Introduction
Triple-negative breast cancer (TNBC) is a type of
breast cancer that does not express estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor-2 (HER-2). TNBC accounts for 15–20% of all cases of breast
cancer and has more aggressive biological characteristics (1,2). For
locally advanced TNBC, the standard treatment is neoadjuvant
chemotherapy (NAC) followed by surgery (3). The primary goal of NAC is to achieve a
pathological complete response (pCR), as most patients with pCR
have a good survival outcome (4).
The pCR rate for patients with TNBC who receive NAC is 30–40%
(5). Increasing evidence has
revealed that TNBC exhibits higher chemosensitivity compared with
other breast cancer subtypes; thus, patients with TNBC are more
likely to achieve a pCR following NAC (6). However, whether NAC can prolong the
survival of patients remains uncertain. Studies have demonstrated
that patients who had achieved pCR after receiving NAC displayed a
significantly improved prognosis. However, subgroup analysis
revealed that the survival rates of patients who did not achieve
pCR were lower compared with those of patients who achieved pCR,
even when compared with the adjuvant chemotherapy group (7–9). This
may be due to the increased risk of recurrence caused by residual
lesions in patients who received NAC but did not achieve pCR
(10). TNBC is a heterogeneous
disease, which comprises subtypes with different biological
behaviors and clinical outcomes (11). Therefore, it is important to develop
strategies that can accurately predict the efficacy of NAC for TNBC
to select candidates with an improved predicted prognosis.
Several molecular markers have been identified to
predict responses to NAC, including p53 (12), cytokeratin ck5/6 (13), epidermal growth factor receptor
(14), Ki67 (15), and methylation of Ras association
domain family 1 isoform A (RASSF1A) and WNT inhibitory
factor-1 (WIF-1) (16) in
serum. However, biomarkers that can accurately predict the response
to NAC in clinical settings remain limited. Protocadherin 17
(PCDH17) is a member of the cadherin superfamily located at
13q21.2 and it is primarily involved in intracellular signaling and
cell-to-cell interactions (17). As
a tumor suppressor gene, PCDH17 exerts an inhibitory effect
on tumor growth during normal physiological processes (18). However, its tumor suppressor function
is lost as a result of hypermethylation in numerous types of
cancer, such as esophageal cancer (17), renal cell carcinoma (19), bladder cancer (20), gastric cancer (21) and breast cancer (22). A previous study indicated that
PCDH17 may be an antagonist of the Wnt/β-catenin signaling
pathway in breast cancer. In breast cancer cells transfected with
PCDH17, the expression levels of active β-catenin and its
downstream target genes c-myc and cyclin D1 are decreased (22). In the cytoplasm and nucleus of breast
cancer cells, the Wnt/β-catenin receptor Frizzled-1 decreases
β-catenin protein expression and increases inhibition of the
Wnt/β-catenin signaling pathway, thus decreasing drug resistance in
chemotherapy (23). PCDH17
expression in breast cancer cells is often inhibited or silenced
upon PCDH17 methylation, and drug-induced demethylation can
reactivate PCDH17 expression (22). An association between PCDH17
methylation and decreased PCDH17 expression has been
observed in most breast cancer cases (22). It was hypothesized that PCDH17
methylation would reduce its inhibitory effect on the Wnt/β-catenin
signaling pathway, leading to elevated chemoresistance; therefore,
the present study investigated the predictive value of
PCDH17 methylation status for NAC efficacy in TNBC.
In the present study, methylation-specific PCR (MSP)
was used to detect the methylation status of PCDH17 in TNBC
specimens prior to NAC. The objective of the study was to assess
whether the methylation status of PCDH17 in TNBC tissues was
associated with the response to NAC.
Materials and methods
Patients and samples
The present study included 280 female patients (mean
age, 50.7 years; range, 27–69 years) with breast cancer who were
treated at the Jining No.1 People's Hospital and the Shandong
Provincial Qianfoshan Hospital Affiliated to Shandong University
(Jining, China) between January 2016 and June 2019. The inclusion
criteria were as follows: i) TNBC (stage IIB or III) diagnosed via
ultrasound-guided core needle biopsy (16G Bard biopsy needle)
performed prior to chemotherapy and confirmed by two experienced
independent pathologists (discordant interpretations were confirmed
by a third pathologist); ii) available paraffin specimens obtained
from biopsy prior to NAC in which tissue DNA could be successfully
extracted at concentrations >50 ng/µl; iii) no contraindications
for chemotherapy and NAC; and iv) no prior history of chemotherapy,
radiotherapy, endocrine therapy or molecular targeted therapy. The
exclusion criteria were: i) Other subtypes of breast cancer or
inflammatory breast cancer; ii) distant metastases detected via
ultrasound, computed tomography or bone scan; iii) response to
chemotherapy could not be assessed because surgery was not
conducted; and iv) incomplete medical record. After biopsy, all
patients received six cycles of TAC regimen (docetaxel + epirubicin
+ cyclophosphamide), followed by surgery. The present study was
approved by the Ethics Committee of the Jining No.1 People's
Hospital [institutional review board approval no. Lun Shenyan No.
2017 (011)]. The study was conducted in accordance with the
provisions of the Declaration of Helsinki and local regulations.
Informed written consent was obtained from all individuals who
participated in the present study.
Clinical staging was performed according to the 7th
edition of the TNM method from the American Joint Commission on
Cancer (24). Physical examination
was combined with molybdenum b-ultrasound and magnetic resonance
imaging results to determine tumor T staging and regional
lymph-node status. Imaging results (and axillary lymph node biopsy
when necessary) determined N staging.
Immunostaining
All patients underwent biopsy gun puncture (C.R.
