Introduction
Oral squamous cell carcinoma (OSCC) is a highly
aggressive neoplasm found in the oral and maxillofacial region,
with approximately 354,864 new cases and 177,384 deaths in 2018,
worldwide (1). The most significant
risk factors are smoking and alcohol consumption (2,3). Despite
recent advances in cancer treatment, such as surgery, radiotherapy
or chemotherapy, the overall five-year survival rate for OSCC
remains less than 50% (4).
Therefore, novel therapeutic methods are required to treat this
disease. Recently, advances in cancer immunotherapy have made
considerable progress. Immunotherapy can be used to manipulate the
development of early carcinogenesis, improve the quality of life
and extend the patient survival time by improving the antitumor
immune functions of the patients (5). Therefore, immunotherapy may be
considered an alternative treatment strategy for OSCC.
Interleukin-21 (IL-21) is a member of the γ chain
cytokine family, which is mainly produced by activated
CD4+ T cells and natural killer (NK) cells (6). IL-21 can regulate the innate and
adaptive immune response, which have been found to serve a diverse
range of important roles in inflammation and tumor progression
(7). For example, IL-21 can
critically regulate B cell function and amplify NK cell function,
and can also regulate potent antitumor activity and facilitate
heightened tumor-specific T lymphocyte function (8). Moreover, IL-21 was revealed to
stimulate the expansion of CD8+ T cells and increase
their cytotoxicity, which in turn promoted the proliferation and
antibody production of T cell-dependent B cells; this induced the
differentiation and activation of NK cells, and reduced the number
of Treg cells in tumors (8,9). Previously, the application of
recombinant human IL-21 to patients or animals has demonstrated
significant antitumor activity in both nonclinical and clinical
studies (10–12). Phase I and II clinical trials of
IL-21 have been conducted in patients with renal cell carcinoma,
non-Hodgkin's lymphoma and malignant melanoma (10–12); the
results indicated that the therapeutic administration of IL-21 had
a high validity and safety profile in patients (13–15).
Thus, the present study aimed to investigate the
expression levels of IL-21 in OSCC tissues and the antitumor
effects of IL-21 in CAL-27 cells in vitro. Moreover, the
role of IL-21 in OSCC immunotherapy was further determined.
Materials and methods
Human OSCC samples
Ethical approval was obtained from the Ethics
Committee of the Yantai Yuhuangding Hospital and informed written
consent was provided from all patients. None of the patients with
OSCC had received any chemotherapy or radiotherapy before excision.
OSCC and adjacent normal tissues (positioned >5 cm away from the
tumor) were obtained from 45 patients with OSCC (29 men and 16
women; age range, 42–79 years; mean age, 59 years) who underwent
surgical resection at the Yantai Yuhuangding Hospital between
October 2016 and April 2018. All samples were confirmed by
pathological examination. The histological grade and tumor stage
were assigned according to the World Health Organization (WHO)
(16) and the International Union
Against Cancer classification system (17).
Reagents
DMEM (cat. no. 11960–044), FBS (cat. no. 12484-010),
penicillin-streptomycin (cat. no. 15140-122), TRIzol®
reagent (cat. no. 15596-026), rabbit anti-IL-21 polyclonal antibody
(cat. no. PA5-34801; 1:1,000), mouse anti-β-actin monoclonal
antibody (cat. no. MA1-744; 1:5,000), horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG antibody (cat. no. 31430;
1:3,000) and HRP-conjugated goat anti-rabbit IgG antibody (cat. no.
31460; 1:3,000) were all obtained from Invitrogen; Thermo Fisher
Scientific, Inc. The rabbit anti-cleaved (c)-caspase-3 antibody
(cat. no. ab2302; 1:2,000), rabbit anti-Bax antibody (cat. no.
ab32503; 1:2,000), rabbit anti-Bcl-2 antibody (cat. no. ab32124;
1:1,000), rabbit anti-JNK1+JNK2+JNK3 antibody (cat. no. ab179461;
1:2,000), rabbit anti-JNK1+JNK2+JNK3 (phospho T183+T183+T221;
1:1,000) antibody (cat. no. ab124956), rabbit anti-AKT1+AKT2+AKT3
antibody (cat. no. ab185633; 1:1,000) and rabbit anti-AKT3 (phospho
S472)+AKT2 (phospho S474)+AKT1 (phospho S473) antibody (cat. no.
ab192623; 1:2,000) were obtained from Abcam. The RevertAid First
Strand cDNA Synthesis kit (cat. no. K1622), SuperSignal™ West Pico
PLUS Chemiluminescent substrate (cat. no. 34580) and the SYBR™
Green PCR Master mix (cat. no. 4334973) were purchased from Thermo
Fisher Scientific, Inc. The Cell Counting Kit-8 (CCK-8; cat. no.
E606335) and the TUNEL apoptosis assay kits (cat. no. E607172) were
purchased from Sangon Biotech Co., Ltd. Finally, the Annexin
V-allophycocyanin (APC)/7′-aminoactinomycin D (AAD) Apoptosis Assay
kit (cat. no. KGA1026) was obtained from Nanjing KeyGen Biotech
Co., Ltd.
Cell culture and reagents
The human tongue squamous cell carcinoma cell line
CAL-27 was obtained from the Central Laboratory of Yantai
Yuhuangding Hospital of Qingdao University (Yantai, China). The
cells were cultured in DMEM, supplemented with 10% FBS and 100 U/ml
penicillin-streptomycin, and maintained in a humidified atmosphere
at 37°C with 5% CO2.
Adenovirus infection
The adenovirus vector (pAd-Track) was supplied by Dr
Wang Shengzhi of Yantai Yuhuangding Hospital (Yantai, China) and
the recombinant adenovirus expressing human IL-21 (Ad-IL-21) was
constructed by Shanghai Shenggong Biology Engineering Technology
Service, Ltd. Ad-IL-21 or Ad-null (pAd-Track null vector) were
cloned into the adenovirus vector. When CAL-27 cells number reached
1×106, cells were infected with 1×108 µg
Ad-null or Ad-IL-21 by polybrene (Sigma) and the control cells were
treated with PBS for 24 h at 37°C.
