Introduction
Prostate cancer (PCa), which is related to the
activity of the androgen receptor (AR), is the second major cause
of cancer-related mortality (10%) among men in the United States in
2019 (1). Androgen deprivation
therapy (ADT) is the primary approach for the treatment of PCa, but
the majority of patients eventually progress to the disease stage
known as castration-resistant PCa (CRPC) (2). Enzalutamide, an AR antagonist, is
currently the most effective treatment for CRPC (3). However, enzalutamide resistance
develops after several months of treatment (4). Therefore, it is important to develop an
effective therapeutic target to overcome enzalutamide
resistance.
Rac1, a small GTPase, has been demonstrated to
regulate the reorganization of the cytoskeleton during cellular
activities, including cell cycle progression, migration, adhesion
and polarization (5). In addition,
Rac1 is commonly upregulated in ovarian cancer, breast cancer,
colon cancer, and non-small cell lung cancer (6–9), and is
associated with a poor prognosis. Rac1 is a key molecular component
in tumorigenesis, invasion and metastasis, and directly activates
various downstream pathways, including p21-activated kinases,
actin-binding LIM kinase and cofilin, to control cellular processes
(10,11). Previous studies have demonstrated
that Rac1 contributes to poor treatment response and drug
resistance in cancer (12,13) and Rac1 inhibition may result in a
reduction in the dosage of cisplatin by 1.5- to 3.0-fold in head
and neck squamous cell carcinoma (14,15).
Reinstatement of doxorubicin can sensitize squamous cell carcinoma
by inhibiting Rac1 expression (16).
In addition, Rac1 inhibition reverses the resistance to cisplatin
and 5-fluorouracil chemotherapy in gastric adenocarcinoma spheroids
and inhibits tumor growth in vivo (17,18).
Therefore, Rac1 may be a valid therapeutic target for patients with
chemotherapy resistance.
In this study, we explored the role of Rac1 in the
development of enzalutamide resistance and investigated the
effectiveness and the associated mechanisms of combination
enzalutamide and Rac1 depleted in CRPC.
Materials and methods
Cell culture and lentivirus
MR49F cells were developed enzalutamide resistant
cells derived from the parent cell line LNCaP, and obtained from Dr
Xiaoqi Liu's lab and maintained in RPMI-1640 medium (Thermo Fisher
Scientific, Inc.) containing 10 µM enzalutamide at 37°C with 5%
CO2 (19). LNCaP and
22RV1 cells were purchased from the American Type Culture
Collection and cultured in RPMI-1640 medium supplemented with 10%
(v/v) fetal bovine serum (FBS) (Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2. Lentiviruses with short hairpin RNA
(shRNA) targeting Rac1 (sh-Rac1; 5′-CCTTCTTAACATCACTGTCTT-3′) and
non-targeting control (sh-Ctl; 5′-GCGCGATAGCGCTAATAATTT-3′) were
purchased from Sigma-Aldrich; Merck KGaA. Rac1 downregulation was
performed using 3×106 MR49F or 22Rv1 cells by
transfecting the pLKO.1/sh-Rac1 (the titer of 105) or
the silencer negative control pLKO.1/sh-Ctl lentiviruses. Following
transfection the cells were cultured with 2 µg/ml puromycin for 3
days before subsequent experiments.
Antibodies and reagents
Antibodies against Rac1 (cat. no. 2465), JNK (cat.
no. 9252), activated transcription factor 2 (ATF2; cat. no. 35031),
E-cadherin (cat. no. 14472), Snail (cat. no. 3879), GAPDH (cat. no.
2118), Bax (cat. no. 14796), Bcl-2 (cat. no. 4223), Ki67 (cat. no.
9129), cleaved-poly-ADP ribose polymerase (PARP; cat. no. 5625),
anti-mouse IgG (cat. no. 7076), and anti-rabbit IgG (cat. no. 7074
and 4412) were purchased from Cell Signaling Technology, Inc.
Enzalutamide (cat. no. S1250) was purchased from Selleck
Chemicals.
Western blotting
Cells (1×104) were plated in 25
cm2 flask at 37°C with 5% CO2. After
incubation at 37°C for 24 h, cells were treated with 5 µl DMSO or
enzalutamide (20 µM) for another 48 h. Then cells were washed
thrice in ice-cold PBS, harvested and cell pellets were resuspended
in RIPA lysis buffer (cat. no. R0278; Sigma-Aldrich; Merck KGaA)
supplemented with a protease and phosphatase inhibitor cocktail and
subjected to sonication. Sonication was performed at 4°C for 10–30
sec on ice until the sample solution was clear. Protein
concentrations were measured using a Pierce bicinchoninic acid
protein assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.).
Equal amounts of proteins (20 µg per sample) were separated using
10% SDS-PAGE and transferred to PVDF membranes. The membranes were
blocked with 5% milk in PBS at room temperature for 1 h and then
incubated at 4°C overnight with the primary antibodies, including
Rac1, JNK, ATF2, E-cadherin, cleaved-PARP, Snail, GAPDH, Bax and
Bcl-2 (1:1,000). Subsequently, the membranes were washed thrice
with PBS and incubated with horseradish peroxidase-conjugated
secondary antibodies (anti-rabbit or anti-mouse IgG; 1:2,000; Cell
Signaling Technology, Inc.) for 30 min at room temperature. Protein
bands were visualized with Super Signal West Dura Extended Duration
Substrate (Thermo Fisher Scientific, Inc.). All data are
representative of ≥3 independent experiments.
Cell viability assay
MR49F cells (3×103 cells/well) were
seeded into 96-well plates, cultured for 12 h and treated with 20
µM enzalutamide at 37°C with 5% CO2 for a certain time.
MTT (10 µl, 5 mg/ml) was added to each well and cells were
incubated for an additional 4 h. Then, 150 µl DMSO was added into
each well to dissolve the formazan crystals, and the optical
density at 570 nm was measured using a microplate reader.
Manual cell counting
MR49F cells (2×104 cells/well) were
seeded into 6-well plates, cultured for 12 h and treated with 20 µM
enzalutamide at 37°C with 5% CO2. After incubation,
cells were washed thrice with PBS, harvested and counted using a
TC10 automated cell counter (cat. no. 145–0010; Bio-Rad
Laboratories, Inc.).
