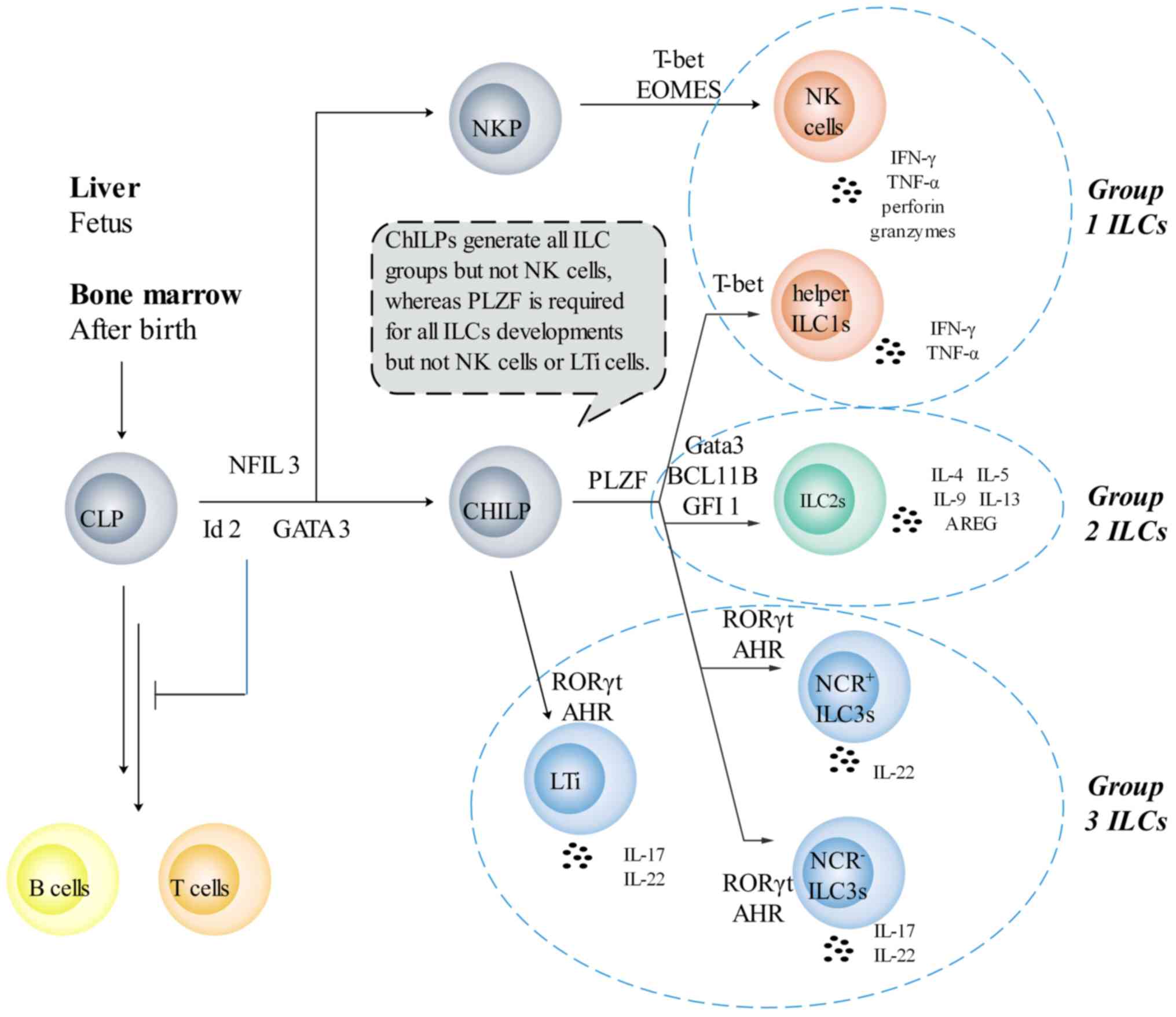
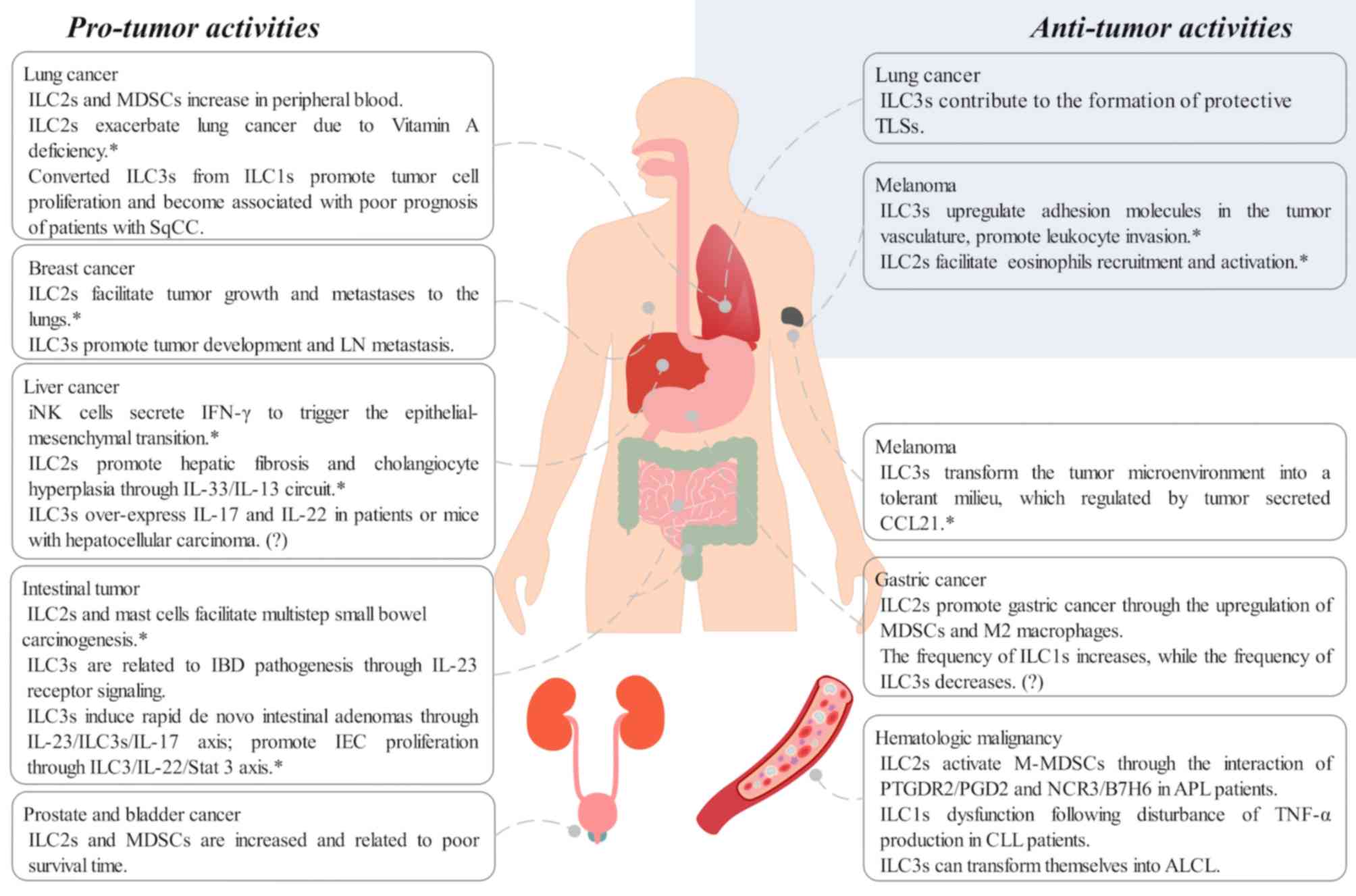
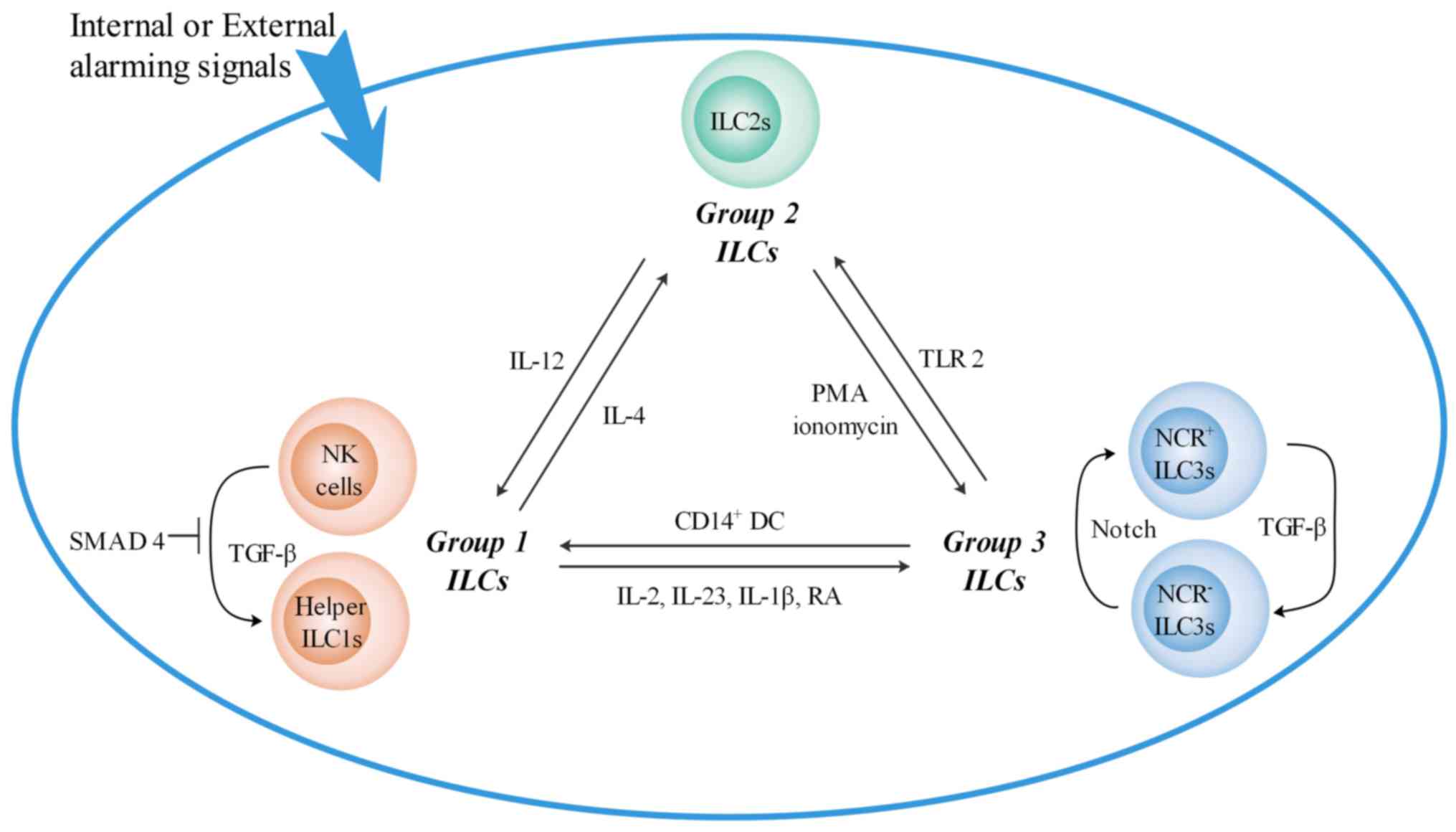
|
1
|
Withers DR: Innate lymphoid cell
regulation of adaptive immunity. Immunology. 149:123–130. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwasaki A and Medzhitov R: Regulation of
adaptive immunity by the innate immune system. Science.
327:291–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eberl G, Colonna M, Di Santo JP and
McKenzie AN: Innate lymphoid cells. Innate lymphoid cells: A new
paradigm in immunology. Science. 348:aaa65662015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ebbo M, Crinier A, Vély F and Vivier E:
Innate lymphoid cells: Major players in inflammatory diseases. Nat
Rev Immunol. 17:665–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar V: Innate lymphoid cells: New
paradigm in immunology of inflammation. Immunol Lett. 157:23–37.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Artis D and Spits H: The biology of innate