Immunohistochemical (IHC)
analysis
The expression levels of IL-21 were analyzed by IHC
using standard staining procedures. Briefly, antigen retrieval was
performed by incubating the sections in 10 mM citric acid buffer
(pH 6.0) at 100°C for 15 min. Subsequently, sections were dewaxed
in xylene I and II at room temperature, for 20 min each, and
rehydrated in a descending ethanol series (absolute ethanol for 5
min, 95% ethanol for 5 min, 90% ethanol for 5 min and 80% ethanol
for 5 min). Following three washes with PBS-Tween (0.05% Tween-20
in PBS), the sections were incubated in 5% BSA (Sangon Biotech Co.,
Ltd.) for 45 min at room temperature. The sections were
subsequently incubated at 4°C overnight with rabbit anti-IL-21
polyclonal antibody diluted in 1X PBST (1:100). Following the
primary antibody incubation, the membranes were washed in PBST and
incubated with a HRP-conjugated goat anti-rabbit IgG secondary
antibody (1:100) at room temperature for 45 min. The slides were
subsequently stained with 3,3′-diaminobenzidine tetrahydrochloride
at room temperature for 10 min. Finally, the sections were
rehydrated with ethanol (80% ethanol for 5 min, 90% ethanol for 5
min, 95% ethanol for 5 min and absolute ethanol for 5 min), cleared
and mounted with neutral tree gum, and counterstained with 0.5%
Harris' hematoxylin at room temperature for 5 min. The images were
screened using a confocal microscope (magnification, ×100 and
×400).
Western blotting
Total protein were extracted from cells or tissues
using TNE lysis buffer [1.0% (vol/vol) Triton X-100, 10 mM
Tris-HCl, pH 7.5, 120 mM NaCl, 25 mM KCl, 1 lg/ml leupeptin, 1
lg/ml pepstatin, 2 lg/ml aprotinin, and 0.5 mM phenylmethylsulfonyl
fluoride] (18). Total protein was
quantified using the BCA method (19) and 30 µg protein/lane was separated
via SDS-PAGE on a 10% gel. The separated proteins were subsequently
transferred onto PVDF membranes (Bio-Rad Laboratories, Inc.) and
blocked in 10% non-fat milk in TBS-Tween (0.05% Tween-20) at room
temperature for 2 h. The membranes were incubated with primary
antibodies against: Polyclonal IL-21 (1:1,000); caspase-3
(1:2,000); Bax (1:2,000); Bcl-2 (1:1,000); JNK1+JNK2+JNK3
(1:2,000); JNK1+JNK2+JNK3 (phospho T183+T183+T221; 1:1,000); rabbit
anti-AKT1+AKT2+AKT3 (1:1,000); AKT3 (phospho S472)+AKT2 (phospho
S474)+AKT1 (phospho S473; 1:2,000) and monoclonal β-actin (1:5,000)
overnight at 4°C. Following the primary antibody incubation, the
membranes were incubated with HRP-conjugated goat anti-mouse IgG
(1:3,000) and HRP-conjugated goat anti-rabbit IgG secondary
antibodies (1:3,000) at room temperature for 1 h. Protein bands
were assessed using chemiluminescence substrates, which is an
enhanced chemiluminescent HRP substrate that enables picogram to
high femtogram-level protein detection (SuperSignal™ West Pico PLUS
Chemiluminescent substrate; Thermo Fisher Scientific, Inc.) and the
expression levels were quantified using Image-Pro Plus software
(version 6.0; Media Cybernetics, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA was reverse
transcribed into cDNA using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. RT was performed as follows: 55°C for 30
min, followed by 95°C for 5 min and 5°C for 5 min. qPCR was
subsequently performed using the SYBR™ Green PCR Master mix and a
LightCycler® 480 system (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocols. The following
primer sequences were used for qPCR: IL-21 forward,
5′-ATCCAGTCCTGGCAACATGG-3′ and reverse, 5′-TGTGGCGATCTTGACCTTGG-3′;
and β-actin forward, 5′-CCACTGGCATCGTGATGGAC-3′ and reverse,
5′-ACGGATGTCCACGTCACACT-3′. The following thermocycling conditions
were used for qPCR: 95°C for 3 min; 40 cycles of 95°C for 30 sec,
60°C for 30 sec, 72°C for 45 sec; and a final extension at 72°C for
1 min. Expression levels were quantified using the
2−ΔΔCq method (20) and
normalized to β-actin.
Cell viability assay
The infected CAL-27 cells were plated into 96-well
plates at a density of 5×104 and cultured for 48 h. Cell
viability was measured using the CCK-8 assay kit (Sangon Biotech
Co., Ltd.), according to the manufacturer's protocol. Briefly, the
cells were washed with DMEM and incubated with 10 µl CCK-8 solution
at 37°C for 4 h. Subsequently, the absorbance was measured at 450
nm with a spectrophotometer (BioTek Instruments, Inc.).
Cell migration assay
The infected CAL-27 cells were plated into 10 cm
dish at a density 5×106 and cultured for 24 h.
Subsequently, the cells were scraped with a 200-µl pipette tip and
washed with PBS three times, prior to being cultured in DMEM
without FBS. The images of the scratch wounds were captured at 0 or
24 h using a confocal microscope (magnification, ×400). The width
of each wound was quantified using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.). The cell migration rate (%) was
calculated as follows: [(Width of wound at 0 h-width of wound at 24
h)/width of wound at 0 h] ×100%.
TUNEL staining
The infected CAL-27 cells were plated into 6-well
plates dish at a density 2×105 and cultured for 48 h.
The TUNEL assay was performed using a TUNEL apoptosis assay kit,
according to the manufacturer's protocol. Briefly, the cells were
fixed in 4% paraformaldehyde at room temperature for 30 min,
permeabilized in 0.5% Triton X-100 on ice for 2 min and incubated
with a dUTP and TDT enzyme reaction mixture in a humidified
atmosphere at 37°C for 60 min. Subsequently, the slides were
incubated with streptavidin-fluorescein at 37°C for 30 min and
counterstained with 5 µg/ml DAPI at room temperature for 5 min. The
slices were mounted with neutral tree gum. TUNEL-positive cells
were observed in ≥5 randomly selected fields of view using a
fluorescent microscope (Bio-Rad Laboratories, Inc.; magnification,
×400). The positive cells generated a bright green
fluorescence.