Colony formation assay
MR49F (sh-Ctl) or MR49F (sh-Rac1) cells
(5×102 cells/well) were seeded into 6-well plates. After
incubation overnight, cells were treated with or without 10 µM
enzalutamide for 14 days. The colonies were fixed in 10% formalin
for 15 min at room temperature, and stained with 1% crystal violet
at room temperature for 30 min and the number of colonies was
counted manually.
Xenograft experiments
The animal experimental protocol was approved by The
Committee of Animal Experiments and Experimental Animal Welfare of
Shanxi Datong University (protocol no. 2019018). Twenty male BALB/c
nude mice (4–6 weeks; weighing 18–20 g; Beijing Vital River
Laboratories Animal Technology Co., Ltd) were housed at a constant
temperature (23°C) and relative humidity (60%) with a fixed 12 h
light-dark cycle, and free access to food and water. After 2 weeks
of feeding in the new environment, mice were divided into the four
groups randomly with 5 mice per group: sh-Ctl, sh-Ctl with
enzalutamide, sh-Rac1, sh-Rac1with enzalutamide group.
22RV1(sh-Ctl) or 22RV1 (sh-Rac1) cells (3×105
cells/mouse) were mixed with 50% Matrigel gel (cat. no. 354248;
Corning, Inc.) and inoculated into pre-castrated nude mice
subcutaneously in the right dorsal flank until the average tumor
size of sh-Ctl and sh-Ctl plus enzalutamide groups grew up to ~100
mm3. The mice maintained at a specific pathogen free
facility with a constant humidity and temperature at 12/12 h
light/dark cycle with free access to food and water. An
enzalutamide suspension (30 mg/kg) was prepared in corn oil and
administered to mice in the sh-Ctl plus enzalutamide and sh-Rac1
plus enzalutamide groups via gavage every 2 days. The
administration volume of per mouse was 0.2 ml. Of note, the coil
was given every 2 days also. The tumor size and body weight were
measured every 2 days by reading Vernier caliper and the tumor
volumes were calculated using the formula V=L × W2/2,
where V is the volume (mm3), L is length (mm) and W is
the width (mm). The wet weight and size of the tumors and body
weights were measured after the the mice were sacrificed on day
24.
Histology and immunofluorescence
staining
Xenograft tumors were dissected, fixed in 10%
formalin overnight at room temperature, embedded in paraffin,
sectioned at 5 µm thick, and mounted on poly-L-lysine-charged glass
slides (cat. no. P0425-72EA; Sigma-Aldrich; Merck KGaA). The
hematoxylin and eosin (H&E) staining was completed in the
Affiliated Tumor Hospital of Shanxi Datong University.
Immunofluorescence staining was performed using an Immunodetection
M.O.M kit (cat. no. PK-2200; Vector Laboratories, Inc.) according
to the manufacturer's protocol. The paraffin-embedded tissue blocks
were cut into 5-µM sections and mounted on glass slides. After the
sections were deparaffinized with xylene and rehydrated in a
descending ethanol series of 70, 80, 90, 95 and 100%, followed by
antigen retrieval (cat. no. H-3300, Vector Laboratories, Inc.),
microwaved on high power to 100°C for 3 min. Then sections were
cooled to room temperature and endogenous peroxidase activity was
blocked by incubation sections with BLOXALL® Endogenous
Peroxidase and Alkaline Phosphatase blocking solution (cat. no.
SP-6000, Vector Laboratories, Inc.) for 10 min at room temperature.
The sections were then incubated in working solution of
M.O.MTM mouse IgG blocking reagent (10 µl of stock
solution in M.O.M diluent) at room temperature for 1 h.
Subsequently, the sections were incubated with anti-Ki67 rabbit
antibody (1:200) in M.O.MTM diluent overnight at 4°C.
After washing by PBS with 0.2% Triton X-100, the sections were
incubated with the anti-rabbit IgG (Alexa Fluor® 488
conjugate, 1:200) at room temperature for 1 h. After washing, the
sections were subsequently stained with DAPI (cat. no. P36931,
Thermo Fisher Scientific, Inc.) at room temperature for 15 min, and
covered with coverslip. the sample sections were viewed using a
light microscope (Axioplan 2; Zeiss, Berlin, Germany).
Cancer cell invasion and migration
assay
MR49F cells (4×104) were seeded into the
upper chamber of a 24-well Transwell plate (cat. no. 3422; Corning,
Inc.). After incubation at 37°C for 4 h, the cells in the upper
chamber were treated with or without 10 µM enzalutamide in
RPMI-1640 medium containing 0.1% FBS, and RPMI-1640 medium with
0.5% FBS, as a chemoattractant, was added into the lower chamber.
Following incubation at 37°C for 72 h, cells that migrated into the
opposite side of the membrane were washed with PBS, fixed in 4%
paraformaldehyde at room temperature for 30 min and stained with
0.25% crystal violet for 15 min at room temperature. Cell migration
was quantified by counting the number of migrated cells in
microscopic fields (magnification, ×100) per filter by the light
microscope, and the mean value per filter was calculated from three
replicate filters.
For the Transwell invasion assay, the upper well of
the Transwell insert was precoated with 50 µl (1 µg/ml) Matrigel
(cat. no. 356234; BD Biosciences) at 37°C in a 5% CO2
incubator for 4 h, and the MR49F cells (4×104) were
seeded into the upper chamber. The subsequent methodology of the
assay was performed as aforementioned.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 7 software (GraphPad Software, Inc.), and the data are
presented as the mean ± SD. One-way ANOVA followed by Tukey's
multiple comparison test was used to determine statistical
significance. P<0.05 was considered to indicate a statistically
significant difference.
