Introduction
Colorectal cancer is the most common
gastrointestinal cancer worldwide. The morbidity of colorectal
cancer has increased over the past 20 years in most countries
(1). The aging of population and the
high-fat and low-fiber diet are the main reasons of colorectal
cancer. The initial onset of colorectal cancer is insidious; in
most cases colorectal cancer has no clinical symptoms and there are
different degrees of delayed diagnosis (2). At present, surgery, chemotherapy and
radiotherapy are the main treatments for colorectal cancer
(3,4). Although the treatment methods are
constantly improving, recurrence and metastasis still occur after
the treatment of colorectal cancer by these methods. Thus,
colorectal cancer still poses a threat to human health (5). Serum tumor markers, such as
carcinoembryonic antigen and carbohydrate antigen 199, have greatly
improved the diagnostic levels; however, they are only used in
postoperative monitoring and they are not suitable for the early
detection of colorectal cancer (6–8).
Therefore, finding new tumor markers is important to improve the
diagnosis and prognosis in colorectal cancer.
LI-cadherin was initially discovered in the liver
and intestinal tract of rats (9). As
a non-classical cadherin, LI-cadherin has its own unique structure.
Unlike the traditional cadherins, which have five E-cadherin
iterons, LI-cadherin has seven independent domains outside the
cells and there are 20 amino acid residues in cytoplasm, indicating
that there is less homology between LI-cadherin and other cadherins
(10). The expression of LI-cadherin
is closely associated with tumors related to the digestive system,
and LI-cadherin expression is often associated with tumor prognosis
(11,12). At present, there is a number of
studies on the LI-cadherin expression in gastric, esophageal and
pancreatic cancers (13). In recent
years, LI-cadherin has been a hotspot in research; however, there
are few studies on LI-cadherin in colorectal cancer.
MicroRNA (miRNA) is an endogenous, 20–23
nucleotide-long, non-coding, single-stranded RNA (14), which plays an important role in
regulation after genes are transcribed, and plays a key role in the
development of the organisms, the differentiation of cells, the
cell signaling, the regulation of the expression of genes, and the
occurrence and development of malignant tumors (15). miRNAs are abnormally expressed in
various malignant tumors. For example, the expression of miR-155 is
upregulated in breast cancer (16),
the expression of miR-221 is upregulated in pancreatic cancer
(17), and the expression of
miR-885-5p is downregulated in hepatoma (18). miRNA is considered to be involved in
the occurrence and development of tumors, and thus, may be regarded
as an oncogene or a tumor suppressor gene. Lu et al
(19) have reported that the tissue
source and differentiation status of various tumors can be more
accurately reflected by the expression profile of miRNA. Therefore,
the expression profile of miRNA can be used for the classification
of various poorly differentiated tumors, indicating that miRNA is
very important in the diagnosis of tumors and the prognosis of
cancer. A study on miR-378e is rare in China and globally. A
relevant study has shown that the expression of miR-378e is
downregulated in colorectal cancer tissue, suggesting that miR-378e
may be considered as a tumor suppressor gene and could be used as a
potential molecular marker for colorectal cancer (20). However, currently there is no other
study to verify this and the specific effect still needs to be
further investigated.
The aim of the present study was to investigate the
expression levels of LI-cadherin and miR-378e in the serum of
patients with colorectal cancer, and to analyze the diagnostic
value and prognostic significance of LI-cadherin and miR-378e in
colorectal cancer.
Subjects and methods
General data
A total of 110 patients who were diagnosed with
colorectal cancer in Weihai Central Hospital (Weihai, China), from
January 2012 to November 2014, were selected and enrolled in the
experimental group. There were 66 males and 44 females included in
the experimental group, 62.13±9.21 years of age. In addition, there
were 69 cases in stages A and B, and 41 cases in stages C and D.;
and there were 45 cases with lymphatic metastasis, and 65 cases
without lymphatic metastasis. At the same time, 90 healthy subjects
who underwent physical examination were selected and enrolled in
the control group. There were 52 males and 38 females included in
the control group, 61.89±9.28 years of age.
Inclusion criteria: Patients who had complete
clinicopathological data; patients who had not received neoadjuvant
chemotherapy, radiotherapy or endocrinotherapy; patients who had
completed some examinations within 2 weeks before the operation,
including hepatorenal function, tumor markers, blood routine and
urine routine examinations.
Exclusion criteria: Patients with other malignant
tumors; patients with severe congenital heart disease; patients
with severe organ lesion or severe organ disease; patients with
autoimmune system disease; women in gestation or lactation period;
patients who did not cooperate with the examinations; patients
whose family refused to sign the informed consent form.
The study was approved by the Ethics Committee of
the Weihai Central Hospital. All participants were informed in
detail on the experiments of this research and had complete
clinical data. Signed written informed consents were obtained from
the participants of this study and/or their guardians.
Collection of serum
After the patients were diagnosed with colorectal
cancer, 2 ml of peripheral blood were collected after having fasted
in the morning. Blood samples were added into anticoagulation tubes
for further analysis. In the control group, 2 ml of fasting venous
blood were collected in the morning. After the blood was coagulated
for 60 min (between 20 and 25°C), centrifugation was carried out
for 10 min at 4°C at a speed of 1,006.2 × g. Next, the supernatant
was collected and placed at −80°C for further analysis. Repeated
freezing and thawing were avoided as much as possible.
