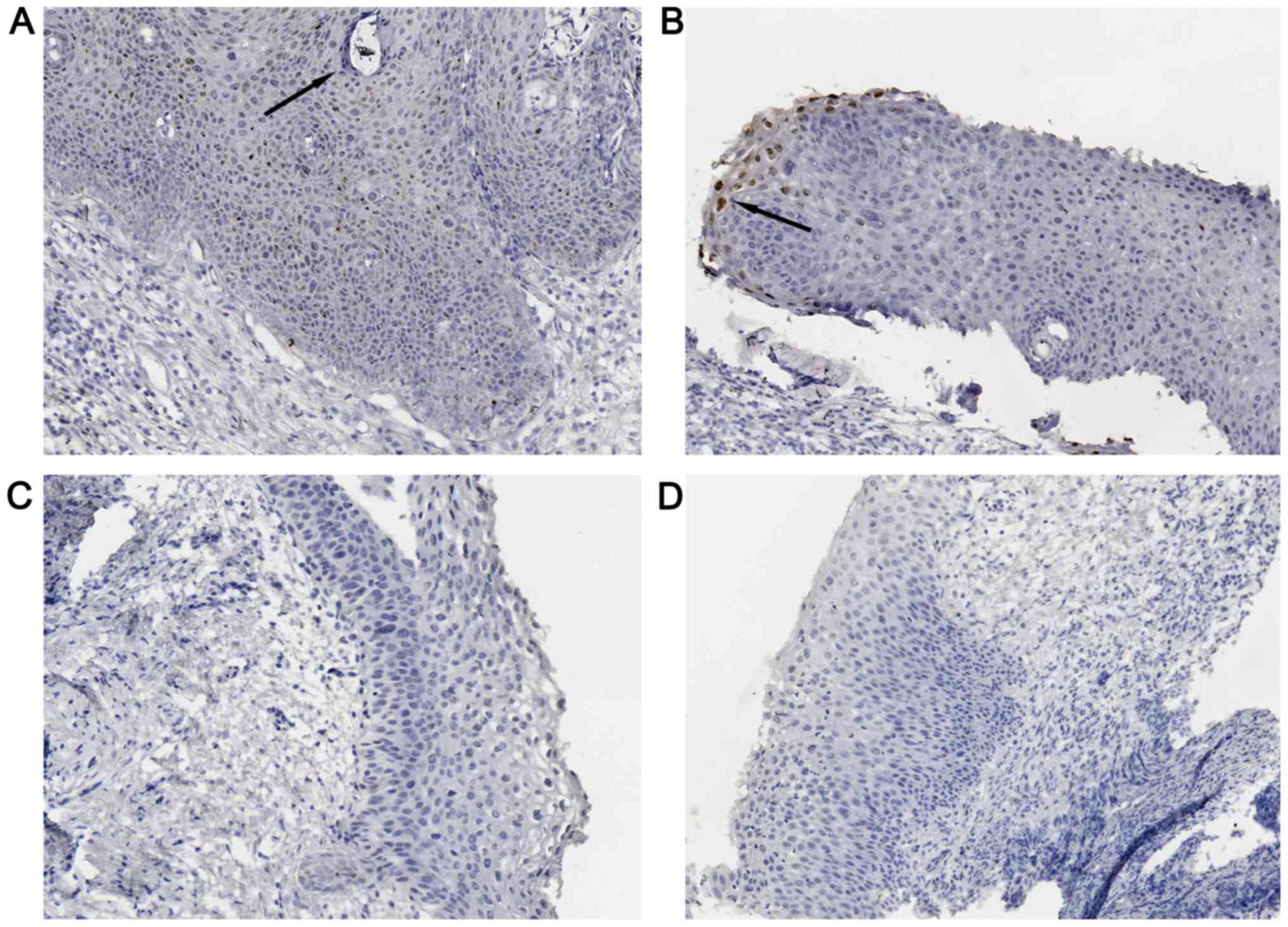
individual end of follow-up for each patient. Representative images
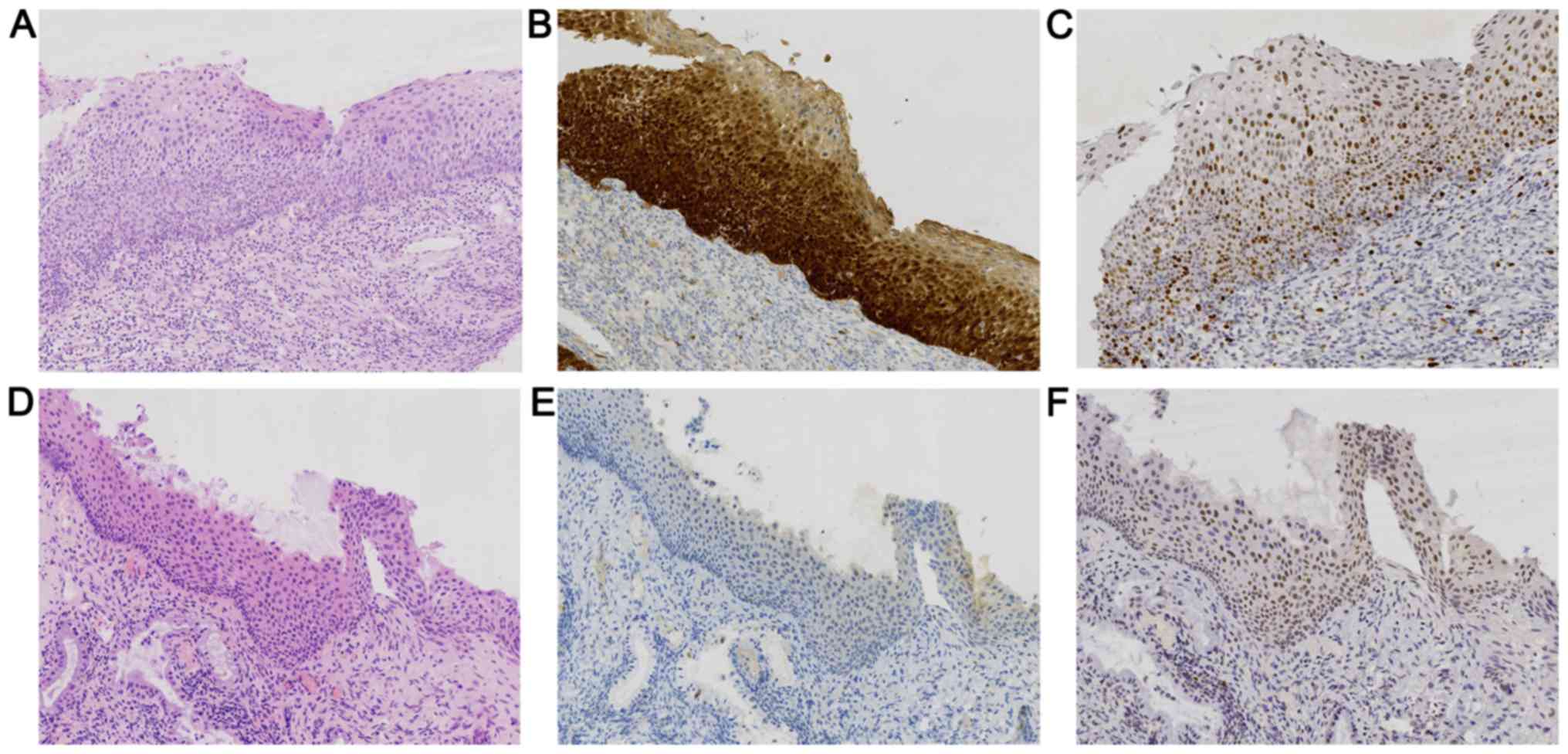
of IHC detection of E6/E7 mRNA are displayed in Fig. 1 and those for p16 and Ki-67 protein
are provided in Fig. 2.
p16INK4a and Ki-67 HPV
E6/E7 mRNA expression and influence on the prognosis of CIN2
Among the 108 CIN2 cases, the positive rate of p16
expression was 75.9% (82/108) and the negative rate of p16 was
24.1% (26/108; Table I). Of the
p16-positive cases, 20 cases were detected to have progression, 28
cases had persistence and 34 cases had regression at the individual
end of follow-up for each patient. In the p16-negative group, no
progression occurred in any of the patients, 8 cases had
persistence and 18 cases exhibited regression. There were
statistically significant differences among these groups as
indicated by Fisher's test (P<0.05). For predicting progression,
the sensitivity of p16 was 100% (20/20+0) and the specificity was
34.6% (18/18+34; Table II).
 | Table I.Association of different variables
with the prognosis of CIN2. |
Table I.
