Introduction
Ovarian cancer is the deadliest type of malignant
tumor in gynecology, and it seriously affects the lives of women
(1). In 2018, 152,000 women died
from ovarian cancer worldwide, and the overall 5-year survival rate
was ~30% (2,3). High-grade serous ovarian cancer (HGSOC)
is the most common form of ovarian cancer, as well as the most
deadly and invasive subtype (4). The
standard treatment for HGSOC is cytoreductive surgery combined with
platinum and paclitaxel-based chemotherapy (4). Although patients are initially
sensitive to chemotherapy, the majority eventually develop drug
resistance, which is associated with increased mortality (5). Thus, the identification of targets to
treat HGSOC resistance and prognostic biomarkers is of great
significance for the treatment of HGSOC.
Hypermethylation of CpG islands in the gene promoter
region causes gene silencing and is an important characteristic of
cancer cells (6). Methyl-CpG binding
domain proteins (MBDs) silence gene expression by binding to
methylated DNA and interacting with histone deacetylases (HDACs)
and histone methyltransferases, thus affecting carcinogenesis
(7,8). The central region of MBD2, a member of
the MBD family, contains two binding domains, MBD and a
transcriptional inhibition domain, which are responsible for
methylation specific binding and transcriptional inhibition
(9). MBD2 usually binds to
methylated promoter CpG islands (clusters of high density CpG
dinucleotides) and acts as a methylation-dependent transcriptional
repressor (10). MBD2 can recruit
the nucleosome remodeling and deacetylase/Mi-2 complex to silence
genes through a methylation-related mechanism (11–14).
However, one study also reported that MBD2 is a transcriptional
activator of the cAMP response promoter (15). These controversial results suggest
that MBD2 serves different roles according to the cell type.
Increasing evidence indicates that MBD2 is associated with the
occurrence and development of a variety of tumors, and its role in
different tumors is also different. For example, MBD2 expression is
higher in hepatocellular carcinoma (HCC), glioblastoma (GBM) and
breast cancer tissues compared with normal tissues (16–18),
whereas it is lower in gastric, cervical and colon cancer tissues
compared with normal tissues (17,19,20). In
addition, high expression of MBD2 is associated with poor prognosis
of patients with HCC (21). A
previous study demonstrated that stable short hairpin RNA
(shRNA)-mediated downregulation of MBD2 inhibits the proliferation
of SK-BR-3, MDA-MB-231 and MDA-MB-435 breast cancer cells cultured
in vitro (22). However, Yuan
et al reported that MBD2 downregulation combined with HDAC-1
promotes the growth and metastasis of colorectal cancer (23).
To the best of our knowledge, the expression of MBD2
in HGSOC has not been studied to date; thus, the aim of the present
study was to investigate the association between MBD2 expression
and the prognosis of patients with HGSOC and platinum
resistance.
Materials and methods
Bioinformatics analysis of MBD2
expression in human OC
The Human Protein Atlas (http://www.proteinatlas.org) is a database that
provides immunohistochemical (IHC) staining data for common
cancers, normal tissues and cell lines; it contains >10 million
IHC images (24–27). The expression data of MBD2 in
different normal tissues can be obtained by entering MBD2 in the
Tissue Atlas module, and the results were displayed on the
webpage.
Patient selection
The study was approved by the Ethics Committee of
Zhejiang Cancer Hospital (approval no. IRB-2015-175) and written
informed consent was obtained from all patients. In total, 131
female patients were enrolled onto the present study (age range,
39–74 years old), including 115 HGSOC (age range, 39–74 years old)
and 16 normal patients (age range, 39–65 years old). The 131 frozen
tissue samples used in this study were provided by the Biobank of
Zhejiang Cancer Hospital (Hangzhou, China). All tissue samples were
collected in the operating room of the Zhejiang Cancer Hospital
(Hangzhou, China) from January 2008 to June 2014. The samples were
fixed and paraffin embedded. Among all samples, there were 115
HGSOC tissue samples (including 1 case of stage I, 11 cases of
stage II, 95 cases of stage III and 8 cases of stage IV) and 16
normal ovarian tissue samples. Normal ovarian tissues samples were
obtained patients with other gynecological benign tumors. The
diagnosis and stage of HGSOC are determined by two independent
experienced pathologists in the Zhejiang Cancer Hospital (Hangzhou,
China). Histological classification and tumor staging were
performed according to World Health Organization histological
classification criteria and the International Federation of
Gynaecology and Obstetrics (FIGO) staging criteria (28).