Experimental reagents and
instruments
TRIzol® kit (Thermo Fisher Scientific,
Inc.); DNase I (Shanghai Shenggong Biology Engineering Technology
Service, Ltd.); cDNA reverse transcription kit (Takara Bio, Inc.);
ultraviolet spectrophotometer (Shanghai Mepuda Instrument Co.,
Ltd.); SYBR Premix Ex Taq™ kit (Takara Bio, Inc.); quantitative PCR
detector (ABI 7300; Shanghai Zhiyan Scientific Instrument Co.,
Ltd.); LI-cadherin enzyme-linked immunosorbent assay (ELISA) test
kit (R&D Systems China Co., Ltd.); BS-1101 enzyme microplate
reader (Beijing Linmao Technology Co., Ltd.).
Detection of miR-378e expression by
reverse transcription-quantitative PCR
Total RNA in serum was extracted using
TRIzol® reagent, according to the manufacturer's
protocol. DNase I (RNase-free) was used to digest the template RNA
in order to eliminate the contamination of genome DNA. An
ultraviolet spectrophotometer was used for the detection of purity
and concentration of total RNA, and agarose gel electrophoresis was
used for the detection of RNA integrity. The concentration of RNA
was adjusted to 500 ng/µl. A reverse transcription kit was used for
the reverse transcription of total RNA into cDNA, in strict
accordance with the manufacturer's instructions. The reaction
conditions were 42°C for 60 min, 95°C for 3 min. SYBR Premix Ex
Taq™ kit and quantitative PCR detector were used for PCR. The
RT-qPCR system was: 20 µl total volume, 10.0 µl of SYBR Premix Ex
Taq™ (2X), 0.4 µl of upstream primers and 0.4 µl of downstream
primers (10 µM), 0.4 µl of ROX reference dye II, 2.0 µl of
template, 6.8 µl of double distilled water (ddH2O). qPCR
reaction conditions were: Pre-denaturation at 95°C for 10 min, 45
cycles of 95°C for 10 sec, 60°C for 30 sec, 72°C for 10 sec; the
procedure was repeated 3 times. The above system was configured
following strictly the manufacturer's instructions. U6 was used as
the internal reference of miR-378e and the U6 primers were
synthesized by Shanghai Shenggong Biology Engineering Technology
Service, Ltd.: Upstream, 5′-CTCGCTTCGGCAGCACA-3′ reverse
transcription and downstream, 5′-AACGCTTCACGAATTTGCGT-3′. The
upstream and downstream primers of miR-378e were synthesized by
Guangzhou Ruibo Co., Ltd.: Upstream, 5′-TTCGAGCCTACTGGACTTGGAG-3′
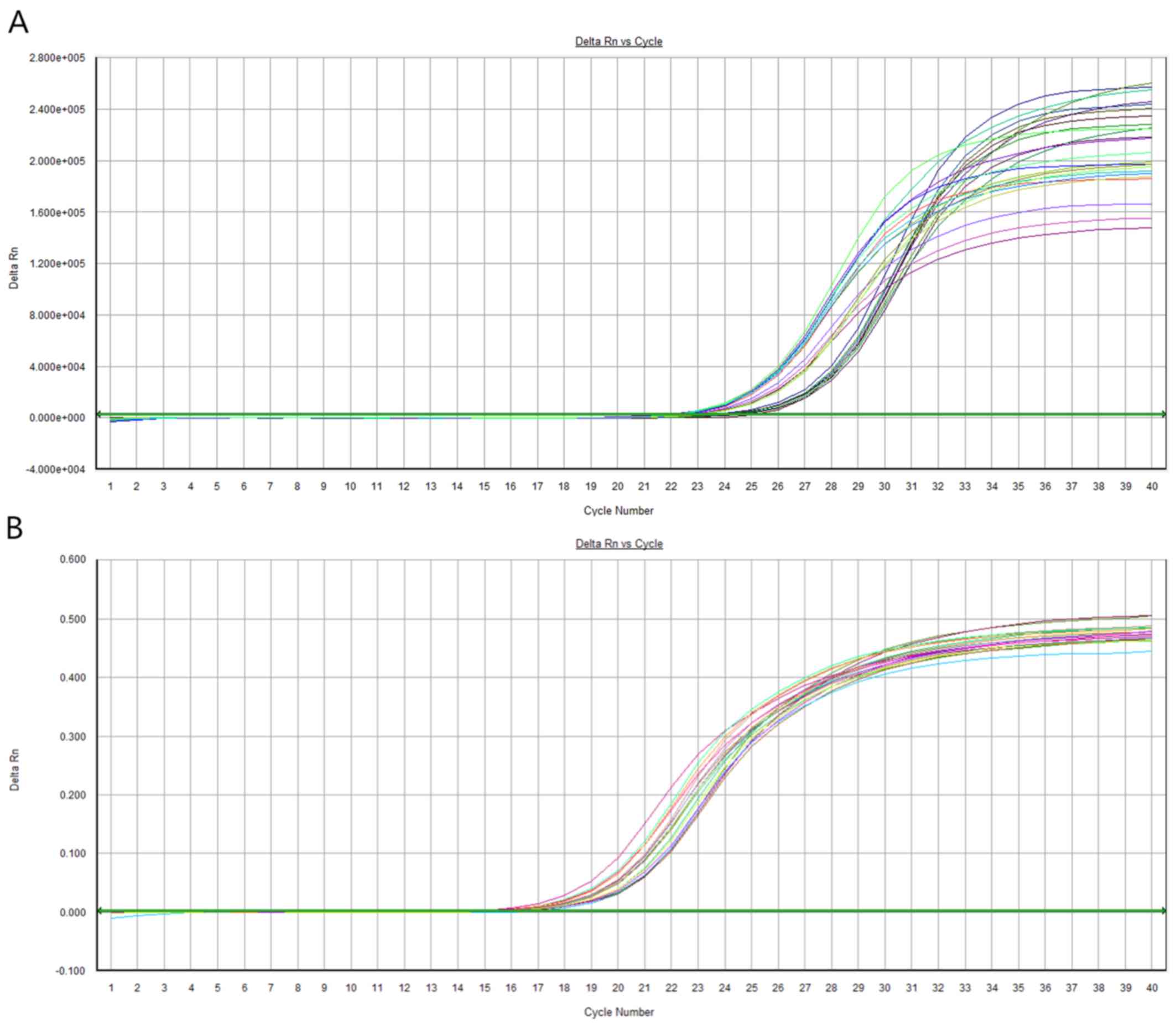
and downstream, 5′-AGGGTCCGAGGTATTCGCACT-3′ (Fig. 1). The 2−ΔCq method
(21) was used to quantify the
relative expression levels of miR-378e in the blood.
