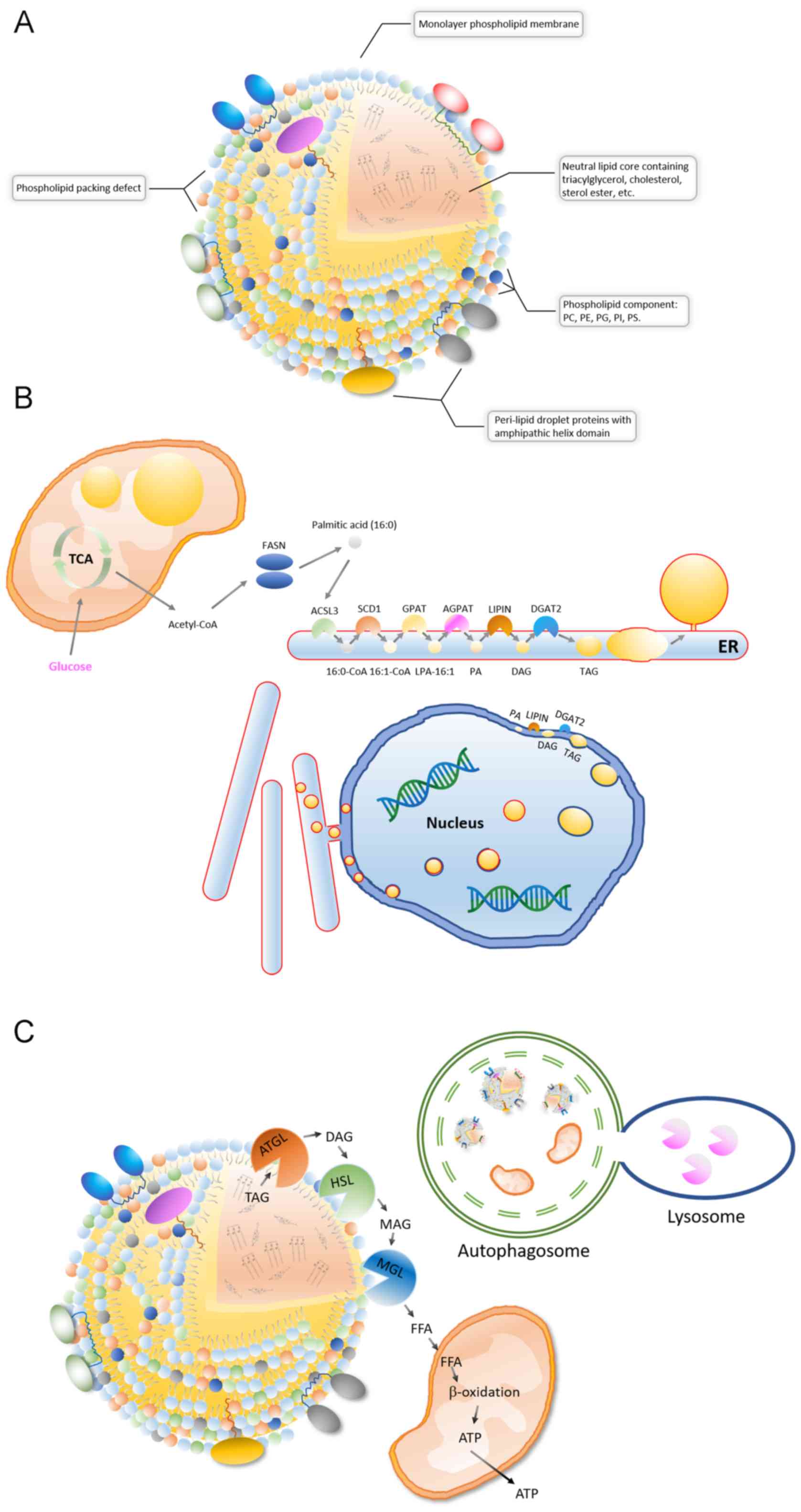
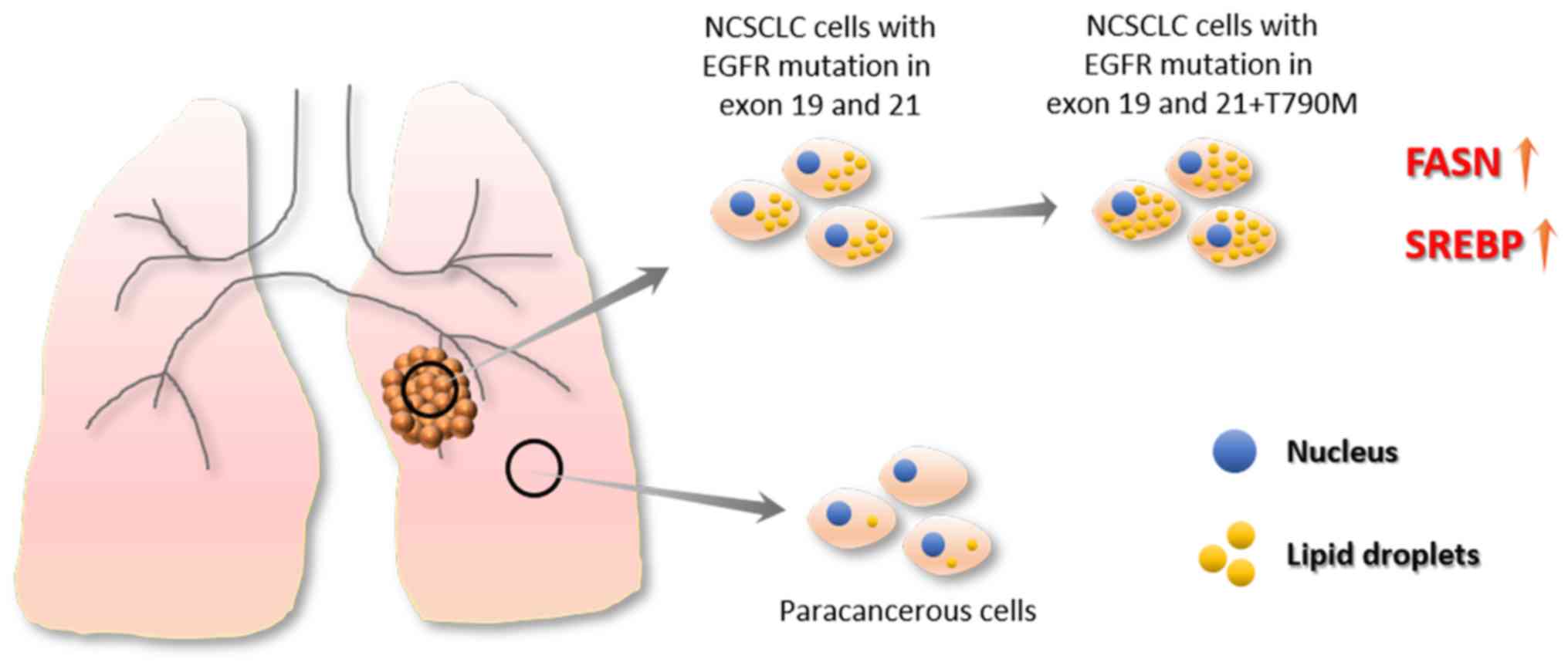
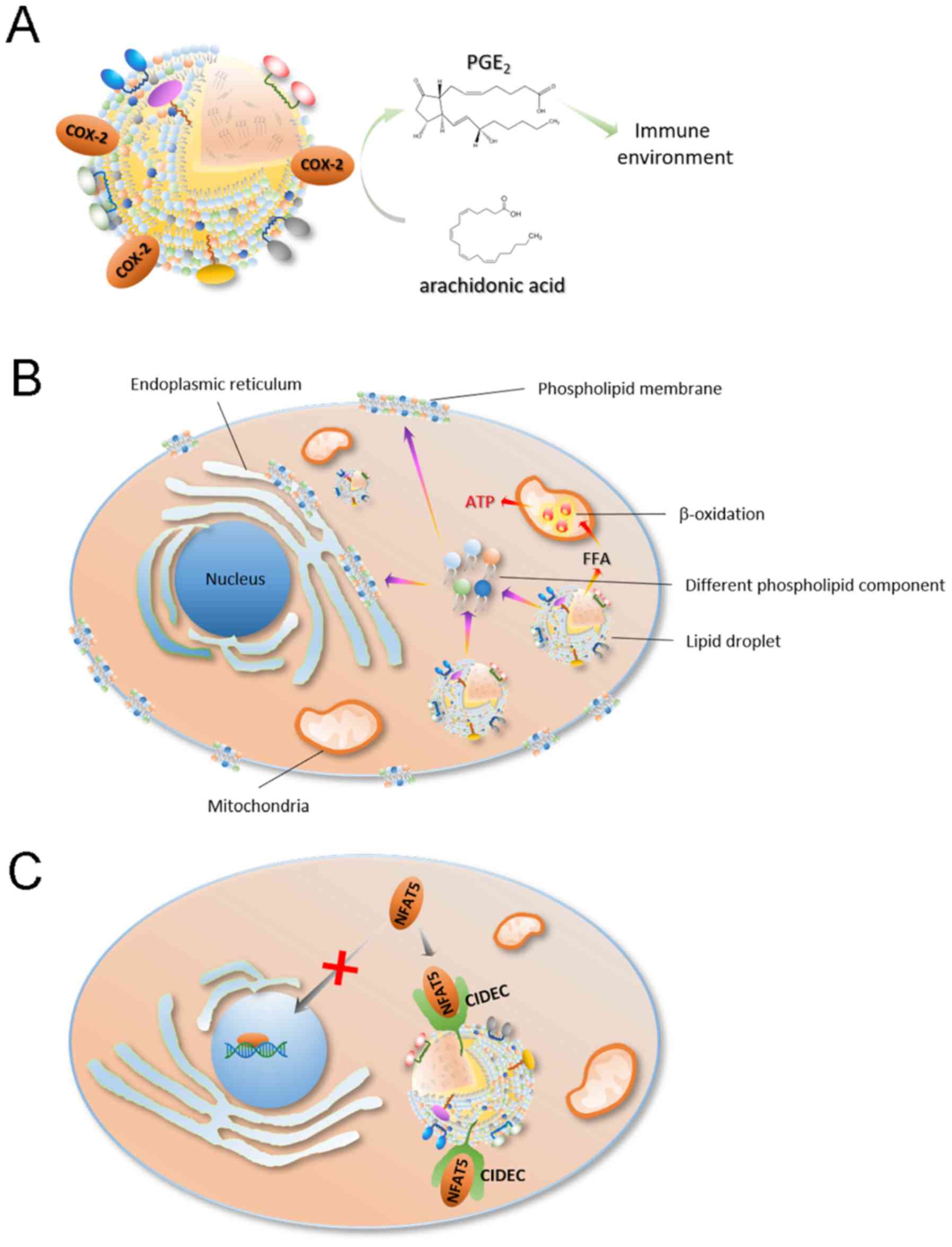
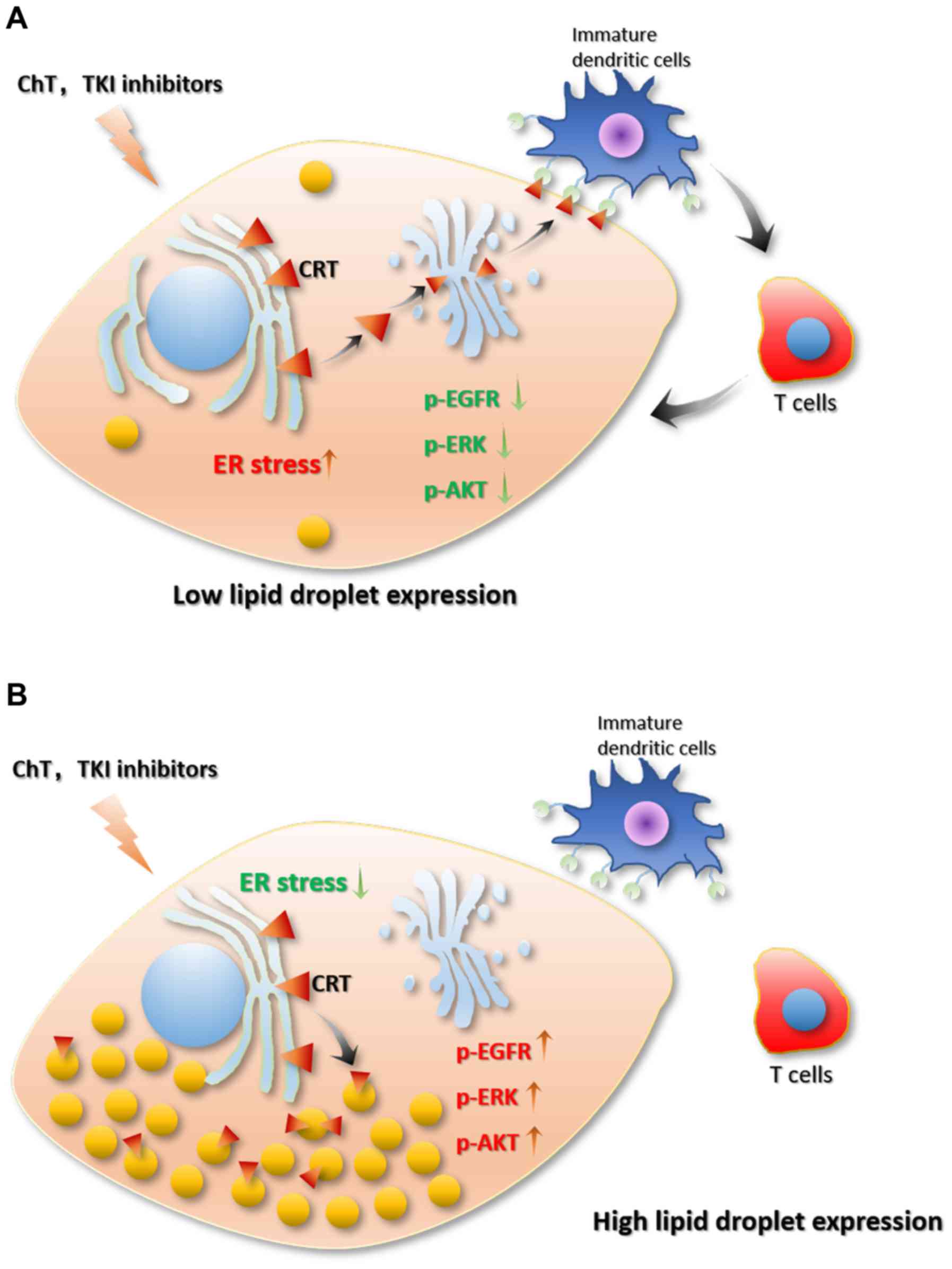
|
1
|
Welte MA: Expanding roles for lipid
droplets. Curr Biol. 25:R470–R481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walther TC, Chung J and Farese RV Jr:
Lipid Droplet Biogenesis. Annu Rev Cell Dev Biol. 33:491–510. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilfling F, Haas JT, Walther TC and Farese
RV Jr: Lipid droplet biogenesis. Curr Opin Cell Biol. 29:39–45.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen S, Valm AM and Lippincott-Schwartz
J: Interacting organelles. Curr Opin Cell Biol. 53:84–91. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olzmann JA and Carvalho P: Dynamics and
functions of lipid droplets. Nat Rev Mol Cell Biol. 20:137–155.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valm AM, Cohen S, Legant WR, Melunis J,
Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E and
Lippincott-Schwartz J: Applying systems-level spectral imaging and
analysis to reveal the organelle interactome. Nature. 546:162–167.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujimoto T, Ohsaki Y, Cheng J, Suzuki M
and Shinohara Y: Lipid droplets: A classic organelle with new
outfits. Histochem Cell Biol. 130:263–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salo VT and Ikonen E: Moving out but
keeping in touch: Contacts between endoplasmic reticulum and lipid
