Introduction
In the last few years, the therapeutic strategy for
primary breast cancer has undergone radical changes. The
possibility to characterize breast cancer histological subtypes
according to molecular phenotypes as well as the adoption of the
technique of axillary sentinel lymph node clearance were among the
causes that brought about the innovations (1).
In the early 2000s, Sørlie introduced the concept of
biomolecular characterization of primary breast cancer, based on
gene expression profile (2).
Subsequently, in 2013, the St Gallen International Expert Consensus
stated that conventional tumor biomarkers such as hormone receptor
status, tumour proliferative activity and Her2/neu gene
amplification permitted a surrogate breast cancer subtype
classification representative of multigene molecular assays
(3). Breast cancers were confirmed
to be classified according to different biomarker characteristics
in 5 subtypes: Luminal A, Luminal B/HER2 negative, Luminal B/HER2
positive, HER2-positive (not luminal), Basal Like-Triple negative
(TNBC). The prognostic-predictive relevance of this
subclassification has been largely confirmed (4).
Screening programs and women's increased awareness
of breast cancer risk are permitting an even earlier diagnosis of
breast cancer, with smaller size primary tumour and lower frequency
of involved axillary lymph nodes at diagnosis (5). Consequently, to perform a radical
surgical clearance of the axilla has been largely debated also the
complete axillary node dissection (ALND) is limited by potentially
important negative side effects and without a ‘per se’ clear
therapeutic efficacy (6). Currently,
sentinel lymph node biopsy (SLNB) has replaced ALND for axillary
staging in patients with early-stage breast cancer (7). However, the reported incidence of node
metastasis at SLNB is generally low which means that for the most
part, women underwent unnecessary invasive axilla surgery (8); furthermore, recent data raises doubts
on the role of SLNB itself as clinical determinant, considering
that small tumors, after diagnosis of positive sentinel node would
not necessarily need further total axillary dissection (5,9).
The purpose of this study is to verify in a large
single-center consecutive series of early breast cancer patients if
biomarker tumor characterization according to the St. Gallen
criteria (3) can play a role in the
decision making process regarding the surgical management of
axillary lymph node and in particular regarding the use of
SLNB.
Materials and methods
We retrospectively analyzed breast cancer cases
consecutively treated with primary surgery at our National Cancer
Research Centre between 2008–2014. Clinical and biological
information from women enrolled in the study were available:
Complete biomarker assay of the primary tumour permitting the
breast cancer subtype classification according to St. Gallen 2013
(3); known clinical status of
axillary nodes at time of primary surgery; in cases with clinically
negative axilla, histological diagnosis of SLNB.
2002/2420 breast cancer patients were enrolled, of
whom, 1297 (64.8%) with clinically positive axillary nodes
underwent complete ALND; conversely, 705 (35,2%) women with
clinically negative axillary nodes underwent SLNB. The clinical
status of axillary nodes was evaluated by the same operator (D.S.).
130/705 cases (14,6%) treated with SLNB showed metastasis at least
in one of the cleared nodes (median number of cleared
nodes=2.3).
All women had their primary breast cancer analyzed
for tumor size (mm in max diameter), estrogen receptor (ER) and
progesterone receptor (PgR) by ISH, tumour proliferative activity
according to Ki-67MIB expression, Her2/neu amplification according
to ISH/FISH approach.
The present study was approved by the Institutional
Review Board of the National Cancer Research Institute ‘Giovanni
Paolo II’. Before undergoing routine surgery, all patients signed
an informed consent form authorizing the use of the removed
biological tissue for research purposes according to ethical
standards.
Sentinel Lymph Node preoperative
assessment and intraoperative biopsy
In order to detect sentinel lymph nodes, a single
tracer was used according to a two-day-protocol. The day before
surgery patients were injected subdermally above the breast lesion
with 100 MBq Tc-99m-labelled human albumin colloid particles at the
Nuclear Medicine Institute of University of Bari. After injection
of Tc-99m-labelled human albumin colloid particles the radioactive
emission of the tracer in sentinel lymph nodes were identified with
a gamma-camera (e.cam, Siemens) and additionally with a hand-held
gamma-detector probe and marked on the skin. In additon, pictures
including the area of injection and emission of tracer were taken.
A hand-held gamma-detector probe was used to identify the sentinel
node and guide the surgeon intraoperatively (10). The definition of the sentinel node
consisted in any hot node with an ex vivo radioactivity
count of ten times or more above the background count with the
probe held perpendicular and in direct contact to the node. γ-probe
counts were obtained on axillary dissection specimens, all
surrounding nodal basins, and sentinel nodes ex vivo.
Laboratory analyses
After surgical removal the breast tissue was
immediately transferred to the Histopathology Unit of our Institute
where all analyses were performed by operators with long standing
experience in biomarker analysis and in intra-extra laboratory QC
programs (11,12). ER, PgR, and Ki-67Mib-1 biomarkers
were analysed by immunohistochemical assays according to standards
already employed by our team (13).