All patients received standard chemotherapy and were
divided into platinum-resistant and platinum-sensitive (including
partially sensitive) groups according to the following criteria: i)
Platinum-resistant group, patients displaying progression or
recurrence <6 months after finishing platinum treatment; and ii)
platinum-sensitive group, those displaying recurrence >6 months
after platinum treatment or those who did not exhibit
recurrence.
Immunohistochemical staining
The immunohistochemical staining kit was purchased
from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. The
tissue samples were fixed with 10% formalin at room temperature for
24 h. Paraffin-embedded tissue samples were cut into 4-µm-thick
sections and placed at 72°C for 30 min, then dewaxed and hydrated
by xylene and ethanol. The sections were dewaxed with xylene twice
for 5 min each time, and the gradient ethanol rehydration (100% for
3 min twice, 95% for 3 min twice, 80% for 3 min) was rinsed with
clean running water for 30 min. Antigens were retrieved by pressure
cooker treatment for 90 sec in 0.01 mmol/l citrate buffer. After
three washes, the slides were placed in 3% hydrogen peroxide
solution for 5–10 min, washed with running water twice and
phosphate buffer saline (PBS) for 5 min, and incubated with rabbit
anti-human MBD2 polyclonal primary antibody (1:100; cat. no.
ab188474; Abcam) overnight at 4°C. After washing with PBS twice for
5 min, slides were incubated with horseradish peroxidase-conjugated
rabbit secondary antibodies (cat. no. PV-6000, OriGene
Technologies, Inc.) at room temperature for 20 min, followed by
washing and 3,3′-diaminobenzidine staining at room temperature for
2 min. The sections were counterstained with haematoxylin for 1 min
at room temperature and washed with running water three times. This
was followed by incubation with 1% hydrochloric acid alcohol for 1
min and dehydration with a gradient of ethanol (80% for 10 sec, 95%
for 10 sec, 100% for 5 min three times), and treated with xylene
for 3 min at room temperature. Images were captured using a BX63
fluorescence microscope with a CCD camera (DP80; Olympus
Corporation) at 200× magnification.
Evaluation of immunohistochemical
staining
The expression of MBD2 detected by
immunohistochemistry was evaluated by calculating the sum of
staining intensity and the proportion of positively-stained cells,
as previously described (29).
Briefly, according to the staining intensity, there were four
levels, including negative (0 point), weak positive (1 point),
intermediate positive (2 points) and strong positive (3 points).
The proportion of positively-stained cells was scored as follows:
<5%, 0 point; 5–25%, 1 point; 26–50%, 2 points; 51–75%, 3
points; and >75%, 4 points. Finally, the sum of the percentage
of positive cells score and the intensity score was calculated to
provide the final score of MBD2 expression, which ranged between 0
and 7. A final score of 0 points indicated no expression, 1–4
points indicated low expression, and 5–7 points indicated high
expression.
