Lipid droplets are organelles with a unique
structure composed of a neutral lipid core and a monolayer
phospholipid membrane embedded with several lipid
droplet-associated proteins. For hundreds of years, lipid droplets
were thought to be the only particles passively storing or released
energy in the cell. However, in the last 20 years, lipid droplets
have been extensively investigated, and lipid droplet biology is
becoming one of the most popular and cutting-edge areas in the
field of organelle study (1–3). Several studies have shown that lipid
droplets are a complex, active and dynamic type of multi-functional
organelle that moves along the cytoskeleton within the cell and
interacts with other organelles, including the endoplasmic
reticulum, mitochondria, nucleus, peroxisomes, autophagosomes and
Golgi, to regulate the metabolism and energy balance of cells
(4–6). In addition, studies have found that
lipid droplets also have an important role in cell proliferation,
apoptosis, metabolism, stress, immunity, membrane transport,
protein degradation, signal transduction and transcriptional
regulation (1,5,7–10). Therefore, lipid droplets have a lot
of physiological functions beyond lipid storage (1). For example, a recent study reported
that lipid droplets contribute to the airway inflammation by
driving pathogenic group 2 innate lymphoid cells (11). Moreover, lipid droplets can decrease
the expression of oxidized phospholipids, such as 4-hydroxynonenal
(HNE) in glia cells, which can protect against oxidative stress in
brain (12). Furthermore, lipid
droplets can provide energy rapidly in brown adipocytes by
interacting with mitochondria under cold stress (13). If the lipid droplets are not present
due to gene editing or inhibition of long-chain fatty acid
synthesis, the cell becomes lipotoxic, which can induce severe
cellular stress and even cell apoptosis (14). A typical feature of cancer cells is
that their metabolism is different from healthy cells. Usually,
energy metabolism, particularly lipid metabolism, in cancer cells
undergoes reprogramming (15–17).
Healthy cells absorb free fatty acid directly for energy
production, and the lipid synthesis is at a low level, but lipid
synthesis is activated and numerous fatty acids and lipids are
formed in tumor cells (15–17). The lipid synthesis pathway in cancer
cells is usually constitutively activated, and the lipid breakdown
process is inhibited (18,19). These excess lipids are often stored
in lipid droplets, resulting in a significantly higher content of
lipid droplets in cancer cells and cancerous tissues compared with
healthy cells and tissues. Therefore, lipid droplet accumulation is
often considered a prominent feature of cancer cells (20,21).
Such intracellular lipid droplet accumulation often leads to
cellular abnormalities, including cellular insulin resistance,
oxidative stress and dysregulated transcription (22–27).
Lung cancer is one of the most common cancer types
in the world, with ~85% of lung cancer cases being non-small cell
lung cancer (NSCLC) in 2018 (28).
It was initially expected that the use of molecularly targeted
anticancer drugs (such as tyrosine kinase inhibitors) could inhibit
tumor growth; however, numerous patients develop resistant tumors
after 1–2 years of treatment (29,30). The
discovery of activating mutations in the epidermal growth factor
receptor (EGFR) in 2004 provided a new avenue the treatment of lung
cancer (31,32), through which individualized treatment
options can be developed by targeting specific genetic mutation
sites. There are activating mutations located in the tyrosine
kinase domain of the EGFR gene, including a 19-exon deletion and a
point mutation in the 21 exon L858R (31–33).
These activating mutations make EGFR highly sensitive to EGFR-TKIs
molecular targeted drugs, such as gefitinib and erlotinib, but
patients also develop resistance to these drugs (34–36).
Previous studies have shown that changes in lipid metabolism in
cancer cells are associated with EGFR-TKI resistance (37–39), and
the inhibition of intracellular lipid droplet synthesis can reverse
the resistance of cancer cells to gefitinib (40). This result suggests that lipid
droplet accumulation in cancer cells may be one of the causes of
EGFR-TKI resistance. The combined use of lipid droplet-targeting
drugs with TKIs in patients with EGFR-TKI resistance may have an
improved therapeutic effect compared with TKI treatment alone. The
present review explains the association between lipid droplet
accumulation and drug resistance in lung cancer from the
perspective of lipid droplet biology.
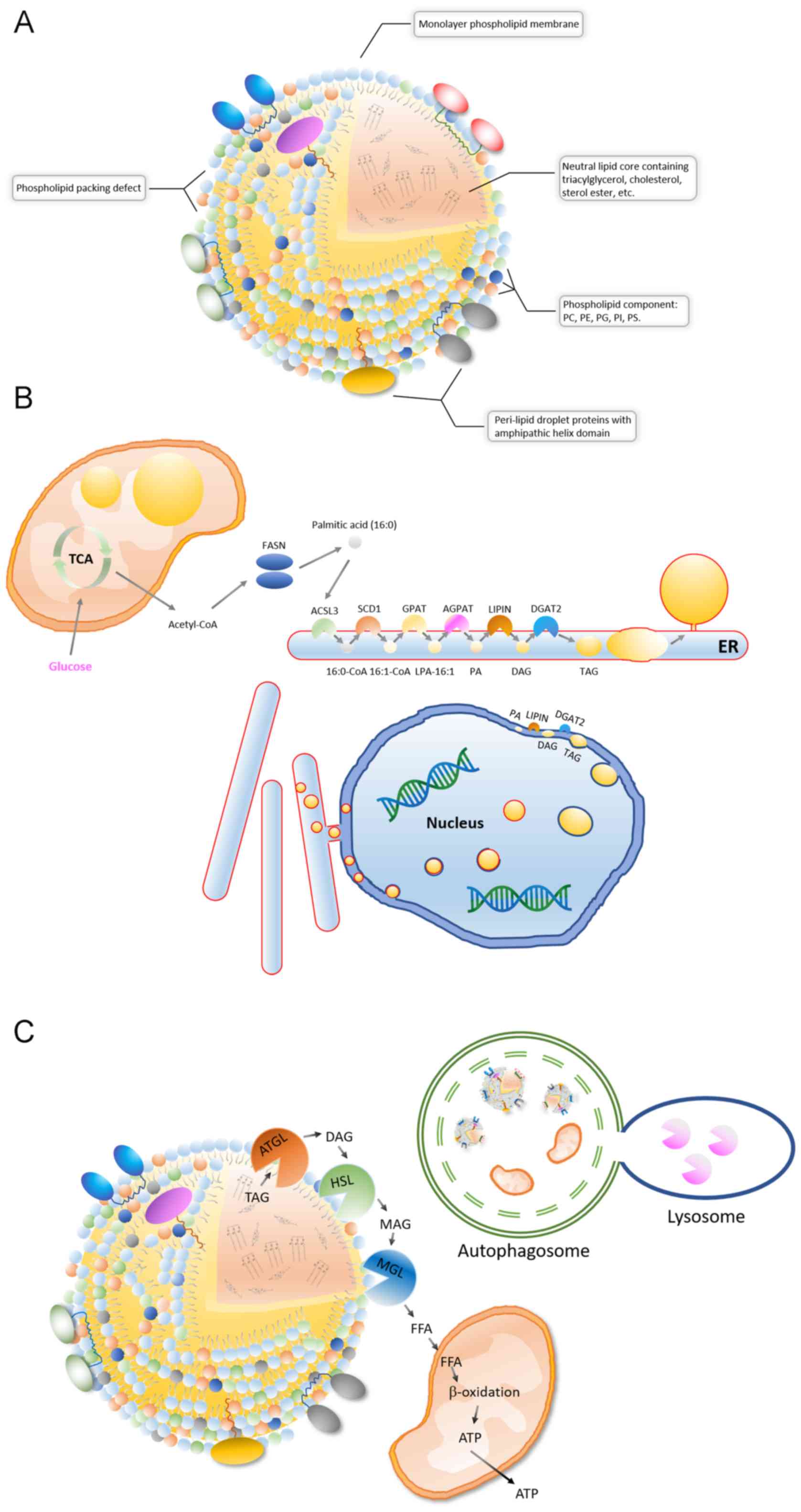
A lipid droplet is a spherical organelle whose core
contains a wide variety of neutral lipids, including cholesteryl
esters, retinyl esters and triglycerides with saturated or
unsaturated chains (2,3,41,42)
(Fig. 1A). The neutral lipid core is
coated by a single layered phospholipid membrane, and its
phospholipid components are diverse, including phosphatidylcholine
(PC), phosphatidylethanolamines, phosphatidylglycerol,
phosphatidylinositol and phosphatidylserine (43) (Fig.
1A). Lipid droplet surfaces are characterized by phospholipid
packing defects, which makes the lipid droplet expose a rougher
interface toward the aqueous cytosol. The phospholipid packing
defects means that neutral lipids are exposed in some areas on the
lipid droplets surface. Therefore, proteins containing large
hydrophobic helical domains can be recruited to the packing defects
of surface (44) (Fig. 1A). Different types of cells, and even
different cells of the same type, have different lipid droplet
sizes, content, lipid components and associated protein types under
different metabolic states (2,3).
Following advances in mass spectrometry, the proteomes of lipid
droplet-associated proteins, as well as the lipid components of
individual droplets, have been resolved. Among them, TG and PC are
the most commonly dominant components of lipid droplets. For
example, although the components of the fatty acid chains of TG and
PC are diverse, the 16:0/18:1/18:1 (fatty acid chains) of TG and
the 18:1/16:0 of PC are dominant in HepG2 cells (45).
Electron microscope and fluorescent images of
intracellular lipid droplets have revealed that lipid droplets are
most often in contact with the endoplasmic reticulum (ER) and
mitochondria (5,8,9). It is
currently accepted that intracellular lipid droplets are produced
in the ER (2,3) (Fig. 1B).
