Introduction
Bladder cancer (BCa) is one of the most common types
of urological cancer, resulting in ~150,000 deaths worldwide every
year (1). Approximately 75% of new
BCa cases present as non-muscle-invasive BCa (NMIBC) and up to 50%
of them will recur with 20% of these recurring cases progressing
(2). Treatment options for BCa vary
based on the stage at diagnosis. For MIBC and recurrent NMIBC,
radical cystectomy (RC) is usually the mainstay of treatment, where
the goal is to provide optimal cancer control by removal of whole
bladder tissue (3). Despite this,
~4-10% of patients are still susceptible to secondary urothelial
tumors following RC and frequently have an adverse prognosis due to
late diagnosis (4). Although
numerous serum and urine biomarkers have been suggested, their
precision and specificity still require further evidence (5). Therefore, identification of a novel
molecular mechanism or biomarker that may provide a novel treatment
option, retard the progression of Bca, improve the prediction of
the prognosis of BCa may provide a significant benefit in terms of
benefiting clinical treatment greatly.
Apurinic/apyrimidinic endodeoxyribonuclease 1 (APE1)
protein, also known as redox factor-1 (Ref-1), was originally
identified as a multifunctional protein involved in DNA base
excision repair (BER) and redox signaling. Previous studies have
indicated that APE1 expression is not only significantly higher in
multiple cancer types, including liver cancer, ovary cancer and
osteosarcoma, but also highly associated with prognosis (6–8).
Although APE1 was suggested as a urinary biomarker (5,9), its
prognostic value in cancer tissue has not been verified with
sufficient cases.
Previously, Sun and Nelson (10) suggested that the DNA repair gene APE1
is able to promote the production of a spectrum of cytokines,
growth factors and proteases, including vascular endothelial growth
factor (VEGF), by the DNA damage secretory program. In the tumor
microenvironment (TME), VEGF is secreted by tumor cells and diffuse
through the tissue contributing to angiogenesis (11). Furthermore, VEGF has a role as an
immunosuppressive gene, inducing a ‘tumor endothelial barrier’ to
promote T-cell arrest, or the loss of T cell motility (12). In BCa, VEGF has been indicated to be
associated with stage and recurrence. Wheeler et al
(13) suggested that VEGF is able to
recruit macrophages and polarize them to the M2 phenotype in the
decidua. This may provide a novel mechanism to how VEGF is able to
worsen the clinical outcomes of BCa through tumor-associated
macrophage (TAM) manipulation. The study of TAMs impacting the
progression and metastasis of cancer has been fruitful.
Alternatively activated M2 macrophages in TME are responsible for
angiogenesis and tissue remodeling and feature an immunosuppressive
phenotype, which may have an important role in tumor progression
(14). In BCa, M2 type macrophages
are usually associated with poor response to BCG therapy and may
significantly limit its efficacy (15). Of note, in MIBC, TAMs mostly
infiltrate into the tumor areas as opposed to stroma-tumor margins
in NMIBC, which is associated with an unfavorable clinical outcome
(16).
Therefore, the present study sought to evaluate the
association among the expression of APE1 and vascular endothelial
growth factor A (VEGFA) and the infiltration of M2 macrophages, as
detected via the M2 marker CD163, as well as their prognostic
significance in BCa. To the best of our knowledge, the present
study was the first to implicate APE1 as a key factor in the DNA
damage response with polarization of TAM, laying a foundation for
elucidating the mechanism of the recurrence of BCa and exploring
the efficacy of immunotherapy for this neoplasm.
Materials and methods
Patients and data collection
A total of 167 patients with BCa subjected to
radical cystectomy at Daping Hospital between January 2013 and
January 2017 were recruited without any restriction by age or sex.
Data collection was closed by May 2019. Those patients who did not
have a reported event of death at the time of closure were included
for calculation of the median follow-up time. Patient follow-up was
usually performed every 3 months in the first year post-surgery and
then every 6 months. The cause of death was determined by chart
review from patients' medical records or from the death
certificate. The follow-up time was 4–78 months and the median
follow-up time was 48 months. Cases with non-urothelial carcinoma
types and patients who died of causes not associated with cancer
were excluded, and cases without precise follow-up were also
excluded, resulting in 127 remaining cases. All subjects were from
a Han Chinese population and were subjected to radical cystectomy
at Daping Hospital (Chongqing, China) with the diagnosis made based
on pathology. The general characteristics of the patients are
summarized in Table SI. The median
age was 64.25 years (range, 39–88 years). There were 45 cases of
NMIBC and 82 cases of MIBC. In total, 10 cases were low-grade
carcinoma and 117 were high-grade carcinoma, and 36 of the latter
were indicated to have lymphovascular invasion (LVI). Regarding the
smoking history, a person who had at least one pack of
cigarettes/day for >1 year in his/her lifetime was regarded as
having a positive smoking history; otherwise, they were considered
as a non-smoker (17). The study was
approved by the Ethics Committee of Daping Hospital (Chongqing,
China).
Pathological evaluation and
immunohistochemistry (IHC)
Tumor tissues were fixed overnight in 4%
paraformaldehyde, dehydrated, embedded in paraffin and sectioned
into 8–10 mm (RM2235; Leica Microsystems). Experienced pathologists
blinded to the clinical outcomes reviewed these pathological
specimens. The tumors were staged according to the 8th AJCC TNM
staging classification (18).