HER2/neu oncogene status was determined according to ASCO/CAP
guidelines (14).
In more detail, the expression of ER, PgR, and of
Ki-67Mib cellular proliferation index were tested by IHC. Estrogen
and progesterone hormone receptors were tested using monoclonal
rabbit anti-human estrogen receptor α (clone SP1; 1:60 dilution;
Dako, Glostrup, Denmark) and monoclonal mouse anti-human
progesterone receptor (clone PgR 636; 1:100 dilution; Dako)
respectively; Ki-67MiB1 was detected using monoclonal mouse
anti-human Ki-67 antigen (clone E3 ubiquitin protein ligase 1
(MIB-1); 1:80 dilution; Dako). ER and PgR receptors were scored as
positive when >1% of tumor cells were present in the tumor
(15). Ki-67MIB1 expression was
considered high when staining was present in >14% of tumor cells
(16).
All samples were analyzed for HER2/neu expression by
IHC using the HerceptTest™ kit (Dako) according to the
manufacturers' protocol. HER2 was scored as 0, 1+,
2+ or 3+; for tumors scored
1+/2+, FISH for gene amplification was
conducted using a dual HER2/Cep17 probe (Path Vysion HER2 DNA Probe
kit; Abbott Molecular, Inc., Des Plaines, IL, USA. Gene
amplification was reported when HER2/CEP17 ratio was ≥2 or when the
mean HER2 copy number was ≥6.
The Laboratory where the biomarkers assay of breast
cancer was performed participated in QC programs for ISH analysis
of Hormone Receptors and for FISH analysis of HER/2neu managed by
Società Italiana Anatomia Patologica and Citologia Diagnostic.
Statistical analysis
The hormone receptor status, the proliferation
activity of Ki-67/MiB-1 expression and the HER2/NEU
expression/amplification of the tumor were analyzed by univariate
analysis. Frequency of cases in subgroups of patients with
different clinical pathological characteristics were compared by
χ2-test.
The frequency of clinically positive nodes and
histologically positive nodes at SLNB were analyzed separately in
the 5 subgroups of tumours by univariate and multivariate logistic
regressions. A generalized linear model was fitted by the glm
function of R (version 3.2.3) (R Core Team 2015. R:A language and
environment for statistical computing. R Foundation for Statistical
Computing, Vienna, Austria. URL http://www.R-project.org/), adjusting for age.
A Regression Tree analysis, which included all the
main clinical pathological characteristics such as continuous
variables in the model, was performed. The results evidenced the
clinico-pathological characteristics and their cut-off values which
significantly separated subgroups of cases (nodes) with different
percentage of positive SLNB. Results were considered as
statistically significant when P<0.05.
Results
A total of 2002 women participating were involved in
this retrospective study, and they were enrolled considering a
consecutive series of patients treated for primary breast cancer at
our Institute. Median age of the series was 59 years old (range
35–83 years old) (Table I). 7.4%, 30
and 34% of women had T1a-b, T1c, and T2 tumors. 81 and 70% of women
resulted positive to ER+ and PgR tumors, respectively.
20% of the cases resulted HER2/neu positive. A diagnosis of
invasive ductal carcinoma was performed in 76,17%; lobular invasive
carcinoma represented 7,19% of cases.
 | Table I.Clinical characteristics of the
series. |
Table I.
Clinical characteristics of the
series.
|
| Axillary clinical
status | Sentinel lynph node
status |
|---|
|
|
|
|
|---|
| Characteristic | Negative n=710 | Positive
n=1294 | P-value | Negative n=570 | Positive n=130 | P-value |
|---|
| Age (years) |
|
|
1.003×10−9 |
|
| 0.0004 |
|
<59 | 417
(42)a | 574 (58) |
| 318 (76.9) | 95 (23.1) |
|
|
≥59 | 293 (28.9) | 720 (71.1) |
| 252 (87.8) | 35 (12.2) |
|
| T diameter |
|
|
2.2×10−16 |
|
| 0.0001 |
| ≤10
mm | 106 (71.1) | 43 (28.9) |
| 100 (95.2) | 5 (4.8) |
|
| >10
and ≤20 mm | 340 (56.7) | 259 (43.3) |
| 281 (84.1) | 53 (15.9) |
|
| >20
mm | 151 (22.4) | 523 (77.6) |
| 112 (75.2) | 37 (24.8) |
|
|
N/A | 113 (19.4) | 469 (80.6) |
| 77 (68.7) | 35 (31.3) |
|
| ER status |
|
| 0.01 |
|
| 0.49 |
|
Negative | 109 (29.9) | 255 (70.1) |
| 91 (84.2) | 17 (15.8) |
|
|
Positive | 601 (36.6) | 1,039 (63.4) |
| 479 (80.9) | 113 (19.1) |
|
| PgR status |
|
| 0.02 |
|
| 0.32 |
|
Negative | 187 (31.5) | 406 (68.5) |
| 154 (84.1) | 29 (15.9) |
|
|
Positive | 523 (37.1) | 888 (62.9) |
| 416 (80.5) | 101 (19.5) |
|
| HER2/neu
amplified |
|
|
9.72×10−6 |
|
| 0.66 |
|
Negative | 606 (37.8) | 996 (62.2) |
| 489 (81.7) | 109 (18.3) |
|
|
Positive | 104 (25.8) | 298 (74.2) |
| 81 (79.4) | 21 (20.6) |
|
| Ki-67/Mib1
express |
|
|
2.1×10−11 |
|
| 0.03 |
|
Negative | 349 (44.4) | 437 (55.6) |
| 292 (84.6) | 53 (15.4) |
|
|
Positive | 361 (29.6) | 857 (70.4) |
| 278 (78.3) | 77 (21.7) |
|
All patients with primary breast cancer included in
the analysis were classified in 5 biomarker subtypes according to
St Gallen 2013 (3) as reported in
Table II. Luminal A and Luminal
B/HER negative subtypes resulted to be more frequent (35.4 and 35%,
respectively); conversely, HER2 positive not Luminal were the less
frequent (8.1%).
