Introduction
Liver cancer, including hepatocellular carcinoma
(HCC), is a common and severe type of cancer worldwide (1). HCC is characterized by a high malignant
degree, infiltration, metastasis and congenital drug resistance
(2). Epidemiological evidence has
demonstrated that there are ~1 million malignant tumor cases of HCC
in the world each year with a mortality rate of ~40% (3). Thus, it is necessary to explore the
pathogenic mechanisms of liver cancer and to improve therapeutic
regimens for patients with liver cancer.
As a common chemotherapy drug, 5-fluorouracil (5-FU)
triggers apoptosis by inhibiting the thymidylic acid synthetase
activity and by binding to DNA and RNA sequences (4). It has been reported that the 5-year
survival rate of advanced HCC is ≤10% in the USA in 2015 (5), whereas 5-FU-mediated adjuvant
chemotherapy effectively reduces the mortality rates of patients
with liver cancer (6). However,
5-FU-induced multi-drug resistance (MDR) often occurs in the
treatment of cancer, such as HCC and colorectal cancer (7–9).
Recently, MDR has been the main obstacle in chemotherapy resulting
in recurrence and metastasis of cancers (10,11).
Thus, it is essential to find safe and effective drugs for
reversing MDR.
Plants used in traditional Chinese medicine (TCM)
exhibit low cytotoxicity and antitumor activity; thus, they may be
useful in clinical application for cancer treatment and prevention
(12). Konjac glucomannan (KGM) is
extracted from Amorphophallus konjac K. Koch, which is a TCM
plant. The products of this plant are listed as health foods by the
World Health Organization (13,14). KGM
is used for obesity treatment, improving lipid metabolism,
laxative, antidiabetic and anti-inflammatory effects (15). For example, KGM improves the
metabolic control as determined by glycaemia, lipidemia and blood
pressure in patients with high-risk type-2 diabetes, suggesting
that KGM may be used as an alternative therapy for type-2 diabetes
mellitus or reduction of blood glucose (16). KGM has also been reported to
efficiently inhibit high fat diet-induced obesity in mice by
attenuating insulin resistance, liver injury and inflammation,
enhancing the antioxidant defense system and modulating the
secretion of adipocytokines and adipogenesis-associated proteins
(17). Additionally, KGM may
potentially decrease the high fat-induced risk of colon
carcinogenesis (18). In addition,
KGM is commonly used in Asia to treat patients with chronic
hepatitis (19) and is a potential
compound against liver cancer (20).
KGM significantly reduces the viability of HepG2 cells and induces
apoptosis-associated morphological changes (20). KGM also upregulates Bax and
downregulates BCL-2 expression, indicating that the inhibitory
activity of KGM on HepG2 cells is influenced by BCL-2/Bax signaling
(20). Thus, KGM may be a promising
drug for treatment of cancer, including HCC.
The present study aimed to investigate the reversal
effects of KGM on the resistance of HepG2/5-FU cells to 5-FU in
vivo and in vitro and to explore the potential
mechanisms of KGM in anti-drug resistance.
Materials and methods
Cell culture
HepG2 and HepG2/5-FU cells were purchased from the
Cell Conservation Center of Xiangya Medicine College, Hunan
University (Hunan, China) and they were bought from American Type
Culture Collection (ATCC, cat. no. ATCC® HB-8065). The
cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (cat. no. 16000-044, Gibco;
Thermo Fisher Scientific, Inc.). The cells were seeded in 6-, 12-
or 24-well plates, diluted to the final concentration of
5.0×105 cells/ml and incubated at 37°C in a humidified
incubator with an atmosphere of 5% CO2 under aseptic
conditions.
Cell viability assay
KGM was purchased from Career Henan Chemical Co.
(cat. no. 37220-17-0; http://www.chemicalbook.com/ProductDetail_EN_450429.htm),
and its purity was 99%; 5-FU was purchased from Sigma Aldrich;
Merck KGaA (cat. no. F6627-1G). 5-FU and KGM were diluted in DMSO
and water, respectively.
Cell viability or proliferation was determined using
an MTT assay. The cells (HepG2 or HepG2/5-FU) were seeded in
96-well plates at a density of 1.0×104 cells/well in 0.1
ml RPMI-1640 medium and were exposed to increasing concentrations
of 5-FU (0.001, 0.005, 0.020, 0.080, 0.320, 1.280, 2.560, 5, 10,
20, 40, 80 or 160 µM) or KGM (0, 2, 4, 8, 10, 100 or 1,000 µg/ml)
for 24 h.
MTT (cat. no. 57360-69-7; Sigma-Aldrich; Merck KGaA)
was dissolved in DMSO to 5 mg/ml; the cells were incubated with MTT
for 4 h at 37°C. The absorbance of the samples was measured using a
microtiter plate reader at 490 nm.
To investigate the reversal effects of KGM on the
viability of HepG2/5-FU cells, the cells were preincubated with KGM
(2 or 6 µg/ml) and 5-FU (0.5 µM) for 24 h at 37°C. The absorbance
of the samples at 490 nm was determined by MTT assay as described
above.
Apoptosis analysis by flow
cytometry
Apoptosis was determined using Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) staining. HepG2/5-FU
cells were seeded in 96-well plates at a density of
5.0×105 cells/well. The cells were treated with 2 or 6
µg/ml KGM for 24 h at 37°C in the presence of 5-FU. Subsequently,
the cells were stained using an Annexin V-FITC/PI Apoptosis
Detection kit (Beyotime Institute of Biotechnology) according to
the manufacturer's instructions. The cells were analyzed using a BD
FACScan flow cytometer (Becton, Dickinson and Company) and
CellQuest acquisition and analysis software v.3.0 (Becton,
Dickinson and Company). Early, late or early + late apoptosis was
assessed and the early apoptotic cells were stained by Annexin
V-FITC and the late apoptotic cells were stained by both Annexin
V-FITC and PI.