Bard, Inc.) before surgery, resulting in four tumor-tissue samples
(1.5–2.0 cm in length) per patient. Samples were immediately fixed
in 10% formalin at room temperature for 24 h and embedded in
paraffin. Some paraffin-embedded tissue blocks were cut into
4-µm-thick continuous slices for hematoxylin and eosin staining
(for the pathological diagnosis of the tumor) and for
immunohistochemistry (to determine ER, PR, HER-2, and Ki67 status).
Hematoxylin and eosin staining was this routinely performed by the
hospital as previously described (25). The streptavidin-perosidase staining
method was also used for immunohistochemistry as previously
described (26).
Monoclonal antibodies against ER (pre-diluted clone
611; cat. no. ORG-8871) and PR (pre-diluted clone 16; cat no.
ORG-8721) (both Leica Microsystems, Inc.) were used to determine
the ER and PR statuses, following previously published procedures
(27,28). HER-2 expression was determined with
the HercepTest kit according to the manufacturer's protocol
(Agilent Technologies, Inc.) (29).
For Ki67, a rabbit monoclonal antibody at a 1:200 dilution was used
(RMA-0542, Fuzhou Maixin Biotech Co., Ltd.), following previously
published procedures (30).
Triple-negative status (31) was defined as ER and PR levels <1%,
as well as HER-2-negative (HercepTest score 0/1+ or gene
amplification ratio <2.2 after in situ hybridization).
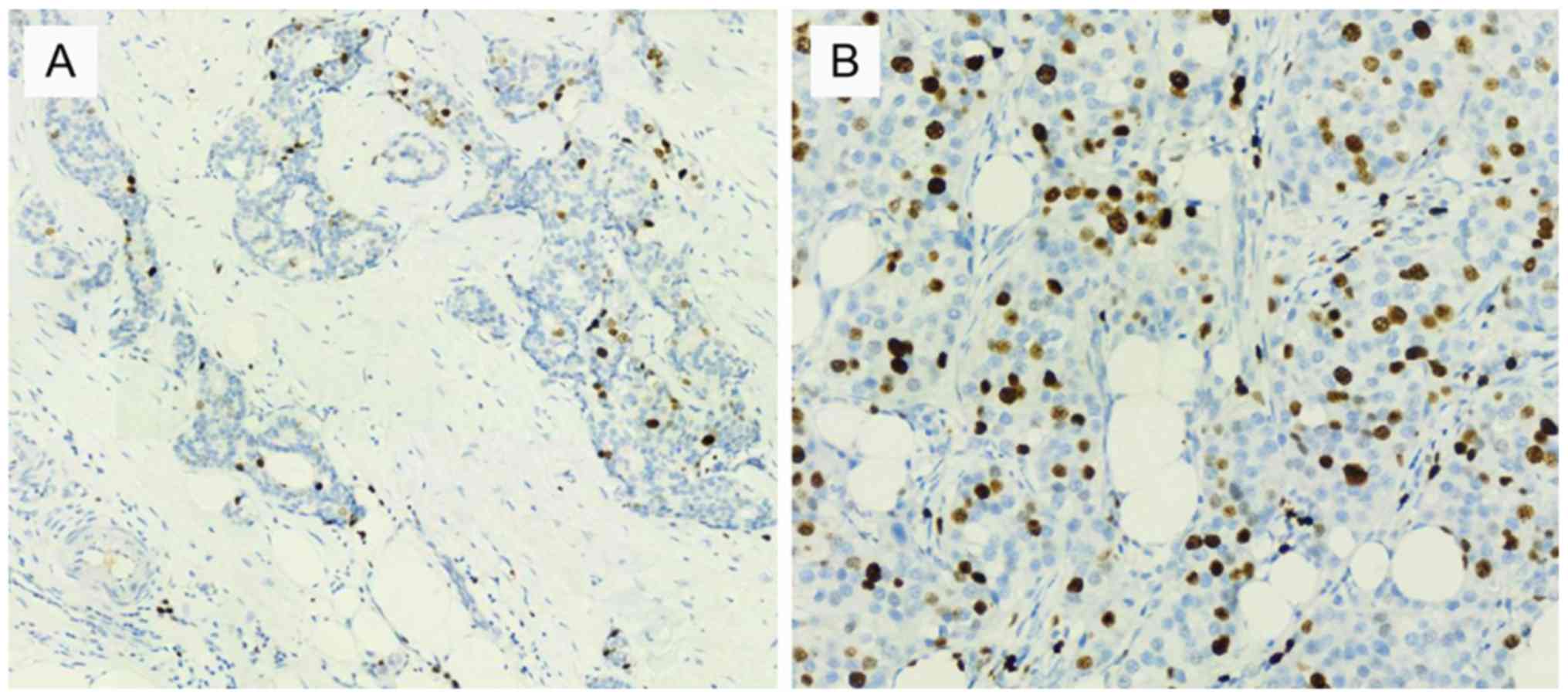
Ki67-positive cells were defined as those with yellow nuclei
(32). The percentage of
Ki67-positive cells from 500 cells was calculated after selection
under five different high-power microscope fields. A light
microscope (magnification, ×200) was used to capture the images.
The threshold for Ki67 expression was 20% (high expression, ≥20%;
low expression, <20%). Two experienced independent pathologists
performed all pathological diagnoses. Consultation with a third
pathologist ruled out inter-observer differences.