lymphoid cells. Nature. 517:293–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spits H and Cupedo T: Innate lymphoid
cells: Emerging insights in development, lineage relationships, and
function. Annu Rev Immunol. 30:647–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kiessling R, Klein E, Pross H and Wigzell
H: ‘Natural’ killer cells in the mouse. II. Cytotoxic cells with
specificity for mouse Moloney leukemia cells. Characteristics of
the killer cell. Eur J Immunol. 5:117–121. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiessling R, Klein E and Wigzell H:
‘Natural’ killer cells in the mouse. I. Cytotoxic cells with
specificity for mouse Moloney leukemia cells. Specificity and
distribution according to genotype. Eur J Immunol. 5:112–117. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mebius RE, Rennert P and Weissman IL:
Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can
differentiate to APC, NK cells, and follicular cells but not T or B
cells. Immunity. 7:493–504. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cella M, Fuchs A, Vermi W, Facchetti F,
Otero K, Lennerz JK, Doherty JM, Mills JC and Colonna M: A human
natural killer cell subset provides an innate source of IL-22 for
mucosal immunity. Nature. 457:722–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moro K, Yamada T, Tanabe M, Takeuchi T,
Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H and Koyasu S:
Innate production of T(H)2 cytokines by adipose tissue-associated
c-Kit(+)Sca-1(+) lymphoid cells. Nature. 463:540–544. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neill DR, Wong SH, Bellosi A, Flynn RJ,
Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al:
Nuocytes represent a new innate effector leukocyte that mediates
type-2 immunity. Nature. 464:1367–1370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saglani S: Innate helper cells: A novel
cell type essential in the initiation of asthma? Thorax.