Western blot analysis
The cells (HepG2 or HepG2/5-FU) were lysed in RIPA
Buffer (Beyotime Institute of Biotechnology), and protein
concentration was measured by bicinchoninic acid assay (Beyotime
Institute of Biotechnology). Total protein (50 µg) was resolved
using 10% SDS-PAGE, electro-transferred to polyvinylidene fluoride
(PVDF) membranes (Beyotime Institute of Biotechnology) and blocked
with 5% non-fat dry milk in Tris-buffered saline, pH 7.5 for 30 min
at the room temperature. The PVDF membranes were incubated with
primary antibodies against MDR1 (1:1,000; cat. no. 13978; Cell
Signaling Technology Inc.); anti-P-gp (1:1,000; cat. no. ab170904;
Abcam); Cyclin A1 (1:1,000; cat. no. 4656; Cell Signaling
Technology Inc.); anti-cyclin B1 (1:1,000; cat. no. 4138; Cell
Signaling Technology Inc.); CDK2 (1:1,000; cat. no. 2546; Cell
Signaling Technology Inc.); Bcl-2 (1:1,000 dilution; cat. no. 4223;
Cell Signaling Technology Inc.); Bax (1:1,000 dilution; cat. no.
14796; Cell Signaling Technology Inc.); GAPDH (1:1,000; cat. no.
MB001; Bioworld, Technology Inc.); cleaved caspase-3 (1:1,000; cat.
no. P42574; Bioworld Technology Inc.); p53 (1:1,000; cat. no. 9282;
Cell Signaling Technology Inc.); E-cadherin (1:1,000; cat. no.
14472; Cell Signaling Technology Inc.); N-cadherin (1:1,000; cat.
no. 13116; Cell Signaling Technology Inc.); AKT (1:1,000; cat. no.
4685; Cell Signaling Technology Inc.) and p-AKT (1:1,000 dilution;
cat. no. 9611; Cell Signaling Technology Inc.) for 5 h at room
temperature and the membranes were washed with PBST
(Tween-20-containing phosphate-buffered saline) and incubated with
horseradish peroxidase (HRP)-conjugated mouse or rabbit IgG
secondary antibodies anti-HRP IgG (1:5,000; cat. no. 7074; Cell
Signaling Technology Inc.) and anti-HRP IgG (1:5,000; cat. no.
7076; Cell Signaling Technology Inc.) for 1 h at 37°C. Protein
bands were detected by an enhanced chemiluminescence kit
(Invitrogen; Thermo Fisher Scientific, Inc.) and quantified using
ImageJ v.1.8.0 software (National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells (HepG2 or
HepG2/5-FU) with TRIzol® reagent (Sangon Biotech Co.,
Ltd.,), and cDNA synthesis was performed using the PrimeScript II
1st Strand cDNA Synthesis kit (Takara Bio, Inc.)
according to the manufacturer's instructions. QPCR was performed
with a SYBR® Green PCR system (Takara Bio, Inc.) in an
ABI 7500 thermal cycler (Thermo Fisher Scientific, Inc.), and the
thermocycling conditions were as follows: 94°C for 5 min; followed
by 35 cycles of 94°C for 30 sec, 58°C for 30 sec and 72°C for 15
sec. The primers used were as follows: GAPDH forward,
5′-GCAGTGGCAAAGTGGAGATT-3′ and reverse, 5′-TGAAGTCGCAGGAGACAACC-3′;
MDR1 forward, 5′-TCACTTCAGTTACCCATCTCG-3′ and reverse,
5′-CACCAATGATTTCCCGTAG-3′; P-gp forward, 5′-ACTTGCAAGGGGACCAGAGA-3′
and reverse, 5′-CCTTCAAGATCCATTCCGACC-3′; cyclin A forward,
5′-TCCATGTCAGTGCTGAGAGGC-3′ and reverse,
5′-GAAGGTCCATGAGACAAGGC-3′; cyclin B1 forward,
5′-ATGCAGCACCTGGCTAAGAA-3′ and reverse, 5′-TTACACCTTTGCCACAGCCT-3′;
CDK2 forward, 5′-CTTTGCTGAGATGGTGACTCG-3′ and reverse,
5′-TCATCCAGGGGAGGTACAACT-3′; and caspase-3 forward,
5′-TGCATACTCCACAGCACCTG-3′ and reverse, 5′-TCTGTTGCCACCTTTCGGTT-3′.
RT-qPCR for each sample was performed in duplicate. The
fold-changes were calculated by relative quantification
(2−ΔΔCq) (21). GAPDH was
used as an internal control.
Tumor growth
BALB/c male nude mice (4-week-old) were purchased
from the Model Animal Research Center of Nanjing University
(Nanjing, China) and divided into 2 mice/group. All animal
experiments were performed in accordance with the relevant
guidelines of the Institutional Animal Care and Use Committee of
Nanjing Medical University (approval number, SYXK 2015-0015).
HepG2/5-FU cells (150 µl, 4.0×106 cells) were injected
subcutaneously into the right flank of athymic mice. The mice were
housed in an isolated, clean, air-conditioned room at 24–26°C and a
relative humidity of ~50% with a 12/12-h light/dark cycle and they
had free access to food and water. The tumors were inspected every
other day. Drug treatment was started when the tumor volume reached
40–50 mm3 (22). The mice
were randomly divided into two groups 5-FU (2 mg/kg) alone and 5-FU
(2 mg/kg) plus KGM (20 mg/kg) groups with two mice in each group.
