Introduction
Solitary fibrous tumor (SFT) is a rare neoplasm that
generally arises from the pleura or, more rarely, from other
serosal membranes. The literature reports different extrapleural
sites, which supports the hypothesis of a submesenchymal origin
instead of a mesothelial origin (1–4).
Extrapleural SFT are fibroblastic/myofibroblastic
neoplasms, rarely metastasizing and with intermediate biological
behavior (5). The wide heterogeneity
of denominations used for this neoplasm is simply the
representation of its much-disputed histogenesis; indeed, various
benign and malignant tumors may share the same growth pattern. The
haemangiopericytoma (HPC)-like features are common to three
categories of neoplasms: Non-HPC neoplasms (e.g., synovial
sarcoma), occasionally displaying HPC-like features; true HPCs
(glomangiopericytoma⁄myopericytoma, infantile myofibromatosis) with
evident myoid-pericytic differentiation; and the SFT group (giant
cell angiofibromas, lipomatous HPCs) (6). The World Health Organization (WHO)
considered HPC as histological variant of SFT and no longer as
separate entities, due to recent findings about its
immunohistochemical and molecular features. Hence, according to
WHO, HPC is currently a cellular phenotypic variant of SFT that is
a single spectrum of mesenchymal tumors. These fibroblastic
lesions, usually pertain to adults and can occur at any site
(7).
Although the etiology of SFT remains still
undisclosed, the pathogenesis is related to the NAB2-STAT6 gene
fusion caused by paracentric inversion on chromosome 12q13.
NAB2-STAT6 gene encodes a chimeric protein which activates ERG1 and
seems to deregulate ERG1-dependent target genes (4,5,7).
From clinical point of view, SFT is a slow-growing
mass in the fifth and sixth decades, with no sex predilection and
may be responsible for paraneoplastic syndromes and in particular,
for hypoglycemia probably caused by the production of an
insulin-like growth factor (6).
Although most SFTs have a good prognosis, about 10–40% of cases
relapse or metastasize (3,4,8). For
instance, the clinically aggressive behavior is related to
pathological criteria of malignancy. However, a clear correlation
between hystology and clinical behavior has not been identified
yet. Furthermore, considering its rarity, there are no guidelines
and the course of SFT remains still unpredictable (3,4,8).
SFT has been described in a wide variety of atypical
extrapleural sites of mesenchymal origin, among which head and neck
regions, para-pharyngeal space, tongue, larynx, and parotid gland.
The presence of SFTs in the sino-nasal cavities are unusual;
indeed, very few cases are described in the literature (3). Immunohistochemical pattern is useful to
differentiate SFT from other soft tissue tumors (9–11). In
particular, these lesions have 2 main characteristics, the solid
spindle and the diffuse sclerosing types, usually within the same
lesion. Though chemo-radiotherapy have been used to control the
tumor, surgery remains the treatment of choice (12). We report a very rare case of a large
extrapleural SFT of the nasal septum undergoing endoscopic
surgery.
Case report
A 55-year-old man presented at ENT Unit of ‘Federico
II’ University complaining right-sided nasal obstruction, discharge
and recurrent epistaxis for 2-years. He reported a history of
cigarette smoking, hypertension, diabetes mellitus type II,
hypercholesterolemia, and previous iliac artery percutaneous
transluminal angioplasty and stenting.
