Introduction
Cholangiocarcinoma (CCA) is a malignancy of bile
duct epithelial cells. By 2002, CCA is the second most common type
of primary liver cancer in most parts of the world (1). CCA has a poor prognosis, with a 5-year
survival rate of 5–10% worldwide between 2012 and 2013 (2,3).
Surgical resection is the most effective treatment for CCA
(1,4). When resection is performed at an early
stage, the 5-year survival rate increases to 25–30% between 1997
and 2010 in Japan (4,5). In non-resectable, recurrent and
metastatic CCA, various chemotherapeutic agents, including
gemcitabine, cisplatin and oxaliplatin, have been used either alone
or in combination (5). Resistance to
chemotherapy, however, constrains the response rate to 0–40% and
median survival to 2–12 months between 1994 and 2002, worldwide
(6–8).
The regulation of redox homeostasis is an essential
factor in maintaining normal cellular functions and ensuring cell
survival (9). High reactive oxygen
species (ROS) levels in cancer cells are a consequence of
alteration of several signaling pathways linked to tumorigenesis,
including stimulation of cellular proliferation, as well as
promotion of mutation and genetic instability (9,10).
Redox-modulating strategies are a potential treatment for patients
with breast, ovarian, lung and pancreatic cancer that may enable
therapeutic selectivity and help in overcoming drug resistance
(11,12). For instance, cancer cells with an
increased level of ROS or decreased antioxidant capacity are more
susceptible to oxidative stress-induced cell death (13). Certain anti-cancer agents, including
arsenic trioxide, anthracyclines and cisplatin, have been
demonstrated to act as ROS-generating agents that cause increased
cellular ROS generation (10). These
anti-cancer agents are candidates for evaluating the preferential
targeting of cancer cells with increased ROS-induced stress
(9,10). The present study focused on
piperlongumine (PL), a phytochemical that acts as an anti-cancer
agent (14–16). PL induces redox dysregulation,
selectively killing cancer cells (including CCA) with incremental
increases in intracellular ROS (14). The increased sensitivity of cells to
PL is associated with the degree of cell transformation (16). In addition, this was demonstrated in
previous studies (15) on
immortalized cholangiocytes and spontaneously immortalized
fibroblasts (15,16). PL-induced ROS generation is dependent
on the activation of MAPKs, including JNK, ERK and p38 (14,15).
Various responses to PL have been reported, which may be due to
differences in the underlying genetics of the antioxidant defense
mechanism in each type of cancer cell (15).
Heme oxygenase-1 (HO-1), an inducible form of HO,
was the first rate-limiting enzyme discovered. In mammalian cells,
HO-1 degrades cellular heme to release free iron, CO and biliverdin
(17). HO-1 is frequently
upregulated in numerous types of tumor, including prostate, renal,
gastric, colon cancer and CCA (17–21).
Upregulation of HO-1 is associated with tumor progression,
including tumor growth, metastasis and chemoresistance (20,21).
Previous studies have demonstrated that the depletion of critical
cytoprotective enzymes in cancer cells (particularly HO-1) enhanced
the chemosensitivity of several anti-cancer agents, including
gemcitabine, cisplatin and bortezomib (20,22,23). The
present study aimed to demonstrate that increased chemosensitivity
of CCA may be achieved by a combination of anti-cancer agents,
specifically PL targeting HO-1. The hypothesis was that HO-1 may be
induced during PL treatment in CCA cell lines and that the
suppression of HO-1 by a chemical inhibitor or specific small
interfering (si)RNA may increase the level of intracellular ROS and
chemosensitivity to PL.
Materials and methods
Materials
Cell culture reagents were from Gibco (Thermo Fisher
Scientific, Inc.). PL, 2,7-dichlorodihydrofluorescein diacetate
(DCFH-DA; cat. no. D6883), trichloroacetic acid (cat. no. T0699),
zinc-protoporphyrin IX (ZnPP; HO-1 inhibitor; cat. no. 691550) and
sulforhodamine B (SRB; cat. no. 51402) were obtained from
Sigma-Aldrich (Merck KGaA). Specific siRNA to HO-1 (siHO-1; cat.
no. sc-35554) and non-targeted negative control siRNA (siCon; cat.
no. sc-37007) were purchased from Santa Cruz Biotechnology, Inc.
DharmaFect 1 siRNA transfection reagent (cat. no. T-2001-20) was
purchased from GE Healthcare Dharmacon, Inc. Primary antibodies
were obtained from Cell Signaling Technology, Inc., including total
Akt (60 kDa) (cat. no. 4685S; 1:1,000), serine 473-phosphorylated
Akt (pAkt; 60 kDa) (cat. no. 4060S; 1:1,000), poly(ADP-ribose)
polymerase (PARP; 116/89 kDa) (cat. no. 9542; 1:1,000), lamin B1
(68 kDa) (cat. no. 13435; 1:1,000) and Bcl-2 (28 kDa) (cat. no.
4223; 1:1,000). Antibodies to HO-1 (32 kDa) (cat. no. sc-136960;
1:500), nuclear factor erythroid 2-related factor 2 (Nrf2; 110 kDa)
(cat. no. sc-365949; 1:500), Bax (23 kDa) (cat. no. sc-526; 1:500),
and β-actin (42 kDa) (cat. no. sc-47778; 1:2,000) were purchased
from Santa Cruz Biotechnology, Inc. Mouse anti-rabbit
IgG-horseradish peroxidase (HRP) (cat. no. NXA931; 1:2,000) and
donkey anti-rabbit IgG-HRP (cat. no. NA934V; 1:2,000) secondary
antibodies were obtained from Cytiva. The Luminata™ Forte Western
HRP substrate detection reagents (cat. no. WBLUF0100) were
purchased from Merck KGaA. The Superscript VILO™ cDNA synthesis kit
(cat. no. 11754-050) was purchased from Invitrogen (Thermo Fisher
Scientific, Inc.). The LightCycler® 480 RT-PCR System
and the LightCycler® 480 SYBR Green I master mix (cat.
no. 04707516001) were from Roche Diagnostics GmbH. Wortmannin (cat.
no. 9951S) was purchased from Cell Signaling Technology, Inc.
Cell culture and transfections
A total of 2 human CCA cell lines (KKU-100 and
KKU-213A) had been established from tumor of patients with CCA with
liver-fluke infection admitted to Srinagarind Hospital, Khon Kaen
University (Khonkaen, Thailand), as described previously by Sripa
et al (24,25). Certificates of analyses were obtained
from the Japanese Collection of Research Bioresources Cell Bank.
Cells were cultured in Ham's F12 medium (cat. no. 21700-075; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 1%
penicillin-streptomycin (cat. no. 15140-122; Gibco; Thermo Fisher
Scientific, Inc.) and 10% FBS (cat. no. 10270-098; Gibco; Thermo
Fisher Scientific, Inc.). Cells were incubated at 37°C in a
humidified atmosphere containing 5% CO2. Cells with
70–80% confluence at 24 h were trypsinized with 0.25% trypsin-EDTA
and subcultured in the same media. Mycoplasma testing with
MycoAlert mycoplasma detection kit (cat. no. LT07-418; Lonza
Rockland, Inc.) was conducted for the cell lines used.
Inhibition of HO-1 was performed by transfecting
HO-1 siRNA into the cell lines KKU-100 and KKU-213A. Cells were
seeded into 6-well plates at a seeding density of
3–4×105 cells/well and incubated overnight. Cells were
transfected with 10 µM of siHO-1 or siCon using DharnaFect 1 siRNA
transfection reagent for 24 or 48 h. The transfection procedure was
performed according to the manufacturer's protocol.
