Introduction
Hypopharyngeal cancer (mostly squamous cell
carcinoma (SCC)) is most common in the head and neck. Treating
patients is difficult and they experience poor life quality due to
problems eating, swallowing, vocalizing, and breathing; moreover,
the prognosis remains particularly ominous, even among the head and
neck cancers (1). These cancers also
present high local invasiveness with neck lymph node and distant
metastases (2), and approaches are
needed to improve adjuvant therapy for hypopharyngeal cancer.
Chemokines are a superfamily of small cytokine-like
proteins that can bind to the transmembrane domain and activate
chemokine receptors, a family of seven transmembrane G
protein-coupled receptors (3). The
chemokine family currently comprises over 40 members that are
subdivided into groups based on their first two N-terminal cysteine
residue motifs and binding to G protein-coupled receptors segmented
based on chemokine subgroups (CXCR, CCR, CX3R1, XCR1) (4). Chemokines have various roles in
vivo, including promoting mitosis and regulating apoptosis,
survival, and angiogenesis (5,6). These
functions are beneficial for tumor progression, and chemokines are
now recognized to be expressed in many tumor types (7).
In particular, the interaction between the CXC
chemokine receptor type 4 (CXCR4) and its ligand stromal derived
factor 1 (SDF-1), also known as CXCL12, has been shown to affect
cell survival, proliferation, and migration in malignant tumors
(8,9). It was recently discovered that the
expression of CXCR4 is involved in the metastasis of breast and
prostate cancer cells cooperating with SDF-1 (10,11).
Thus, SDF-1/CXCR4 plays a role in the tumorigenesis of many cancers
(12,13).
Studies of SDF-1/CXCR4 have focused mainly on head
and neck cancer. Research models conducted in head and neck SCC
(HNSCC) cell lines or HNSCC nude mice have shown that the
expression of SDF-1/CXCR4 promotes cell motility, proliferation,
and metastasis via the upregulation of various pathways, such as
ERK 1/2, NF-kB, and matrix metalloproteinase (MMP) (14–18). In
addition, some clinical data have suggested that
SDF-1/CXCR4-positive HNSCC tumors have high metastatic potential
and poor outcomes (14,19–22).
However, research on its correlation with other tumor-promoting
factors is lacking.
CD147, also known as extracellular matrix
metalloproteinase inducer (EMMPRIN), is a member of the
immunoglobulin superfamily that is highly expressed in cancer
cells. It causes a variety of malignant tumors, including HNSCC
(23,24). Studies have attempted to elucidate
the mechanisms underlying CD147-induced tumorigenesis in various
types of cancer (3,25), and the number of studies on the
contribution of CD147 to the progression of HNSCC is increasing. We
have previously reported that CD147 increases cell invasiveness,
proliferation, and drug resistance via interaction with its ligand,
cyclophilin A, in HNSCC cells [including in a hypopharyngeal cell
line; (26)]. Moreover, CD147
expression is associated with cervical lymph node metastases in
patients with tongue SCC (27).
However, the role of CD147 in HNSCC tumorigenesis and its
underlying mechanisms are not fully understood. In particular,
although the relationship between CD147 and cytokines has been
reported in various diseases (28,29),
many uncertainties remain in patients with cancer. The present
study was aimed to investigate the mechanisms of tumor progression
by SDF-1/CXCR4 in patients with hypopharyngeal cancer, thus
assessing their association with CD147.
Materials and methods
Cell lines and cell culture
We purchased HSC-3, a human tongue SCC cell line,
from the Japanese Collection of Research Bioresources Cell Bank
(Osaka, Japan). FaDu, the cells from a human hypopharyngeal SCC
cell line, were kindly gifted by the Department of Cell Biology and
Morphology, Akita University Graduate School of Medicine (Akita,
Japan); we used both cell lines for in vitro studies. All
cells were maintained in the Dulbecco's modified Eagle's medium
(DMEM; Merck KGaA) supplemented with 10% fetal bovine serum in a
humidified atmosphere containing 5% CO2 at 37°C.
Western blotting
We detected protein expression using western blot
analysis with actin as an internal control. We lysed cell lines in
detergent containing 1% NP-40, 150 mmol/l NaCl, 1 mmol/l EDTA, 0.1
mmol/l phenylmethylsulfonyl fluoride, 1 µg/ml leupeptin, and 1
µg/ml aprotinin and determined the protein levels using the Bio-Rad
Protein Assay method (Bio-Rad Laboratories). We separated 40 µg of
the total protein on 8% SDS-PAGE gels and transferred them to
nitrocellulose membranes using a semidry transfer machine (Bio-Rad
Laboratories). Next, we blocked membranes with 5% skimmed milk/TBS
with Tween-20 solution for 1 h at room temperature, and incubated
with primary antibodies in 5% skimmed milk in TBS-T overnight at
4°C. After washing with TBS-T three times, we incubated the
membranes for 1 h with horseradish-peroxidase-conjugated secondary
antibody (Bio-Rad Laboratories) 1:3,000 diluted in 5% skimmed milk
in TBS-T. We rinsed the filters with TBS-T three times and
developed the blot using Luminol Reagent (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) by autoradiography. The band intensities were
analyzed using the ImageJ software (U. S. National Institutes of
Health). We used the following primary antibodies: Rabbit
anti-CXCR4 (1:1,000; Bioss), rabbit anti-CD147 (1:1,000; Santa Cruz
Biotechnology), and mouse anti-β-actin (1:5,000; Merck
Millipore).