66:834–835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saenz SA, Siracusa MC, Perrigoue JG,
Spencer SP, Urban JF Jr, Tocker JE, Budelsky AL, Kleinschek MA,
Kastelein RA, Kambayashi T, et al: IL25 elicits a multipotent
progenitor cell population that promotes T(H)2 cytokine responses.
Nature. 464:1362–1366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koyasu S, Moro K, Tanabe M and Takeuchi T:
Natural helper cells: A new player in the innate immune response
against helminth infection. Adv Immunol. 108:21–44. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fuchs A, Vermi W, Lee JS, Lonardi S,
Gilfillan S, Newberry RD, Cella M and Colonna M: Intraepithelial
type 1 innate lymphoid cells are a unique subset of IL-12- and
IL-15-responsive IFN-γ-producing cells. Immunity. 38:769–781. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spits H, Artis D, Colonna M, Diefenbach A,
Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius
RE, et al: Innate lymphoid cells-a proposal for uniform
nomenclature. Nat Rev Immunol. 13:145–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vivier E, Artis D, Colonna M, Diefenbach
A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ,
Mebius RE, et al: Innate lymphoid cells: 10 years on. Cell.
174:1054–1066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morvan MG and Lanier LL: NK cells and
cancer: You can teach innate cells new tricks. Nat Rev Cancer.
16:7–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guillerey C and Smyth MJ: NK cells and
cancer immunoediting. Curr Top Microbiol Immunol. 395:115–145.
2016.PubMed/NCBI
|
|
22
|
Klose CS and Artis D: Innate lymphoid
cells as regulators of immunity, inflammation and tissue
homeostasis. Nat Immunol. 17:765–774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simoni Y, Fehlings M, Kløverpris HN,
McGovern N, Koo SL, Loh CY, Lim S, Kurioka A, Fergusson JR, Tang
CL, et al: Human innate lymphoid cell subsets possess tissue-type
based heterogeneity in phenotype and frequency. Immunity.
46:148–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spits H, Bernink JH and Lanier L: NK cells
and type 1 innate lymphoid cells: Partners in host defense. Nat
Immunol. 17:758–764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bernink JH, Peters CP, Munneke M, te Velde
AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand
N, Buskens CJ, et al: Human type 1 innate lymphoid cells accumulate
in inflamed mucosal tissues. Nat Immunol. 14:221–229. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monticelli LA, Sonnenberg GF, Abt MC,
Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ,
Yang CY, Sathaliyawala T, et al: Innate lymphoid cells promote
lung-tissue homeostasis after infection with influenza virus. Nat
Immunol. 12:1045–1054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gieseck RL III, Wilson MS and Wynn TA:
Type 2 immunity in tissue repair and fibrosis. Science. 18:62–76.
2018.
|
|
28
|
Goc J, Hepworth MR and Sonnenberg GF:
Group 3 innate lymphoid cells: Regulating host-commensal bacteria
interactions in inflammation and cancer. Int Immunol. 28:43–52.
2016.PubMed/NCBI
|
|
29
|
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa
SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ and
Ouyang W: Interleukin-22 mediates early host defense against
attaching and effacing bacterial pathogens. Nat Med. 14:282–289.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klose CS, Kiss EA, Schwierzeck V, Ebert K,
Hoyler T, d'Hargues Y, Göppert N, Croxford AL, Waisman A, Tanriver
Y and Diefenbach A: A T-bet gradient controls the fate and function
of CCR6-RORγt+ innate lymphoid cells. Nature. 494:261–265. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buonocore S, Ahern PP, Uhlig HH, Ivanov
II, Littman DR, Maloy KJ and Powrie F: Innate lymphoid cells drive
interleukin-23-dependent innate intestinal pathology. Nature.
464:1371–1375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim HY, Lee HJ, Chang YJ, Pichavant M,
Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, et
al: Interleukin-17-producing innate lymphoid cells and the NLRP3
inflammasome facilitate obesity-associated airway hyperreactivity.