droplets. Curr Opin Cell Biol. 57:64–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schuldiner M and Bohnert M: A different
kind of love - lipid droplet contact sites. Biochim Biophys Acta
Mol Cell Biol Lipids 1862B. 1188–1196. 2017. View Article : Google Scholar
|
|
10
|
Welte MA and Gould AP: Lipid droplet
functions beyond energy storage. Biochim Biophys Acta Mol Cell Biol
Lipids 186B. 1260–1272. 2017. View Article : Google Scholar
|
|
11
|
Karagiannis F, Masouleh SK, Wunderling K,
Surendar J, Schmitt V, Kazakov A, Michla M, Hölzel M, Thiele C and
Wilhelm C: Lipid-Droplet Formation Drives Pathogenic Group 2 Innate
Lymphoid Cells in Airway Inflammation. Immunity. 52:620–634 e626.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bailey AP, Koster G, Guillermier C, Hirst
EM, MacRae JI, Lechene CP, Postle AD and Gould AP: Antioxidant Role
for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell.
163:340–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rambold AS, Cohen S and
Lippincott-Schwartz J: Fatty acid trafficking in starved cells:
Regulation by lipid droplet lipolysis, autophagy, and mitochondrial
fusion dynamics. Dev Cell. 32:678–692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jarc E and Petan T: Lipid Droplets and the
Management of Cellular Stress. Yale J Biol Med. 92:435–452.
2019.PubMed/NCBI
|
|
15
|
Pavlova NN and Thompson CB: The Emerging
Hallmarks of Cancer Metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sciacovelli M and Frezza C: Metabolic
reprogramming and epithelial-to-mesenchymal transition in cancer.