Grading was performed following the 1998 WHO/ISUP consensus
classification (19). The presence
of LVI was assessed in all specimens. IHC staining for APE1, VEGFA
and CD163 was conducted utilizing the following antibodies: APE1
(1:2,000 dilution; mouse monoclonal; cat. no. ab194; Abcam), VEGFA
(1:150 dilution; mouse monoclonal; cat. no. ab52917; Abcam) and
CD163 (1:150 dilution; mouse monoclonal; cat. no. TA506388;
Origene). Goat antibodies conjugated to horseradish peroxidase
(HRP) (working solution, Kit-5030; MXB) was used as a secondary
antibody, 3,3-diaminobenzidine was used as a chromogenic substrate
and the sections were counterstained with hematoxylin. The results
were analyzed by an experienced pathologist (HX) blinded to the
clinical outcomes. APE1 and VEGFA expression were graded based on
the staining intensity of the nuclei and cytoplasm, respectively,
in the tumor area. Necrotic areas were ignored. Grade 0 had the
weakest staining and grade 3 had the strongest staining. In the
evaluation of APE1 expression, grade 0–2 was considered to indicate
low expression, while grade 3 was considered to indicate high
expression. For VEGFA expression, grade 0–1 was considered low
expression, while grade 2–3 was considered as high expression. For
CD163, the percentage of stained macrophages in the tumor stroma
compared to the total number of nucleated stromal cells was scored
using a scale from 0 to 100% and graded as follows: 0, 0–5%; 1,
6–30%; 2, 31–50%; and 3, >50%. For evaluation purposes, grade
0–1 is considered as low TAM ratio, inversely grade 2–3 is
considered as high ratio. The cutoff is set by mainly taking the
median grade value in consideration.
Multiple immunofluorescence
staining
Multiple immunofluorescence staining was performed
on paraffin-embedded tissues. Sections of 4 µm thickness were cut
from selected BCa tissues. The slides were deparaffinized in
xylene, rehydrated and washed with tap water prior to boiling in
Tris-EDTA buffer (pH 9, 643901; Klinipath) for epitope
retrieval/microwave treatment. Endogenous peroxidase was blocked
using Antibody Diluent/Block (cat. no. 72424205; Perkin Elmer) and
incubated at room temperature. Protein blocking was performed using
Antibody Diluent/Block at 37°C for 1 h. The slides were incubated
with primary antibodies to CD163 (1:150 dilution; cat. no. ZM0428;
Zsbio), APE1 (1:2,000 dilution; cat. no. ab194; Abcam) and VEGFA
(1:100 dilution; cat. no. ab52917; Abcam) for 1 h at 37°C, and then
incubated with antibody to CD34 (cat. no. kit-0004; MXB) at 4°C
overnight. Next, incubation with Opal Polymer HRP Ms+Rb (cat. no.
2414515; Perkin Elmer) was performed at 37°C for 10 min. Tyramide
signal amplification (TSA) visualization was performed with the
Opal eight-color IHC kit (cat. no. NEL797B001KT; Perkin Elmer),
containing fluorophores DAPI, Opal 690 (CD34), Opal 650 (VEGFA),
Opal 570 (APE1) and Opal 520 (CD163), as well as a TSA Coumarin
system (cat. no. NEL703001KT; Perkin Elmer).
Slides were scanned using the Perkin Elmer Vectra
(Vectra 3.0.5; Perkin Elmer). Multispectral images were unmixed
using spectral libraries built from images of single stained
tissues for each reagent using the inform Advanced Image Analysis
software (inForm 2.3.0; Perkin Elmer). A selection of 5–10
representative original multispectral images was used to train the
inForm software (tissue segmentation, cell segmentation,
phenotyping tool and positivity score). All the settings applied to
the training images were saved within an algorithm to allow the
inForm software (PerkinElmer; v2.3.0) batch analysis of multiple
original multispectral images of the same tissue (20).
Statistical analysis
Data analysis was performed using SPSS (version 22;
IBM, Corp.) and GraphPad Prism 7 (GraphPad Software, Inc.).
Cumulative survival probabilities were estimated using the
Kaplan-Meier method and differences between survival rates were
tested for significance using the log-rank test. Univariate and
multivariate Cox regression analyses were calculated using SPSS.
Hazard ratios (HRs) and 95% confidence intervals (95% CI) were used
to present the associations of dependent and independent variables
with the risk of mortality. The χ2 test was employed to
assess differences between two groups. All tests were two-sided
with a 95% CI and P<0.05 was considered to indicate statistical
significance. Receiver operating characteristic (ROC) analysis was
conducted for further evidence. Correlation of APE1, VEGFA and
CD163 expression in muscle invasive bladder cancer tissues with
multiple immunofluorescence staining was analyzed with Pearson
correlation.