The ER surface contains various lipid synthase enzymes, including
acyl-CoA synthetase long chain family member 3, stearoyl-CoA
desaturase (SCD1), glycerol-3-phosphate acyltransferases (GPATs),
acylglycerol-3-phosphate-o-acyltransferases, phosphatidate
phosphatases and diacylglycerol-o-acyltransferases (DGATs)
(2,3). TG is produced by the catalysis of these
enzymes and is stored in the middle of the bilayer membrane of the
ER in a len structure. Like TG accumulation, lipid droplets
separate from the ER into the cytoplasm in a budding manner
(2,3). These newly formed lipid droplets can
interact with the ER and complete the transport of lipids from the
ER to the lipid droplets through a bridge structure (8,9). In
addition, TG synthases, such as GPAT4 and DGAT2, can be transferred
to the surface of lipid droplets from the ER via direct contact,
therefore TGs can be synthesized on the surface of the lipid
droplets and then transferred to its lipid core (46,47). It
should be noted that lipid droplets are different from vesicles,
which are a bilayer-phospholipid-membrane organelle derived from
ER, in terms of structure. For example, vesicles consist of a
phospholipid bilayer with an aqueous core, whereas lipid droplets
consist of phospholipid monolayer and neutral lipid core (46). Several previous studies have reported
that the surface proteins of lipid droplets can transfer during
lipid droplets contact with other organelles (5,9,10,46).
Therefore, both vesicles and lipid droplets are involved in
transportation of proteins. Newly synthesized TG can be stored in
lipid droplets or released into the blood as very low-density
lipoproteins in the liver and transferred to other tissue cells
(48,49). A previous study has shown that the
nuclear inner membrane also has lipid synthesis activity and is the
site where phosphatidic acid and DAG accumulate (50) (Fig.
1B). DGAT2 on the inner nuclear membrane converts DAG into TG
and stores TG in the middle of the nuclear membrane bilayer of
phospholipids, which is then released into the nucleoplasm by
budding (50). In addition, the
lipid droplets in the lumen of the ER can be transferred to the
nuclear membrane. The lipid droplets coated with the inner nuclear
membrane are then released into the nucleus, the coated nuclear
membrane breaks and then the encapsulated lipid droplets are
released (51). Recent findings
suggest that lipid droplets are also produced inside the
mitochondria (52) (Fig. 1B). After knocking out tissue-specific
sorting nexin Snx31 in urothelial umbrella cells, multivesicular
bodies (MVBs) were no longer observed and lipid droplets were
produced inside the mitochondria, which were further degraded by
autophagy (52). This result
suggests that mitochondria also have lipogenic activity. Therefore,
the endoplasmic reticulum, nucleus and mitochondria are able to
synthesize lipid droplets.
The role of lipid droplets in cancer is a novel
field of research; however, there is evidence suggesting that
increased lipid droplet content is associated with tumor
aggressiveness and chemotherapy resistance (77–81).
Numerous tumorigenic proteins, including PI3K, ERK1, ERK2 and
caveolins, were found to be recruited in lipid droplets (80,82–84). In
addition, lipid droplets are associated with maturation of immature
dendritic cells and the activation of T cells, suggesting that
lipid droplets are also associated with tumor development and
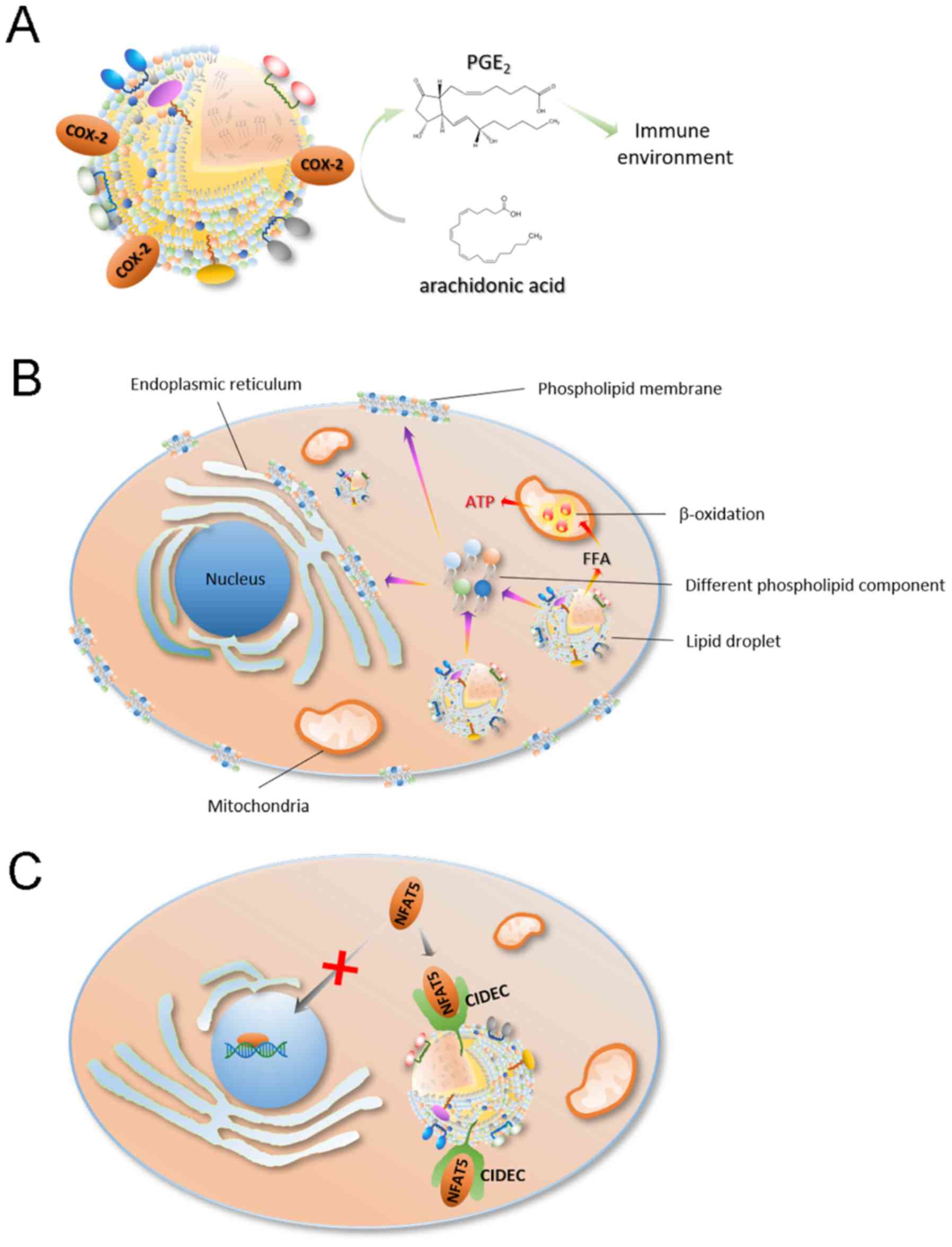
progression (85,86). It is well known that lipid droplets
are important sites for intracellular lipid metabolism and are
closely associated with the production of various lipids and their
derivatives (80,87). Some lipid metabolites, such as
arachidonic acid [an important substrate for prostaglandin (PGE)2
synthesis] and eicosanoids, are important for the immune process as
PGE2 can inhibit the proliferation of T cells (86,87). For
example, Accioly et al (79)
reported that lipid droplets in colon adenocarcinoma cell lines and
colon cancer biopsy tissues were significantly higher compared with
these those in normal cells and paired normal tissues. In addition,
high lipid droplet content is associated with high PGE2 synthesis
(88). It was found that
cyclooxygenase (COX)-2 (a key enzyme in PGE2 synthesis) is
recruited in lipid droplets, which led PGE2 synthesis in lipid
droplets (78) (Fig. 3A). PGE2 is the most highly expressed
PGE and is abundant in tissues containing a number glands, such as
the colon, lung, breast and brain tissue (89–92).
PGE2 plays an important role in promoting tumor growth (93), as it can promote tumor cell
proliferation and migration (92).
As a site of lipid storage, lipid droplets can
provide the necessary lipid components for cell proliferation, such
as cell membrane components like phospholipids, through lipophagy
(94–96) (Fig.
3B). In the process of cancer cell proliferation, lipid
droplets provide both cell membrane components and a large amount
of energy, thereby maintaining cancer cell proliferation and
survival (78,97–100)
(Fig. 3B). For example, in the
process of colon cancer cell proliferation, where the cell energy
demand is large, the rich lipid droplet content in these cell is
compatible with this demand (100).
Notably, Penrose et al (100) also observed an association between
increased lipid droplets and EGFR signaling in colon cancer
(100). Colon cancer cell
proliferation is regulated by EGFR signaling, stimulating an
increase in LD density in an EGFR expression and
activation-dependent manner, and increasing individual cellular
capacity for lipid synthesis (100). This effect is enhanced by the
EGFR-induced PI3K/mTOR pathway and PGE2 synthesis and negatively
regulated by forkhead box protein O1/ NAD-dependent protein
deacetylase sirtuin-6-mediated tumor suppressor inactivation
(100).
Lipid droplets can also regulate the proliferation
of cancer cells by transcriptional regulation (Fig. 3C). Nuclear factor of activated
T-cells (NFAT) 5 is a member of the NFAT protein family and has a
DNA binding domain similar to the Rel-homology region of NF-κB
(101). NFAT5 has an important role
in embryonic development, cell proliferation, immune response and
the cellular stress response (101). In addition, NFAT5 plays an
important role in the development, invasion and proliferation of
tumor cells (102). For example,
NFAT5 can promote the proliferation and invasion of renal cancer
cells, and promote the infiltration of melanoma (103–107),
which suggests that NFAT5 may also be able to promote tumor cell
proliferation and infiltration in other types of cancer.
Furthermore, Meng et al (108) found that NFAT5 regulates lung
cancer cell proliferation, migration and infiltration (108). Previous studies have found that
cell-death inducing DFF45-like effector c (CIDEC) recruited to the
surface of lipid droplets contains a protein domain that binds to
NFAT5, which can bind to NFAT5 on the surface of lipid droplets and
prevent its transcriptional function in the nucleus (22).