Drug treatments
A stock concentration of 50 mM PL, 5 mM ZnPP and 2
mM wortmannin was prepared in DMSO and stored in aliquots at −20°C
until use. Various concentrations of PL (0.01, 0.1, 1, 10, 25, 50
and 100 µM) or ZnPP (0, 1, 5 and 10 µM) were diluted with cell
culture media for subsequent experiments. The vehicle control was
DMSO with 0.001% concentration used in the preparation of the PL,
ZnPP or wortmannin working solutions. For the combination treatment
of PL and ZnPP or PL and wortmannin, cells were pre-treated with
ZnPP (0, 1, 2.5, 5.0, 10, 25, 50 and 100 µM) for 3 h or wortmannin
(1, 2 and 5 µM) for 2 h. Then, ZnPP or wortmannin were removed and
cultured for 24 h or were removed prior to being treated with
various concentrations of PL (0, 0.01, 0.1, 1, 10, 25, 50 and 100
µM; or 0, 10 and 20 µM, respectively) for 24 h. For the PL
treatment after transfection with siHO-1 or siCon, transfected
cells were seeded at 5×103 cells per well into a 96 well
plate. At 24 h after seeding, cells were treated with a range of
concentrations of PL from 0, 5, 10 and 20 µM for 24- h. Treated
cells were subsequently tested for cell viability at 48 h,
intracellular ROS and assessed by reverse
transcription-quantitative PCR (RT-qPCR) and western blot
analysis.
Cell viability
KKU-100 and KKU-213A cells were seed at
5×103 cells per well into a 96-well plate. At 24 h after
seeding, the CCA cell lines were treated with drug as
aforementioned and incubated for 24 h. Cell viability was measured
using an SRB assay capable of determining cell density based on the
measurement of cellular protein content, performed according to
Voigt with slight modifications (26). In brief, cells were fixed with 10%
trichloroacetic acid for overnight, washed 5 times with distilled
water and stained with 0.4% SRB in 1% acetic acid for 30 min.
Plates were washed 5 times with 1% acetic acid, air-dried and then
solubilized the protein-bound dye with 100 µl of unbuffered 10 mM
Tris solution (pH=10). The absorbance was measured at 564 nm using
a microplate reader (Bio-Rad Laboratories, Inc.). The cell
viability was calculated as follows: Cell viability (%) = [optical
density at 564 nm (OD564) in treatment wells]/(OD546 in control
wells) × 100. Each experiment was performed independently in
triplicate. The half-maximal inhibitory concentration
(IC50) values were calculated using GraphPad Prism
software (version 5.0; GraphPad Software, Inc.).
Measurement of intracellular
accumulation of ROS
The production of intracellular ROS was detected
using the DCFH-DA fluorescence assay, as previously described
(10). In brief, KKU-100 cells were
used for detecting ROS production. Thus, transfected KKU-100 cells
(2×105 cells) were seeded into 6-well plates and stored
overnight for 30 h post-transfection. Cells were then treated with
PL at 0, 10 and 20 µM for 12 h. Following treatment, the
intracellular ROS assay was performed by incubation with 20 µM of
DCFH-DA in a humidified atmosphere with 5% CO2 at 37°C
for 30 min. After washing with 1X PBS, cells were trypsinized and
re-suspended in 1X PBS. The fluorescence intensity of the DCFH-DA
was determined with a flow cytometer.
RT-qPCR
KKU-100 and KKU-213A cells that underwent drug
treatments or transfection were harvested using TRIzol reagent for
RNA preparation. In brief, total RNA was extracted using TRIzol
reagent (cat. no. 15596; Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. First-stand
complementary (c)DNA was synthesized using a Superscript VILO™ cDNA
synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.) with 2
µg total RNA according to the manufacturer's protocol. The basal
mRNA expression of 11 antioxidant-associated genes including
nuclear factor erythroid 2-related factor 2 (Nrf2), NADPH quinone
oxidoreductase-1 (NQO-1), heme oxygenase-1 (HO-1), superoxide
dismutase 2 (SOD2), glutathione S-transferase P1 (GSTP1), aldo-keto
reductase 1 subunits C-1 and 3 (AKR1C1 and AKR1C3),
γ-glutamylcysteine synthetase catalytic subunit (GCLC) and
γ-glutamylcysteine synthetase modifier subunit (GCLM), Parkinson
disease protein 7 precursor (PARK7), thioredoxin (TXN) was
investigated in the two CCA cell lines, which have differential
responses to PL; KKU-100 (CCA less sensitive to PL) and KKU-213A
(CCA highly sensitive to PL). The sequences of the primers used are
listed in Table I. Real-time PCR was
performed using the LightCycler® 480 RT-PCR System and
the LightCycler® 480 SYBR-Green I master mix (Roche
Diagnostics GmbH). The thermocycling conditions were as follows,
according to the LightCycler 480 manufacturer's instructions: 95°C
for 5 min; 95°C for 10 sec, annealing at 60°C for 10 sec and
extension at 72°C for 10 sec, for 45 cycles. The specificity of
each of the PCR products was confirmed via melting curve analysis.
Relative mRNA expression was obtained following normalization to
endogenous human β-actin and quantified using the 2−∆∆Cq
methods (27).
 | Table I.Sequences of the primers used for
reverse transcription-quantitative PCR. |
Table I.
Sequences of the primers used for
reverse transcription-quantitative PCR.
| Gene | Forward (5–3) | Reverse (5–3) |
|---|
| Nrf2 |
TACTCCCAGGTTGCCCACA |
CATCTACAAACGGGAATGTCTGC |
| GCLM |
GACAAAACACAGTTGGAACAGC |
CAGTCAAATCTGGTGGCATC |
| GCLC |
ATGCCATGGGATTTGGAAT |
AGATATACTGCAGGCTTGGAATG |
| AKR1C1 |
CATGCCTGTCCTGGGATTT |
AGAATCAATATGGCGGAAGC |
| AKR1C3 |
CATTGGGGTGTCAAACTTCA |
CCGGTTGAAATACGGATGAC |
| HO-1 |
CAACATCCAGCTCTTTGAGGA |
GGGCAGAATCTTGCACTTTG |
| NQO1 |
GATATTCCAGTTCCCCCTGC |
TTCTTACTCCGGAAGGGTCC |
| TXN |
GAGAGCAAGACTGCTTTTCA |
CAGAGAGGGAATGAAAGAAAG |
| GSTP1 |
TACACCAACTATGAGGCGGG |
AGCGAAGGAGATCTGGTCTC |
| SOD2 |
GTTGGCCAAGGGAGATGTTAC |
AGCAACTCCCCTTTGGGTTC |
| PARK7 |
CGAGCTGGGATTAAGGTCA |
CATATGGTCCCTCTTTTTTTGC |
| β-actin |
GATCAGCAAGCAGGAGTATGACG |
AAGGGTGTAACGCAACTAAGTCATAG |
Protein preparation
The cell lines KKU-100 and KKU-213A were cultured in
6-well plates at 3–4×105 cells/well for 24 h at 37°C in
a humidified atmosphere containing 5% CO2. Cells were
then treated with siRNA and/or various concentrations of PL (0, 5,
10 or 20 µM) for 0, 1, 3, 6 or 24 h. Following incubation, the
whole-cell lysate was harvested as previously described by Thongsom
et al (15). Nuclear and
cytoplasmic fractions were extracted using a nuclear extraction kit
(cat. no. 90498; Chemicon; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The whole-cell lysate and
nuclear and cytoplasmic fractions were then tested for their
respective protein concentrations using a Pierce® BCA
protein assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol.
SDS-PAGE and western blot
analysis
Protein samples from the whole-cell lysate, nuclear
or cytoplasmic fraction with a mass of 20 µg were separated using
10% SDS-PAGE. The proteins were transferred to a nitrocellulose
membrane and blocked for 1 h with 5% (w/v) skimmed milk in 1X PBS
supplemented with 0.05% Tween-20 (PBST). The membranes were then
incubated overnight at 4°C with primary antibodies at dilutions of
1:500 for HO-1, Bax and Nrf2 proteins; 1:1,000 for PARP, Bcl-2,
pAkt (Ser473), total Akt and Lamin B1 proteins; or 1:2,000 for
β-actin protein in PBST. The membranes were then incubated with
HRP-conjugated secondary antibodies at a dilution of 1:2,000 for 1
h at room temperature. Luminata™ Forte Western HRP substrate (Merck
KGaA) was applied for protein detection. The densities of the bands
for HO-1, cleaved PARP, Bcl-2, Bax, Nrf2, Akt and pAkt were
determined using Image J software version 1.52v (National
Institutes of Health) and normalized to β-actin for whole cell
lysate or normalized to lamin B1 for nuclear extracts. The
respective ratio of each protein to β-actin, the ratio of pAkt/Akt,
the ratio of cleaved PARP/Bcl-2 and ratio of Bcl-2/Bax were
calculated.