The drugs were administered every 2 days i.p. The tumor growth was
determined every other day, and the tumor volume was calculated
using the following formula: Volume = (longest diameter × shortest
diameter2) / 2 (22). On day 13, the
tumor-bearing mice were sacrificed using the cervical dislocation
method and the tumors were weighed.
Statistical analysis
The data are presented as the mean ± standard
deviation. All assays were performed as three independent
experiments. Data were analyzed using Student's t-test or one-way
ANOVA followed by Tukey's honestly significant difference test
using SPSS 17.0 software (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of 5-FU and KGM on the
viability of HepG2 and HepG2/5-FU cells
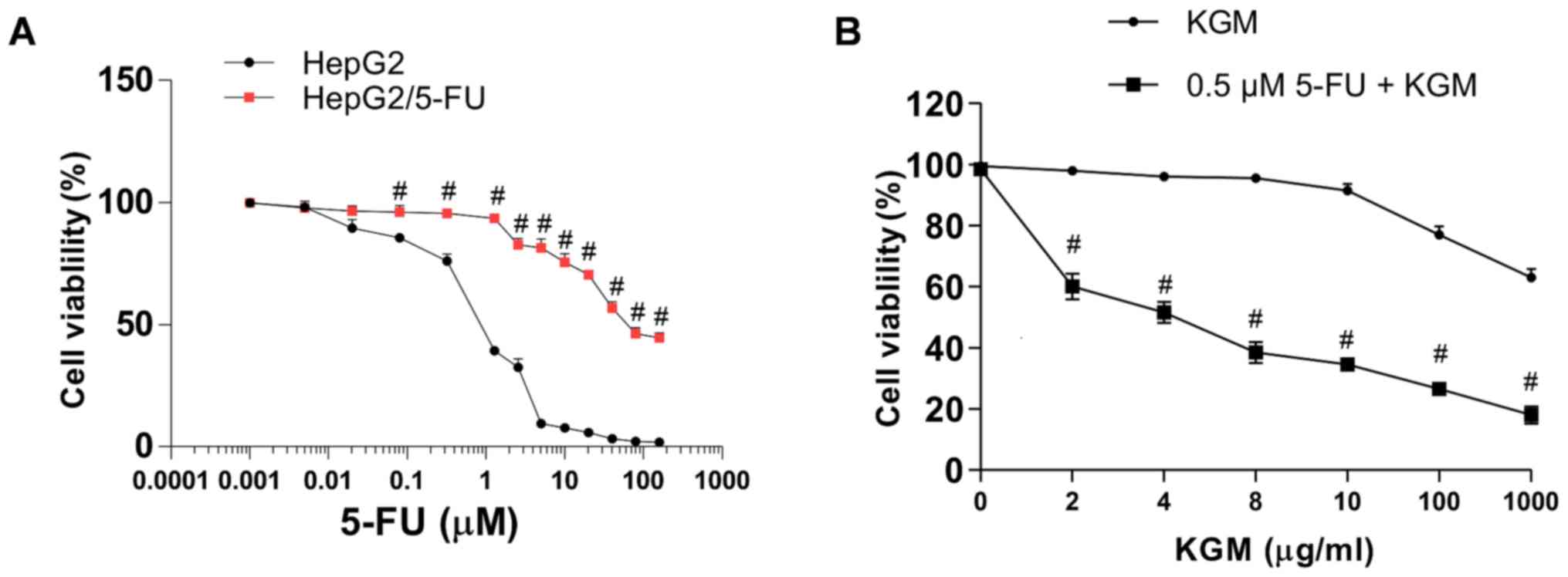
MTT assays were performed to measure the effects of
5-FU and KGM on the viability of liver cancer cells. The results
demonstrated that 5-FU exhibited cytotoxic activity on the HepG2
and HepG2/5-FU cells with 50% inhibitory concentration
(IC50) values of 1.03 and 60.02 µM, respectively
(Fig. 1A). By contrast, the
IC50 of KGM was 1,500.41 µg/ml in HepG2 cells (Fig. 1B). Thus, 5-FU exhibited different
inhibition in drug-sensitive and MDR cancer cells. In addition,
5-FU exhibited no effects on the HepG2/5-FU cell viability at ≤0.6
µM (Fig. 1A), and KGM exhibited no
cytotoxic activity on HepG2/5-FU cells at ≤8 µg/ml (Fig. 1B). Thus, to retain the 5-FU-resistant
properties of HepG2/5-FU, the cells were cultured in medium
containing 0.2 µM 5-FU.