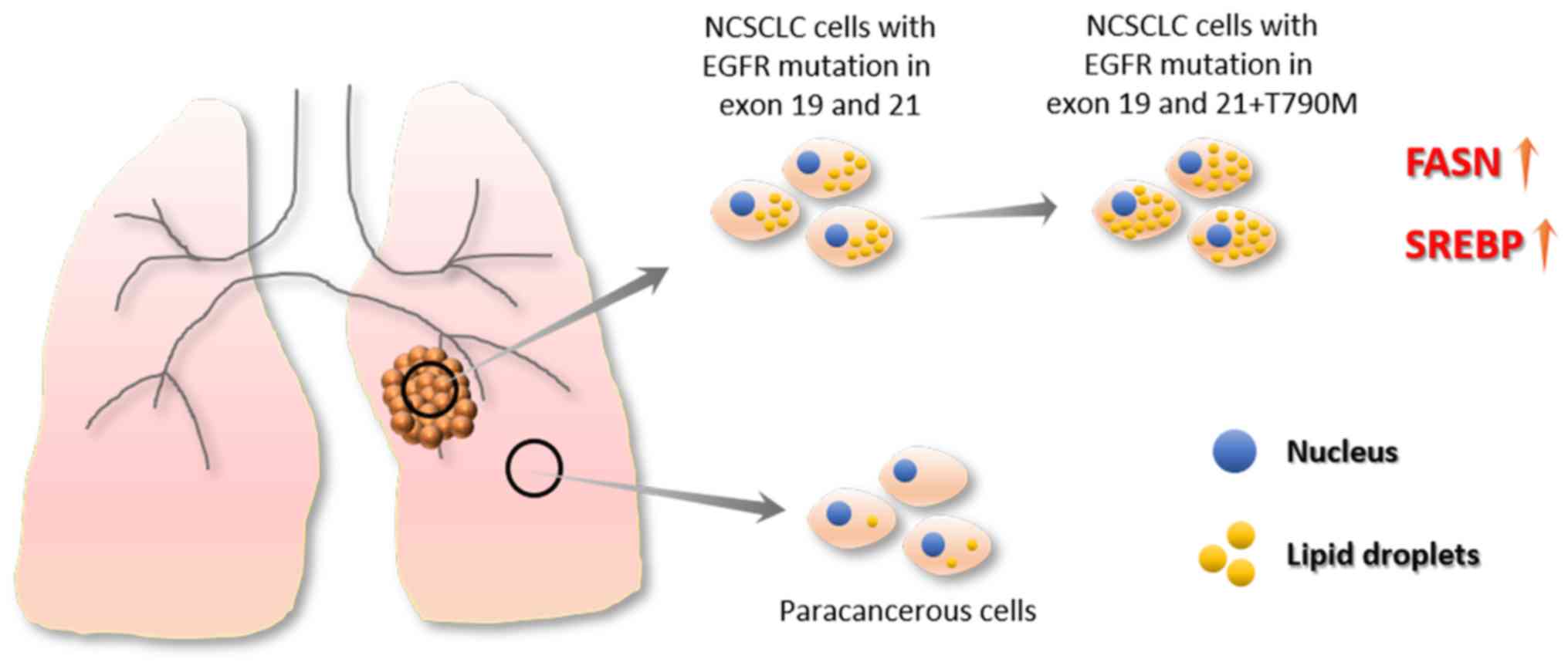
There are a limited number of reports investigating
the association between of drug resistance and lipid droplets in
cancer cells. A recent study by Huang et al (40) revealed an association between lipid
metabolism rearrangement and EGFR-TKI resistance in NSCLC cancer
cells (40) (Fig. 4). The authors found that NSCLC tumor
tissue had higher lipid droplet content compared with
para-cancerous tissue, and TKI treatments could further increase
lipid droplet content in tumor tissue. In addition, the
gefitinib-resistant cell lines, HCC827GR (T790M), H1975 and PC9GR,
expressed more lipid droplets compared with EGFR-TKI-sensitive cell
lines, HCC827 and PC-9, which suggests an association between lipid
droplet content and drug resistance (40). In addition, after treatment with
oleic acid, even EGFR-TKI-sensitive cell lines showed decreased
sensitivity to EGFR-TKI and upregulated p-EGFR. This result
indicates that oleic acid treatment abolished the inhibitory effect
of gefitinib on the activation of the p-EGFR/p-AKT/p-ERK signaling
pathway (40). When injecting
combined gefitinib and oleic acid treated cells into a HCC827 mice
to establish a xenograft tumor model, oleic acid attenuated
gefitinib-induced apoptosis and reduced the cytotoxicity of
gefitinib. Then, the authors treated the cells with
(20S)-protopanaxatriol (g-PPT) (targeting SCD1, inhibiting lipid
synthesis) to effectively reduce the number of intracellular lipid
droplets (40). Notably, the authors
found that EGFR-TKI-resistant mutant cell lines (HCC827GR and
H1975) had increased sensitivity to EGFR-TKI after the number of
cellular lipid droplets decreased (40). Moreover, the combined use of
gefitinib and g-PPT could inhibit cancer cell proliferation and
downregulate p-EGFR expression levels (40). In addition, the combined use of
gefitinib and g-PPT inhibited the activation of the
p-EGFR/p-AKT/p-ERK signaling pathway and promoted apoptosis in
vitro and in vivo. These results indicate that the
inhibition of lipid droplet formation can reverse gefitinib
resistance caused by EGFR-TKI resistant mutations and enhance the
cytotoxicity of gefitinib, thereby promoting gefitinib-induced
apoptosis (40). In addition, some
evidence has shown that mutations in EGFR may be derived from
changes in cellular metabolism (110–112).
Therefore, changes in lipid metabolism may promote the complex
development of NSCLC and promote drug resistance in cancer cells.
Huang et al (40) provides a
novel perspective for the treatment of drug-resistant NSCLC
(40). Drug targeting lipid droplets
can reduce drug resistance during the treatment of drug-resistant
NSCLC to enhance the cytotoxicity of drugs on cancer cells
(40).
In summary, the rearrangement of lipid metabolism in
cancer cells induces lipid droplet accumulation, which could impair
the therapeutic effect of drugs by inhibiting drug-induced
apoptosis. Lipid droplets provide lipid substrates and energy for
the proliferation of cancer cells; therefore, lipid droplet
accumulation is disadvantageous for the treatment of cancer.
Chemotherapy drugs, programmed death (PD)-ligand
1/PD-1, or TKI drugs for NSCLC treatment are all based on the
principle of promoting cancer cell stress and apoptosis (113,114).
Previous studies have shown that lipid droplets are associated with
cellular stress and apoptosis processes. Firstly, there is an
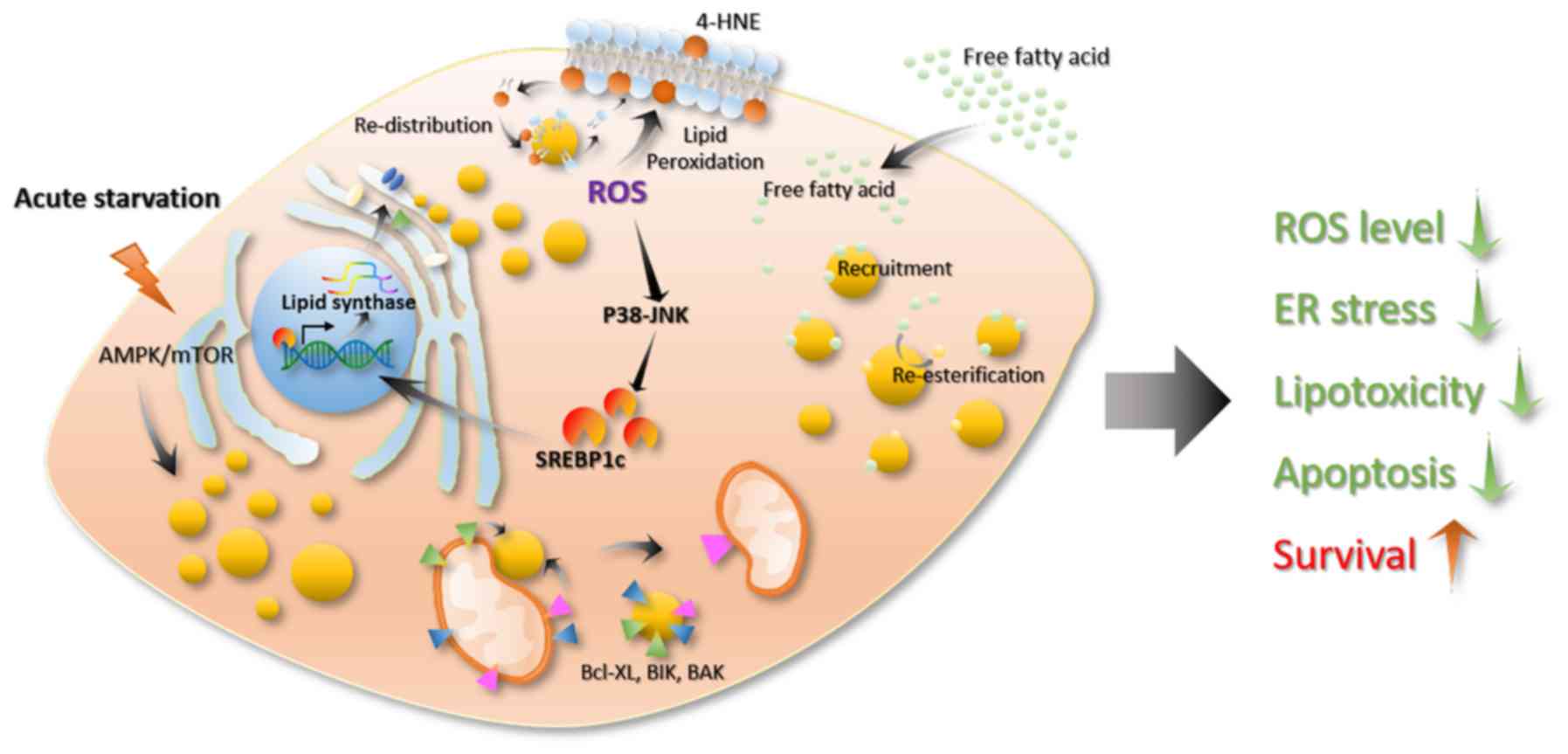
association between lipid droplets and cell stress. When stress
occurs, such as a lack of nutrients or elevated levels of reactive
oxygen species (ROS), cells will produce a large number of lipid
droplets. In addition, increased lipid droplet levels lead to
resistance to cell stress and maintains cell homeostasis (14). During evolution, cells have
established an energy-sensing system to accommodate for the
uncertainty of the nutrient supply (115). AMP-activated protein kinase (AMPK)
and the mammalian target of rapamycin complex 1 (mTORC1) are highly
conserved among different species and are central to cellular
metabolic regulation (115–118). AMPK can be activated by nutrients,
genotoxins, xenobiotics and oxidative stress (115). Activation of AMPK inhibits the
synthesis of fatty acids, sterols and triglycerides (116,119–121)
and promotes glycolysis, mitochondrial activity and fatty acid
oxidation (120). On the contrary,
mROTC1 regulates the synthesis of fatty acids, sterols and
glycolipids by regulating SREBP transcription factors (122–124).
