As one of the deadliest diseases, cancer represents
a growing clinical challenge worldwide (1), despite surgical, chemotherapeutic and
radiotherapeutic advances (2,3). A
reason for the poor prognosis in cancer is the lack of efficient
pre-progression diagnostic methods and effective prognostic
indicators to guide clinical treatment. Therefore, it is important
and necessary to identify suitable biomolecules for the timely
detection of cancer to inform clinical decisions.
The lncRNA growth arrest-specific transcript 5
(GAS5) is a novel tumor-suppressor lncRNA, which is downregulated
in mammary carcinoma, stomach cancer, lung carcinoma, prostate
cancer and other malignant tumors (16–18). A
summary of research progression regarding the value of GAS5 in the
diagnosis and treatment of different types of cancer is provided in
the present study. In addition, the published mechanisms by which
GAS5 regulates genes involved in tumor genesis are explored and the
potential clinical application of GAS5 is reviewed.
GAS5 was initially described by a report screening
for potential tumor-suppressors whose expression levels were
increased during growth stagnation (19). GAS5 is located on chromosome 1q25,
with ~630 nucleotides (20). It is a
5′-terminal oligopyrimidine RNA comprised of 12 non-conserved exons
(21). GAS5 introns are transcribed
into 10 box C/D small nucleolar RNA (snoRNA) molecules and 2 mature
lncRNAs (GAS5a and GAS5b) (21,22).
Although GAS5 has a short open reading frame, it encodes no protein
and functions as a snoRNA host gene (23).
GAS5 biologically functions through its introns,
which encode a number of snoRNAs (22). Although the exact functions of GAS5
remain unknown, it encodes several snoRNAs regulating ribosomal RNA
synthesis (22). The transcription
products of GAS5 are widely found in tissues, but show instability
in actively dividing cells (24).
The spliced GAS5 RNA expression is decreased in growing cells, but
increased in the growth stagnation period (25). Mourtada-Maarabouni et al
(25) initially investigated the
bioactivity of GAS5 in human T cells and demonstrated that GAS5
overexpression induces apoptosis and decreases the number of cells
in the S-stage, while the silencing of GAS5 leads to the opposite
results. These findings suggested that GAS5 is necessary to inhibit
the growth of T cells (25). Several
studies have been performed to elucidate the function of GAS5 in
cancer. Notably, GAS5 expression levels are downregulated in
several human solid tumors and such aberrant expression is
negatively associated with tumor size, disease stage and prognosis
(24,26,27). In
addition, GAS5 has been shown to affect cell cycle progression and
is essential for normal growth stagnation (28). High GAS5 expression levels inhibit
cell cycle progression, whereas GAS5 suppression reduces apoptosis
and promotes accelerated cell division (29,30),
which will be described in detail later in this review.
Evidence has revealed that GAS5 is abnormally
expressed in various human malignancies (16–18).