KGM potentiates 5-FU-induced
cytotoxicity and inhibits P-gp and MDR expression in HepG2/5-FU
cells
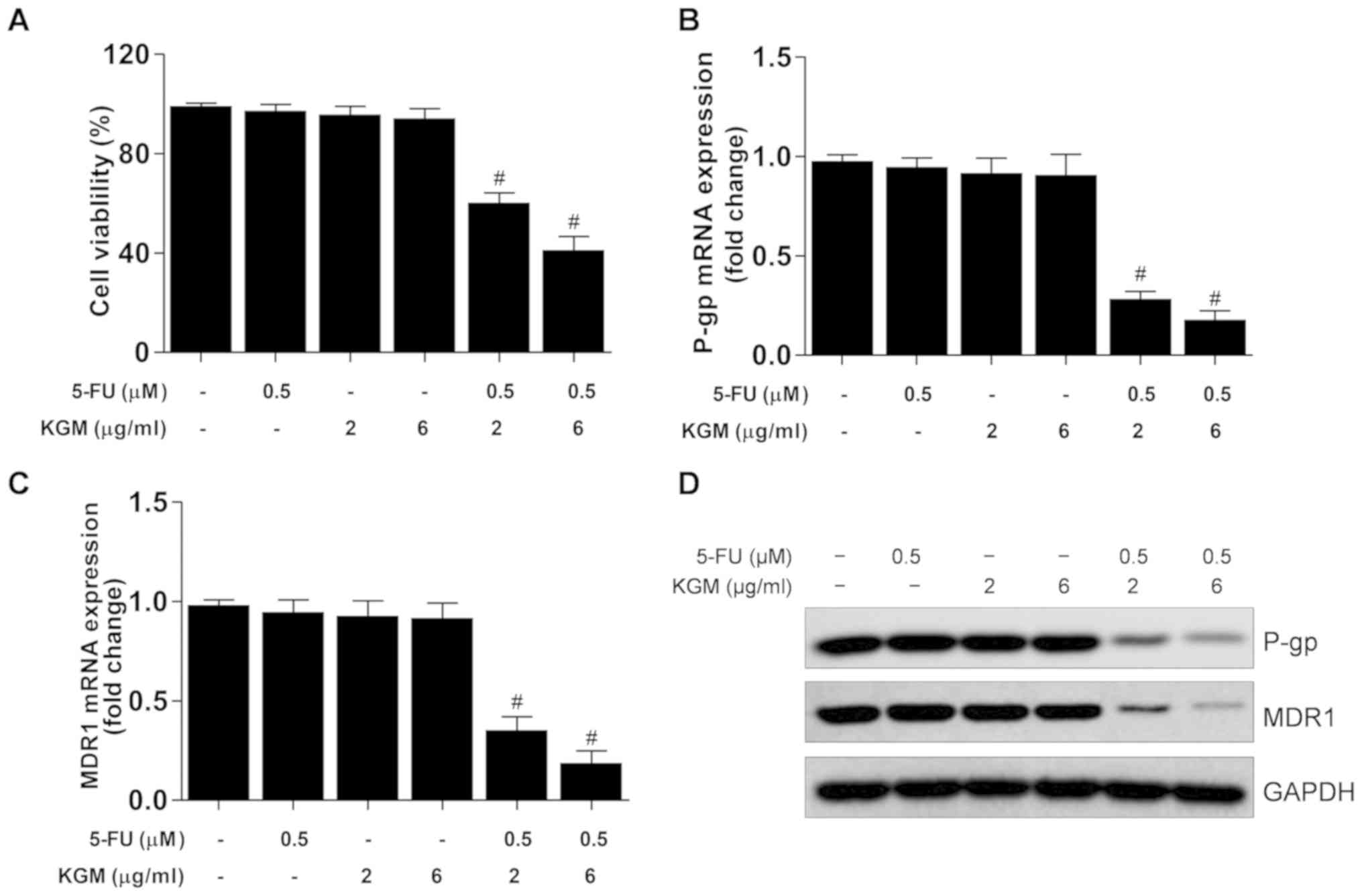
To investigate the reversal effects of KGM on
HepG2/5-FU cells, the cells were incubated with 0.5 µM 5-FU and/or
2 or 6 µg/ml KGM for 24 h. The results demonstrated that 0.5 µM
5-FU exhibited no cytotoxic activity on HepG2/5-FU cells (Fig. 1A), and that 2 or 6 µg/ml KGM also
exhibited no cytotoxicity to this drug-resistant cell line
(Figs. 1B and 2A). However, in the presence of 0.5 µM
5-FU, 2 and 6 µg/ml KGM significantly decreased HepG2/5-FU cell
viability (Figs. 1B and 2A). In addition, the mRNA and protein
levels of P-gp and MDR were significantly downregulated by KGM in
the presence of 0.5 µM 5-FU compared with 5-FU or KGM treatment
alone (Fig. 2B-D).
KGM suppresses the cell cycle and
increases apoptosis in HepG2/5-FU cells
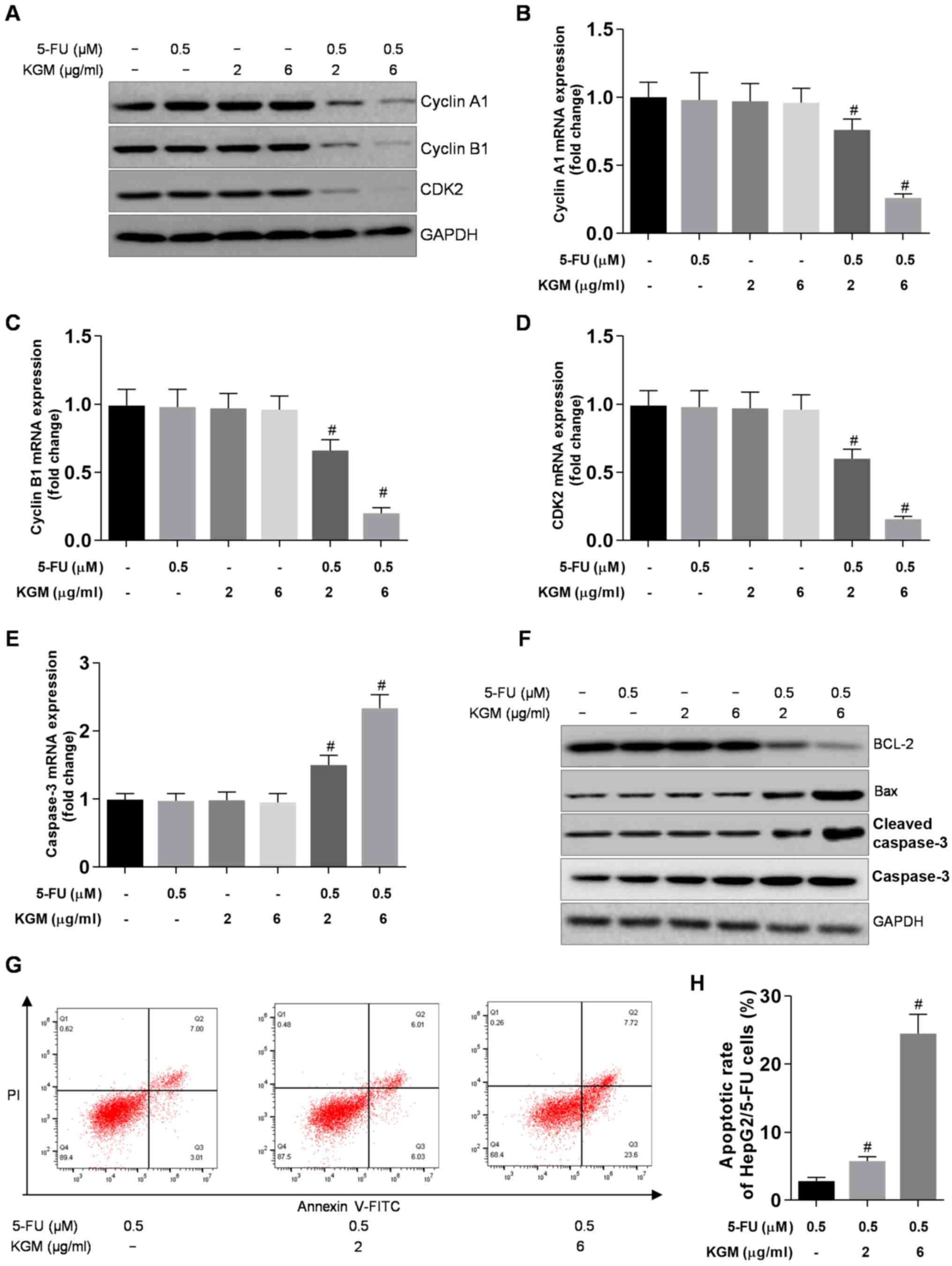
To investigate the effects of KGM on HepG2/5-FU cell
proliferation, the expression levels of the cell cycle-related
genes cyclin A, cyclin B1 and CDK2 were determined. The results
demonstrated that no significant differences were observed between
the protein or mRNA levels of cyclin A, cyclin B1 and CDK2 in
HepG2/5-FU cells following incubation with 0.5 µM 5-FU or 2 µg/ml
KGM alone and the control groups. By contrast, increasing
concentrations of KGM in the presence of 0.5 µM 5-FU significantly
reduced the protein and mRNA levels of cyclin A, cyclin B1 and CDK2
in HepG2/5-FU compared KGM treatment alone (Fig. 3A-D).
The expression of the proapoptotic gene caspase-3
was upregulated in HepG2/5-FU cells after KGM treatment in the
presence of 0.5 µM 5-FU compared with 5-FU or KGM treatment alone
(Fig. 3E). Additionally, the protein
levels of the antiapoptotic gene BCL-2 level were reduced, whereas
the protein levels of the proapoptotic genes Bax and caspase-3 were
increased after KGM treatment in the presence of 0.5 µM 5-FU in
HepG2/5-FU cells compared with 5-FU or KGM treatment alone
(Fig. 3F).