Results
Expression of APE1, VEGFA and
CD163+ TAMs in BCa tissues
IHC was used to estimate the expression of APE1 and
VEGFA, as well as the ratio of CD163+ TAMs, and the
association between each of them with the clinicopathological
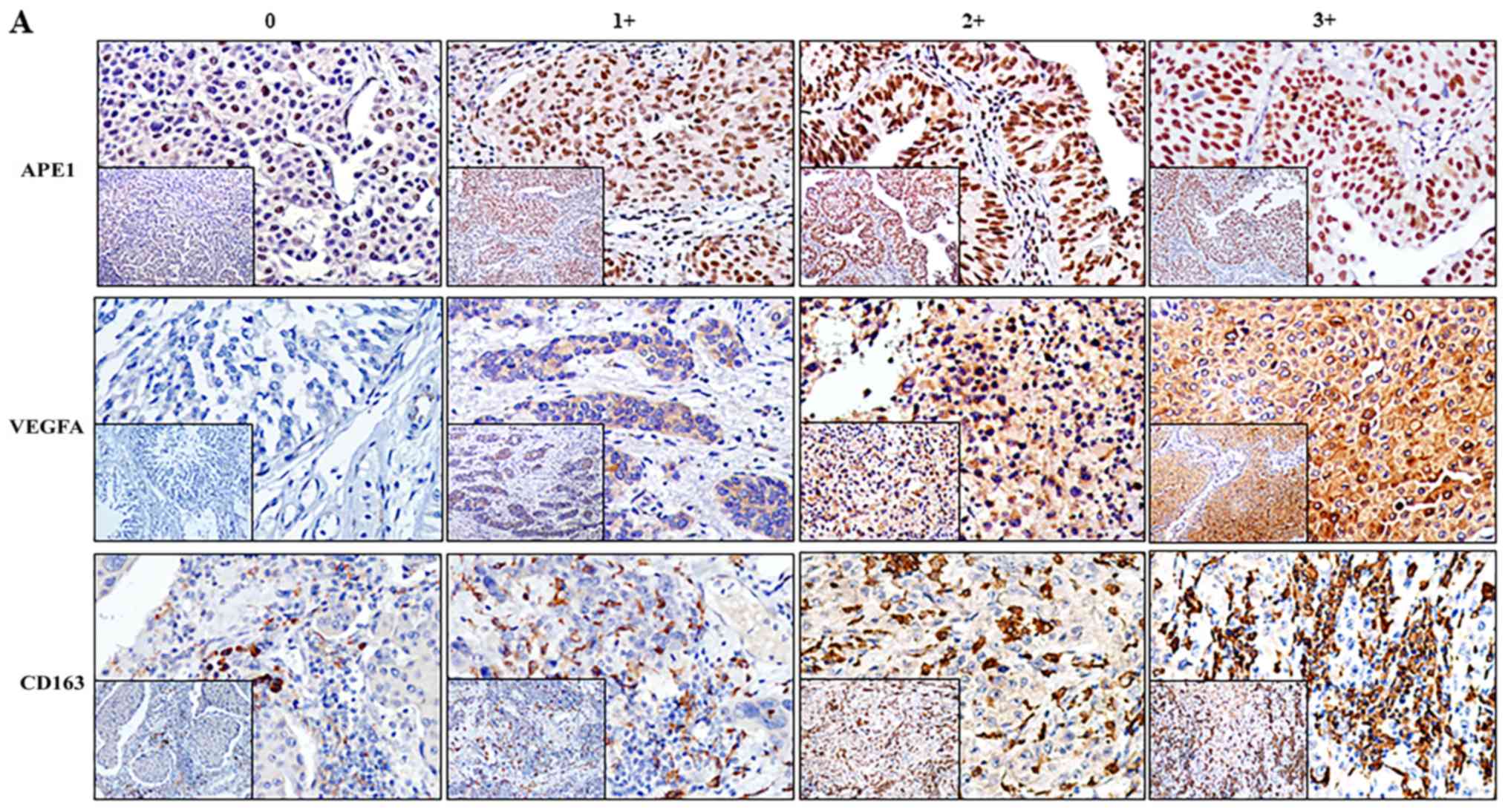
characteristics was determined. APE1 was mainly localized in the
nuclei with a certain amount residing in the cytoplasm, whereas
VEGFA appeared in the cytoplasm only (Fig. 1). Among the 127 patients, 62 (48.82%)
and 67 (52.76%) exhibited elevated levels of APE1 and VEGFA,
respectively. The APE1 levels appeared to be associated with LVI
(P=0.019) but not with the tumor stage or grade (Table SII). The levels of VEGFA were not
associated with the tumor stage, grade or LVI. CD163+
TAMs were present in the tumor stroma and tumor islets. The tumor
stroma included the papillary axis, lymphoid aggregates and the
stroma. In certain specimens, staining for CD163 was positive in
the cytoplasm and membrane of tumor cells. Of the 127 cases, 62
(48.82%) had a high grade regarding CD163+ TAMs but this
was not associated with the tumor stage, grade or LVI.
APE1 and VEGFA expression is
correlated with the CD163+ TAM ratio in BCa
The correlation between the expression of APE1 and
VEGFA and the proportion of CD163+ M2 macrophages was
then assessed. The results suggested that high expression of APE1
in tumor cells was positively correlated with the grade regarding
CD163+ TAMs (r=0.196, P=0.027), as well as with VEGFA
expression (r=0.208, P=0.018). Furthermore, VEGFA expression was
strongly correlated with the grade regarding CD163+ TAMs
(r=0.408, P<0.001; Table SIII).