Western blotting
Western blotting was performed as previously
described (17). Tissues were lysed
in ice-cold cell lysis buffer (Beyotime Institute of Biotechnology)
containing protease inhibitors (Thermo Fisher Scientific, Inc.),
and the protein concentration in tissue extracts was quantified
using the bicinchoninic acid protein assay kit (Beyotime Institute
of Biotechnology). Equal amounts of protein (25 µg/lane) from each
extract were denatured and separated using a 10% polyacrylamide gel
(Beyotime Institute of Biotechnology), and then transferred by
electrophoresis onto a nitrocellulose membrane (Thermo Fisher
Scientific, Inc.). The membranes were blocked with 5% non-fat dried
milk in PBST for 1 h at room temperature and incubated with primary
antibodies against MBD2 (1:1,000; cat. no. ab188474; Abcam) and
β-actin (1:3,000; cat. no. EM21002; Hangzhou Huaan Biological
Technology, Ltd.) at 4°C overnight. Membranes were then incubated
with horseradish peroxidase-conjugated anti-mouse (1:3,000; cat.
no. 170-6516; Bio-Rad Laboratories, Inc.). or anti-rabbit secondary
antibodies (1:3,000; cat. no. 170-6515; Bio-Rad Laboratories,
Inc.). The membranes were developed using SuperSignal West Pico
Substrate and CL-XPosure Film (Thermo Fisher Scientific, Inc.).