 | Table II.Biomarker classification of 2002
primary breast cancers according to Goldirsch et al
(3). |
Table II.
Biomarker classification of 2002
primary breast cancers according to Goldirsch et al
(3).
| Biomarker
subtypes | N (%) |
|---|
| Luminal A:
ER+ PgR+, MiB-1 low, HER2/NEU neg | 709 (35.37) |
| Luminal B/HER2
negative: ER+, PgR+, MiB-1 high, HER2/NEU
neg | 701 (34.98) |
| Luminal B/HER2
positive: ER+, PgR+, MiB-1 high/low, HER2/NEU
+++ | 239 (11.92) |
| HER2 positive (not
luminal): ER−, PgR−, HER2/NEU +++ | 163 (8.13) |
| Triple negative:
ER−, PgR−, HER2/NEU neg. | 192 (9.6) |
Axillary nodes clinical status
Regarding the assessment of axillary lymph node
clinical status 1297/2002 (64.8%) women treated for primary breast
cancer at our Institute had clinically positive axillary nodes and
were selected to receive complete ALND. This subset of patients was
significantly younger, with tumor diameter significantly smaller
and biomarker asset expressing more aggressiveness in comparison to
women with node negative axilla (Table
I).
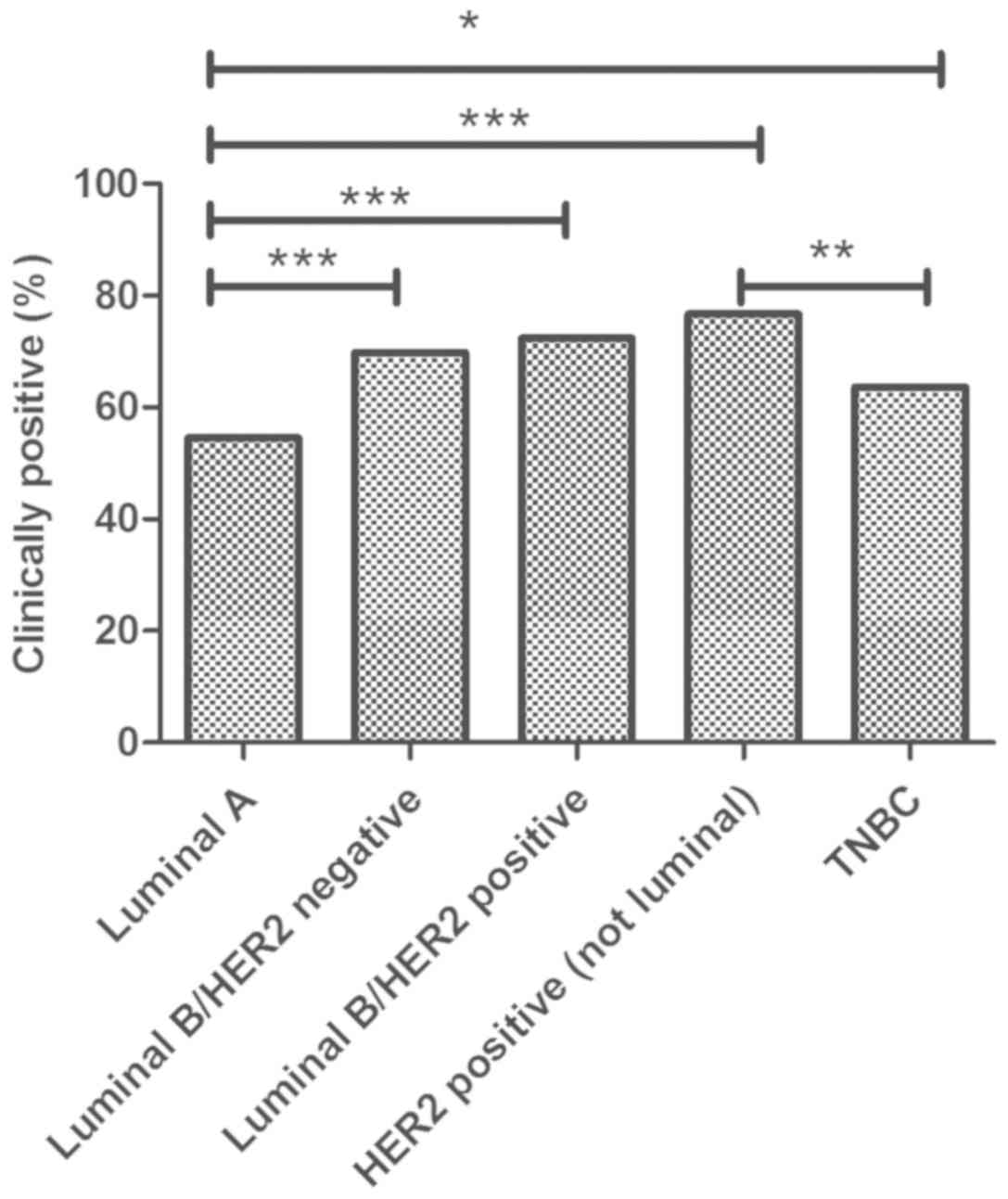
When biomarker subtyping was considered (Fig. 1), Luminal B/HER2 tumors showed the
higher percentage (76.7%) of women with clinically positive axilla,
TNBC the lowest with 63.6% of the cases. The percentage of
clinically positive nodes resulted significantly different between
TNBC and HER2/neu positive subgroups (P<0.01).
To assess the relationships among biomarker subtypes
and axillary nodal status independently from other
clinico-pathological variables of ascertained clinical relevance,
we performed a logistic regression analysis with clinical nodal