Matrigel invasion and cell migration
assays
We evaluated cell invasion and migration using in
vitro using Matrigel-coated semipermeable modified Boyden
inserts with a pore size of 8 µm (BectonDickinson/Biocoat). We
plated cells in duplicate at a density of 5×103
cells/well for the invasion assay or at 3×104 cells/well
for the migration assay. Plating was carried out on serum-free DMEM
with SDF-1 (0.1 µg/ml; Pepro Tech), AMD3100 (10 ng/ml; Abcam),
anti-CD147 function-blocking antibody (10 µg/ml, UM-8D6, cat. no.
10R-CD147aHU; Research Diagnostics), for which the blocking
activity has been published (30,31), or
a combination of SDF-1 and AMD3100 for the migration assay or
anti-CD147 function-blocking antibody for the invasion assay in the
inserts. We plated the cells in 96-well plates to serve as loading
controls. Both the insert and the holding well were filled with the
same medium composition, but without serum. The insert contained no
serum, whereas the lower well contained 10% FBS that served as a
chemoattractant. After a 24-h treatment at 37°C in a 5%
CO2 incubator, we gently wiped away the cells in the
insert using a cotton swab. Cells on the reverse side of the insert
were fixed and stained with Diff-Quik® (Sysmex)
according to the manufacturer's instructions. Cells plated in
24-well plates were subjected to
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
assays, and we normalized the cell numbers across the groups. We
also adjusted the number of invading or migrating cells
accordingly.
Proliferation assay
FaDu cells were plated in triplicate at a density of
3×104 cells/well and allowed to seed overnight in a
12-well plate. Cells were then treated with SDF-1 (0.1 µg/ml),
anti-CD147 function-blocking antibody (10 µg/ml), or a combination
of SDF-1 and anti-CD147 function-blocking antibody in DMEM with 10%
FBS. At selected time-points, we trypsinized the cells and stained
them with trypan blue, and viable cells were counted using a
hemocytometer.
Statistical analysis
Statistical analyses were performed using Statcel 3
(OMS Publishing). One-way ANOVA with post-hoc Tukey test was used
to assess the statistically significant differences in
proliferation, invasion, and migration studies. Data are presented
as the mean ± SD from experiments that were repeated at least three
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
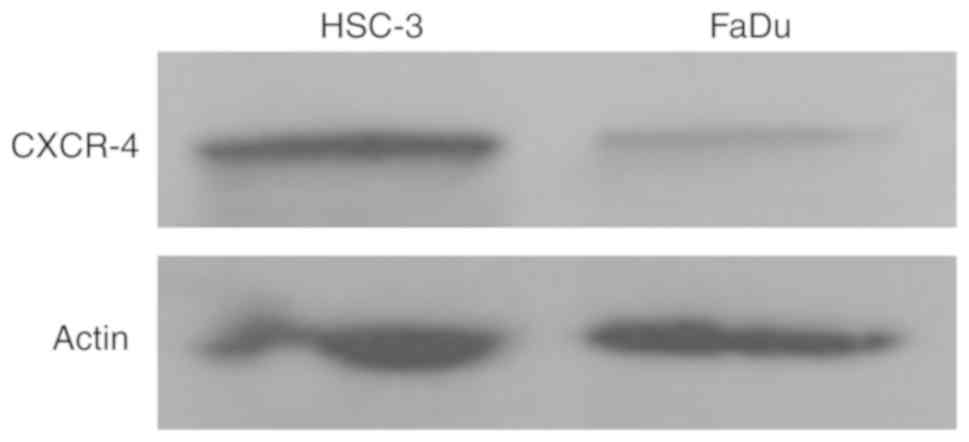
Hypopharyngeal SCC cell expresses
CXCR4
To investigate the function of CXCR4 in
hypopharyngeal SCC, we measured the expression of CXCR4 in FaDu
cells (established from hypopharyngeal SCC) by western blotting. At
the same time, we also analyzed the expression of CXCR4 in HSC-3 (a
cell line established from SCC of the tongue) as a control
(32). Our results showed that FaDu
cells express CXCR4 protein; however, the expression level was weak
compared to that in the tongue SCC cell line HSC-3 (Fig. 1).
SDF-1/CXCR4 increases cell migration
in hypopharyngeal SCC cells
As tumor cell invasion and migration are important
during the tumor progression cascade, controlling cell mobility is
viewed as a target for clinical tumor suppression (33,34). To
understand the mechanisms underlying hypopharyngeal SCC cell
invasion, we seeded FaDu cells into Boyden chambers and stimulated
them with SDF-1.
The invasiveness of FaDu cells increased when the
cells were co-cultured with SDF-1 (P<0.05) is shown in Fig. 2A and B. To determine whether this
increased migration was mediated by an SDF-1-CXCR4 interaction, we
added AMD3100, a CXCR4 antagonist (35). The AMD3100 treatment reversed the
increased migration of FaDu cells (P<0.05), suggesting that
SDF-1 induces hypopharyngeal SCC cell migration by stimulating
CXCR4 (Fig. 2A and B). In addition,
we evaluated the ability of SDF-1 to induce FaDu cell proliferation
because during tumor progression, an increase in cell number is as
important as cell mobility.