NAC and surgery
Written informed consent for chemotherapy treatment
was obtained from all patients with confirmed diagnosis of TNBC
detected by inpatient biopsy. All patients were tested for liver
function(serum enzymology test) (33), electrocardiogram and echocardiography
to evaluate their tolerance to chemotherapy. Oral dexamethasone
tablets were given 3 days prior to NAC. Treatment with TAC regimen
(days per cycle; 6 cycles) consisted of: Day 1, epirubicin
hydrochloride at 75 mg/m2 i.v.; day 1, cyclophosphamide
at 500 mg/m2 i.v.; and day 2, docetaxel at 75
mg/m2 i.v. Omeprazole and ondansetron were prescribed as
supportive care (for gastroprotection and antiemesis,
respectively). Upon completion of chemotherapy, appropriate
granulocyte colony-stimulating factor therapy was given to increase
the number of blood cells based on blood test results. All patients
underwent modified radical mastectomy after chemotherapy.
Pathological assessment after NAC
completion
The efficacy of chemotherapy was evaluated as
previously reported (34).
Pathological evaluation of the postoperative tissue sections of all
patients who received NAC was performed according to the
Miller-Payne grading criteria for pathological response (35): Grade 1, no reduction in the overall
cellularity of tumor cells; grade 2, loss of tumor cells <30%;
grade 3, loss of tumor cells between 30 and 90%; grade 4, >90%
loss of tumor cells; and grade 5, no residual invasive cancer
observed under microscope, but ductal carcinoma in situ may
be present. In the present study, grades 1–4 were defined as
non-pCR, and grade 5 was defined as pCR.
Methylation detection using MSP
DNA extraction from formalin-fixed paraffin-embedded
(FFPE) tissues was performed in strict accordance with the
manufacturer's protocol of the FFPE DNA extraction kit (Omega
Bio-Tek, Inc.; cat. no. D3399-01). If the resulting DNA
concentration detected using NanoDrop 2000 (Thermo Fisher
Scientific, Inc.) was <50 ng/µl, extraction was repeated to
ensure the DNA concentration was >50 ng/µl. Bisulfite treatment
and DNA purification was performed according to the manufacturer's
protocol of the Methylation-Gold kit [Zymo Research Corp.; cat. no.
D5005S (10)]. The obtained DNA
samples were stored at −20°C until further use. The treated DNA was
amplified using methylated and unmethylated PCR primers. According
to the principle of MSP, two pairs of primers, one pair for the
unmethylated DNA strand (U primers) and one pair for the methylated
DNA strand (M primers), were designed using the software Methyl
Primer Express v1.0 (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The primers (Table I) were
synthesized by Wuhan Servicebio Technology Co., Ltd. The 25 µl MSP
system contained 2.5 µl 10X PCR buffer, 2.5 µl 2.5 mM dNTPs, 2 µl
10 µM primers, 3 µl genomic DNA, 0.1 µl of 5 U/µl Takara Taq Hot
Start DNA polymerase (Takara Biotechnology Co., Ltd.; cat. no.
R007A), and 14.9 µl double-distilled H2O.
 | Table I.Primers used for M and U sequences in
PCDH17. |
Table I.
Primers used for M and U sequences in
PCDH17.
| Primer name | Sequence
(5′-3′) | Length, bp | Tm,
°C | Base no. |
|---|
| H-PCDH17
(M)-F |
GGAGAGAAGTTTTTGTTCGCGG | 108 | 63.0 | 22 |
| H-PCDH17
(M)-R |
AATAAATCTTCGCCTCTATTCGTAAA |
| 60.3 | 26 |
| H-PCDH17
(U)-F |
TGTTTGGAGAGAAGTTTTTGTTTGTG | 112 | 62.2 | 26 |
| H-PCDH17
(U)-R |
ATAAATCTTCACCTCTATTCATAAAACACAC |
| 61.5 | 31 |
PCR amplification conditions were as follows:
Pre-denaturation at 95°C for 5 min, 40 cycles of denaturation at
95°C for 30 sec, annealing at 55°C for 30 sec and extension at 72°C
for 30 sec, and final extension at 72°C for 5 min. The PCR products
were separated by electrophoresis on a 2% agarose gel. After
separation, the gel was placed in a gel imager in which the bands
were observed and imaged. Placental DNA treated with SssI
methyltransferase (New England Biolabs, Inc.) was subjected to
bisulfite treatment and served as a positive control for methylated
DNA, whereas placental DNA not treated with methyltransferase was
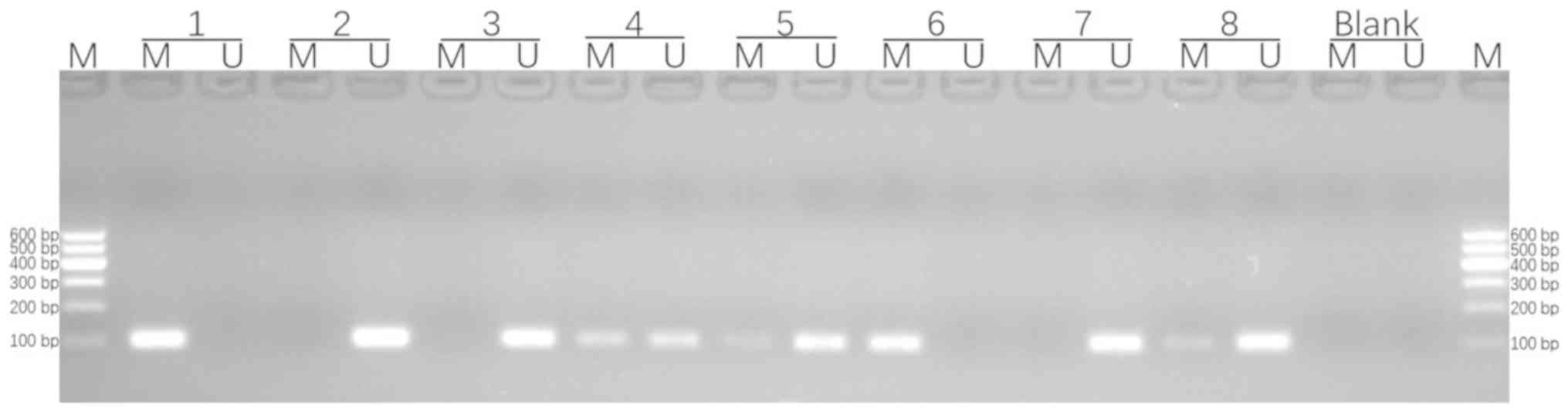
used as a positive control for unmethylated DNA (36). A blank control group was included, in
which distilled water was used as the template for the MSP. The
status of methylation was defined as positive if only the M band
was amplified (single pattern) or if both U and M bands were
simultaneously amplified (mixed pattern). The sample was deemed to
be unmethylated if only the U band was amplified. All methylation
assays were repeated twice to ensure reproducibility.
