Introduction
Chemokines are a large family of cytokines serving
important roles in the immune system, such as the regulation of
cell trafficking to inflammatory sites (1). By regulating the activity of cells in
homeostasis and signal transduction, chemokines are involved in
numerous physiological and pathological processes such as
angiogenesis, embryonic development, wound healing, organ
sclerosis, tumour growth and autoimmune disease (2,3).
Chemokine (C-X-C motif) ligand 17 (CXCL17) was first
identified in 2006 and is also known as vascular endothelial growth
factor-correlated chemokine-1 or dendritic cell and monocyte
chemokine-like protein (4,5). CXCL17 is widely expressed in mucosal
tissues and is considered a mucous chemokine (6,7).
Moreover, this chemokine has potential anti-inflammatory and
antibacterial effects and is related to the homeostasis of the
intramucosal environment (6,8). Matsui et al (8) reported that the expression of CXCL17
was common in colon and breast carcinoma cell lines, some gastric
cell lines, non-small cell lung cancer and pancreatic carcinoma
cell lines, but not in melanoma cell lines. However, in
vivo, the significance of CXCL17 expression in different types
of tumour is inconsistent. Previous studies involving cancer types,
such as endometrial cancer (9),
hepatocellular cancer (10), lung
cancer (8) and colon cancer
(11), suggested that CXCL17 was
upregulated in tumour tissue, which was associated with
oncogenicity and poor prognosis. However, CXCL17 expression has
also been reported to be downregulated in pancreatic cancer
(12) and gastric cancer (13).
Maravillas-Montero et al (14) suggested that the levels of orphan
receptor G-coupled protein receptor 35 (GPR35) in mucous membranes
correlated with the expression of CXCL17. The same study also
confirmed that GPR35 was the receptor for CXCL17, and this was
renamed CXCR8 (14). The
relationship between both proteins and the associated and
signalling axis have since been studied in tumours. For instance,
Guo et al (15) reported that
both CXCL17 and CXCR8 were highly expressed in breast cancer
tissues and cell lines, but no correlation was observed between the
expression of the two proteins. However, high expression of CXCR8
correlated with histological grade and high expression of Ki67
(15). CXCL17 is associated with
shorter overall survival (OS) and represents an independent
predictive factor of poor prognosis. In addition, the same study
also identified how the CXCL17/CXCR8 signalling axis may play a
role in activating the ERK pathway and contribute to the
proliferation and migration of breast cancer cells (15). Khandelwal et al (16) examined the role of CXCL17 and CXCR8
in the pathogenesis of cutaneous squamous cell carcinoma (CSCC) and
revealed that these were significantly expressed in CSCC cell
lines. Moreover, stimulation with CXCL17 significantly induced CSCC
cell proliferation and migration. Therefore, CXCL17/CXCR8
signalling in CSCC could represent a potentially novel target for
the treatment of non-melanoma skin cancer (16).
As CXCL17 is a mucous chemokine that is highly
expressed in several tumour types, this chemokine may serve an
important role in the pathophysiological processes of colon cancer.
However, the biological role of CXCL17 in the onset and progression
of colon cancer remains unknown. Therefore, the aim of the present
study was to examine the significance of both CXCL17 and its
receptor in colon cancer by investigating their expression patterns
in Chinese patients. The association of these two proteins with
clinicopathological parameters and survival rates was also
investigated. Furthermore, CXCL17 and CXCR8 co-expression in colon
cancer tissue was assessed in order to determine the prognostic
value of the combined high expression of both proteins.
Materials and methods
Clinical samples and microarray
A commercial tissue microarray (TMA) was obtained
from The National Human Genetic Resources Sharing Service Platform
(Shanghai Outdo Biotech Co. Ltd.). The TMA samples were from 101
patients with sporadic colon cancer who had undergone surgery and
79 healthy tumour-adjacent samples were included for comparative
analysis. The colon tissues outside the tumour loci were selected
as healthy tumour-adjacent samples that exhibited no tumour texture
histologically. Clinicopathological data included patient age
(43–91 years old), sex (male:female, 50:61), tumour type, lymph
node metastasis, histological grade and TNM stage (17). The survival data were obtained from
the supplier of the TMA by follow-up via telephone consultation.
The ‘event’ for the purposes of survival analysis was defined as
colon cancer-related mortality. Follow-up information was available
for 101 patients with colon cancer. The average follow-up period
was 61.96±3.72 months (range, 2–97 months; between September 2007
and July 2015). During the follow-up, mortality occurred in 52
cases. OS was calculated as the time between the first day of
diagnosis and colon cancer related mortality or the last known
follow-up. The median OS was 89.0 months. All patients provided
specimens with written informed consent and approval from the
Ethics Committee in The Taizhou Hospital of Zhejiang Province was
obtained in this study.
Data on CXCL17 and GPR35 mRNA
expression from TCGA
Data on CXCL17 and GPR35 mRNA expression levels in
colon cancer tissues from American patients including survival
information were obtained from The Cancer Genome Atlas (TCGA)
(https://portal.gdc.cancer.gov) in March
2020 (Data on ‘CXCR8’ as the key word was not available from TCGA).
The results without follow-up were excluded and the means of the
repeated detection outcomes were used for statistical analysis.
Immunohistochemistry (IHC)
staining
The sections (4 µm thick) wereobtained from 10%
formalin-fixed for 24 h and paraffin-embedded TMA blocks and used
for IHC. The sections were washed in xylene to remove the paraffin
and rehydrated in a graded alcohol series of 100, 100, 90, 80 and
70%, followed by PBS washing. Antigen retrieval was achieved by
heating sections in EDTA antigen retrieval solution for 20 min at
99°C. Endogenous peroxidase activity was blocked using 3%
H2O2/methanol for 20 min at 37°C. After
blocking non-specific protein binding with 10% normal goat serum
(Boster Biological Technology co.ltd) for 30 min at 37°C, the
sections were incubated with primary antibodies against CXCL17
(1:50; cat. no. 422208; R&D Systems, Inc.) and CXCR8 (1:750;
cat. no. DF4973; Affinity Biosciences), respectively, overnight at
4°C. After leaving at room temperature for 30 min and rinsing three
times in PBS, the sections were incubated with secondary antibody
(ready to use, Dako K8002, Agilent Technologies, Inc.) for 30 min
at 37°C. The 3,3′-diaminobenzidine substrate was applied to the
sections, which were then counterstained for 1 min at room
temperature with haematoxylin, dehydrated and mounted. Negative
controls were run in parallel and PBS was used instead of primary
antibody.
Evaluation of IHC
Immunostaining was evaluated under a light
microscope (magnification, ×200) by two pathologists blinded to the
experimental conditions and clinicopathological information.