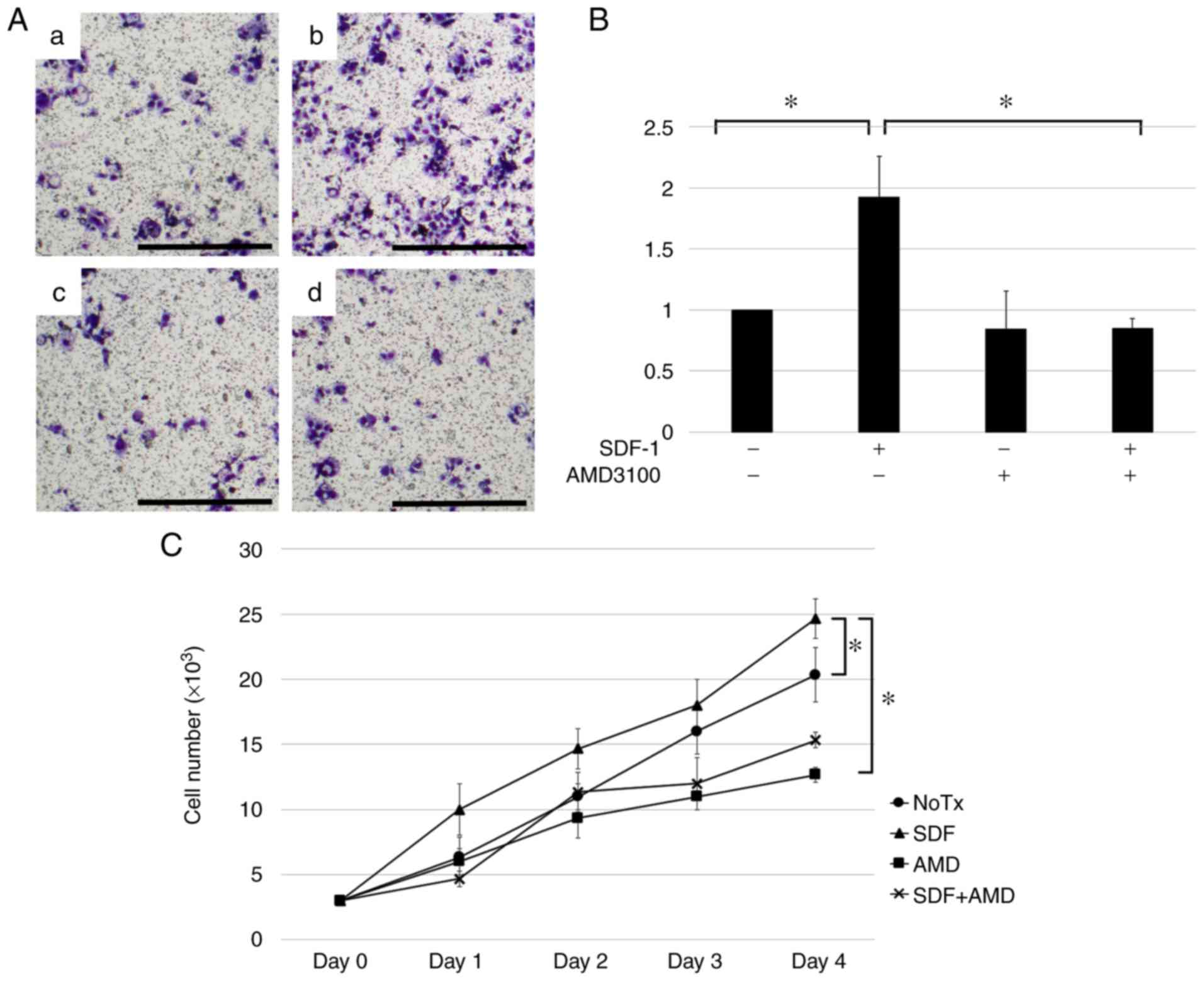 | Figure 2.SDF-1 induces hypopharyngeal SCC cell
migration via its interaction with CXCR4. Cell migration and
proliferation ability of FaDu, a hypopharyngeal SCC cell, were
evaluated after adding SDF-1 (0.1 µg/ml), AMD3100 (10 ng/ml), a
combination of the agents or none. (A) Representative image of the
migration assay results. FaDu cells were plated in the inserts in
serum-free medium. Untreated cells were used as controls. (a)
Control; (b) SDF-1; (c) AMD3100; and (d) combination of the agents.
Scale bar, 100 µm. (B) Results are presented as fold-changes in
migration relative to those in control cells. FaDu cell migration
increased in response to SDF-1 stimulation, whereas the addition of
AMD3100 reversed this increase. Experiments were repeated three
times, and fold migratory relative to control was expressed as the
mean ± standard deviation. *P<0.05. (C) Proliferation assay of
hypopharyngeal SCC cells treated with SDF-1 (0.1 µg/ml) and/or
AMD3100 (10 ng/ml). Cells were plated in 12-well plates in DMEM
with 10% FBS, and after 24 h, the growth media was replaced with
media containing each agent. Cells were harvested and counted by
vital dye exclusion. Cell counts on days 1, 2, 3 and 4 from three
independent experiments are presented as the mean ± standard
deviation. *P<0.05. SCC, squamous cell carcinoma; CXCR4, CXC
chemokine receptor type 4; SCC, squamous cell carcinoma; SDF-1,
stromal cell-derived factor 1. |
On day 4 the cells treated with SDF-1 increased
significantly in number as compared to the untreated cells
(P<0.05) (Fig. 2C). To determine
whether this increase in proliferation ability was mediated by an
SDF-1-CXCR4 interaction, we added AMD3100. AMD3100 treatment
reversed the increase in proliferation of FaDu cells (P<0.05),
suggesting that SDF-1 induces hypopharyngeal SCC cell proliferation
by stimulating CXCR4 (Fig. 2C).