KGM also significantly promoted HepG2/5-FU
apoptosis; KGM increased the apoptotic rate (1.85±0.21) in cells
treated with 0.5 µM 5-FU to 5.06±0.62 and 26.91±1.73% in cells
treated with 0.5 µM 5-FU and 2 or 6 µg/ml KGM, respectively
(Fig. 3G and H).
KGM reverses the resistance of
HepG2/5-FU cells to 5-FU in a mouse xenograft model by inhibiting
p53 expression and AKT phosphorylation
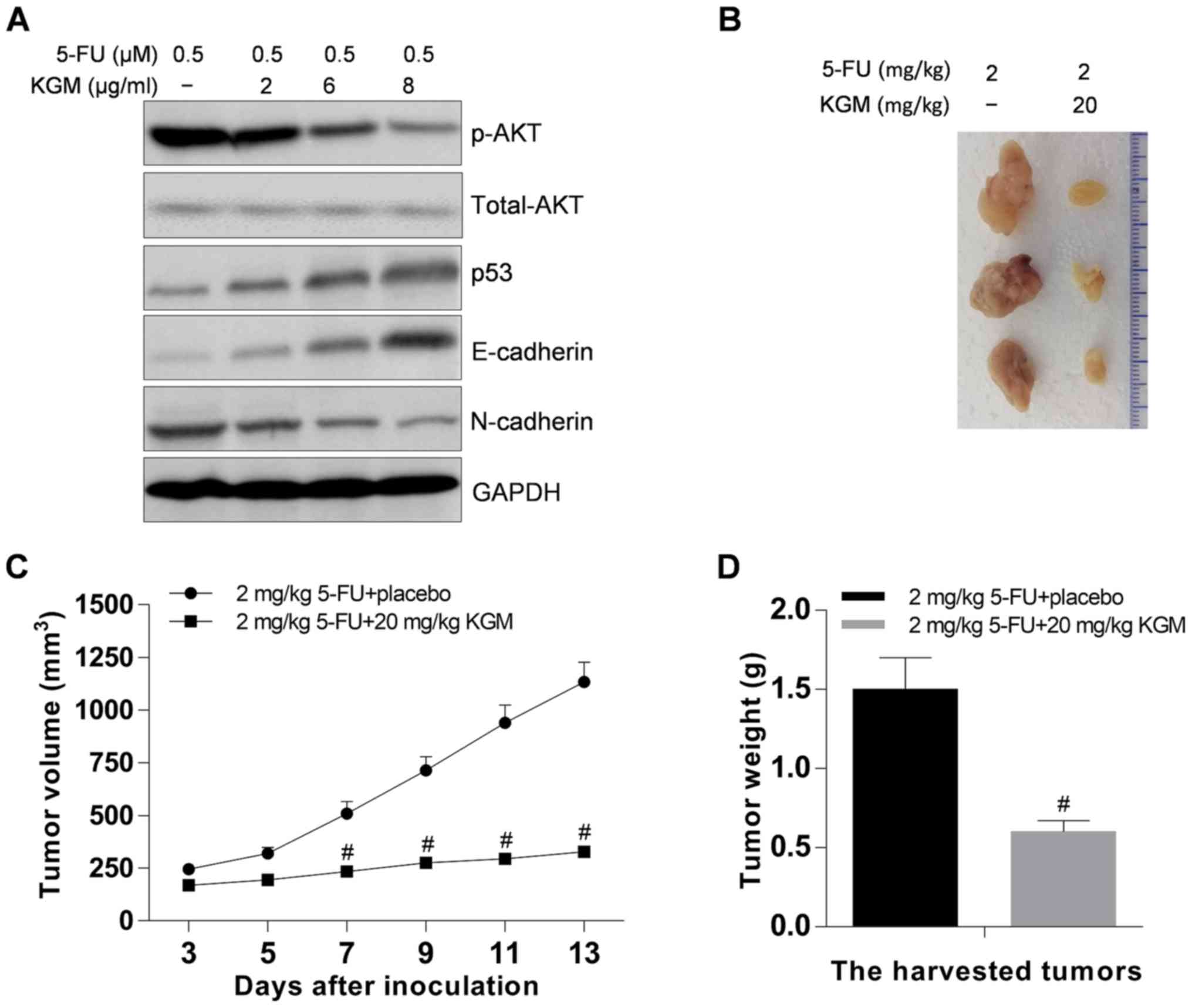
To explore the potential mechanisms of KGM in
reversing effects of MDR, p53 expression and AKT phosphorylation in
KGM-incubated HepG2/5-FU cells was investigated. The results
demonstrated that KGM significantly upregulated p53 and E-cadherin
and decreased phosphorylated AKT and N-cadherin expression levels
in HepG2/5-FU cells in the presence of 5-FU (Fig. 4A).
The reversal effects of KGM on the resistance of
HepG2/5-FU cells to 5-FU were also investigated in vivo. The
results demonstrated that KGM inhibited the HepG2/5-FU tumor growth
in vivo (Fig. 4B); in the
control group, the volume of tumors 13 days after 5-FU-treatment
were ~4.69-fold higher compared with those at the start of
treatment, whereas the volume of tumors 13 days after KGM and 5-FU
treatment were <1.94-fold higher compared with those at the
start of treatment (Fig. 4C). In
addition, the weight of the tumors was significantly lower in the
KGM-treated group compared with that in the control group (Fig. 4D).
Discussion
KGM has been used for its antiobesity, lipid
metabolism-improving, laxative, antidiabetic and anti-inflammatory
effects (15). KGM has also been
reported to attenuate colon carcinogenesis and liver cancer
(18,20). The present study investigated the
reversal effects of KGM on the resistance of HepG2/5-FU cells to
5-FU in vivo and in vitro, and explored the potential
mechanisms of KGM in MDR. The present study demonstrated that 5-FU
exhibited no cytotoxic activity on HepG2/5-FU cells at ≤1 µM, and
KGM exhibited no cytotoxic activity HepG2/5-FU cells at the
concentration of ≤8 µg/ml. The present study demonstrated that KGM