Statistical analysis
All experiments were performed 2–3 times and the
results are presented as the mean ± standard error of the mean.
Statistical analyses were performed using GraphPad Prism software
(version 5.0; GraphPad Software, Inc.). The Student's t-test was
used for between-group statistical analyses. One-way and two-way
analysis of variance followed by Bonferroni's correction were
applied for statistical analysis of multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression levels of phase II
detoxification enzymes and antioxidant proteins in CCA cell
lines
Nrf2-mediated cytoprotective genes are thought to be
a primary antioxidant defense mechanism in mammalian cells. The
process eliminates harmful ROS or carcinogens, particularly phase
II detoxification enzymes and antioxidant proteins (10). In the present study, basal mRNA
expression of 11 antioxidant-associated genes was investigated in
two CCA cell lines previously (15)
indicated to have differential responses to PL: KKU-100 (CCA less
sensitive to PL) and KKU-213A (CCA highly sensitive to PL). The
results demonstrated that mRNA expression levels of phase II
detoxification enzymes, including NADPH quinone oxidoreductase-1
(NQO-1), heme oxygease-1 (HO-1), superoxide dismutase 2 (SOD2),
glutathione S-transferase P1 (GSTP1) and aldo-keto reductase 1
subunits C-1 and 3 (AKR1C1 and AKR1C3) were significantly higher in
KKU-100 compared with KKU-213A (Fig.
1). Furthermore, the basal expression levels of Nrf2 and other
Nrf2-mediated cytoprotective genes, including PARK7, thioredoxin
TXN and particularly enzymes involved in glutathione synthesis,
including GCLC and GCLM, were not significantly different. These
results indicated that the mechanism of action for antioxidant
defense may depend on the genetic background of each CCA cell
line.
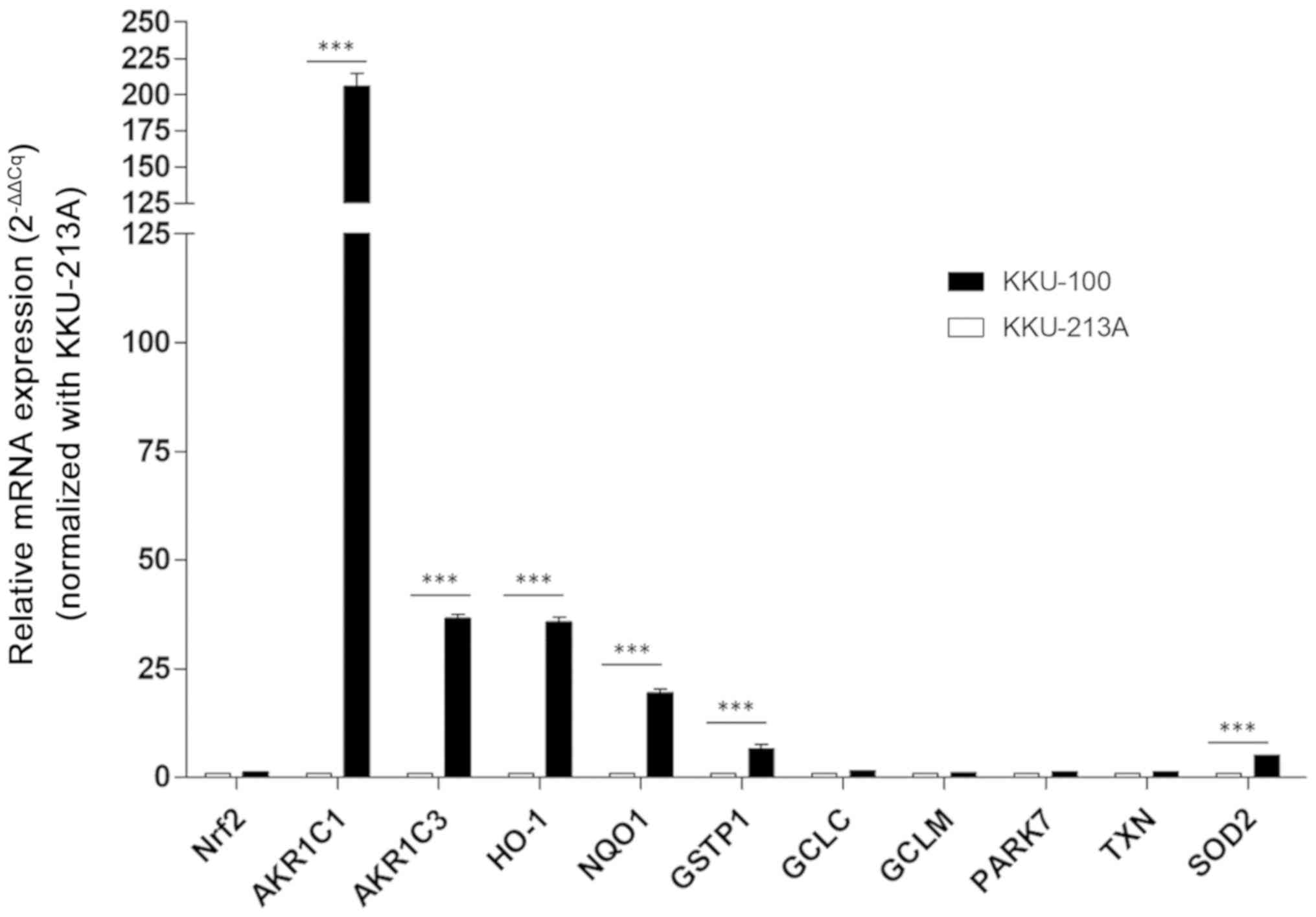 | Figure 1.Antioxidant expression profiles in
the cholangiocarcinoma KKU-100 and KKU-213A cell lines. mRNA
expression of all antioxidant-associated genes was assessed using
reverse transcription-quantitative PCR with normalization to
β-actin used as the reference gene. mRNA expression of all genes
was calculated using the 2−ΔΔCq method. Values are
expressed as the mean ± standard error of the mean of two
independent experiments. ***P<0.001 vs. KKU-213A. Nrf2, nuclear
factor erythroid 2-related factor 2; AKR1C1/3, aldo-keto reductase
1 subunits C-1/3; HO-1, heme oxygenase 1; NQO1, NADPH quinone
oxidoreductase-1; GSTP1, glutathione S-transferase P1; GCLC,
γ-glutamylcysteine synthetase catalytic subunit; GCLM,
γ-glutamylcysteine synthetase modifier subunit; PARK7, Parkinson
disease protein 7 precursor; TXN, thioredoxin; SOD2, superoxide
dismutase 2. |
Induction of HO-1 expression following
PL treatment
To evaluate the role of Nrf2-mediated cytoprotective
genes in the responses to PL treatment, CCA cell lines were treated
with 10 µM PL or DMSO (control) for 6 h. The respective mRNA
expression of Nrf2-mediated cytoprotective genes (AKR1C1, AKR1C3,
NQO-1, HO-1, GCLC and GCLM) was determined using RT-qPCR. The
results revealed that the expression levels of HO-1, GCLC and GCLM
were significantly increased in KKU-213A; however, only HO-1
expression was significantly increased in KKU-100 following PL
treatment. Therefore, HO-1 was the only Nrf2-mediated
cytoprotective gene whose expression was increased in both CCA cell
lines following PL treatment (Fig.
2A).