GAS5 expression levels are downregulated in numerous malignant
tumors, suggesting that GAS5 may have anticancer activity. In
addition, GAS5 overexpression contributes to growth inhibition in
various tumor cell lines in vitro (16–18). In
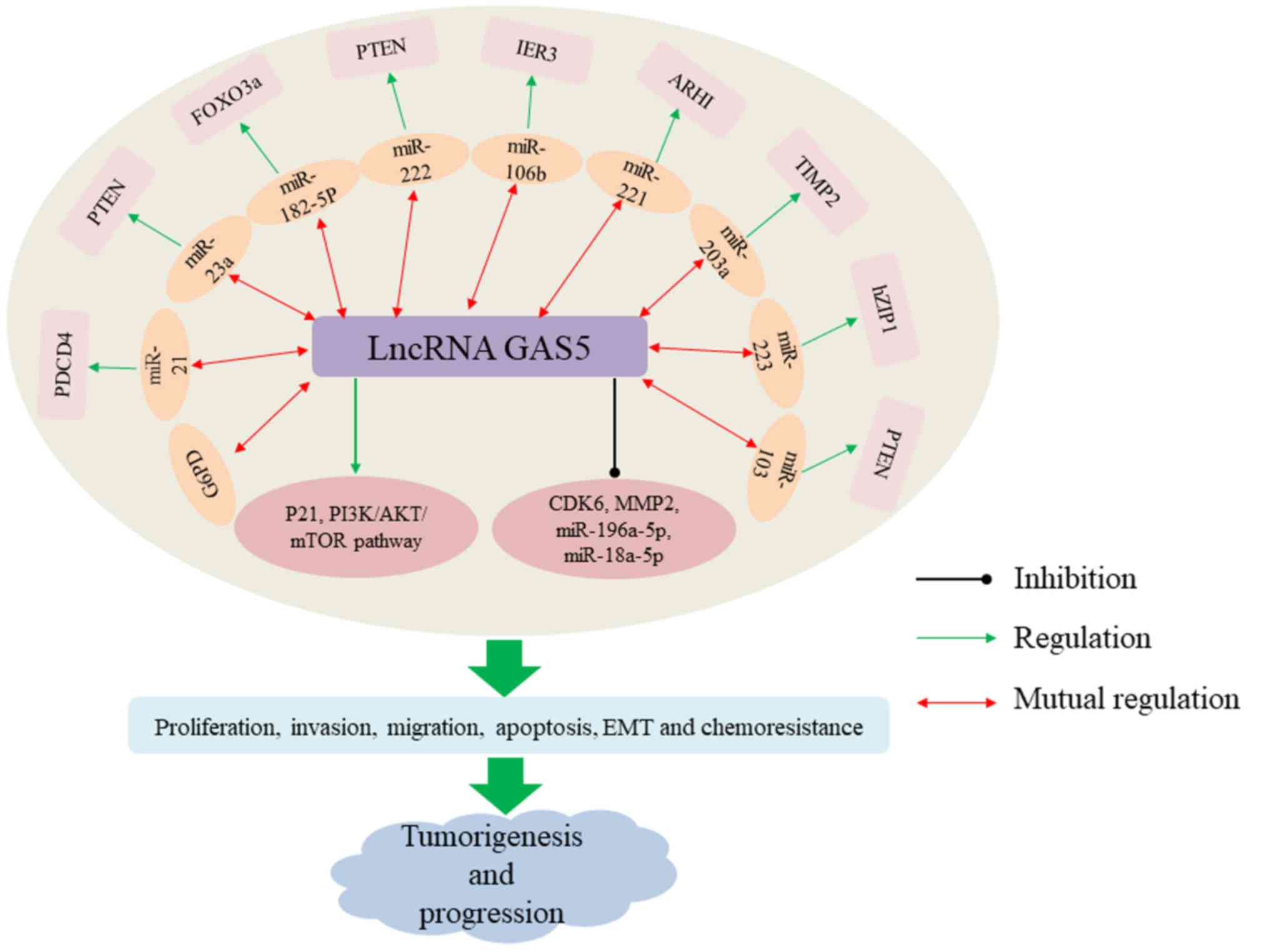
this section, findings regarding GAS5 expression, clinical
significance in various types of human cancer (Tables I and II) and the modulatory mechanisms of GAS5
in malignant tumors are discussed (Fig.
1).
In comparison with non-cancerous tissue samples,
cancer tissue specimens exhibit markedly reduced GAS5 transcription
levels (16). The decrease in GAS5
expression levels suggests a plausible function of GAS5 in
tumorigenesis. GAS5 is commonly downregulated in breast cancer
tissue samples and cells, and this downregulation is associated
with a large tumor volume, advanced tumor lymph node metastasis and
estrogen receptor negativity (31).
GAS5 could be used as a microRNA (miR)-23a sponge, which promotes
autophagy and enhances the formation of autophagosomes after GAS5
overexpression (31). GAS5
downregulates phosphatase and tensin homolog (PTEN) induced by
lapatinib in SKBR-3/Tr cells, suggesting that GAS5 may be a
plausible target for overcoming drug resistance in mammary
carcinoma (32). The decrease in
GAS5 expression levels attenuates the responses of breast cancer
cells to apoptosis stimulation (32). Additionally, the degree of mammary
carcinoma cell death is associated with the expression levels of
GAS5, suggesting that GAS5 is associated with patient prognosis
(33). GAS5 may negatively regulate
miR-21 via the RNA-induced silencing complex (RISC) in breast
cancer (33). It is hypothesized
that miR-21 and GAS5 modulate each other.
PCa is the second most prevalent cancer in men and
the sixth leading cause of cancer-associated mortality in men in
2008 worldwide (34). Compared with
normal prostate tissue or primary prostate cancer, metastatic
prostate cancer cells show markedly reduced GAS5 expression levels
(35). In addition, further studies
have shown that high expression levels of GAS5 promote basal cell
apoptosis of prostate cancer cells and enhance the effect of
apoptosis stimulation (36,37). High GAS5 expression levels enhance
cancer cell death induced by UV-C irradiation and chemotherapeutic
drugs, while GAS5 downregulation attenuates these effects. GAS5
binds to the corresponding region of androgen receptor and
suppresses transcription of this receptor (38). Therefore, improving the expression
levels of cellular GAS5 alongside the administration of
chemotherapeutics could provide an improved strategy for treating
advanced PCa. Since androgen receptor is needed for the survival of
prostate cancer cells, GAS5 downregulation may increase
pro-survival signals via the androgen receptor pathway (39). The aforementioned findings indicate
that GAS5 may have an antitumor function in human prostate
cancer.
Melanoma is an aggressive disease with an increasing
incidence rate and is the leading cause of skin cancer-associated
death in the United States (52).