Association of different variables
with the prognosis of CIN2.
|
|
| Prognosis of
CIN2 |
|
|---|
|
|
|
|
|
|---|
| Feature | Total | Progression | Persistence | Regression | P-value |
|---|
| p16 |
|
|
|
| 0.002 |
| (+) | 82 (75.9) | 20 (18.5) | 28 (25.9) | 34 (31.5) |
|
| (−) | 26 (24.1) | 0 (0) | 8 (7.4) | 18 (16.7) |
|
| Ki-67 staining
(%) |
|
|
|
| 0.027 |
|
<5 | 8 (7.4) | 0 (0) | 2 (1.8) | 6 (5.6) |
|
| 6-25 | 54 (50.0) | 5 (4.6) | 20 (18.5) | 29 (26.9) |
|
|
>25 | 46 (42.6) | 15 (13.9) | 14 (13.0) | 17 (15.7) |
|
| HPVE6/E7 |
|
|
|
| 0.014 |
|
(+) | 69 (63.9) | 18 (16.7) | 19 (17.6) | 32 (29.6) |
|
|
(−) | 39 (36.1) | 2 (1.9) | 17 (15.7) | 20 (18.5) |
|
| Gland
involvement |
|
|
|
| 0.191 |
|
Yes | 14 (13.0) | 6 (5.6) | 5 (4.6) | 3 (2.8) |
|
| No | 94 (87.0) | 14 (13.0) | 31 (28.7) | 49 (45.3) |
|
| Age (years) |
|
|
|
| 0.082 |
|
≤30 | 27 (25.0) | 6 (5.6) | 9 (8.3) | 12 (11.1) |
|
|
>30 | 81 (75.0) | 14 (13.0) | 27 (25.0) | 40 (37.0) |
|
 | Table II.Value of p16 and HPVE6/E7 expression
and association with subsequent CIN3. |
Table II.
Value of p16 and HPVE6/E7 expression
and association with subsequent CIN3.
| Expression
status | Total positive
cases | Subsequent
CIN3 | Sensitivity
(%) | Specificity
(%) | Positive predictive
value (%) | Negative predictive
value (%) |
|---|
| P16+ | 82 (75.9) | 20 (18.5) | 100 | 34.6 | 37.0 | 100.0 |
| HPVE6/E7+ | 69 (63.9) | 18 (16.7) | 90.0 | 38.5 | 36.0 | 90.9 |
| P16+HPVE6/E7+ | 59 (54.6) | 18 (16.7) | 90.0 | 34.2 | 41.9 | 100.0 |
The positive rate of HPV E6/E7 mRNA expression was
63.9% (69/108) and the negative rate was 36.1% (39/108; Table I). In the HPV E6/E7 mRNA-positive
group, 18 cases were determined to have progression, 19 cases had
persistence and 32 cases exhibited regression. In the HPV E6/E7
mRNA-negative group, 2 cases were detected to have progression, 17
cases had persistence and 20 cases exhibited regression. There were
statistically significant differences among these groups according
to Fisher's test (P<0.05). For predicting progression, the
sensitivity of HPV E6/E7 mRNA was 90.0% (18/18+2) and the
specificity was 38.5% (20/20+32; Table
II).
For 59/108 cases, p16 and HPV E6/E7 mRNA were
positive in the same patient. The rate of progression to HSIL/CIN3
for p16+/HPV E6/E7 mRNA+ cases was 16.7% (18/108). For predicting
progression, the sensitivity and specificity of p16+/HPV E6/E7
mRNA+ was 90.0 and 34.2%, respectively (Table II).
Regarding staining for Ki-67, none of the eight
cases with <5% Ki-67 was detected to have progression, while