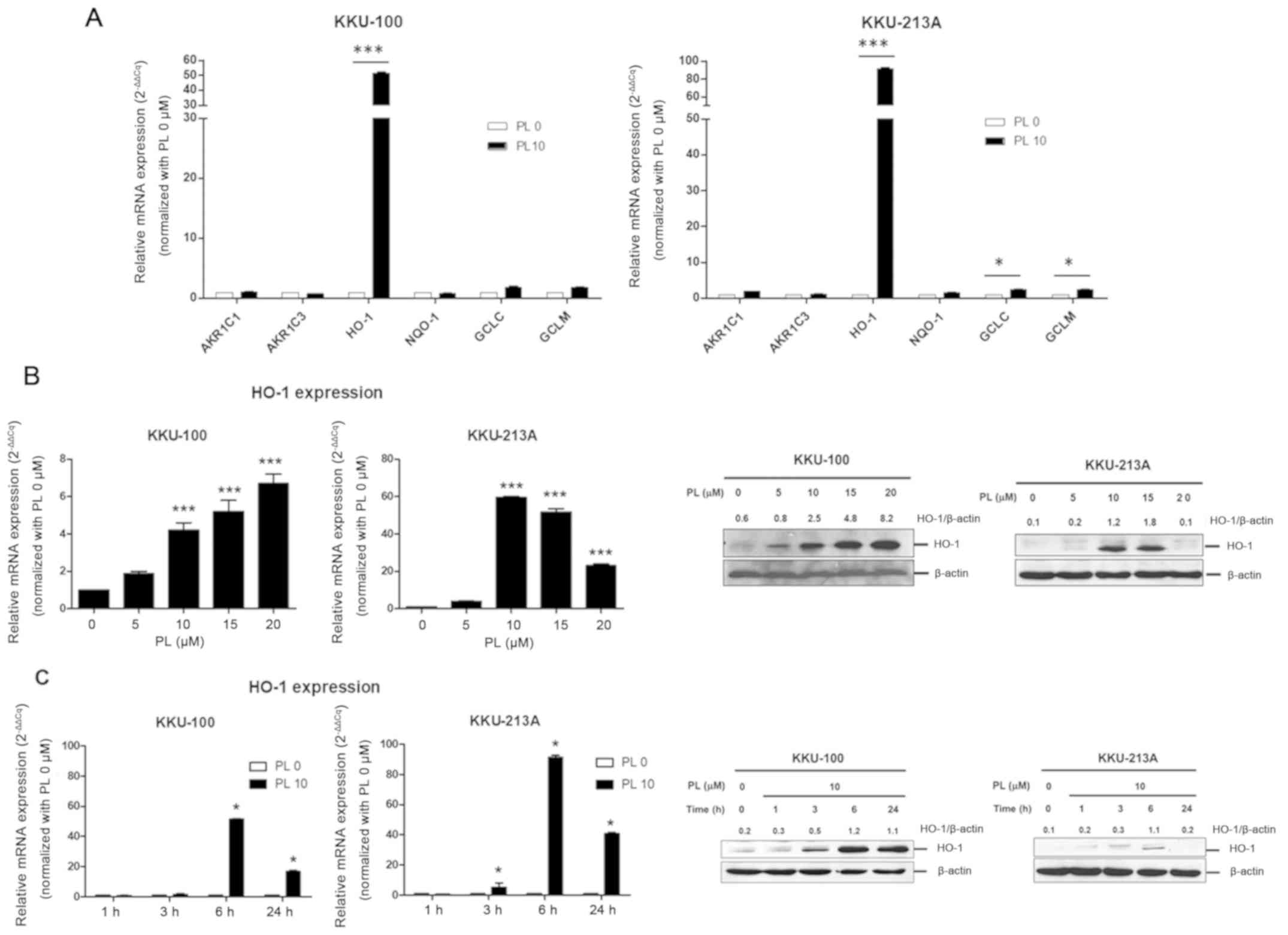 | Figure 2.PL induces HO-1 expression in
cholangiocarcinoma cell lines. (A) KKU-100 and KKU-213A were
treated with PL at 10 µM or DMSO (control) for 6 h and the mRNA
expression of AKR1C1, AKR1C3, NQO1, HO-1, GGCL and GCLM was
determined using RT-qPCR. Results were normalized using β-actin as
the reference gene. Relative mRNA expression was calculated using
the 2−∆∆Cq method. KKU-100 and KKU-213A treated with (B)
PL (at 0, 5, 10, 15 or 20 µM) for 24 h or (C) 10 µM PL for 0,1, 3,
6 or 24 h. The relative mRNA and protein expression of HO-1 was
determined using RT-qPCR (2−∆∆Cq) and western blot
analysis, respectively. Values are expressed as the mean ± standard
error of the mean of 3 independent experiments. Protein expression
is presented as the mean of two independent experiments. *P<0.05
and ***P<0.001 vs. PL 0 µM. PL, piperlongumine; AKR1C1 and 3,
aldo-keto reductase 1 subunits C-1 and 3; NQO1, NADPH quinone
oxidoreductase-1; HO-1, heme oxygenase 1; GCLC, γ-glutamylcysteine
synthetase catalytic subunit; GCLM, γ-glutamylcysteine synthetase
modifier subunit; RT-qPCR, reverse transcription-quantitative
PCR. |
Subsequently, HO-1 expression in the CCA cell lines
in the presence of PL at various concentrations (0, 5,10, 15 and 20
µM) for 24 h or 10 µM of PL for various lengths of time (0, 1, 3, 6
and 24 h) was examined. The expression of HO-1 at the mRNA and
protein levels in KKU-100 and KKU-213A cell lines following PL
treatment was altered in a dose-dependent manner; however, while
HO-1 expression increased in KKU-100, expression of HO-1 in
KKU-213A peaked at 10 µM and subsequently decreased (Fig. 2B). In addition, the induction of HO-1
expression increased in response to PL treatment for different
durations, particularly at 6 h and then declined following 24 h of
PL treatment (Fig. 2C). These
results indicated that PL preferentially triggered the induction of
HO-1 expression in CCA cell lines and that the activation of HO-1
expression may be an early antioxidant defense in response to PL
treatment.
HO-1 silencing promotes PL-induced CCA cell death by
increasing ROS accumulation. Subsequently, it was investigated
whether HO-1 acts as a key antioxidant defense for protecting from
PL-mediated ROS generation in CCA cells. ZnPP, a chemical HO-1
inhibitor, was utilized to inhibit HO-1 activity in KKU-100 and
KKU-213A. The effect of HO-1 inhibition was then evaluated with
respect to PL-induced cytotoxicity. As ZnPP acts as an enzymatic
substrate of HO-1, it competes with heme for HO-1, which leads to
decreasing levels of CO and bilirubin that act as an antioxidant
defense (28). As previous studies
have demonstrated that ZnPP is a cytotoxic agent and exhibits
anti-tumor activity (29–31), the cytotoxicity of ZnPP was examined.