Nat Med. 20:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scandella E, Bolinger B, Lattmann E,
Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T and
Ludewig B: Restoration of lymphoid organ integrity through the
interaction of lymphoid tissue-inducer cells with stroma of the T
cell zone. Nat Immunol. 9:667–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh N, Baby D, Rajguru JP, Patil PB,
Thakkannavar SS and Pujari VB: Inflammation and cancer. Ann Afr
Med. 18:121–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang S, Tian Z, Wu Y, van Velkinburgh JC
and Ni B: Pivotal roles of ILCs in hepatic diseases. Int Rev
Immunol. 34:509–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun C, Sun H, Zhang C and Tian Z: NK cell
receptor imbalance and NK cell dysfunction in HBV infection and
hepatocellular carcinoma. Cell Mol Immunol. 12:292–302. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han X, Huang T and Han J: Cytokines
derived from innate lymphoid cells assist Helicobacter
hepaticus to aggravate hepatocellular tumorigenesis in viral
transgenic mice. Gut Pathog. 11:232019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McHedlidze T, Waldner M, Zopf S, Walker J,
Rankin AL, Schuchmann M, Voehringer D, McKenzie AN, Neurath MF,
Pflanz S and Wirtz S: Interleukin-33-dependent innate lymphoid
cells mediate hepatic fibrosis. Immunity. 39:357–371. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Razumilava N, Gores GJ, Walters S,
Mizuochi T, Mourya R, Bessho K, Wang YH, Glaser SS, Shivakumar P
and Bezerra JA: Biliary repair and carcinogenesis are mediated by
IL-33-dependent cholangiocyte proliferation. J Clin Invest.
124:3241–3251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao
Y, Wang X and Sun B: Interleukin-22 promotes human hepatocellular
carcinoma by activation of STAT3. Hepatology. 54:900–909. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Geremia A, Arancibia-Cárcamo CV, Fleming
MP, Rust N, Singh B, Mortensen NJ, Travis SP and Powrie F: IL-23-
responsive innate lymphoid cells are increased in inflammatory
bowel disease. J Exp Med. 208:1127–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fuchs A and Colonna M: Innate lymphoid
cells in homeostasis, infection, chronic inflammation and tumors of
the gastrointestinal tract. Curr Opin Gastroenterol. 29:581–587.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Castleman MJ, Dillon SM, Purba CM,
Cogswell AC, Kibbie JJ, McCarter MD, Santiago ML, Barker E and
Wilson CC: Commensal and pathogenic bacteria indirectly induce
IL-22 but Not IFNγ production from human colonic ILC3s via multiple
mechanisms. Front Immunol. 10:6492019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Man SM: Inflammasomes in the
gastrointestinal tract: Infection, cancer and gut microbiota
homeostasis. Nat Rev Gastroenterol Hepatol. 15:721–737. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chan IH, Jain R, Tessmer MS, Gorman D,
Mangadu R, Sathe M, Vives F, Moon C, Penaflor E, Turner S, et al:
Interleukin-23 is sufficient to induce rapid de novo gut
tumorigenesis, independent of carcinogens, through activation of
innate lymphoid cells. Mucosal Immunol. 7:842–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kirchberger S, Royston DJ, Boulard O,
Thornton E, Franchini F, Szabady RL, Harrison O and Powrie F:
Innate lymphoid cells sustain colon cancer through production of
interleukin-22 in a mouse model. J Exp Med. 210:917–931. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bergmann H, Roth S, Pechloff K, Kiss EA,
Kuhn S, Heikenwälder M, Diefenbach A, Greten FR and Ruland J:
Card9-dependent IL-1β regulates IL-22 production from group 3
innate lymphoid cells and promotes colitis-associated cancer. Eur J
Immunol. 47:1342–1353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saadalla AM, Osman A, Gurish MF, Dennis
KL, Blatner NR, Pezeshki A, McNagny KM, Cheroutre H, Gounari F and
Khazaie K: Mast cells promote small bowel cancer in a tumor
stage-specific and cytokine-dependent manner. Proc Natl Acad Sci
USA. 115:1588–1592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chang WJ, Du Y, Zhao X, Ma LY and Cao GW:
Inflammation- related factors predicting prognosis of gastric
cancer. World J Gastroenterol. 20:4586–4596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bie Q, Zhang P, Su Z, Zheng D, Ying X, Wu
Y, Yang H, Chen D, Wang S and Xu H: Polarization of ILC2s in
peripheral blood might contribute to immunosuppressive
microenvironment in patients with gastric cancer. J Immunol Res.