5/54 cases in the 6–25% Ki-67 group and 15/46 cases in the >25%
Ki-67 group exhibited progression. There were statistically
significant differences among the groups (P<0.05).
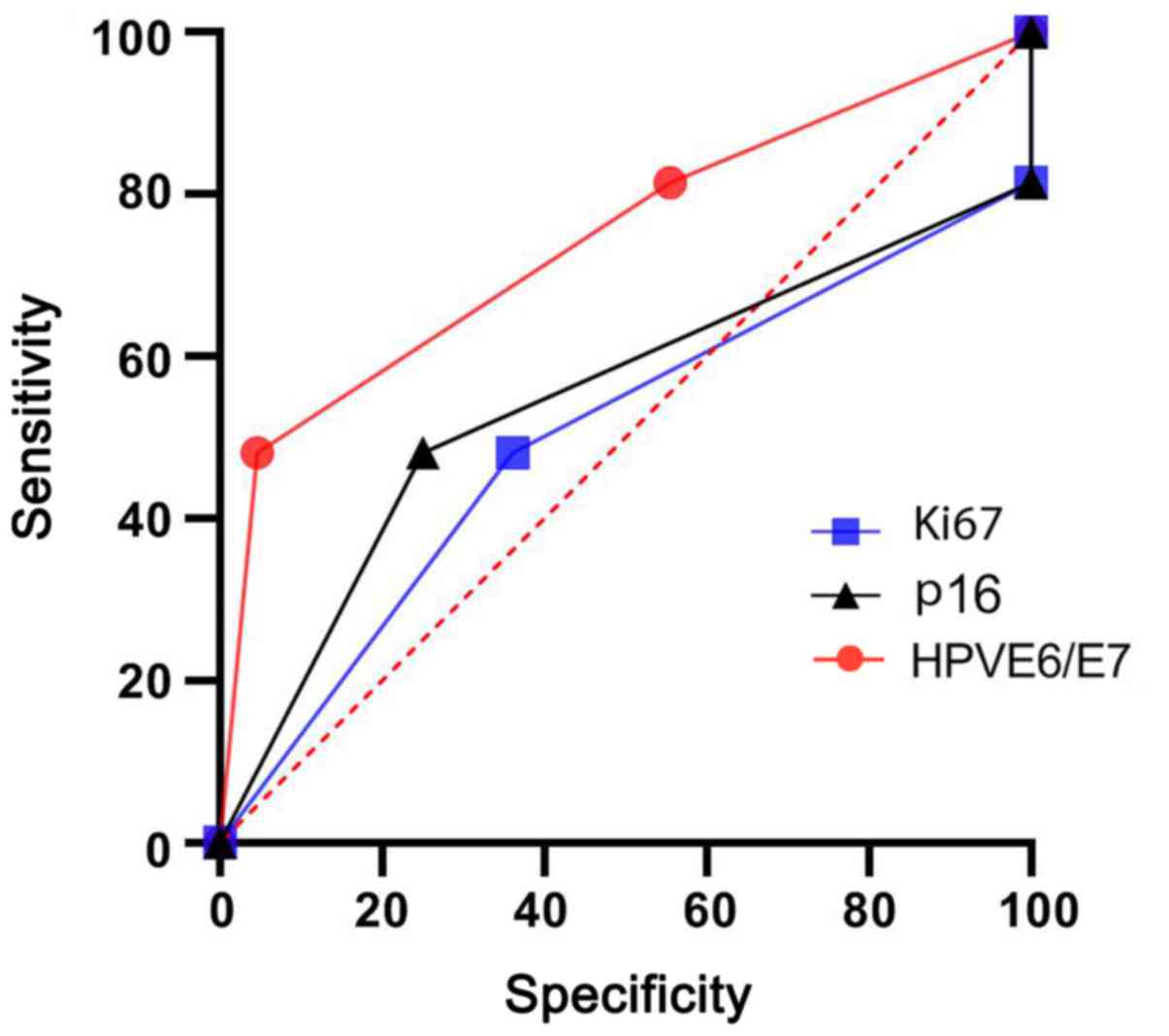
According to the association of HPV E6/E7 mRNA, P16,
Ki-67 with the progression to CIN3 results, the ROCs were plotted.
The AUC of HPV E6/E7 mRNA, P16 and Ki-67 was 0.745, 0.546 and
0.501, respectively (Fig. 3).
Influence of age and gland involvement
on the prognosis of patients with CIN2
All 108 cases were divided into 2 groups based on
age. Of the patients aged ≤30 years, 6/27 cases exhibited
progression, and among those aged >30 years, 14/81 cases had
progression (P>0.05 according to Fisher's test). Furthermore, 14
cases had gland involvement, while 94 cases had no gland
involvement (P>0.05).
Discussion
The name and grading system of CIN has been
officially formalized since 2003 (16). However, this naming system has been
controversial, as the diagnostic repeatability for CIN2 is poor and
there inevitably exists inter-observer variability, resulting in
numerous CIN2 lesions that are frequently misclassified (over- or
underdiagnosed). CIN2 represents an unclear biological entity and
is an admixture of transient lesions and true pre-cancer lesions
(10). In 2012, the American Society
of Pathologists and the American Society of Colposcopy and Cervical
Pathology generated a report from the Lower Anogenital Squamous
Terminology (LAST) project, suggesting that cervical lesions should
be divided into LSIL and HSIL (10).
This system was also endorsed by the 2014 World Health Organization
Classification of Tumors of Female Reproductive Organs (17). Among them, CIN1 was included in LSIL,
CIN3 was included in HSIL and for the morphologically equivocal
CIN2, p16 was considered as the reference index. CIN2 with a
p16-positive status belongs to HSIL and p16-negative lesions,
formerly classified as CIN2, may be downgraded to LSIL to simplify
clinical management (10).
The management of CIN2 is still a problem for
gynecologists. In the past, certain studies have demonstrated that
CIN2 has spontaneously regressed in young females. The results of
the present study are similar to those of most published reports
(14), with a 48.1% spontaneous
regression rate of CIN2 during the least 6 months of follow-up in
patients, independent of their age. Therefore, a conservative
follow-up approach may be considered to reduce unnecessary cone
excision for these patients.