The results demonstrated that >10 µM ZnPP suppressed CCA cell
viability, with <50% viable cells remaining in the KKU-100
following 3 h of treatment, whereas <50% cell viability in the
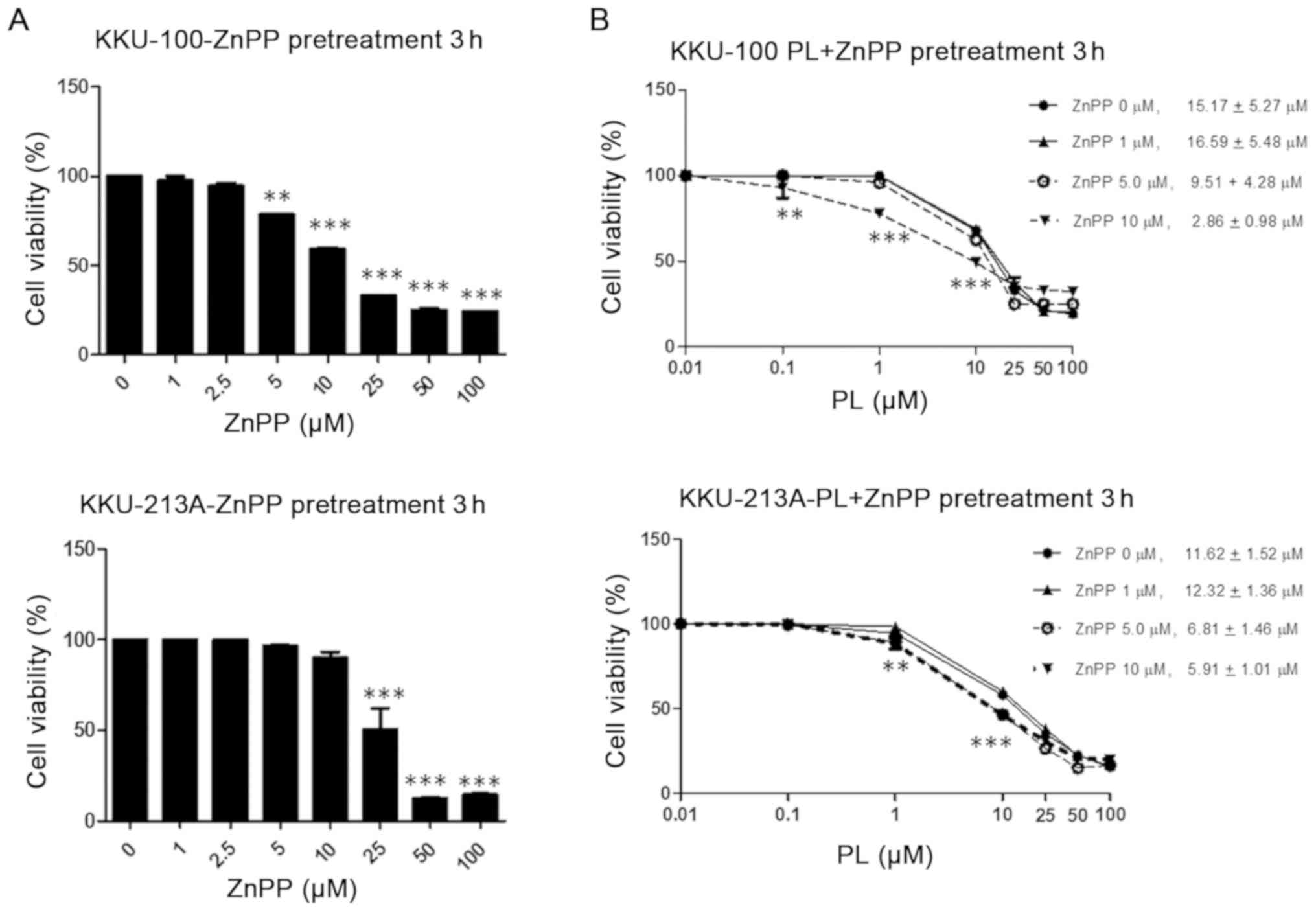
KKU-213A was observed at ZnPP >25 µM (Fig. 3A). Subsequently, cell lines were
pre-treated with 0–10 µM ZnPP for 3 h in combination with 0–100 µM
PL treatment to examine the effect of PL during HO-1 suppression by
ZnPP. The respective IC50 values of PL were reduced
compared with the combination of ZnPP at 5 and 10 µM in both
KKU-100 and KKU-213A cells (Fig.
3B).
Following this, HO-1 knockdown was used to elucidate
the role of HO-1 in the sensitivity of CCA cell lines to PL. The
mRNA expression levels of HO-1 in both KKU-100 and KKU-213A were
significantly decreased following transfection with HO-1 siRNA at
the time-points of 24 and 48 h (Fig.
4A); however, suppression of HO-1 was not influenced by CCA
cell growth (Fig. 4B). The knockdown
of HO-1 significantly enhanced the anti-tumor activity of PL in a
dose-dependent manner for both KKU-100 and KKU-213A (Fig. 4C). In addition, the combination of
HO-1-silencing with PL treatment at 20 µM resulted in a significant
increase in the accumulation of intracellular ROS. This result
indicated that the effective dose of PL to induce ROS accumulation
in KKU-100 was 20 µM (Fig. 4D).
Response to combination treatments in KKU-100 at 12 h was
detectable via western blot analysis, which confirmed the
upregulation of apoptotic proteins (cleaved PARP) and
downregulation of anti-apoptotic proteins (Bcl-2; Fig. 4E and F). Furthermore, the increase of
PL-induced CCA apoptosis through HO-1 suppression was clearly
demonstrated by a high ratio of cleaved PARP/Bcl-2 and a low ratio
of Bcl-2/Bax (Fig. 4F). Therefore,
inhibition of HO-1 promoted PL-mediated ROS generation, leading to
PL-induced CCA cell apoptosis.
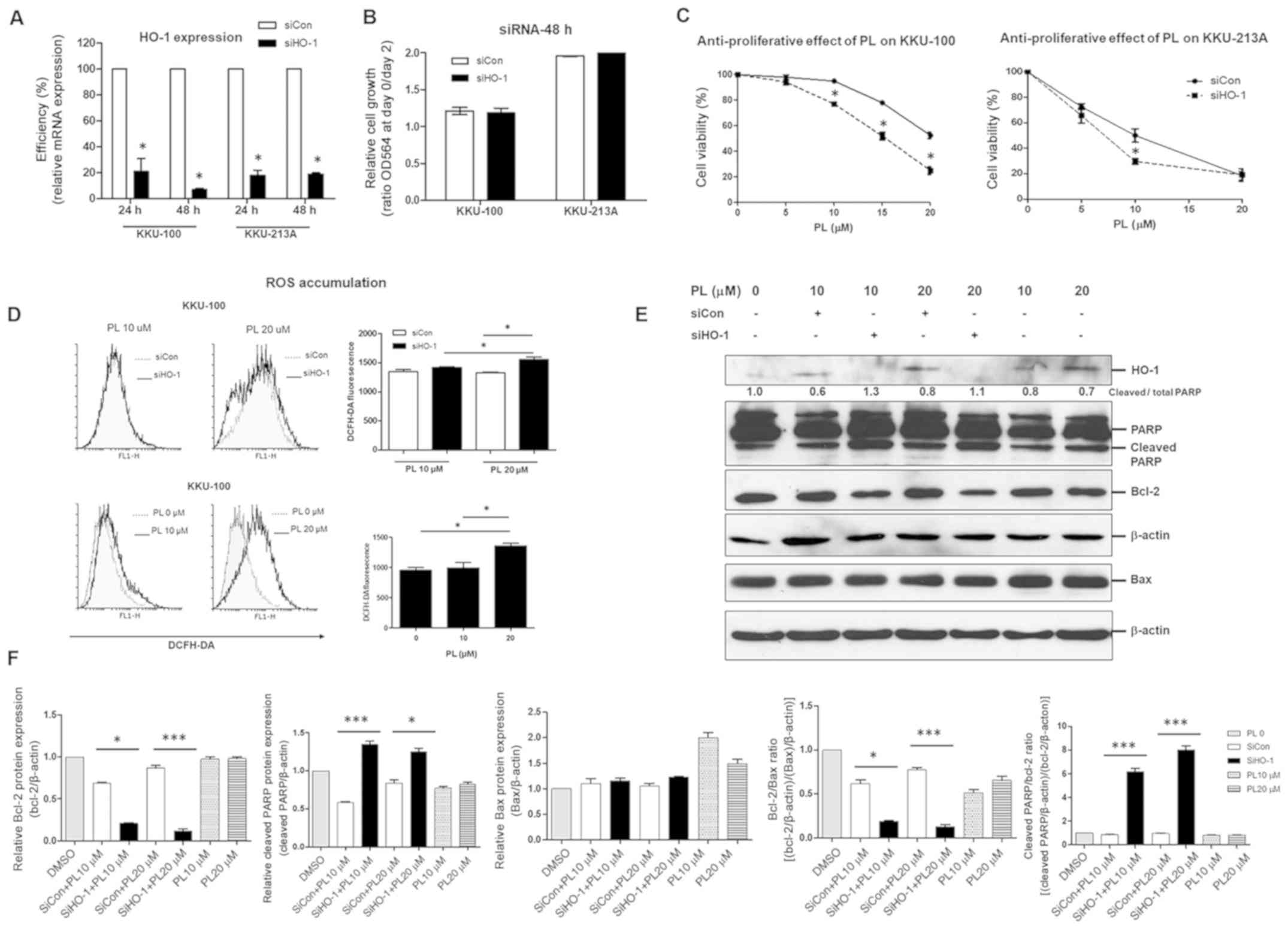 | Figure 4.Knockdown of HO-1 by siRNA sensitizes
cholangiocarcinoma cell lines to PL. KKU-100 and KKU-213A were
transfected with siHO-1 or siCon for 24 and 48 h. (A) The
efficiency of siHO-1 knockdown at 24 and 48 h relative to siCon was
determined using reverse transcription-quantitative PCR. (B)
Proliferative effect at 48 h and (C) the anti-proliferative effect
of PL at various concentrations at 24 h were determined. (D)
Reactive oxygen species accumulation at 3 (left graph) and 12 h
(right graph). (E) Apoptotic and anti-apoptotic proteins at 12 h
were determined and compared between HO-1-knockdown cells and
controls. (F) Relative protein levels of cleaved PARP and Bcl-2,
Bax and the Bcl-2/Bax ratio and cleaved PARP/Bcl-2 ratios were
determined. Values are expressed as the mean ± standard error of
the mean of 3 independent experiments. *P<0.05 and ***P<0.001
vs. siCon. HO-1, heme oxygenase 1; siRNA, small interfering RNA;
PL, piperlongumine; siHO-1, HO-1 siRNA; siCon, siRNA control
(scrambled); PARP, poly(ADP-ribose) polymerase; DCFH-DA,
2,7-dichlorodihydrofluorescein diacetate; OD564, optical density at
564 nm. |
PL induces Nrf2-mediated HO-1
expression via activation of the PI3K/Akt pathway
Previous studies have reported that the PI3K/Akt
pathway acts as a survival signal against multiple apoptotic
insults and is hypothesized to be a major upstream signaling event
prior to the induction of Nrf2-mediated HO-1 expression (32,33).