Although targeted therapy and immunotherapy have markedly improved
melanoma treatment and patient quality of life, currently there are
few suitable drugs for the treatment of this disease (53). Therefore, further assessment of the
metastatic progression in melanoma is needed to develop novel
targeted therapeutics. Overexpression of GAS5 inhibits the
malignant potential of melanoma SK-Mel-110 cells, partly by
reducing MMP2 expression levels and activity (54). One previous study showed that GAS5
has physical effects, with G6PD expression reducing GAS5-associated
cell apoptosis, inducing G1/S progression and altering
reactive oxygen species levels in melanoma cells (55). These findings indicate that abnormal
downregulation of GAS5 has a function in melanoma development.
Esophageal cancer (EC) ranks 8th and 6th in
incidence and mortality, respectively, among malignancies
worldwide, and the majority of EC cases are ESCC (56,57).
Functionally, GAS5 overexpression inhibits ESCC cell proliferation
and reduces the migratory and invasive abilities of ESCC cells by
inactivating the PI3K/Akt/mTOR signaling pathway (58). In addition, GAS5 represents a
miR-196a target and functions as a tumor suppressor gene in ESCC
(59). Furthermore, GAS5 is often
downregulated in ESCC and associated with clinical stage. The
decrease in GAS5 expression levels in ESCC is associated with
miR-196a upregulation (59). GAS5
suppresses tumor growth in ESCC both in cell culture and animal
models (59). Finally, miR-196a may
downregulate GAS5 via the RISC (59). The aforementioned findings suggest
that GAS5 downregulation contributes to ESCC development.
GAS5 expression levels are also associated with
malignancy and metastasis in patients with NSCLC. Indeed, GAS5
upregulation leads to growth stagnation in NSCLC cells and promotes
cell apoptosis both in cell and animal models (60). Meanwhile, GAS5 downregulation
promotes tumor cell growth (60). In
72 NSCLC specimens, GAS5 expression levels were downregulated
compared with those in adjacent noncancerous lung tissue samples.
Downregulated GAS5 expression levels were associated with tumor
size and lymph node metastasis (61). The aforementioned findings indicate
that GAS5 may also serve as an NSCLC biomarker.
Low GAS5 expression levels and high miR-196a-5p
expression levels are associated with increased tumor volume and
more advanced International Federation of Gynecology and Obstetrics
stage (III–IV) in ovarian cancer (62,63).
GAS5 downregulation is associated with increased survival, faster
proliferation and reduced apoptosis rate of ovarian cancer cells,
as well as increased tumor volume in rats (63). GAS5 can directly bind to and regulate
miR-196a-5p. miR-196a-5p expression is elevated in ovarian cancer
tissues and cell lines, suggesting that it could promote ovarian
cancer (63). High GAS5 expression
levels promote apoptosis in ovarian cancer cells (64). These results indicate that reduced
GAS5 expression levels may be an indicator of poor prognosis in
ovarian cancer and a plausible target for diagnosing and treating
this malignancy.
GAS5 is downregulated in multiple malignancies,
including CC. A previous study showed that GAS5-knockdown can
promote malignancy in CC cells (65), while GAS5 overexpression increases
the resistance of CC cells to cisplatin by modulating Akt
phosphorylation (66). In addition,
according to bioinformatic prediction, GAS5 is a molecular sponge
of miR-106b; reduced GAS5 and elevated miR-106b expression levels
were found in clinical CC tissue samples from patients insensitive
to radiotherapy (67,68). GAS5 regulates IER3 via miR-106b, and
could enhance radiosensitivity in CC cells by suppressing miR-106b
both in cell and animal models (69). Together, these findings suggest that
GAS5 may be a novel plausible target for cervical cancer
treatment.
In comparison with non-cancerous cerebral tissues,
gliomas show reduced GAS5 expression levels (70), which are associated with a longer
survival time (71). It has been
demonstrated that GAS5 expression levels are elevated under stress
in glioma cells induced by DNA damage (72). Previously, Zhao et al
(73) confirmed GAS5 exerts
antitumor effects in glioma cells via direct targeting of miR-222.
GAS5 inhibits glioma cell proliferation both in cell and animal
models, as well as cell migration and invasion. In addition, GAS5
downregulates miR-18a-5p, which in turn reduces GAS5 expression
levels (74). It has also been found
that GAS5 influences the biological properties of glioma cells by
suppressing miR-18a-5p, thereby altering neogenin expression
(74). These data indicate that GAS5
may present a novel therapeutic target for glioma treatment.
Elevated GAS5 expression levels suppress
proliferation, migration and epithelial mesenchymal transformation
(EMT) in osteosarcoma cells (75).
GAS5 shows direct binding to miR-221, decreasing the expression
levels of miR-221 and upregulating ARHI. Additionally, a role for
GAS5 in reducing tumor growth in osteosarcoma has been demonstrated
in vivo (75). Competing with
miR-221, GAS5 suppresses growth in osteosarcoma cells via
miR-221/ARHI signaling (75). GAS5
may represent a tumor suppressor in osteosarcoma, isolating
miR-203a from TIMP2 through sponge action (76). These findings suggest that abnormal
GAS5 downregulation may promote tumor growth via miR-221/ARHI
signaling.