FEBS J. 284:3132–3144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ward PS and Thompson CB: Metabolic
reprogramming: A cancer hallmark even warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ou J, Miao H, Ma Y, Guo F, Deng J, Wei X,
Zhou J, Xie G, Shi H, Xue B, et al: Loss of abhd5 promotes
colorectal tumor development and progression by inducing aerobic
glycolysis and epithelial-mesenchymal transition. Cell Rep.
9:1798–1811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zagani R, El-Assaad W, Gamache I and
Teodoro JG: Inhibition of adipose triglyceride lipase (ATGL) by the
putative tumor suppressor G0S2 or a small molecule inhibitor
attenuates the growth of cancer cells. Oncotarget. 6:28282–28295.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirsch HA, Iliopoulos D, Joshi A, Zhang Y,
Jaeger SA, Bulyk M, Tsichlis PN, Shirley Liu X and Struhl K: A
transcriptional signature and common gene networks link cancer with
lipid metabolism and diverse human diseases. Cancer Cell.
17:348–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patterson AD, Maurhofer O, Beyoglu D, Lanz
C, Krausz KW, Pabst T, Gonzalez FJ, Dufour JF and Idle JR: Aberrant
lipid metabolism in hepatocellular carcinoma revealed by plasma
metabolomics and lipid profiling. Cancer Res. 71:6590–6600. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ueno M, Shen WJ, Patel S, Greenberg AS,
Azhar S and Kraemer FB: Fat-specific protein 27 modulates nuclear
factor of activated T cells 5 and the cellular response to stress.