Western blot results were analyzed using Image Lab 3.0 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
A Pearson Chi-square (χ2) test or
Fisher's exact test was used to analyze the associations between
MBD2 expression and clinicopathological features, including age,
FIGO stage, degree of differentiation, cancer antigen-125 (CA-125),
lymph node metastasis and postoperative residual disease size. The
associations between platinum resistance and MBD2 expression, as
well as clinical features were analyzed using χ2 test or
Fisher's exact test and multiple logistic regression. The relapse
date was defined as the time when new lesions were indentified by
radiographic analysis in patients with HGSOC after the completion
of surgery, so as to calculate the relapse-free survival (RFS) from
the date of surgical resection to the date of relapse. The
Kaplan-Meier method was also used, and Cox proportional hazard
regression, log-rank test and Breslow test were used for univariate
and multivariate survival analyses of RFS. The unpaired Student's
t-test or Fisher's exact test was used to compare the mean of two
groups. Analyses were performed using SPSS version 18.0 (SPSS,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
MBD2 expression and
clinicopathological characteristics of patients with HGSOC
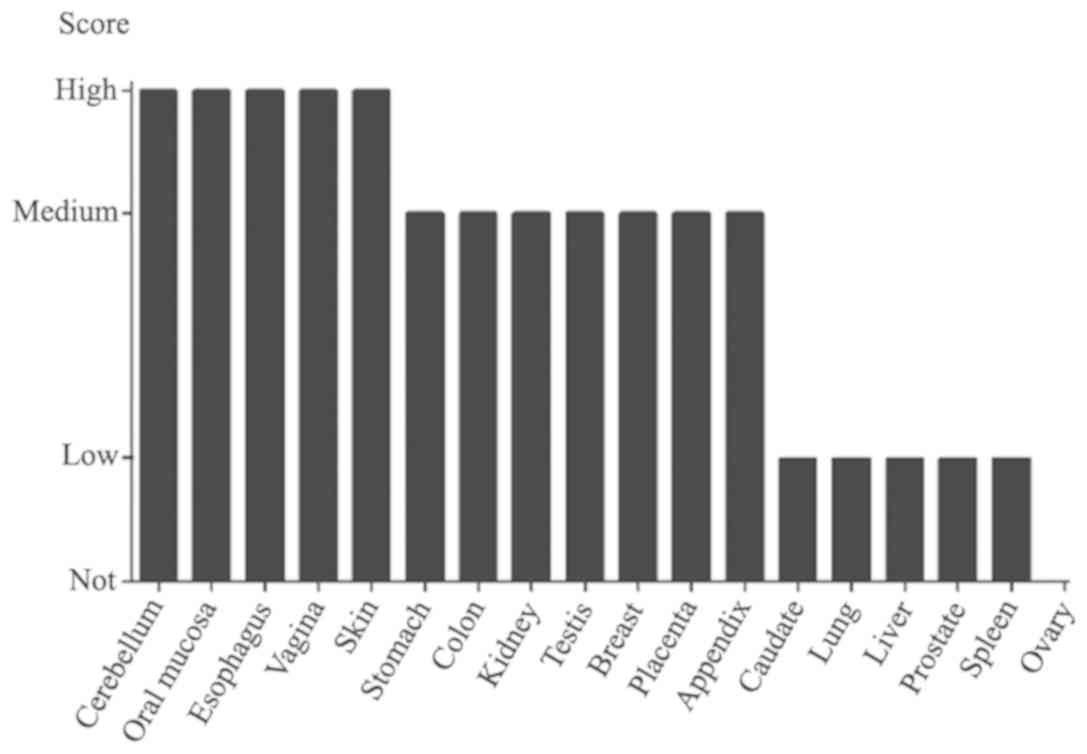