Previous studies have shown that GAS5 expression in
PC is markedly decreased compared with that in non-cancerous
pancreatic ductal cells. Overexpression of GAS5 enhances the
expression of cytokine signal transduction factor 3 and inhibits
proliferation, migration, gemcitabine resistance, stem-like
characteristics and EMT in PC cells via direct binding to and
downregulation of miR-221 (77).
Furthermore, the expression levels of CDK6 are reduced by GAS5 in
cell and animal models (77). CDK6
downregulation partly decreases GAS5-siRNA-associated tumor cell
growth (78). The aforementioned
results indicate that decreased GAS5 expression levels may promote
growth and metastasis in PC by negatively regulating CDK6.
GAS5 expression levels in bladder cancer cells and
clinical samples are low and inversely associated with disease
stage, and elevated GAS5 expression levels inhibit bladder cancer
cell proliferation (79). By
interacting with the transcription factor E2F transcription factor
4, GAS5 downregulates EZH2 at the mRNA level and promotes apoptosis
in bladder cancer cells, and GAS5 serves a role in GA-associated
apoptosis in bladder cancer (79).
Additionally, GAS5 exerts its effects in part via CDK6 regulation,
as GAS5 downregulation increases CDK6 mRNA and protein expression
levels, and since inhibition of GAS5 leads to significantly
decreased G0/G1 phase and markedly increased
S phase in bladder cancer cells (80). These data suggest that GAS5 may
represent a novel target for treating bladder cancer.
In comparison with non-cancerous cells, RCC cells
show significantly downregulated GAS5 expression levels (81). GAS5 overexpression decreases the
level of malignancy in RCC cells (82). A study showed that GAS5 serves as a
competing endogenous RNA for miR-223; the inhibition of GAS5
attenuates the activity of miR-223 inhibitor on cell growth,
apoptosis and invasion. Additionally, GAS5 downregulation
facilitates tumor growth in vivo, which is abolished by
hZIP1 overexpression (82). The
aforementioned data indicates that GAS5 may present a novel target
for RCC treatment.
GAS5 expression levels are markedly reduced in PTC
tissue samples and cells. GAS5 overexpression suppresses PTC growth
in cell and animal models. In addition, GAS5 is considered a target
of miR-222-3p, which is abnormally elevated in PTC cells (83). These data indicate that GAS5 may
present a novel target for treating PTC.
GAS5 is highly expressed in both MYCN-amplified and
non-amplified neuroblastoma cells, and GAS5 downregulation leads to
elevated cell proliferation and reduced apoptosis and cell cycle
arrest in neuroblastoma cells (84).
In addition, deletion of GAS5 upregulates p53, BRCA1 protein and
growth arrest and DNA-damage-inducible protein α, which seem to
simultaneously regulate cell cycle arrest (84). These data indicate that abnormal GAS5
downregulation may promote neuroblastoma progression by inducing
cell cycle arrest.
The expression levels of GAS5 in HCC tissues is
decreased compared with normal adjacent tissues, suggesting poor
prognosis of HCC patients (86).
Additionally, GAS5 suppresses hepatoma cell growth in association
with vimentin regulation (86).
Another study showed that GAS5 serves an anticancer role in HCC by
negatively regulating miR-21 and its target protein PDCD4 and PTEN
to alter the migratory and invasive features of HCC cells (87). In addition, Tao et al
(88) reported that GAS5 may act as
a proto-oncogene in HCC. Therefore, GAS5 may potentially be a novel
target for HCC treatment.
GAS5 is downregulated in a number of human
malignancies. GAS5 can induce apoptosis and inhibit the
proliferative and metastatic properties of tumors. The exact
molecular mechanisms by which GAS5 affects cancer development
remain unclear. GAS5 may serve a role in tumorigenesis by
regulating multiple tumor-associated molecules. Tumor inhibition by
GAS5 has been described for numerous malignancies, such as breast
cancer, prostate cancer, CRC, stomach cancer, melanoma, ESCC,
NSCLC, ovarian cancer, cervical cancer, gliomas, osteosarcoma,
pancreatic cancer, bladder cancer, RCC, PTC, neuroblastoma,
endometrial cancer and HCC, indicating a role for this lncRNA as a
tumor suppressor. In this review, the potential of GAS5 in
diagnosing and treating different cancer types, and its potential
clinical application, were summarized, pointing to a new direction
for GAS5 research.
Not applicable.
The present study was funded by The Provincial
Natural Science Foundation of Hunan (grant nos. 2019JJ50500 and
2017JJ2226), The Hunan Provincial Key Laboratory of Tumor
Microenvironment Responsive Drug Research [Hunan Provincial Science
and Technology Department Document (grant number 2019–56)], The
Scientific Research Fund of Hunan Provincial Education Department
(grant no. 17C1402), The Hunan Province Cooperative Innovation
Center for Molecular Target New Drug Study (grant no. 2014-405) and
The Undergraduate Research-based Learning and Innovative
Experimental Program of University of South China (grant nos.