plus 5-FU attenuated the drug resistance of HepG2/5-FU cells by
inhibiting HepG2/5-FU proliferation and increasing HepG2/5-FU
apoptosis. In addition, KGM significantly downregulated the
expression of cyclin A, cyclin B1, CDK2, and BCL-2, and upregulated
the expression of Bax and cleaved caspase-3. KGM also increased the
p53 expression, inhibited AKT phosphorylation and reversed the MDR
of HepG2/5-FU cells in vivo resulting in suppression of
tumor growth.
5-FU remains a commonly used chemotherapeutic drug
in cancer treatment and clinical studies (23). The antineoplastic efficacy of 5-FU is
attributed to its ability to increase DNA damage, which results in
cell cycle arrest and apoptosis (24). However, the clinical efficacy of 5-FU
decreases due to the drug resistance of cancer cells (24). Thus, further studies are necessary
for overcoming the drug resistance of cancer cells leading to the
increasing potency of cancer therapy. In the present study, the
5-FU IC50 values were significantly higher in HepG2/5-FU
cells compared with those in HepG2 cells, suggesting that
HepG2/5-FU is a 5-FU-resistant cell line. In addition, increasing
concentrations of KGM significantly attenuated the resistance of
HepG2/5-FU cells to 5-FU by downregulating the expression of P-gp
and MDR1 protein, which serve an important role in 5-FU resistance
(25). The present study also
investigated the effects of KGM on the cell cycle and apoptosis of
HepG2/5-FU cells; co-treatment with KGM and 5-FU significantly
downregulated the positive regulators of cell proliferation
including cyclin A, cyclin B1 and CDK2 at the protein and mRNA
level, suggesting that KGM may suppress HepG2/5-FU cell
proliferation. In addition, KGM + 5-FU increased the expression of
the apoptosis-promoting proteins Bax and cleaved-caspase-3,
decreased the expression of the antiapoptotic protein BCL-2 and
elevated apoptosis in HepG2/5-FU cells. Thus, KGM may reverse 5-FU
resistance in HepG2/5-FU cells by suppressing cell proliferation
and enhancing apoptosis of these cells.