Detection of LI-cadherin expression by
ELISA
Operation steps: Blank well (the blank control well
was the same as earlier steps; however, no enzyme labeling reagents
and samples were added), standard well and the well of sample to be
tested were respectively set. Standard sample (50 µl) was
accurately added into the reaction well in which the enzyme label
was coated. Firstly, 40 µl of the sample dilution were added into
the well, and then, 10 µl of the sample to be tested were added
into it (the final sample was diluted 5 times). The reaction well
was sealed with a sealing film and incubated in a water bath or an
incubator for 30 min. After the sealing film was removed, the
liquid was discarded. The well was dried with absorbent paper and
was filled with washing liquid. After standing for 30 sec, this
step was repeated 5 times, and the reaction well was dried. In
addition to the blank well, 50 µl of the enzyme labeling reagent
were added into each well, and the mixture was incubated at 37°C
for 30 min. Next, the wells were washed. Substrate A (50 µl) and
substrate B were added into each well, and the color was developed
at 37°C for 15 min in the dark. Stop solution (50 µl) was added
into each well, and the absorbance (OD value) of each well was
measured at a wavelength of 450 nm in 25 min using a BS-1101 enzyme
microplate reader. The expression level of LI-cadherin was
calculated.
Follow-up and observation
indicators
Patients with colorectal cancer were followed up by
hospital re-examination and telephone calls. The survival time of
the patients was recorded at 1, 2 and 3 years after leaving
hospital. The differences in the expression levels of LI-cadherin
and miR-378e between the experimental and the control group were
observed. The association of the expression levels of LI-cadherin
and miR-378e with the clinical stage and the differentiation degree
was analyzed according to the clinicopathological features of the
patients with colorectal cancer. The value of the single and the
combined detection of LI-cadherin and miR-378e, as well as their
prognostic significance were analyzed.
Statistical analysis
SPSS 19.0 software (IBM Corp.) was used for the
statistical analysis of the experimental data. Enumeration data
were expressed as percentages (%) and Chi-square (χ2)
test was used for their comparison between groups. Measurement data
were expressed as the mean ± SD and t-test was used for their
comparison between two groups. ROC curve analysis was used to
assess the sensitivity and specificity of the single and the
combined detection. Kaplan-Meier analysis and log-rank test were
used for survival analysis. Pearson's correlation coefficient was
used to analyze bivariate normal distribution data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of the general data between
the two groups
The general clinical baseline data of the
experimental and control group were collected, including sex, age,
body mass index, average height, history of smoking, hypertension,
alcohol consumption, hemoglobin (HB), platelet (PLT), white blood
cells (WBC), red blood cells (RBC), alanine transaminase (ALT) and
aspartate transaminase (AST). The results of their statistical
analysis revealed that there was no statistically significant
difference in the data between the two groups (P>0.05) (Table I).
 | Table I.Comparison of the general data between
the two groups [n (%), mean ± SD]. |
Table I.
Comparison of the general data between
the two groups [n (%), mean ± SD].
| Characteristics | Experimental group
(n=110) | Control group
(n=90) | χ2/t | P-value |
|---|
| Sex |
|
| 0.101 | 0.751 |
| Male | 66 (60.00) | 52 (57.78) |
|
|
|
Female | 44 (40.00) | 38 (42.22) |
|
|
| Age (years) |
62.13±9.21 |
61.89±9.28 | 0.183 | 0.855 |
| Body mass index
(kg/m2) |
19.79±3.21 |
19.83±3.57 | 0.083 | 0.934 |
| Average height
(cm) | 167.34±4.43 | 166.62±4.64 | 1.119 | 0.264 |
| History of
smoking |
|
| 0.758 | 0.384 |
|
Yes | 63 (57.27) | 57 (63.33) |
|
|
| No | 47 (42.73) | 33 (36.67) |
|
|
| Hypertension |
|
| 0.419 | 0.517 |
|
Yes | 39 (35.45) | 28 (31.11) |
|
|
| No | 71 (64.55) | 62 (68.89) |
|
|
| Alcohol
consumption |
|
| 1.115 | 0.291 |
|
Yes | 81 (73.64) | 72 (80.00) |
|
|
| No | 29 (26.36) | 18 (20.00) |
|
|
| HB (gm/dl) |
12.11±1.81 |
12.24±2.01 | 0.481 | 0.631 |
| PLT
(×109/l) |
155.78±21.87 |
158.31±22.09 | 0.810 | 0.419 |
| WBC
(×109/l) |
7.13±2.34 |
7.24±2.17 | 0.342 | 0.733 |
| RBC
(×1012/l) |
4.43±0.76 |
4.32±0.81 | 0.989 | 0.324 |
| ALT (U/l) |
23.14±10.32 |
22.89±10.12 | 0.172 | 0.864 |
| AST (U/l) |
19.38±7.69 |
19.67±6.65 | 0.282 | 0.778 |
Comparison of the expression levels of
LI-cadherin and miR-378e in the serum of the two groups
The expression levels of LI-cadherin and miR-378e in
the serum of all research subjects were detected. As shown in
Table II, the expression level of
LI-cadherin in the control group was higher than that in the
experimental group, and the difference was statistically
significant (P<0.001); the expression level of miR-378e in the
control group was significantly higher than that in the
experimental group (P<0.001).
 | Table II.Comparison of LI-cadherin and
miR-378e serum expression levels between the two groups (mean ±
SD). |
Table II.