Immunostaining was evaluated using a semi-quantitative scoring
system, according to the percentage of stained cells (0, <10%;
1, 10–29%; 2, 30–49%; 3, 50–74%; 4, ≥75%.) and the intensity of
immunoreactivity (1, weak; 2, moderate; 3, strong staining). The
final immunostaining score was determined by multiplying the
intensity score with the score for the percentage of positively
stained cells, which ranged between 0–12 (18). Agreement on the final scores between
the two pathologists was ~95%. In cases where different scores were
obtained, the IHC scoring was repeated by both pathologists until
the same score was achieved.
Receiver operating curve (ROC) analysis was
performed to determine an optimal cut-off value for the final
immunostaining scores of CXCL17 and CXCR8 expression. Because of a
large difference between the expression of CXCL17 and CXCR8, the
expression levels of each were graded by different criteria. The
area under the curve (AUC) with the largest area for depth of
invasion had the greatest Youden's index at 5 of the final scores
for CXCL17. Therefore, low and high expression levels of CXCL17
were defined by a final score of <6 and ≥6, respectively (data
not shown). Moreover, the AUC with the largest area for
histological grade had the largest Youden's index at 7 of the final
scores for CXCR8. Thus, low and high expression levels of CXCR8
were defined by a final score of ≤6 and >6, respectively (data
not shown).
Statistical analysis
Analyses were performed with SPSS 16.0 (SPSS, Inc.).
Spearman correlation analysis and Pearson's χ2 test were
used to analyse the association between CXCL17 and CXCR8 expression
and clinicopathological parameters, as well as the association
between CXCL17 and CXCR8 expression in colon cancer tissue.
Survival probabilities were estimated using the Kaplan-Meier method
and the log rank test. Univariate and multivariate Cox proportional
hazards regression models were applied to assess the association
between potential confounding variables and prognosis. In order to
avoid missing important information, multivariate analysis included
variables that had P<0.1 in univariate analysis. Survival
analysis was conducted on the data from TCGA by Kaplan-Meier method
and log rank test based on both medians and means of mRNA
expression. P<0.05 was considered to indicate a statistically
significant difference.
Results
CXCL17 and CXCR8 expression in colon
cancer
In order to investigate the expression levels of
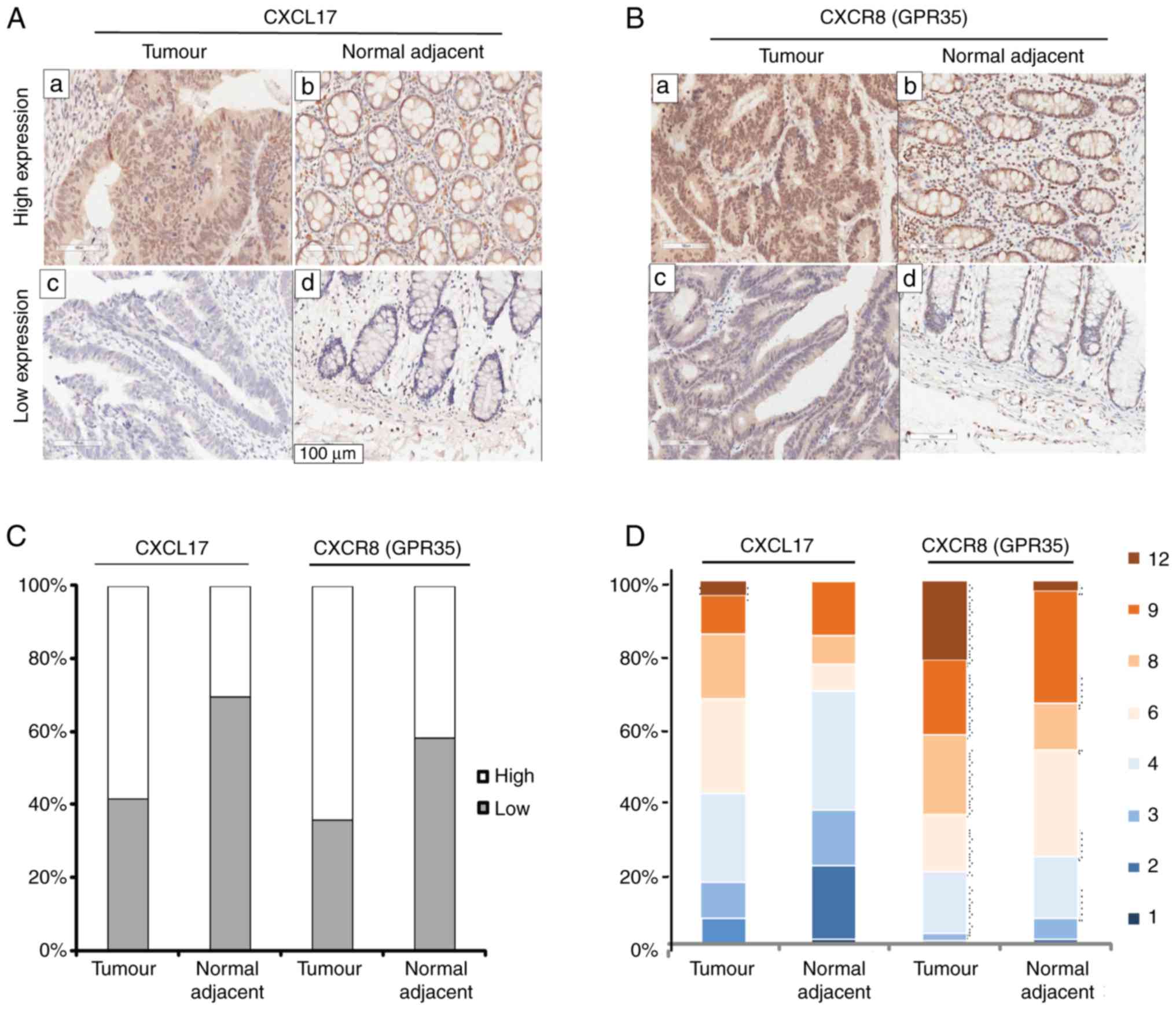
CXCL17 and CXCR8 in colon cancer tissues, IHC staining for CXCL17
and CXCR8 was performed on a commercial colon cancer TMA with
complete pathological and survival information. CXCL17 expression
was mainly detected in the cytoplasm of colon cancer cells, but
also in the nuclei and occasionally in the cell membrane.
Immunoreactivity for CXCR8 was focused in the nuclei and cytoplasm
of colon cancer cells compared with the healthy tumour-adjacent
tissues, high expression levels of both CXCL17 (P=0.003) and CXCR8
(P=0.001) were more common in colon cancer tissues (Fig. 1C). Furthermore, high expression of
CXCL17 was observed in 58.4% (59/101) of colon cancer tissues and
in 30.4% (24/79) of healthy tumour-adjacent tissues. High
expression levels of CXCR8 reached 66.4% (65/101) in colon cancer
tissues and 41.8% (33/79) in healthy tumour-adjacent tissues.