Our results suggest that the SDF-1/CXCR4 axis plays
a key role in hypopharyngeal SCC cell mobility and proliferation,
and may contribute to tumor progression.
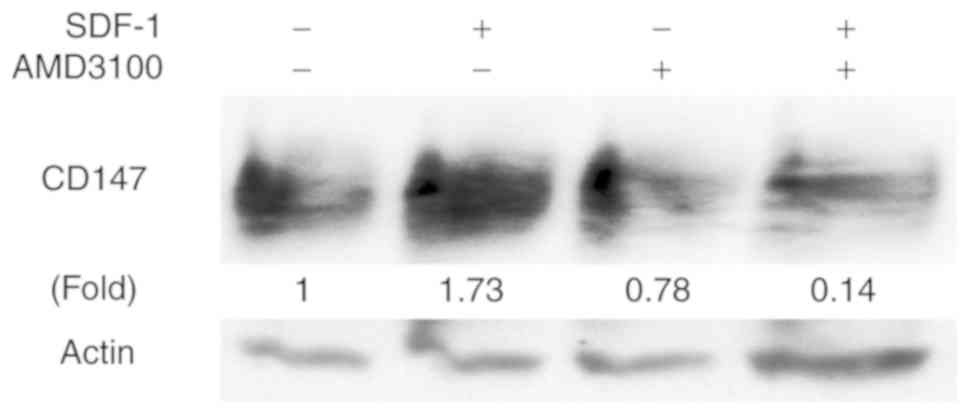
SDF-1/CXCR4 induces expression of
CD147 in hypopharyngeal SCC cell
CD147 expression is associated with tumor
progression and poor prognosis in various types of solid tumors
(including HNSCC). We previously found that CD147 mediates
invasion, proliferation, and drug resistance in FaDu cells
(26). Thus, for this study we
hypothesized that CD147 is associated with CXCR4-induced
tumorigenesis. We treated FaDu cells with SDF-1, and confirmed by
western blotting that CD147 protein expression was up-regulated in
these cells. In addition, AMD3100 suppressed the CD147 upregulation
induced by SDF-1. Our results suggest that SDF-1/CXCR4 regulates
CD147 expression in hypopharyngeal SCC (Fig. 3).
CD147 mediates SDF-1/CXCR4-induced
hypopharyngeal SCC cell invasion
The results shown in Figs. 2A-B and 3 indicate that SDF-1/CXCR4 induce cell
mobility and CD147 expression in the hypopharyngeal SCC cell line
FaDu. But, whether CD147 was associated with the
SDF-1/CXCR4-induced increment of mobility of the cells was unclear.
Thus, we planned an invasion assay with FaDu cells using a
function-blocking antibody of CD147 in the presence or absence of
SDF-1. As shown in Fig. 4, SDF-1
induced invasiveness, and this effect was significantly diminished
by addition of the CD147 function-blocking antibody. This suggests
that SDF-1/CXCR4 induces invasion in hypopharyngeal SCC and that
CD147 may play a role in SDF-1/CXCR4 induced tumorigenicity.
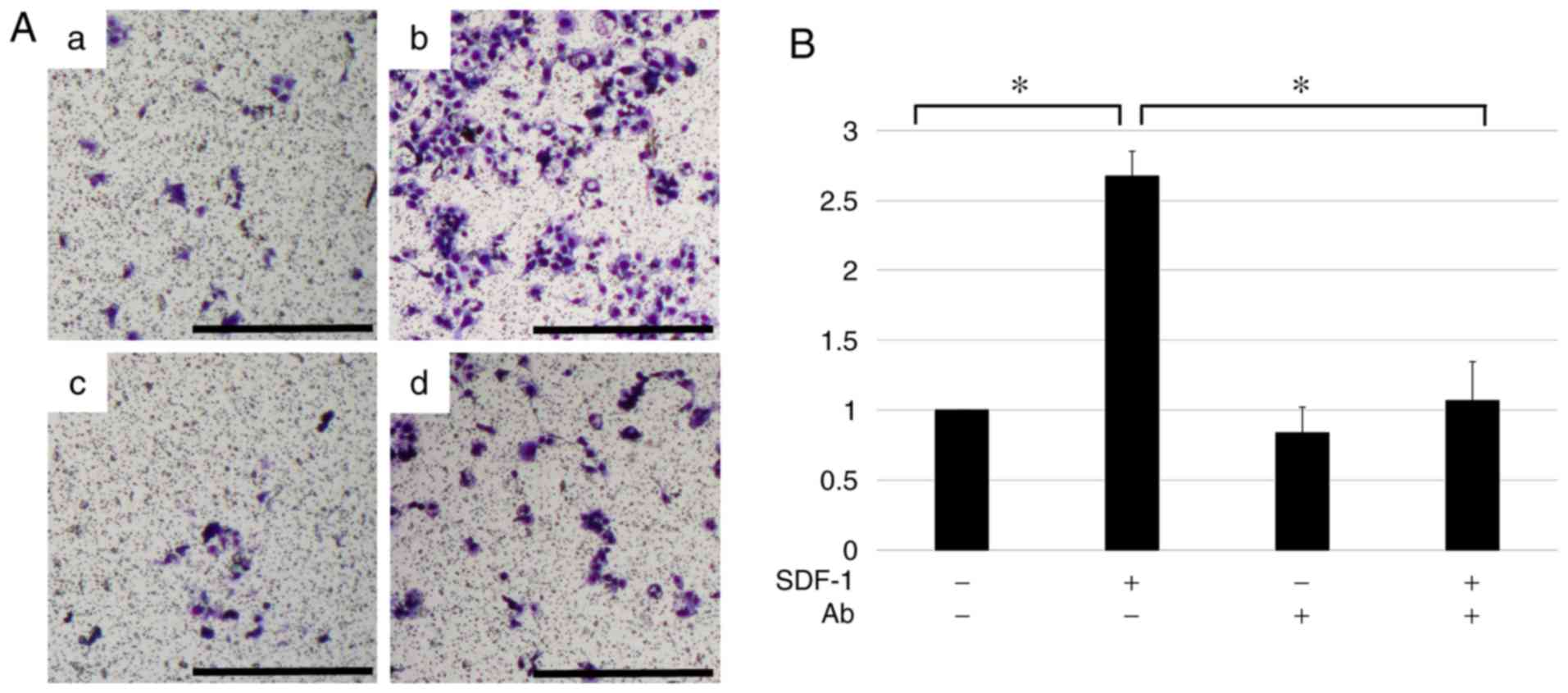 | Figure 4.CD147 plays a critical role in
SDF-1-induced hypopharyngeal SCC cell invasion. Cell invasiveness
of FaDu, a hypopharyngeal SCC cell, was evaluated using an invasion
assay. Cells were plated on inserts with or without SDF-1 (0.1
µg/ml), CD147 function-blocking antibody (10 µg/ml), or a
combination of both agents in serum-free media with 10% FBS, with
the lower well serving as a chemoattractant; the CD147
function-blocking antibody decreased head and neck squamous cell
carcinoma invasion during SDF-1 treatment. (A) Representative image
of the invasion assay results. Untreated cells were used as
controls. (a) Control; (b) SDF-1; (c) CD147 function-blocking
antibody; and (d) combination of the agents. Scale bar, 100 µm. (B)
Results are presented as fold-changes in invasion relative to those
in control cells. The experiment was repeated three times, and fold
migratory relative to control was expressed as the mean ± standard
deviation. *P<0.05. CD147, cluster of differentiation-147; SCC,
squamous cell carcinoma; SDF-1, stromal cell-derived factor 1. |
Discussion
The importance of SDF-1/CXCR4 in cancer progression