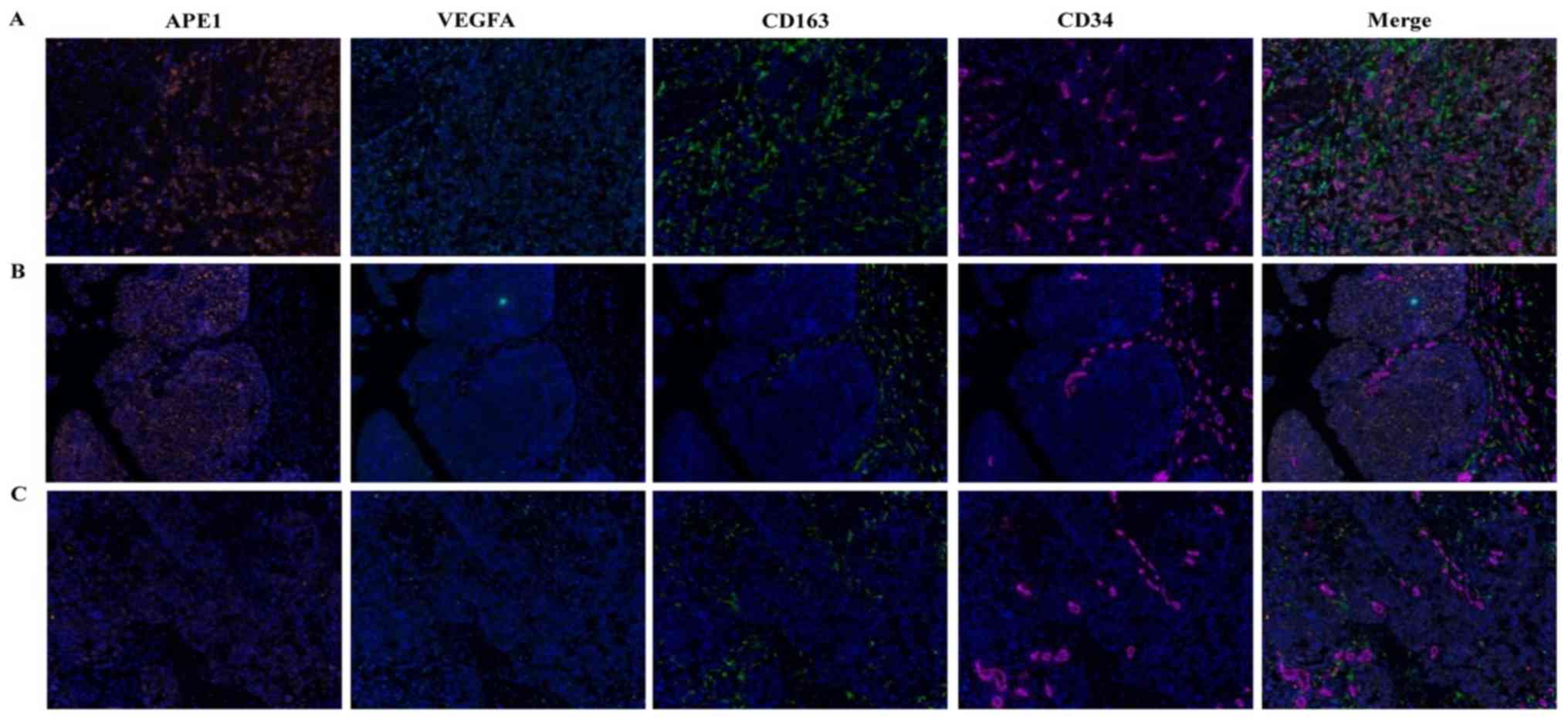
To further assess the expression of APE1 and VEGFA in BCa tissues
and their correlation with the CD163+ TAM ratio,
multiple immunofluorescence staining for APE1, VEGFA, CD163 and
CD34 was performed. The results suggested that APE1 and VEGFA were
co-expressed in tumor cells, and CD163+ TAMs were mostly
distributed around the microvasculature of tumor stroma or between
tumor cells (Fig. 2). Further
analysis using inForm software suggested that APE1 expression is
positively correlated with VEGFA and CD163 expression (Table SIV).