Flow cytometric analysis of
apoptosis
Briefly, the infected cells (5×105
cells/well) were harvested with 0.25% trypsinization and
centrifugated at 300 × g for 5 min at room temperature, then rinsed
twice with PBS and resuspended in 500 µl binding buffer solution at
a density of 1×105 cells/ml. The cells were subsequently
stained with 5 µl Annexin V-APC and 5 µl 7-AAD using the Annexin
V-APC/AAD Apoptosis Assay kit at room temperature for 15 min in the
dark, according to the manufacturer's protocol. Apoptotic cells
were analyzed using a CytoFLEX flow cytometer and CytExpert
software (version 2.0; Beckman Coulter, Inc.) within 1 h.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp.). Data are presented as the mean ± standard
deviation, and all experiments were performed in triplicate.
Statistical differences between the groups were analyzed with the
paired Student's t-test or a one-way ANOVA, followed by a Tukey's
post hoc test for multiple comparisons. A χ2 test was
used to determine the association between the expression levels of
IL-21 and OSCC clinical and histopathological features. P<0.05
was considered to indicate a statistically significant
difference.
Results
IL-21 expression levels are decreased
in OSCC tissues
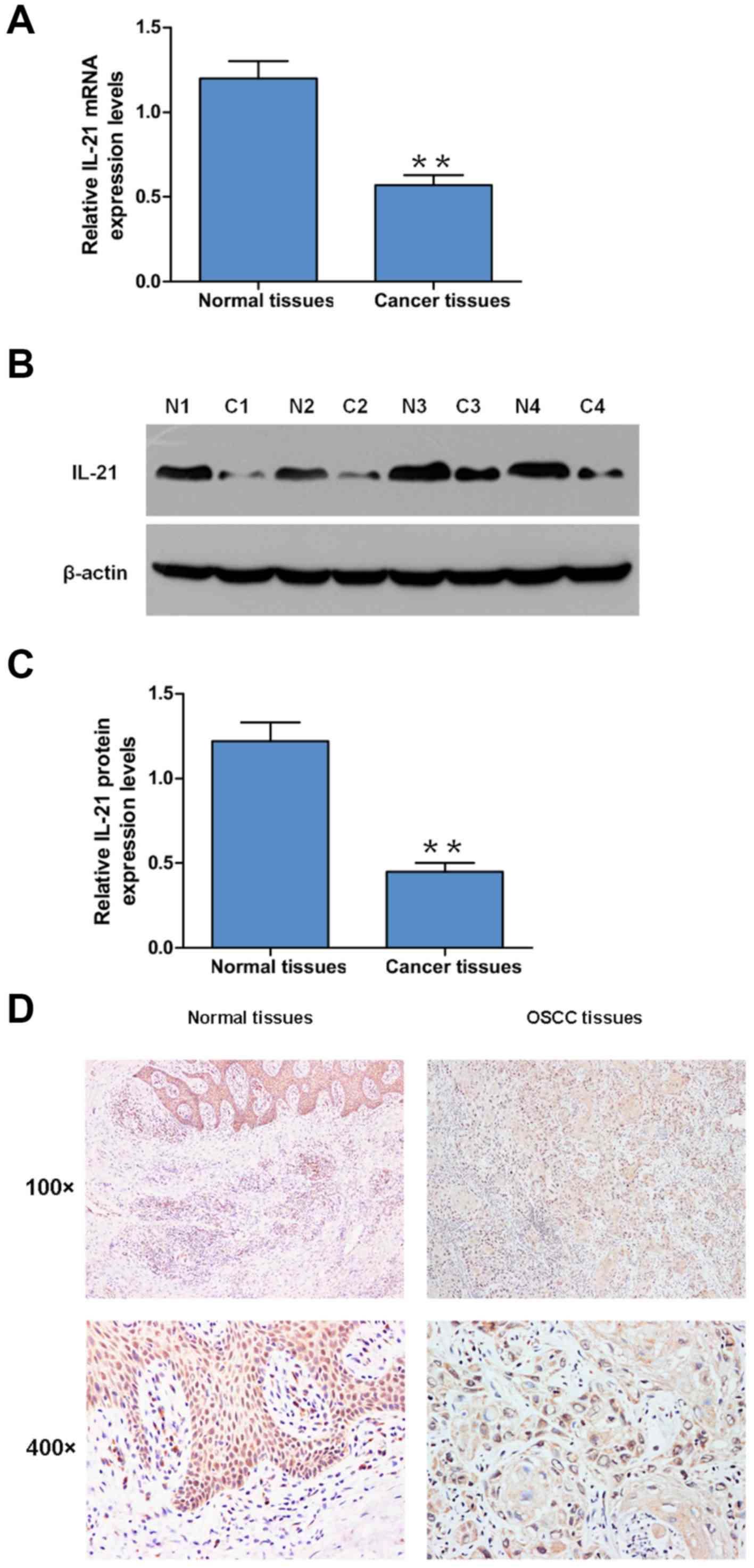
The expression levels of IL-21 in OSCC and adjacent
normal tissues were analyzed using RT-qPCR and western blotting.
The results indicated that both the mRNA and protein expression
levels of IL-21 were significantly decreased in OSCC tissues
compared with the adjacent normal tissues (Fig. 1A-C). Furthermore, IHC analysis was
performed to analyze IL-21 expression levels; the positive rate of
IL-21 detection was 20% (9/45) in OSCC tissues and 62% (28/45) in
the adjacent tissues (Fig. 1D). The
positive rate of IL-21 detection decreased in the OSCC tumor tissue
compared with the adjacent normal tissues (Fig. 1D). The associations between the
expression levels of IL-21 and the clinicopathological features of
OSCC are presented in Table I.
IL-21-negative tumors were significantly associated with the TNM
status, pathological grade and lymphocytic infiltration (Table I). Overall, these findings indicated
that IL-21 expression levels may be decreased in OSCC tissues,
which may be positively associated with the malignant progression
of OSCC.
 | Table I.Association between interleukin-21
expression and the clinical and histopathological features of
patients with oral squamous cell carcinoma (n=45). |
Table I.