Results
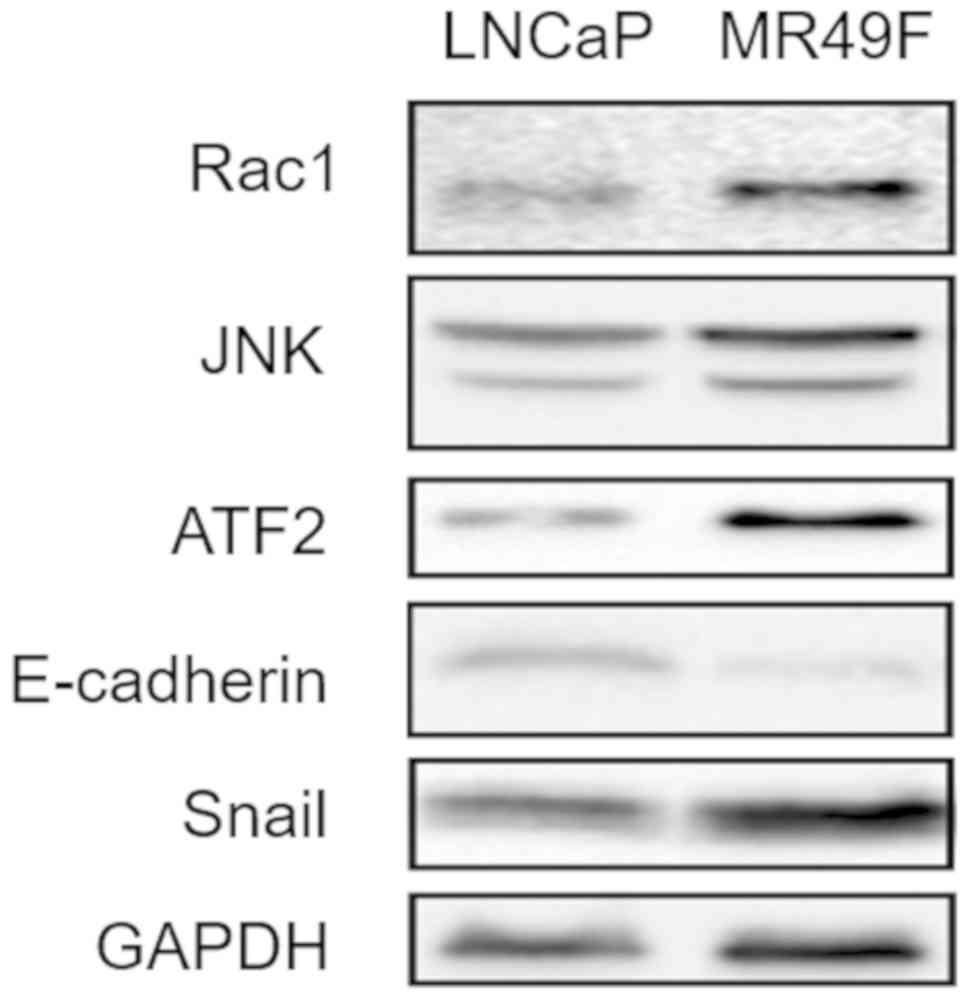
Identification of highly expressed
Rac1 in enzalutamide-resistant PCa cell lines (19)
To investigate the molecular mechanisms underlying
enzalutamide resistance in CRPC, western blotting was performed
using LNCaP and MR49F cells. The results demonstrated that Rac1,
JNK and ATF2 protein expression levels were upregulated in MR49F
cells compared with those in LNCaP cells (Fig. 1). In addition, the protein expression
levels of E-cadherin, an epithelial-mesenchymal transition (EMT)
marker (20), were lower in the
enzalutamide resistance cells. In contrast, Snail, another EMT
marker, was much higher in MR49F cells compared with LNCaP cells.
These results suggested that the Rac1/JNK/ATF2 signaling pathway
may be activated in enzalutamide-resistant PCa cells.
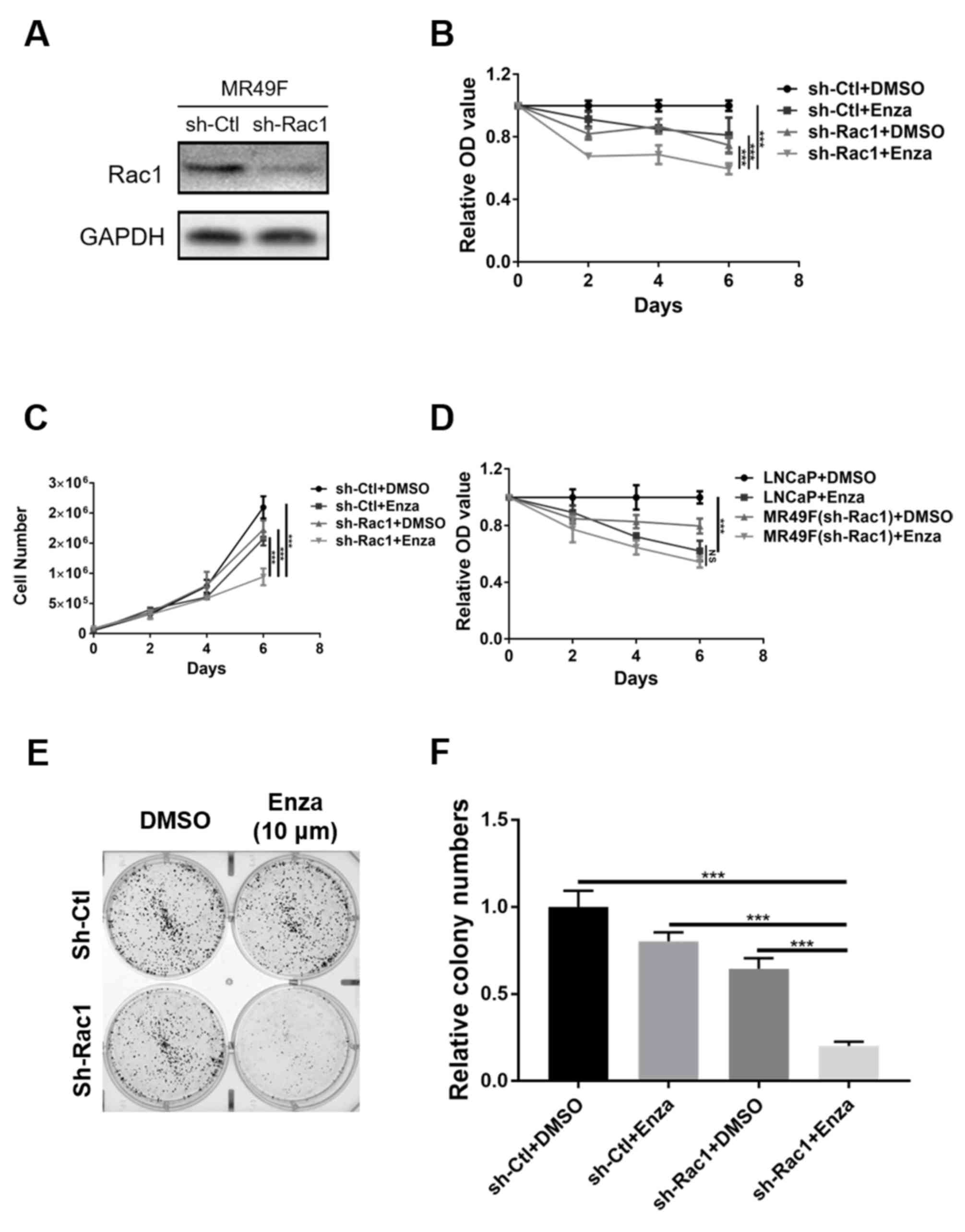
Rac1 knockdown enhances the
sensitivity of enzalutamide-resistant PCa cells to
enzalutamide
To further assess the role of Rac1 in the
development of enzalutamide resistance in CRPC, MR49F cells were
infected with a lentivirus expressing shRNA to knock down Rac1
(Fig. 2A). The results demonstrated
that the treatment of Rac1-knockdown MR49F cells with enzalutamide
significantly inhibited cell proliferation, compared with
monotherapy with enzalutamide or Rac1-deleted (Fig. 2B and C). To clarify whether there was
Rac1-independent enzalutamide resistance, the rates of LNCaP cell
proliferation following enzalutamide treatment with were compared
with that of Rac1-knockdown MR49F cells treatment with
enzalutamide. The results suggested that there were no significant
difference between the two cell types (Fig. 2D). Additionally, treatment with
enzalutamide also significantly inhibited colony formation in
Rac1-knockdown MR49F cells compared to Rac1-deleted MR49F cells and
MR49F (sh-Ctl) treated with enzalutamide alone (Fig. 2E and F). Of note, treatment of
Rac1-knockdown MR49F cells with enzalutamide significantly induced
cleaved-PARP and Bax expression, and downregulated Bcl-2 expression
(Fig. 3E). Collectively, the present
results suggested that knockdown of Rac1 was sufficient to induce
the re-sensitization of enzalutamide-resistant PCa cells to
enzalutamide.