For the diagnosis of cervical lesions, p16 is an
important marker. In the present study, the positive rate of p16
was 75.9% (82/108), whereas the negative rate was 24.1% (27/108).
Of the p16-positive cases, 20 cases were detected to have
progression; however, in the negative group, no progression
occurred. There were statistically significant differences among
these groups (P<0.05). The group with p16-negative CIN2 biopsies
only had regression or persistence but no progression. Conversely,
CIN2 cases positive for p16 were at a higher risk of evolving into
HSIL/CIN3 lesions. The sensitivity of p16 was 100%, the specificity
was 34.6%, the positive predictive value was 37% and the negative
predictive value was 100%. These results indicate that expression
levels of p16 are closely associated with the outcome of CIN2
lesions and support the idea that p16 is able to identify cases
more likely to have definitive pre-cancer features at the time of
follow-up (15,18,19).
The sensitivity of p16 for predicting future
progression to CIN3 was the highest (100%), demonstrating that p16
positive may progress to HSIL. When the negative predictive value
of p16 was the highest (100%), a negative status for p16 only led
to regression or persistence and did not lead to progression. The
result of the present study was consistent with the recommendation
of the LAST study (20). Another
study exploring the value of p16 immunostaining for improving the
diagnostic accuracy in cervical biopsy tissues indicated that p16
was more sensitive and less specific in diagnosing lesions as CIN2
or worse as compared with routine histopathological assessments
(21). However, this may not be the
best way to choose a cutoff value where the sensitivity is 100%.
This is a limitation of the present study.
The following two utilities have been recognized for
p16: First, p16 may be used to distinguish benign or reactive
changes from precursor lesions associated with HR-HPV (19). Second, p16 may be used to separate
LSIL from HSIL. The results are consistent with those of several
studies (12,22,23) that
have evaluated the role of p16 as a predictive marker for the
outcome of LSIL (20–22). In general, lesions with LSIL status
with diffuse positive p16 staining had a significantly higher
tendency to progress to high-grade lesions than p16-negative
lesions.
Although p16-positive CIN2 lesions exhibited a
significantly higher tendency to persist or progress than
p16-negative lesions, 31.5% (34/108) of p16-positive CIN2 lesions
had regressed at follow-up. If all p16-positive CIN2 lesions are
considered as high-grade lesions, this may lead to overtreatment of
patients in this group. For p16-negative cases, the primary
approach should be regular observation with follow-up. However, it
has been reported that a small percentage of p16-negative CIN2
lesions progress to CIN3, so additional diagnostic and prognostic
markers may still be required (24).
HPV E6/E7, which are translated into E6 and E7
proteins, may directly reflect the malignant transformation
information of host cells and serve as an important biomarker. As
one of the early coding products in the HPV genome after HPV
infection, E6 and E7 proteins react with human tumor suppressor
gene products, p53 and retinoblastoma protein (pRb), respectively,
and block the p53-dependent apoptotic pathway, leading to
carcinogenesis (24). HR-HPV E6/E7
mRNA is an indicator of the active state of the HPV oncogene and
the expression of HR-HPV E6/E7 mRNA indicates that the virus has
started the process of integration and carcinogenesis, leading to
the occurrence of HSIL. It may be a good assessment factor for
monitoring HPV infection and predicting the progression of lesions
to more severe cervical lesions and even cervical cancer. HPV E6/E7
mRNA has been suggested as a novel screening method for cervical
lesions (25). The identification of
HPV mRNA is of particular clinical appeal, as it is well
established that transcriptionally active HR-HPV with dysregulated
expression of oncogenes E6 and E7 is necessary for neoplastic
transformation (26).
In the present study, the positive rate of HPV E6/E7
mRNA was 63.9% (69/108), whereas the negative rate was 36.1%
(39/108) in CIN2 lesions. This positive rate is lower than that
reported in a previous study (8). In
the HPV E6/E7 mRNA-positive group, 18 cases were detected to have
progression, whereas in the negative group, only 2 cases were
detected to have progression. The differences in expression levels
of HPV E6/E7 mRNA were statistically significant among the
different prognosis groups of CIN2 lesions (P<0.05). The
progression rate in the HPV E6/E7 mRNA-positive group was higher
than that in the negative group. This indicates that HPV E6/E7 mRNA
may be used as a clinical predictive marker to identify the risk of
developing more aggressive lesions from CIN2. A prospective study
that used liquid-based cervical cytology samples collected over 24
months reported that 91% of cases with an LSIL cytological result
and E6/E7-positive status were detected as having progressed, while
85% of E6/E7-negative cases had regressed (27). For predicting progression, the
sensitivity of HPV E6/E7 mRNA was 90% and the specificity was
38.5%, whereas the positive predictive value and negative
predictive value was 36 and 90.9%, respectively. The sensitivity of
HPV E6/E7 mRNA for predicting future CIN3 was high (up to 90%). HPV
E6/E7 mRNA-positive patients may have an increased risk of
progression to HSIL, whereas patients with negative predictive
values (up to 90.9%) may be more likely to have regression or
persistence, while having a lower risk of progression. HPV E6/E7
mRNA was more sensitive and less specific for predicting the
outcome of CIN2 but performed worse than routine histopathological
assessments. This result is similar to the study by Frega et
al (28), where HPV E6/E7 mRNA
was indicated to have a high sensitivity and negative predictive
value, which may be used to predict the recurrence of cervical
lesions.