Association between interleukin-21
expression and the clinical and histopathological features of
patients with oral squamous cell carcinoma (n=45).
| Variables | Total number of
patients, n | Positive | Positive rate
(%) | χ2 | P-value |
|---|
| Sex |
|
|
|
|
|
|
Male | 29 | 17 | 58.62 | 0.024 | 0.878 |
|
Female | 16 | 9 | 56.25 |
|
|
| Age, years |
|
|
|
|
|
|
≥60 | 24 | 14 | 58.33 | 0.007 | 0.936 |
|
<60 | 21 | 12 | 57.14 |
|
|
| Tumor size, cm |
|
|
|
|
|
| ≥3 | 24 | 13 | 54.17 | 0.275 | 0.600 |
|
<3 | 21 | 13 | 61.90 |
|
|
| TNM stage |
|
|
|
|
|
| I or
II | 14 | 13 | 92.85 | 8.270 | 0.004b |
| III or
IV | 31 | 13 | 41.94 |
|
|
|
Differentiation |
|
|
|
|
|
|
Well | 13 | 11 | 84.62 | 3.961 | 0.047a |
|
Moderate/poor | 32 | 15 | 46.87 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
Negative | 12 | 11 | 91.67 | 5.926 | 0.015a |
|
Positive | 33 | 15 | 45.45 |
|
|
IL-21 inhibits the proliferation and
migration of OSCC cells in vitro
To verify the roles of IL-21 in OSCC, a recombinant
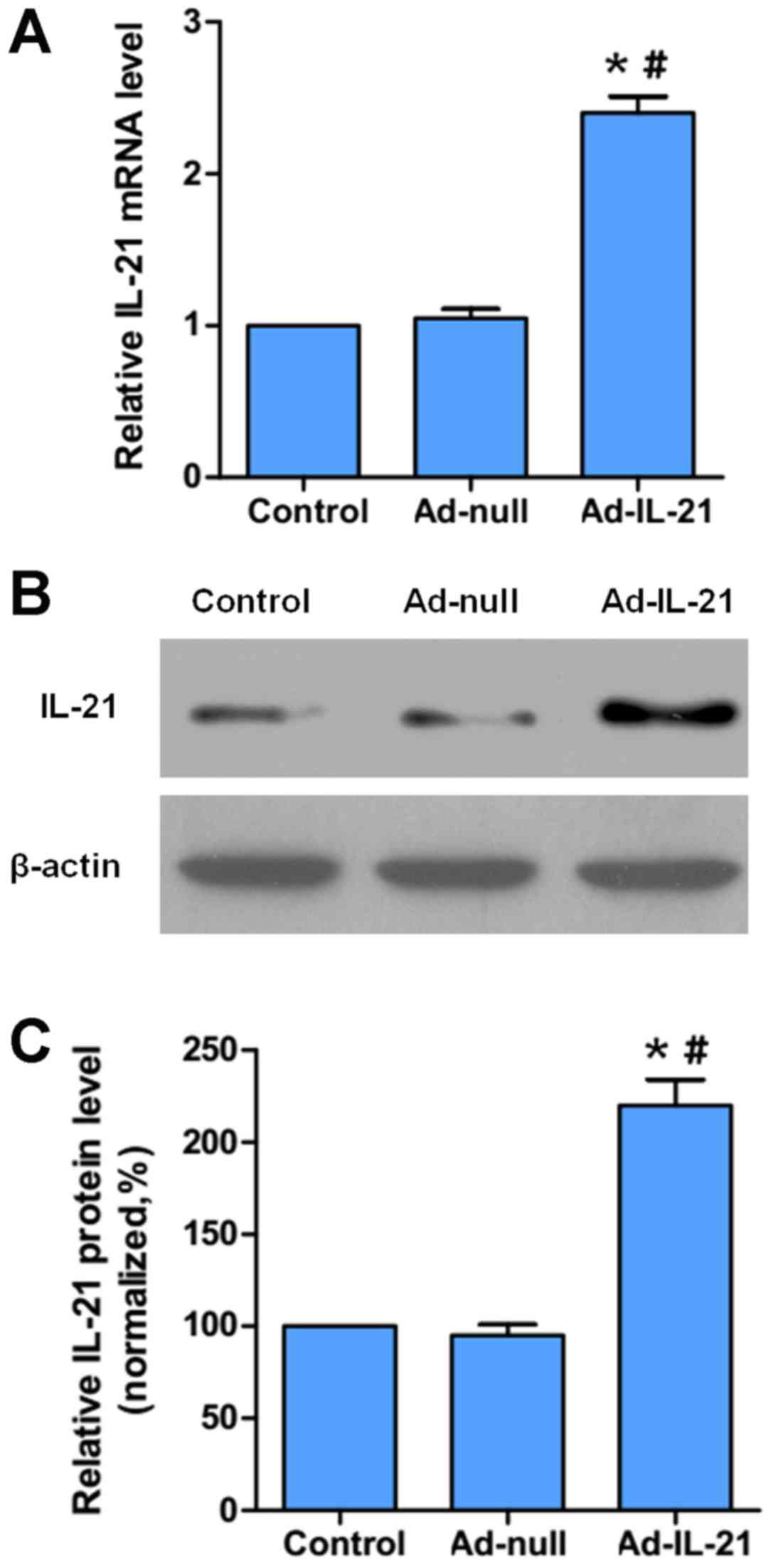
Ad was constructed to overexpress IL-21 (Ad-IL-21), which was used
to infect CAL-27 cells in vitro. Initially, the expression
levels of IL-21 mRNA and protein were analyzed using RT-qPCR
(Fig. 2A) and western blotting
respectively (Fig. 2B and C) to
demonstrate the proof of successful infection. The results
demonstrated that Ad-IL-21 infection significantly increased the
mRNA and protein expression levels of IL-21 compared with the
control and Ad-null cells (Fig.