Comparison of LI-cadherin and
miR-378e serum expression levels between the two groups (mean ±
SD).
| Groups | LI-cadherin
(ng/ml) | miR-378e |
|---|
| Experimental group
(n=110) | 4.11±1.57 | 4.53±1.88 |
| Control group
(n=90) | 7.34±1.86 | 8.59±2.12 |
| t | −10.88 | 15.63 |
| P-value | <0.001 | <0.001 |
Relationship between the LI-cadherin
and miR-378e expression levels and the clinicopathological
characteristics of the patients in the experimental group
The clinicopathological characteristics of 110
patients diagnosed with colorectal cancer were recorded. According
to the results of the statistical analysis, LI-cadherin was not
significantly associated with sex, age or histological type
(P>0.05); however, LI-cadherin was significantly associated with
the pathogenic site, the lymphatic metastasis, depth of
infiltration, degree of differentiation and clinical stage
(P<0.05). The expression level of miR-378e was not associated
with sex, age, pathogenic site, lymphatic metastasis, degree of
differentiation, clinical stage or histological type (P>0.05);
however, miR-378e was significantly associated with the depth of
infiltration (P<0.05) (Table
III).
 | Table III.Relationship between the LI-cadherin
and miR-378e expression levels and the clinicopathological
characteristics of the patients in the experimental group (mean ±
SD). |
Table III.
Relationship between the LI-cadherin
and miR-378e expression levels and the clinicopathological
characteristics of the patients in the experimental group (mean ±
SD).
|
Characteristics | Cases | LI-cadherin
(ng/ml)) | t | P-value | miR-378e | t | P-value |
|---|
| Sex |
|
| 0.136 | 0.892 |
| 0.304 | 0.761 |
|
Male | 66 | 4.09±1.58 |
|
| 4.43±1.92 |
|
|
|
Female | 44 | 4.12±1.53 |
|
| 4.51±1.76 |
|
|
| Age (years) |
|
| 0.665 | 0.507 |
| 0.345 | 0.730 |
|
≤50 | 34 | 3.97±1.55 |
|
| 4.45±1.87 |
|
|
|
>50 | 76 | 4.12±1.63 |
|
| 4.54±1.79 |
|
|
| Site |
|
| 2.851 | 0.005 |
| 1.102 | 0.272 |
|
Rectum | 52 | 4.17±1.65 |
|
| 4.37±1.85 |
|
|
|
Colon | 58 | 3.46±1.87 |
|
| 4.65±1.71 |
|
|
| Lymphatic
metastasis |
|
| 2.341 | 0.020 |
| 0.488 | 0.626 |
|
Yes | 45 | 3.47±1.74 |
|
| 4.38±1.68 |
|
|
| No | 65 | 4.02±1.54 |
|
| 4.50±1.79 |
|
|
| Depth of
infiltration |
|
| 3.114 | 0.002 |
| 3.419 | <0.001 |
|
T1+T2 | 42 | 4.11±1.21 |
|
| 4.01±1.57 |
|
|
|
T3+T4 | 68 | 3.76±1.86 |
|
| 4.87±2.12 |
|
|
| Degree of
differentiation |
|
| 10.86 | <0.001 |
| 0.348 | 0.728 |
| High
and medium | 71 | 4.21±1.76 |
|
| 4.61±1.98 |
|
|
|
Low | 39 | 2.01±1.19 |
|
| 4.52±1.85 |
|
|
| Clinical stage |
|
| 8.886 | <0.001 |
| 0.281 | 0.779 |
|
A+B | 69 | 4.10±1.65 |
|
| 4.47±1.99 |
|
|
|
C+D | 41 | 2.25±1.43 |
|
| 4.54±1.69 |
|
|
| Histological
type |
|
| 1.246 | 0.214 |
| 0.695 | 0.488 |
| Tubular
adenocarcinoma | 58 | 3.69±1.78 |
|
| 4.51±1.53 |
|
|
|
Non-tubular
adenocarcinoma | 52 | 3.98±1.67 |
|
| 4.67±1.87 |
|
|
Comparison of the value of single