has been reported, especially in the metastasis and proliferation
of cancer cells. The association between solid cancers and
SDF-1/CXCR4 interaction was first reported as a chemokine receptor
that is highly expressed in primary and metastatic breast cancer
lesions (10). In subsequent
studies, signaling via SDF-1/CXCR4 in various solid tumors has been
established as a chemokine receptor pathway that plays an important
role in cancer progression and malignancy. SDF-1/CXCR4 has roles in
oncogenesis, proliferation, metastasis, and angiogenesis in many
cancers types, including lung cancer, melanoma, esophageal cancer,
ovarian cancer, glioblastoma, and basal cell carcinoma cells
(8,9,12,13,36,37).
Analyses using molecular biology techniques have
revealed that communication between CXCR4 and SDF-1 activates
multiple signaling pathways to enhance tumor cell invasion and
distant metastasis (38). In
addition, CXCR4 cooperates with other transcription factors, such
as NF-κB and Nanog, to help maintain the stemness of cancer stem
cells as well as to induce metastatic behavior (39,40).
Therefore, SDF-1/CXCR4 is considered a new drug target for cancer
treatment.
Research on sSDF-1/CXCR4 molecules is also
undergoing in patients with head and neck cancer. CXCR4 promotes
migration and invasion of oral cancer cells (18) and SDF-1/CXCR4 is involved in the
metastasis of laryngeal and hypopharyngeal cancers (14). For this process, MMP production via
the ERK signaling pathway is essential, and this mechanism of
SDF-1/CXCR4 molecules in patients with head and neck cancer has
been elucidated (14). Studies have
reported that CXCR4 expression in primary tumors is involved not
only in the local recurrence of head and neck cancer (22) but also in cervical lymph node
metastasis and distant metastasis of hypopharyngeal cancer
(14). Moreover, SDF-1/CXCR4
expression has been shown to be a negative prognostic factor after
postoperative radiotherapy for head and neck cancer (21,41).
Therefore, clarifying the mechanisms of SDF-1/CXCR4
is important to overcome the therapeutic resistance of HNSCC.
Through our experiments, we confirmed CXCR4
expression in FaDu cells. A study has confirmed that FaDu cells
express CXCR4 but not its ligand SDF-1 (42). Thus, we used exogenous SDF-1 for
experiments on the interaction between SDF-1 and CXCR4.
In this study, we found that CXCR4 was involved in
FaDu cell invasion and proliferation.
This was similar to the result of a study that used
oral cancer cells (16). On the
other hand, it was reported that CXCR4 was not involved in cell
proliferation in HEp-2, an SCC of the larynx cell line (43). Therefore, these reports indicate that
CXCR4 has different effects depending on the sub-location even
within the same type of head and neck cancer and that detailed
examination is necessary depending on the tumor site.