In mammalian cells, acute nutritional restrictions promote lipid
droplet formation (98). This
process is associated with the autophagy induced by mTORC1
inactivation (125). Through this
process, cells can preserve several lipid precursors and energy to
enable cells to survive in a state of energy deficiency (Fig. 5) (126–128).
Excess lipids cause cellular lipo-toxicity when cells take up large
quantities of lipids exceeding their storage or depletion
capacities (129). Then, the lipid
synthesis pathway is activated, transforming the free unesterified
fatty acids into neutral lipids, such as TGs and sterols, to store
in the lipid core of the lipid droplets (Fig. 5) (130). The production of lipid droplets
decreases the cellular lipid toxicity caused by excess lipids and
maintains cell homeostasis (14). In
addition, ROS is an important oxidative factor in cells, and
excessive ROS can cause oxidative stress. ROS refers to a class of
molecules derived from molecular oxygen and free radicals. As ROS
contain an unpaired electron, they become a potent oxidant that
reacts quickly with other pairs of electrons. ROS in cells are
mainly derived from the mitochondria, which is a by-product of
fatty acid oxidation, mainly in the form of hydrogen peroxide in
the cytoplasm (14). Usually, the
intracellular ROS levels in cells are high when the lipid content
is also high (131). ROS are
important signaling molecules that act as a ligands or activate
multiple cellular signaling pathways, such as heat shock factor
protein 1, NF-κB, p53, PI3K-AKT and ERK/JNK/p38, thereby affecting
cellular carbohydrate metabolism, mitochondrial function, apoptosis
and necrosis, proliferation and cancer cell infiltration (132–135).
At present, studies have found that ROS affect the formation and
degradation of cellular lipids (12,23,64,136).
Increased ROS levels can damage mitochondria, reduce mitochondrial
activity and activate c-Jun-N-terminal Kinase (JNK) and SREBP,
which could lead to lipid droplet accumulation in glial cells
(23). A decrease in mitochondrial
activity further increases intracellular ROS levels, thereby
further activating the SREBP pathway and promoting lipid droplet
formation (Fig. 5) (137–139).
In addition, studies have reported that ROS molecules regulate the
recruitment of lipase to the surface of lipid droplets and the
recognition of lipid droplets by autophagic vacuoles (136,140).
Some studies have shown that lipid droplets can protect cells from
ROS-induced cell damage (78,98,131,141).
For example, Bailey et al (12) reported that ROS can oxidize
phospholipid membranes to increase the content of 4-hydroxynonenal
(HNE) (a product of lipid oxidation that causes protein damage) on
cell the membrane, whereas 4-HNE can promote ROS production, which
leads to positive feedback that causes cell damage. In this
process, lipid droplets can re-distribute the oxidized lipids of
membranes, thereby reducing the oxidized lipid content of the cell
membrane and lowering the levels of 4-HNE to promote cell survival
(Fig. 5) (12,142).
Lipid droplets can also resist cellular stress by interacting with
other organelles. For example, by analyzing the traces of several
organelles in cells under starvation and normal conditions, it can
be observed that, in a state of starvation, the lipid droplets
contact the mitochondria at a higher frequency and they are closer
together (6), which is compatible
with increased energy supply. For example, lipid droplets can bind
to mitochondria, which promotes the utilization of lipids (6).
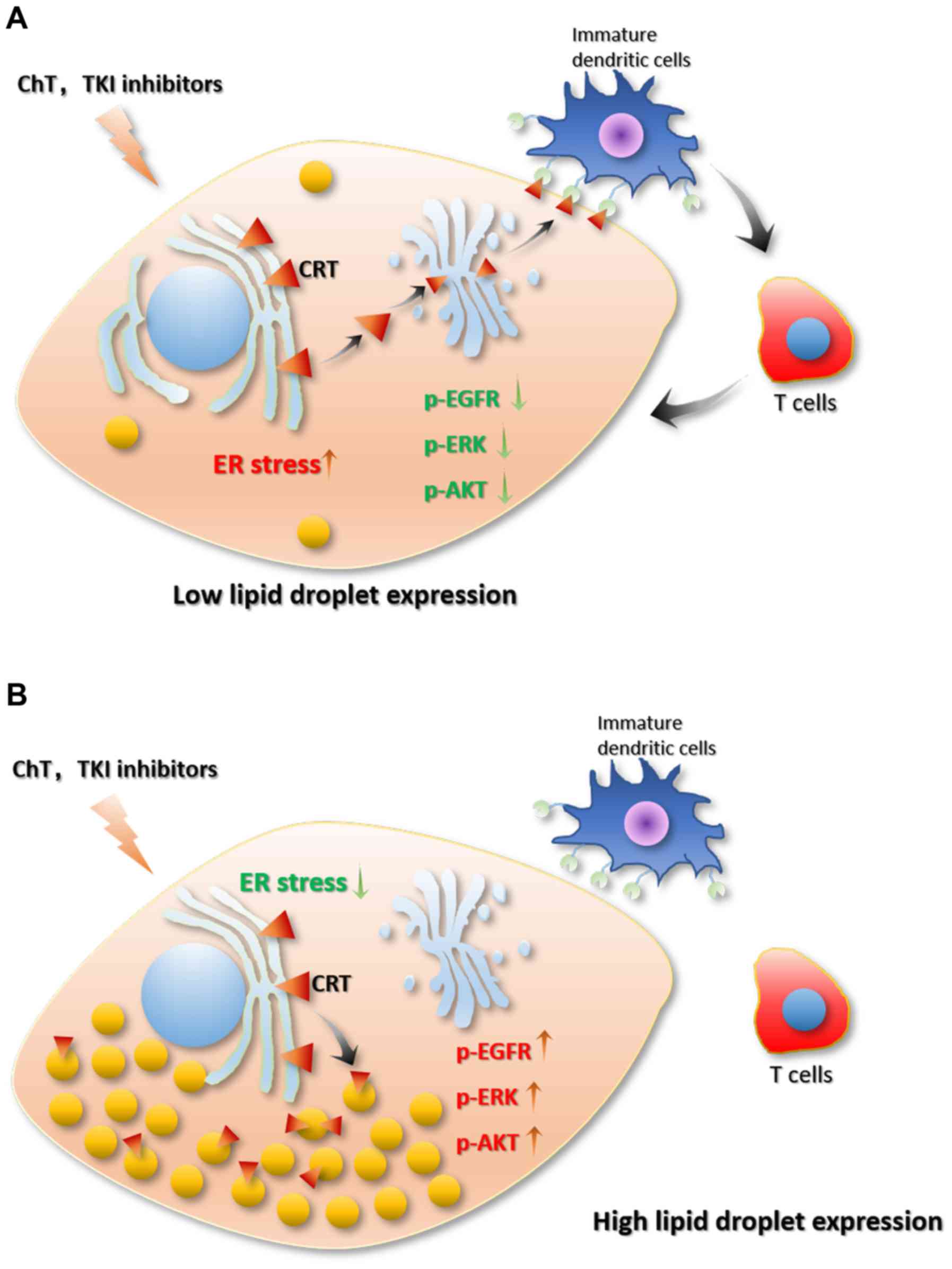
Caspase-12 serves an important role in the
apoptosis-mediated ER stress pathway (143). Cells with a higher lipid droplet
content have enhanced resistance against ROS and lipo-toxicity by
decreasing the release of calcium ions from the ER and inhibiting
the activation of caspase-12 mediated by ER stress. This is
important for apoptosis. In the case of immunotherapy, the high
levels of lipid droplets can recruit CRT (144), thereby blocking the transfer of CRT
from the Golgi to the cell membrane (Fig. 5) (144). Low levels of CRT on the cell
membrane inhibit the maturation of immature dendritic cells, as the
maturation of immature dendritic cells is activated by the binding
between CD91 on the surface of immature dendritic cells and CRT on
the surface of cancer cells (99).
Caspase-8 is important for CRT exposure (145). However, lipid droplet accumulation
leads to a downregulation of caspase-8, which causes a decrease in
CRT exposure and prevents cancer cells from being recognized by
immune cells (99). As
aforementioned, the presence of COX-2 in the lipid droplets
promotes the production of PGE2 (79), thereby producing an immunosuppressive
environment (Fig. 5) (146). Furthermore, CIDEC can be recruited
to the surface of the lipid droplet. The CIDEC amino acids 174–192
are lipophilic, allowing them to bind to lipid droplets. However,
this domain also plays an important role in the interaction with
caspase-9 (147,148). Therefore, CIDEC binding to lipid
droplets impacts the role of CIDEC in the apoptosis process
(Fig. 5). A recent study showed that
lipid droplets can be in contact with the mitochondria and transfer
apoptosis-associated proteins, such as BAX, BCL-2 and BCL-Xl, from
the surface of the mitochondria to the surface of the lipid. This
process could prevent mitochondrial damage and inhibit apoptosis
(Fig. 5) (149). Moreover, a study has shown that
bacterial lipid droplets can bind to DNA via the surface protein,
microorganism lipid droplet small protein (MLDS), which plays an
important role in stabilizing bacterial genetic material and
promotes the survival of bacteria under stress conditions (150). In addition, it is noteworthy that
the nuclear inner membrane also engages in lipid synthesis
activities and produces nuclear lipid droplets in eukaryotes,
whereas nuclear lipid droplets regulate the synthesis of cellular
phospholipids, and maintain cell membranes homeostasis (50). In summary, lipid droplets can enhance
cell stress resistance, reduce stress-induced cell death, maintain
cell homeostasis and promote cell survival by stabilizing excess
free lipids. This provides cell membrane components and energy,
re-distributes peroxidized lipids and inhibits caspase
activation.