status such as dependent variable and tumor size, age at diagnosis
and subtypes included in the model. The multivariate analysis
demonstrated that Luminal A subtype was significantly associated
(OR 0.62; 95% CI 0.49–0.78; P<10−9) with a lower
probability of clinically positive axillary nodes while the
subgroup of HER2 positive/not Luminal with the highest (OR 1.71;
95% CI: 1.12–2.65; P<0.012) (Table
III).
 | Table III.Logistic regression with independent
variable the clinically positive lymph node status. The five
biomarker profiles were analyzed with respect to clinically
positive nodal status, when adjusted for tumor size and age
(median: 59 years). |
Table III.
Logistic regression with independent
variable the clinically positive lymph node status. The five
biomarker profiles were analyzed with respect to clinically
positive nodal status, when adjusted for tumor size and age
(median: 59 years).
| Biomarker
profile | Odds ratio (95%
CI) | P-value |
|---|
| Luminal A | 0.62
(0.49÷0.78) | <0.001 |
| Luminal B/HER2
negative | 1.52
(1.11÷2.10) | 0.009 |
| Luminal B/HER2
positive | 1.62
(1.11÷2.39) | 0.011 |
| HER2+
(not luminal) | 1.71
(1.12÷2.65) | 0.012 |
| Triple
negative | 0.71
(0.49÷1.04) | 0.070 |
SLNB status
All women with clinically negative axillary nodes
(n=705) underwent SLNB. The detection rate of a sentinel lymph node
biopsy assay was >95%. 130 out of 705 (18.5%) cases showed
metastasis at least in one of the surgically cleared nodes (median
number of cleared nodes=2.3). The subgroup of patients having at
least one metastatic node at SLNB was older (P<0.0004) and the
node itself was larger in diameter (P<0.0001) than those without
(Table I).