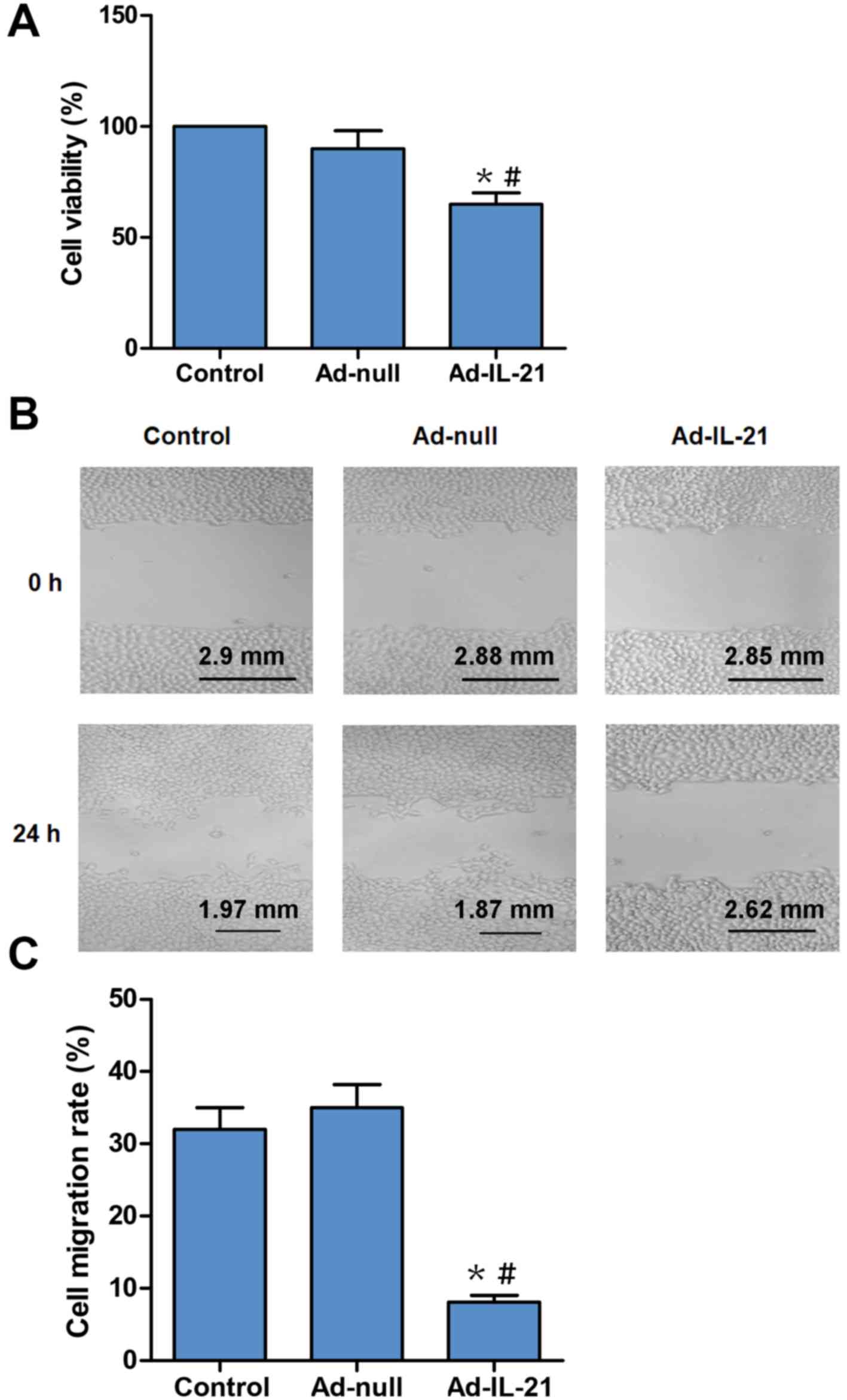
2A-C). Subsequently, cell proliferation was analyzed using the
CCK-8 method. The Ad-IL-21 group exhibited a significant decrease
in cell viability compared with the control or the Ad-null group
(Fig. 3A). Thus, it was suggested
that IL-21 overexpression may inhibit the proliferation of CAL-27
cells. Furthermore, the role of IL-21 in cell migration was
confirmed using a wound-healing assay. The results revealed that
the cell migratory activity of the Ad-IL-21 group was significantly
decreased at 24 h compared with the control or the Ad-null groups
(Fig. 3B and C). Therefore, these
results revealed that IL-21 overexpression may inhibit the
proliferation and migration of CAL-27 cells in vitro.
IL-21 induces the apoptosis of OSCC
cells in vitro
TUNEL staining was performed to determine the role
of IL-21 in cell apoptosis. The number of apoptotic cells was
significantly increased in the Ad-IL-21 group compared with the
control or Ad-null groups (Fig. 4A and
B). In addition, the expression levels of the pro-apoptotic
proteins, c-caspase-3 and Bax, and the anti-apoptotic protein
Bcl-2, were investigated. Western blotting indicated that the