Studies have indicated that the expression level of
HPVE6/E7 is correlated with the severity of cervical lesions. As
the lesion grade increased, the positive rate of E6/E7 mRNA
exhibited an increasing trend (29).
Methylation assays (as a surrogate marker for the accumulation of
genetic and epigenetic changes as a result of persisting effects of
HPV E6/E7 mRNA expression in high-grade CIN) are highly efficient
at detecting advanced CIN lesions (30). HPV E6/E7 mRNA may have an important
role in the diagnostic evaluation and prediction of prognosis, but
at present, HPV E6/E7 mRNA as a predictive marker to identify the
risk of developing more aggressive lesions in CIN2 has been rarely
reported.
In the present study, 59 of the 108 cases were
positive for p16 and HPV E6/E7 combined. The sensitivities of P16+,
HPVE6/E7+ and P16+HPVE6/E7+ status for predicting future CIN3 were
similar (100% vs. 90% vs. 90%). The specificity of the three
indicators was also similar (34.6% vs. 38.5% vs. 34.2%), as were
the positive predictive value and the negative predictive value.
The simultaneous presence of HPV E6/E7 mRNA and p16 protein may
reflect the regulation of p16 by HPV E6/E7 in the development of
cervical cancer and pre-cancerous lesions. When HPV virus is
capable of transcriptional activity, pRb in HPV-positive cells, in
combination with HPVE7 protein, may render E2F transcription factor
continuously activated. This process leads to the failure of pRb to
combine with p16 protein, resulting in excessive deposition and
overexpression of p16 protein in cells (8).
Ki-67 is a nuclear antigen associated with cell
proliferation and its expression is associated with the
pathogenesis of pre-cancerous lesions. In the present study, a
statistically significant association of higher expression of Ki-67
with a higher risk of disease progression was observed (P<0.05).
For instance, none of the cases with <5% Ki-67 staining
progressed, while 15/46 of the cases with >25% Ki-67 staining
progressed. This result is similar to that reported by Baak et
al (31).
When the ROC curves of HPVE6/E7 mRNA, p16 and Ki-67
were evaluated, the AUC of HPV E6/E7 mRNA, p16 and Ki-67 protein
was 0.745, 0.546 and 0.501, respectively. Of the three factors, the
AUC of HPVE6/E7 was the largest with the highest prognostic value
for the progression of CIN2, and the AUC of p16 and Ki67 was
relatively lower. Of note, only indicators with an AUC of >0.5
may be considered to be of sufficient prognostic value, while the
two indicators p16 and Ki67 were not sufficient for prognostic
evaluation. This is consistent with the suggestion that HPVE6/E7
mRNA may ultimately be superior to p16 in the identification of
HR-HPV infection (8). It is also
consistent with another study that reported that the two
combination algorithms of the HPVE6/E7 mRNA detection and p16
detection were superior to those of p16 alone in predicting
prognosis (7).
In an earlier study, Loopik et al (9) suggested that the regressive likelihood
is greater in females aged <25 years, suggesting a conservative
approach option for managing CIN2. A large systematic review and
meta-analysis by Tainio et al (14) suggested that there were higher rates
of regression and lower rates of progression of histologically
confirmed CIN2 lesions, particularly in females aged ≤30. These
studies indicated that in younger patients, HPV may be eliminated
by the immune system over a certain period. However, the present
study was not in accordance with the above-mentioned ones. When the
cohort of the present study was stratified by age, there were no
significant differences between ages in terms of outcome of CIN2
(P>0.05). The biopsy resection may have stimulated an immune
response and altered the course of the disease. The follow-up time
was relatively short. The range of lesions may be small and
limited. The treatment of CIN2 lesions includes follow-up and
treatment. Based on the design of the present study, only patients
with follow-up data were selected. Thus, the question as to whether
age should be a predictive indicator requires further
investigation.