Furthermore, Lee et al (34)
demonstrated that PL directly binds to cysteine residues by a thiol
modification within kelch-like ECH-associated protein (Keap1) and
that this direct binding promoted nuclear translocation of Nrf2 and
subsequent upregulation of HO-1 expression. The
activation/phosphorylation of Akt in PL-treated CCA cell lines was
determined in order to elucidate the role of PI3K/Akt in PL-induced
Nrf2 activation and HO-1 expression. HO-1 and Akt phosphorylation
increased in response to PL treatment (Fig. 5A). Nuclear Nrf2 was also observed in
a dose-dependent manner (5, 10 and 20 µM; Fig 5A). To demonstrate the association
between PI3K/Akt signaling and HO-1 expression, various
concentrations (1, 2 and 5 µM) of wortmannin, a specific inhibitor
of PI3K, were used to inhibit PI3K/Akt activation. The level of
HO-1 expression was then determined via western blot analysis.
Wortmannin significantly inhibited Akt phosphorylation and
PL-induced HO-1 expression in CCA cells in a dose-dependent manner
(Fig. 5B). These results indicated
that PL-induced HO-1 expression is stimulated via Nrf2/PI3K/Akt
activation.
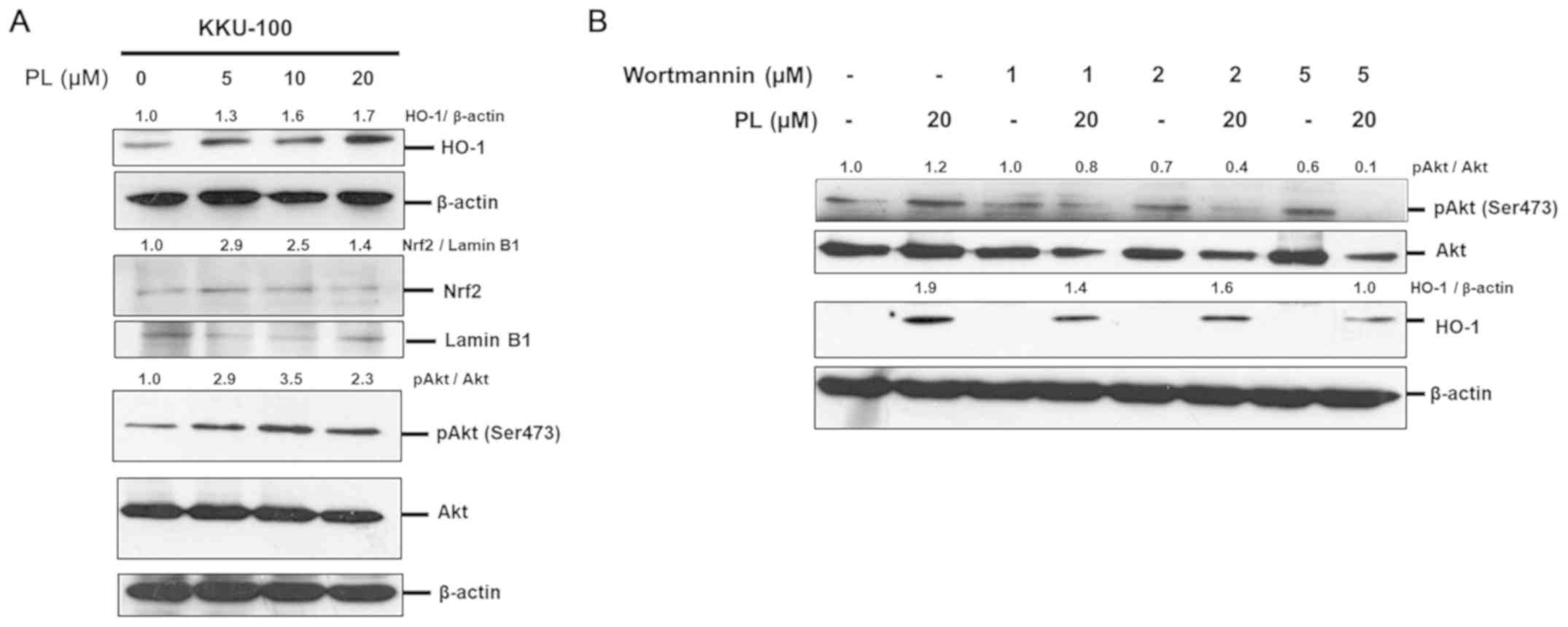 | Figure 5.PL induces HO-1 expression via
PI3K/Akt activation. (A) KKU-100 was treated with various
concentrations of PL (0, 5, 10 or 20 µM) for 3 h. Whole-cell
lysates (for HO-1, Akt and β-actin) and nuclear extracts (for Nrf2
and Lamin B1) were analyzed via western blotting and probed with
antibodies specific to HO-1, Akt, pAkt (Ser473), Nrf2 and β-actin.
(B) The effects of the Akt signaling inhibitor wortmannin on
PL-induced HO-1 expression were determined. KKU-100 was pre-treated
with various concentrations of wortmannin (1, 2 and 5 µM) for 2 h
and treated with 20 µM PL for an additional 24 h. The expression of
HO-1, Akt, pAkt and β-actin were determined via western blot
analysis. Values are expressed as the mean of 2 independent
experiments. PL, piperlongumine; pAkt, phosphorylated Akt; HO-1,
heme oxygenase 1; Nrf2, nuclear factor erythroid 2-related factor
2. |
Discussion
PL exerts an anti-tumor effect on various types of
cancer, including glioblastoma, lung and CCA (14,15,35). PL
inhibits tumor growth via the induction of ROS accumulation and the
activation of MAPKs (including JNK, ERK and p38) (14,15) or
the inhibition of the PI3K/Akt pathway (35,36).