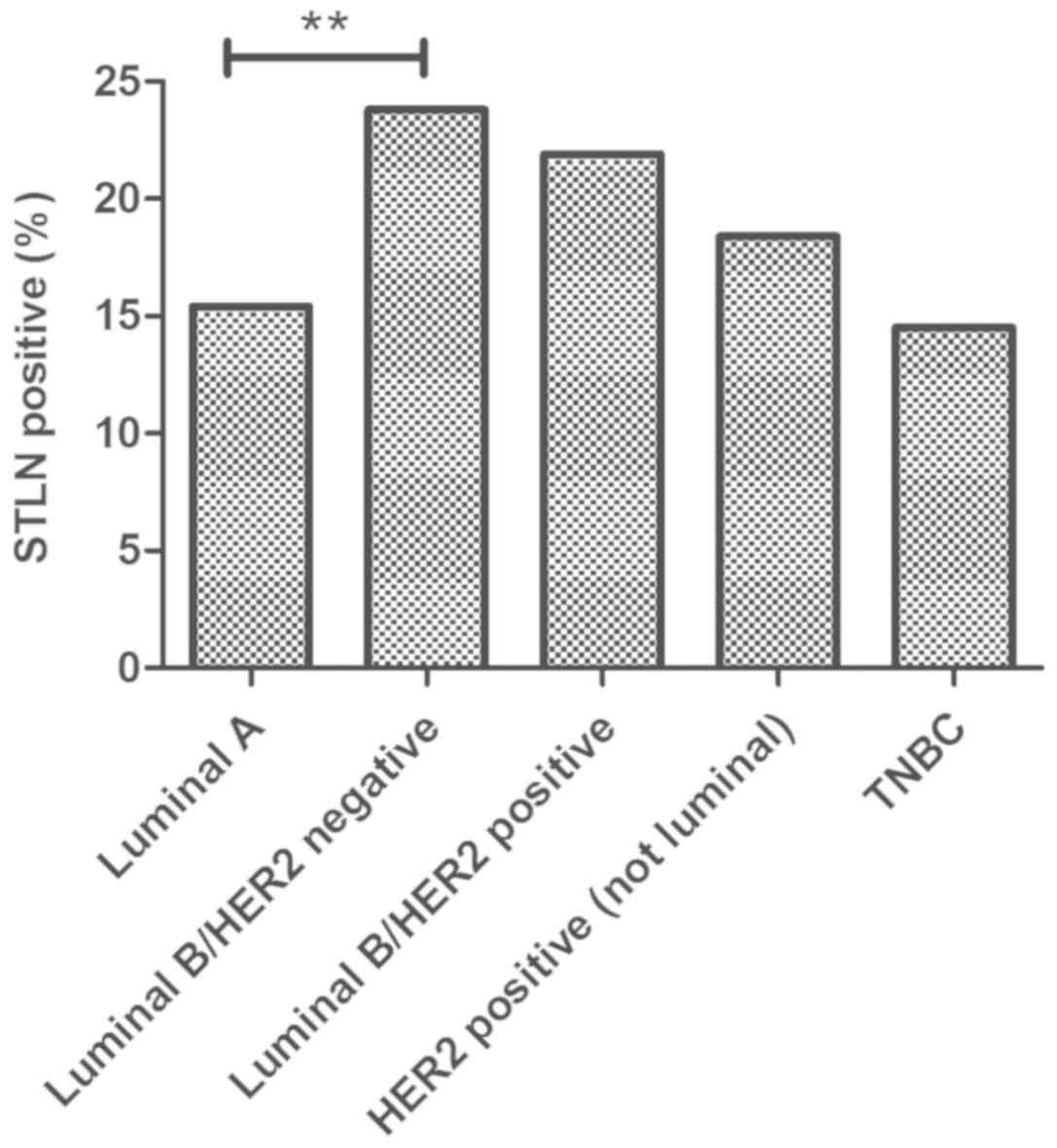
Moreover, the histological SLNB status of nodes was
analyzed with respect to biomarker subtypes. Our series of women
affected by breast cancer and treated with SLNB (n=705), showed a
differently distributed percentage of cases with positive sentinel
lymph node in the 5 subgroups (Fig.
2). In particular, the percentage resulted higher in Luminal
B/HER2 negative tumors (23.8%) and lower in TNBC (14.5%). The
probability of finding metastatic nodes at SLNB resulted
significantly different between Luminal A and Luminal B/HER2
negative subgroups (15.4% vs. 23.8%, respectively; P<0.01).
Moreover, the relationships between breast cancer
subtypes and SLNB status independently from other
clinico-pathological variables of ascertained clinical relevance
were investigated by logistic regression analysis considering node
positivity as an dependent variable and with tumor size, age at
diagnosis, biomarker subtypes included in the model (Table IV). The subtype Luminal B/ HER2
negative showed a strong trend (OR 1.73; 95% CI: 0.94–3.09;
P<0.06) towards an association with a higher probability for
positive SLNB. No additional statistical associations were
verified.
 | Table IV.Logistic regression with independent
variable Axillary Lymph nodal status. The 5 biomarker profiles were
analyzed with respect to clinically positive nodal status, when
adjusted for tumor size and age (median: 59 years). |
Table IV.
Logistic regression with independent
variable Axillary Lymph nodal status. The 5 biomarker profiles were
analyzed with respect to clinically positive nodal status, when
adjusted for tumor size and age (median: 59 years).
| Genomic biomarker
profiles | Odds ratio (95%
CI) | P-value |
|---|
| Luminal A | 0.82
(0.52÷1.29) | 0.39 |
| Luminal B/HER2
negative | 1.73
(0.94÷3.09) | 0.06 |
| Luminal B/HER2
positive | 1.63
(0.75÷3.28) | 0.18 |
| HER2+
(not luminal) | 0.76
(0.25÷1.87) | 0.58 |
| Triple
negative | 0.58
(0.24÷1.21) | 0.18 |
Considering the limited power of biomarker subtyping
in predicting SLNB status, we employed a regression tree analysis
using age, tumor size, ER expression, PgR expression, Ki-67/Mib1
expression as continuous variables, and with HER2/neu status
included in the model (Fig. 3).