AKT signaling is involved in the development of MDR
of cancers (26–29). For example, inhibition of AKT
overcomes 5-FU resistance in SNU-C5/5-FU cells (30). Activated AKT promotes Wnt/β-catenin
signaling and activates the antiapoptotic protein mdm2 resulting in
the development of cancer, such as ovarian and prostate cancer
(31,32). Similarly, AKT activation positively
regulates the NF-κB pathway, enhancing cancer cell survival and
resistance to apoptosis (32).
Timosaponin A-III reverses MDR in human chronic myelogenous
leukemia K562/ADM cells by downregulating MDR1 and multidrug
resistance associated protein 1 expression and inhibiting the
PI3K/AKT pathway (28). Activation
of the PI3K/AKT/Snail pathway contributes to the
epithelial-mesenchymal transition-induced resistance to sorafenib
in HCC cells (29). AKT signaling is
involved in Annexin A2-mediated MDR in gastric cancer (27). These findings suggest that the AKT
pathway serves an essential role in the MDR of cancer cells. The
present study demonstrated that AKT signaling was involved in MDR
of HepG2/5-FU cells, and co-treatment with KGM and 5-FU
significantly reversed the MDR of HepG2/5-FU by inactivating AKT.
Previous studies have reported that the tumor suppressor gene p53
participates in chemotherapy resistance and cancer progression
(33,34). The present study observed that
co-treatment with KGM and 5-FU increased p53 expression compared
with 5-FU alone, suggesting that p53 may be involved in the
reversal effects exhibited by KGM to 5-FU resistance in HepG2/5-FU
cells. Taken together, the results of the present study
demonstrated the beneficial activity of KGM in the MDR of
HepG2/5-FU cells by suppressing AKT signaling and elevating p53
expression.
In conclusion, KGM reversed the MDR of HepG2/5-FU
cells in vitro and in vivo, inhibited the HepG2/5-FU
cell proliferation and increased apoptosis. In addition, KGM plus
5-FU significantly repressed AKT signaling and promoted p53
expression. Additionally, KGM inhibited HepG2/FU cell proliferation
in vivo, resulting in the suppression of tumor growth. Thus,
KGM may be a promising drug for anti-MDR in clinical treatment.
Acknowledgements
The authors would like to thank Professor Zhijian
Pan (the Affiliated Hospital of Hangzhou Normal University,
Hangzhou, China) for technical assistance.
Funding
This study was supported by the Science and
Technology Project of Traditional Chinese Medicine Administration
Bureau of Zhejiang Province (grant no. 2017A101).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
YX conceived and designed the experiments. BC, XX,
KZ, LL and YY performed the experiments and analyzed the data. YY
and BC drafted the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the relevant guidelines of the Institutional Animal Care and
Use Committee of Nanjing Medical University (approval no. SYXK
2015-0015).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Finn RS, Zhu AX, Farah W, Almasri J, Zaiem
F, Prokop LJ, Murad MH and Mohammed K: Therapies for advanced stage
hepatocellular carcinoma with macrovascular invasion or metastatic
disease: A systematic review and meta-analysis. Hepatology.
67:422–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh AK, Kumar R and Pandey AK:
Hepatocellular Carcinoma: Causes, Mechanism of Progression and
Biomarkers. Curr Chem Genomics Transl Med. 12:9–26. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Luo Q, Li Y, Deng S, Li X and Wei
S: A systematic assessment of the quality of systematic
reviews/meta-analyses in radiofrequency ablation versus hepatic
resection for small hepatocellular carcinoma. J Evid Based Med.
7:103–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirashima Y and Shirao K: Predicting drug
efficacy-fluorinated pyrimidines (fluorouracil, S-1 and
capecitabine)]. Gan To Kagaku Ryoho. 39:1603–1607. 2012.(In
Japanese). PubMed/NCBI
|
|
5
|
Udali S, Guarini P, Moruzzi S, Ruzzenente
A, Tammen SA, Guglielmi A, Conci S, Pattini P, Olivieri O,
Corrocher R, et al: Global DNA methylation and hydroxymethylation
differ in hepatocellular carcinoma and cholangiocarcinoma and
relate to survival rate. Hepatology. 62:496–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chau I and Cunningham D: Chemotherapy in
colorectal cancer: New options and new challenges. Br Med Bull.
64:159–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Duan B, Guo Y, Zhou R, Sun J, Bie B,
Yang S, Huang C, Yang J and Li Z: Baicalein sensitizes
hepatocellular carcinoma cells to 5-FU and Epirubicin by activating
apoptosis and ameliorating P-glycoprotein activity. Biomed
Pharmacother. 98:806–812. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: Mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Chen J, Yuan W, Ruan H, Shu Y, Ji
J, Wu L, Tang Q, Zhou Z, Zhang X, et al: Nuclear Factor I/B
Promotes Colorectal Cancer Cell Proliferation,
Epithelial-Mesenchymal Transition and 5-fluorouracil Resistance.
Cancer Sci. 110:86–98. 2019.PubMed/NCBI
|
|
10
|
Koretz RL: Adjuvant chemotherapy increased
survival in colorectal cancer patients with low recurrence risk.