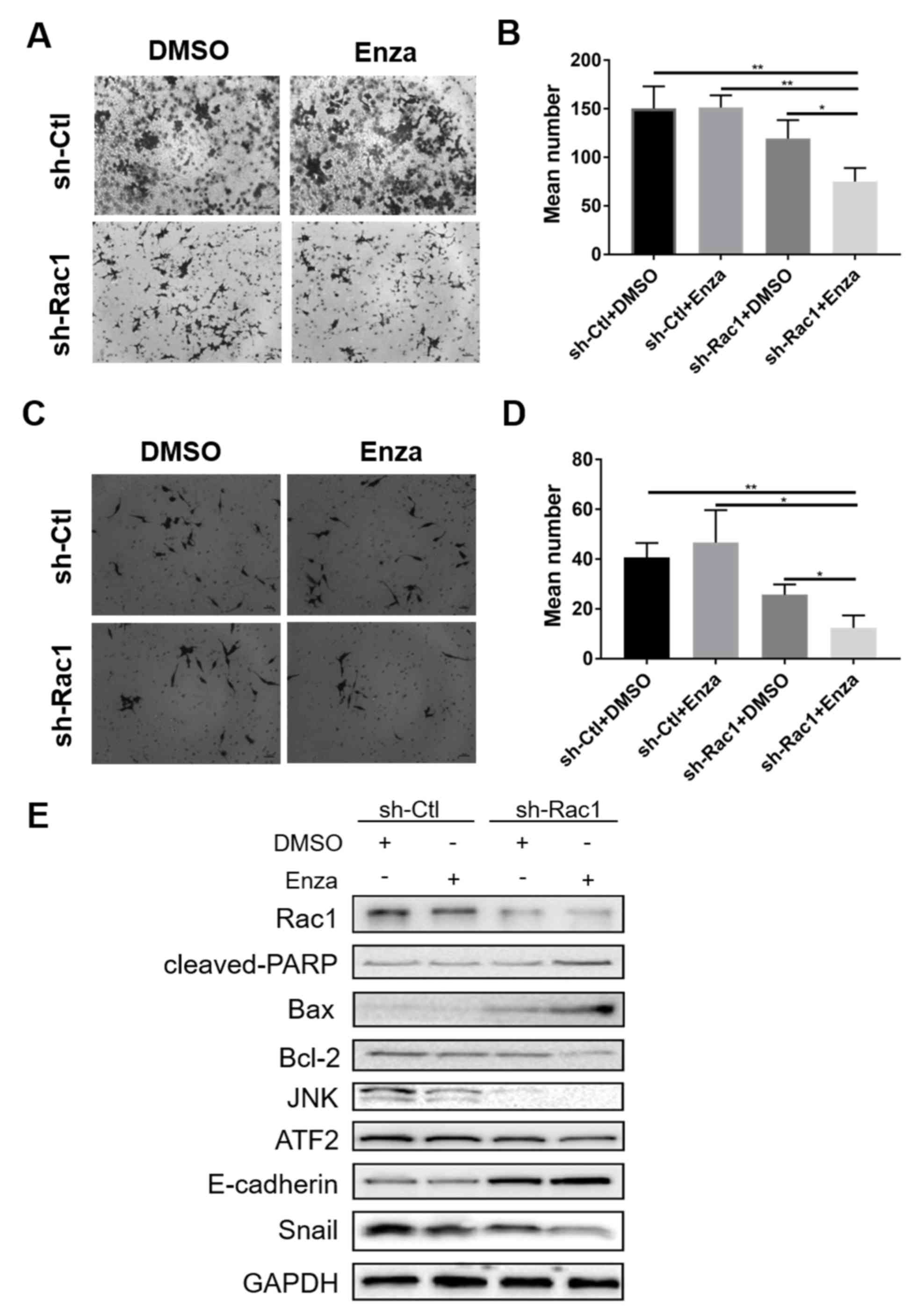
Knockdown of Rac1 attenuates the
migration and invasion of enzalutamide-resistant PCa cells
The effects of knocking down Rac1 on MR49F cell
migration and invasion were examined. The results demonstrated that
enzalutamide treatment significantly inhibited the migratory and
invasive abilities of MR49F cells following Rac1 knockdown
(Fig. 3A-D). To study the underlying
mechanisms of the enzalutamide-induced inhibitory effect on cell
migration and invasion in enzalutamide-resistant PCa cells, western
blotting was performed; treatment with enzalutamide in
Rac1-knockdown MR49F cells upregulated of E-cadherin expression to
a higher degree compared with Rac1-knockdown cells treated with
DMSO or sh-Ctl-transfected cells treated with enzalutamide
(Fig. 3E). Furthermore, Snail
expression was decreased in Rac1-knockdown cells treated with
enzalutamide compared with the other groups. In addition, the
expression levels of the downstream proteins of Rac1, including JNK
and ATF2, were also decreased in Rac1-knockdown MR49F cells treated
with enzalutamide. Therefore, these results suggested that knocking
down Rac1 attenuated the migratory and invasive abilities of
enzalutamide-resistant PCa cells via the upregulation of E-cadherin
and downregulation of Snail expression.