A query from The Human Protein Atlas database
demonstrated that MBD2 was not expressed in normal ovarian tissues
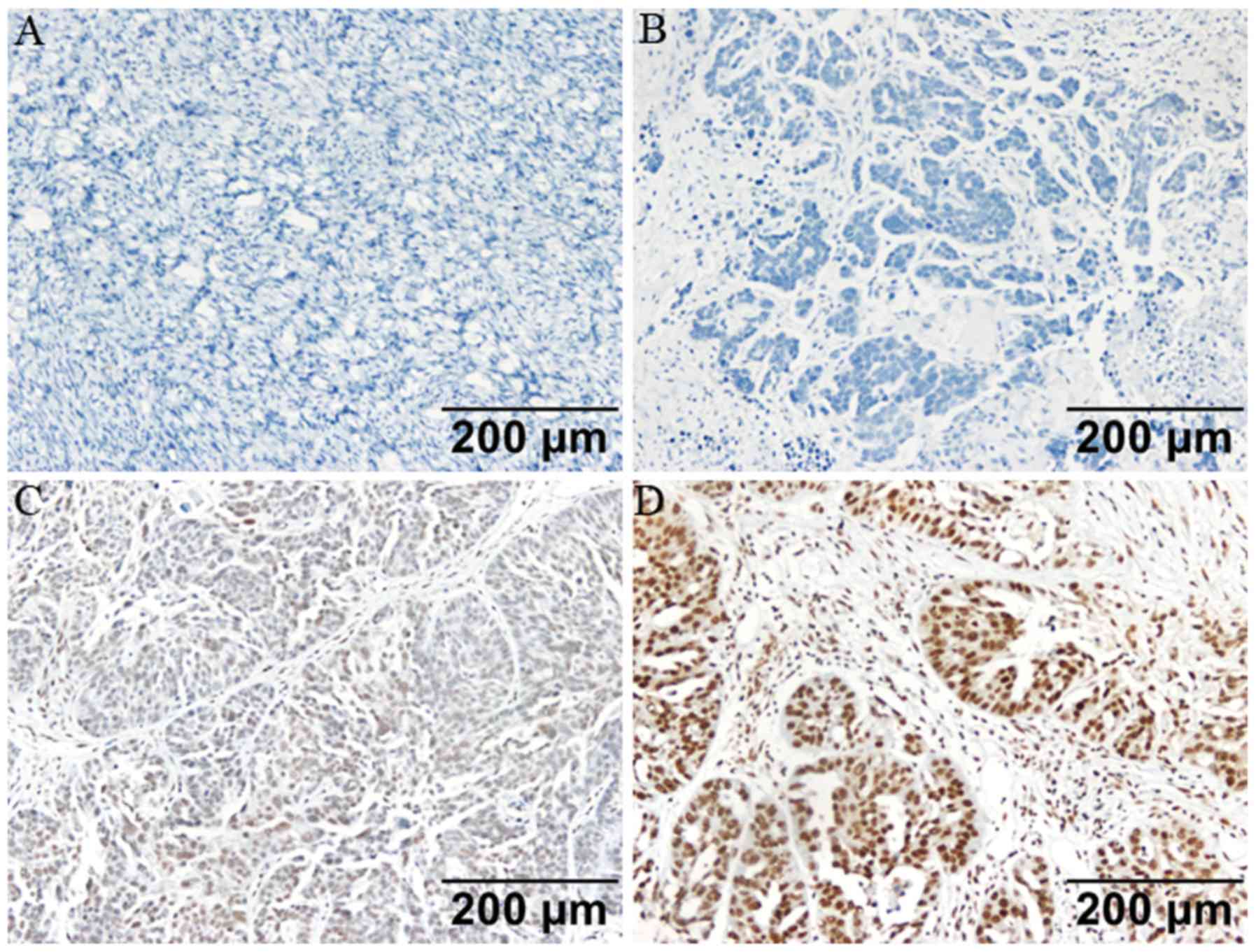
(Fig. 1). The expression of MBD2 in
115 HGSOC tissue samples and 16 normal ovarian tissue samples from
the patients with normal ovariectomy was investigated by
immunohistochemistry (Fig. 2). The
results demonstrated that MBD2 was expressed in 73 HGSOC samples
(63.5%), but not expressed in 42 HGSOC samples (36.5%). No MBD2
expression was detected in all 16 normal ovarian tissue samples
(100%). Fisher's exact test demonstrated that the expression of
MBD2 in HGSOC tissues was significantly higher compared with that
in normal ovarian tissues (P<0.001, Table I). In addition, among the 115 HGSOC
samples, there was no significant association between MBD2
expression and age, FIGO stage, histological grade, CA-125, lymph
node metastasis or postoperative residual tumor size (P>0.05),
whereas MBD2 expression was significantly associated with platinum
resistance (P=0.001; Table II). The
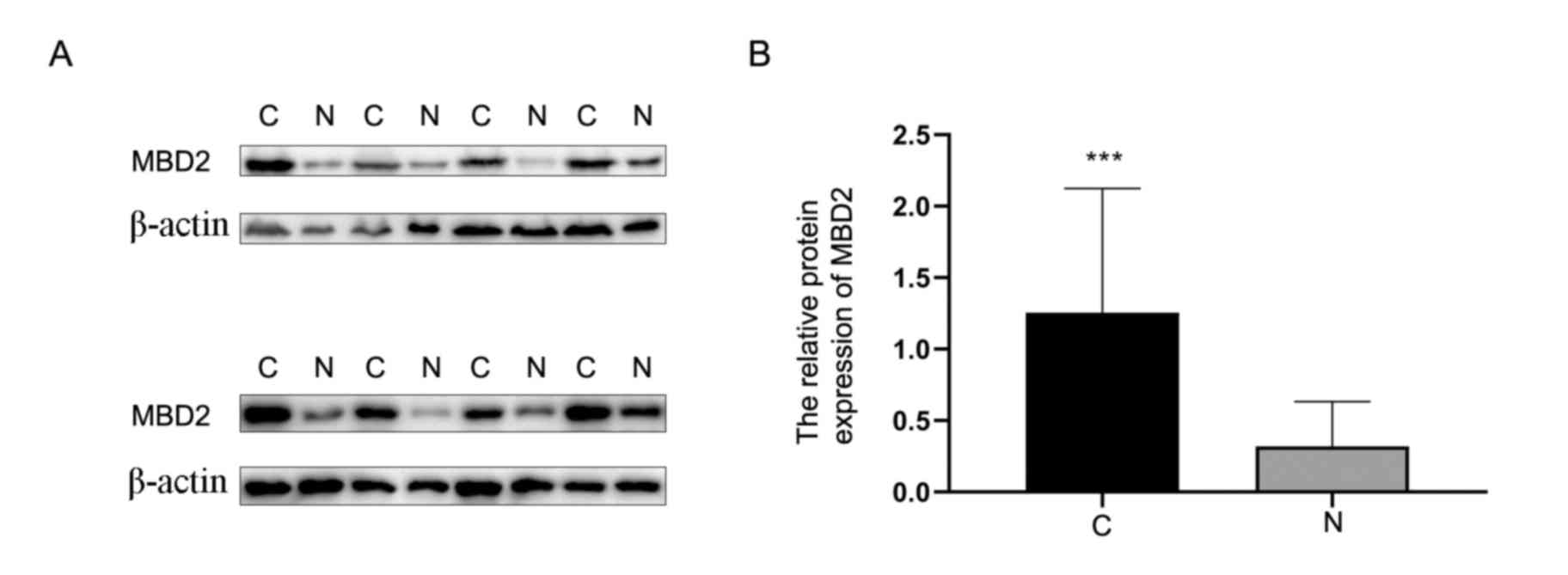
expression of MBD2 was also detected by western blotting in eight
HGSOC tissues and eight normal ovarian tissues. The results
demonstrated that the expression of MBD2 was significantly higher
in HGSOC tissues compared with that in normal tissues (Fig. 3).