Fig. 1A and B showed high and low
expression of CXCL17 and CXCR8 in colon cancer and healthy
tumour-adjacent tissues, respectively. Fig. 1D shows the percentage of the final
staining scores of the two proteins in colon cancer and healthy
tumour-adjacent tissues. In general, the immunostaining of CXCR8
was much stronger than that of CXCL17.
Association between the expression
levels of CXCL17 and CXCR8 and the clinicopathological
characteristics of colon cancer cases
The relationship between CXCL17 and CXCR8 expression
levels and the clinicopathological parameters of colon cancer cases
was then assessed using Pearson's χ2 test (Table I). No significant associations were
identified between the expression of CXCL17 and CXCR8 and patient
age, sex, tumour type, lymph nodes metastasis status, histological
grade and TNM stage.
 | Table I.Association between the expression of
CXCL17 and CXCR8 and the clinicopathological characteristics of
colon cancer cases. |
Table I.
Association between the expression of
CXCL17 and CXCR8 and the clinicopathological characteristics of
colon cancer cases.
|
| CXCL17 | CXCR8 |
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | Low, n=42 | High, n=59 |
P-valuea | χ2
a | Low, n=36 | High, n=65 |
P-valuea | χ2
a |
|---|
| Age, years |
|
≤67 | 23 | 26 | 0.289 | 1.123 | 17 | 32 | 0.847 | 0.037 |
|
>67 | 19 | 33 |
|
| 19 | 33 |
|
|
| Sex |
|
Male | 17 | 33 | 0.126 | 2.345 | 15 | 35 | 0.241 | 1.375 |
|
Female | 25 | 26 |
|
| 21 | 30 |
|
|
| Tumour
typeb |
| TA | 21 | 29 | 0.971 | 0.059 | 19 | 31 | 0.327 | 2.233 |
| A | 15 | 23 |
|
| 15 | 23 |
|
|
| CC | 5 | 7 |
|
| 2 | 10 |
|
|
| Node metastasis,
n |
| 0 | 19 | 38 | 0.404 | 1.812 | 26 | 35 | 0.101 | 4.579 |
|
1-3 | 13 | 17 |
|
| 6 | 24 |
|
|
|
>3 | 6 | 4 |
|
| 4 | 6 |
|
|
| Histological
grade |
| 1 | 6 | 16 | 0.300 | 2.405 | 10 | 12 | 0.112 | 4.377 |
| 2 | 27 | 33 |
|
| 23 | 37 |
|
|
| 3 | 9 | 10 |
|
| 3 | 16 |
|
|
| Depth of
invasionb |
|
T1-2 | 1 | 5 | 0.067 | 5.414 | 2 | 4 | 0.518 | 1.316 |
| T3 | 29 | 46 |
|
| 25 | 50 |
|
|
| T4 | 12 | 7 |
|
| 9 | 10 |
|
|
| TNM stage |
|
I–II | 23 | 38 | 0.329 | 0.954 | 26 | 35 | 0.071 | 3.271 |
|
III–IV | 19 | 21 |
|
| 10 | 30 |
|
|
Association between the expression of
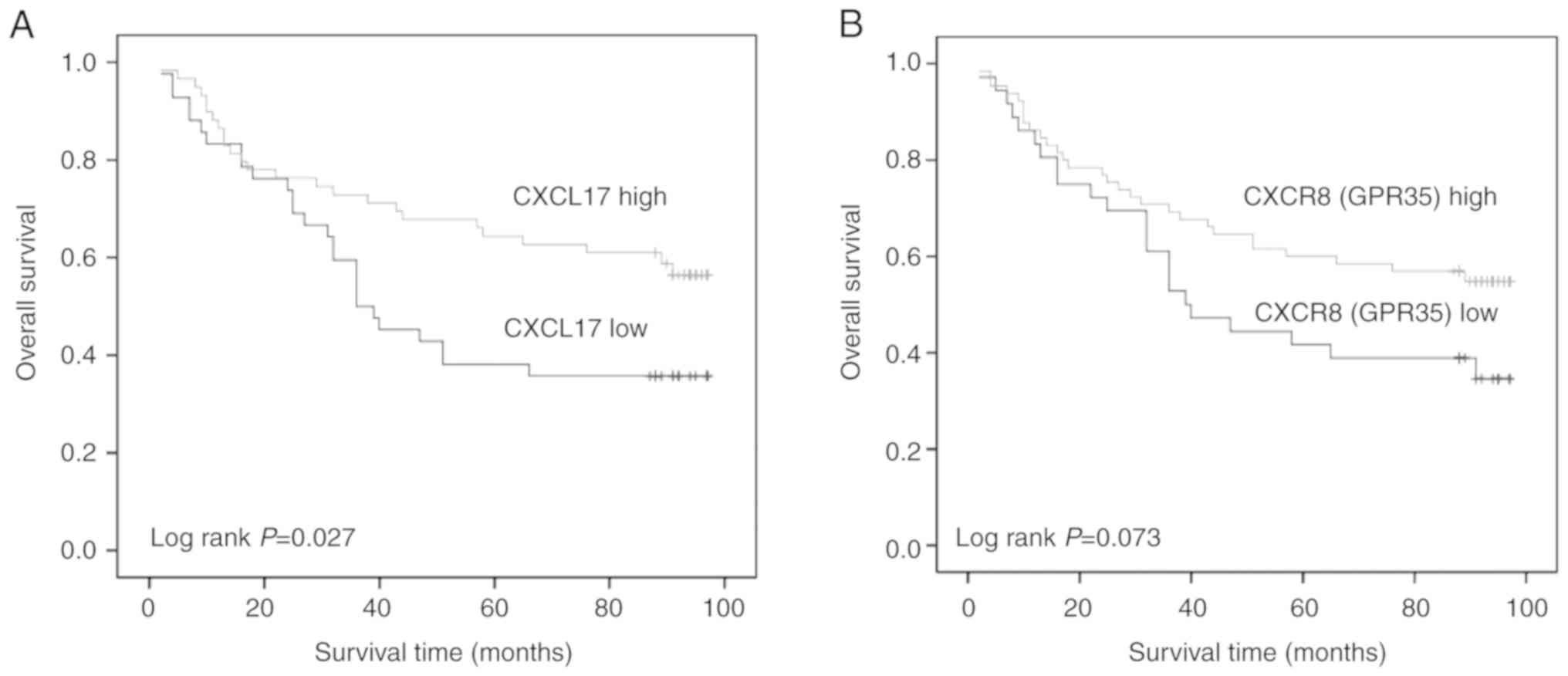
CXCL17 and CXCR8 and OS of patients with colon cancer
The association of CXCL17 and CXCR8 expression
levels with OS in patients with colon cancer was evaluated using
Kaplan-Meier analysis. Patients with a higher CXCL17 expression
levels had longer OS (log rank; P=0.027). A similar trend was
observed for CXCR8; however, when compared with patients with lower
CXCR8 expression, no significant differences were observed (log
rank; P=0.073; Fig. 2).