APE1 and CD163 are independent
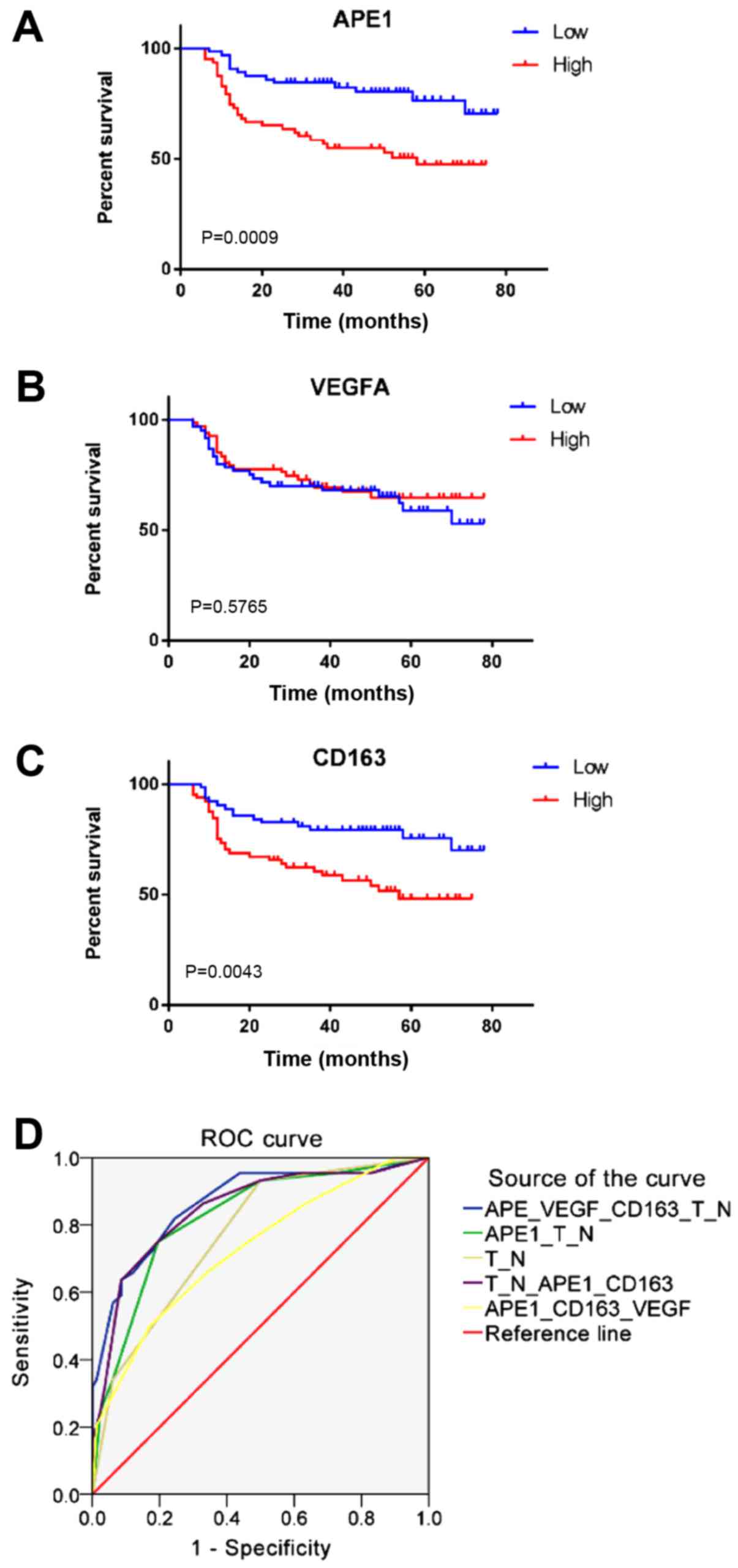
prognostic factors for OS in BCa
KM analysis revealed that high APE1 expression and a
high CD163+ TAM ratio were associated with a shorter OS
(Fig. 3). A univariate Cox
regression analysis for the expression of APE1, VEGFA,
CD163+ TAMs and clinical characteristics was performed,
indicating that the T-stage (HR=8.279, 95% CI: 2.948–23.255,
P<0.001), N-stage (HR=4.265, 95% CI: 2.262–8.045, P<0.001),
LVI (HR=2.974, 95% CI: 1.642–5.387, P<0.001), APE1 expression
(HR=2.797, 95% CI: 1.486–5.267, P=0.001) and CD163+ TAMs
(HR=2.425, 95% CI: 1.300–4.521, P=0.005) were associated with
prognosis. In addition, a multivariate analysis for OS was
performed by combining the marker panel and clinical variables,
suggesting that the T-stage (HR=8.279, 95% CI: 2.948–23.255,
P<0.001), N-stage (HR=4.265, 95% CI: 2.262–8.045, P<0.001),
APE1 expression (HR=2.797, 95% CI: 1.486–5.267, P=0.001) and
CD163+ TAMs (HR=2.425, 95% CI: 1.300–4.521, P=0.005)
were independent prognostic factors (Table I). Area under the curve are shown in
Table SV.
 | Table I.Univariate and multivariate COX
analysis of the prognosis of IHC marker for OS. |
Table I.
Univariate and multivariate COX
analysis of the prognosis of IHC marker for OS.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Agea | 1.492 | 0.829–2.687 | 0.182 |
|
|
|
| Sexb | 1.047 | 0.413–2.654 | 0.923 |
|
|
|
|
Smokingc | 0.913 | 0.479–1.741 | 0.783 |
|
|
|
| LVId | 2.974 | 1.642–5.387 | <0.001 |
|
|
|
|
T-stagee | 8.279 | 2.948–23.255 | <0.001 | 12.857 | 3.883–42.575 | <0.001 |
|
N-stagef | 4.265 | 2.262–8.045 | <0.001 |
|
|
|
| Gradeg | 2.201 | 0.530–9.135 | 0.277 |
|
|
|
| APE1h | 2.797 | 1.486–5.267 | 0.001 | 3.644 | 1.863–7.129 | <0.001 |
| VEGFAi | 0.860 | 0.479–1.544 | 0.613 | 0.410 | 0.217–0.773 | 0.006 |
| CD163j | 2.425 | 1.300–4.521 | 0.005 | 2.883 | 1.480–5.617 | 0.002 |
Discussion
Multiple nomograms and models have been constructed
in the past to predict the outcomes of Bca (21). Accurate risk stratification may
provide a marked benefit for patients with NMIBC that qualify for
early RC, as well as for patients with MIBC, who gain from
perioperative chemotherapy. The identification of new
characteristics will substantially increase the fidelity of such
models.