There was no palpable latero-cervical
lymphadenopathy and the ultrasound of the neck was negative. Nasal
endoscopy revealed a rough reddish bleeding mass with implantation
point on the septum occupying the right nasal fossa. Computed
Tomography (CT) scan confirmed the presence of the right nasal mass
without invading the anterior cranial fossa, signs of bone
destruction and remodeling, or distant metastases. Magnetic
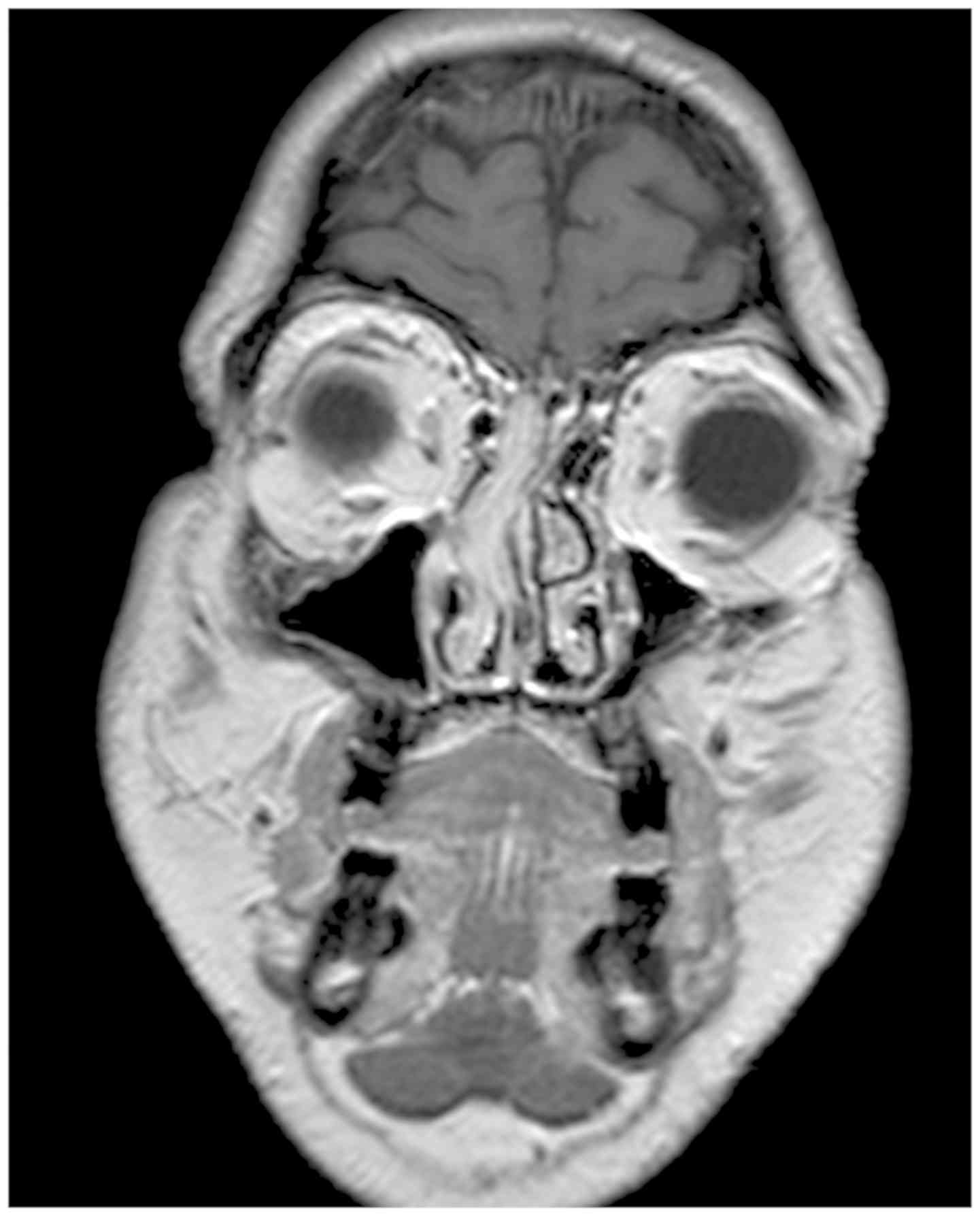
resonance imaging (MRI) showed a lesion in the right nasal fossa
with a hypointense signal on T1-weighted images and hyperintense
T2-weighted right-sided nasal mass (Fig.
1) (13). The neoplasm showed a
prominent and non-homogeneous enhancement after gadolinium
injection.
Due to the abundant vascularization of the lesion
revealed by imaging, angiographic study was performed. It showed an
arterial supply from the anterior ethmoidal artery, so a selective
embolization was performed.
A diagnostic biopsy of the mass was carried out
under local anesthesia. Histologic investigation revealed a
low-grade mesenchymal neoplasm, morphologically and
immunophenotypically accordant with the diagnosis of nasal SFT.
Subsequently, the patient underwent surgery under general
anesthesia to remove the lesion, endonasal endoscopic approach was
performed. The insertion of tumor on the right anterior-superior
region of the nasal septum was identified and the mass was
completely removed, achieving a mucosal flap with
sub-muchoperichondrium incision.