A univariate Cox regression model was used to
determine the influence of each clinicopathological variable on OS
(Table II). Univariate analysis
indicated that lymph node metastasis and TNM stage were
significantly associated with prognosis in patients with colon
cancer (RR=2.618; 95% CI, 1.514–4.527; P=0.001). However, although
there appeared to be a relationship between histological grade and
prognosis in patients with colon cancer, the differences in risk
ratio (RR) were not statistically significant (RR=2.017; 95% CI,
0.949–4.290; P=0.068). In particular, high expression of CXCL17
reduced the RR in patients with colon cancer (RR=0.545; 95% CI,
0.316–0.942; P=0.030). While high expression of CXCR8 had a similar
trend to that of CXCL17, there was no statistical significance
(RR=0.610; 95% CI, 0.353–1.057; P=0.078).
 | Table II.Cox regression univariate analysis of
clinicopathological characteristics, CXCL17 and CXCR8 expression
and overall survival in colon cancer. |
Table II.
Cox regression univariate analysis of
clinicopathological characteristics, CXCL17 and CXCR8 expression
and overall survival in colon cancer.
| Clinicopathological
characteristic | n | RR (95% CI) | P-value |
|---|
| Age (≤67 vs. >67
years) | 101 | 1.199
(0.695–2.070) | 0.514 |
| Sex (male vs.
female) | 101 | 1.070
(0.621–1.843) | 0.809 |
| Tumour type (TA/A
vs. CC) | 100 | 1.490
(0.862–2.577) | 0.153 |
| Node metastasis
(yes vs. no) | 101 | 2.618
(1.514–4.527) | 0.001 |
| Histological grade
(1 vs. 2/3) | 101 | 2.017
(0.949–4.290) | 0.068 |
| Depth of invasion
(T1/2/3 vs. T4) | 100 | 1.488
(0.778–2.842) | 0.230 |
| TNM stage (I/II vs.
III/IV) | 101 | 2.618
(1.514–4.527) | 0.001 |
| CXCL17 (low vs.
high) | 101 | 0.545
(0.316–0.942) | 0.030 |
| CXCR8 (low vs.
high) | 101 | 0.610
(0.353–1.057) | 0.078 |
Multivariate Cox regression analysis demonstrated
that TNM stage were independent prognostic factors for OS in
patients with colon cancer (RR=2.793; 95% CI, 1.590–4.906;
P<0.001), while histological grade and high CXCL17 expression
were not. Moreover, CXCR8 expression was also an independent
prognostic factor for OS (Table
III); however, no statistical significance was identified using
Cox regression univariate analysis (Table II).
 | Table III.Cox regression multivariate analysis
of CXCL17 and CXCR8 expression and overall survival in colon
cancer. |
Table III.
Cox regression multivariate analysis
of CXCL17 and CXCR8 expression and overall survival in colon
cancer.
| Clinicopathological
characteristic | n | RR (95% CI) | P-value |
|---|
| TNM stage (I/II vs.
III/IV) | 101 | 2.793
(1.590–4.906) | <0.001 |
| Histological grade
(1 vs. 2/3) | 101 | 1.804
(0.833–3.905) | 0.135 |
| CXC17 (low vs.
high) | 101 | 0.723
(0.401–1.302) | 0.280 |
| CXCR8 (low vs.
high) | 101 | 0.543
(0.298–0.987) | 0.045 |
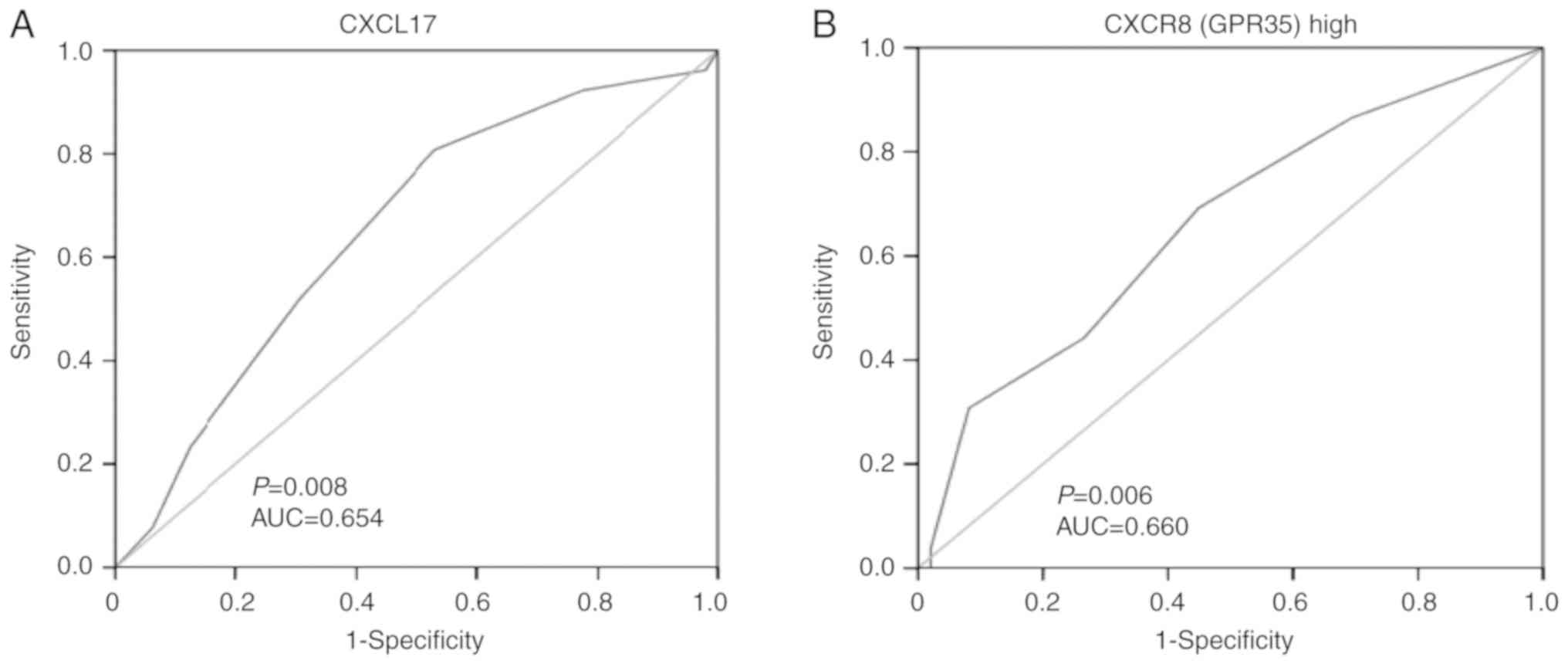
ROC analysis was performed to evaluate the
prognostic significance of the expression of the two proteins in
surgically treated patients with colon cancer. According to the
analysed results by SPSS, the AUC for CXCL17 was 0.654, and CXCL17
expression at a score of 7 had the largest sum of sensitivity and
specificity (Youden's index). Sensitivity reached 0.808 and
specificity was 0.469. In addition, the AUC for CXCR8 was 0.660,
and CXCR8 expression at a score of 8.5 had the greatest Youden's
index with, sensitivity at 0.692 and specificity at 0.551 (Fig. 3).