 | Table I.Expression of methyl-CpG binding
domain protein 2 in HGSOC and normal ovarian tissue samples. |
Table I.
Expression of methyl-CpG binding
domain protein 2 in HGSOC and normal ovarian tissue samples.
|
| MBD2
expression |
|---|
|
|
|
|---|
| Variables | Low | High | P-value |
|---|
| HGSOC | 42 | 73 | <0.001 |
| Normal | 16 | 0 |
|
 | Table II.Associations between the expression
level of MBD2 and clinicopathological characteristics. |
Table II.
Associations between the expression
level of MBD2 and clinicopathological characteristics.
|
| MBD2
expression |
|---|
|
|
|
|---|
| Variables | Low | High | P-value |
|---|
| Age, years |
|
| 0.157a |
|
≤54 | 19 | 43 |
|
|
>54 | 23 | 30 |
|
| FIGO stage |
|
| 0.756b |
|
I–II | 5 | 7 |
|
|
III–IV | 37 | 66 |
|
| Histological
grade |
|
| 1.000b |
| G2 | 5 | 9 |
|
| G3 | 34 | 63 |
|
|
Null | 3 | 1 |
|
| CA-125, U/ml |
|
| 0.078a |
|
≤500 | 6 | 21 |
|
|
>500 | 36 | 52 |
|
| Lymph node
metastasis |
|
| 0.884a |
| No | 19 | 32 |
|
|
Yes | 23 | 41 |
|
| Residual tumor
size, cm |
|
| 0.547a |
| ≤1 | 31 | 50 |
|
|
>1 | 11 | 23 |
|
| Platinum
resistance |
|
| 0.001b |
|
Present | 2 | 22 |
|
|
Absent | 39 | 47 |
|
|
Null | 1 | 4 |
|
Associations between platinum
resistance and clinicopathological characteristics of patients with
HGSOC
It was demonstrated that postoperative residual
tumor size (P=0.030) and MBD2 expression (P=0.001) were
significantly associated with platinum resistance. However,
platinum resistance was not associated with age, FIGO stage,
histological grade, CA-125 or lymph node metastasis (P>0.05;
Table III). Multivariate logistic
regression analysis confirmed that >1 cm of residual disease and
high expression of MBD2 were significantly associated with platinum
resistance (P<0.05; Table
IV).
 | Table III.Associations between chemotherapy
resistance and clinicopathological characteristics. |
Table III.