2017XJYZ040 and 2018XJXZ348).
Not applicable.
XY wrote the original draft. The manuscript was
revised by ZX, XL and RG. The authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zugazagoitia J, Guedes C, Ponce S, Ferrer
I, Molina-Pinelo S and Paz-Ares L: Current challenges in cancer
treatment. Clin Ther. 38:1551–1566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma Y, Yang Y, Wang F, Moyer MP, Wei Q,
Zhang P, Yang Z, Liu W, Zhang H, Chen N, et al: Long non-coding RNA
CCAL regulates colorectal cancer progression by activating Wnt/β
catenin signalling pathway via suppression of activator protein 2α.
Gut. 65:1494–1504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruan X, Li P, Cangelosi A, Yang L and Cao
H: A long non-coding RNA, lncLGR, regulates hepatic glucokinase
expression and glycogen storage during fasting. Cell Rep.
14:1867–1875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Veneziano D, Nigita G and Ferro A:
Computational approaches for the analysis of ncRNA through deep
sequencing techniques. Front Bioeng Biotechnol. 3:772015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Theis M, Paszkowski-Rogacz M, Weisswange
I, Chakraborty D and Buchholz F: Targeting human long noncoding
transcripts by endoribonucleaseprepared sirnas. J Biomol Screen.
20:1018–1026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X and Yan GY: Novel human
lncRNA-disease association inference based on lncRNA expression
profiles. Bioinformatics. 29:2617–2624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Yan CC, Luo C, Ji W, Zhang Y and
Dai Q: Constructing lncRNA functional similarity network based on
lncRNA-disease associations and disease semantic similarity. Sci
Rep. 5:113382015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmitt AM and Chang HY: Long noncoding
rnas in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Chen S, Yang G, Gu F, Li M, Zhong
B, Hu J, Hoffman A and Chen M: Long noncoding RNA HOTAIR as an
independent prognostic marker in cancer: A meta-analysis. PLoS One.
9:e1055382014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue Y, Teng YQ, Zhou JD and Rui YJ:
Prognostic value of long non-coding RNA MALAT1 in various
carcinomas: Evidence from nine studies. Tumour Biol. 37:1211–1215.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mourtada-Maarabouni M, Pickard MR, Hedge
VL, Farzaneh F and Williams GT: GAS5, a non-protein-coding RNA,
controls apoptosis and is downregulated in breast cancer. Oncogene.
28:195–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun M, Jin FY, Xia R, Kong R, Li JH, Xu
TP, Liu YW, Zhang EB, Liu XH and De W: Decreased expression of long
non-coding RNA GAS5 indicates a poor prognosis and promotes cell
proliferation in gastric cancer. BMC Cancer. 14:3192014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pickard MR, Mourtada-Maarabouni M and
Williams GT: Long non-coding RNA GAS5 regulates apoptosis in
prostate cancer cell lines. Biochim Biophys Acta. 1832:1613–1623.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coccia EM, Cicala C, Charlesworth A,
Ciccarelli C, Rossi GB, Philipson L and Sorrentino V: Regulation
and expression of a growth arrest-specific gene (gas5) during
growth, differentiation, and development. Mol Cell Biol.
12:3514–3521. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fleming JV, Fontanier N, Harries DN and
Rees WD: The growth arrest genes gas5, gas6, and CHOP-10 (gadd153)
are expressed in the mouse preimplantation embryo. Mol Reprod Dev.
48:310–316. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schneider C, King RM and Philipson L:
Genes specifically expressed at growth arrest of mammalian cells.
Cell. 54:787–793. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith CM and Steitz JA: Classification of
gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member
of the 5′-terminal oligopyrimidine gene family reveals common
features of snoRNA host genes. Mol Cell Biol. 18:6897–6909. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma C, Shi X, Zhu Q, Li Q, Liu Y, Yao Y and
Song Y: The growth arrest-specific transcript 5 (GAS5): A pivotal
tumor suppressor long non-coding RNA in human cancers. Tumour Biol.
37:1437–1444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F
and Song Y: A critical role for the long non-coding RNA GAS5 in
proliferation and apoptosis in non-small-cell lung cancer. Mol
Carcinog. 54 (Suppl 1):E1–E12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mourtada-Maarabouni M, Hedge VL, Kirkham
L, Farzaneh F and Williams GT: Growth arrest in human T-cells is
controlled by the non-coding RNA growth-arrest-specific transcript
5 (GAS5). J Cell Sci. 121:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun M, Jin FY, Xia R, Kong R, Li JH, Xu
TP, Liu YW, Zhang EB, Liu XH and De W: Decreased expression of long
non-coding RNA GAS5 indicates a poor prognosis and promotes cell
proliferation in gastric cancer. BMC Cancer. 14:3192014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin D, He X, Zhang E, Kong R, De W and
Zhang Z: Long non-coding RNA GAS5 affects cell proliferation and
predicts a poor prognosis in patients with colorectal cancer. Med
Oncol. 31:2532014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3:ra82010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Z, Wang W, Jiang J, Bao E, Xu D, Zeng
Y, Tao L and Qiu J: Downregulation of GAS5 promotes bladder cancer
cell proliferation, partly by regulating CDK6. PLoS One.