The presence of LVI, defined as the presence of
tumor cells in the lymphatic vessel and in vascular walls, has been
reported to be of prognostic value in patients with Bca. Evidence
suggests that LVI is a characteristic of biologically and
clinically aggressive BCa and may be of prognostic value. In the
present study, 34 out of 127 patients were identified to have LVI,
32 of which had MIBC and 2 had NMIBC (Table SII). In addition, the presence of
LVI is associated with an unfavorable prognosis. This is consistent
with other studies (22).
Aromatic amines are well-known risk factors for BCa
(23). Interaction between
environmental exposure and genetic susceptibility may markedly
increase DNA damage and thus trigger urothelial precancerous
events; therefore, prompt elimination of such damage counteracts
malignant transformation. The BER pathway has a critical role in
DNA repair mechanisms by removing nucleobase modifications by
oxidation, alkylation and deamination, and also repairs backbone
single-strand DNA breaks (24). The
enzyme APE1 has a mainframe role in BER by correcting
apurinic/apyrimidinic sites, which are pre-mutagenic lesions able
to stall DNA replication forks. Studies have indicated above-basal
expression levels of APE1 and other DNA repair pathway proteins in
high-grade BCa (25). Choi et
al (5) proposed that APE1 may be
used as a non-invasive urinary biomarker for BCa; however, its
sensitivity and specificity are not satisfying for immediate
clinical results. Recently, Fishel et al (26) reported that APE1 was overexpressed in
human BCa tissue and that APE1 redox signaling-specific inhibitor
APX3330 was able to attenuate the proliferation of BCa cells and
increase their apoptosis. However, the potential capability of APE1
expression levels to differentiate between NMIBC and MIBC has not
been previously reported. The present study suggested an even
distribution of APE1 expression in both NMIBC and MIBC, with no
significant difference between the two (P=0.711). Furthermore, no
significant difference in APE1 expression was determined between
high-grade and low-grade BCa (P=0.744). However, high APE1
expression was associated with the presence of LVI and unfavorable
prognosis in patients with BCa. APE1 expression was also positively
correlated with VEGFA and CD163+ TAM ratio in BCa
tissue.
Previous studies have indicated that high expression
of APE1 in lung cancer and osteosarcoma is associated with
upregulated VEGF through hypoxia-inducible factor-1α activation and
retardation of APE1 results in a significant drop in VEGF
expression (27). Of note, Pignot
et al (28) analyzed the
angiogenic pathways in human transitional cell carcinoma of the
bladder using a large-scale real-time reverse transcription-PCR
approach, indicating that VEGFA, MET, C-X-C motif chemokine
receptor 4 and interleukin-8 were significantly overexpressed in
tumor samples, but only VEGFA overexpression was an independent
prognostic factor for overall and disease-free survival. This did
not align with the present analysis of VEGFA expression at the
protein level, as no significant prognostic value for VEGFA was
determined in BCa; perhaps a further study with more cases will
better indicate the correlation between transcriptional levels and
protein expression of VEGFA in BCa.
VEGF and its receptors have profound effects on the
early development and differentiation of both vascular endothelial
and hematopoietic progenitors. Kloepper et al (29) further suggested that when subjecting
glioblastoma to anti-VEGF/angiopoietin-2 blockade, TAMs along the
M1 to M2 continuum are being reprogrammed toward the M1 phenotype
to provide a greater survival benefit. This suggested that VEGF may
have a novel role in immunosuppression by M2 polarization.
Macrophage infiltration is associated with
significantly better cancer-specific survival in patients with
CD163+ TAM-associated tumors. A meta-analysis of 1,400
cases of BCa including 13 studies further indicated that only
CD163+ TAMs, not CD68+ TAMs, were closely
associated with OS, recurrence-free survival and progression-free
survival (30). Consistent with the
present results, macrophage infiltration was indicated to be
associated with the prognosis of BCa. The present study
demonstrated through IHC and multiple immunofluorescences staining
that a high CD163+ macrophage ratio is associated with
an unfavorable prognosis.
Recently, Hudson et al (31) published a comparative study of 19
glioma specimens with recurrence after treatment. Through screening
of 96 gene expressions, APE1 expression in tumor tissues was
significantly increased after treatment. In addition, genes linked
to immunosuppression, invasion and metastasis (glycoprotein nmb,
C-C motif chemokine ligand 5 and killer cell lectin-like receptor
C1) and an M2 phenotype (CD163) were also detected. The polarized
genes were significantly upregulated. In 2018, Yan et al
(32) reported that the
APE1-specific redox inhibitor APX3330 directly affected the
polarization of M2 macrophages in the role of neurological recovery
after stroke in type I diabetic rats. However, the specific
mechanism by which APE1 regulates M2 polarization has remained
elusive. The present results confirmed a positive correlation
between APE1, VEGFA and CD163 at the protein level, suggesting that
APE1 may be involved in the regulation of macrophage polarization
through VEGFA.