We highlighted a decisional tree with marker
cut-offs indicating 5 subgroups of patients (nodes) significantly
different in view of the probability of having positive nodes at
SLNB; the probability resulted significantly different in tumors
with <38 mm compared to those with tumor diameter >38 mm
(P<0.001). Specifically speaking the probability was the highest
(24%) in women with tumour size >38 mm (representing 1.7% of
cases of the overall series) and the lowest (2%) in those with
tumor size <10 mm (14.9% of the overall series). Tumors with
size >10 and <38 mm were further split in two nodes according
to PgR status (higher or lower than 50% of positive tumour
cells).
Discussion
The nodal dissemination represents a complex process
in which a vicious cycle boosts both the cancer cells and the
bystander microenvironment: The recent biological deconvolution of
this reciprocal education uncovered peculiar biomarkers to be
pivotal in facilitating the malignant cells undisturbed lymph-node
invasion. Nodal involvement exemplifies a paradigmatic setting with
prognostic and therapeutic consequences in several malignancies
(17–20).
Therefore, we were interested in verifying weather
biomarker subtyping could impact on the surgical approach to
axillary nodes management in breast cancer. To this end, we
considered the clinical status of the axilla, which, if positive,
implies an ALND. In clinically nodal negative disease, SLNB is
routinely performed. However, SLNB low positive rate often prompts
unnecessary surgery (21). Thus,
there is an urgent need of largely validated markers able to
predict SLNB positivity and, confidently, spare invasive approach,
also supporting the overall clinical management.
Current literature is lacking in data concerning the
search for markers predictive of node status at SLNB. Age confirms
to be a predictive marker for axillary node involvement either at
axillary dissection or at SLNB (22). In addition, the size of the sentinel
node, and the body mass index has been also reported to predict
SLNB status (22,23). High differentiation grade (24), mitotic activity (25,26) and
lympho-vascular invasion (27) have
been also reported.
More recently, the attention has been focused on
biomarker subtyping of breast cancer and axillary node involvement
demonstrating a strong association with node involvement at ALND
(28); conversely, the question is
still debated as regards the association with SLNB status with a
significant relationship reported only for a lower probability of
positive nodes in TNBC (24,29). These last studies were performed in
limited series of Asian patients and were characterized by a lack
of information regarding biological predictors.
We analyzed the relationships between tumour
biomarker subtyping and axillary status in a consecutive series of
2002 women with breast cancer, prospectively collected for this
purpose. All these assays and histopathological diagnosis were
performed in laboratories actively participating in QC programs.
The patients cohort characteristics matched clinical and biological
determinants, as can be seen in studies already published (15,16).
For breast cancer biomarker subtyping, we used the
classification described by Goldirsch et al (3) that has been largely confirmed to be of
prognostic-predictive relevance (4).
The frequency in our series of patients of various breast cancer
subtypes are reported in Table I;
when compared to the Yanagawa observation on 363 patients with
breast cancer classified according to same criteria (30), we reported a lower percentage of
HER2/neu positive tumors (20% vs. 30%, in our and Janagawa series,
respectively).
When considering clinical-pathological factors
predicting clinical status of the axillary nodes (Table I), our experience also confirmed that
tumour size was appeared to be the most powerful factor predicting
clinical involvement of axillary nodes. The same strong
relationship was confirmed regarding the age of women at diagnosis
showing that during clinical observation earlier onset was
associated with significantly higher probability of node
involvement. Clinical involvement of axillary nodes was also more
significantly frequent in hormone receptor negative and HER2/neu
amplified tumors. However, in a multivariate analysis, only the
Ki-67-Mib expression resulted significantly predictive for
statistics (OR 0.99; 0.98–0.99 95% CI; P<0.005).
Table I reports data
on SLNB status as well. In an univariate analysis, age at diagnosis
(P<0.0004) and tumor size (P<0.0001) were confirmed
significantly associated with presence of involved nodes at SLNB.
Regarding the multivariate analysis of biomarkers significantly
associated with SLNB status, we already published that only
PgR-positive status confirmed to be associated independently from
other variables with SLNB status (OR: 0.37; 95% CI: 0.17–0.72;
P<0.005) (31). In this study we
further investigated this point with a regression tree analysis.
Interestingly, we were able to confirm the clinical relevance of
tumor size and of PgR. (Fig. 3). In
fact, we were able to identify tumors moving to it a probability of
close to 80% to have involved nodes at SLNB (tumor diameter >38
mm), to less than 3% (tumors diameter <10 mm); moreover, in
tumors with a diameter comprised between 10 and 38 mm, PgR is able
to split in two different subgroups. This is the only model
demonstrating how it could be possible to manage the SLNB assay
according to a combination of tumor size and PgR status data.