overexpression of IL-21 could significantly increase the expression
levels of c-caspase-3 and Bax, while decrease the expression levels
of Bcl-2, and also significantly increase the Bax/Bcl-2 ratio
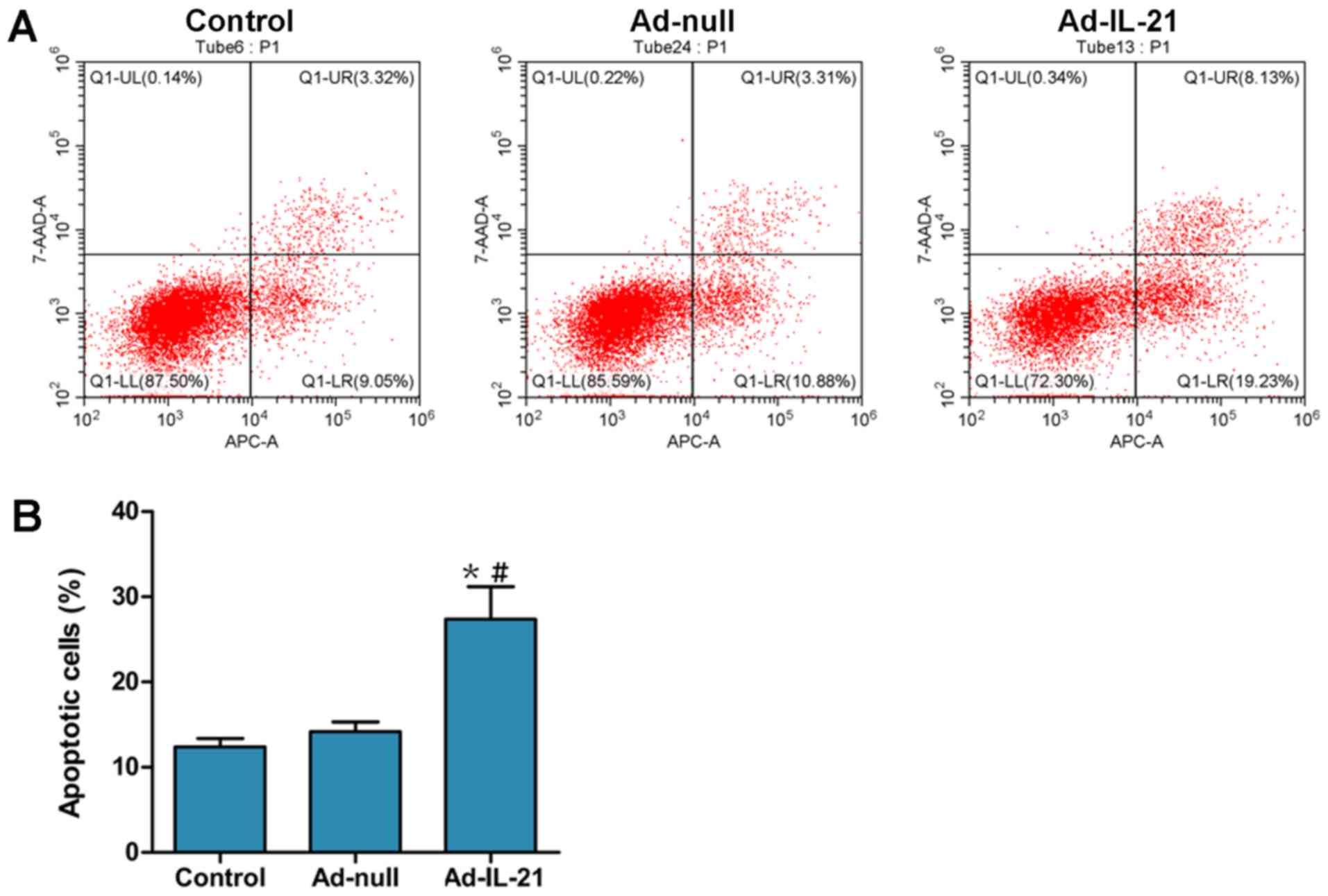
compared with the control and Ad-null groups (Fig. 4C and D). Apoptosis was also
investigated using flow cytometric analysis (Fig. 5); the number of apoptotic cells in
the Ad-IL-21 group was significantly increased compared with the
control or Ad-null groups. These results suggested that IL-21 may
promote the apoptosis of CAL-27 cells in vitro.
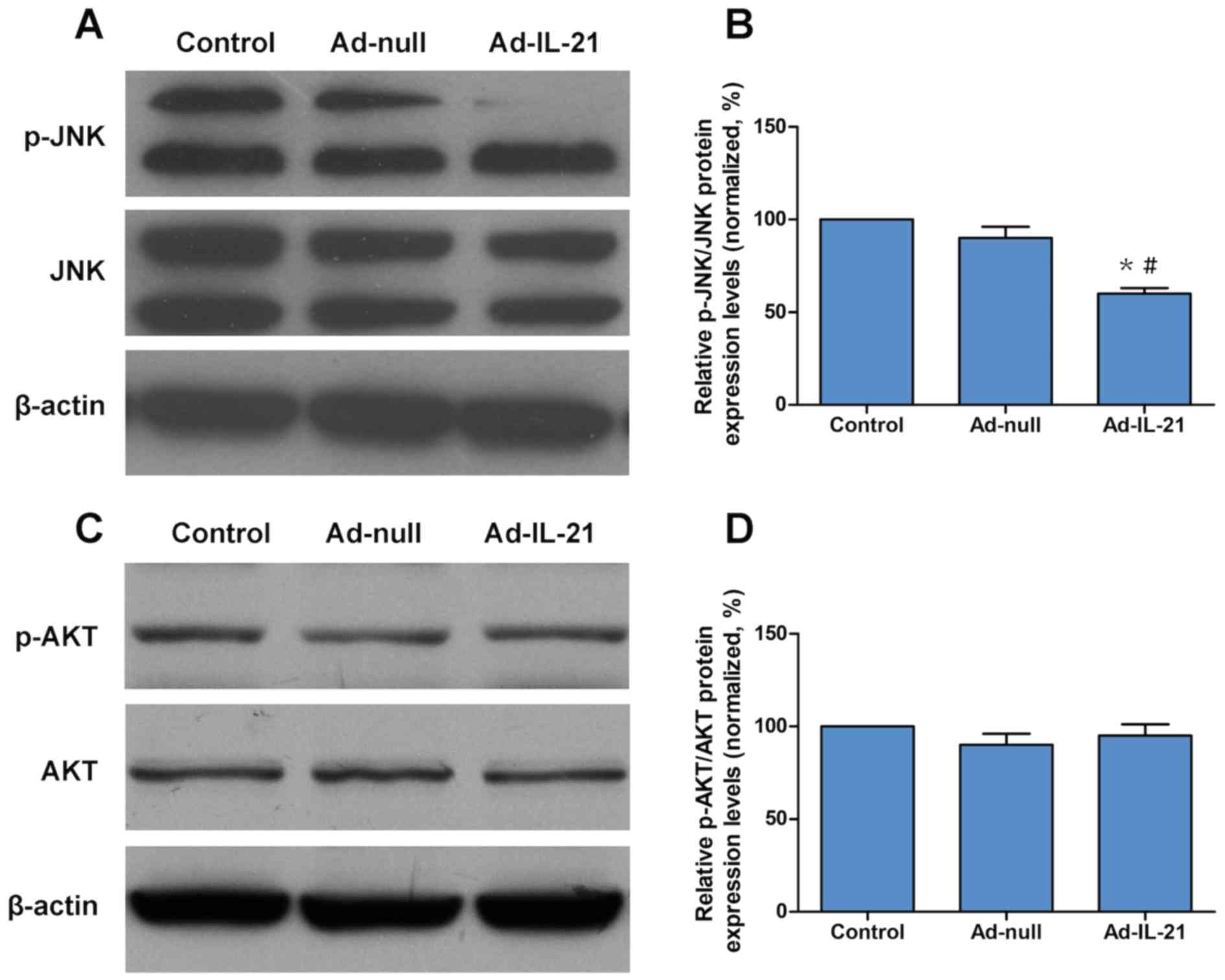
IL-21 inhibits the activation of the
JNK signaling pathway
To determine the molecular mechanism of IL-21 in
CAL-27 cells, the activation of JNK and AKT was analyzed using
western blotting. The results indicated that IL-21 overexpression
significantly decreased the phosphorylation levels of JNK compared
with the control and Ad-null groups (Fig. 6A and B), but had no effect on the
phosphorylation levels of AKT (Fig. 6C
and D). Therefore, the inhibitory effect of IL-21 in CAL-27
cells may function through the JNK signaling pathway.