Annals of Internal Medicine. 148:JC3–6. 2008. View Article : Google Scholar
|
|
11
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qurishi Y, Hamid A, Majeed R, Hussain A,
Qazi AK, Ahmed M, Zargar MA, Singh SK and Saxena AK: Interaction of
natural products with cell survival and signaling pathways in the
biochemical elucidation of drug targets in cancer. Future Oncol.
7:1007–1021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tester RF and Al-Ghazzewi FH: Beneficial
health characteristics of native and hydrolysed konjac
(Amorphophallus konjac) glucomannan. J Sci Food Agric.
96:3283–3291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chua M, Baldwin TC, Hocking TJ and Chan K:
Traditional uses and potential health benefits of Amorphophallus
konjac K. Koch ex N.E.Br. J Ethnopharmacol. 128:268–278. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Behera SS and Ray RC: Konjac glucomannan,
a promising polysaccharide of Amorphophallus konjac K. Koch
in health care. Int J Biol Macromol. 92:942–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vuksan V, Jenkins DJ, Spadafora P,
Sievenpiper JL, Owen R, Vidgen E, Brighenti F, Josse R, Leiter LA
and Bruce-Thompson C: Konjac-mannan (glucomannan) improves glycemia
and other associated risk factors for coronary heart disease in
type 2 diabetes. A randomized controlled metabolic trial. Diabetes
Care. 22:913–919. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhai X, Lin D, Zhao Y, Li W and Yang X:
Enhanced anti-obesity effects of bacterial cellulose combined with
konjac glucomannan in high-fat diet-fed C57BL/6J mice. Food Funct.
9:5260–5272. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu WT and Chen HL: Effects of konjac
glucomannan on putative risk factors for colon carcinogenesis in
rats fed a high-fat diet. J Agric Food Chem. 59:989–994. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Villaverde AF, Benlloch S, Berenguer M,
Miguel Rayón J, Pina R and Berenguer J: Acute hepatitis of
cholestatic type possibly associated with the use of glucomannan
(Amorphophalus konjac). J Hepatol. 41:1061–1062. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sawai S, Mohktar MS, Safwani WKZW and
Ramasamy TS: Suppression of the Viability and Proliferation of
HepG2 Hepatocellular Carcinoma Cell Line by Konjac Glucomannan.
Anticancer Agents Med Chem. 18:1258–1266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rajeevan MS, Ranamukhaarachchi DG, Vernon
SD and Unger ER: Use of real-time quantitative PCR to validate the
results of cDNA array and differential display PCR technologies.
Methods. 25:443–451. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fiebig HH, Berger DP, Winterhalter BR and
Plowman J: In vitro and in vivo evaluation of US-NCI compounds in
human tumor xenografts. Cancer Treat Rev. 17:109–117. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasada S, Miyata Y, Tsutani Y, Tsuyama N,
Masujima T, Hihara J and Okada M: Metabolomic analysis of dynamic
response and drug resistance of gastric cancer cells to
5-fluorouracil. Oncol Rep. 29:925–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang HY, Zhao L, Yang Z, Zhao Q, Qiang L,
Ha J, Li ZY, You QD and Guo QL: Oroxylin A reverses multi-drug
resistance of human hepatoma BEL7402/5-FU cells via downregulation
of P-glycoprotein expression by inhibiting NF-κB signaling pathway.
Mol Carcinog. 51:185–195. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang ZD, Li Y, Fan Q, Zhao B, Tan B and
Zhao XF: Annexin A2 is implicated in multi-drug-resistance in
gastric cancer through p38MAPK and AKT pathway. Neoplasma.
61:627–637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen JR, Jia XH, Wang H, Yi YJ, Wang JY
and Li YJ: Timosaponin A-III reverses multi-drug resistance in
human chronic myelogenous leukemia K562/ADM cells via
downregulation of MDR1 and MRP1 expression by inhibiting PI3K/Akt
signaling pathway. Int J Oncol. 48:2063–2070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong J, Zhai B, Sun W, Hu F, Cheng H and
Xu J: Activation of phosphatidylinositol 3-kinase/AKT/snail
signaling pathway contributes to epithelial-mesenchymal
transition-induced multi-drug resistance to sorafenib in
hepatocellular carcinoma cells. PLoS One. 12:e01850882017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim EJ, Kang GJ, Kang JI, Boo HJ, Hyun JW,
Koh YS, Chang WY, Kim YR, Kwon JM, Maeng YH, et al: Over-activation
of AKT signaling leading to 5-Fluorouracil resistance in
SNU-C5/5-FU cells. Oncotarget. 9:19911–19928. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Do TV, Kubba LA, Antenos M, Rademaker AW,
Sturgis CD and Woodruff TK: The role of activin A and Akt/GSK
signaling in ovarian tumor biology. Endocrinology. 149:3809–3816.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Q, Yan B, Hu X, Li XB, Zhang J and
Fang J: Luteolin inhibits invasion of prostate cancer PC3 cells
through E-cadherin. Mol Cancer Ther. 8:1684–1691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He L, Zhu H, Zhou S, Wu T, Wu H, Yang H,
Mao H, SekharKathera C, Janardhan A, Edick AM, et al: Wnt pathway
is involved in 5-FU drug resistance of colorectal cancer cells. Exp
Mol Med. 50:1012018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sui X, Kong N, Wang X, Fang Y, Hu X, Xu Y,
Chen W, Wang K, Li D, Jin W, et al: JNK confers 5-fluorouracil
resistance in p53-deficient and mutant p53-expressing colon cancer
cells by inducing survival autophagy. Sci Rep. 4:46942014.
View Article : Google Scholar : PubMed/NCBI
|