Sensitivity to PL varies among different cancer cell lines,
including breast cancer and CCA cell lines (15,34). The
present study aimed to investigate the underlying mechanisms of
PL-induced HO-1 expression. Several previous studies have
demonstrated that HO-1 has a powerful cytoprotective effect against
various apoptotic insults in normal and cancer cells (19–23). The
basal HO-1 expression levels of each type of cancer cell indicate
its sensitivity to chemotherapeutic agents (20,21).
Similar observations were reported for CCA cells treated with PL,
as cells with a low basal level of HO-1 expression were more
sensitive to PL compared with those with high HO-1 expression
(15). In addition, in the present
study, HO-1 inducible expression was evident following 3 and 6 h of
PL treatment in all CCA cell lines. This result indicated that
induction of HO-1 expression serves a vital role in the early
protection against the PL-induced reaction to oxidative stress. The
results of the present study are consistent with those of studies
on well-known chemotherapeutic agents (including gemcitabine and
cisplatin), which mediate oxidative stress and lead to induced HO-1
expression in CCA and laryngeal squamous cell cancer (20,23).
The PI3K/Akt pathway is involved in cellular
survival, metastasis and drug resistance in various types of cancer
including CCA, pancreatic and oral cancer (37–41).
Development of acquired resistance to radiation, chemotherapy
and/or targeted therapy has been revealed to be associated with the
induction of the PI3K/Akt pathway (41,42). In
addition, PI3K/Akt signaling is a key pathway for the activation of
HO-1 through the Nrf2/Keap pathway. Certain studies have
demonstrated that PL bears two electrophilic α,β-unsaturated
carbonyl groups that cause oxidation or covalent modification of
cysteine residues within Keap1. PL directly binds to cysteine
residues within Keap1 by thiol modification (34). This direct binding was observed to
promote nuclear translocation of Nrf2 and subsequent upregulation
of HO-1 expression (34,40). Inhibition of this pathway has been
proposed to increase the chemosensitivity of CCA (38,43). The
present study revealed that PL treatment resulted in Akt
phosphorylation and Nrf2 activation, suggesting that PL induced
HO-1 expression via PI3K/Akt/Nrf2 activation. However, these
results are preliminary and should be confirmed in a future study.
In contrast to PL-induced antioxidant defense in CCA, PL stimulated
oxidative stress via ROS generation and induced CCA apoptosis
through the activation of the ROS/JNK/ERK pathway (15). These results indicated that in an
oxidative stress environment, i.e. that caused by PL, CCA cells
upregulate anti-oxidant defenses by inducing PI3K/Akt-mediated HO-1
expression and enhance the anti-apoptotic capacity in order to
protect against PL-induced ROS generation. As Keap1 was not
assessed in PL-induced Nrf2 activation and HO-1 expression in the
present study, investigating the effect of PL on the Nrf2/Keap1
pathway in CCA is required for further study.
HO-1 is a powerful antioxidant enzyme in
Nrf2-mediated cytoprotective responses and serves a role in the
malignant transformation of cancer cells (10). High levels of HO-1 are associated
with the progression of CCA and its therapeutic resistance
(20,21). Concurrently, suppression of HO-1
activity has been observed to enhance the chemosensitivity of
cancer cells to various chemotherapeutic agents including cisplatin
and gemcitabine (20,23,44). The
results of the present study demonstrated that inhibition of HO-1
activity using chemical inhibitors or specific siRNA to HO-1
increased the anti-tumor activity of PL against CCA cell lines
through an increase in intracellular ROS accumulation and increased
CCA cell death via upregulation of apoptotic proteins. In the
present study, the increase of intracellular ROS was not
significantly different between siCon KKU-100 cells treated with PL
at 10 and 20 µM. This may be due to the insufficient concentration
of PL, as the IC50 value of PL in KKU-100 is 15.9 µM at
24 h (15), or PL may have altered
the activity of a redox-sensitive enzyme/transcription protein. In
summary, the results of the present study suggest that induction of
HO-1 expression may provide a significant antioxidant defense to PL
treatment and the level of basal HO-1 expression indicates the
efficiency of PL for treating CCA. It should be noted that the CCA
cell lines used in the present study were established from tumors
of patients with CCA with liver-fluke infections. Therefore, the
results may not be generalizable to patients with non-liver
fluke-associated CCAs. From a technical point, the experiments
should ideally be performed three times. However, the determination
of protein expression via western blot analysis was performed only
twice. This limitation could influence the interpretation of the
findings; thus, future studies are required to confirm.
In conclusion, in a PL-induced oxidative stress
environment, PI3K/Akt-mediated HO-1 activation served an important
role in antioxidant defenses, thereby protecting CCA from
PL-induced apoptosis. The results demonstrated that suppression of
HO-1 resulted in increased intracellular ROS generation and CCA
cell apoptosis induced by PL. The present results provide strong
evidence that the mechanism of PL-induced HO-1 expression in CCA
and inhibition of HO-1 may be a potential strategy for increasing
the chemosensitivity of CCA to PL.
Acknowledgements
The authors would like to thank Mr. Bryan Roderick
Hamman (Aegis of the Khon Kaen University Publication Clinic, Khon
Kaen, Thailand) for his assistance in translating the manuscript
into English.
Funding
The present study was supported by the Young
Research Grants, Thailand Research Fund (grant no. MRG6080055 to
CT).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CT and SW conceived and designed the present study.
CT performed the experiments. CT, KT and SW analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al British Society of Gastroenterology, :
Guidelines for the diagnosis and treatment of cholangiocarcinoma:
An update. Gut. 61:1657–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhudhisawasdi V, Talabnin C, Pugkhem A,
Khuntikeo N, Seow OT, Chur-in S, Pairojkul C and Wongkham S:
Evaluation of postoperative adjuvant chemotherapy for intrahepatic
cholangiocarcinoma patients undergoing R1 and R2 resections. Asian
Pac J Cancer Prev. 13:169–174. 2012.PubMed/NCBI
|
|
3
|
Dhanasekaran R, Hemming AW, Zendejas I,
George T, Nelson DR, Soldevila-Pico C, Firpi RJ, Morelli G, Clark V
and Cabrera R: Treatment outcomes and prognostic factors of
intrahepatic cholangiocarcinoma. Oncol Rep. 29:1259–1267. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagino M, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Takahashi Y and Nimura Y: Evolution of surgical
treatment for perihilar cholangiocarcinoma: A single-center 34-year
review of 574 consecutive resections. Ann Surg. 258:129–140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagorney DM, Donohue JH, Farnell MB,
Schleck CD and Ilstrup DM: Outcomes after curative resections of
cholangiocarcinoma. Arch Surg. 128:871–879. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thongprasert S: The role of chemotherapy
in cholangiocarcinoma. Ann Oncol. 16 (Suppl 2):ii93–ii96. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fodale V, Pierobon M, Liotta L and
Petricoin E: Mechanism of cell adaptation: When and how do cancer
cells develop chemoresistance? Cancer J. 17:89–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: an evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pelicano H, Carney D and Huang P: ROS
stress in cancer cells and therapeutic implications. Drug Resist
Updat. 7:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan J, Lo M, Dockery P, Mahon S, Karp CM,
Buckley AR, Lam S, Gout PW and Wang YZ: The xc-cystine/glutamate
antiporter as a potential therapeutic target for small-cell lung
cancer: Use of sulfasalazine. Cancer Chemother Pharmacol.