Lung cancer is one of the most common types of
cancer worldwide, which accounted for 13.49% of total cancer cases
in 2018, and the 5-year survival rate of lung cancer is only 18%
(28). Several EGFR tyrosine kinase
inhibitors (TKIs), including gefitinib, erlotinib, afatinib and
osimertinib are used for the treatment of lung cancer; however,
persistent drug resistance suppresses the cure rate (29,30).
Compared with healthy cells, cancer cells undergo a rearrangement
of their metabolism, during which changes in lipid metabolism cause
lipid droplets to accumulate. As highly dynamic organelles, lipid
droplets participate in a variety of biological processes,
especially the regulation of energy metabolism, cell proliferation,
apoptosis, stress resistance, immune response and other processes
associated with cancer cell proliferation (1–3,9,80).
Therefore, the role of lipid droplets cannot be ignored when
investigating novel treatments for cancer. NSCLC tissues have
higher lipid droplet content compared with para-cancerous tissues,
and EGFR-TKI resistant cells contain more lipid droplets compared
with non-resistant cells. Intracellular lipid droplets not only
provide cell membrane phospholipid components for cancer cell
proliferation but also provide a large supply of energy, which is
especially important for cancer cells with rapid metabolism and
proliferation. In addition, treatment with common chemotherapeutic
drugs and TKI drugs (such as gefitinib) will further stimulate the
formation of lipid droplets, and the accumulated lipid droplets
will weaken the cytotoxicity of the drug and inhibit drug-mediated
apoptosis, which affects the clinical treatment benefit.
Furthermore, lipid droplets can inhibit ER stress and the
activation of caspase-8 and caspase-12. Lipid droplets can also
recruit CRT, thereby inhibiting the transfer of CRT from the ER to
the Golgi apparatus and cell membrane, which affects the
recognition of cancer cells by immune cells. In addition, the
presence of COX-2 on the surface of lipid droplets can promote the
synthesis of PGE2, which interferes with the immune environment,
and induces inhibition of the antitumor responses. The metabolic
process of lipid synthesis and oxidation is enhanced in cancer
cells, which causes a greater generation of ROS. However, lipid
droplets can attenuate lipid peroxidation of cell membrane
components and can remove proteins, such as Bcl-XL, BIK and BAK,
which could induce apoptosis from the mitochondria by contact.
Therefore, lipid droplets offer an important contribution to cell
stress resistance and the maintenance of cell homeostasis.
There are currently no effective inhibitors of
lipid droplet accumulation, but targets in the lipid droplet
formation process have been identified resulting in inhibited lipid
formation, such as some anti-inflammatory drugs, such as sulindac,
celecoxib and aspirin (155–157).
FASN is a rate-limiting enzyme in that functions in the de
novo synthesis of lipids. The inhibitor TVB-3664 inhibits the
de novo synthesis of fatty acids by targeting FASN (158,159).
In addition, inhibitors against SCD1, such as g-PPT, can reduce the
synthesis of polyunsaturated fatty acids, thereby inhibiting the
synthesis of TG and reducing lipid droplet content (40). The combination of g-PPT and gefitinib
effectively reduces drug resistance and promotes the apoptosis of
cancer cells (40). Therefore, in
the future, a variety of lipid-targeting inhibitors, such as
PLIN2-targeting inhibitors, should be developed, particularly as
PLIN2 has an important role in the formation and stabilization of
lipid droplets by regulating lipid droplet lipolysis and autophagy
(160). By reducing the amount of
lipid droplets in tumor cells, the protective effect of lipid
droplets on cells can be impaired, and drug cytotoxicity and
drug-mediated apoptosis can be promoted.
In general, in the treatment of drug-resistant
NSCLC, a combined approach should be considered. Using drugs
targeting lipid metabolism to reduce lipid droplet content would
promote the sensitivity of tumor cells to drugs and enhance
treatment effects.
The authors of the present study would like to
thank Dr Yi Jin, from the College of Animal Science, Huazhong
Agricultural University (Wuhan, China) for his help in researching
the progress of lipid droplets and oxidative stress.
No funding was received.
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
CJ researched the topic literation, designed the
study and drafted the initial manuscript. CJ and PY participated in
writing and editing the manuscript critically. CJ and PY prepared
the images. Both authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Welte MA: Expanding roles for lipid
droplets. Curr Biol. 25:R470–R481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walther TC, Chung J and Farese RV Jr:
Lipid Droplet Biogenesis. Annu Rev Cell Dev Biol. 33:491–510. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilfling F, Haas JT, Walther TC and Farese
RV Jr: Lipid droplet biogenesis. Curr Opin Cell Biol. 29:39–45.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen S, Valm AM and Lippincott-Schwartz
J: Interacting organelles. Curr Opin Cell Biol. 53:84–91. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olzmann JA and Carvalho P: Dynamics and
functions of lipid droplets. Nat Rev Mol Cell Biol. 20:137–155.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valm AM, Cohen S, Legant WR, Melunis J,
Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E and
Lippincott-Schwartz J: Applying systems-level spectral imaging and
analysis to reveal the organelle interactome. Nature. 546:162–167.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujimoto T, Ohsaki Y, Cheng J, Suzuki M
and Shinohara Y: Lipid droplets: A classic organelle with new
outfits. Histochem Cell Biol. 130:263–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salo VT and Ikonen E: Moving out but
keeping in touch: Contacts between endoplasmic reticulum and lipid
droplets. Curr Opin Cell Biol. 57:64–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schuldiner M and Bohnert M: A different
kind of love - lipid droplet contact sites. Biochim Biophys Acta
Mol Cell Biol Lipids 1862B. 1188–1196. 2017. View Article : Google Scholar
|
|
10
|
Welte MA and Gould AP: Lipid droplet
functions beyond energy storage. Biochim Biophys Acta Mol Cell Biol
Lipids 186B. 1260–1272. 2017. View Article : Google Scholar
|
|
11
|
Karagiannis F, Masouleh SK, Wunderling K,
Surendar J, Schmitt V, Kazakov A, Michla M, Hölzel M, Thiele C and
Wilhelm C: Lipid-Droplet Formation Drives Pathogenic Group 2 Innate
Lymphoid Cells in Airway Inflammation. Immunity. 52:620–634 e626.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bailey AP, Koster G, Guillermier C, Hirst
EM, MacRae JI, Lechene CP, Postle AD and Gould AP: Antioxidant Role
for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell.
163:340–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rambold AS, Cohen S and
Lippincott-Schwartz J: Fatty acid trafficking in starved cells:
Regulation by lipid droplet lipolysis, autophagy, and mitochondrial
fusion dynamics. Dev Cell. 32:678–692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jarc E and Petan T: Lipid Droplets and the
Management of Cellular Stress. Yale J Biol Med. 92:435–452.
2019.PubMed/NCBI
|
|
15
|
Pavlova NN and Thompson CB: The Emerging
Hallmarks of Cancer Metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sciacovelli M and Frezza C: Metabolic
reprogramming and epithelial-to-mesenchymal transition in cancer.
FEBS J. 284:3132–3144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ward PS and Thompson CB: Metabolic
reprogramming: A cancer hallmark even warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ou J, Miao H, Ma Y, Guo F, Deng J, Wei X,
Zhou J, Xie G, Shi H, Xue B, et al: Loss of abhd5 promotes
colorectal tumor development and progression by inducing aerobic
glycolysis and epithelial-mesenchymal transition. Cell Rep.
9:1798–1811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zagani R, El-Assaad W, Gamache I and
Teodoro JG: Inhibition of adipose triglyceride lipase (ATGL) by the
putative tumor suppressor G0S2 or a small molecule inhibitor
attenuates the growth of cancer cells. Oncotarget. 6:28282–28295.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirsch HA, Iliopoulos D, Joshi A, Zhang Y,
Jaeger SA, Bulyk M, Tsichlis PN, Shirley Liu X and Struhl K: A
transcriptional signature and common gene networks link cancer with
lipid metabolism and diverse human diseases. Cancer Cell.
17:348–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patterson AD, Maurhofer O, Beyoglu D, Lanz
C, Krausz KW, Pabst T, Gonzalez FJ, Dufour JF and Idle JR: Aberrant
lipid metabolism in hepatocellular carcinoma revealed by plasma
metabolomics and lipid profiling. Cancer Res. 71:6590–6600. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ueno M, Shen WJ, Patel S, Greenberg AS,
Azhar S and Kraemer FB: Fat-specific protein 27 modulates nuclear
factor of activated T cells 5 and the cellular response to stress.
J Lipid Res. 54:734–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu L, Zhang K, Sandoval H, Yamamoto S,
Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, et al: Glial
lipid droplets and ROS induced by mitochondrial defects promote
neurodegeneration. Cell. 160:177–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Covington JD, Coen PM, Burk DH, Obanda DN,
Ebenezer PJ, Tam CS, Goodpaster BH, Ravussin E and Bajpeyi S:
Intramyocellular Lipid Droplet Size Rather than Total Lipid Content
Is Related to Insulin Sensitivity after 8 Weeks of Overfeeding.
Diabetes. 64:A11. 2015.
|
|
25
|
Nielsen J, Christensen AE, Nellemann B and
Christensen B: Lipid droplet size and location in human skeletal
muscle fibers are associated with insulin sensitivity. Am J Physiol
Endocrinol Metab. 313:E721–E730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Satapati S, Kucejova B, Duarte JAG,
Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu
X, et al: Mitochondrial metabolism mediates oxidative stress and
inflammation in fatty liver. J Clin Invest. 125:4447–4462. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HY, Kwon WY, Kim YA, Oh YJ, Yoo SH,
Lee MH, Bae JY, Kim JM and Yoo YH: Polychlorinated biphenyls
exposure-induced insulin resistance is mediated by lipid droplet
enlargement through Fsp27. Arch Toxicol. 91:2353–2363. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Remon J, Morán T, Majem M, Reguart N,
Dalmau E, Márquez-Medina D and Lianes P: Acquired resistance to
epidermal growth factor receptor tyrosine kinase inhibitors in
EGFR-mutant non-small cell lung cancer: A new era begins. Cancer
Treat Rev. 40:93–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Juchum M, Günther M and Laufer SA:
Fighting cancer drug resistance: Opportunities and challenges for
mutation-specific EGFR inhibitors. Drug Resist Updat. 20:12–28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wen C, Xu G, He S, Huang Y, Shi J, Wu L
and Zhou H: Screening Circular RNAs Related to Acquired Gefitinib
Resistance in Non-small Cell Lung Cancer Cell Lines. J Cancer.