Interestingly, studies published while our data were being
concluded (24,28,29)
uncovered the clinico-pathological characteristics to be relevant
in the management of lymph node metastasis in breast cancer. To our
knowledge, our study substantially extends these findings by
providing deeper insight into the largest, monoinstitutional, and
consecutive cohort of Caucasian patients. Furthermore, we also
employed stringent st Gallen criteria for subtype categorization.
These criteria are of paramount importance in describing mammary
carcinoma characteristics.
Collectively, we uncovered the age at diagnosis and
the tumor size to strong and unequivocal predictors of clinically
evident axillary node involvement in our 2002 Caucasian patients
cohort. Additionally, tumor biomarkers subtyiping according to
Goldirsch classification (3) allowed
us to identify Luminal A tumors with a significantly lower
probability of clinically evident nodes (OR 0.62).
Conversely, in earlier phases of nodal invasion,
characterized by clinically negative axilla, requiring SLNB, tumor
size represents a stronger predictor of nodal metastasis. Moreover,
we found a lack of clinical utility of additional biomarkers in
this scenario. Remarkably, by a regression tree analysis we could
dissect subgroups of tumors with different SLNB nodal involvement
potential. This approach pinpoints biomarker cut-offs for tumour
size and PgR status by stratifying four groups of women with
variable likelihood of SLNB node involvement ranging from 75 to 3%.
This approach hold great clinical promise, prompting statistically
powered studies aiming to SLNB optimization.
Acknowledgements
Not applicable.
Funding
The current study was supported by Ministry of
Health of Italian Government, Funds RC 2017 (grant no. 191/2017)
and by the Apulian Regional Project ‘Medicina di Precisione’ (grant
no. 929/2018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the last author on reasonable request.
Authors contributions
SD and AVP designed the study; RA, CD and MDG
collected data and organized the db analysis; MIP and FAZ provided
all pathological samples, and analyzed biological data; GR provided
all information concerning SLNB; SDS and ST conducted statistical
analyses; AVP wrote the manuscript. AA and NS analyzed and
interpreted data, revised and edited the work. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The retrospective study was approved by IAB of the
Institute. Before undergoing routine surgery, all patients signed
an informed consent form authorizing the use of the removed
biological tissue and personal data for research purposes according
to ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moo TA, Sanford R, Dang C and Morrow M:
Overview of breast cancer therapy. PET Clin. 13:339–354. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen International expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vasconcelos I, Hussainzada A, Berger S,
Fietze E, Linke J, Siedentopf F and Schoenegg W: The St. Gallen
surrogate classification for breast cancer subtypes successfully
predicts tumor presenting features, nodal involvement, recurrence
patterns and disease free survival. Breast. 29:181–185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jatoi I, Benson JR and Toi M:
De-escalation of axillary surgery in early breast cancer. Lancet
Oncol. 17:e430–e441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Veronesi U, Viale G, Paganelli G, Zurrida
S, Luini A, Galimberti V, Veronesi P, Intra M, Maisonneuve P, Zucca
F, et al: Sentinel lymph node biopsy in breast cancer: Ten-year
results of a randomized controlled study. Ann Surg. 251:595–600.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lyman GH, Somerfield MR and Giuliano AE:
Sentinel lymph node biopsy for patients with early-stage breast
cancer: 2016 American Society of clinical oncology clinical
practice guideline update summary. J Oncol Pract. 13:196–198. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan M, Abdi MA and Falkson C: Axillary
management in breast cancer patients: A comprehensive review of the
key trials. Clin Breast Cancer. 18:e1251–e1259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gentilini O and Veronesi U: Abandoning
sentinel lymph node biopsy in early breast cancer? A new trial in
progress at the European Institute of Oncology of Milan (SOUND:
Sentinel node vs. observation after axillary UltraSouND). Breast.
21:678–681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Veronesi U, Paganelli G, Viale G, Luini A,
Zurrida S, Galimberti V, Intra M, Veronesi P, Maisonneuve P, Gatti
G, et al: Sentinel-lymph-node biopsy as a staging procedure in
breast cancer: Update of a randomised controlled study. Lancet
Oncol. 7:983–990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paradiso A, Miller K, Marubini E,
Pizzamiglio S and Verderio P: The need for a quality control of the
whole process of immunohistochemistry human epidermalgrowth factor
receptor 2/neu determination: A United Kingdom National External
Quality Assessment Service/Italian Network for quality assessment
of tumor biomarkers pilot experience. J Clin Oncol. 25:e27–e28.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paradiso A, Volpe S, Iacobacci A, Marubini
E, Verderio P, Costa A, Daidone MG, Marchetti A, Mottolese M,
Amadori D, et al Italian network for quality assessment of tumor
biomarkers, : Quality control for biomarker determination in
oncology: The experience of the Italian network for quality
assessment of tumor biomarkers (INQAT). Int J Biol Markers.