2014:9231352014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Salimi M, Wang R, Yao X, Li X, Wang X, Hu
Y, Chang X, Fan P, Dong T and Ogg G: Activated innate lymphoid cell
populations accumulate in human tumour tissues. BMC Cancer.
18:3412018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Trabanelli S, Curti A, Lecciso M, Salomé
B, Riether C, Ochsenbein A, Romero P and Jandus C: CD127+ innate
lymphoid cells are dysregulated in treatment naïve acute myeloid
leukemia patients at diagnosis. Haematologica. 100:e257–e260. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Munneke JM, Björklund AT, Mjösberg JM,
Garming-Legert K, Bernink JH, Blom B, Huisman C, van Oers MH, Spits
H, Malmberg KJ and Hazenberg MD: Activated innate lymphoid cells
are associated with a reduced susceptibility to graft-versus-host
disease. Blood. 124:812–821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Trabanelli S, Chevalier MF,
Martinez-Usatorre A, Gomez- Cadena A, Salomé B, Lecciso M,
Salvestrini V, Verdeil G, Racle J, Papayannidis C, et al:
Tumour-derived PGD2 and NKp30-B7H6 engagement drives an
immunosuppressive ILC2-MDSC axis. Nat Commun. 8:5932017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
de Weerdt I, van Hoeven V, Munneke JM,
Endstra S, Hofland T, Hazenberg MD and Kater AP: Innate lymphoid
cells are expanded and functionally altered in chronic lymphocytic
leukemia. Haematologica. 101:e461–e464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schleussner N, Merkel O, Costanza M, Liang
HC, Hummel F, Romagnani C, Durek P, Anagnostopoulos I, Hummel M,
Jöhrens K, et al: The AP-1-BATF and -BATF3 module is essential for
growth, survival and TH17/ILC3 skewing of anaplastic large cell
lymphoma. Leukemia. 32:1994–2007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stein JV and Nombela-Arrieta C: Chemokine
control of lymphocyte trafficking: A general overview. Immunology.
116:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jovanovic IP, Pejnovic NN, Radosavljevic
GD, Pantic JM, Milovanovic MZ, Arsenijevic NN and Lukic ML:
Interleukin-33/ST2 axis promotes breast cancer growth and
metastases by facilitating intratumoral accumulation of
immunosuppressive and innate lymphoid cells. Int J Cancer.
134:1669–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Irshad S, Flores-Borja F, Lawler K,
Monypenny J, Evans R, Male V, Gordon P, Cheung A, Gazinska P, Noor
F, et al: RORγt+ innate lymphoid cells promote lymph node
metastasis of breast cancers. Cancer Res. 77:1083–1096. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Carrega P, Loiacono F, Di Carlo E,
Scaramuccia A, Mora M, Conte R, Benelli R, Spaggiari GM, Cantoni C,
Campana S, et al: NCR(+)ILC3 concentrate in human lung cancer and
associate with intratumoral lymphoid structures. Nat Commun.
6:82802015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Koh J, Kim HY, Lee Y, Park IK, Kang CH,
Kim YT, Kim JE, Choi M, Lee WW, Jeon YK and Chung DH:
IL23-producing human lung cancer cells promote tumor growth via
conversion of innate lymphoid cell 1 (ILC1) into ILC3. Clin Cancer
Res. 25:4026–4037. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wu Y, Yan Y, Su Z, Bie Q, Chen X, Barnie
PA, Guo Q, Wang S and Xu H: Enhanced circulating ILC2s and MDSCs
may contribute to ensure maintenance of Th2 predominant in patients
with lung cancer. Mol Med Rep. 15:4374–4381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cui W, Zhang W, Yuan X, Liu S, Li M, Niu
J, Zhang P and Li D: Vitamin A deficiency execrates Lewis lung
carcinoma via induction of type 2 innate lymphoid cells and
alternatively activates macrophages. Food Sci Nutr. 7:1288–1294.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bruchard M and Ghiringhelli F: Deciphering
the roles of innate lymphoid cells in cancer. Front Immunol.
10:6562019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Eisenring M, vom Berg J, Kristiansen G,
Saller E and Becher B: IL-12 initiates tumor rejection via lymphoid
tissue-inducer cells bearing the natural cytotoxicity receptor
NKp46. Nat Immunol. 11:1030–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lotfi R, Lee JJ and Lotze MT: Eosinophilic
granulocytes and damage-associated molecular pattern molecules
(DAMPs): Role in the inflammatory response within tumors. J
Immunother. 30:16–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ikutani M, Yanagibashi T, Ogasawara M,
Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y,
Takaki S and Takatsu K: Identification of innate IL-5-producing
cells and their role in lung eosinophil regulation and antitumor
immunity. J Immunol. 188:703–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Moskalenko M, Pan M, Fu Y, de Moll EH,
Hashimoto D, Mortha A, Leboeuf M, Jayaraman P, Bernardo S, Sikora
AG, et al: Requirement for innate immunity and CD90+
NK1.1− lymphocytes to treat established melanoma with
chemo-immunotherapy. Cancer Immunol Res. 3:296–304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shields JD, Kourtis IC, Tomei AA, Roberts
JM and Swartz MA: Induction of lymphoidlike stroma and immune
escape by tumors that express the chemokine CCL21. Science.