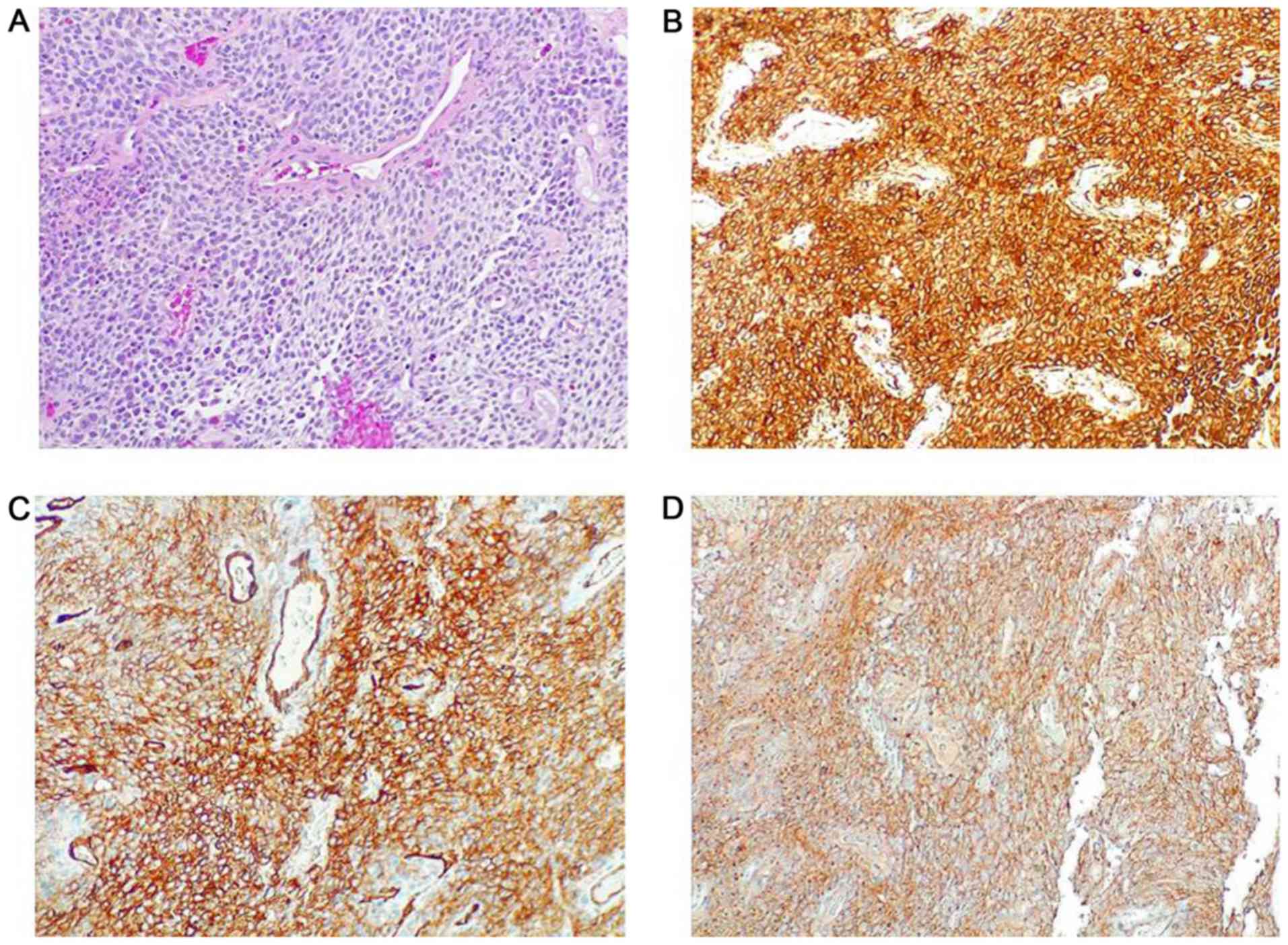
Histology revealed a homogeneous and diffuse
proliferation of spindle cells that alternated hypercellular and
less cellular areas with collagenized stroma, characterized by mild
pleomorphism and more than occasional mitoses; necrosis was absent.
The tumour showed a prominent vascular network consisting of
branching vessels with a slightly thickened wall. The
immunohistochemistry showed positivity of neoplastic cells for
vimentin, CD34 and CD99 and negativity for S100 protein, desmin,
pan-cytokeratin, smooth muscle actin and LCA. The proliferation
index Ki-67 was less than 5%. The morphology and immunophenotype
strongly suggested a solitary fibrous tumour (Fig. 2).
The postoperative course did not show complications
and the patient was discharged within 24 h. After 2 years of
follow-up, no relapse was observed at endoscopy and imaging
studies.