CXCL17 expression is positively
associated with the expression of CXCR8 and CXCL17/CXCR8
co-expression is an independent prognostic factor in colon
cancer
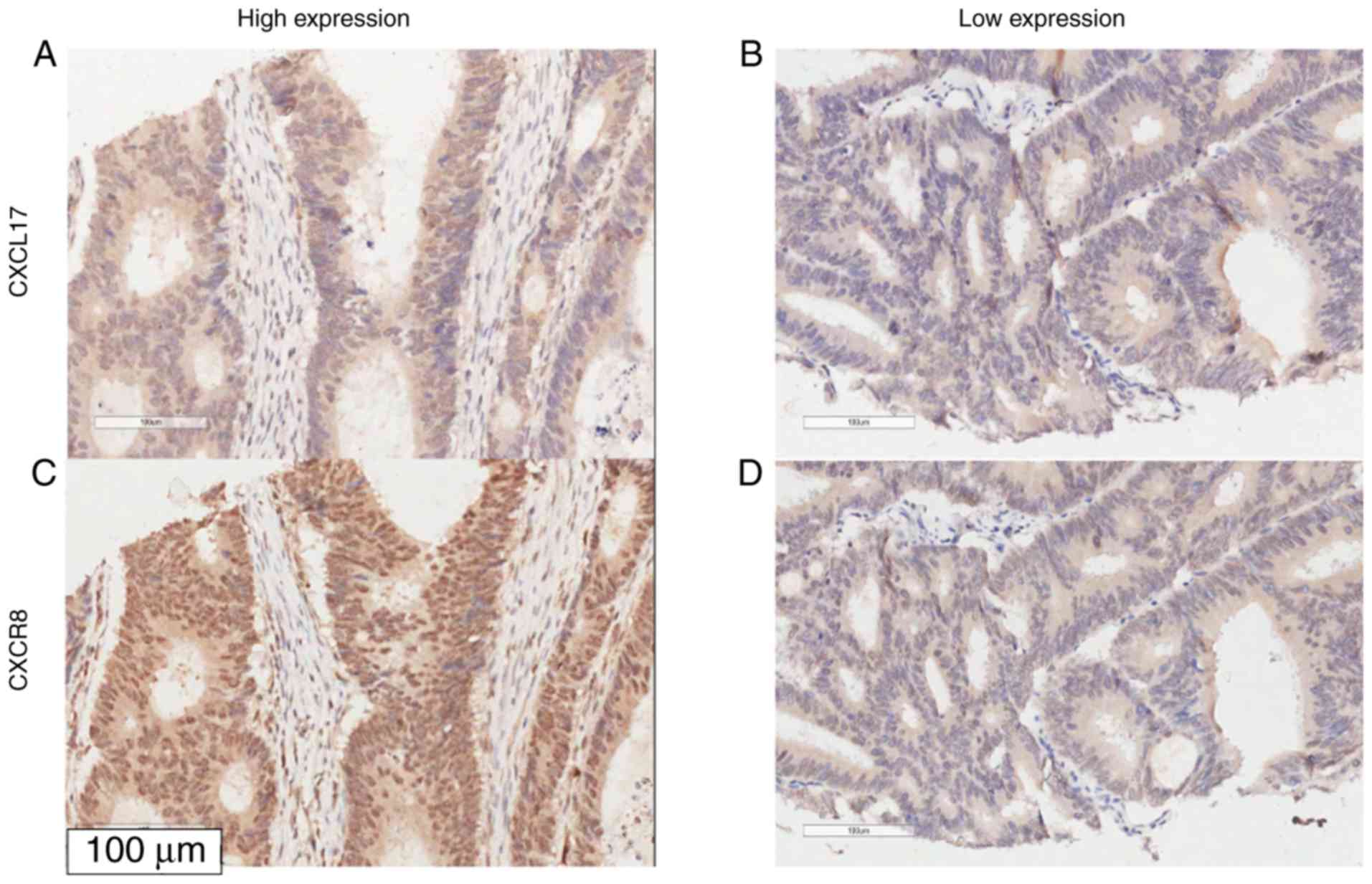
The association between CXCL17 and CXCR8 expression
in colon cancer tissues was assessed using Spearman rank
correlation analysis. The expression of CXCR8 was positively
correlated with the expression of CXCL17 in colon cancer samples
(Ρ=0.295; P=0.003, χ2=11.317), but not in healthy
tumour-adjacent samples (Table IV
and Fig. 4).
 | Table IV.Correlation between CXCL17 and CXCR8
expression in colon cancer samples and healthy tumour-adjacent
samples. |
Table IV.
Correlation between CXCL17 and CXCR8
expression in colon cancer samples and healthy tumour-adjacent
samples.