detection and combined detection of LI-cadherin and miR-378e in the
diagnosis of colorectal cancer
As presented in Table
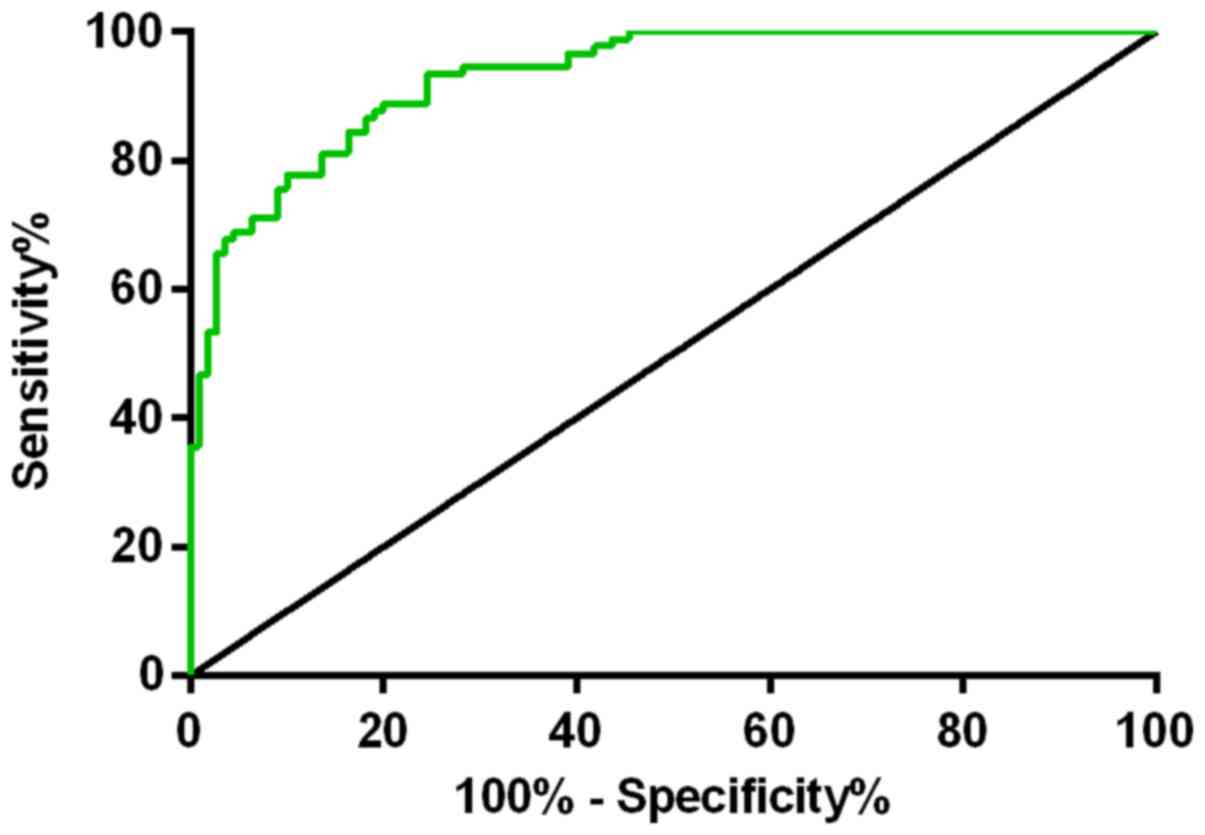
IV, the sensitivity and specificity of LI-cadherin were 84 and
80%, respectively, and the optimal critical value was 5.76. The
sensitivity and specificity of miR-378e were 89 and 80%,
respectively, and the optimal critical value was 6.06. The
sensitivity and specificity of the combined diagnosis were 86 and
94%, respectively. miR-378e detection had the highest sensitivity,
and the combined detection had the highest specificity. The Youden
indexes were 0.82, 0.68 and 0.64 for the combined detection,
miR-378e, and LI-cadherin, respectively. The larger the Youden
index was, the better the effect of detection was, and the higher
the authenticity. ROC curves are shown in Figs. 2 and 3).
 | Table IV.Comparison of the single detection
and the combined detection of LI-cadherin and miR-378e in the
diagnosis of colorectal cancer. |
Table IV.
Comparison of the single detection
and the combined detection of LI-cadherin and miR-378e in the
diagnosis of colorectal cancer.
| Items | Sensitivity
(%) | Specificity
(%) | Youden index | Optimal
threshold |
|---|
| LI-cadherin | 84 | 80 | 0.64 | 5.76 |
| miR-378e | 89 | 80 | 0.68 | 6.06 |
| LI-cadherin +
miR-378e | 86 | 94 | 0.82 | – |
Survival of patients in the
experimental group
Relationship between the expression of
LI-cadherin and prognosis
The survival data of the experimental group were
analyzed and the optimal threshold (5.76) of the expression level
of LI-cadherin was taken as the limit. There were 88 cases in the
low-expression group, in which the value of LI-cadherin was
<5.76, and 22 cases in the high-expression group, in which the
value of LI-cadherin was ≥5.76. The deadline of the follow-up
period was November 20, 2017. The survival rate in the
high-expression group was 63.64%, and the survival rate in the
low-expression group was 39.77%. As shown in Fig. 4, the survival rate of patients in the
LI-cadherin high-expression group was significantly higher than
that in the low-expression group (P<0.05).
Relationship between the expression of miR-378e
and prognosis
The survival data of the experimental group were
analyzed, and the optimal threshold (6.06) of the expression level
of miR-378e was taken as the limit. There were 90 cases in the
low-expression group, in which the value of miR-378e was <6.06,
and 20 cases in the high-expression group, in which the value of
miR-378e was ≥6.06. The deadline of the follow-up period was
November 20, 2017. The survival rate in the high-expression group
was 65.00%, and the survival rate in the low-expression group was
40.00%. As shown in Fig. 5, the
survival rate of patients in the miR-378e high-expression group was
significantly higher than that in the low expression group
(P<0.05).