J Lipid Res. 54:734–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu L, Zhang K, Sandoval H, Yamamoto S,
Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, et al: Glial
lipid droplets and ROS induced by mitochondrial defects promote
neurodegeneration. Cell. 160:177–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Covington JD, Coen PM, Burk DH, Obanda DN,
Ebenezer PJ, Tam CS, Goodpaster BH, Ravussin E and Bajpeyi S:
Intramyocellular Lipid Droplet Size Rather than Total Lipid Content
Is Related to Insulin Sensitivity after 8 Weeks of Overfeeding.
Diabetes. 64:A11. 2015.
|
|
25
|
Nielsen J, Christensen AE, Nellemann B and
Christensen B: Lipid droplet size and location in human skeletal
muscle fibers are associated with insulin sensitivity. Am J Physiol
Endocrinol Metab. 313:E721–E730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Satapati S, Kucejova B, Duarte JAG,
Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu
X, et al: Mitochondrial metabolism mediates oxidative stress and
inflammation in fatty liver. J Clin Invest. 125:4447–4462. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HY, Kwon WY, Kim YA, Oh YJ, Yoo SH,
Lee MH, Bae JY, Kim JM and Yoo YH: Polychlorinated biphenyls
exposure-induced insulin resistance is mediated by lipid droplet
enlargement through Fsp27. Arch Toxicol. 91:2353–2363. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Remon J, Morán T, Majem M, Reguart N,
Dalmau E, Márquez-Medina D and Lianes P: Acquired resistance to
epidermal growth factor receptor tyrosine kinase inhibitors in
EGFR-mutant non-small cell lung cancer: A new era begins. Cancer
Treat Rev. 40:93–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Juchum M, Günther M and Laufer SA:
Fighting cancer drug resistance: Opportunities and challenges for
mutation-specific EGFR inhibitors. Drug Resist Updat. 20:12–28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wen C, Xu G, He S, Huang Y, Shi J, Wu L
and Zhou H: Screening Circular RNAs Related to Acquired Gefitinib
Resistance in Non-small Cell Lung Cancer Cell Lines. J Cancer.
11:3816–3826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin Y, Higashisaka K, Shintani T, Maki A,
Hanamuro S, Haga Y, Maeda S, Tsujino H, Nagano K, Fujio Y, et al:
Progesterone receptor membrane component 1 leads to erlotinib
resistance, initiating crosstalk of Wnt/β-catenin and NF-κB
pathways, in lung adenocarcinoma cells. Sci Rep. 10:47482020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen C, Liu WR, Zhang B, Zhang LM, Li CG,
Liu C, Zhang H, Huo YS, Ma YC, Tian PF, et al: lncRNA H19
downregulation confers erlotinib resistance through upregulation of
PKM2 and phosphorylation of AKT in EGFR-mutant lung cancers. Cancer
Lett. 486:58–70. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu JY, Wu SG, Yang CH, Chang YL, Chang YC,
Hsu YC, Shih JY and Yang PC: Comparison of gefitinib and erlotinib
in advanced NSCLC and the effect of EGFR mutations. Lung Cancer.
72:205–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee CK, Brown C, Gralla RJ, Hirsh V,
Thongprasert S, Tsai CM, Tan EH, Ho JC, Chu T, Zaatar A, et al:
Impact of EGFR inhibitor in non-small cell lung cancer on
progression-free and overall survival: A meta-analysis. J Natl
Cancer Inst. 105:595–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kasahara K, Arao T, Sakai K, Matsumoto K,
Sakai A, Kimura H, Sone T, Horiike A, Nishio M, Ohira T, et al:
Impact of serum hepatocyte growth factor on treatment response to
epidermal growth factor receptor tyrosine kinase inhibitors in
patients with non-small cell lung adenocarcinoma. Clin Cancer Res.
16:4616–4624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang Q, Wang Q, Li D, Wei X, Jia Y, Zhang
Z, Ai B, Cao X, Guo T and Liao Y: Co-administration of
20(S)-protopanaxatriol (g-PPT) and EGFR-TKI overcomes EGFR-TKI
resistance by decreasing SCD1 induced lipid accumulation in
non-small cell lung cancer. J Exp Clin Canc Res. 38:1292019.
View Article : Google Scholar
|
|
41
|
Grillitsch K, Connerth M, Köfeler H, Arrey
TN, Rietschel B, Wagner B, Karas M and Daum G: Lipid
particles/droplets of the yeast Saccharomyces cerevisiae revisited:
Lipidome meets proteome. Biochim Biophys Acta. 1811:1165–1176.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bartz R, Li WH, Venables B, Zehmer JK,
Roth MR, Welti R, Anderson RG, Liu P and Chapman KD: Lipidomics
reveals that adiposomes store ether lipids and mediate phospholipid
traffic. J Lipid Res. 48:837–847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vrablik TL, Petyuk VA, Larson EM, Smith RD
and Watts JL: Lipidomic and proteomic analysis of Caenorhabditis
elegans lipid droplets and identification of ACS-4 as a lipid
droplet-associated protein. Biochim Biophys Acta. 1851:1337–1345.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prévost C, Sharp ME, Kory N, Lin Q, Voth
GA, Farese RV Jr and Walther TC: Mechanism and Determinants of
Amphipathic Helix-Containing Protein Targeting to Lipid Droplets.
Dev Cell. 44:73–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao Y, Chen Z, Wu Y, Tsukui T, Ma X,
Zhang X, Chiba H and Hui SP: Separating and Profiling
Phosphatidylcholines and Triglycerides from Single Cellular Lipid
Droplet by In-Tip Solvent Microextraction Mass Spectrometry. Anal
Chem. 91:4466–4471. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wilfling F, Thiam AR, Olarte MJ, Wang J,
Beck R, Gould TJ, Allgeyer ES, Pincet F, Bewersdorf J, Farese RV Jr
and Walther TC: Arf1/COPI machinery acts directly on lipid droplets
and enables their connection to the ER for protein targeting.
Elife. 3:e016072014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang H, Becuwe M, Housden BE, Chitraju C,
Porras AJ, Graham MM, Liu XN, Thiam AR, Savage DB, Agarwal AK, et
al: Seipin is required for converting nascent to mature lipid
droplets. Elife. 5:e165822016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sturley SL and Hussain MM: Lipid droplet
formation on opposing sides of the endoplasmic reticulum. J Lipid
Res. 53:1800–1810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gibbons GF, Islam K and Pease RJ:
Mobilisation of triacylglycerol stores. Biochim Biophys Acta.