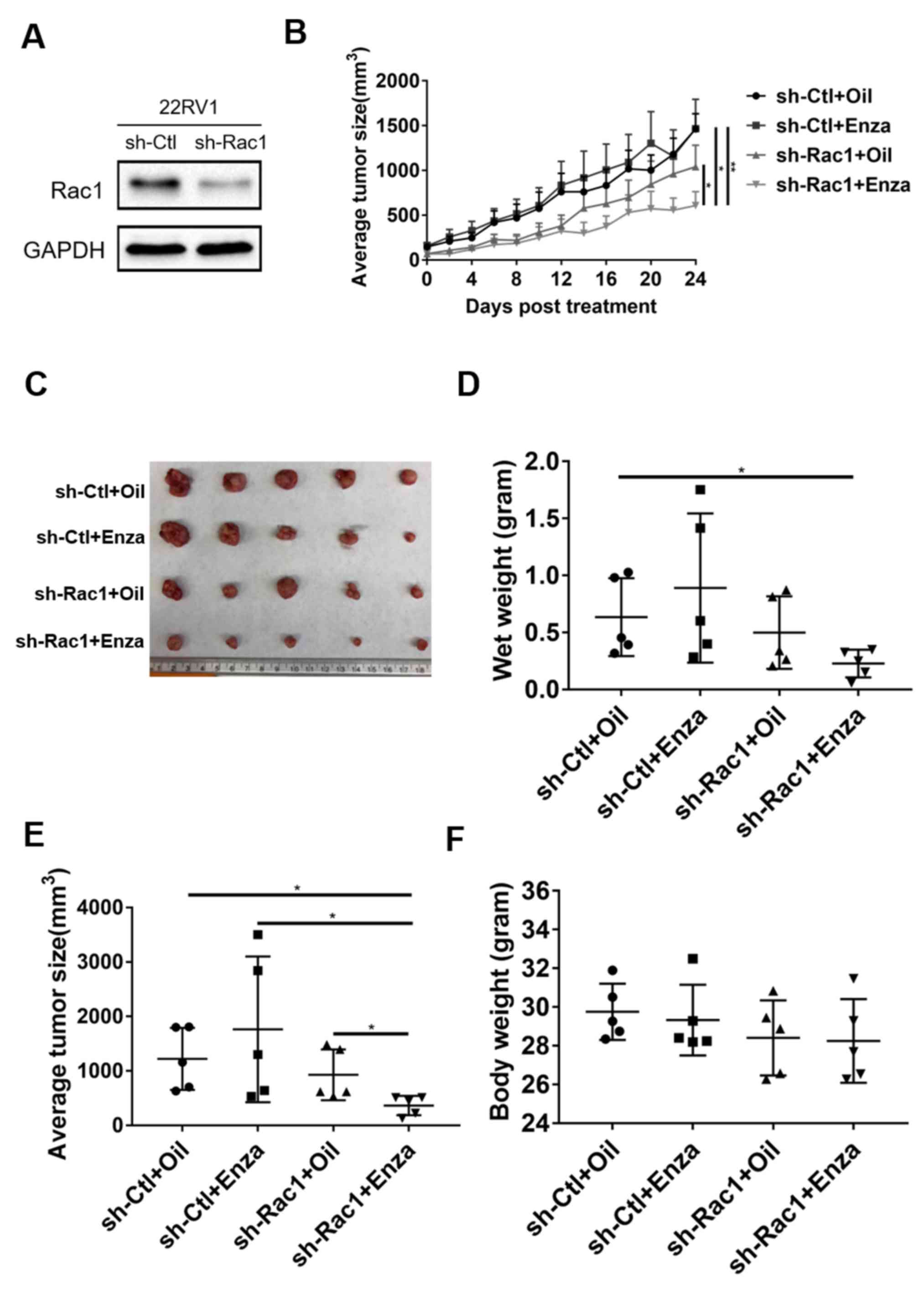
Depletion of Rac1 enhances the
efficacy of enzalutamide in enzalutamide-resistant xenograft
tumors
To assess the effects of enzalutamide on the
regulation of Rac1 in vivo, a 22RV1-derived xenograft mice
model was established (Fig. 4A). The
results demonstrated that Rac1 depletion inhibited tumor growth in
Rac1-knockdown 22RV1 cell xenograft mice, indicating that knockdown
of Rac1 reduced enzalutamide resistance and restored cell
sensitivity to enzalutamide (Fig. 4B and
C). While both wet weights and sizes of the tumors were reduced
by Rac1 knockdown alone (sh-Rac1 group) or monotherapy of
enzalutamide (sh-Ctl plus enzalutamide group), the effect was more
significant with Rac1 knockdown plus enzalutamide treatment
(Fig. 4D and E). On day 24, the mean
tumor volume was reduced in Rac1-knockdown 22RV1 cell xenograft
mice treated with enzalutamide (365.047±157.164 mm3)
compared with the control mice (1,221.633±509.891 mm3;
Fig. 4E). In addition, the volume of
largest tumor was in the monotherapy of enzalutamide group
(3,501.142 mm3). However, no significant differences
were observed in the body weights of mice among the different
groups (Fig. 4F), suggesting that
the side effects were not aggravated when using a combined
treatment of Rac1 knockdown and enzalutamide.