Correlation analysis of LI-cadherin and
miR-378e
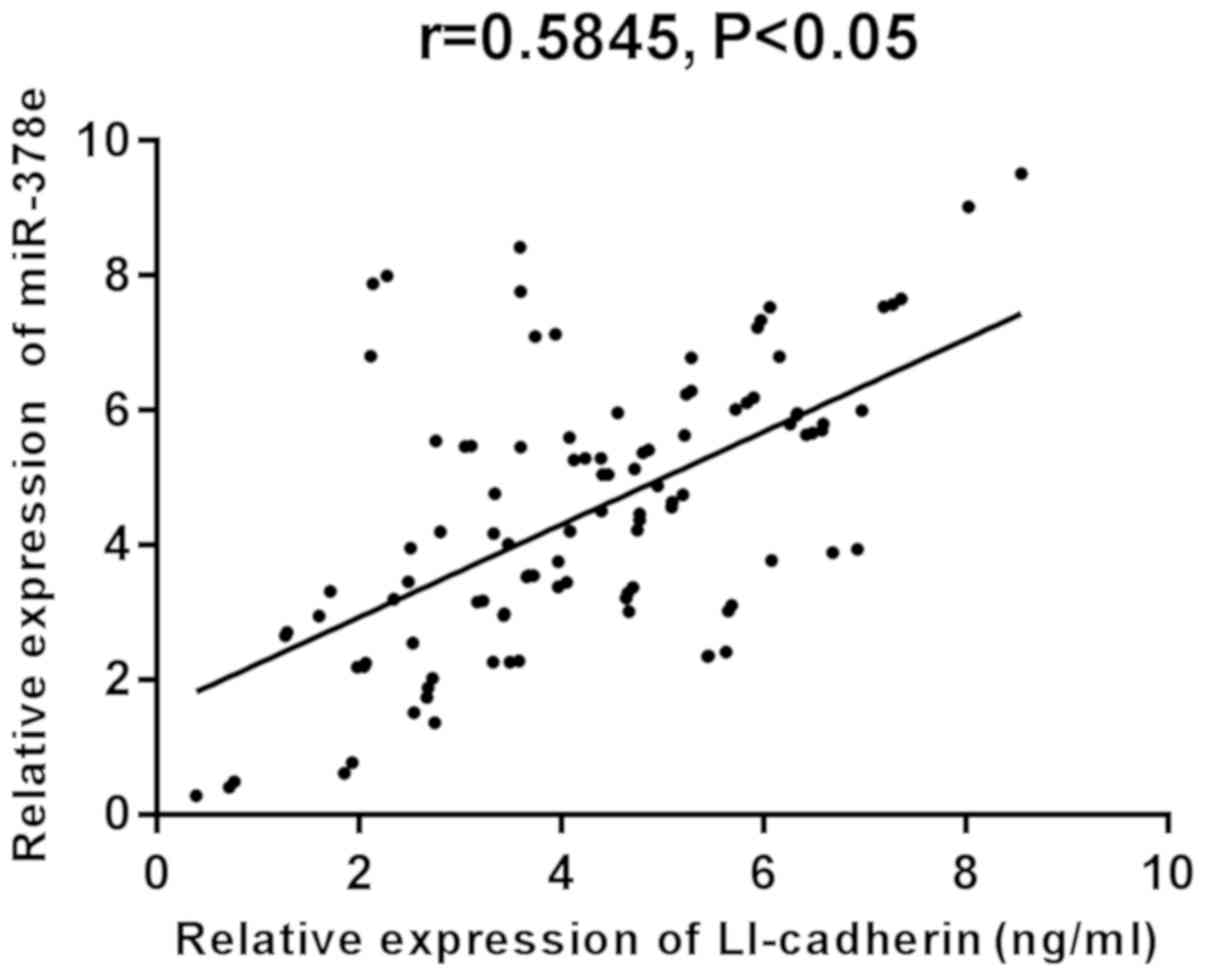
The results of Pearson's correlation coefficient
analysis revealed that there was a significantly positive
correlation between LI-cadherin and miR-378e in the experimental,
as well as the control group (r=0.5845 and 0.6356, respectively;
P<0.05) (Figs. 6 and 7).
Discussion
Colorectal cancer is one of the most common
malignant tumors with high morbidity, that ranks second among
female malignant tumors and third among male malignant tumors
worldwide (22). Colorectal cancer
originates from the epithelial cells in colon or rectum of the
digestive tract, which is also one of the main culprits that are
responsible for the cancer-related deaths in humans (23,24).
Therefore, the accurate diagnosis of colorectal cancer is crucial.
In addition, the occurrence, growth, infiltration and metastasis of
tumors are extremely complex processes, and genes and factors that
affect their processes have become hotspots in the researche of
cancer (1,25). Moreover, the therapeutic efficacy of
colorectal cancer and the improvement of patients' prognosis mainly
depend on early diagnosis and treatment (26).
LI-cadherin is a new member of the cadherin
superfamily. The functional characteristics and the unique
structure differentiate LI-cadherin from the classical cadherins
(27). LI-cadherin regulates the
adhesion function of cells through the complex structure of the
cytoplasmic region, its diverse functions, and the interaction with
calnexin (9). Relevant studies have
shown that silencing LI-cadherin can increase the expression levels
of MMP-2 and MMP-9 and downregulate the protein level of
galectin-3, which is a substrate of MMP-2 and MMP-9 (28). Downregulation of the LI-cadherin
expression can promote the invasion of cancer cells by enhancing
the expression of MMP-2 and MMP-9, activating and degrading the
ingredients of extracellular matrix, which can also facilitate the
adhesion and migration of cancer cells by altering the expression
of galectin-3 (28). In recent
years, miRNA has been a research hotspot in the field of molecular
biology and genetics, and mature miRNA molecules have been shown to
combine with Argonaute protein and other molecules, forming an
RNA-induced silencing complex in cells. They can match and combine
with the untranslated region of target mRNA 3′ end completely or
incompletely to induce the degradation of target mRNA or block its
post-transcriptional translation (29), to involve in a series of biological
processes, including cell proliferation, apoptosis,
differentiation, metabolism, development and tumor metastasis
(20).