1483:37–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Romanauska A and Kohler A: The Inner
Nuclear Membrane Is a Metabolically Active Territory that Generates
Nuclear Lipid Droplets. Cell. 174:700–715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Soltysik K, Ohsaki Y, Tatematsu T, Cheng
JL and Fujimoto T: Nuclear lipid droplets derive from a lipoprotein
precursor and regulate phosphatidylcholine synthesis. Nat Commun.
10:4732019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liao Y, Tham DKL, Liang FX, Chang J, Wei
Y, Sudhir PR, Sall J, Ren SJ, Chicote JU, Arnold LL, et al:
Mitochondrial lipid droplet formation as a detoxification mechanism
to sequester and degrade excessive urothelial membranes. Mol Biol
Cell. 30:2969–2984. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zimmermann R, Strauss JG, Haemmerle G,
Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger
G, Eisenhaber F, Hermetter A, et al: Fat mobilization in adipose
tissue is promoted by adipose triglyceride lipase. Science.
306:1383–1386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Reid BN, Ables GP, Otlivanchik OA,
Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua
SC Jr and Huang LS: Hepatic overexpression of hormone-sensitive
lipase and adipose triglyceride lipase promotes fatty acid
oxidation, stimulates direct release of free fatty acids, and
ameliorates steatosis. J Biol Chem. 283:13087–13099. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Holm C, Kirchgessner TG, Svenson KL,
Fredrikson G, Nilsson S, Miller CG, Shively JE, Heinzmann C,
Sparkes RS, Mohandas T, et al: Hormone-sensitive lipase: Sequence,
expression, and chromosomal localization to 19 cent-q13.3. Science.
241:1503–1506. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Karlsson M, Contreras JA, Hellman U,
Tornqvist H and Holm C: cDNA cloning, tissue distribution, and
identification of the catalytic triad of monoglyceride lipase.
Evolutionary relationship to esterases, lysophospholipases, and
haloperoxidases. J Biol Chem. 272:27218–27223. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Young SG and Zechner R: Biochemistry and
pathophysiology of intravascular and intracellular lipolysis. Genes
Dev. 27:459–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zechner R, Zimmermann R, Eichmann TO,
Kohlwein SD, Haemmerle G, Lass A and Madeo F: FAT SIGNALS--lipases
and lipolysis in lipid metabolism and signaling. Cell Metab.
15:279–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jaworski K, Sarkadi-Nagy E, Duncan RE,
Ahmadian M and Sul HS: Regulation of triglyceride metabolism. IV.
Hormonal regulation of lipolysis in adipose tissue. Am J Physiol
Gastrointest Liver Physiol. 293:G1–G4. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Settembre C and Ballabio A: Lysosome:
Regulator of lipid degradation pathways. Trends Cell Biol.
24:743–750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Aboumrad MH, Horn RC Jr and Fine G:
Lipid-secreting mammary carcinoma. Report of a case associated with
Paget's disease of the nipple. Cancer. 16:521–525. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ramos CV and Taylor HB: Lipid-rich
carcinoma of the breast. A clinicopathologic analysis of 13
examples. Cancer. 33:812–819. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang WC, Li X, Liu J, Lin J and Chung
LWK: Activation of androgen receptor, lipogenesis, and oxidative
stress converged by SREBP-1 is responsible for regulating growth
and progression of prostate cancer cells. Mol Cancer Res.
10:133–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Baenke F, Peck B, Miess H and Schulze A:
Hooked on fat: The role of lipid synthesis in cancer metabolism and
tumour development. Dis Model Mech. 6:1353–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zaytseva YY, Harris JW, Mitov MI, Kim JT,
Butterfield DA, Lee EY, Weiss HL, Gao T and Evers BM: Increased
expression of fatty acid synthase provides a survival advantage to
colorectal cancer cells via upregulation of cellular respiration.
Oncotarget. 6:18891–18904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cai Y, Crowther J, Pastor T, Abbasi Asbagh
L, Baietti MF, De Troyer M, Vazquez I, Talebi A, Renzi F, Dehairs
J, et al: Loss of Chromosome 8p Governs Tumor Progression and Drug
Response by Altering Lipid Metabolism. Cancer Cell. 29:751–766.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Röhrig F and Schulze A: The multifaceted
roles of fatty acid synthesis in cancer. Nat Rev Cancer.
16:732–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Porstmann T, Santos CR, Griffiths B, Cully
M, Wu M, Leevers S, Griffiths JR, Chung YL and Schulze A: SREBP
activity is regulated by mTORC1 and contributes to Akt-dependent
cell growth. Cell Metab. 8:224–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu Y: Fatty acid oxidation is a dominant
bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic
Dis. 9:230–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hager MH, Solomon KR and Freeman MR: The
role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab
Care. 9:379–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang H, Xi Q and Wu G: Fatty acid synthase
regulates invasion and metastasis of colorectal cancer via Wnt
signaling pathway. Cancer Med. 5:1599–1606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gansler TS, Hardman W III, Hunt DA,
Schaffel S and Hennigar RA: Increased expression of fatty acid
synthase (OA-519) in ovarian neoplasms predicts shorter survival.
Hum Pathol. 28:686–692. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fujimoto M, Yoshizawa A, Sumiyoshi S,
Sonobe M, Menju T, Hirata M, Momose M, Date H and Haga H:
Adipophilin expression in lung adenocarcinoma is associated with
apocrine-like features and poor clinical prognosis: An
immunohistochemical study of 328 cases. Histopathology. 70:232–241.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang XD, Li W, Zhang N, Hou YL, Niu ZQ,
Zhong YJ, Zhang YP and Yang SY: Identification of adipophilin as a
potential diagnostic tumor marker for lung adenocarcinoma. Int J
Clin Exp Med. 7:1190–1196. 2014.PubMed/NCBI
|
|
77
|
Rak S, De Zan T, Stefulj J, Kosović M,
Gamulin O and Osmak M: FTIR spectroscopy reveals lipid droplets in
drug resistant laryngeal carcinoma cells through detection of
increased ester vibrational bands intensity. Analyst (Lond).
139:3407–3415. 2014. View Article : Google Scholar
|
|
78
|
Qiu B, Ackerman D, Sanchez DJ, Li B,
Ochocki JD, Grazioli A, Bobrovnikova-Marjon E, Diehl JA, Keith B
and Simon MC: HIF2α-Dependent Lipid Storage Promotes Endoplasmic
Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer
Discov. 5:652–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Accioly MT, Pacheco P, Maya-Monteiro CM,
Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA,
Bozza PT and Viola JP: Lipid bodies are reservoirs of
cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon
cancer cells. Cancer Res. 68:1732–1740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bozza PT and Viola JP: Lipid droplets in
inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids.