328:749–752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chevalier MF, Trabanelli S, Racle J,
Salomé B, Cesson V, Gharbi D, Bohner P, Domingos-Pereira S,
Dartiguenave F, Fritschi AS, et al: ILC2-modulated T cell-to-MDSC
balance is associated with bladder cancer recurrence. J Clin
Invest. 127:2916–2929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Crome SQ, Nguyen LT, Lopez-Verges S, Yang
SY, Martin B, Yam JY, Johnson DJ, Nie J, Pniak M, Yen PH, et al: A
distinct innate lymphoid cell population regulates tumor-associated
T cells. Nat Med. 23:368–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Punt S, Fleuren GJ, Kritikou E, Lubberts
E, Trimbos JB, Jordanova ES and Gorter A: Angels and demons: Th17
cells represent a beneficial response, while neutrophil IL-17 is
associated with poor prognosis in squamous cervical cancer.
Oncoimmunology. 4:e9845392015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bonelli M, Shih HY, Hirahara K, Singelton
K, Laurence A, Poholek A, Hand T, Mikami Y, Vahedi G, Kanno Y and
O'Shea JJ: Helper T cell plasticity: Impact of extrinsic and
intrinsic signals on transcriptomes and epigenomes. Curr Top
Microbiol Immunol. 381:279–326. 2014.PubMed/NCBI
|
|
76
|
Gaffen SL, Jain R, Garg AV and Cua DJ: The
IL-23-IL-17 immune axis: From mechanisms to therapeutic testing.
Nat Rev Immunol. 14:585–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mucida D, Husain MM, Muroi S, van Wijk F,
Shinnakasu R, Naoe Y, Reis BS, Huang Y, Lambolez F, Docherty M, et
al: Transcriptional reprogramming of mature CD4+ helper T cells
generates distinct MHC class II-restricted cytotoxic T lymphocytes.
Nat Immunol. 14:281–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gao Y, Souza-Fonseca-Guimaraes F, Bald T,
Ng SS, Young A, Ngiow SF, Rautela J, Straube J, Waddell N, Blake
SJ, et al: Tumor immunoevasion by the conversion of effector NK
cells into type 1 innate lymphoid cells. Nat Immunol. 18:1004–1015.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cortez VS, Ulland TK, Cervantes-Barragan
L, Bando JK, Robinette ML, Wang Q, White AJ, Gilfillan S, Cella M
and Colonna M: SMAD4 impedes the conversion of NK cells into
ILC1-like cells by curtailing non-canonical TGF-β signaling. Nat
Immunol. 18:995–1003. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chea S, Perchet T, Petit M, Verrier T,
Guy-Grand D, Banchi EG, Vosshenrich CA, Di Santo JP, Cumano A and
Golub R: Notch signaling in group 3 innate lymphoid cells modulates
their plasticity. Sci Signal. 9:ra452016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Viant C, Rankin LC, Girard-Madoux MJ,
Seillet C, Shi W, Smyth MJ, Bartholin L, Walzer T, Huntington ND,
Vivier E and Belz GT: Transforming growth factor-β and Notch
ligands act as opposing environmental cues in regulating the
plasticity of type 3 innate lymphoid cells. Sci Signal. 9:ra462016.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bal SM, Bernink JH, Nagasawa M, Groot J,
Shikhagaie MM, Golebski K, van Drunen CM, Lutter R, Jonkers RE,
Hombrink P, et al: IL-1β, IL-4 and IL-12 control the fate of group
2 innate lymphoid cells in human airway inflammation in the lungs.