|
|
|
| CXCR8, n (%) |
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Gene | Tissue |
| Low | High | ρa |
P-valueb | χ2b |
| CXCL17, n (%) | Tumour | Low | 22 (52.4) | 20 (47.6) | 0.295 | 0.003 | 8.781 |
|
|
| High | 14 (23.7) | 45 (76.3) |
|
|
|
|
|
Tumour-adjacent | Low | 32 (58.2) | 23 (41.8) | −0.001 | 0.990 | <0.001 |
|
|
| High | 14 (58.3) | 10 (41.7) |
|
|
|
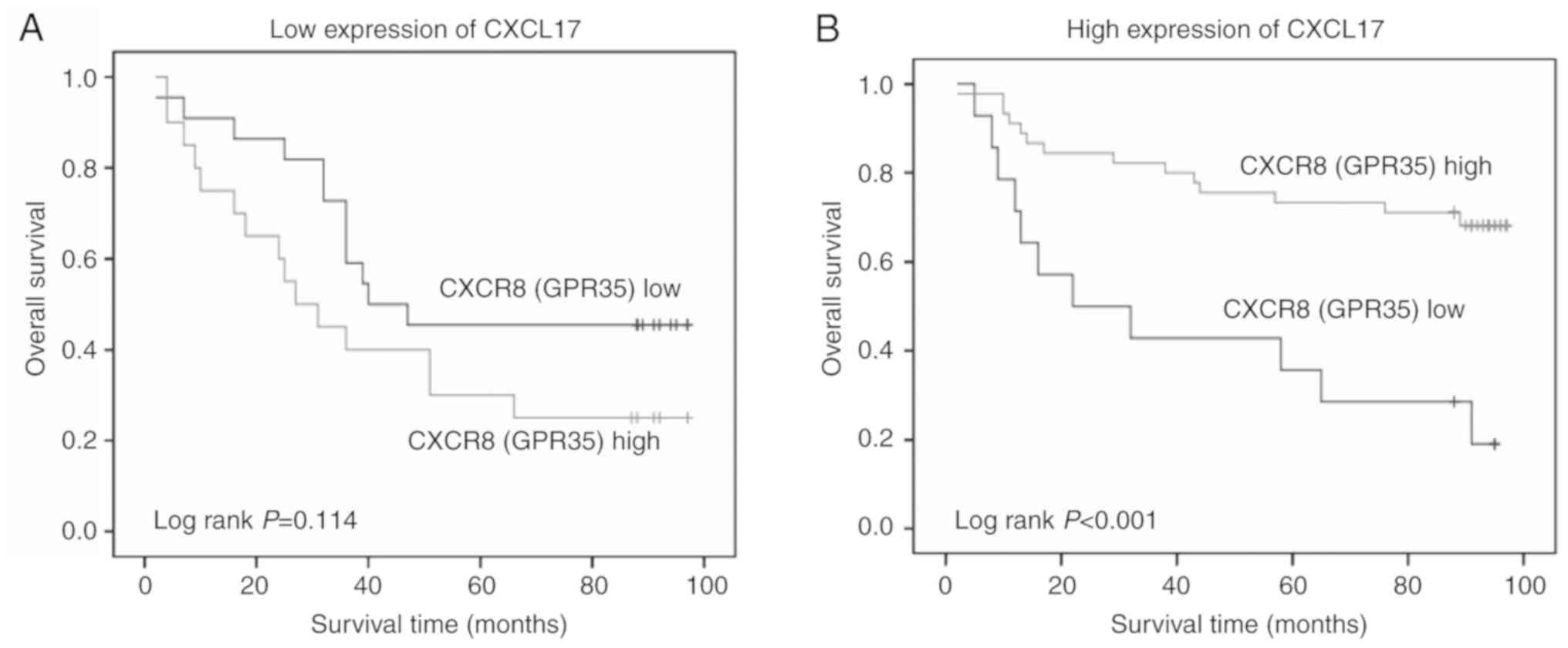
Based on the positive association between CXCL17 and
CXCR8 expression and their high expression levels in colon cancer
tissues, the association between different expression levels of
CXCR8 and OS was investigated in patients with colon cancer
stratified according to their CXCL17 expression. A longer OS was
observed in patients with a high CXCR8 expression in the high
CXCL17 expression group compared with those with low expression of
CXCR8 (P<0.001). However, there was no significant association
in low CXCR17 expression group (P=0.114; Fig. 5).
To evaluate the prognostic value of combined high
expression levels of CXCL17 and CXCR8, one low expression or both
low expression of two proteins was referred to as ‘non-combined
expression’. However, both high expression of two proteins was
referred to as ‘combined expression’. The association between
combined expression and clinicopathological characteristics in
colon cancer cases was evaluated accordingly. However, the results
only showed a small negative association between the depth of tumor
invasion and combined expressions of CXCL17 and CXCR8, where
P=−0.240 and P=0.018 by Spearman's correlation analysis (data not
shown), but P=0.055 using the Pearson χ2 test (data not
shown). However, multivariate Cox regression analysis suggested
that the combined expression of CXCL17 and CXCR8 was a significant,
independent prognostic factor for OS in patients with colon cancer
(P=0.001; Table V).
 | Table V.Cox regression multivariate analysis
of CXCL17 and CXCR8 co-expression and overall survival in colon
cancer. |
Table V.
Cox regression multivariate analysis
of CXCL17 and CXCR8 co-expression and overall survival in colon
cancer.
| Clinicopathological
characteristic | n | RR (95% CI) | P-value |
|---|
| TNM stage (I/II vs.
III/IV) | 101 | 2.375
(1.367–4.127) | 0.002 |
| Histological grade
(1 vs. 2/3) | 101 | 1.769
(0.826–3.789) | 0.142 |
| Expression of
CXCL17 and CXCR8 (non-combined vs. combined) | 101 | 0.350
(0.189–0.650) | 0.001 |
The association between combined CXCL17/CXCR8
expression and OS was then evaluated in subgroups of patients with
colon cancer stratified according to TNM stage. Patients with
combined high expression of CXCL17 and CXCR8 presented a longer OS,
compared with those without combined high expression in the
subgroups with a TNM stage of I–II (P=0.001). However, this was not
observed in patients with a TNM stage of III–IV (Table VI).
 | Table VI.Association between combined
expression of CXCL17 and CXCR8 and survival status in subgroups of
patients with colon cancer stratified by TNM stage. |
Table VI.
Association between combined
expression of CXCL17 and CXCR8 and survival status in subgroups of
patients with colon cancer stratified by TNM stage.
|
|
| Expression of
CXCL17 and CXCR8, n (%) |
|
|
|---|
|
|
|
|
|
|
|---|
| TNM stage | Outcome | Non-combined | Combined |
P-valuea | χ2 |
|---|
| I–II | Dead | 19 (59.4) | 5 (17.2) | 0.001 | 11.317 |
|
| Alive | 13 (40.6) | 24 (82.8) |
|
|
| III–IV | Dead | 19 (79.2) | 9 (56.2) | 0.121 | 2.401 |
|
| Alive | 5 (20.8) | 7 (43.8) |
|
|
The mRNA expression of CXCL17 and CXCR8 (GPR35) were
not detected in 101 colon cancer samples and 79 healthy
tumour-adjacent samples. However, in the present study, data on
CXCL17 and GPR35 mRNA expression levels in colon cancer tissues
from American patients were obtained from TCGA. Of the 432 patients
included in the final data, mortality occurred in 87 patients.
Kaplan-Meier analysis indicated no significant associations between
the mRNA expression levels of CXCL17 and GPR35 and OS in patients
with colon cancer; although patients with high mRNA expression
levels of CXCL17 and GPR35 tended to have a longer OS time compared
with those with low expression of CXCL17 and GPR35 (Fig. S1).
Discussion
In the present study, the expression levels of
CXCL17 and CXCR8 were higher in colon cancer tissues compared with
healthy tumour-adjacent tissues. This finding was consistent with
previous studies on CXCL17 in colon cancer (8), as well as CXCR8 in breast cancer
(15) and lung cancer (19). Recent studies performed at the mRNA
level suggested that both CXCL17 (20) and CXCR8 (21) were ectopically expressed in primary
colon cancer tissues and regional positive lymph nodes. High
expression levels of CXCR8 variants 2/3 and CXCL17 mRNA in regional
metastatic lymph nodes are indicators of poor prognosis in patients
with colon cancer, based on the analysis of survival data.