17:201–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paradiso A, Scarpi E, Malfettone A, Addati
T, Giotta F, Simone G, Amadori D and Mangia A: Nuclear NHERF1
expression as a prognostic marker in breast cancer. Cell Death Dis.
4:e9042013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lambein K, Van Bockstal M, Denys H and
Libbrecht L: 2013 update of the American society of clinical
oncology/college of American pathologists guideline for human
epidermal growth factor receptor 2 testing: Impact on
immunohistochemistry-negative breast cancers. J Clin Oncol.
32:1856–1857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hammond MH, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American society of clinical oncology/college of
American pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Medri L, Volpi A, Nanni O, Vecci AM,
Mangia A, Schittulli F, Padovani F, Giunchi DC, Zito A, Amadori D,
et al: Prognostic relevance of mitotic activity in patients with
node-negative breast cancer. Mod Pathol. 16:1067–1075. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stacker SA, Williams SP, Karnezis T,
Shayan R, Fox SB and Achen MG: Lymphangiogenesis and lymphatic
vessel remodelling in cancer. Nat Rev Cancer. 14:159–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Farnsworth RH, Achen MG and Stacker SA:
The evolving role of lymphatics in cancer metastasis. Curr Opin
Immunol. 53:64–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Argentiero A, De Summa S, Di Fonte R,
Iacobazzi RM, Porcelli L, Da Vià M, Brunetti O, Azzariti A,
Silvestris N and Solimando AG: Gene expression comparison between
the lymph node-positive and -negative reveals a peculiar immune
microenvironment signature and a theranostic role for WNT targeting
in pancreatic ductal adenocarcinoma: A pilot study. Cancers
(Basel). 11:9422019. View Article : Google Scholar
|
|
20
|
Tjan-Heijnen V and Viale G: The lymph node
and the metastasis. N Engl J Med. 378:2045–2046. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galimberti V, Cole BF, Zurrida S, Viale G,
Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, et
al: IBCSG 23-01 randomised controlled trial comparing axillary
dissection versus no axillary dissection in patients with
sentinel-node micrometastases. Lancet Oncol. 14:297–305. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sato K, Tamaki K, Shigekawa T, Tsuda H,
Kosuda S, Kusano S, Hiraide H and Mochizuki H: Clinicopathologic
and technical factors associated with the uptake of radiocolloid by
sentinel nodes in patients with breast cancer. Surg Today.
33:403–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schwartz ASK, Leo C, Rufibach K, Varga Z,
Fink D and Gabriel N: Does increased tumor burden of sentinel nodes
in breast cancer affect detection procedure? Eur J Surg Oncol.
39:266–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding J, Jiang L and Wu W: Predictive Value
of clinicopathological characteristics for sentinel lymph Node
Metastasis in early breast cancer. Med Sci Monit. 23:4102–4108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thangarajah F, Malter W, Hamacher S,
Schmidt M, Krämer S, Mallmann P and Kirn V: Predictors of sentinel
lymph node metastases in breast cancer-radioactivity and Ki-67.
Breast. 30:87–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozemir IA, Orhun K, Eren T, Baysal H,
Sagiroglu J, Leblebici M, Ceyran AB and Alimoglu O: Factors
affecting sentinel lymph node metastasis in Turkish breast cancer
patients: Predictive value of Ki-67 and the size of lymph node.
Bratisl Lek Listy. 117:436–441. 2016.PubMed/NCBI
|
|
27
|
Malter W, Hellmich M, Badian M, Kirn V,
Mallmann P and Krämer S: Factors predictive of sentinel lymph node
involvement in primary breast cancer. Anticancer Res. 38:3657–3662.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He ZY, Wu SG, Yang Q, Sun JY, Li FY, Lin Q
and Lin HX: Breast cancer subtype is associated with axillary lymph
node metastasis: A retrospective cohort study. Medicine
(Baltimore). 94:e22132015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao F, Yao R, Peng L, Zhao JL, Liang ZY
and Sun Q: Predictive clinicopathological characteristics affecting
sentinel lymph node metastasis in early breast cancer patients.
Transl Cancer Res. 6:968–975. 2017. View Article : Google Scholar
|
|
30
|
Yanagawa M, Ikemot K, Kawauchi S, Furuya
T, Yamamoto S, Oka M, Oga A, Nagashima Y and Sasaki K: Luminal A
and luminal B (HER2 negative) subtypes of breast cancer consist of
a mixture of tumors with different genotype. BMC Res Notes.
5:3762012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simone G, Diotaiuti S, Digennaro M,
Sambiasi D, De Summa S, Tommasi S, Altieri R, Mangia A, Dantona C
and Paradiso A: Comment on ‘Renewed interest in the progesterone
receptor in breast cancer’. Br J Cancer. 117:e12017. View Article : Google Scholar : PubMed/NCBI
|