Discussion
SFT is a slow-growing, well-encapsulated spindle
cell tumor resembling fibro-sarcoma. However, unlike fibrosarcoma,
SFT did not show any sign of metastasis or infiltration. Increasing
evidence suggested a mesenchymal origin of these tumors (1). Extrapleural localization (including the
oral cavity and orbit) is rare and head and neck involvement is
exceptional, approximately 5–27% (3). SFT rarely involves the nasal fossa and
the paranasal sinuses, so SFT is sometimes not distinguishable from
other mesenchymal lesions (3,9–11). To our knowledge, a limited number of
SFT involving sino-nasal cavities have been described. A review of
English literature has identified about 92 cases of sino-nasal
area, most of them provided by single case report (3). Males and females are affected equally.
Diagnosis of sino-nasal SFT is based on nasal endoscopy and
imaging, CT assesses tumor extension and bone resorption, whereas
MRI assesses orbit or endocranial extension. Particularly, CT shows
a well-delineate isodense mass with heterogeneous contrast
enhancement, whereas MR shows a hypo- or isointense on T1-weighted
mass and hypo- or, hyperintense on T2-weighted mass, with
heterogeneous contrast enhancement after contrast (13). The gold standard of therapy is
surgery, endoscopic sinus surgery with elevation and resection of
the periosteum of the tumor-contacting bone allows a complete
resection. Preoperative embolization of the mass may decrease
bleeding, although some authors reported rare inta/post-operative
bleeding (14). Additional
therapeutic options are sole radiotherapy, chemotherapy or
embolization (14). SFTs are
capsulated tumors, composed of cytologically bland spindle cells
set in a collagenous stroma. Immunohistochemical analysis shows
cells positive for CD34, vimentin and frequently bcl-2, and
negative for keratin, desmin and S100 protein (1,3,5,15).
Disease progression, relapse or metastasis, can be
rarely found. Pathologic criteria for malignancy are not well
determinated. The WHO classifies SFT as malignant based on the
presence of hypercellularity, increased mitoses (>4 mitoses per
10 high power fields), cytological atypia, tumor necrosis, and/or
infiltrative margins (3). For
instance, histo-pathology seems to be an imperfect predictor of
clinical behavior. Precisely, some tumors with morphologic
parameters suggestive of malignancy would behave as benign mass.
Recurrence and metastases may lack atypical histologic features
(3).
In addition, other models considering a combination
of various patient characteristics as age (≥55 years), tumor size
(>10 or ≥15 cm), and mitotic index (>4 mitoses per 10 high
power fields), or tumor characteristics as mitotic rate,
cellularity, and pleomorphism have been proposed as behavior
predictor of SFTs (3). Recent
studies point out the predictive role of telomerase reverse
transcriptase (TERT) promoter mutations as molecular marker of
prognosis in SFT high-risk patients. The combination of TERT
promoter mutations and other clinicopathologic risk features might
improve the accuracy of predicting outcomes in patients with SFT
(7).
In this report we presented a very rare case of SFT
involving the nasal septum undergoing surgical endoscopy and
disease free after 24 months follow up.
Although previous authors reported clinical cases of
nasal SFT, these cases did not present an exclusive nasal septal
localization and did not perform embolization before endoscopic
surgery. Other surgeons performed an open surgical technique.
Furthermore, in the report of Zielinska-Kazmierska and in the
report of Chauhan the patient did not perform MRI (16–18).
Our patient reported a 2 years history of symptoms
in line with literature, in which the symptoms before diagnosis
ranged from 25 days to 24 months, with an average of 9.9 months
(3).
Interestingly, some of these tumors are recognized
to be associated with systemic hypoglycemia (6).
Unlikely, our patient was suffering from diabetes,
therefore it could be hypothesized that the disease was, as well,
related to impaired glucose metabolism or we can speculate that
therapy with hypoglycemic agents could have caused hypoglycemia as
risk factor for STF.
In conclusion, in our opinion the diagnosis of
sino-nasal STF is challenging and the prognosis is even more
demanding. We, therefore, proposed integrating the assessment of
TERT promoter mutational status to improve risk prediction in
patients with sino-nasal STF, in addition to nasal endoscopy,
imaging and histopathological examination. We think that a careful
evaluation of blood glucose levels is also helpful.
Furthermore, in our experience, preoperative
embolization has been useful not only to optimize the surgical
resection but also as additional therapeutic option per se,
although many authors do not consider it necessary for the purpose
of controlling intraoperative bleeding (14).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
EC conceived and designed this case report. MC, EP,
CDN and EC wrote the initial draft of the report. EP, MC, GM, MM
and CDN collected data and wrote the initial draft. AMDL, SA and MI
acquired the data in the diagnostic imaging. MM acquired staining
and histological images. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent for surgery was obtained
from the patient.