8:e739912013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu X, Fang Y, Wang Z, Xie J, Zhan Q, Deng
X, Chen H, Jin J, Peng C, Li H, et al: Downregulation of gas5
increases pancreatic cancer cell proliferation by regulating CDK6.
Cell Tissue Res. 354:891–896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu J, Wang Y, Wang X, Zhou D, Wang X, Zhou
M and He Z: Effect of the lncRNA GAS5-miR-23a-ATG3 axis in
regulating autophagy in patients with breast cancer. Cell Physiol
Biochem. 48:194–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Zhai L, Wang H, Liu C, Zhang J, Chen
W and Wei Q: Downregulation of LncRNA GAS5 causes trastuzumab
resistance in breast cancer. Oncotarget. 7:27778–27786. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C,
Xu M, Wu F and Mo YY: Negative regulation of lncRNA GAS5 by miR-21.
Cell Death Differ. 20:1558–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pickard MR, Mourtada-Maarabouni M and
Williams GT: Long non-coding RNA GAS5 regulates apoptosis in
prostate cancer cell lines. Biochim Biophys Acta. 1832:1613–1623.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo G, Liu D, Huang C, Wang M, Xiao X,
Zeng F, Wang L and Jiang G: LncRNA GAS5 inhibits cellular
proliferation by targeting P27Kip1. Mol Cancer Res. 15:789–799.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie X, Dai J, Huang X, Fang C and He W:
MicroRNA-145 inhibits proliferation and induces apoptosis in human
prostate carcinoma by upregulating long non-coding RNA GAS5. Oncol
Lett. 18:1043–1048. 2019.PubMed/NCBI
|
|
38
|
Abbah SA, Lam CX, Ramruttun AK, Goh JC and
Wong HK: Fusion performance of low-dose recombinant human bone
morphogenetic protein 2 and bone marrow-derived multipotent stromal
cells in biodegradable scaffolds: A comparative study in a large
animal model of anterior lumbar interbody fusion. Spine.
36:1752–1759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Su X, Kong Z, Fu F, Zhang P, Wang
D, Wu H, Wan X and Li Y: An androgen reduced transcript of LncRNA
GAS5 promoted prostate cancer proliferation. PLoS One.
12:e01823052017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cheng K, Zhao Z, Wang G, Wang J and Zhu W:
lncRNA GAS5 inhibits colorectal cancer cell proliferation via the
miR-182-5p/FOXO3a axis. Oncol Rep. 40:2371–2380. 2018.PubMed/NCBI
|
|
42
|
Yang Y, Shen Z, Yan Y, Wang B, Zhang J,
Shen C, Li T, Ye C, Gao Z, Peng G, et al: Long non-coding RNA GAS5
inhibits cell proliferation, induces G0/G1 arrest and apoptosis,
and functions as a prognostic marker in colorectal cancer. Oncol
Lett. 13:3151–3158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miyahara R, Niwa Y, Matsuura T, Maeda O,
Ando T, Ohmiya N, Itoh A, Hirooka Y and Goto H: Prevalence and
prognosis of gastric cancer detected by screening in a large
Japanese population: Data from a single institute over 30 years. J
Gastroenterol Hepatol. 22:1435–1442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zabaleta J: Multifactorial etiology of
gastric cancer. Methods Mol Biol. 863:411–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vogelaar IP, van der Post RS, Bisseling
TM, van Krieken JHJ, Ligtenberg MJ and Hoogerbrugge N: Familial
gastric cancer: Detection of a hereditary cause helps to understand
its etiology. Hered Cancer Clin Pract. 10:182012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Correa P and Schneider BG: Etiology of
gastric cancer: What is new? Cancer Epidemiol Biomarkers Prev.
14:1865–1868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo X, Deng K, Wang H, Xia J, Shan T,
Liang Z, Yao L and Jin S: GAS5 Inhibits gastric cancer cell
proliferation partly by modulating CDK6. Oncol Res Treat.
38:362–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu X, Jiao T, Wang Y, Su W, Tang Z and
Han C: Long non-coding RNA GAS5 acts as a molecular sponge to
regulate miR-23a in gastric cancer. Minerva Med. Nov 9–2016.(Epub
ahead of print).
|
|
49
|
Sun M, Jin FY, Xia R, Kong R, Li JH, Xu
TP, Liu YW, Zhang EB, Liu XH and De W: Decreased expression of long
non-coding RNA GAS5 indicates a poor prognosis and promotes cell
proliferation in gastric cancer. BMC Cancer. 14:3192014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang N, Wang AY, Wang XK, Sun XM and Xue
HZ: GAS5 is downregulated in gastric cancer cells by promoter
hypermethylation and regulates adriamycin sensitivity. Eur Rev Med
Pharmacol Sci. 20:3199–3205. 2016.PubMed/NCBI
|
|
51
|
Li Y, Gu J and Lu H: The GAS5/miR-222 axis
regulates proliferation of gastric cancer cells through the PTEN/
Akt/ mTOR pathway. Dig Dis Sci. 62:3426–3437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun J, Carr MJ and Khushalani NI:
Principles of targeted therapy for melanoma. Surg Clin North Am.