Statistical analysis
Data were presented as numbers and percentages and
were analyzed using SPSS 17.0 (SPSS, Inc.). Patients were divided
into negative PCDH17 methylation or positive PCDH17
methylation groups. The difference of clinicopathological
parameters between the two groups were analyzed using a
χ2 test. The association between PCDH17
methylation status and efficacy of NAC was analyzed using
univariate and multivariate logistic regression. The selection or
rejection of variables for the multivariate analysis was based on
evidence from the literature. Factors with P<0.2 in the
univariate logistic regression and factors in the literature
clinically considered to be closely associated with the efficacy of
neoadjuvant chemotherapy were included in the multivariate analysis
model. Multivariate logistic regression analysis applies the
forward method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
All 280 patients with TNBC received NAC and surgery.
Of these participants, 30.4% (85/280) were at stage IIB, while
69.6% (195/280) were at stage III (Table II). A total of 250 cases of invasive
ductal carcinoma were observed (89.3%); the remaining 30 samples
(10.7%) were mucinous adenocarcinoma (n=7), myeloid carcinoma
(n=13), poorly differentiated adenocarcinoma (n=4) and chemoplastic
carcinoma (n=6). Immunohistochemical examination (Fig. 1) revealed 215 samples (76.8%) with
high Ki67 expression and 65 samples (23.2%) with low Ki67
expression (Table II).
 | Table II.Associations between negative (n=52)
and positive (n=228) PCDH17 methylation and
clinicopathological parameters in 280 patients with triple-negative
breast cancer. |
Table II.
Associations between negative (n=52)
and positive (n=228) PCDH17 methylation and
clinicopathological parameters in 280 patients with triple-negative
breast cancer.
| Parameter | n (%) | Negative
PCDH17 methylation | Positive
PCDH17 methylation | χ2
value | P-value |
|---|
| Age, years |
|
<50 | 118 (42.1) | 23 (44.2) | 95 (41.7) | 0.114 | 0.735 |
|
≥50 | 162 (57.9) | 29 (55.8) | 133 (58.3) |
|
|
| Menopausal
status |
|
Pre | 136 (48.6) | 28 (53.8) | 108 (47.4) | 0.711 | 0.399 |
| yPost | 144 (51.4) | 24 (46.2) | 120 (52.6) |
|
|
| T stage |
| T2 | 68 (24.3) | 7 (13.4) | 61 (26.8) | 5.894 | 0.052 |
| T3 | 174 (62.1) | 34 (65.4) | 140 (61.4) |
|
|
| T4 | 38 (13.6) | 11 (21.2) | 27 (11.8) |
|
|
| Lymph node
metastasis |
| No | 52 (18.6) | 12 (23.1) | 40 (17.5) | 0.857 | 0.355 |
|
Yes | 228 (81.4) | 40 (76.9) | 188 (82.5) |
|
|
| TNM stage |
|
IIB | 85 (30.4) | 10 (19.2) | 75 (32.9) | 3.739 | 0.053 |
|
III | 195 (69.6) | 42 (80.8) | 153 (67.1) |
|
|
| Histological
grade |
| I | 57 (20.4) | 11(21.2) | 46 (20.2) | 1.494 | 0.474 |
| II | 133 (47.5) | 21 (40.4) | 112 (49.1) |
|
|
|
III | 90 (32.1) | 20 (38.5) | 70 (30.7) |
|
|
| Pathological
type |
| Other
types | 30 (10.7) | 4 (7.7) | 26 (11.4) | 0.610 | 0.435 |
|
IDC | 250 (89.3) | 48 (92.3) | 202 (88.6) |
|
|
| Ki67 |
| Low
expression | 65 (23.2) | 10 (19.2) | 55 (24.1) | 0.568 | 0.451 |
| High
expression | 215 (76.8) | 42 (80.8) | 173 (75.9) |
|
|
| Radiotherapy |
| No | 43 (15.4) | 7 (13.5) | 36 (15.8) | 0.177 | 0.674 |
|
Yes | 237 (84.6) | 45 (86.5) | 192 (84.2) |
|
|
| pCR |
| No | 173 (61.8) | 17 (32.7) | 156 (68.4) | 22.893 | 0.001 |
|
Yes | 107 (38.2) | 35 (67.3) | 72 (31.6) |
|
|
Associations between PCDH17 methylation and
clinicopathological parameters. DNA was successfully extracted from
the tissues of all 280 patients. The concentrations of all DNA
samples were >50 ng/µl. Fig. 2
includes the methylated positive control, the demethylated positive
control, the blank control and methylation status of protocadherin
17 in triple-negative breast cancer tissues detected via
methylation-specific PCR (3 and 7 were unmethylated; 4, 5, 6 and 8
were methylated). Methylation analyses by MSP revealed that of the
280 patients with TNBC, 228 (81.4%) were positive for PCDH17
methylation and 52 (18.6%) were negative for PCDH17
methylation. The results of the associations between methylation
status of PCDH17 and clinicopathological features are shown
in Table II. Clinicopathological
parameters, such as age, menopausal status, histological tumor
grade, T stage, TNM stage, pathological tumor type, lymph node
metastasis, radiotherapy and Ki67 status, were not significantly
different in patients with different PCDH17 methylation
status (P>0.05; Table II).