Discussion
The CD4+ T-cell population is the main
source of IL-21 (21); within this
population, T follicular helper cells and Th17 cells have been
found to produce the highest amounts of IL-21, whereas the
production from NK cells was observed to be slightly lower
(21). However, IL-21 has been
revealed to be an important regulator of the differentiation,
proliferation, survival and activation of NK cells (22–24).
IL-21 reportedly serves multiple functions, such as lymphocytes
proliferation, cytotoxicity of CD8+ T cells and natural killer (NK)
cells, B-cell differentiation and Th17 development, in multiple
lymphoid organs and myeloid populations, in addition to within
epithelial cells (25). Recently,
the therapeutic efficacy of IL-21 as single-agent immunotherapy has
been tested in phase I and II clinical trials to treat different
types of cancers including melanoma, renal cell carcinoma and
metastatic colorectal cancer (13).
A modest clinical outcome was observed when patients with stage IV
malignant melanoma who did not receive prior treatment were treated
with IL-21 at a dose of 30 mg/kg/d in 5-day cycles. The overall
response rate of this study according to RECIST was about 8%, with
one of 14 patients achieving a complete response and one patient
having a confirmed partial response (26). Current research is focusing on the
potential of IL-21 in combination with targeted therapy. Such as
cetuximab, an antibody of epidermal growth factor receptor (EGFR)
(27). In a phase I trial, the
combination of IL-21 and cetuximab achieved the best response in
60% of patients with stage IV colorectal cancer (28). However, IL-21 appears to have a major
role in promoting the inflammation-induced development of colon
cancer, thus the aforementioned clinical trial was terminated
(28).
In the present study, IL-21 expression levels were
detected in the OSCC tissues and the results revealed that IL-21
expression levels were significantly decreased in OSCC tissues,
which was closely associated with the clinical stage of the tumor
and lymph node metastasis. These findings suggested that the
expression of IL-21 may be defective during tumor progression.
Consistent with these results, Dutta et al (29) also reported that the levels of IL-21
mRNA transcripts decreased in OSCC tissues and a similar trend of
transcript expression of IL-21 was also observed in peripheral
blood of patients with OSCC. Subsequently, the role of IL-21 on the
proliferation, migration and apoptosis of OSCC cells was
investigated in vitro. IL-21 overexpression was revealed to
inhibit cell proliferation and migration, whilst promoting cell
apoptosis in CAL-27 cells. Another study on laryngeal cancer cells
also demonstrated that when IL-21 was overexpressed in Hep-2 cells,
the proliferation and invasion of Hep-2 cells decreased (30). In hepatocellular carcinoma (HCC),
exogenous IL-21 may reinvigorate NK cells in patients with
HBV-associated HCC, resulting in significantly increased levels of
cytotoxicity, degranulation and cytokine expression, and the
reinvigoration of NK function by IL-21 is mediated by the STAT1
signaling pathway (31). In various
solid and hematological tumors, IL-21 improves effector T cell and
NK cell cytotoxic activity and IL-21 was also discovered to inhibit
cancer cell viability by stimulating interferon-γ production and by
increasing the granularity of NK cells (32,33).
Therefore, IL-21 may be an effective agent for cancer immunotherapy
(34).
In conclusion, the present study indicated that
IL-21 may exert a potent inhibitory effect over OSCC growth in
vitro. Future studies should aim to investigate the role of
IL-21 in OSCC animal models in order to determine its potential as
an immunotherapeutic target for OSCC.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Natural
Science Foundation of Shandong Province (grant no. ZR2016HM72), the
National Natural Science Foundation of China (grant no. 81701246)
and the Medical and Health Development Program of Shandong Province
(grant no. 2016WS0711).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and SW designed the study; HL, PL, DX and XW
performed the experiments and analyzed the data; DS performed the
immunohistochemical analysis and constructed the figures; and JY
and SW collected the human OSCC samples, confirmed the histological
grade and tumor stage and drafted the initial manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics
Committee of the Yantai Yuhuangding Hospital (Yantai, China;
approval no. 2015-127). All procedures performed in the study
involving human participants were in accordance with the ethical
standards of the Institutional and National Research Committee and
with the Declaration of Helsinki (1964) and its later amendments or
comparable ethical standards (35,36).
Patient consent for publication
The present study followed the tenets of the
Declaration of Helsinki and informed written consent was obtained
from all patients and control subjects following the explanation of
the nature and possible consequences of the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ribeiro IL, de Medeiros JJ, Rodrigues LV,
Valenca AM and Lima Neto Ede A: Factors associated with lip and
oral cavity cancer. Rev Bras Epidemiol. 18:618–629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Varoni EM, Lodi G and Iriti M: Ethanol
versus phytochemicals in wine: Oral cancer risk in a light drinking
perspective. Int J Mol Sci. 16:17029–17047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mascitti M, Orsini G, Tosco V,
Monterubbianesi R, Balercia A, Putignano A, Procaccini M and
Santarelli A: An overview on current non-invasive diagnostic
devices in oral oncology. Front Physiol. 9:15102018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polverini PJ, D'Silva NJ and Lei YL:
Precision therapy of head and neck squamous cell carcinoma. J Dent
Res. 97:614–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dinesh P and Rasool M: Multifaceted role
of IL-21 in rheumatoid arthritis: Current understanding and future
perspectives. J Cell Physiol. 233:3918–3928. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zuniga EI, Macal M, Lewis GM and Harker
JA: Innate and adaptive immune regulation during chronic viral
infections. Annu Rev Virol. 2:573–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Croce M, Rigo V and Ferrini S: IL-21: A
pleiotropic cytokine with potential applications in oncology. J
Immunol Res. 2015:6965782015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davis MR, Zhu Z, Hansen DM, Bai Q and Fang
Y: The role of IL-21 in immunity and cancer. Cancer Lett.