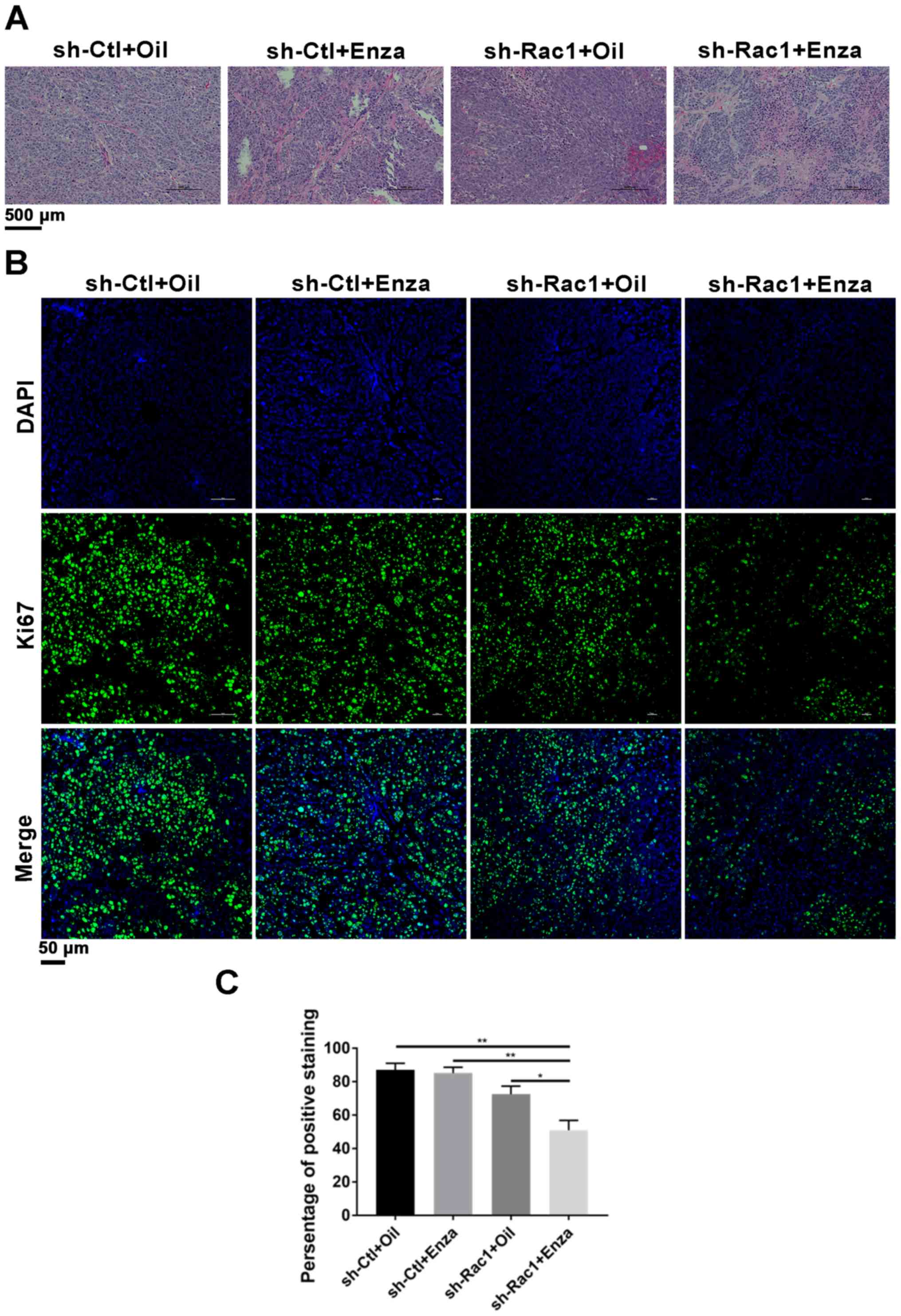
H&E staining of the xenograft tumors identified
numerous mitotic cells in tumor tissues in the control group,
suggesting that cell proliferation was active (Fig. 5A). By contrast, a large number of
apoptotic bodies with condensed cytoplasm and pyknotic nuclei were
detected in Rac1 knockdown cell-derived tumors from mice treated
with enzalutamide. To determine whether Rac1 knockdown alone or its
combination with enzalutamide suppressed tumor proliferation and
promoted apoptosis, tumor sections were analyzed by
immunofluorescence staining with Ki67 (Fig. 5B and C). Immunofluorescence staining
showed that the positive ratios of the Ki-67 protein in the
Rac1-delepted plus the enzalutamide group were 50.893±10.221%,
which is less than the control (87.001±6.915%), the enzalutamide
(85.092±5.997%) and the Rac1-depleted groups (72.465±8.313%).
Collectively, the in vivo results, which were in line with
the cell-based experiments, suggested that Rac1 knockdown reversed
enzalutamide-resistance in CRPC, and thus provided a novel
therapeutic strategy for the treatment for CRPC.
Discussion
PCa is one of the most common cancer types among men
in the United States (1), and
enzalutamide, which was approved by the US Food and Drug
Administration in 2018, is the most frequently used first-line
treatment for patients with metastatic CRPC (21). However, the development of
enzalutamide resistance limits the utilization of enzalutamide for
treating patients with CRPC (22–24).
Multiple signaling pathways have been repcontribute to enzalutamide
resistance, and inhibiting these pathways has been demonstrated to
enhance enzalutamide efficacy (19,25–28).
Rac1 serves a major role in the regulation of cytoskeleton
organization and cell adhesion, and its upregulation is associated
with cancer and poor prognosis (11,29,30).
Furthermore, Rac1 can induce EMT by enhancing the expression of JNK
(11,29,30). The
results of the present study suggested that Rac1 was upregulated in
enzalutamide-resistant MR49F cells, thus indicating that abnormal
Rac1 may be a key contributing factor in enzalutamide resistance.
Furthermore, knockdown of Rac1 re-sensitized MR49F cells to
enzalutamide, which prevented further cell proliferation and colony
formation. Knockdown of Rac1 enhanced the efficacy of enzalutamide
in enzalutamide-resistant xenograft tumors. Mechanistically, it was
demonstrated that knocking down Rac1 inhibited the expression
levels of downstream proteins JNK and ATF2. Therefore, the present
study provided a potential strategy for the treatment of patients
with enzalutamide-resistant CRPC. Future studies will examine the
effects of Rac1 knockdown on the phosphorylation of JNK and assess
whether activation of the Rac1/JNK signaling pathway may serve a
role in the acquisition of enzalutamide resistance in CRPC
cells.