Pathological response after NAC
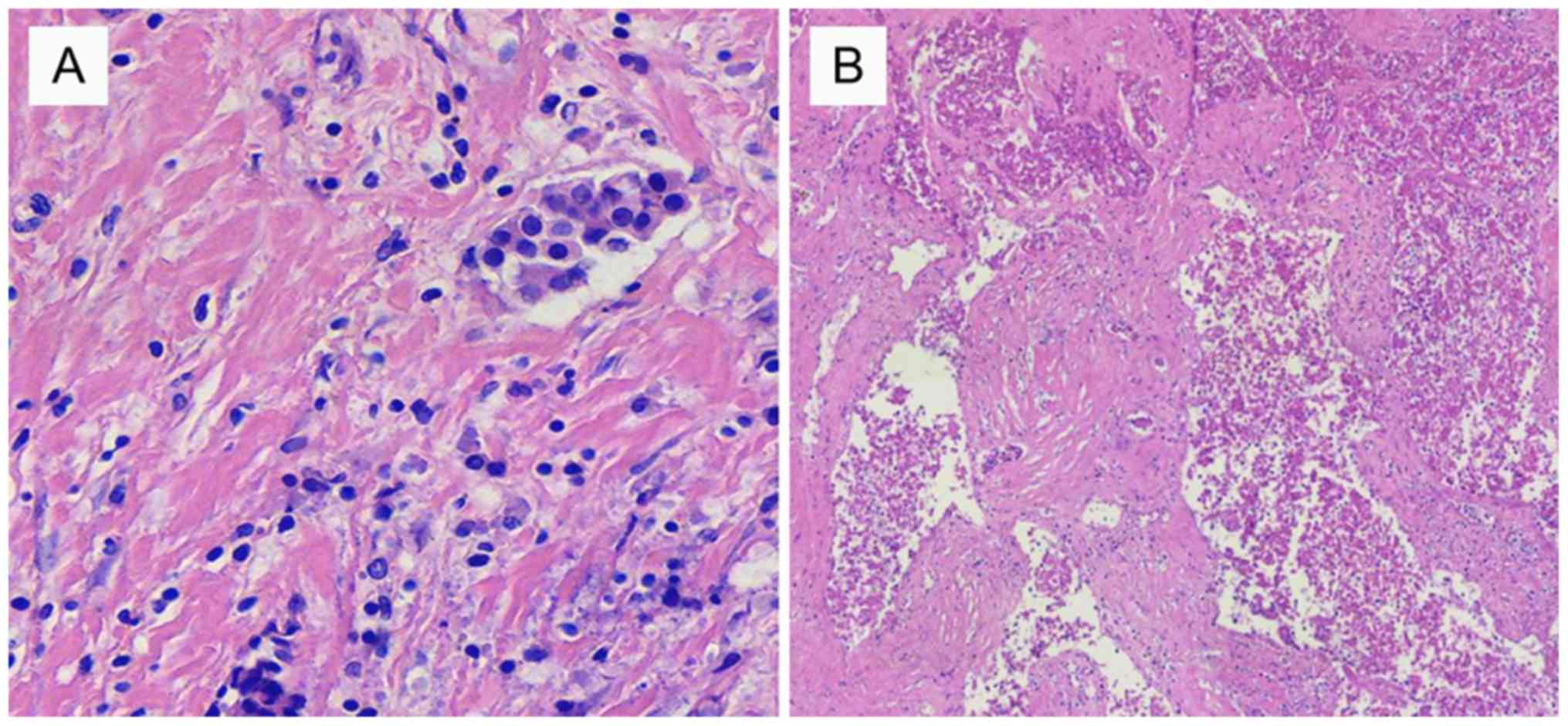
According to the pathological response criteria, 107
patients achieved pCR and 173 patients did not achieve pCR.
Fig. 3 shows the representative
images of pCR and non-pcr. The pCR rate was 38.2%. Among the 52
patients negative for PCDH17 methylation, 35 achieved pCR,
resulting in a pCR rate of 67.3%. Among the 228 patients positive
for PCDH17 methylation, 72 achieved pCR, resulting in a pCR
rate of 31.6%, the significant result presented in Table II for the pCR being significantly
different depending on the PCDH17 methylation status.
Univariate regression analysis across both groups of
patients revealed that patients who were negative for PCDH17
methylation and those with a high Ki67 expression had significantly
higher pCR rates [odds ratio (OR), 4.46, P=0.001; OR=2.78, P=0.002,
respectively; Table III]. Factors
with P<0.2 in univariate regression analysis were included in
multivariate logistic regression analysis. In addition to these
factors, age and lymph node metastasis, which are considered
clinically relevant to neoadjuvant chemotherapy, were also included
in the multivariate regression analysis (32,37). The
results revealed that patients who were negative for PCDH17
methylation and those with high Ki67 expression had significantly
higher pCR rates (OR=4.48, P=0.001; OR=3.04, P=0.001, respectively;
Table III).
 | Table III.Univariate and multivariate logistic
regressions of factors associated with pathological complete
response. |
Table III.
Univariate and multivariate logistic
regressions of factors associated with pathological complete
response.
| Parameters | Univariate analysis
OR (95% CI) | Multivariate
analysis P-value | OR (95% CI) | P-value |
|---|
| Age, years |
| ≥50 vs.