82:243–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nieva C, Marro M, Santana-Codina N, Rao S,
Petrov D and Sierra A: The lipid phenotype of breast cancer cells
characterized by Raman microspectroscopy: Towards a stratification
of malignancy. PLoS One. 7:e464562012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fujimoto T, Kogo H, Ishiguro K, Tauchi K
and Nomura R: Caveolin-2 is targeted to lipid droplets, a new
‘membrane domain’ in the cell. J Cell Biol. 152:1079–1085. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yu W, Bozza PT, Tzizik DM, Gray JP,
Cassara J, Dvorak AM and Weller PF: Co-compartmentalization of MAP
kinases and cytosolic phospholipase A2 at cytoplasmic
arachidonate-rich lipid bodies. Am J Pathol. 152:759–769.
1998.PubMed/NCBI
|
|
84
|
Yu W, Cassara J and Weller PF:
Phosphatidylinositide 3-kinase localizes to cytoplasmic lipid
bodies in human polymorphonuclear leukocytes and other
myeloid-derived cells. Blood. 95:1078–1085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Melo RCN and Weller PF: Lipid droplets in
leukocytes: Organelles linked to inflammatory responses. Exp Cell
Res. 340:193–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Heller S, Cable C, Penrose H, Makboul R,
Biswas D, Cabe M, Crawford SE and Savkovic SD: Intestinal
inflammation requires FOXO3 and prostaglandin E2-dependent
lipogenesis and elevated lipid droplets. Am J Physiol Gastrointest
Liver Physiol. 310:G844–G854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rigas B, Goldman IS and Levine L: Altered
eicosanoid levels in human colon cancer. J Lab Clin Med.
122:518–523. 1993.PubMed/NCBI
|
|
90
|
Wang D and Dubois RN: Cyclooxygenase-2: A
potential target in breast cancer. Semin Oncol. 31 (Suppl 3):64–73.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hambek M, Baghi M, Wagenblast J, Schmitt
J, Baumann H and Knecht R: Inverse correlation between serum PGE2
and T classification in head and neck cancer. Head Neck 29 (Spec).
244–248. 2007. View Article : Google Scholar
|
|
92
|
McLemore TL, Hubbard WC, Litterst CL, Liu
MC, Miller S, McMahon NA, Eggleston JC and Boyd MR: Profiles of
prostaglandin biosynthesis in normal lung and tumor tissue from
lung cancer patients. Cancer Res. 48:3140–3147. 1988.PubMed/NCBI
|
|
93
|
Yan M, Myung SJ, Fink SP, Lawrence E,
Lutterbaugh J, Yang P, Zhou X, Liu D, Rerko RM, Willis J, et al:
15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism
of resistance to celecoxib chemoprevention of colon tumors. Proc
Natl Acad Sci USA. 106:9409–9413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Martinez-Lopez N and Singh R: Autophagy
and Lipid Droplets in the Liver. Annu Rev Nutr. 35:215–237. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Schulze RJ, Sathyanarayan A and Mashek DG:
Breaking fat: The regulation and mechanisms of lipophagy. Biochim
Biophys Acta Mol Cell Biol Lipids 1862B. 1178–1187. 2017.
View Article : Google Scholar
|
|
96
|
Zechner R, Madeo F and Kratky D: Cytosolic
lipolysis and lipophagy: Two sides of the same coin. Nat Rev Mol
Cell Biol. 18:671–684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Herms A, Bosch M, Reddy BJ, Schieber NL,
Fajardo A, Rupérez C, Fernández-Vidal A, Ferguson C, Rentero C,
Tebar F, et al: AMPK activation promotes lipid droplet dispersion
on detyrosinated microtubules to increase mitochondrial fatty acid
oxidation. Nat Commun. 6:71762015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cabodevilla AG, Sánchez-Caballero L,
Nintou E, Boiadjieva VG, Picatoste F, Gubern A and Claro E: Cell
survival during complete nutrient deprivation depends on lipid
droplet-fueled β-oxidation of fatty acids. J Biol Chem.
288:27777–27788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cotte AK, Aires V, Fredon M, Limagne E,
Derangère V, Thibaudin M, Humblin E, Scagliarini A, de Barros JP,
Hillon P, et al: Lysophosphatidylcholine acyltransferase 2-mediated
lipid droplet production supports colorectal cancer
chemoresistance. Nat Commun. 9:3222018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Penrose H, Heller S, Cable C, Makboul R,
Chadalawada G, Chen Y, Crawford SE and Savkovic SD: Epidermal
growth factor receptor mediated proliferation depends on increased
lipid droplet density regulated via a negative regulatory loop with
FOXO3/Sirtuin6. Biochem Biophys Res Commun. 469:370–376. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Müller MR and Rao A: NFAT, immunity and
cancer: A transcription factor comes of age. Nat Rev Immunol.
10:645–656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jauliac S, López-Rodriguez C, Shaw LM,
Brown LF, Rao A and Toker A: The role of NFAT transcription factors
in integrin-mediated carcinoma invasion. Nat Cell Biol. 4:540–544.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
103
|
Germann S, Gratadou L, Zonta E, Dardenne
E, Gaudineau B, Fougère M, Samaan S, Dutertre M, Jauliac S and
Auboeuf D: Dual role of the ddx5/ddx17 RNA helicases in the control
of the pro-migratory NFAT5 transcription factor. Oncogene.
31:4536–4549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chen M, Sinha M, Luxon BA, Bresnick AR and
O'Connor KL: Integrin alpha6beta4 controls the expression of genes
associated with cell motility, invasion, and metastasis, including
S100A4/metastasin. J Biol Chem. 284:1484–1494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kim DH, Kim KS and Ramakrishna S: NFAT5
promotes in vivo development of murine melanoma metastasis. Biochem
Biophys Res Commun. 505:748–754. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guo K and Jin F: NFAT5 promotes
proliferation and migration of lung adenocarcinoma cells in part
through regulating AQP5 expression. Biochem Biophys Res Commun.