In the present study, the clinicopathological
characteristics of the research subjects in the experimental and
the control group were compared, and the two groups were shown to
be comparable. Through immunoblotting experiment analysis, Bernhard
et al (30) found that
LI-cadherin may be a potential biomarker when colon cancer cells
secrete into plasma. In the present study, by detecting the
expression levels of LI-cadherin and miR-378e in the serum, it was
shown that the expression levels of LI-cadherin and miR-378e in the
control group were significantly higher than those in the
experimental group (P<0.001), which indicates that LI-cadherin
and miR-378e are expressed at low levels in colorectal cancer. When
the expression of LI-cadherin is reduced in patients with colon
cancer, tumors tend to be poorly differentiated and have the
characteristics of proliferation, invasion and metastasis (13). However, Gao et al (20) have reported that miR-378e is
significantly downregulated in colorectal cancer tissues,
suggesting that miR-378e may play a similar role in cancer
inhibition in the occurrence and development of tumors. Cadherin
not only plays an important role in the intercellular adhesion of
epithelial cells, but also maintains the intact form of the
epithelial cell lines of tumors. Once the intact form of the
epithelial cell lines is lost, cancer cell lines will have the
ability to invade (31). The
expression of LI-cadherin is correlated with the dedifferentiation
level of tumors, lymph node metastasis, and TNM stage and
progression of tumors. The prognosis of patients with tumors is
closely related to these factors (31). Our results revealed that LI-cadherin
was significantly associated with the pathogenic site, the
lymphatic metastasis, depth of infiltration, degree of
differentiation, and clinical stage (P<0.05). This indicates
that cancer cells whose differentiation degree is good can maintain
the good expression ability of LI-cadherin, while those with poor
differentiation degree have lower expression ability. The
expression level of LI-cadherin is related to lymph node
metastasis, indicating that LI-cadherin can be used as an indicator
to estimate the prognosis of patients with colorectal cancer, and
has some value in predicting the survival time of patients with
colorectal cancer and the occurrence and development of this
disease. However, miR-378e is only related to the depth of
infiltration, which suggests that the infiltration of colorectal
cancer may induce the expression of miR-378e, indicating that
miR-378e can be considered as a tumor suppressor gene. Up to our
knowledge, there are still few relevant studies on the specific
mechanisms of miR-378e, thus, further in-depth research is needed.
According to the results of the present study, miR-378e had the
highest sensitivity, and the combined detection had the highest
specificity. The Youden index was the highest for the combined
detection, followed by that of the miR-378e detection, and that of
LI-cadherin single detection. Therefore, the combined detection is
more valuable than the single detection in the diagnosis of
colorectal cancer. In order to further investigate the correlation
of LI-cadherin expression level with the miR-378e expression level
in colorectal cancer, a series of animal experiments and clinical
experiments need to be carried out. The analysis of the survival of
the patients in the experimental group, revealed that the high
expression of LI-cadherin is associated to the high survival rate
of the patients, and the low LI-cadherin expression is associated
to a lower survival rate, suggesting that LI-cadherin can be used
as a biomarker to predict the prognosis of patients with colorectal
cancer, which is similar to the research results of Takamura et
al (32). There are few studies
on the relationship betweeen miR-378e and tumor prognosis.
Donnarumma et al (33) have
reported that patients with breast cancer, whose miR-378 level is
low, have a long overall survival time, suggesting that miR-378e
has an effect on the prognosis of the disease (P<0.01). The
results of the present study demonstrated that patients with high
expression of miR-378e have high survival rate, and patients with
low miR-378e expression have a lower survival rate, indicating that
miR-378e can also be used as a biomarker for the prognosis of
patients with colorectal cancer. Different miRNAs have different
expression levels in tumors, thus, more studies in this direction
need to be carried out. In the present study, there was a
significantly positive correlation between LI-cadherin and miR-378e
in both groups, indicating that the changes in the expression of
LI-cadherin may be related to miR-378e. Currently, there is little
research on the correlation between LI-cadherin and miR-378e,
therefore future studies need to be conducted to verity this
result.
In the present study the expression levels of
LI-cadherin and miR-378e, as well as and their prognostic value in
colorectal cancer, were investigated in a comprehensive way to
provide future reference for clinical researches. However, the
specific mechanisms of LI-cadherin and miR-378e and their effects
on different cancers need to be further investigated. The
relationship between clinicopathological factors and prognosis need
to be analyzed multifactorially to estimate the prognosis of
patients more accurately.
In conclusion, LI-cadherin and miR-378e expression
levels may contribute to the understanding of the occurrence,
development and biological behavior of colorectal cancer.
LI-cadherin and miR-378e expression levels have positive diagnostic
value for colorectal cancer and can be used as biomarkers for the
prognosis of colorectal cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW interpreted the data and drafted the manuscript.
XW and ZHL performed PCR. JF and WX collected and analyzed the
general data. XW and ZXL were responsible for ELISA. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weihai Central Hospital (Weihai, China). All participants had
complete clinical data. Signed written informed consents were
obtained from the participants of this study and/or their
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kwon CH, Park HJ, Choi JH, Lee JR, Kim HK,
Jo HJ, Kim HS, Oh N, Song GA and Park DY: Snail and serpinA1
promote tumor progression and predict prognosis in colorectal
cancer. Oncotarget. 6:20312–20326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koerner J, Brunner T and Groettrup M:
Inhibition and deficiency of the immunoproteasome subunit LMP7
suppress the development and progression of colorectal carcinoma in
mice. Oncotarget. 8:50873–50888. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun X, Wang T, Zhang C, Ning K, Guan ZR,
Chen SX, Hong TT and Hua D: S100A16 is a prognostic marker for
colorectal cancer. J Surg Oncol. 117:275–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bagaria B, Sood S, Sharma R and Lalwani S:
Comparative study of CEA and CA19-9 in esophageal, gastric and
colon cancers individually and in combination (ROC curve analysis).