<50 | 1.07
(0.66–1.75) | 0.785 | 1.20
(0.70–2.03) | 0.507 |
| Menopausal
status |
| Post
vs. pre | 1.35
(0.83–2.20) | 0.222 |
|
|
| T stage |
| 0.227 |
| 0.318 |
| T3 vs.
T2 | 1.70
(0.93–3.10) | 0.086 | 1.56
(0.84–3.02) | 0.155 |
| T4 vs.
T2 | 1.57
(0.68–3.60) | 0.292 | 1.16
(0.47–2.86) | 0.749 |
| Lymph node
metastasis |
| Yes vs.
no | 0.81
(0.44–1.50) | 0.501 | 0.86
(0.44–1.68) | 0.654 |
| TNM stage |
| III vs.
IIB | 1.39
(0.81–2.37) | 0.231 |
|
|
| Histological
grade |
| 0.410 |
| 0.249 |
| II vs.
I | 0.65
(0.35–1.23) | 0.183 | 0.57
(0.29–1.13) | 0.108 |
| III vs.
I | 0.72
(0.37–1.42) | 0.347 | 0.59
(0.29–1.24) | 0.165 |
| Pathological
type |
| IDC vs.
other types | 0.79
(0.37–1.70) | 0.542 |
|
|
| Ki67 |
| High
expression vs. low expression | 2.78
(1.45–5.32) | 0.002 | 3.04
(1.53–6.03) | 0.001 |
| PCDH17
methylation |
| No vs.
yes | 4.46
(2.35–8.49) | 0.001 | 4.48
(2.27–8.81) | 0.001 |
Discussion
The efficacy of NAC in the treatment of breast
cancer has been widely recognized in recent years. NAC is able to
reduce micrometastases and improve long-term survival, as well as
increase the chance of breast-conserving surgery (38). However, numerous urgent problems
remain to be solved in the clinical application of NAC for breast
cancer. For example, as breast cancer is highly heterogeneous at
the molecular level, tumors of the same clinical stage and
histomorphology do not exhibit the same molecular changes, leading
to differences in tumor response to therapies (39). It has been demonstrated that 20% of
locally advanced breast cancer is not sensitive to chemotherapy,
and NAC will delay the opportunity for timely local treatment
(40). Therefore, it is important to
individualize the use of NAC to improve the pCR rate and to
predict, monitor and accurately evaluate treatment efficacy.
Epigenetic regulation refers to the regulation of
the frequency, speed or levels of gene expression through processes
such as DNA methylation and post-translational modifications of
chromatin histones without altering the genomic DNA sequence, which
ultimately lead to phenotypic changes (41). Epigenetic regulation is closely
associated with malignant tumors and can affect the sensitivity of
tumors to NAC and thereby influence the resistance to chemotherapy
drugs (42). Hu et al
(16) evaluated the methylation of
RASSF1A and WIF-1 in the serum of patients with
locally advanced breast cancer, and revealed that the degree of
methylation of these genes had significant clinical utility in
predicting the efficacy of the NAC regimen TAC. Xie et al
(43) found that the differentially
methylated sites in the promoter regions of ABCG2 and
DNMT3b could be the specific methylation sites that promoted
the differential expression of the ABCG2 gene, providing
novel targets for reversing the ABCG2-mediated multidrug
resistance. The DNA methylation status of a number of genes could
therefore help to predict the efficacy of NAC in patients with
malignant tumors. The present study aimed to investigate whether
the methylation status of PCDH17 could predict the efficacy
of NAC. The present study evaluated 280 patients with TNBC who
received NAC and identified that 81.4% of patients were positive
for PCDH17 methylation. Univariate and multivariate
regression analysis across both groups of patients with different
methylation status of PCDH17 revealed that patients who were
negative for PCDH17 methylation had significantly higher pCR
rates. The current data indicated that the methylation status of
PCDH17 may effectively predict the efficacy of NAC in
patients with TNBC.
The present study demonstrated the predictive value
of PCDH17 methylation status for NAC, providing a candidate
for reliable screening of treatment success under clinical
conditions. Furthermore, the current findings suggested that
adjusting PCDH17 methylation may improve NAC effects and
therefore should contribute to efforts aimed at developing novel
treatment strategies. The limitations of the present study include
small sample sizes and body mass index that could not be taken into
account since data regarding this factor were not collected. Based
on the results of the present study, more reliable clinical data
should be obtained by increasing the sample size and including
multicenter collaboration, in which the value of PCDH17
methylation for predicting the prognosis of patients receiving NAC
can be further validated to confirm its clinical utility. In
conclusion, the present data indicate that PCDH17
methylation status may be used to predict the response to NAC in
patients with TNBC and may serve as a predictive factor for NAC
efficacy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Development Plan of Jining No.1 People's Hospital (grant
no. 2014jnjc14).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZF designed the study and analyzed the data. DDK
carried out the experiments. LL participated in specimen collection
and pathological diagnosis. WW performed the surgery. SBW carried
out acquisition of data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Jining No.1 People's Hospital, Jinging, China
[institutional review board approval no. Lun Shenyan No. 2017
(011)]. All procedures performed in the present study involving
human participants were in accordance with the ethical standards of
the Institutional and National Research Committee and with the 1964
Declaration of Helsinki and its later amendments or comparable
ethical standards. Written informed consent was obtained from all
individual participants included in the present study. When
receiving ultrasound-guided breast tumor puncture, patients were
informed that tissue samples and relevant data would be used for
future research work, and provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Wang S, Wei X, Zhang S, Song Z, Chen
X and Zhang J: Role of inhibitor of yes-associated protein 1 in
triple-negative breast cancer with taxol-based chemoresistance.