EMT is a complex biological process during which
epithelial cells acquire the metastatic and invasive phenotypes of
mesenchymal cells (31,32). EMT is highly associated with drug
resistance, which results in the failure of standard chemotherapy
(33). Previous studies have also
revealed that EMT induces enzalutamide resistance in PCa (34–36). In
addition, it has been demonstrated that EMT is associated with the
upregulation of Rac1 (8,17). Leng et al (9), have reported that Rac1 upregulation
promotes EMT via the PAK1 and p38/MAPK pathways, resulting in poor
prognosis. The activation of the Rac1/β-catenin signaling pathway
can also lead to cell invasion by regulating the expression levels
of Snail and matrix metalloproteinase-9 (37) and inhibiting the Rac1/β-catenin
pathway that suppresses cell EMT (38,39). The
results of the present study indicated that the knockdown of Rac1
resulted in the downregulation of Snail and the upregulation of
E-cadherin expression, suggesting that Rac1 promoted cell invasion
and migration that in turn resulted in drug resistance. Therefore,
targeted depletion of Rac1 may enhance the efficacy of enzalutamide
in enzalutamide-resistant cells.
In conclusion, the results of the present study
suggested that the aberrant expression of Rac1 contributed to
enzalutamide resistance. In addition, the proliferation of
enzalutamide-resistant cells was significantly inhibited by Rac1
knockdown combined with enzalutamide in vivo and in
vitro. Therefore, this novel combinatorial therapeutic strategy
may be used to overcome enzalutamide resistance in CRPC.
Acknowledgements
The authors would like to thank Dr Xiaoqi Liu of the
University of Kentucky for providing technical support.
Funding
The study was supported by the Ph.D. Initiation
Grant of Shanxi Datong University (grant nos. 2017-B-21 and
2018-B-16), the Crosswise Tasks of the First Affiliated Hospital of
Shanxi Datong University (grant no. HX-201938), the Natural Science
Foundation of Shanxi (grant nos. 201801D121035, 201901D211427 and
201901D211428) and the Scientific and Technological Innovation
Programs of Higher Education Institutions in Shanxi (grant no.
2019L0735).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XC, LY and FF designed the experiments. XC, LY, YB,
GQ, YL and BL conducted the experiments. XC wrote the manuscript.
YL, BL and FF provided technical support and supervised the
progress of the experiments. XC and LY analyzed the statistical
data and prepared the figures. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Committee of
Animal Experiments and Experimental Animal Welfare of Shanxi Datong
University (protocol no. 2019018), Datong, Shanxi, P. R. China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hotte SJ and Saad F: Current management of
castrate-resistant prostate cancer. Curr Oncol. 17 (Suppl
2):S72–S79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siemens DR, Klotz L, Heidenreich A,
Chowdhury S, Villers A, Baron B, van Os S, Hasabou N, Wang F, Lin P
and Shore ND: Efficacy and safety of enzalutamide vs bicalutamide
in younger and older patients with metastatic castration resistant
prostate cancer in the TERRAIN trial. J Urol. 199:147–154. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beer TM, Armstrong AJ, Rathkopf DE, Loriot
Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J,
Chowdhury S, et al: Enzalutamide in metastatic prostate cancer
before chemotherapy. N Engl J Med. 371:424–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cardama GA, Alonso DF, Gonzalez N, Maggio
J, Gomez DE, Rolfo C and Menna PL: Relevance of small GTPase Rac1
pathway in drug and radio-resistance mechanisms: Opportunities in
cancer therapeutics. Crit Rev Oncol Hematol. 124:29–36. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Y, Fu F, Lv J, Wang M, Li Y, Zhang J
and Wang C: Identification of potential key genes for HER-2
positive breast cancer based on bioinformatics analysis. Medicine
(Baltimore). 99:e184452020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia L, Lin J, Su J, Oyang L, Wang H, Tan
S, Tang Y, Chen X, Liu W, Luo X, et al: Diallyl disulfide inhibits
colon cancer metastasis by suppressing Rac1-mediated
epithelial-mesenchymal transition. Onco Targets Ther. 12:5713–5728.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Liao Q, Han Y, Chen J, Liu Z, Ling
H, Zhang J, Yang W, Oyang L, Xia L, et al: Rac1 overexpression is
correlated with epithelial mesenchymal transition and predicts poor
prognosis in non-small cell lung cancer. J Cancer. 7:2100–2109.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leng R, Liao G, Wang H, Kuang J and Tang
L: Rac1 expression in epithelial ovarian cancer: Effect on cell EMT
and clinical outcome. Med Oncol. 32:3292015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bid HK, Roberts RD, Manchanda PK and
Houghton PJ: RAC1: An emerging therapeutic option for targeting
cancer angiogenesis and metastasis. Mol Cancer Ther. 12:1925–1934.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Engers R, Ziegler S, Mueller M, Walter A,
Willers R and Gabbert HE: Prognostic relevance of increased Rac
GTPase expression in prostate carcinomas. Endocr Relat Cancer.
14:245–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hofbauer SW, Krenn PW, Ganghammer S,
Asslaber D, Pichler U, Oberascher K, Henschler R, Wallner M,
Kerschbaum H, Greil R and Hartmann TN: Tiam1/Rac1 signals
contribute to the proliferation and chemoresistance, but not
motility, of chronic lymphocytic leukemia cells. Blood.
123:2181–2188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Chen G, Sun L, Zhang Y, Han J, Dai
Y, He J, Shi S and Chen B: TUFT1 Promotes triple negative breast
cancer metastasis, stemness, and chemoresistance by up-regulating
the Rac1/β-catenin pathway. Front Oncol. 9:6172019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arnold CR, Abdelmoez A, Thurner G, Debbage
P, Lukas P, Skvortsov S and Skvortsova II: Rac1 as a
multifunctional therapeutic target to prevent and combat cancer
metastasis. Oncoscience. 1:513–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Skvortsov S, Dudás J, Eichberger P,
Witsch-Baumgartner M, Loeffler-Ragg J, Pritz C, Schartinger VH,
Maier H, Hall J, Debbage P, et al: Rac1 as a potential therapeutic
target for chemo-radioresistant head and neck squamous cell
carcinomas (HNSCC). Br J Cancer. 110:2677–2687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hazar-Rethinam M, Merida de Long L, Gannon
OM, Boros S, Vargas AC, Dzienis M, Mukhopadhyay P, Saenz-Ponce N,
Dantzic DDE, Simpson F and Saunders NA: RacGAP1 is a novel
downstream effector of E2F7-dependent resistance to doxorubicin and
is prognostic for overall survival in squamous cell carcinoma. Mol
Cancer Ther. 14:1939–1950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon C, Cho SJ, Chang KK, Park DJ, Ryeom
SW and Yoon SS: Role of Rac1 pathway in epithelial-to-mesenchymal
transition and cancer stem-like cell phenotypes in gastric
adenocarcinoma. Mol Cancer Res. 15:1106–1116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng RJ, Zheng CW, Gu JE, Zhang HX, Xie L,
Xu LY and Li EM: RAC1 inhibition reverses cisplatin resistance in
esophageal squamous cell carcinoma and induces downregulation of
glycolytic enzymes. Mol Oncol. 13:2010–2030. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Farah E, Li C, Cheng L, Kong Y, Lanman NA,
Pascuzzi P, Lorenz GR, Zhang Y, Ahmad N, Li L, et al: NOTCH
signaling is activated in and contributes to resistance in
enzalutamide-resistant prostate cancer cells. J Biol Chem.