Moreover, the prognostic value of CXCR8 and CXCL17 is enhanced when
combined with carcinoembryonic antigen mRNA expression. However,
these studies investigated CXCL17 and CXCR8 at the mRNA level in
regional lymph nodes in European patients with colon cancer
(20). In the present study, data on
CXCL17 and CXCR8 mRNA expression levels in colon cancer tissues
from American patients were obtained from TCGA in March 2020.
Although Kaplan-Meier analysis indicated no significant
associations between the mRNA expression levels of CXCL17 and CXCR8
and OS in patients with colon cancer; patients with high mRNA
expression levels of CXCL17 and GPR35 (CXCR8) tended to have a
longer OS compared with those with low expression of CXCL17 and
GPR35, which had a similar tendency to the current study of
proteins expression. Ohlsson et al (11) reported that CXCL17 was also highly
expressed in primary tumours compared with healthy colon tissues by
IHC; however, further findings related to the clinical significance
of CXCL17 and its association with survival were not reported,
which could be attributed to the small cohort size (11).
To the best of the authors' knowledge, the present
study was the first to investigate CXCL17 and CXCR8 co-expression
using TMA from a relatively large number of cases of colon cancer,
including healthy tumour-adjacent tissues. Although the association
observed between the expression levels of CXCL17 and CXCR8 and the
clinicopathological features was not significant in the current
study, the results suggested that patients with high expression of
either CXCL17 or CXCR8 presented longer survival times compared
with patients with low expression of either of the two genes. In
previous studies (11,15,22),
high expression of CXCL17 was also mostly observed in cancer
tissues, such as breast cancer, and the prognosis of patients with
breast cancer with high expression of CXCL17 was poor (15). The prognostic value of high
expression levels of CXCL17 in hepatocellular carcinoma (HCC) has
been reported to be similar to that in breast cancer (15,23). A
recent study revealed that CXCL17 in HCC mediates the invasion of
malignant cells (22). In addition,
high GPR35 expression correlates with drug resistance in lung
cancer (19). Compared with studies
on other malignancies, high CXCL17 and CXCR8 expression in the
present study was associated with different outcomes, as patients
with high expression had an improved prognosis. Moreover, increased
CXCL17 expression in different tissues and organs can largely vary
under distinct pathophysiological conditions (4–6),
suggesting that this chemokine serves a specific biological role in
specific tissues.
Further univariate and multivariate COX regression
analyses indicated that lymph node metastasis and clinical staging
were independent prognostic factors in colon cancer. However, the
expression of CXCL17 was not a statistically significant
independent prognostic factor when using multivariate analysis. In
addition, CXCR8, which had a slightly higher P-value compared with
the statistical significance threshold in univariate analysis, was
a strong independent prognostic factor in the multivariate
analysis. This trend should be further investigated using studies
that involve a larger number of cases. A possible reason why CXCR8
was a strong independent prognostic factor when using multivariate
analysis is that CXCL17 exhibits its biological effects via
interaction with CXCR8.
The association between the CXCL17 and CXCR8
expression levels and the potential significance of their combined
high expression was also examined. The expression levels of the two
proteins were associated in cancer tissues, but there was no
significant association between combined high CXCL17/CXCR8
expression and clinicopathological variables. Nonetheless, patients
with combined high expression of the two proteins presented longer
survival times compared with those with non-combined expression.
However, the present data indicated that the expression of the two
proteins did not both increase or decrease simultaneously,
therefore there was not a totally dependent relationship between
the two proteins. Prior to the identification of CXCR8 as a
receptor of CXCL17, endogenous ligands of CXCR8 have been reported
to include kynurenic acid, lodoxamide (14,24,25) and
lysophosphatidic acid (26). Walczak
et al (25) demonstrated that
kynurenic acid synthesis increased in healthy and cancer colorectal
cells. Recent studies have also hypothesised whether CXCR8 is the
receptor of CXCL17. It has been reported that THP-1 cells
pre-treated with a CXCR8 antagonist have no significant effects on
migration to the gradient CXCL17, and the migration of THP-1 cells
stimulated by CXCL17 was not dependent on CXCR8 (27,28).
However, the present results suggested a close functional
association between CXCL17 and CXCR8 in colon cancer, which is
consistent with previous studies supporting their ligand-receptor
relationship (14–16).
The present study indicated that the CXCL17/CXCR8
axis may have a protective biological role in colon cancer, and it
is possible that CXCL17 promotes the antitumour immunity in
patients with colon cancer. However, it has been reported that
CXCL17-responding myeloid-derived cells cause a notable enhancement
of xenograft tumour formation (8).
Hiraoka et al (12) injected
CMS5a fibrosarcoma cells and CT26 colon cancer cells stably
expressing CXCL17 and/or intercellular adhesion molecule 2 (ICAM2)
into BALB/c mice, which resulted in slower tumour growth compared
with mice injected with wild-type cells; indeed, tumours failed to
develop in the former group. Moreover, Hiraoka et al
(12) concluded that tumour growth
was inhibited by immune surveillance by cytotoxic T cell-mediated
cytolysis in the context of CXCL17 and ICAM2 during the early
stages of pancreatic carcinogenesis. However, the antitumor immune
reaction changed from an immune response to immune tolerance
between the stages of intraductal papillary mucinous adenoma and
intraductal papillary mucinous carcinoma (12). In line with these findings, the
present study demonstrated that the combined high expression of
CXCL17 and CXCR8 in patients with early TNM staging presented
longer survival times compared with those without combined high
expressions of CXCL17 and CXCR8. However, this was not the case in
patients with late TNM staging. These findings support an
antitumour role for CXCL17/CXCR8 signalling in the early stages of
colon cancer.
In conclusion, the results of the present study
indicated that CXCL17/CXCR8 signalling is involved in colon cancer.
While the underlying biological mechanism is yet to be elucidated,
these findings suggested that CXCL17 and CXCR8 may serve as useful
biomarkers for improved prognosis of colon cancer. However, further
experimental validation is required to verify the protective effect
of CXCL17/CXCR8 signalling in colon cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Liaoning Science
and Technology Projects (grant no. 2014022040).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
XL and HY contributed to the conception and design
of the study. HY, YL and XB performed IHC investigation,
statistical analysis and prepared figures and tables. XB, ZY and YL
interpreted the results. XL, HY and ZY drafted and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The commercial tissue microarray specimens were
collected from Taizhou Hospital by Shanghai Outdo Biotech Co., Ltd.
All patients provided specimens with written informed consent and
approval from the Ethics Committee in The Taizhou Hospital of
Zhejiang Province was obtained in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CXCL17
|
C-X-C motif chemokine ligand 17
|
|
CXCR8
|
C-X-C motif chemokine receptor 8
|
|
GPR35
|
G-coupled protein receptor 35
|
|
OS
|
overall survival
|
|
RR
|
relative risk
|
References
|
1
|
Sokol CL and Luster AD: The chemokine
system in innate immunity. Cold Spring Harb Perspect Biol.