100:175–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen L, Yang H, Xiao Y, Tang X, Li Y, Han
Q, Fu J, Yang Y and Zhu Y: Lentiviral-mediated overexpression of
long non-coding RNA GAS5 reduces invasion by mediating MMP2
expression and activity in human melanoma cells. Int J Oncol.
48:1509–1518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen L, Yang H, Yi Z, Jiang L, Li Y, Han
Q, Yang Y, Zhang Q, Yang Z, Kuang Y, et al: LncRNA GAS5 regulates
redox balance and dysregulates the cell cycle and apoptosis in
malignant melanoma cells. J Cancer Res Clin Oncol. 145:637–652.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Arnold M, Soerjomataram I, Ferlay J and
Forman D: Global incidence of oesophageal cancer by histological
subtype in 2012. Gut. 64:381–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang G, Sun J, Zhao H and Li H: Long
non-coding RNA (lncRNA) Growth Arrest Specific 5 (GAS5) suppresses
esophageal squamous cell carcinoma cell proliferation and migration
by inactivating phosphatidylinositol 3-kinase (PI3K)/AKT Mammalian
Target of Rapamycin (mTOR) signaling pathway. Med Sci Monit.
24:7689–7696. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang K, Li J, Xiong G, He G, Guan X, Yang
K and Bai Y: Negative regulation of lncRNA GAS5 by miR-196a
inhibits esophageal squamous cell carcinoma growth. Biochem Biophys
Res Commun. 495:1151–1157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mei Y, Si J, Wang Y, Huang Z, Zhu H, Feng
S, Wu X and Wu L: Long non-coding RNA GAS5 suppresses tumorigenesis
by inhibiting miR-23a expression in non-small cell lung cancer.
Oncol Res. 25:1027–1037. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F
and Song Y: A critical role for the long non-coding RNA GAS5 in
proliferation and apoptosis in non-small-cell lung cancer. Mol
Carcinog. 54 (Suppl 1):E1–E12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
McComas KN, Torgeson AM, Ager BJ,
Hellekson C, Burt LM, Maurer KA, Werner TL and Gaffney DK: The
variable impact of positive lymph nodes in cervical cancer:
Implications of the new FIGO staging system. Gynecol Oncol.
156:85–92. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhao H, Yu H, Zheng J, Ning N, Tang F,
Yang Y and Wang Y: Lowly-expressed lncRNA GAS5 facilitates
progression of ovarian cancer through targeting miR-196-5p and
thereby regulating HOXA5. Gynecol Oncol. 151:345–355. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gao J, Liu M, Zou Y, Mao M, Shen T, Zhang
C, Song S, Sun M, Zhang S, Wang B, et al: Long non-coding RNA
growth arrest-specific transcript 5 is involved in ovarian cancer
cell apoptosis through the mitochondria-mediated apoptosis pathway.
Oncol Rep. 34:3212–3221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cao S, Liu W, Li F, Zhao W and Qin C:
Decreased expression of lncRNA GAS5 predicts a poor prognosis in
cervical cancer. Int J Clin Exp Pathol. 7:6776–6783.
2014.PubMed/NCBI
|
|
66
|
Wen Q, Liu Y, Lyu H, Xu X, Wu Q, Liu N,
Yin Q, Li J and Sheng X: Long non-coding RNA GAS5, which acts as a
tumor suppressor via microRNA 21, regulates cisplatin resistance
expression in cervical cancer. Int J Gynecol Cancer. 27:1096–1108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ma D, Zhang YY, Guo YL, Li ZJ and Geng L:
Profiling of microRNA-mRNA reveals roles of microRNAs in cervical
cancer. Chin Med J (Engl). 125:4270–4276. 2012.PubMed/NCBI
|
|
68
|
Zheng L, Zhang Y, Liu Y, Zhou M, Lu Y,
Yuan L, Zhang C, Hong M, Wang S and Li X: MiR-106b induces cell
radioresistance via the PTEN/PI3K/AKT pathways and p21 in
colorectal cancer. J Transl Med. 13:2522015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gao J, Liu L, Li G, Cai M, Tan C, Han X
and Han L: LncRNA GAS5 confers the radio sensitivity of cervical
cancer cells via regulating miR-106b/IER3 axis. Int J Biol
Macromol. 126:994–1001. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang X, Sun S, Pu JK, Tsang AC, Lee D,
Man VO, Lui WM, Wong ST and Leung GK: Long non-coding RNA
expression profiles predict clinical phenotypes in glioma.