Cancer Sci. 110:561–567. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bardia A, Parton M, Kümmel S, Estévez LG,
Huang CS, Cortés J, Ruiz-Borrego M, Telli ML, Martin-Martorell P,
López R, et al: Paclitaxel with inhibitor of apoptosis antagonist,
LCL161, for localized triple-negative breast cancer, prospectively
stratified by gene signature in a biomarker-driven neoadjuvant
trial. J Clin Oncol. 20:JCO20177483922018.
|
|
3
|
Murphy BL, Day CN, Hoskin TL, Habermann EB
and Boughey JC: Neoadjuvant chemotherapy use in breast cancer is
greatest in excellent responders: Triple-negative and
HER2+ subtypes. Ann Surg Oncol. 25:2241–2248. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Hilli Z, Choong G, Keeney MG, Visscher
DW, Ingle JN, Goetz MP and Jakub JW: Metaplastic breast cancer has
a poor response to neoadjuvant systemic therapy. Breast Cancer Res
Treat. 176:709–716. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang RX, Chen S, Huang L, Zhou Y and Shao
ZM: Monitoring serum VEGF in neoadjuvant chemotherapy for patients
with triple-negative breast cancer: A new strategy for early
prediction of treatment response and patient survival. Oncologist.
24:753–761. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herrero-Vicent C, Guerrero A, Gavilá J,
Gozalbo F, Hernández A, Sandiego S, Algarra MA, Calatrava A,
Guillem-Porta V and Ruiz-Simón A: Predictive and prognostic impact
of tumour infiltrating lymphocytes in triple-negative breast cancer
treated with neoadjuvant chemotherapy. Ecancermedicalscience.
11:7592017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding Y, Ding K, Yu K, Zou D, Yang H, He X,
Mo W, Yu X and Ding X: Prognosis and endocrine therapy selection
for patients with low hormone receptor-positive breast cancer
following neoadjuvant chemotherapy: A retrospective study of 570
patients in China. Oncol Lett. 18:6690–6696. 2019.PubMed/NCBI
|
|
8
|
Sharma P, López-Tarruella S, García-Saenz
JA, Khan QJ, Gómez HL, Prat A, Moreno F, Jerez-Gilarranz Y,
Barnadas A, Picornell AC, et al: Pathological response and survival
in triple-negative breast cancer following neoadjuvant carboplatin
plus docetaxel. Clin Cancer Res. 24:5820–5829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akimoto E, Kadoya T, Kajitani K, Emi A,
Shigematsu H, Ohara M, Masumoto N and Okada M: Role of 18F-PET/CT
in predicting prognosis of patients with breast cancer after
neoadjuvant chemotherapy. Clin Breast Cancer. 18:45–52. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Resende U, Cabello C, Ramalho SOB and
Zeferino LC: Prognostic assessment of breast carcinoma submitted to
neoadjuvant chemotherapy with pathological non-complete response.
BMC Cancer. 19:6012019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jézéquel P, Kerdraon O, Hondermarck H,
Guérin-Charbonnel C, Lasla H, Gouraud W, Canon JL, Gombos A, Dalenc
F, Delaloge S, et al: Identification of three subtypes of
triple-negative breast cancer with potential therapeutic
implications. Breast Cancer Res. 21:652019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim T, Han W, Kim MK, Lee JW, Kim J, Ahn
SK, Lee HB, Moon HG, Lee KH, Kim TY, et al: Predictive significance
of p53, Ki-67, and Bcl-2 expression for pathologic complete
response after neoadjuvant chemotherapy for triple-negative breast
cancer. J Breast Cancer. 18:16–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdelrahman AE, Rashed HE, Abdelgawad M
and Abdelhamid MI: Prognostic impact of EGFR and cytokeratin 5/6
immunohistochemical expression in triple-negative breast cancer.
Ann Diagn Pathol. 28:43–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sobral-Leite M, Lips EH, Vieira-Monteiro
HA, Giacomin LC, Freitas-Alves DR, Cornelissen S, Mulder L,
Wesseling J, Schmidt MK and Vianna-Jorge R: Evaluation of the EGFR
polymorphism R497K in two cohorts of neoadjuvantly treated breast
cancer patients. PLoS One. 12:e01897502017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, He C, Han D, Zhou M, Wang Q, Tian
J, Li L, Xu F, Zhou E and Yang K: The predictive value of Ki-67
before neoadjuvant chemotherapy for breast cancer: A systematic
review and meta-analysis. Future Oncol. 13:843–857. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu YL, Li GL and Lin ZhX: Values of serum
RASSF1A and WIF-1 methylation could evaluate the efficacy of
neoadjuvant chemotherapy for advanced breast cancer. Jiyinzuxue Yu
Yingyong Shengwuxue. 37:4937–4942. 2018.(In Chinese).
|
|
17
|
Haruki S, Imoto I, Kozaki K, Matsui T,
Kawachi H, Komatsu S, Muramatsu T, Shimada Y, Kawano T and Inazawa
J: Frequent silencing of protocadherin 17, a candidate tumour
suppressor for esophageal squamous cell carcinoma. Carcinogenesis.
31:1027–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Liu J, Li X and Li JC:
PCDH17 gene promoter demethylation and cell cycle arrest by
genistein in gastric cancer. Histol Histopathol. 27:217–224.
2012.PubMed/NCBI
|
|
19
|
Lin YL, Gui SL, Guo H, Ma JG and Li WP:
Protocadherin17 promoter methylation is a potential predictive
biomarker in clear cell renal cell carcinoma. Med Sci Monit.