7:a0163032015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rotondi M, Chiovato L, Romagnani S, Serio
M and Romagnani P: Role of chemokines in endocrine autoimmune
diseases. Endocr Rev. 28:492–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weinstein EJ, Head R, Griggs DW, Sun D,
Evans RJ, Swearingen ML, Westlin MM and Mazzarella R: VCC-1, a
novel chemokine, promotes tumor growth. Biochem Biophys Res Commun.
350:74–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pisabarro MT, Leung B, Kwong M, Corpuz R,
Frantz GD, Chiang N, Vandlen R, Diehl LJ, Skelton N, Kim HS, et al:
Cutting edge: Novel human dendritic cell- and monocyte-attracting
chemokine-like protein identified by fold recognition methods. J
Immunol. 176:2069–2073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burkhardt AM, Tai KP, Flores-Guiterrez JP,
Vilches-Cisneros N, Kamdar K, Barbosa-Quintana O, Valle-Rios R,
Hevezi P, Zuñiga J, Selman M, et al: CXCL17 is a mucosal chemokine
elevated in idiopathic pulmonary fibrosis that exhibits broad
antimicrobial activity. J Immunol. 188:6399–6406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hernández-Ruiz M and Zlotnik A: Mucosal
chemokines. J Interferon Cytokine Res. 37:62–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsui A, Yokoo H, Negishi Y,
Endo-Takahashi Y, Chun NA, Kadouchi I, Suzuki R, Maruyama K,
Aramaki Y, Semba K, et al: CXCL17 expression by tumor cells
recruits CD11b+Gr1 high F4/80-cells and promotes tumor progression.
PLoS One. 7:e440802012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saghir FS, Rose IM, Dali AZ, Shamsuddin Z,
Jamal AR and Mokhtar NM: Gene expression profiling and
cancer-related pathways in type I endometrial carcinoma. Int J
Gynecol Cancer. 20:724–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mu X, Chen Y, Wang S, Huang X, Pan H and
Li M: Overexpression of VCC-1 gene in human hepatocellular
carcinoma cells promotes cell proliferation and invasion. Acta
Biochim Biophysica Sinica. 41:631–637. 2009. View Article : Google Scholar
|
|
11
|
Ohlsson L, Hammarström ML, Lindmark G,
Hammarstrom S and Sitohy B: Ectopic expression of the chemokine
CXCL17 in colon cancer cells. Br J Cancer. 114:697–703. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiraoka N, Yamazaki-Itoh R, Ino Y,
Mizuguchi Y, Yamada T, Hirohashi S and Kanai Y: CXCL17 and ICAM2
are associated with a potential anti-tumor immune response in early
intraepithelial stages of human pancreatic carcinogenesis.
Gastroenterology. 140:310–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mao Y, Zhao Q, Yin S, Ding X and Wang H:
Genome-wide expression profiling and bioinformatics analysis of
deregulated genes in human gastric cancer tissue after gastroscopy.
Asia Pac J Clin Oncol. 14:e29–e36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maravillas-Montero JL, Burkhardt AM,
Hevezi PA, Carnevale CD, Smit MJ and Zlotnik A: Cutting edge:
GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. J
Immunol. 194:29–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo YJ, Zhou YJ, Yang XL, Shao ZM and Ou
ZL: The role and clinical significance of the CXCL17-CXCR8 (GPR35)
axis in breast cancer. Biochem Biophys Res Commun. 493:1159–1167.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khandelwal AR, Alam MM, Moore-Medlin T,
Savage HA and Nathan CAO: Role of the CXCL17-CXCR8 (GPR35) axis in
cutaneous squamous cell carcinoma. Cancer Res. 792019.PubMed/NCBI
|
|
17
|
Lan YT, Yang SH, Chang SC, Liang WY, Li
AF, Wang HS, Jiang JK, Chen WS, Lin TC and Lin JK: Analysis of the
seventh edition of american joint committee on colon cancer
staging. Int J Colorectal Dis. 27:657–663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Xiao Q, Bai X, Yu Z, Sun M, Zhao H,
Mi X, Wang E, Yao W, Jin F, et al: Activation of STAT3 is involved
in malignancy mediated by CXCL12-CXCR4 signaling in human breast
cancer. Oncol Rep. 32:2760–2768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Han T, Tong W, Zhao J and Qiu X:
Overexpression of GPR35 confers drug resistance in NSCLC cells by
β-arrestin/Akt signaling. Onco Targets Ther. 11:6249–6257. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rashad Y, Olsson L, Israelsson A, Öberg Å,
Lindmark G, Hammarström ML, Hammarström S and Sitohy B: Lymph node
CXCL17 messenger RNA: A new prognostic biomarker for colon cancer.
Tumour Biol. 40:10104283187992512018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ali H, AbdelMageed M, Olsson L, Israelsson
A, Lindmark G, Hammarström ML, Hammarström S and Sitohy B: Utility
of G protein-coupled receptor 35 expression for predicting outcome
in colon cancer. Tumour Biol. 41:10104283198588852019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Li H, Zhen Z, Ma X, Yu W, Zeng H
and Li L: CXCL17 promotes cell metastasis and inhibits autophagy
via the LKB1-AMPK pathway in hepatocellular carcinoma. Gene.
690:129–136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Yan J, Xu J, Liu CQ, Zhen ZJ, Chen
HW, Ji Y, Wu ZP, Hu JY, Zheng L and Lau WY: CXCL17 expression
predicts poor prognosis and correlates with adverse immune
infiltration in hepatocellular carcinoma. PLoS One. 9:e1100642014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Deavers M, Patenia R, Bassett RL
Jr, Mueller P, Ma Q, Wang E and Freedman RS: Monocyte/macrophage
and T-cell infiltrates in peritoneum of patients with ovarian
cancer or benign pelvic disease. J Transl Med. 4:302006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walczak K, Dabrowski W, Langner E, Zgrajka
W, Piłat J, Kocki T, Rzeski W and Turski WA: Kynurenic acid
synthesis and kynurenine aminotransferases expression in colon
derived normal and cancer cells. Scand J Gastroenterol. 46:903–912.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oka S, Ota R, Shima M, Yamashita A and
Sugiura T: GPR35 is a novel lysophosphatidic acid receptor. Biochem
Biophys Res Commun. 395:232–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Binti Mohd Amir NAS, Mackenzie AE, Jenkins
L, Boustani K, Hillier MC, Tsuchiya T, Milligan G and Pease JE:
Evidence for the Existence of a CXCL17 receptor distinct from
GPR35. J Immunol. 201:714–724. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park SJ, Lee SJ, Nam SY and Im DS: GPR35
mediates lodoxamide-induced migration inhibitory response but not
CXCL17-induced migration stimulatory response in THP-1 cells; is
GPR35 a receptor for CXCL17? Br J Pharmacol. 175:154–161. 2018.
View Article : Google Scholar : PubMed/NCBI
|