In the univariate linear regression analysis
performed in the present study, VEGFA was indicated to have no
association with OS but it was a confounding factor in the
multivariate analysis. This is possibly due to several reasons: i)
The number of cases was too low to accurately present the
association; ii) VEGFA may not be a decisive factor on OS in BCa,
but may still be a contributing factor due to its association with
other meaningful factors, including APE1 and CD163 expression.
Further studies on this mechanism will be required to draw a
conclusion.
The present study demonstrated that high APE1
expression is associated with the presence of LVI in BCa. APE1
expression was also determined to be positively associated with
VEGFA expression and increased infiltration of CD163+
TAM. LVI, high APE1 expression and a high ratio of
CD163+ TAM in BCa may lead to a reduced survival time of
patients. The results may add valuable insight to optimize models
predicting BCa prognosis, consequently guiding perioperative
treatments that benefit patients the most. The present study also
indicated that there may be a mechanism by which high APE1
expression contributes to an increase of M2-type TAMs by definition
as CD163+, possibly involving regulation of VEGFA
expression through its redox function. However, the present results
are limited in that only patients subjected to radical cystectomy
were recruited, and studies including patients with broader
recruitment criteria (not only restricting to patients who
underwent cystectomy but, for example, also those who underwent
transurethral resection) will provide a better analysis. In
addition, only paraffin-embedded tissues were assessed and there
was a lack of fresh tissues, which may limit the conclusions of the
present study. Further prospective studies with standardized
protocols should be performed to fully assess the impact of LVI,
APE1 and VEGFA expression, as well as the CD163+ TAM
ratio, on the outcomes for patients with BCa.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81772704 and 81972398 to
JJ) and the Clinical Medical Research Personnel Training Program of
Army Medical University (grant no. 2018XLC3076 to LW).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors contributions
LAW contributed to the conception and design,
acquisition of data, analysis and interpretation of the data and
IHC of the tissue and was a major contributor in writing the
manuscript. BY was a major contributor to the analysis and
interpretation of the data. TT contributed to the acquisition of
data. YY, JX and LL contributed to the IHC analysis of the tissues.
DZ contributed to the writing and critical revision of the
manuscript, as well as towards the conception of the study. HX is
an experienced pathologist and contributed to reviewing and
analyzing the results of IHC and multiple immunofluorescence. LW
contributed to the retrieval of funding for the study and revision
of the manuscript. JJ contributed to the conception and design,
writing and revision of the manuscript and retrieval of funding for
the study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study involved the use of patient tissues and
data. Approval was acquired from the Ethics Committee of the
Research Institute of Surgery and Daping Hospital, Army Medical
University (Chongqing, China), serial number 2018 No.25. Written
informed consent was obtained from a patient representative who is
involved in this study for the usage of the lesion tissue for
research purposes was acquired at the time-point of surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cambier S, Sylvester RJ, Collette L,
Gontero P, Brausi MA, van Andel G, Kirkels WJ, Silva FC,
Oosterlinck W, Prescott S, et al: EORTC nomograms and risk groups
for predicting recurrence, progression, and disease-specific and
overall survival in non-muscle-invasive stage Ta-T1 urothelial
bladder cancer patients treated with 1–3 years of maintenance
Bacillus Calmette-Guérin. Eur Urol. 69:60–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gakis G, Efstathiou J, Lerner SP, Cookson
MS, Keegan KA, Guru KA, Shipley WU, Heidenreich A, Schoenberg MP,
Sagaloswky AI, et al International Consultation on Urologic
Disease-European Association of Urology Consultation on Bladder
Cancer 2012, : ICUD-EAU International Consultation on Bladder
Cancer 2012: Radical cystectomy and bladder preservation for
muscle-invasive urothelial carcinoma of the bladder. Eur Urol.
63:45–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gakis G, Black PC, Bochner BH, Boorjian
SA, Stenzl A, Thalmann GN and Kassouf W: Systematic Review on the
Fate of the Remnant Urothelium after Radical Cystectomy. Eur Urol.
71:545–557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi S, Shin JH, Lee YR, Joo HK, Song KH,
Na YG, Chang SJ, Lim JS and Jeon BH: Urinary APE1/Ref-1: A
potential bladder cancer biomarker. Dis Markers. 2016:72765022016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Q, Wei X, Zhou ZW, Wang SN, Jin H, Chen
KJ, Luo J, Westover KD, Wang JM, Wang D, et al: GADD45α sensitizes
cervical cancer cells to radiotherapy via increasing cytoplasmic
APE1 level. Cell Death Dis. 9:5242018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu GS, Li M, Xu CX and Wang D: APE1
stimulates EGFR-TKI resistance by activating Akt signaling through
a redox-dependent mechanism in lung adenocarcinoma. Cell Death Dis.