CD147 is associated with various physiological
actions in cells, mainly via the production and activation of MMP
(44). These are also beneficial for
tumor progression. The correlation between the expression of CD147
in tumors and poor prognosis has been widely observed in
individuals with solid tumors including HNSCC (45). It has been reported that CD147 is
involved in MMP production, cell invasion, cell proliferation, and
EMT in head and neck cancer cells (26,46).
Moreover, we have reported that CD147 expression in tongue cancer
patients is positively correlated with lymph node metastasis
(23). Thus, the involvement of
CD147 in cancer progression has been gradually established.
Furthermore, the role of CD147 as a therapeutic target for cancer
has been studied, and its antitumor effects are expected for head
and neck cancer (47,48).
It is known that a targeting strategy is very
important and epidermal growth factor receptor (EGFR) antibody is
already in clinical use for head and neck cancer (49). In addition, recent studies have
reported that the antitumor effect is further enhanced by
simultaneously targeting multiple tumorigenic factors (34,50). We
also have reported that head and neck cancer progression can be
suppressed synergistically by combining the inhibition of EGFR and
CD147 (51).
These results suggest that because of the complex
pathways of cancer progression, inhibition of a single
tumor-related factor is not sufficient for tumor control. For a
more effective antitumor effect, it is necessary to simultaneously
inhibit a plurality of factors related to the factor of
interest.
In this study, we showed that CD147 expression was
enhanced after the stimulation of CXCR4 by SDF-1. This enhanced
expression of CD147 was abrogated by AMD3100, an inhibitor of
CXCR4. In addition, the enhancement of cell infiltration induced by
SDF-1/CXCR4 was suppressed by inhibiting CD147. These results
indicate that CD147 acts as a mediator of the SDF-1/CXCR4 pathway
and that it is involved in the invasion induced by SDF-1/CXCR4 in
hypopharyngeal cancer cells.
These results suggest a possibility that not only
would SDF-1/CXCR4 be a therapeutic target, but also that targeting
CD147 simultaneously with SDF-1/CXCR4 may be a new and effective
strategy for treating hypopharyngeal cancer.
On the other hand, the inhibition of infiltration
induced by the SDF-1/CXCR4 was only partial after CD147 inhibition.
This suggests that one of the SDF-1/CXCR4-induced tumor progression
pathways is via CD147. Elucidation of these pathways is required
for more powerful control of head and neck cancer progression.
Acknowledgements
Not applicable.
Funding
The present study was supported by Grant-in-Aid for
Scientific Research (C) (grant no. 18K09337) from The Ministry of
Education, Culture, Sports, Science and Technology, Japan.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS conceived and designed the experiments. SS, ST
and YK performed the experiments. SS analyzed data and contributed
to writing of the manuscript. TY performed data analysis and
interpretation. All authors read and approved the final
manuscript.
Ethics approval and consent to
publication
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CD147
|
cluster of differentiation-147
|
|
CXCR4
|
CXC chemokine receptor type 4
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
SCC
|
squamous cell carcinoma
|
|
SDF-1
|
stromal cell-derived factor 1
|
References
|
1
|
Carvalho AL, Pintos J, Schlecht NF,
Oliveira BV, Fava AS, Curado MP, Kowalski LP and Franco EL:
Predictive factors for diagnosis of advanced-stage squamous cell
carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg.
128:313–318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ho AS, Kim S, Tighiouart M, Gudino C, Mita
A, Scher KS, Laury A, Prasad R, Shiao SL, Ali N, et al: Association
of quantitativemetastatic lymph node burden with survival in
hypopharyngeal and laryngeal cancer. JAMA Oncol. 4:985–989. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Proudfoot AEI: Chemokine receptors:
Multifaceted therapeutic targets. Nat Rev Immunol. 2:106–115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou Y, Larsen PH, Hao C and Yong VW:
CXCR4 is a major chemokine receptor on glioma cells and mediates
their survival. J Biol Chem. 277:49481–49487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burger JA and Kipps TJ: CXCR4: A key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 107:1761–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scala S, Ottaiano A, Ascierto PA, Cavalli
M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G and
Castello G: Expression of CXCR4 predicts poor prognosis in patients
with malignant melanoma. Clin Cancer Res. 11:1835–1841. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barbero S, Bonavia R, Bajetto A, Porcile
C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T and
Schettini G: Stromal cell-derived factor 1alpha stimulates human
glioblastoma cell growth through the activation of both
extracellular signal-regulated kinases 1/2 and Akt. Cancer Res.
63:1969–1974. 2003.PubMed/NCBI
|
|
10
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taichman RS, Cooper C, Keller ET, Pienta
KJ, Taichman NS and McCauley LK: Use of the stromal cell-derived
factor-1/CXCR4 pathway in prostate cancer metastasis to bone.