Patient consent for publication
Written informed consent for publication of the
present report was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yalcin CE and Tihan T: Solitary fibrous
tumor/hemangiopericytoma dichotomy revisited: A restless family of
neoplasms in the CNS. Adv Anat Pathol. 23:104–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ronchi A, Cozzolino I, Zito Marino F,
Accardo M, Montella M, Panarese I, Roccuzzo G, Toni G, Franco R and
De Chiara A: Extrapleural solitary fibrous tumor: A distinct entity
from pleural solitary fibrous tumor. An update on clinical,
molecular and diagnostic features. Ann Diagn Pathol. 34:142–150.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thompson LDR and Lau SK: Sinonasal tract
solitary fibrous tumor: A clinicopathologic study of six cases with
a comprehensive review of the literature. Head Neck Pathol.
12:471–480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Demicco EG, Park MS, Araujo DM, Fox PS,
Bassett RL, Pollock RE, Lazar AJ and Wang WL: Solitary fibrous
tumor: A clinicopathological study of 110 cases and proposed risk
assessment model. Mod Pathol. 25:1298–1306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robinson DR, Wu YM, Kalyana-Sundaram S,
Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, et al:
Identification of recurrent NAB2-STAT6 gene fusions in solitary
fibrous tumor by integrative sequencing. Nat Genet. 45:180–185.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gengler C and Guillou L: Solitary fibrous
tumour and haemangiopericytoma: Evolution of a concept.
Histopathology. 48:63–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bahrami A, Lee S, Schaefer IM, Boland JM,
Patton KT, Pounds S and Fletcher CD: TERT promoter mutations and
prognosis in solitary fibrous tumor. Mod Pathol. 29:1511–1522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang SC and Huang HY: Solitary fibrous
tumor: An evolving and unifying entity with unsettled issues.
Histol Histopathol. 34:313–314. 2019.PubMed/NCBI
|
|
9
|
Cantone E, Borzillo V, Di Lullo AM, Marano
L, Guadagno E, Mansueto G, Di Franco R, Cammarota F, Catalano L,
Muto P and Iengo M: Cyberknife® system: A new
therapeutic strategy for sinonasal solitary extramedullary
plasmacytomae. J Biol Regul Homeost Agents. 3:763–768. 2017.
|
|
10
|
Cantone E, Di Lullo AM, Marano L, Guadagno
E, Mansueto G, Capriglione P, Cammarota F, Catalano L and Iengo M:
Strategy for the treatment and follow-up of sinonasal solitary
extramedullary plasmacytoma: A case series. J Med Case Rep.
11:2192017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cantone E, Cavaliere M, Di Lullo AM,
Guadagno E and Iengo M: Immunohistochemical patterns in the
differential diagnosis of rhinopharyngeal granulocytic sarcoma.
Oncol Lett. 12:2777–2781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jurado-Ramos A, Ropero Romero F, Cantillo
Baños E and Salas Molina J: Minimally invasive endoscopic
techniques for treating large, benign processes of the nose,
paranasal sinus, and pterygomaxillary and infratemporal fossae:
Solitary fibrous tumour. J Laryngol Otol. 123:457–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhanlong M, Haibin S, Xiangshan F,
Jiacheng S and Yicheng N: Variable solitary fibrous tumor
locations: CT and MR imaging features. Medicine (Baltimore).
95:e30312016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rizzo S, Giunta AAM and Pennacchi A:
Sinonasal and rhinopharyngeal solitary fibrous tumour: A case
report and review of the literature. Acta Otorhinolaryngol Ital.
35:455–458. 2015.PubMed/NCBI
|
|
15
|
Lo Muzio L, Mascolo M, Capodiferro S,
Favia G and Maiorano E: Solitary fibrous tumor of the oral cavity:
The need for an extensive sampling for a correct diagnosis. J Oral
Pathol Med. 36:538–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chauhan SS, Krishnan J and Heffner DK:
Solitary fibrous tumor of nasal cavity in patient with
long-standing history of cocaine inhalation. Arch Pathol Lab Med.
128:e1–e4. 2004.PubMed/NCBI
|
|
17
|
Mathew GA, Ashish G, Tyagi AK,
Chandrashekharan R and Paul RR: Solitary fibrous tumor of nasal
cavity: A case report. Iran J Otorhinolaryngol. 27:307–312.
2015.PubMed/NCBI
|
|
18
|
Zielińska-Kaźmierska B, Grodecka J and
Szyszkowski A: Solitary fibrous tumor of the nasal cavity and
paranasal sinuses: A case report. J Oral Biol Craniofac Res.
5:112–116. 2015. View Article : Google Scholar : PubMed/NCBI
|