11:3816–3826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin Y, Higashisaka K, Shintani T, Maki A,
Hanamuro S, Haga Y, Maeda S, Tsujino H, Nagano K, Fujio Y, et al:
Progesterone receptor membrane component 1 leads to erlotinib
resistance, initiating crosstalk of Wnt/β-catenin and NF-κB
pathways, in lung adenocarcinoma cells. Sci Rep. 10:47482020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen C, Liu WR, Zhang B, Zhang LM, Li CG,
Liu C, Zhang H, Huo YS, Ma YC, Tian PF, et al: lncRNA H19
downregulation confers erlotinib resistance through upregulation of
PKM2 and phosphorylation of AKT in EGFR-mutant lung cancers. Cancer
Lett. 486:58–70. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu JY, Wu SG, Yang CH, Chang YL, Chang YC,
Hsu YC, Shih JY and Yang PC: Comparison of gefitinib and erlotinib
in advanced NSCLC and the effect of EGFR mutations. Lung Cancer.
72:205–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee CK, Brown C, Gralla RJ, Hirsh V,
Thongprasert S, Tsai CM, Tan EH, Ho JC, Chu T, Zaatar A, et al:
Impact of EGFR inhibitor in non-small cell lung cancer on
progression-free and overall survival: A meta-analysis. J Natl
Cancer Inst. 105:595–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kasahara K, Arao T, Sakai K, Matsumoto K,
Sakai A, Kimura H, Sone T, Horiike A, Nishio M, Ohira T, et al:
Impact of serum hepatocyte growth factor on treatment response to
epidermal growth factor receptor tyrosine kinase inhibitors in
patients with non-small cell lung adenocarcinoma. Clin Cancer Res.
16:4616–4624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang Q, Wang Q, Li D, Wei X, Jia Y, Zhang
Z, Ai B, Cao X, Guo T and Liao Y: Co-administration of
20(S)-protopanaxatriol (g-PPT) and EGFR-TKI overcomes EGFR-TKI
resistance by decreasing SCD1 induced lipid accumulation in
non-small cell lung cancer. J Exp Clin Canc Res. 38:1292019.
View Article : Google Scholar
|
|
41
|
Grillitsch K, Connerth M, Köfeler H, Arrey
TN, Rietschel B, Wagner B, Karas M and Daum G: Lipid
particles/droplets of the yeast Saccharomyces cerevisiae revisited:
Lipidome meets proteome. Biochim Biophys Acta. 1811:1165–1176.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bartz R, Li WH, Venables B, Zehmer JK,
Roth MR, Welti R, Anderson RG, Liu P and Chapman KD: Lipidomics
reveals that adiposomes store ether lipids and mediate phospholipid
traffic. J Lipid Res. 48:837–847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vrablik TL, Petyuk VA, Larson EM, Smith RD
and Watts JL: Lipidomic and proteomic analysis of Caenorhabditis
elegans lipid droplets and identification of ACS-4 as a lipid
droplet-associated protein. Biochim Biophys Acta. 1851:1337–1345.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prévost C, Sharp ME, Kory N, Lin Q, Voth
GA, Farese RV Jr and Walther TC: Mechanism and Determinants of
Amphipathic Helix-Containing Protein Targeting to Lipid Droplets.
Dev Cell. 44:73–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao Y, Chen Z, Wu Y, Tsukui T, Ma X,
Zhang X, Chiba H and Hui SP: Separating and Profiling
Phosphatidylcholines and Triglycerides from Single Cellular Lipid
Droplet by In-Tip Solvent Microextraction Mass Spectrometry. Anal
Chem. 91:4466–4471. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wilfling F, Thiam AR, Olarte MJ, Wang J,
Beck R, Gould TJ, Allgeyer ES, Pincet F, Bewersdorf J, Farese RV Jr
and Walther TC: Arf1/COPI machinery acts directly on lipid droplets
and enables their connection to the ER for protein targeting.
Elife. 3:e016072014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang H, Becuwe M, Housden BE, Chitraju C,
Porras AJ, Graham MM, Liu XN, Thiam AR, Savage DB, Agarwal AK, et
al: Seipin is required for converting nascent to mature lipid
droplets. Elife. 5:e165822016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sturley SL and Hussain MM: Lipid droplet
formation on opposing sides of the endoplasmic reticulum. J Lipid
Res. 53:1800–1810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gibbons GF, Islam K and Pease RJ:
Mobilisation of triacylglycerol stores. Biochim Biophys Acta.
1483:37–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Romanauska A and Kohler A: The Inner
Nuclear Membrane Is a Metabolically Active Territory that Generates
Nuclear Lipid Droplets. Cell. 174:700–715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Soltysik K, Ohsaki Y, Tatematsu T, Cheng
JL and Fujimoto T: Nuclear lipid droplets derive from a lipoprotein
precursor and regulate phosphatidylcholine synthesis. Nat Commun.
10:4732019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liao Y, Tham DKL, Liang FX, Chang J, Wei
Y, Sudhir PR, Sall J, Ren SJ, Chicote JU, Arnold LL, et al:
Mitochondrial lipid droplet formation as a detoxification mechanism
to sequester and degrade excessive urothelial membranes. Mol Biol
Cell. 30:2969–2984. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zimmermann R, Strauss JG, Haemmerle G,
Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger
G, Eisenhaber F, Hermetter A, et al: Fat mobilization in adipose
tissue is promoted by adipose triglyceride lipase. Science.
306:1383–1386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Reid BN, Ables GP, Otlivanchik OA,
Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua
SC Jr and Huang LS: Hepatic overexpression of hormone-sensitive
lipase and adipose triglyceride lipase promotes fatty acid
oxidation, stimulates direct release of free fatty acids, and
ameliorates steatosis. J Biol Chem. 283:13087–13099. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Holm C, Kirchgessner TG, Svenson KL,
Fredrikson G, Nilsson S, Miller CG, Shively JE, Heinzmann C,
Sparkes RS, Mohandas T, et al: Hormone-sensitive lipase: Sequence,
expression, and chromosomal localization to 19 cent-q13.3. Science.
241:1503–1506. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Karlsson M, Contreras JA, Hellman U,
Tornqvist H and Holm C: cDNA cloning, tissue distribution, and
identification of the catalytic triad of monoglyceride lipase.
Evolutionary relationship to esterases, lysophospholipases, and
haloperoxidases. J Biol Chem. 272:27218–27223. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Young SG and Zechner R: Biochemistry and
pathophysiology of intravascular and intracellular lipolysis. Genes
Dev. 27:459–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zechner R, Zimmermann R, Eichmann TO,
Kohlwein SD, Haemmerle G, Lass A and Madeo F: FAT SIGNALS--lipases
and lipolysis in lipid metabolism and signaling. Cell Metab.
15:279–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jaworski K, Sarkadi-Nagy E, Duncan RE,
Ahmadian M and Sul HS: Regulation of triglyceride metabolism. IV.
Hormonal regulation of lipolysis in adipose tissue. Am J Physiol
Gastrointest Liver Physiol. 293:G1–G4. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Settembre C and Ballabio A: Lysosome:
Regulator of lipid degradation pathways. Trends Cell Biol.
24:743–750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Aboumrad MH, Horn RC Jr and Fine G:
Lipid-secreting mammary carcinoma. Report of a case associated with
Paget's disease of the nipple. Cancer. 16:521–525. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ramos CV and Taylor HB: Lipid-rich
carcinoma of the breast. A clinicopathologic analysis of 13
examples. Cancer. 33:812–819. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang WC, Li X, Liu J, Lin J and Chung
LWK: Activation of androgen receptor, lipogenesis, and oxidative
stress converged by SREBP-1 is responsible for regulating growth
and progression of prostate cancer cells. Mol Cancer Res.
10:133–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Baenke F, Peck B, Miess H and Schulze A:
Hooked on fat: The role of lipid synthesis in cancer metabolism and
tumour development. Dis Model Mech. 6:1353–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zaytseva YY, Harris JW, Mitov MI, Kim JT,
Butterfield DA, Lee EY, Weiss HL, Gao T and Evers BM: Increased
expression of fatty acid synthase provides a survival advantage to
colorectal cancer cells via upregulation of cellular respiration.
Oncotarget. 6:18891–18904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cai Y, Crowther J, Pastor T, Abbasi Asbagh
L, Baietti MF, De Troyer M, Vazquez I, Talebi A, Renzi F, Dehairs
J, et al: Loss of Chromosome 8p Governs Tumor Progression and Drug
Response by Altering Lipid Metabolism. Cancer Cell. 29:751–766.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Röhrig F and Schulze A: The multifaceted
roles of fatty acid synthesis in cancer. Nat Rev Cancer.
16:732–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Porstmann T, Santos CR, Griffiths B, Cully
M, Wu M, Leevers S, Griffiths JR, Chung YL and Schulze A: SREBP
activity is regulated by mTORC1 and contributes to Akt-dependent
cell growth. Cell Metab. 8:224–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu Y: Fatty acid oxidation is a dominant
bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic
Dis. 9:230–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hager MH, Solomon KR and Freeman MR: The
role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab
Care. 9:379–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang H, Xi Q and Wu G: Fatty acid synthase
regulates invasion and metastasis of colorectal cancer via Wnt
signaling pathway. Cancer Med. 5:1599–1606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gansler TS, Hardman W III, Hunt DA,
Schaffel S and Hennigar RA: Increased expression of fatty acid
synthase (OA-519) in ovarian neoplasms predicts shorter survival.