21:2870–2876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Costa VL, Henrique R, Danielsen SA, Eknaes
M, Patrício P, Morais A, Oliveira J, Lothe RA, Teixeira MR, Lind
GE, et al: TCF21 and PCDH17 methylation: An innovative panel
of biomarkers for a simultaneous detection of urological cancers.
Epigenetics. 6:1120–1130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu X, Sui X, Li L, Huang X, Rong R, Su X,
Shi Q, Mo L, Shu X, Kuang Y, et al: Protocadherin 17 acts as a
tumour suppressor inducing tumour cell apoptosis and autophagy, and
is frequently methylated in gastric and colorectal cancers. J
Pathol. 229:62–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin X, Xiang T, Mu J, Mao H, Li L, Huang
X, Li C, Feng Y, Luo X, Wei Y, et al: Protocadherin 17 functions as
a tumor suppressor suppressing Wnt/β-catenin signaling and cell
metastasis and is frequently methylated in breast cancer.
Oncotarget. 7:51720–51732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Zhang X, Wu X, Li W, Su P, Cheng
H, Xiang L, Gao P and Zhou G: Interference of Frizzled 1 (FZD1)
reverses multidrug resistance in breast cancer cells through the
Wnt/β-catenin pathway. Cancer Lett. 323:106–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu L, Liang Z, Li S and Ma J: Signaling
via the CXCR5/ERK pathway is mediated by CXCL13 in mice with breast
cancer. Oncol Lett. 15:9293–9298. 2018.PubMed/NCBI
|
|
26
|
Yu DF, Jiang SJ, Pan ZP, Cheng WD, Zhang
WJ, Yao XK, Li YC and Lun YZ: Expression and clinical significance
of Sirt1 in colorectal cancer. Oncol Lett. 11:1167–1172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. Arch Pathol Lab Med. 134:907–922. 2010.PubMed/NCBI
|
|
28
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members, : Strategies for
subtypes - dealing with the diversity of breast cancer: Highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cuadros M and Villegas R: Systematic
review of HER2 breast cancer testing. Appl Immunohistochem Mol
Morphol. 17:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gallardo A, Garcia-Valdecasas B, Murata P,
Teran R, Lopez L, Barnadas A and Lerma E: Inverse relationship
between Ki67 and survival in early luminal breast cancer:
Confirmation in a multivariate analysis. Breast Cancer Res Treat.
167:31–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
von Minckwitz G, Schneeweiss A, Loibl S,
Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S,
Gerber B, et al: Neoadjuvant carboplatin in patients with
triple-negative and HER2-positive early breast cancer (GeparSixto;
GBG 66): A randomised phase 2 trial. Lancet Oncol. 15:747–756.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang HW, Jung H, Hyeon J, Park YH, Ahn
JS, Im YH, Nam SJ, Kim SW, Lee JE, Yu JH, et al: A nomogram to
predict pathologic complete response (pCR) and the value of
tumor-infiltrating lymphocytes (TILs) for prediction of response to
neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast
Cancer Res Treat. 173:255–266. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duan YF, Sun DL, Chen J, Zhu F and An Y:
MicroRNA-29a/b/c targets iNOS and is involved in protective remote
ischemic preconditioning in an ischemia-reperfusion rat model of
non-alcoholic fatty liver disease. Oncol Lett. 13:1775–1782. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ogston KN, Miller ID, Payne S, Hutcheon
AW, Sarkar TK, Smith I, Schofield A and Heys SD: A new histological
grading system to assess response of breast cancers to primary
chemotherapy: Prognostic significance and survival. Breast.
12:320–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shintia C, Endang H and Diani K:
Assessment of pathological response to neoadjuvant chemotherapy in
locally advanced breast cancer using the Miller-Payne system and
TUNEL. Malays J Pathol. 38:25–32. 2016.PubMed/NCBI
|
|
36
|
Li Ch, Li HJ, Chen Q, Zhang XM and Li CL:
Expression of ASPH gene in invasion breast cancer and its clinical
significance in promoter methylation. Sichuan Da Xue Xue Bao Yi Xue
Ban. 49:54–58. 2018.(In Chinese). PubMed/NCBI
|
|
37
|
Hamy AS, Belin L, Bonsang-Kitzis H, Paquet
C, Pierga JY, Lerebours F, Cottu P, Rouzier R, Savignoni A, Lae M,
et al: Pathological complete response and prognosis after
neoadjuvant chemotherapy for HER2-positive breast cancers before
and after trastuzumab era: Results from a real-life cohort. Br J
Cancer. 114:44–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arnaout A, Boileau JF and Brackstone M:
Surgical considerations in locally advanced breast cancer patients
receiving neoadjuvant chemotherapy. Curr Opin Support Palliat Care.
8:39–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen F, Zhang Z and Pu F: Role of
stanniocalcin-1 in breast cancer. Oncol Lett. 18:3946–3953.
2019.PubMed/NCBI
|
|
40
|
Kong D, Wang MH, Yang J and Liang Li:
Association of T-cadherin levels with the response to neoadjuvant
chemotherapy in locally advanced breast cancer. Oncotarget.
8:13747–13753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hernandez SJ, Dolivo DM and Dominko T:
PRMT8 demonstrates variant-specific expression in cancer cells and
correlates with patient survival in breast, ovarian and gastric
cancer. Oncol Lett. 13:1983–1989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Azad N, Zahnow CA, Rudin CM and Baylin SB:
The future of epigenetic therapy in solid tumours--lessons from the
past. Nat Rev Clin Oncol. 10:256–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xie N, Mou L, Yuan J, Liu W, Deng T, Li Z,
Jing Y and Hu Z: Modulating drug resistance by targeting BCRP/ABCG2
using retrovirus-mediated RNA interference. PLoS One.
9:e1034632014. View Article : Google Scholar : PubMed/NCBI
|