Cancer Res. 62:1832–1837. 2002.PubMed/NCBI
|
|
12
|
Jiang YP, Wu XH, Shi B, Wu WX and Yin GR:
Expression of chemokine CXCL12 and its receptor CXCR4 in human
epithelial ovarian cancer: An independent prognostic factor for
tumor progression. Gynecol Oncol. 103:226–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen GS, Yu HS, Lan CC, Chow KC, Lin TY,
Kok LF, Lu MP, Liu CH and Wu MT: CXC chemokine receptor CXCR4
expression enhances tumorigenesis and angiogenesis of basal cell
carcinoma. Br J Dermatol. 154:910–918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan CT, Chu CY, Lu YC, Chang CC, Lin BR,
Wu HH, Liu HL, Cha ST, Prakash E, Ko JY and Kuo ML: CXCL12/CXCR4
promotes laryngeal and hypopharyngeal squamous cell carcinoma
metastasis through MMP-13-dependent invasion via the ERK1/2/AP-1
pathway. Carcinogenesis. 29:1519–1527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Samara GJ, Lawrence DM, Chiarelli CJ,
Valentino MD, Lyubsky S, Zucker S and Vaday GG: CXCR4-mediated
adhesion and MMP-9 secretion in head and neck squamous cell
carcinoma. Cancer Lett. 214:231–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong JS, Pai HK, Hong KO, Kim MA, Kim JH,
Lee JI, Hong SP and Hong SD: CXCR-4 knockdown by small interfering
RNA inhibits cell proliferation and invasion of oral squamous cell
carcinoma cells. J Oral Pathol Med. 38:214–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rehman AO and Wang CY: CXCL12/SDF-1 alpha
activates NF-kappaB and promotes oral cancer invasion through the
Carma3/Bcl10/Malt1 complex. Int J Oral Sci. 1:105–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu T, Wu Y, Helman JI, Wen Y, Wang C and
Li L: CXCR4 Promotes oral squamous cell carcinoma migration and
invasion through inducing expression of MMP-9 and MMP-13 via the
ERK signaling pathway. Mol Cancer Res. 9:161–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishikawa T, Nakashiro KI, Hara S, Klosek
SK, Li C, Shintani S and Hamakawa H: CXCR4 expression is associated
with lymph-node metastasis of oral squamous cell carcinoma. Int J
Oncol. 28:61–66. 2006.PubMed/NCBI
|
|
20
|
Katayama A, Ogino T, Bandoh N, Nonaka S
and Harabuchi Y: Expression of CXCR4 and its down-regulation by
IFN-gamma in head and neck squamous cell carcinoma. Clin Cancer
Res. 11:2937–2946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De-Colle C, Mönnich D, Welz S, Boeke S,
Sipos B, Fend F, Mauz PS, Tinhofer I, Budach V, Jawad JA, et al:
SDF-1/CXCR4 expression in head and neck cancer and outcome after
postoperative radiochemotherapy. Clin Transl Radiat Oncol. 5:28–36.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knopf A, Bahadori L, Fritsche K, Piontek
G, Becker CC, Knolle P, Krüger A, Bier H and Li Y: Primary
tumor-associated expression of CXCR4 predicts formation of local
and systemic recurrency in head and neck squamous cell carcinoma.
Oncotarget. 8:112739–112747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caudroy S, Polette M, Tournier JM, Burlet
H, Toole B, Zucker S and Birembaut P: Expression of the
extracellular matrix metalloproteinase inducer (EMMPRIN) and the
matrix metalloproteinase-2 in bronchopulmonary and breast lesions.
J Histochem Cytochem. 47:1575–1580. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki S, Sato M, Senoo H and Ishikawa K:
Direct cell-cell interaction enhances pro-MMP-2 production and
activation in co-culture of laryngeal cancer cells and fibroblasts:
Involvement of EMMPRIN and MT1-MMP. Exp Cell Res. 293:259–266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hibino T, Sakaguchi M, Miyamoto S,
Yamamoto M, Motoyama A, Hosoi J, Shimokata T, Ito T, Tsuboi R and
Huh NH: S100A9 is a novel ligand of EMMPRIN that promotes melanoma
metastasis. Cancer Res. 73:172–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takahashi M, Suzuki S and Ishikawa K:
Cyclophilin A-EMMPRIN interaction induces invasion of head and neck
squamous cell carcinoma. Oncol Rep. 27:198–203. 2012.PubMed/NCBI
|
|
27
|
Suzuki S, Honda K, Nanjo H, Iikawa N,
Tsuji T, Kawasaki Y, Yamazaki K, Sato T, Saito H, Shiina K and
Ishikawa K: CD147 expression correlates with lymph node metastasis
inT1-T2 squamous cell carcinoma of the tongue. Oncol Lett.
14:4670–4676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi WP, Ju D, Li H, Yuan L, Cui J, Luo D,
Chen ZN and Bian H: CD147 promotes CXCL1 expression and modulates
liver fibrogenesis. Int J Mol Sci. 19:11452018. View Article : Google Scholar
|
|
29
|
Peng C, Zhang S, Lei L, Zhang X, Jia X,
Luo Z, Huang X, Kuang Y, Zeng W, Su J and Chen X: Epidermal CD147
expression plays a key role in IL-22-induced psoriatic dermatitis.
Sci Rep. 7:441722017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bordador LC, Li X, Toole B, Chen B, Regezi
J, Zardi L, Hu Y and Ramos DM: Expression of emmprin by oral
squamous cell carcinoma. Int J Cancer. 85:347–352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koga K, Nabeshima K, Aoki M, Kawakami T,
Hamasaki M, Toole BP, Nakayama J and Iwasaki H: Emmprin in
epithelioid sarcoma: Expression in tumor cell membrane and
stimulation of MMP-2 production in tumor-associated fibroblasts.
Int J Cancer. 120:761–768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishikawa T, Nakashiro KI, Klosek SK, Goda
H, Hara S, Uchida D and Hamakawa H: Hypoxia enhances CXCR4
expression by activating HIF-1 in oral squamous cell carcinoma.