64:463–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montero AJ, Diaz-Montero CM, Deutsch YE,
Hurley J, Koniaris LG, Rumboldt T, Yasir S, Jorda M, Garret-Mayer
E, Avisar E, et al: Phase 2 study of neoadjuvant treatment with
NOV-002 in combination with doxorubicin and cyclophosphamide
followed by docetaxel in patients with HER-2 negative clinical
stage II–IIIc breast cancer. Breast Cancer Res Treat. 132:215–223.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu JM, Pan F, Li L, Liu OR, Chen Y, Xiong
XX, Cheng K, Yu SB, Shi Z, Yu CH, et al: Piperlongumine selectively
kills glioblastoma multiforme cells via reactive oxygen species
accumulation dependent JNK and p38 activation. Biochem Biophys Res
Commun. 437:87–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thongsom S, Suginta W, Lee KJ, Choe H and
Talabnin C: Piperlongumine induces G2/M phase arrest and apoptosis
in cholangiocarcinoma cells through the ROS-JNK-ERK signaling
pathway. Apoptosis. 22:1473–1484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raj L, Ide T, Gurkar AU, Foley M, Schenone
M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al:
Selective killing of cancer cells by a small molecule targeting the
stress response to ROS. Nature. 475:231–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maines MD and Abrahamsson PA: Expression
of heme oxygenase-1 (HSP32) in human prostate: normal,
hyperplastic, and tumor tissue distribution. Urology. 47:727–733.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodman AI, Choudhury M, da Silva JL,
Schwartzman ML and Abraham NG: Overexpression of the heme oxygenase
gene in renal cell carcinoma. Proc Soc Exp Biol Med. 214:54–61.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin H, Fang J, Liao L, Maeda H and Su Q:
Upregulation of heme oxygenase-1 in colorectal cancer patients with
increased circulation carbon monoxide levels, potentially affects
chemotherapeutic sensitivity. BMC Cancer. 14:4362014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kongpetch S, Kukongviriyapan V, Prawan A,
Senggunprai L, Kukongviriyapan U and Buranrat B: Crucial role of
heme oxygenase-1 on the sensitivity of cholangiocarcinoma cells to
chemotherapeutic agents. PLoS One. 7:e349942012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kongpetch S, Puapairoj A, Ong CK,
Senggunprai L, Prawan A, Kukongviriyapan U, Chan-On W, Siew EY,
Khuntikeo N, Teh BT, et al: Haem oxygenase 1 expression is
associated with prognosis in cholangiocarcinoma patients and with
drug sensitivity in xenografted mice. Cell Prolif. 49:90–101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furfaro AL, Piras S, Passalacqua M,
Domenicotti C, Parodi A, Fenoglio D, Pronzato MA, Marinari UM,
Moretta L, Traverso N, et al: HO-1 up-regulation: A key point in
high-risk neuroblastoma resistance to bortezomib. Biochim Biophys
Acta. 1842:613–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv X, Song DM, Niu YH and Wang BS:
Inhibition of heme oxygenase-1 enhances the chemosensitivity of
laryngeal squamous cell cancer Hep-2 cells to cisplatin. Apoptosis.
21:489–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sripa B, Leungwattanawanit S, Nitta T,
Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C and Miwa M:
Establishment and characterization of an opisthorchiasis-associated
cholangiocarcinoma cell line (KKU-100). World J Gastroenterol.
11:3392–3397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sripa B, Seubwai W, Vaeteewoottacharn K,
Sawanyawisuth K, Silsirivanit A, Kaewkong W, Muisuk K, Dana P,
Phoomak C, Lert-Itthiporn W, et al: Functional and genetic
characterization of three cell lines derived from a single tumor of
an Opisthorchis viverrini-associated cholangiocarcinoma patient.
Hum Cell. Mar 23–2020.(Epub ahead of print). doi:
10.1007/s13577-020-00334-w. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Voigt W: Sulforhodamine B assay and
chemosensitivity. Methods Mol Med. 110:39–48. 2005.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Labbé RF, Vreman HJ and Stevenson DK: Zinc
protoporphyrin: A metabolite with a mission. Clin Chem.
45:2060–2072. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Avery JE, Hannafon BN, Lind SE and
Ding WQ: Zinc protoporphyrin suppresses cancer cell viability
through a heme oxygenase-1-independent mechanism: The involvement
of the Wnt/β-catenin signaling pathway. Biochem Pharmacol.
85:1611–1618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu YS, Li HS, Qi DF, Zhang J, Jiang XC,
Shi K, Zhang XJ and Zhang XH: Zinc protoporphyrin IX enhances
chemotherapeutic response of hepatoma cells to cisplatin. World J
Gastroenterol. 20:8572–8582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng CC, Guan SS, Yang HJ, Chang CC, Luo
TY, Chang J and Ho AS: Blocking heme oxygenase-1 by zinc
protoporphyrin reduces tumor hypoxia-mediated VEGF release and
inhibits tumor angiogenesis as a potential therapeutic agent
against colorectal cancer. J Biomed Sci. 23:182016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin D, Rojo AI, Salinas M, Diaz R,
Gallardo G, Alam J, Galarreta CM and Cuadrado A: Regulation of heme
oxygenase-1 expression through the phosphatidylinositol
3-kinase/Akt pathway and the Nrf2 transcription factor in response
to the antioxidant phytochemical carnosol. J Biol Chem.
279:8919–8929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen HH, Chen YT, Huang YW, Tsai HJ and
Kuo CC: 4-Ketopinoresinol, a novel naturally occurring ARE
activator, induces the Nrf2/HO-1 axis and protects against
oxidative stress-induced cell injury via activation of PI3K/AKT
signaling. Free Radic Biol Med. 52:1054–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HN, Jin HO, Park JA, Kim JH, Kim JY,
Kim B, Kim W, Hong SE, Lee YH, Chang YH, et al: Heme oxygenase-1
determines the differential response of breast cancer and normal
cells to piperlongumine. Mol Cells. 38:327–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang F, Mao Y, You Q, Hua D and Cai D:
Piperlongumine induces apoptosis and autophagy in human lung cancer
cells through inhibition of PI3K/Akt/mTOR pathway. Int J
Immunopathol Pharmacol. 28:362–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou L, Li M, Yu X, Gao F and Li W:
Repression of hexokinases II-mediated glycolysis contributes to
piperlongumine-induced tumor suppression in non-small cell lung
cancer cells. Int J Biol Sci. 15:826–837. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yokoi K, Kobayashi A, Motoyama H, Kitazawa
M, Shimizu A, Notake T, Yokoyama T, Matsumura T, Takeoka M and
Miyagawa SI: Survival pathway of cholangiocarcinoma via AKT/mTOR
signaling to escape RAF/MEK/ERK pathway inhibition by sorafenib.
Oncol Rep. 39:843–850. 2018.PubMed/NCBI
|
|
38
|
Yoon H, Min JK, Lee JW, Kim DG and Hong
HJ: Acquisition of chemoresistance in intrahepatic
cholangiocarcinoma cells by activation of AKT and extracellular
signal-regulated kinase (ERK)1/2. Biochem Biophys Res Commun.
405:333–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yothaisong S, Dokduang H, Techasen A,
Namwat N, Yongvanit P, Bhudhisawasdi V, Puapairoj A, Riggins GJ and
Loilome W: Increased activation of PI3K/AKT signaling pathway is
associated with cholangiocarcinoma metastasis and PI3K/mTOR
inhibition presents a possible therapeutic strategy. Tumour Biol.
34:3637–3648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen HH, Chen YT and Huang YW:
4-Ketopinoresinol, a novel naturally occurring ARE activator,
induces the Nrf2/HO-1 axis and protects against oxidative
stress-induced cell injury via activation of PI3K/AKT signaling.
Free Radic Biol Med. 52:1054–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arlt A, Gehrz A, Muerkoster S, Vorndamm J,
Kruse ML, Folsch UR and Schäfer H: Role of NF-kappaB and Akt/PI3K
in the resistance of pancreatic carcinoma cell lines against
gemcitabine-induced cell death. Oncogene. 22:3243–3251. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang WC and Hung MC: Induction of Akt
activity by chemotherapy confers acquired resistance. J Formos Med
Assoc. 108:180–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leelawat K, Narong S, Udomchaiprasertkul
W, Leelawat S and Tungpradubkul S: Inhibition of PI3K increases
oxaliplatin sensitivity in cholangiocarcinoma cells. Cancer Cell
Int. 9:32009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Berberat PO, Dambrauskas Z, Gulbinas A,
Giese TG, Kunzli B, Autschbach F, Meuer S, Büchler MW and Friess H:
Inhibition of heme oxygenase-1 increases responsiveness of
pancreatic cancer cells to anticancer treatment. Clin Cancer Res.
11:3790–3798. 2005. View Article : Google Scholar : PubMed/NCBI
|