465:644–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kuper C, Beck FX and Neuhofer W:
NFAT5-mediated expression of S100A4 contributes to proliferation
and migration of renal carcinoma cells. Front Physiol. 5:2932014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Meng X, Li Z, Zhou S, Xiao S and Yu P:
miR-194 suppresses high glucose-induced non-small cell lung cancer
cell progression by targeting NFAT5. Thorac Cancer. 10:1051–1059.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tomin T, Fritz K, Gindlhuber J, Waldherr
L, Pucher B, Thallinger GG, Nomura DK, Schittmayer M and
Birner-Gruenberger R: Deletion of Adipose Triglyceride Lipase Links
Triacylglycerol Accumulation to a More-Aggressive Phenotype in A549
Lung Carcinoma Cells. J Proteome Res. 17:1415–1425. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang J, Song F, Zhao X, Jiang H, Wu X,
Wang B, Zhou M, Tian M, Shi B, Wang H, et al: EGFR modulates
monounsaturated fatty acid synthesis through phosphorylation of
SCD1 in lung cancer. Mol Cancer. 16:1272017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Makinoshima H, Takita M, Matsumoto S,
Yagishita A, Owada S, Esumi H and Tsuchihara K: Epidermal growth
factor receptor (EGFR) signaling regulates global metabolic
pathways in EGFR-mutated lung adenocarcinoma. J Biol Chem.
289:20813–20823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
De Rosa V, Iommelli F, Monti M, Fonti R,
Votta G, Stoppelli MP and Del Vecchio S: Reversal of Warburg Effect
and Reactivation of Oxidative Phosphorylation by Differential
Inhibition of EGFR Signaling Pathways in Non-Small Cell Lung
Cancer. Clin Cancer Res. 21:5110–5120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu Y, Liu S, Wu C, Huang W, Xu B, Lian S,
Wang L, Yue S, Chen N and Zhu Z: PD-1-Mediated PI3K/Akt/mTOR,
Caspase 9/Caspase 3 and ERK Pathways Are Involved in Regulating the
Apoptosis and Proliferation of CD4+ and CD8+
T Cells During BVDV Infection in vitro. Front Immunol. 11:4672020.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Amri J, Molaee N and Karami H; J A, :
Up-Regulation of MiRNA-125a-5p Inhibits Cell Proliferation and
Increases EGFR-TKI Induced Apoptosis in Lung Cancer Cells. Asian
Pac J Cancer Prev. 20:3361–3367. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Efeyan A, Comb WC and Sabatini DM:
Nutrient-sensing mechanisms and pathways. Nature. 517:302–310.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Garcia D and Shaw RJ: AMPK: Mechanisms of
Cellular Energy Sensing and Restoration of Metabolic Balance. Mol
Cell. 66:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Aramburu J, Ortells MC, Tejedor S, Buxade
M and Lopez-Rodriguez C: Transcriptional regulation of the stress
response by mTOR. Sci Signal. 7:re22014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Muoio DM, Seefeld K, Witters LA and
Coleman RA: AMP-activated kinase reciprocally regulates
triacylglycerol synthesis and fatty acid oxidation in liver and
muscle: Evidence that sn-glycerol-3-phosphate acyltransferase is a
novel target. Biochem J. 338:783–791. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jeon SM, Chandel NS and Hay N: AMPK
regulates NADPH homeostasis to promote tumour cell survival during
energy stress. Nature. 485:661–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wendel AA, Lewin TM and Coleman RA:
Glycerol-3-phosphate acyltransferases: Rate limiting enzymes of
triacylglycerol biosynthesis. Biochim Biophys Acta. 1791:501–506.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Düvel K, Yecies JL, Menon S, Raman P,
Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S,
et al: Activation of a metabolic gene regulatory network downstream
of mTOR complex 1. Mol Cell. 39:171–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yecies JL, Zhang HH, Menon S, Liu S,
Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS,
Lee CH, et al: Akt stimulates hepatic SREBP1c and lipogenesis
through parallel mTORC1-dependent and independent pathways. Cell
Metab. 14:21–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Owen JL, Zhang Y, Bae SH, Farooqi MS,
Liang G, Hammer RE, Goldstein JL and Brown MS: Insulin stimulation
of SREBP-1c processing in transgenic rat hepatocytes requires p70
S6-kinase. Proc Natl Acad Sci USA. 109:16184–16189. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Nguyen TB, Louie SM, Daniele JR, Tran Q,
Dillin A, Zoncu R, Nomura DK and Olzmann JA: DGAT1-Dependent Lipid
Droplet Biogenesis Protects Mitochondrial Function during
Starvation-Induced Autophagy. Dev Cell. 42:9–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Seo AY, Lau PW, Feliciano D, Sengupta P,
Gros MAL, Cinquin B, Larabell CA and Lippincott-Schwartz J: AMPK
and vacuole-associated Atg14p orchestrate mu-lipophagy for energy
production and long-term survival under glucose starvation. Elife.
6:e216902017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Henne WM, Reese ML and Goodman JM: The
assembly of lipid droplets and their roles in challenged cells.
EMBO J. 37:e989472018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hariri H, Rogers S, Ugrankar R, Liu YL,
Feathers JR and Henne WM: Lipid droplet biogenesis is spatially
coordinated at ER-vacuole contacts under nutritional stress. EMBO
Rep. 19:57–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Schaffer JE: Lipotoxicity: When tissues
overeat. Curr Opin Lipidol. 14:281–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Petan T, Jarc E and Jusovic M: Lipid
Droplets in Cancer: Guardians of Fat in a Stressful World.
Molecules. 23:19412018. View Article : Google Scholar
|
|
131
|
Herms A, Bosch M, Ariotti N, Reddy BJ,
Fajardo A, Fernández-Vidal A, Alvarez-Guaita A, Fernández-Rojo MA,
Rentero C, Tebar F, et al: Cell-to-cell heterogeneity in lipid
droplets suggests a mechanism to reduce lipotoxicity. Curr Biol.
23:1489–1496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Perillo B, Di Donato M, Pezone A, Di Zazzo
E, Giovannelli P, Galasso G, Castoria G and Migliaccio A: ROS in
cancer therapy: The bright side of the moon. Exp Mol Med.
52:192–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li R, Jia Z and Trush MA: Defining ROS in
Biology and Medicine. React Oxyg Species (Apex). 1:9–21.