Hum Pathol. 28:686–692. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fujimoto M, Yoshizawa A, Sumiyoshi S,
Sonobe M, Menju T, Hirata M, Momose M, Date H and Haga H:
Adipophilin expression in lung adenocarcinoma is associated with
apocrine-like features and poor clinical prognosis: An
immunohistochemical study of 328 cases. Histopathology. 70:232–241.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang XD, Li W, Zhang N, Hou YL, Niu ZQ,
Zhong YJ, Zhang YP and Yang SY: Identification of adipophilin as a
potential diagnostic tumor marker for lung adenocarcinoma. Int J
Clin Exp Med. 7:1190–1196. 2014.PubMed/NCBI
|
|
77
|
Rak S, De Zan T, Stefulj J, Kosović M,
Gamulin O and Osmak M: FTIR spectroscopy reveals lipid droplets in
drug resistant laryngeal carcinoma cells through detection of
increased ester vibrational bands intensity. Analyst (Lond).
139:3407–3415. 2014. View Article : Google Scholar
|
|
78
|
Qiu B, Ackerman D, Sanchez DJ, Li B,
Ochocki JD, Grazioli A, Bobrovnikova-Marjon E, Diehl JA, Keith B
and Simon MC: HIF2α-Dependent Lipid Storage Promotes Endoplasmic
Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer
Discov. 5:652–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Accioly MT, Pacheco P, Maya-Monteiro CM,
Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA,
Bozza PT and Viola JP: Lipid bodies are reservoirs of
cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon
cancer cells. Cancer Res. 68:1732–1740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bozza PT and Viola JP: Lipid droplets in
inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids.
82:243–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nieva C, Marro M, Santana-Codina N, Rao S,
Petrov D and Sierra A: The lipid phenotype of breast cancer cells
characterized by Raman microspectroscopy: Towards a stratification
of malignancy. PLoS One. 7:e464562012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fujimoto T, Kogo H, Ishiguro K, Tauchi K
and Nomura R: Caveolin-2 is targeted to lipid droplets, a new
‘membrane domain’ in the cell. J Cell Biol. 152:1079–1085. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yu W, Bozza PT, Tzizik DM, Gray JP,
Cassara J, Dvorak AM and Weller PF: Co-compartmentalization of MAP
kinases and cytosolic phospholipase A2 at cytoplasmic
arachidonate-rich lipid bodies. Am J Pathol. 152:759–769.
1998.PubMed/NCBI
|
|
84
|
Yu W, Cassara J and Weller PF:
Phosphatidylinositide 3-kinase localizes to cytoplasmic lipid
bodies in human polymorphonuclear leukocytes and other
myeloid-derived cells. Blood. 95:1078–1085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Melo RCN and Weller PF: Lipid droplets in
leukocytes: Organelles linked to inflammatory responses. Exp Cell
Res. 340:193–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Heller S, Cable C, Penrose H, Makboul R,
Biswas D, Cabe M, Crawford SE and Savkovic SD: Intestinal
inflammation requires FOXO3 and prostaglandin E2-dependent
lipogenesis and elevated lipid droplets. Am J Physiol Gastrointest
Liver Physiol. 310:G844–G854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rigas B, Goldman IS and Levine L: Altered
eicosanoid levels in human colon cancer. J Lab Clin Med.
122:518–523. 1993.PubMed/NCBI
|
|
90
|
Wang D and Dubois RN: Cyclooxygenase-2: A
potential target in breast cancer. Semin Oncol. 31 (Suppl 3):64–73.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hambek M, Baghi M, Wagenblast J, Schmitt
J, Baumann H and Knecht R: Inverse correlation between serum PGE2
and T classification in head and neck cancer. Head Neck 29 (Spec).
244–248. 2007. View Article : Google Scholar
|
|
92
|
McLemore TL, Hubbard WC, Litterst CL, Liu
MC, Miller S, McMahon NA, Eggleston JC and Boyd MR: Profiles of
prostaglandin biosynthesis in normal lung and tumor tissue from
lung cancer patients. Cancer Res. 48:3140–3147. 1988.PubMed/NCBI
|
|
93
|
Yan M, Myung SJ, Fink SP, Lawrence E,
Lutterbaugh J, Yang P, Zhou X, Liu D, Rerko RM, Willis J, et al:
15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism
of resistance to celecoxib chemoprevention of colon tumors. Proc
Natl Acad Sci USA. 106:9409–9413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Martinez-Lopez N and Singh R: Autophagy
and Lipid Droplets in the Liver. Annu Rev Nutr. 35:215–237. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Schulze RJ, Sathyanarayan A and Mashek DG:
Breaking fat: The regulation and mechanisms of lipophagy. Biochim
Biophys Acta Mol Cell Biol Lipids 1862B. 1178–1187. 2017.
View Article : Google Scholar
|
|
96
|
Zechner R, Madeo F and Kratky D: Cytosolic
lipolysis and lipophagy: Two sides of the same coin. Nat Rev Mol
Cell Biol. 18:671–684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Herms A, Bosch M, Reddy BJ, Schieber NL,
Fajardo A, Rupérez C, Fernández-Vidal A, Ferguson C, Rentero C,
Tebar F, et al: AMPK activation promotes lipid droplet dispersion
on detyrosinated microtubules to increase mitochondrial fatty acid
oxidation. Nat Commun. 6:71762015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cabodevilla AG, Sánchez-Caballero L,
Nintou E, Boiadjieva VG, Picatoste F, Gubern A and Claro E: Cell
survival during complete nutrient deprivation depends on lipid
droplet-fueled β-oxidation of fatty acids. J Biol Chem.
288:27777–27788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cotte AK, Aires V, Fredon M, Limagne E,
Derangère V, Thibaudin M, Humblin E, Scagliarini A, de Barros JP,
Hillon P, et al: Lysophosphatidylcholine acyltransferase 2-mediated
lipid droplet production supports colorectal cancer
chemoresistance. Nat Commun. 9:3222018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Penrose H, Heller S, Cable C, Makboul R,
Chadalawada G, Chen Y, Crawford SE and Savkovic SD: Epidermal
growth factor receptor mediated proliferation depends on increased
lipid droplet density regulated via a negative regulatory loop with
FOXO3/Sirtuin6. Biochem Biophys Res Commun. 469:370–376. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Müller MR and Rao A: NFAT, immunity and
cancer: A transcription factor comes of age. Nat Rev Immunol.
10:645–656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jauliac S, López-Rodriguez C, Shaw LM,
Brown LF, Rao A and Toker A: The role of NFAT transcription factors
in integrin-mediated carcinoma invasion. Nat Cell Biol. 4:540–544.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
103
|
Germann S, Gratadou L, Zonta E, Dardenne
E, Gaudineau B, Fougère M, Samaan S, Dutertre M, Jauliac S and
Auboeuf D: Dual role of the ddx5/ddx17 RNA helicases in the control
of the pro-migratory NFAT5 transcription factor. Oncogene.
31:4536–4549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chen M, Sinha M, Luxon BA, Bresnick AR and
O'Connor KL: Integrin alpha6beta4 controls the expression of genes
associated with cell motility, invasion, and metastasis, including
S100A4/metastasin. J Biol Chem. 284:1484–1494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kim DH, Kim KS and Ramakrishna S: NFAT5
promotes in vivo development of murine melanoma metastasis. Biochem
Biophys Res Commun. 505:748–754. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guo K and Jin F: NFAT5 promotes
proliferation and migration of lung adenocarcinoma cells in part
through regulating AQP5 expression. Biochem Biophys Res Commun.
465:644–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kuper C, Beck FX and Neuhofer W:
NFAT5-mediated expression of S100A4 contributes to proliferation
and migration of renal carcinoma cells. Front Physiol. 5:2932014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Meng X, Li Z, Zhou S, Xiao S and Yu P:
miR-194 suppresses high glucose-induced non-small cell lung cancer
cell progression by targeting NFAT5. Thorac Cancer. 10:1051–1059.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tomin T, Fritz K, Gindlhuber J, Waldherr
L, Pucher B, Thallinger GG, Nomura DK, Schittmayer M and
Birner-Gruenberger R: Deletion of Adipose Triglyceride Lipase Links
Triacylglycerol Accumulation to a More-Aggressive Phenotype in A549
Lung Carcinoma Cells. J Proteome Res. 17:1415–1425. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang J, Song F, Zhao X, Jiang H, Wu X,
Wang B, Zhou M, Tian M, Shi B, Wang H, et al: EGFR modulates
monounsaturated fatty acid synthesis through phosphorylation of
SCD1 in lung cancer. Mol Cancer. 16:1272017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Makinoshima H, Takita M, Matsumoto S,
Yagishita A, Owada S, Esumi H and Tsuchihara K: Epidermal growth
factor receptor (EGFR) signaling regulates global metabolic
pathways in EGFR-mutated lung adenocarcinoma. J Biol Chem.