Using gland involvement as a predictive marker to
identify the risk of developing more aggressive lesions in patients
with CIN2 status has been suggested. In the present study, 14 cases
had gland involvement and 94 cases had no glands involvement. There
were no statistically significant differences between patients with
and without gland involvement in terms of progression (P>0.05).
Thus, in the present study, no association between gland
involvement and prognosis of CIN2 lesions was determined,
suggesting that it may not serve as a predictive marker.
Of note, the present study had certain limitations.
First, it was a prospective study and only untreated cases of CIN2
were reviewed, which may have resulted in a certain degree of bias
in the experimental methods. Further studies with a larger number
of patients are required to confirm the present results.
The detection of HPV E6/E7 mRNA may provide
important predictive information for the prognosis of CIN2, however
p16 and Ki-67 proteins alone may provide little value.
Acknowledgements
The authors would like to thank Dr James Byrd,
Department of Gastroenterology, Hepatology and Nutrition Anderson
Cancer Center, Houston, TX, USA for his assistance with manuscript
editing.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XZ, YX, TM and DS were involved in performing
experiments. YX prepared the materials and XZ contributed to data
acquisition and drafted and wrote the manuscript. DS designed and
supervised the study. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Peking University People's Hospital (Beijing, China) and informed
consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Christoph G, Stephan P, Camilla N, Rahhal
J, Hefler L, Tempfer CB, Heinze G, Stary G, Reinthaller A and
Speiser P: Treatment of cervical intraepithelial neoplasia with
topical imiquimod: A randomized controlled trial. Obstet Gynecol.
120:152–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aps G, Mag G and Da-Silva VD: Evaluation
of Telomerase (hTert), Ki67 and p16ink4a expressions in low and
high-grade cervical intraepithelial lesions. Rev Col Bras Cir.
44:131–139. 2017.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koeneman MM, Roy Fpm K, Nijman HW,
Brigitte Fm S, Toon VG and Arnold-Jan K: Natural history of
high-grade cervical intraepithelial neoplasia: A review of
prognostic biomarkers. Expert Rev Mol Diagn. 15:5272015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu QX and Zhang ZY: High-risk Human
papillomavirus genotypes in cervical lesions and vaccination
challenges in China. Asian Pac J Cancer Prev. 16:2193–2197. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evans MF, Peng Z, Clark KM, Adamson CSC,
Ma XJ, Wu X, Wang H, Luo Y and Cooper K: HPV E6/E7 RNA in situ
hybridization signal patterns as biomarkers of three-tier cervical
intraepithelial neoplasia grade. PLoS One. 9:e911422014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mirghani H, Casiraghi O, Amen F, He M, Ma
XJ, Saulnier P, Lacroix L, Drusch F, Lakdhar A and Saint Guily JL:
Diagnosis of HPV-driven head and neck cancer with a single test in
routine clinical practice. Mod Pathol. 28:1518–1527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mirghani H, Casiraghi O, Guerlain J, Amen
F, He MX, Ma XJ, Luo Y, Mourareau C, Drusch F, Lakdhar AB, et al:
Diagnosis of HPV driven oropharyngeal cancers: Comparing p16 based
algorithms with the RNAscope HPV-test. Oral Oncology. 62:101–108.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mills AM, Dirks DC, Poulter MD, Mills SE
and Stoler MH: HR-HPV E6/E7 mRNA in situ hybridization: Validation
against PCR, DNA in situ hybridization, and p16
immunohistochemistry in 102 samples of cervical, vulvar, anal, and
head and neck Neoplasia. Am J Surg Pathol. 41:607–615. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loopik DL, Doucette S, Bekkers RL and
Bentley JR: Regression and progression predictors of CIN2 in women
younger than 25 years. J Low Genit Tract Dis. 20:213–217. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Darragh TM, Colgan TJ, Cox TJ, Heller DS,
Henry MR, Luff RD, McCalmont T, Nayar R, Palefsky JM, Stoler MH, et
al: The lower anogenital squamous terminology standardization
project for HPV-associated lesions: Background and consensus
recommendations from the College of American Pathologists and the
American Society for Colposcopy and Cervical Pathology. Int J
Gynecol Pathol. 32:76–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christine B, Guglielmo R, Miriam R,
Nicolas W, Marc A, Mark S and Magnus von Knebel D: The clinical
impact of using p16(INK4a) immunochemistry in cervical
histopathology and cytology: An update of recent developments. Int
J Cancer. 136:2741–2751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiaobo Z and Danhua S: p16INK4a and Ki-67
measurement predict progression of cervical low-grade squamous
intraepithelial lesion. Int J Clin Exp Pathol. 11:4109–4116.