2016.PubMed/NCBI
|
|
134
|
Ramzan R, Vogt S and Kadenbach B:
Stress-mediated generation of deleterious ROS in healthy
individuals - role of cytochrome c oxidase. J Mol Med
(Berl). 98:651–657. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yang S and Lian G: ROS and diseases: Role
in metabolism and energy supply. Mol Cell Biochem. 467:1–12. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhang Z, Zhao S, Yao Z, Wang L, Shao J,
Chen A, Zhang F and Zheng S: Autophagy regulates turnover of lipid
droplets via ROS-dependent Rab25 activation in hepatic stellate
cell. Redox Biol. 11:322–334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Müller G, Wied S, Jung C and Over S:
Hydrogen peroxide-induced translocation of glycolipid-anchored
(c)AMP-hydrolases to lipid droplets mediates inhibition of
lipolysis in rat adipocytes. Br J Pharmacol. 154:901–913. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Blas-García A, Apostolova N, Ballesteros
D, Monleón D, Morales JM, Rocha M, Victor VM and Esplugues JV:
Inhibition of mitochondrial function by efavirenz increases lipid
content in hepatic cells. Hepatology. 52:115–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sekiya M, Hiraishi A, Touyama M and
Sakamoto K: Oxidative stress induced lipid accumulation via SREBP1c
activation in HepG2 cells. Biochem Biophys Res Commun. 375:602–607.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Krawczyk SA, Haller JF, Ferrante T,
Zoeller RA and Corkey BE: Reactive Oxygen Species Facilitate
Translocation of Hormone Sensitive Lipase to the Lipid Droplet
During Lipolysis in Human Differentiated Adipocytes. Plos One.
7:e349042012. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Velázquez AP, Tatsuta T, Ghillebert R,
Drescher I and Graef M: Lipid droplet-mediated ER homeostasis
regulates autophagy and cell survival during starvation. J Cell
Biol. 212:621–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Welte MA: How Brain Fat Conquers Stress.
Cell. 163:269–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann
N Y Acad Sci. 1010:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Turró S, Ingelmo-Torres M, Estanyol JM,
Tebar F, Fernández MA, Albor CV, Gaus K, Grewal T, Enrich C and Pol
A: Identification and characterization of associated with lipid
droplet protein 1: A novel membrane-associated protein that resides
on hepatic lipid droplets. Traffic. 7:1254–1269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Panaretakis T, Kepp O, Brockmeier U,
Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N,
Pierron G, van Endert P, et al: Mechanisms of pre-apoptotic
calreticulin exposure in immunogenic cell death. EMBO J.
28:578–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kalinski P: Regulation of immune responses
by prostaglandin E2. J Immunol. 188:21–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Gao G, Chen FJ, Zhou L, Su L, Xu D, Xu L
and Li P: Control of lipid droplet fusion and growth by CIDE family
proteins. Biochim Biophys Acta Mol Cell Biol Lipids 1862B.
1197–1204. 2017. View Article : Google Scholar
|
|
148
|
Liu K, Zhou S, Kim JY, Tillison K, Majors
D, Rearick D, Lee JH, Fernandez-Boyanapalli RF, Barricklow K,
Houston MS, et al: Functional analysis of FSP27 protein regions for
lipid droplet localization, caspase-dependent apoptosis, and
dimerization with CIDEA. Am J Physiol Endocrinol Metab.
297:E1395–E1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Bischof J, Salzmann M, Streubel MK, Hasek
J, Geltinger F, Duschl J, Bresgen N, Briza P, Haskova D, Lejskova
R, et al: Clearing the outer mitochondrial membrane from harmful
proteins via lipid droplets. Cell Death Discov. 3:170162017.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhang C, Yang L, Ding Y, Wang Y, Lan L, Ma
Q, Chi X, Wei P, Zhao Y, Steinbüchel A, et al: Bacterial lipid
droplets bind to DNA via an intermediary protein that enhances
survival under stress. Nat Commun. 8:159792017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wang G, Li Y, Yang Z, Xu W, Yang Y and Tan
X: ROS mediated EGFR/MEK/ERK/HIF-1α Loop Regulates Glucose
metabolism in pancreatic cancer. Biochem Biophys Res Commun.
500:873–878. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang TH, Chen CC, Huang KY, Shih YM and
Chen CY: High levels of EGFR prevent sulforaphane-induced reactive
oxygen species-mediated apoptosis in non-small-cell lung cancer
cells. Phytomedicine. 64:1529262019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Bollu LR, Katreddy RR, Blessing AM, Pham
N, Zheng B, Wu X and Weihua Z: Intracellular activation of EGFR by
fatty acid synthase dependent palmitoylation. Oncotarget.
6:34992–35003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ali A, Levantini E, Teo JT, Goggi J,
Clohessy JG, Wu CS, Chen L, Yang H, Krishnan I, Kocher O, et al:
Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated
non-small cell lung cancer. EMBO Mol Med. 10:e83132018. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Thun MJ, Henley SJ and Patrono C:
Nonsteroidal anti-inflammatory drugs as anticancer agents:
Mechanistic, pharmacologic, and clinical issues. J Natl Cancer
Inst. 94:252–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Thun MJ, Jacobs EJ and Patrono C: The role
of aspirin in cancer prevention. Nat Rev Clin Oncol. 9:259–267.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ali R, Toh HC and Chia WK; ASCOLT Trial
Investigators, : The utility of Aspirin in Dukes C and High Risk
Dukes B Colorectal cancer - The ASCOLT study: Study Protocol for a
randomized controlled trial. Trials. 12:2612011. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Jafari N, Drury J, Morris AJ, Onono FO,
Stevens PD, Gao T, Liu J, Wang C, Lee EY, Weiss HL, et al: De novo
fatty acid synthesis-driven sphingolipid metabolism promotes
metastatic potential of colorectal cancer. Mol Cancer Res. Aug
28–2018.(Epub ahead of print).
https://doi.org/10.1158/1541-7786.MCR-18-0199. PubMed/NCBI
|
|
159
|
Heuer TS, Ventura R, Mordec K, Lai J,
Fridlib M, Buckley D and Kemble G: FASN Inhibition and Taxane
Treatment Combine to Enhance Anti-tumor Efficacy in Diverse
Xenograft Tumor Models through Disruption of Tubulin Palmitoylation
and Microtubule Organization and FASN Inhibition-Mediated Effects
on Oncogenic Signaling and Gene Expression. EBioMedicine. 16:51–62.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Tsai TH, Chen E, Li L, Saha P, Lee HJ,
Huang LS, Shelness GS, Chan L and Chang BH: The constitutive lipid
droplet protein PLIN2 regulates autophagy in liver. Autophagy.
13:1130–1144. 2017. View Article : Google Scholar : PubMed/NCBI
|