9:11112018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S, He L, Dai N, Guan W, Shan J, Yang
X, Zhong Z, Qing Y, Jin F, Chen C, et al: Serum APE1 as a
predictive marker for platinum-based chemotherapy of non-small cell
lung cancer patients. Oncotarget. 7:77482–77494. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin JH, Choi S, Lee YR, Park MS, Na YG,
Irani K, Lee SD, Park JB, Kim JM, Lim JS, et al: APE1/Ref-1 as a
serological biomarker for the detection of bladder cancer. Cancer
Res Treat. 47:823–833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y and Nelson PS: Molecular pathways:
Involving microenvironment damage responses in cancer therapy
resistance. Clin Cancer Res. 18:4019–4025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Georganaki M, van Hooren L and Dimberg A:
Vascular targeting to increase the efficiency of immune checkpoint
blockade in cancer. Front Immunol. 9:30812018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wheeler KC, Jena MK, Pradhan BS, Nayak N,
Das S, Hsu CD, Wheeler DS, Chen K and Nayak NR: VEGF may contribute
to macrophage recruitment and M2 polarization in the decidua. PLoS
One. 13:e01910402018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sica A, Allavena P and Mantovani A: Cancer
related inflammation: The macrophage connection. Cancer Lett.
267:204–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takayama H, Nishimura K, Tsujimura A,
Nakai Y, Nakayama M, Aozasa K, Okuyama A and Nonomura N: Increased
infiltration of tumor associated macrophages is associated with
poor prognosis of bladder carcinoma in situ after intravesical
bacillus Calmette-Guerin instillation. J Urol. 181:1894–1900. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sjödahl G, Lövgren K, Lauss M, Chebil G,
Patschan O, Gudjonsson S, Månsson W, Fernö M, Leandersson K,
Lindgren D, et al: Infiltration of CD3+ and
CD68+ cells in bladder cancer is subtype specific and
affects the outcome of patients with muscle-invasive tumors. Urol
Oncol. 32:791–797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Zhang T, Wu J, Wen J, Tao D, Wan T
and Zhu W: Prognosis and risk factors of patients with upper
urinary tract urothelial carcinoma and postoperative recurrence of
bladder cancer in central China. BMC Urol. 19:242019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Epstein JI, Amin MB, Reuter VR and Mostofi
FK; Bladder Consensus Conference Committee, : The World Health
Organization/International Society of Urological Pathology
consensus classification of urothelial (transitional cell)
neoplasms of the urinary bladder. Am J Surg Pathol. 22:1435–1448.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Edge SB, Greene FL, Schilsky RL,
Gaspar LE and Washington M: AJCC Cancer Staging Manual. Springer.
(New York, NY). 2017. View Article : Google Scholar
|
|
20
|
Gorris MA, Halilovic A, Rabold K, van
Duffelen A, Wickramasinghe IN, Verweij D, Wortel IM, Textor JC, de
Vries IJ and Figdor CG: Eight-color multiplex immunohistochemistry
for simultaneous detection of multiple immune checkpoint molecules
within the tumor microenvironment. J Immunol. 200:347–354. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Hong YK, Zhuang DW, He XJ and Lin
ME: Bladder cancer survival nomogram: Development and validation of
a prediction tool, using SEER and TCGA databases. Medicine
(Baltimore). 98:e177252019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim H, Kim M, Kwak C, Kim HH and Ku JH:
Prognostic significance of lymphovascular invasion in radical
cystectomy on patients with bladder cancer: A systematic review and
meta-analysis. PLoS One. 9:e892592014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S, et al: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zharkov DO: Base excision DNA repair. Cell
Mol Life Sci. 65:1544–1565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Evans AR, Limp-Foster M and Kelley MR:
Going APE over ref-1. Mutat Res. 461:83–108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fishel ML, Xia H, McGeown J, McIlwain DW,
Elbanna M, Craft AA, Kaimakliotis HZ, Sandusky GE, Zhang C, Pili R,
et al: Anti-tumor activity and mechanistic characterization of
APE1/Ref-1 inhibitors in bladder cancer. Mol Cancer Ther.
18:1947–1960. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang D, Zhong ZY, Li MX, Xiang DB and Li
ZP: Vector-based Ape1 small interfering RNA enhances the
sensitivity of human osteosarcoma cells to endostatin in vivo.
Cancer Sci. 98:1993–2001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pignot G, Bieche I, Vacher S, Güet C,
Vieillefond A, Debré B, Lidereau R and Amsellem-Ouazana D:
Large-scale real-time reverse transcription-PCR approach of
angiogenic pathways in human transitional cell carcinoma of the
bladder: Identification of VEGFA as a major independent prognostic
marker. Eur Urol. 56:678–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kloepper J, Riedemann L, Amoozgar Z, Seano
G, Susek K, Yu V, Dalvie N, Amelung RL, Datta M, Song JW, et al:
Ang-2/VEGF bispecific antibody reprograms macrophages and resident
microglia to anti-tumor phenotype and prolongs glioblastoma
survival. Proc Natl Acad Sci USA. 113:4476–4481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thorsson V, Gibbs DL, Brown SD, Wolf D,
Bortone DS, Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA,
et al: The Immune Landscape of Cancer. Immunity. 48:812–830.e814.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hudson AL, Parker NR, Khong P, Parkinson
JF, Dwight T, Ikin RJ, Zhu Y, Chen J, Wheeler HR and Howell VM:
Glioblastoma Recurrence Correlates With Increased APE1 and
Polarization Toward an Immuno-Suppressive Microenvironment. Front
Oncol. 8:3142018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan T, Venkat P, Chopp M, Zacharek A, Yu
P, Ning R, Qiao X, Kelley MR and Chen J: APX3330 promotes
neurorestorative effects after stroke in type one diabetic rats.
Aging Dis. 9:453–466. 2018. View Article : Google Scholar : PubMed/NCBI
|