Cancer Biol Med. 10:148–157. 2013.PubMed/NCBI
|
|
7
|
Tan E, Gouvas N, Nicholls RJ, Ziprin P,
Xynos E and Tekkis PP: Diagnostic precision of carcinoembryonic
antigen in the detection of recurrence of colorectal cancer. Surg
Oncol. 18:15–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flamini E, Mercatali L, Nanni O, Calistri
D, Nunziatini R, Zoli W, Rosetti P, Gardini N, Lattuneddu A,
Verdecchia GM, et al: Free DNA and carcinoembryonic antigen serum
levels: An important combination for diagnosis of colorectal
cancer. Clin Cancer Res. 12:6985–6988. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartolmäs T, Hirschfeld-Ihlow C, Jonas S,
Schaefer M and Gessner R: LI-cadherin cis-dimerizes in the plasma
membrane Ca (2+) independently and forms highly dynamic
trans-contacts. Cell Mol Life Sci. 69:3851–3862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gessner R and Tauber R: Intestinal cell
adhesion molecules. Liver-intestine cadherin. Ann N Y Acad Sci.
915:136–143. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ordóñez NG: Cadherin 17 is a novel
diagnostic marker for adenocarcinomas of the digestive system. Adv
Anat Pathol. 21:131–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Snow AN, Mangray S, Lu S, Clubwala R, Li
J, Resnick MB and Yakirevich E: Expression of cadherin 17 in
well-differentiated neuroendocrine tumours. Histopathology.
66:1010–1021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Gao X, Wei F, Zhang X, Yu J, Zhao
H, Sun Q, Yan F, Yan C, Li H, et al: The hOGG1 Ser326Cys
polymorphism contributes to digestive system cancer susceptibility:
Evidence from 48 case-control studies. Tumour Biol. 36:1029–1038.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Romero-Cordoba SL, Salido-Guadarrama I,
Rodriguez- Dorantes M and Hidalgo-Miranda A: miRNA biogenesis:
Biological impact in the development of cancer. Cancer Biol Ther.
15:1444–1455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang S, Zhang HW, Lu MH, He XH, Li Y, Gu
H, Liu MF and Wang ED: MicroRNA-155 functions as an OncomiR in
breast cancer by targeting the suppressor of cytokine signaling 1
gene. Cancer Res. 70:3119–3127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner
MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ and Schmittgen
TD: Expression profiling identifies microRNA signature in
pancreatic cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magrelli A, Azzalin G, Salvatore M,
Viganotti M, Tosto F, Colombo T, Devito R, Di Masi A, Antoccia A,
Lorenzetti S, et al: Altered microRNA expression patterns in
hepatoblastoma patients. Transl Oncol. 2:157–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao L, Tian Y, Zhao Z, Zhang L, He X and
Pei Y: Expression and clinical significance of miR-224 and miR-378e
in colorectal cancer tissues. Tianjin Med J. 8:737–739, 20113.
|
|
21
|
Tang R, Liang L, Luo D, Feng Z, Huang Q,
He R, Gan T, Yang L and Chen G: Downregulation of miR-30a is
associated with poor prognosis in lung cancer. Med Sci Monit.
21:2514–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alnabulsi A and Murray GI: Integrative
analysis of the colorectal cancer proteome: Potential clinical
impact. Expert Rev Proteomics. 13:917–927. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Z, Gao S, Wang D, Song D and Feng Y:
Colorectal cancer cells are resistant to anti-EGFR monoclonal
antibody through adapted autophagy. Am J Transl Res. 8:1190–1196.
2016.PubMed/NCBI
|
|
25
|
Dimofte G, Târcoveanu E, Taraşi M, Panait
C, Lozneanu G, Nicolescu S, Porumb V and Grigoraş O: Mean number of
lymph nodes in colonic cancer specimen: Possible quality control
index for surgical performance. Chirurgia (Bucur). 106:759–764.
2011.PubMed/NCBI
|
|
26
|
Stojkovic Lalosevic M, Stankovic S,
Stojkovic M, Markovic V, Dimitrijevic I, Lalosevic J, Petrovic J,
Brankovic M, Pavlovic Markovic A and Krivokapic Z: Can preoperative
CEA and CA19-9 serum concentrations suggest metastatic disease in
colorectal cancer patients? Hell J Nucl Med. 20:41–45.
2017.PubMed/NCBI
|
|
27
|
Li R, Yang HQ, Xi HL, Feng S and Qin RH:
Inhibition of CDH17 gene expression via RNA interference reduces
proliferation and apoptosis of human MKN28 gastric cancer cells.
Int J Oncol. 50:15–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu Q, Shen W, Zhou H, Dong W and Gao D:
Knockdown of LI-cadherin alters expression of matrix
metalloproteinase-2 and −9 and galectin-3. Mol Med Rep.
13:4469–4474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bernhard OK, Greening DW, Barnes TW, Ji H
and Simpson RJ: Detection of cadherin-17 in human colon cancer
LIM1215 cell secretome and tumour xenograft-derived interstitial
fluid and plasma. Biochim Biophys Acta. 1834:2372–2379. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van der Wurff AA, Vermeulen SJ, van der
Linden EP, Mareel MM, Bosman FT and Arends JW: Patterns of alpha-
and beta-catenin and E-cadherin expression in colorectal adenomas
and carcinomas. J Pathol. 182:325–330. 1997. View Article : Google Scholar
|
|
32
|
Takamura M, Ichida T, Matsuda Y, Kobayashi
M, Yamagiwa S, Genda T, Shioji K, Hashimoto S, Nomoto M, Hatakeyama
K, et al: Reduced expression of liver-intestine cadherin is
associated with progression and lymph node metastasis of human
colorectal carcinoma. Cancer Lett. 212:253–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Donnarumma E, Fiore D, Nappa M, Roscigno
G, Adamo A, Iaboni M, Russo V, Affinito A, Puoti I, Quintavalle C,
et al: Cancer-associated fibroblasts release exosomal microRNAs
that dictate an aggressive phenotype in breast cancer. Oncotarget.
8:19592–19608. 2017. View Article : Google Scholar : PubMed/NCBI
|