289:20813–20823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
De Rosa V, Iommelli F, Monti M, Fonti R,
Votta G, Stoppelli MP and Del Vecchio S: Reversal of Warburg Effect
and Reactivation of Oxidative Phosphorylation by Differential
Inhibition of EGFR Signaling Pathways in Non-Small Cell Lung
Cancer. Clin Cancer Res. 21:5110–5120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu Y, Liu S, Wu C, Huang W, Xu B, Lian S,
Wang L, Yue S, Chen N and Zhu Z: PD-1-Mediated PI3K/Akt/mTOR,
Caspase 9/Caspase 3 and ERK Pathways Are Involved in Regulating the
Apoptosis and Proliferation of CD4+ and CD8+
T Cells During BVDV Infection in vitro. Front Immunol. 11:4672020.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Amri J, Molaee N and Karami H; J A, :
Up-Regulation of MiRNA-125a-5p Inhibits Cell Proliferation and
Increases EGFR-TKI Induced Apoptosis in Lung Cancer Cells. Asian
Pac J Cancer Prev. 20:3361–3367. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Efeyan A, Comb WC and Sabatini DM:
Nutrient-sensing mechanisms and pathways. Nature. 517:302–310.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Garcia D and Shaw RJ: AMPK: Mechanisms of
Cellular Energy Sensing and Restoration of Metabolic Balance. Mol
Cell. 66:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Aramburu J, Ortells MC, Tejedor S, Buxade
M and Lopez-Rodriguez C: Transcriptional regulation of the stress
response by mTOR. Sci Signal. 7:re22014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Muoio DM, Seefeld K, Witters LA and
Coleman RA: AMP-activated kinase reciprocally regulates
triacylglycerol synthesis and fatty acid oxidation in liver and
muscle: Evidence that sn-glycerol-3-phosphate acyltransferase is a
novel target. Biochem J. 338:783–791. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jeon SM, Chandel NS and Hay N: AMPK
regulates NADPH homeostasis to promote tumour cell survival during
energy stress. Nature. 485:661–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wendel AA, Lewin TM and Coleman RA:
Glycerol-3-phosphate acyltransferases: Rate limiting enzymes of
triacylglycerol biosynthesis. Biochim Biophys Acta. 1791:501–506.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Düvel K, Yecies JL, Menon S, Raman P,
Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S,
et al: Activation of a metabolic gene regulatory network downstream
of mTOR complex 1. Mol Cell. 39:171–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yecies JL, Zhang HH, Menon S, Liu S,
Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS,
Lee CH, et al: Akt stimulates hepatic SREBP1c and lipogenesis
through parallel mTORC1-dependent and independent pathways. Cell
Metab. 14:21–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Owen JL, Zhang Y, Bae SH, Farooqi MS,
Liang G, Hammer RE, Goldstein JL and Brown MS: Insulin stimulation
of SREBP-1c processing in transgenic rat hepatocytes requires p70
S6-kinase. Proc Natl Acad Sci USA. 109:16184–16189. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Nguyen TB, Louie SM, Daniele JR, Tran Q,
Dillin A, Zoncu R, Nomura DK and Olzmann JA: DGAT1-Dependent Lipid
Droplet Biogenesis Protects Mitochondrial Function during
Starvation-Induced Autophagy. Dev Cell. 42:9–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Seo AY, Lau PW, Feliciano D, Sengupta P,
Gros MAL, Cinquin B, Larabell CA and Lippincott-Schwartz J: AMPK
and vacuole-associated Atg14p orchestrate mu-lipophagy for energy
production and long-term survival under glucose starvation. Elife.
6:e216902017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Henne WM, Reese ML and Goodman JM: The
assembly of lipid droplets and their roles in challenged cells.
EMBO J. 37:e989472018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hariri H, Rogers S, Ugrankar R, Liu YL,
Feathers JR and Henne WM: Lipid droplet biogenesis is spatially
coordinated at ER-vacuole contacts under nutritional stress. EMBO
Rep. 19:57–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Schaffer JE: Lipotoxicity: When tissues
overeat. Curr Opin Lipidol. 14:281–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Petan T, Jarc E and Jusovic M: Lipid
Droplets in Cancer: Guardians of Fat in a Stressful World.
Molecules. 23:19412018. View Article : Google Scholar
|
|
131
|
Herms A, Bosch M, Ariotti N, Reddy BJ,
Fajardo A, Fernández-Vidal A, Alvarez-Guaita A, Fernández-Rojo MA,
Rentero C, Tebar F, et al: Cell-to-cell heterogeneity in lipid
droplets suggests a mechanism to reduce lipotoxicity. Curr Biol.
23:1489–1496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Perillo B, Di Donato M, Pezone A, Di Zazzo
E, Giovannelli P, Galasso G, Castoria G and Migliaccio A: ROS in
cancer therapy: The bright side of the moon. Exp Mol Med.
52:192–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li R, Jia Z and Trush MA: Defining ROS in
Biology and Medicine. React Oxyg Species (Apex). 1:9–21.
2016.PubMed/NCBI
|
|
134
|
Ramzan R, Vogt S and Kadenbach B:
Stress-mediated generation of deleterious ROS in healthy
individuals - role of cytochrome c oxidase. J Mol Med
(Berl). 98:651–657. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yang S and Lian G: ROS and diseases: Role
in metabolism and energy supply. Mol Cell Biochem. 467:1–12. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhang Z, Zhao S, Yao Z, Wang L, Shao J,
Chen A, Zhang F and Zheng S: Autophagy regulates turnover of lipid
droplets via ROS-dependent Rab25 activation in hepatic stellate
cell. Redox Biol. 11:322–334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Müller G, Wied S, Jung C and Over S:
Hydrogen peroxide-induced translocation of glycolipid-anchored
(c)AMP-hydrolases to lipid droplets mediates inhibition of
lipolysis in rat adipocytes. Br J Pharmacol. 154:901–913. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Blas-García A, Apostolova N, Ballesteros
D, Monleón D, Morales JM, Rocha M, Victor VM and Esplugues JV:
Inhibition of mitochondrial function by efavirenz increases lipid
content in hepatic cells. Hepatology. 52:115–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sekiya M, Hiraishi A, Touyama M and
Sakamoto K: Oxidative stress induced lipid accumulation via SREBP1c
activation in HepG2 cells. Biochem Biophys Res Commun. 375:602–607.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Krawczyk SA, Haller JF, Ferrante T,
Zoeller RA and Corkey BE: Reactive Oxygen Species Facilitate
Translocation of Hormone Sensitive Lipase to the Lipid Droplet
During Lipolysis in Human Differentiated Adipocytes. Plos One.
7:e349042012. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Velázquez AP, Tatsuta T, Ghillebert R,
Drescher I and Graef M: Lipid droplet-mediated ER homeostasis
regulates autophagy and cell survival during starvation. J Cell
Biol. 212:621–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Welte MA: How Brain Fat Conquers Stress.
Cell. 163:269–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann
N Y Acad Sci. 1010:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Turró S, Ingelmo-Torres M, Estanyol JM,
Tebar F, Fernández MA, Albor CV, Gaus K, Grewal T, Enrich C and Pol
A: Identification and characterization of associated with lipid
droplet protein 1: A novel membrane-associated protein that resides
on hepatic lipid droplets. Traffic. 7:1254–1269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Panaretakis T, Kepp O, Brockmeier U,
Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N,
Pierron G, van Endert P, et al: Mechanisms of pre-apoptotic
calreticulin exposure in immunogenic cell death. EMBO J.
28:578–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kalinski P: Regulation of immune responses
by prostaglandin E2. J Immunol. 188:21–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Gao G, Chen FJ, Zhou L, Su L, Xu D, Xu L
and Li P: Control of lipid droplet fusion and growth by CIDE family
proteins. Biochim Biophys Acta Mol Cell Biol Lipids 1862B.
1197–1204. 2017. View Article : Google Scholar
|
|
148
|
Liu K, Zhou S, Kim JY, Tillison K, Majors
D, Rearick D, Lee JH, Fernandez-Boyanapalli RF, Barricklow K,
Houston MS, et al: Functional analysis of FSP27 protein regions for
lipid droplet localization, caspase-dependent apoptosis, and
dimerization with CIDEA. Am J Physiol Endocrinol Metab.
297:E1395–E1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Bischof J, Salzmann M, Streubel MK, Hasek
J, Geltinger F, Duschl J, Bresgen N, Briza P, Haskova D, Lejskova
R, et al: Clearing the outer mitochondrial membrane from harmful
proteins via lipid droplets. Cell Death Discov. 3:170162017.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhang C, Yang L, Ding Y, Wang Y, Lan L, Ma
Q, Chi X, Wei P, Zhao Y, Steinbüchel A, et al: Bacterial lipid
droplets bind to DNA via an intermediary protein that enhances
survival under stress. Nat Commun. 8:159792017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wang G, Li Y, Yang Z, Xu W, Yang Y and Tan
X: ROS mediated EGFR/MEK/ERK/HIF-1α Loop Regulates Glucose
metabolism in pancreatic cancer. Biochem Biophys Res Commun.
500:873–878. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang TH, Chen CC, Huang KY, Shih YM and
Chen CY: High levels of EGFR prevent sulforaphane-induced reactive
oxygen species-mediated apoptosis in non-small-cell lung cancer
cells. Phytomedicine. 64:1529262019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Bollu LR, Katreddy RR, Blessing AM, Pham
N, Zheng B, Wu X and Weihua Z: Intracellular activation of EGFR by
fatty acid synthase dependent palmitoylation. Oncotarget.
6:34992–35003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ali A, Levantini E, Teo JT, Goggi J,
Clohessy JG, Wu CS, Chen L, Yang H, Krishnan I, Kocher O, et al:
Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated
non-small cell lung cancer. EMBO Mol Med. 10:e83132018. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Thun MJ, Henley SJ and Patrono C:
Nonsteroidal anti-inflammatory drugs as anticancer agents:
Mechanistic, pharmacologic, and clinical issues. J Natl Cancer
Inst. 94:252–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Thun MJ, Jacobs EJ and Patrono C: The role
of aspirin in cancer prevention. Nat Rev Clin Oncol. 9:259–267.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ali R, Toh HC and Chia WK; ASCOLT Trial
Investigators, : The utility of Aspirin in Dukes C and High Risk
Dukes B Colorectal cancer - The ASCOLT study: Study Protocol for a
randomized controlled trial. Trials. 12:2612011. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Jafari N, Drury J, Morris AJ, Onono FO,
Stevens PD, Gao T, Liu J, Wang C, Lee EY, Weiss HL, et al: De novo
fatty acid synthesis-driven sphingolipid metabolism promotes
metastatic potential of colorectal cancer. Mol Cancer Res. Aug
28–2018.(Epub ahead of print).
https://doi.org/10.1158/1541-7786.MCR-18-0199. PubMed/NCBI
|
|
159
|
Heuer TS, Ventura R, Mordec K, Lai J,
Fridlib M, Buckley D and Kemble G: FASN Inhibition and Taxane
Treatment Combine to Enhance Anti-tumor Efficacy in Diverse
Xenograft Tumor Models through Disruption of Tubulin Palmitoylation
and Microtubule Organization and FASN Inhibition-Mediated Effects
on Oncogenic Signaling and Gene Expression. EBioMedicine. 16:51–62.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Tsai TH, Chen E, Li L, Saha P, Lee HJ,
Huang LS, Shelness GS, Chan L and Chang BH: The constitutive lipid
droplet protein PLIN2 regulates autophagy in liver. Autophagy.
13:1130–1144. 2017. View Article : Google Scholar : PubMed/NCBI
|