Oncol Rep. 21:707–712. 2009.PubMed/NCBI
|
|
33
|
Asakage T, Yokose T, Mukai K, Tsugane S,
Tsubono Y, Asai M and Ebihara S: Tumor thickness predicts cervical
metastasis in patients with stage I/II carcinoma of the tongue.
Cancer. 82:1443–1448. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koppikar P, Choi SH, Egloff AM, Cai Q,
Suzuki S, Freilino M, Nozawa H, Thomas SM, Gooding WE, Siegfried JM
and Grandis JR: Combined inhibition of c-Src and epidermal growth
factor receptor abrogates growth and invasion of head and neck
squamous cell carcinoma. Clin Cancer Res. 14:4284–4291. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jung YH, Lee DY, Cha W, Kim BH, Sung MW,
Kim KH and Ahn SH: Antitumor effect of CXCR4 antagonist AMD3100 on
the tumorigenic cell line of BHP10-3 papillary thyroid cancer
cells. Head Neck. 38:1479–1486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Spano JP, Andre F, Morat L, Sabatier L,
Besse B, Combadiere C, Deterre P, Martin A, Azorin J, Valeyre D, et
al: Chemokine receptor CXCR4 and early-stage non-small cell lung
cancer: Pattern of expression and correlation with outcome. Ann
Oncol. 15:613–617. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koishi K, Yoshikawa R, Tsujimura T,
Hashimoto-Tamaoki T, Kojima S, Yanagi H, Yamamura T and Fujiwara Y:
Persistent CXCR4 expression after preoperative chemoradiotherapy
predicts early recurrence and poor prognosis in esophageal cancer.
World J Gastroenterol. 12:7585–7590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou W, Guo S, Liu M, Burow ME and Wang G:
Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr Med Chem.
26:3026–3041. 2017. View Article : Google Scholar
|
|
39
|
Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB,
Ko YG, Lee JS, Lee SJ, Lee JC and Park MJ: Upregulation of CXCR4 is
functionally crucial for maintenance of stemness in drug-resistant
non-small cell lung cancer cells. Oncogene. 32:209–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Es-Haghi M, Soltanian S and Dehghani H:
Perspective: Cooperation of Nanog, NF-κΒ, and CXCR4 in a regulatory
network for directed migration of cancer stem cells. Tumour Biol.
37:1559–1565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De-Colle C, Menegakis A, Mönnich D, Welz
S, Boeke S, Sipos B, Fend F, Mauz PS, Tinhofer I, Budach V, et al:
SDF-1/CXCR4 expression is an independent negative prognostic
biomarker in patients with head and neck cancer after primary
radiochemotherapy. Radiother Oncol. 126:125–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wolff HA, Rolke D, Rave-Fränk M, Schirmer
M, Eicheler W, Doerfler A, Hille A, Hess CF, Matthias C, Rödel RM
and Christiansen H: Analysis of chemokine and chemokine receptor
expression in squamous cell carcinoma of the head and neck (SCCHN)
cell lines. Radiat Environ Biophys. 50:145–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu C, Pan Y, He B, Wang B, Xu Y, Qu L,
Bao Q, Tian F and Wang S: Inhibition of CD147 gene expression via
RNA interference reduces tumor cell invasion, tumorigenicity and
increases chemosensitivity to cisplatin in laryngeal carcinoma Hep2
cells. Oncol Rep. 25:425–432. 2011.PubMed/NCBI
|
|
44
|
Vu TH and Werb Z: Matrix
metalloproteinases: Effectors of development and normal physiology.
Genes Dev. 14:2123–2133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rosenthal EL, Shreenivas S, Peters GE,
Grizzle WE, Desmond R and Gladson CL: Expression of extracellular
matrix metalloprotease inducer in laryngeal squamous cell
carcinoma. Laryngoscope. 113:1406–1410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Suzuki S, Toyoma S, Tsuji T, Kawasaki Y
and Yamada T: CD147 mediates transforming growth factor-β1-induced
epithelial-mesenchymal transition and cell invasion in squamous
cell carcinoma of the tongue. Exp Ther Med. 17:2855–2860.
2019.PubMed/NCBI
|
|
47
|
Landras A, de Moura CR, Jouenne F, Lebbe
C, Menashi S and Mourah S: CD147 is a promising target of tumor
progression and a prognostic biomarker. Cancers (Basel). 11:2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dean NR, Newman JR, Helman EE, Zhang W,
Safavy S, Weeks DM, Cunningham M, Snyder LA, Tang Y, Yan L, et al:
Anti-EMMPRIN monoclonal antibody as a novel agent for therapy of
head and neck cancer. Clin Cancer Res. 15:4058–4065. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vermorken JB, Herbst RS, Leon X, Amellal N
and Baselga J: Overview of the efficacy of cetuximab in recurrent
and/or metastatic squamous cell carcinoma of the head and neck in
patients who previously failed platinum-based therapies. Cancer.
112:2710–2719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nozawa H, Howell G, Suzuki S, Zhang Q, Qi
Y, Klein-Seetharaman J, Wells A, Grandis JR and Thomas SM: Combined
inhibition of PLC{gamma}-1 and c-Src abrogates epidermal growth
factor receptor-mediated head and neck squamous cell carcinoma
invasion. Clin Cancer Res. 14:4336–4344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Suzuki S and Ishikawa K: Combined
inhibition of EMMPRIN and epidermal growth factor receptor prevents
the growth and migration of head and neck squamous cell carcinoma
cells. Int J Oncol. 44:912–917. 2014. View Article : Google Scholar : PubMed/NCBI
|