Neurobiol Dis. 48:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang XQ, Sun S, Lam KF, Kiang KM, Pu JK,
Ho AS, Lui WM, Fung CF, Wong TS and Leung GK: A long non-coding RNA
signature in glioblastoma multiforme predicts survival. Neurobiol
Dis. 58:123–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu Q, Sun S, Yu W, Jiang J, Zhuo F, Qiu
G, Xu S and Jiang X: Altered expression of long non-coding RNAs
during genotoxic stress-induced cell death in human glioma cells. J
Neurooncol. 122:283–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhao X, Wang P, Liu J, Zheng J, Liu Y,
Chen J and Xue Y: Gas5 exerts tumor-suppressive functions in human
glioma cells by targeting miR-222. Mol Ther. 23:1899–1911. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu Q, Yu W, Zhu S, Cheng K, Xu H, Lv Y,
Long X, Ma L, Huang J, Sun S, et al: Long non-coding RNA GAS5
regulates the proliferation, migration, and invasion of glioma
cells by negatively regulating miR-18a-5p. J Cell Physiol.
234:757–768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ye K, Wang S, Zhang H, Han H, Ma B and Nan
W: Long non-coding RNA GAS5 suppresses cell growth and
epithelial-mesenchymal transition in osteosarcoma by regulating the
miR-221/ARHI pathway. J Cell Biochem. 118:4772–4781. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang Y and Kong D: LncRNA GAS5 represses
osteosarcoma cells growth and metastasis via sponging MiR-203a.
Cell Physiol Biochem. 45:844–855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liu B, Wu S, Ma J, Yan S, Xiao Z, Wan L,
Zhang F, Shang M and Mao A: lncRNA GAS5 reverses EMT and tumor stem
cell-mediated gemcitabine resistance and metastasis by targeting
miR-221/SOCS3 in pancreatic cancer. Mol Ther Nucleic Acids.
13:472–482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lu X, Fang Y, Wang Z, Xie J, Zhan Q, Deng
X, Chen H, Jin J, Peng C, Li H, et al: Downregulation of gas5
increases pancreatic cancer cell proliferation by regulating CDK6.
Cell Tissue Res. 354:891–896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang M, Guo C, Wang L, Luo G, Huang C, Li
Y, Liu D, Zeng F, Jiang G and Xiao X: Long non-coding RNA GAS5
promotes bladder cancer cells apoptosis through inhibiting EZH2
transcription. Cell Death Dis. 9:2382018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu Z, Wang W, Jiang J, Bao E, Xu D, Zeng
Y, Tao L and Qiu J: Downregulation of GAS5 promotes bladder cancer
cell proliferation, partly by regulating CDK6. PLoS One.
8:e739912013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu L, Pang X, Shang W, Xie H, Feng Y and
Feng G: Long non-coding RNA GAS5 sensitizes renal cell carcinoma to
sorafenib via miR-21/SOX5 pathway. Cell Cycle. 18:257–263. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Dong X, Kong C, Liu X, Bi J, Li Z, Li Z,
Zhu Y and Zhang Z: GAS5 functions as a ceRNA to regulate hZIP1
expression by sponging miR-223 in clear cell renal cell carcinoma.
Am J Cancer Res. 8:1414–1426. 2018.PubMed/NCBI
|
|
83
|
Zhang XF, Ye Y and Zhao SJ: LncRNA Gas5
acts as a ceRNA to regulate PTEN expression by sponging miR-222-3p
in papillary thyroid carcinoma. Oncotarget. 9:3519–3530. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mazar J, Rosado A, Shelley J, Marchica J
and Westmoreland TJ: The long non-coding RNA GAS5 differentially
regulates cell cycle arrest and apoptosis through activation of
BRCA1 and p53 in human neuroblastoma. Oncotarget. 8:6589–6607.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Guo C, Song WQ, Sun P, Jin L and Dai HY:
LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in
endometrial cancer cells. J Biomed Sci. 22:1002015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chang L, Li C, Lan T, Wu L, Yuan Y, Liu Q
and Liu Z: Decreased expression of long non-coding RNA GAS5
indicates a poor prognosis and promotes cell proliferation and
invasion in hepatocellular carcinoma by regulating vimentin. Mol
Med Rep. 13:1541–1550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hu L, Ye H, Huang G, Luo F, Liu Y, Liu Y,
Yang X, Shen J, Liu Q and Zhang J: Long noncoding RNA GAS5
suppresses the migration and invasion of hepatocellular carcinoma
cells via miR-21. Tumour Biol. 37:2691–2702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tao R, Hu S, Wang S, Zhou X, Zhang Q, Wang
C, Zhao X, Zhou W, Zhang S, Li C, et al: Association between indel
polymorphism in the promoter region of lncRNA GAS5 and the risk of
hepatocellular carcinoma. Carcinogenesis. 36:1136–1143. 2015.
View Article : Google Scholar : PubMed/NCBI
|