2018.PubMed/NCBI
|
|
13
|
Wilbur DC and Nayar R: The Bethesda system
for reporting cervical cytology: A historical perspectiv. Acta
Cytologica. 61:359–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tainio K, Athanasiou A, Tikkinen KAO,
Aaltonen R, Cárdenas J, Hernándes, Glazer-Livson S, Jakobsson M,
Joronen K, Kiviharju M, et al: Clinical course of untreated
cervical intraepithelial neoplasia grade 2 under active
surveillance: Systematic review and meta-analysis. BMJ.
360:k4992018. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miralpeix E, Genovés J, Maria SSJ, Mancebo
G, Lloveras B, Bellosillo B, Alameda F and Carreras R: Usefulness
of p16INK4a staining for managing histological
high-grade squamous intraepithelial cervical lesions. Mod Pathol.
30:304–310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tavassoéli FA and Devilee P: Pathology and
genetics of tumours of the breast and female genital organs.
(Lyon). World Health Organization Classification of Tumors.
4322003.
|
|
17
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO classification of tumous of female reproductive
organs. (Fourth edition). 3072014.
|
|
18
|
Miyamoto S, Hasegawa J, Morioka M, Hirota
Y, Kushima M and Sekizawa A: The association between p16 and Ki-67
immunohistostaining and the progression of cervical intraepithelial
neoplasia grade 2. Int J Gynaecol Obstet. 134:45–48. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maniar KP, Sanchez B, Paintal A, Gursel DB
and Nayar R: Role of the biomarker p16 in downgrading-IN 2
diagnoses and predicting higher-grade lesions. Am J Surg Pathol.
39:1708–1718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nuño T and García F: The lower anogenital
squamous terminology project and its implications for clinical
care. Obstet Gynecol Clin North Am. 40:225–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galgano MT, Castle PE, Atkins KA, Brix WK,
Nassau SR and Stoler MH: Using biomarkers as objective standards in
the diagnosis of cervical biopsies. Am J Surg Pathol. 34:1077–1087.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guang-Dong L, Sellors JW, Hai-Kui S, Xun
Z, Yan-Ping B, Jose J, Wen C, Fang-Hui Z, Yan S, Zhi C, et al:
p16INK4A immunohistochemical staining and predictive value for
progression of cervical intraepithelial neoplasia grade 1: A
prospective study in China. Int J Cancer. 134:1715–1724. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maryam R, Andrée S, Oligny LL, Patey N,
Dormoy-Raclet V, Ducruet T and Bouron-Dal Soglio D: Assessment of
correlation between p16INK4a staining, specific subtype of human
papillomavirus, and progression of LSIL/CIN1 lesions: First
comparative study. Am J Clin Pathol. 142:104–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun L, Zhang L, Krigman HR and Hagemann
IS: p16 immunohistochemistry is not always required for accurate
diagnosis of grade 2 squamous intraepithelial lesions. J Low Genit
Tract Dis. 22:104–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan Y and Shen Z: The clinical value of
HPV E6/E7 and STAT3 mRNA detection in cervical cancer screening.
Pathol Res Pract. 214:767–775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gernot H, Mahmood M, Kerstin ID,
Pischinger, Thomas WR, Christian FS, Kubista E and Czerwenka KF:
Physical state and expression of HPV DNA in benign and dysplastic
cervical tissue: Different levels of viral integration are
correlated with lesion grade. Gynecol Oncol. 92:873–880. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Holm R, Kraus I, Skomedal H, Langerød A,
Kristensen GB and Lyng H: Human papillomavirus DNA and e6/e7 mRNA
status in relation to survival of patients treated for cervical
squamous cell carcinoma. Open Virol J. 2:74–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frega A, Sesti F, Lombardi D, Votano S,
Sopracordevole F, Catalano A, Milazzo GN, Lombardo R, Assorgi C,
Olivola S, et al: Assessment of HPV-mRNA test to predict recurrent
disease in patients previously treated for CIN 2/3. J Clin Virol.
60:39–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coquillard G, Palao B and Patterson BK:
Quantification of intracellular HPV E6/E7 mRNA expression increases
the specificity and positive predictive value of cervical cancer
screening compared to HPV DNA. Gynecol Oncol. 120:89–93. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steenbergen RDM, Snijders PJF, Heideman
DLAM and Meijer CJLM: Clinical implications of (Epi)genetic changes
in HPV-induced cervical precancerous lesions. Nat Rev Cancer.
14:395–405. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baak JPA, Arnold-Jan K, Garland SM,
Skaland I, Janssen EAM, Tabrizi S, Fagerheim S, Robboy S and Nilsen
ST: Combined p53 and retinoblastoma protein detection identifies
persistent and regressive cervical high-grade squamous
intraepithelial lesions. Am J Surg Pathol. 29:1062–1066.
2005.PubMed/NCBI
|