358:107–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen C, Liu X and Ren Y: Interleukin 21
treatment in a murine model as a novel potential cytokine
immunotherapy for colon cancer. Adv Clin Exp Med. 27:583–589. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Ma Y, Xu Y and Maerkeya K: TIM-3
expression identifies a distinctive PD-1(+) follicular helper T
cell subset, with reduced interleukin 21 production and B cell help
function in ovarian cancer patients. Int Immunopharmacol.
57:139–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang LN, Cui YX, Ruge F and Jiang WG:
Interleukin 21 and its receptor play a role in proliferation,
migration and invasion of breast cancer cells. Cancer Genomics
Proteomics. 12:211–221. 2015.PubMed/NCBI
|
|
13
|
Bhatia S, Curti B, Ernstoff MS, Gordon M,
Heath EI, Miller WH Jr, Puzanov I, Quinn DI, Flaig TW,
VanVeldhuizen P, et al: Recombinant interleukin-21 plus sorafenib
for metastatic renal cell carcinoma: A phase 1/2 study. J
Immunother Cancer. 2:22014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhatt S, Sarosiek KA and Lossos IS:
Interleukin 21-its potential role in the therapy of B-cell
lymphomas. Leuk Lymphoma. 58:17–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Timmerman JM, Byrd JC, Andorsky DJ, Yamada
RE, Kramer J, Muthusamy N, Hunder N and Pagel JM: A phase I
dose-finding trial of recombinant interleukin-21 and rituximab in
relapsed and refractory low grade B-cell lymphoproliferative
disorders. Clin Cancer Res. 18:5752–5760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katabi N and Lewis JS: Update from the 4th
edition of the world health organization classification of head and
neck tumours: What is new in the 2017 WHO blue book for tumors and
tumor-like lesions of the neck and lymph nodes. Head Neck Pathol.
11:48–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Head and Neck Cancer Study Group (HNCSG),
; Monden N, Asakage T, Kiyota N, Homma A, Matsuura K, Hanai N,
Kodaira T, Zenda S, Fujii H, et al: A review of head and neck
cancer staging system in the TNM classification of malignant tumors
(eighth edition). Jpn J Clin Oncol. 49:589–595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun ZP, Gong L, Huang SH, Geng Z, Cheng L
and Chen ZY: Intracellular trafficking and secretion of cerebral
dopamine neurotrophic factor in neurosecretory cells. J Neurochem.
117:121–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olson B: Assays for determination of
protein concentration. Curr Protoc Pharmacol. 73:A 3A 1–A 3A 32.
2016. View
Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tangye SG and Ma CS: Regulation of the
germinal center and humoral immunity by interleukin-21. J Exp Med.
217:e201916382020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gharibi T, Majidi J, Kazemi T,
Dehghanzadeh R, Motallebnezhad M and Babaloo Z: Biological effects
of IL-21 on different immune cells and its role in autoimmune
diseases. Immunobiology. 221:357–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Ye LJ, Ren HL, Huyan T, Li J, Shi JL
and Huang QS: Multiple effects of IL-21 on human NK cells in ex
vivo expansion. Immunobiology. 220:876–888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romee R, Leong JW and Fehniger TA:
Utilizing cytokines to function-enable human NK cells for the
immunotherapy of cancer. Scientifica (Cairo).
2014:2057962014.PubMed/NCBI
|
|
25
|
Spolski R and Leonard WJ: Interleukin-21:
A double-edged sword with therapeutic potential. Nat Rev Drug
Discov. 13:379–395. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davis ID, Brady B, Kefford RF, Millward M,
Cebon J, Skrumsager BK, Mouritzen U, Hansen LT, Skak K, Lundsgaard
D, et al: Clinical and biological efficacy of recombinant human
interleukin-21 in patients with stage IV malignant melanoma without
prior treatment: A phase IIa trial. Clin Cancer Res. 15:2123–2129.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim N, Cho D, Kim H, Kim S, Cha YJ,
Greulich H, Bass A, Cho HS and Cho J: Colorectal
adenocarcinoma-derived EGFR mutants are oncogenic and sensitive to
EGFR-targeted monoclonal antibodies, cetuximab and panitumumab. Int
J Cancer. 146:2194–2200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steele N, Anthony A, Saunders M, Esmarck
B, Ehrnrooth E, Kristjansen PE, Nihlen A, Hansen LT and Cassidy J:
A phase 1 trial of recombinant human IL-21 in combination with
cetuximab in patients with metastatic colorectal cancer. Br J
Cancer. 106:793–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dutta A, Banerjee A, Saikia N, Phookan J,
Baruah MN and Baruah S: Negative regulation of natural killer cell
in tumor tissue and peripheral blood of oral squamous cell
carcinoma. Cytokine. 76:123–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Q, Qiao X, Li D, Chen B, Zhang L,
Yuan C and Lin H: Expression and association of IL-21, FBXL20 and
tumour suppressor gene PTEN in laryngeal cancer. Saudi J Biol Sci.
26:2048–2051. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin Y, Sun Z, Geng J, Yang L, Song Z, Song
H, Wang J and Tang J: IL-21 reinvigorates exhausted natural killer
cells in patients with HBV-associated hepatocellular carcinoma in
STAT1-depedent pathway. Int Immunopharmacol. 70:1–8. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seo H, Jeon I, Kim BS, Park M, Bae EA,
Song B, Koh CH, Shin KS, Kim IK, Choi K, et al: IL-21-mediated
reversal of NK cell exhaustion facilitates anti-tumour immunity in
MHC class I-deficient tumours. Nat Commun. 8:157762017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leonard WJ, Lin JX and O'Shea JJ: The γ c
family of cytokines: Basic biology to therapeutic ramifications.
Immunity. 50:832–850. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Floros T and Tarhini AA: Anticancer
cytokines: Biology and clinical effects of interferon-α2,
interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin Oncol.
42:539–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bruce-Chwatt LJ: Declaration of helsinki.
Recommendations guiding doctors in clinical research. WHO Chron.
19:31–32. 1965.PubMed/NCBI
|
|
36
|
Woods S and McCormack P: Disputing the
ethics of research: The challenge from bioethics and patient
activism to the interpretation of the declaration of helsinki in
clinical trials. Bioethics. 27:243–250. 2013. View Article : Google Scholar : PubMed/NCBI
|