Nat Immunol. 17:636–645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lim AI, Menegatti S, Bustamante J, Le
Bourhis L, Allez M, Rogge L, Casanova JL, Yssel H and Di Santo JP:
IL-12 drives functional plasticity of human group 2 innate lymphoid
cells. J Exp Med. 213:569–583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ohne Y, Silver JS, Thompson-Snipes L,
Collet MA, Blanck JP, Cantarel BL, Copenhaver AM, Humbles AA and
Liu YJ: IL-1 is a critical regulator of group 2 innate lymphoid
cell function and plasticity. Nat Immunol. 17:646–655. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Silver JS, Kearley J, Copenhaver AM,
Sanden C, Mori M, Yu L, Pritchard GH, Berlin AA, Hunter CA, Bowler
R, et al: Inflammatory triggers associated with exacerbations of
COPD orchestrate plasticity of group 2 innate lymphoid cells in the
lungs. Nat Immunol. 17:626–635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bernink JH, Krabbendam L, Germar K, de
Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD,
Villaudy J, Buskens CJ, et al: Interleukin-12 and −23 control
plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in
the intestinal lamina propria. Immunity. 43:146–160. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Crellin NK, Trifari S, Kaplan CD,
Satoh-Takayama N, Di Santo JP and Spits H: Regulation of cytokine
secretion in human CD127(+) LTi-like innate lymphoid cells by
Toll-like receptor 2. Immunity. 33:752–764. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Huang Y, Guo L, Qiu J, Chen X, Hu-Li J,
Siebenlist U, Williamson PR, Urban JF Jr and Paul WE:
IL-25-responsive, lineage-negative KLRG1(hi) cells are
multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat
Immunol. 16:161–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Pietra G, Vitale C, Pende D, Bertaina A,
Moretta F, Falco M, Vacca P, Montaldo E, Cantoni C, Mingari MC, et
al: Human natural killer cells: News in the therapy of solid tumors
and high-risk leukemias. Cancer Immunol Immunother. 65:465–476.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Baumeister SH, Freeman GJ, Dranoff G and
Sharpe AH: Coinhibitory pathways in immunotherapy for cancer. Annu
Rev Immunol. 34:539–573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Khalil DN, Smith EL, Brentjens RJ and
Wolchok JD: The future of cancer treatment: Immunomodulation, CARs
and combination immunotherapy. Nat Rev Clin Oncol. 13:273–290.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Mallett G, Laurence A and Amarnath S:
Programmed cell death-1 receptor (PD-1)-mediated regulation of
innate lymphoid cells. Int J Mol Sci. 20(pii): E28362019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tumino N, Martini S, Munari E, Tumino N,
Martini S, Munari E, Scordamaglia F, Besi F, Mariotti FR, Bogina G,
et al: Presence of innate lymphoid cells in pleural effusions of
primary and metastatic tumors: Functional analysis and expression
of PD-1 receptor. Int J Cancer. 145:1660–1668. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang T, Zheng N, Luo Q, Jiang L, He B,
Yuan X and Shen L: Probiotics lactobacillus reuteri abrogates
immune checkpoint blockade-associated colitis by inhibiting group 3
innate lymphoid cells. Front Immunol. 10:12352019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Turchinovich G, Ganter S, Bärenwaldt A and
Finke D: NKp46 Calibrates tumoricidal potential of type 1 innate
lymphocytes by regulating TRAIL expression. J Immunol.
200:3762–3768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zaiss DMW, Gause WC, Osborne LC and Artis
D: Emerging functions of amphiregulin in orchestrating immunity,
inflammation, and tissue repair. Immunity. 42:216–226. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lu B, Yang M and Wang Q: Interleukin-33 in
tumorigenesis, tumor immune evasion, and cancer immunotherapy. J
Mol Med (Berl). 94:535–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hanash AM, Dudakov JA, Hua G, O'Connor MH,
Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, et
al: Interleukin-22 protects intestinal stem cells from
immune-mediated tissue damage and regulates sensitivity to graft
versus host disease. Immunity. 37:339–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lim C and Savan R: The role of the
IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev.
25:257–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Markota A, Endres S and Kobold S:
Targeting interleukin-22 for cancer therapy. Hum Vaccin Immunother.
14:2012–2015. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Song Y and Yang JM: Role of interleukin
(IL)-17 and T-helper (Th)17 cells in cancer. s. 493:1–8. 2017.
|
|
103
|
Kathania M, Khare P, Zeng M, Cantarel B,
Zhang H, Ueno H and Venuprasad K: Itch inhibits IL-17-mediated
colon inflammation and tumorigenesis by ROR-γt ubiquitination. Nat
Immunol. 17:997–1004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kim J, Kim W, Moon UJ, Kim HJ, Choi HJ,
Sin JI, Park NH, Cho HR and Kwon B: Intratumorally establishing
type 2 innate lymphoid cells blocks tumor growth. J Immunol.
196:2410–2423. 2016. View Article : Google Scholar : PubMed/NCBI
|