Associations between chemotherapy
resistance and clinicopathological characteristics.
|
| Platinum
resistance |
|---|
|
|
|
|---|
| Variable | Present | Absent | P-value |
|---|
| Age, years |
|
| 0.806a |
|
≤54 | 13 | 49 |
|
|
>54 | 11 | 37 |
|
| FIGO stage |
|
| 0.065b |
|
I–II | 0 | 12 |
|
|
III–IV | 24 | 74 |
|
| Histological
grade |
|
| 0.498b |
| G2 | 4 | 10 |
|
| G3 | 19 | 73 |
|
|
Null | 1 | 3 |
|
| CA-125, U/ml |
|
| 0.710b |
| ≤
500 | 5 | 21 |
|
| >
500 | 19 | 65 |
|
| Lymph node
metastasis |
|
| 0.106a |
| No | 7 | 41 |
|
|
Yes | 17 | 45 |
|
| Residual tumor
size |
|
| 0.030a |
| ≤1
cm | 13 | 66 |
|
| >1
cm | 11 | 20 |
|
| MBD2
expression |
|
| 0.001b |
|
Low | 2 | 39 |
|
|
High | 22 | 47 |
|
 | Table IV.Multivariate logistic analysis of the
association between chemotherapy resistance and clinicopathological
characteristics. |
Table IV.
Multivariate logistic analysis of the
association between chemotherapy resistance and clinicopathological
characteristics.
| Variable | β | SE | Exp(β) | 95% CI | P-value |
|---|
| Residual tumor
(>1 cm) | −1.028 | 0.515 | 0.358 | 0.130–0.981 | 0.046a |
| MBD2 expression
(High) | −2.212 | 0.777 | 0.109 | 0.024–0.502 | 0.004b |
Prognostic value of MBD2 expression in
ovarian cancer
Kaplan-Meier univariate analysis demonstrated that
MBD2 expression, FIGO stage, CA-125, lymph node metastasis and
residual tumor size were significantly associated with relapse-free
survival (RFS) in patients with HGSOC (P<0.05; Table V). Multivariate Cox regression
analysis demonstrated that FIGO stage, CA-125, lymph node
metastasis, residual tumor size and MBD2 expression were
significantly associated with RFS (P<0.05; Table VI). Therefore, late FIGO, CA-125
>500, lymph node metastasis, >1 cm of postoperative residual
disease and high expression of MBD2 were identified as risk factors
for recurrence of HGSOC.
 | Table V.Univariate survival analysis of
relapse-free survival of patients with ovarian cancer. |
Table V.
Univariate survival analysis of
relapse-free survival of patients with ovarian cancer.
| Variable | n | Mean ± SE | 95% CI | P-value |
|---|
| Age, years |
|
|
| 0.978b |
|
≤54 | 62 | 21.760±3.049 | 15.783–27.737 |
|
|
>54 | 48 | 24.375±3.607 | 17.306–31.444 |
|
| FIGO stage |
|
|
|
<0.001a |
|
I–II | 12 | 67.917±9.288 | 49.712–86.121 |
|
|
III–IV | 98 | 17.991±1.859 | 14.347–21.635 |
|
| Histological
grade |
|
|
| 0.488a |
| G2 | 14 | 20.643±5.808 | 9.260–32.026 |
|
| G3 | 92 | 24.140±2.725 | 18.799–29.480 |
|
|
Null | 4 |
|
|
|
| CA-125, U/ml |
|
|
| 0.022a |
|
500 | 26 | 32.138±5.746 | 20.877–43.400 |
|
|
>500 | 84 | 19.698±2.306 | 15.179–24.217 |
|
| Lymph node
metastasis |
|
|
|
<0.001a |
| No | 48 | 33.089±4.547 | 24.177–42.001 |
|
|
Yes | 62 | 15.414±1.888 | 11.713–19.115 |
|
| Residual tumor
size, cm |
|
|
| 0.002a |
| ≤1 | 79 | 27.309±3.163 | 21.109–33.508 |
|
|
>1 | 31 | 13.645±2.664 | 8.423–18.867 |
|
| MBD2
expression |
|
|
| 0.011b |
|
Low | 41 | 28.786±4.221 | 20.514–37.059 |
|
|
High | 69 | 18.662±2.408 | 13.942–23.381 |
|
 | Table VI.Multivariate survival analysis of
relapse-free survival of patients with ovarian cancer. |
Table VI.