294:8543–8554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aruga N, Kijima H, Masuda R, Onozawa H,
Yoshizawa T, Tanaka M, Inokuchi S and Iwazaki M:
Epithelial-mesenchymal transition (EMT) is correlated with
patient's prognosis of lung squamous cell carcinoma. Tokai J Exp
Clin Med. 43:5–13. 2018.PubMed/NCBI
|
|
21
|
Wen L, Valderrama A, Costantino ME and
Simmons S: Real-world treatment patterns in patients with
castrate-resistant prostate cancer and bone metastases. Am Health
Drug Benefits. 12:142–149. 2019.PubMed/NCBI
|
|
22
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-V7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Althaus A and Kibel A: Words of wisdom.
Re: Enzalutamide in metastatic prostate cancer before chemotherapy.
Eur Urol. 67:1742015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu R, Lu C, Mostaghel EA, Yegnasubramanian
S, Gurel M, Tannahill C, Edwards J, Isaacs WB, Nelson PS, Bluemn E,
et al: Distinct transcriptional programs mediated by the
ligand-dependent full-length androgen receptor and its splice
variants in castration-resistant prostate cancer. Cancer Res.
72:3457–3462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong Y, Cheng L, Mao F, Zhang Z, Zhang Y,
Farah E, Bosler J, Bai Y, Ahmad N, Kuang S, et al: Inhibition of
cholesterol biosynthesis overcomes enzalutamide resistance in
castration-resistant prostate cancer (CRPC). J Biol Chem.
293:14328–14341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Cheng L, Li J, Farah E, Atallah
NM, Pascuzzi PE, Gupta S and Liu X: Inhibition of the wnt/β-catenin
pathway overcomes resistance to enzalutamide in
castration-resistant prostate cancer. Cancer Res. 78:3147–3162.
2018.PubMed/NCBI
|
|
27
|
Bai Y, Zhang Z, Cheng L, Wang R, Chen X,
Kong Y, Feng F, Ahmad N, Li L and Liu X: Inhibition of enhancer of
zeste homolog 2 (EZH2) overcomes enzalutamide-resistance in
castration-resistance prostate cancer. J Biol Chem. 294:9911–9923.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Liu J, Cheng L, Li C, Zhang Z, Bai
Y, Wang R, Han T, Huang C, Kong Y, et al: Inhibition of
noncanonical Wnt pathway overcomes enzalutamide resistance in
castration-resistant prostate cancer. Prostate. 80:256–266. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin S, Buel GR, Nagiec MJ, Han MJ, Roux
PP, Blenis J and Yoon SO: ERK2 regulates epithelial-to-mesenchymal
plasticity through DOCK10-dependent Rac1/FoxO1 activation. Proc
Natl Acad Sci USA. 116:2967–2976. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao J, Jie Q, Li G, Li Y, Liu B, Li H,
Luo J, Qin X, Li Z and Wei Y: Rac1 promotes the survival of H9c2
cells during serum deficiency targeting JNK/c-JUN/Cyclin-D1 and
AKT2/MCL1 pathways. Int J Med Sci. 15:1062–1071. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mohammadinejad R, Biagioni A, Arunkumar G,
Shapiro R, Chang KC, Sedeeq M, Taiyab A, Hashemabadi M, Pardakhty
A, Mandegary A, et al: EMT signaling: Potential contribution of
CRISPR/Cas gene editing. Cell Mol Life Sci. 2020.(ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raoof S, Mulford IJ, Frisco-Cabanos H,
Nangia V, Timonina D, Labrot E, Hafeez N, Bilton SJ, Drier Y, Ji F,
et al: Targeting FGFR overcomes EMT-mediated resistance in EGFR
mutant non-small cell lung cancer. Oncogene. 38:6399–6413. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tucci M, Zichi C, Buttigliero C, Vignani
F, Scagliotti GV and Di Maio M: Enzalutamide-resistant
castration-resistant prostate cancer: Challenges and solutions.
OncoTargets Ther. 11:7353–7368. 2018. View Article : Google Scholar
|
|
35
|
Liu Q, Tong D, Liu G, Xu J, Do K, Geary K,
Zhang D, Zhang J, Zhang Y, Li Y, et al: Metformin reverses prostate
cancer resistance to enzalutamide by targeting TGF-β1/STAT3
axis-regulated EMT. Cell Death Dis. 8:e30072017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ware KE, Somarelli JA, Schaeffer D, Li J,
Zhang T, Park S, Patierno SR, Freedman J, Foo WC, Garcia-Blanco MA
and Armstrong AJ: Snail promotes resistance to enzalutamide through
regulation of androgen receptor activity in prostate cancer.
Oncotarget. 7:50507–50521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan M, Xu Y, Hong F, Gao X, Xin G, Hong H,
Dong L and Zhao X: Rac1/β-catenin signalling pathway contributes to
trophoblast cell invasion by targeting snail and MMP9. Cell Physiol
Biochem. 38:1319–1332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Zhu J, Liu Y, Duan C, Chang R and
Zhang C: MicroRNA-331-3p inhibits epithelial-mesenchymal transition
by targeting ErbB2 and VAV2 through the Rac1/PAK1/β-catenin axis in
non-small-cell lung cancer. Cancer Sci. 110:1883–1896.
2019.PubMed/NCBI
|
|
39
|
Du GS, Qiu Y, Wang WS, Peng K, Zhang ZC,
Li XS, Xiao WD and Yang H: Knockdown on aPKC-ι inhibits
epithelial-mesenchymal transition, migration and invasion of
colorectal cancer cells through Rac1-JNK pathway. Exp Mol Pathol.
107:57–67. 2019. View Article : Google Scholar : PubMed/NCBI
|