Multivariate survival analysis of
relapse-free survival of patients with ovarian cancer.
| Variable | β | SE | HR | 95% CI |
P-valuea |
|---|
| FIGO stage
(III–IV) | 1.786 | 0.618 | 5.965 | 1.776–20.041 | 0.004 |
| CA-125
(>500) | 0.678 | 0.281 | 1.971 | 1.135–3.421 | 0.016 |
| Lymph node
metastasis (Yes) | 0.450 | 0.227 | 1.568 | 1.004–2.448 | 0.048 |
| Residual tumor size
(>1 cm) | 0.477 | 0.234 | 1.610 | 1.017–2.549 | 0.042 |
| MBD2 expression
(High) | 0.525 | 0.222 | 1.691 | 1.093–2.615 | 0.018 |
Discussion
MBD2 is closely associated with the hypermethylation
of CpG islands in some cancer types, such as breast and prostate
cancer (9,30). And it has been reported to function
as a tumor activator (31,32). Knockout of MBD2 upregulates the
expression of tumor suppressor genes, such as p16 and p14 (33,34).
MBD2 overexpression has been detected in a variety of solid tumors,
including HCC, breast cancer, GBM and chronic myeloid leukemia
(16–18,35).
MBD2 promotes breast cancer progression by mediating tumor
suppressor gene silencing (22,36). Zhu
et al (16) reported that
MBD2 overexpression leads to silencing of cerebral angiogenesis
inhibitor 1 to promote the growth of GBM. Similar mechanisms have
been reported in other cancers, such as HCC and gastrointestinal
stromal tumors (17,37). However, MBD2 functions in a cell- and
tissue-specific manner, and recent studies demonstrated that MBD2
has antitumor effects (21,38). MBD2 is downregulated in lung
adenocarcinoma, and low expression of MBD2 is associated with poor
prognosis (38). In vitro
experiments further demonstrated that MBD2 inhibits metastasis
(38). The results of the present
study demonstrated that MBD2 was expressed at high levels in HGSOC,
although the underlying mechanism remains unclear.
Approximately 20–30% of patients with ovarian cancer
develop platinum resistance during chemotherapy (39). In the current study, 21.8% of
patients developed platinum resistance, which is consistent with a
recent study (39). Huang et
al (40) performed a
transcriptome analysis of platinum-sensitive and drug-resistant
ovarian cancer cell lines and found differences in the expression
of MBD2 between the two populations. Yu et al (41) used methyl-capture sequencing to
precipitate methylated DNA from the recombinant methyl CpG binding
domain of MBD2 for next generation sequencing. The results
demonstrated that drug-resistant cells had lower levels of CpG
methylation. The results of the present study revealed that MBD2
upregulation was related to platinum resistance in HGSOC, although
the underlying mechanism needs to be further studied.
The results of the present study suggested that the
recurrence of HGSOC was associated with numerous factors, such as
late FIGO stage, lymph node metastasis and high expression of MBD2.
The postoperative recurrence of epithelial ovarian cancer is
related to clinical stage (42). The
results of the present study demonstrated that postoperative
recurrence of HGSOC was significantly correlated with advanced FIGO
stage, which was consistent with a previous report (43). A higher tumor stage was associated
with larger tumors, which may lead to local invasion. Surgical
excision in these cases is difficult and may lead to injury, which
increases the potential for cancer cells to invade blood vessels
and lymphoid tissues, increases the depth of infiltration and
promotes tumor metastasis (44,45). The
present study also demonstrated that lymph node metastasis was a
risk factor for recurrence of HGSOC, which was consistent with a
previous study (46). One possible
reason for this finding is that lymph node metastasis is the most
common type of ovarian cancer metastasis, which also increases the
risk of recurrence (47,48). The results of the present study
demonstrated that a postoperative residual tumor size ≤1 cm
prevented postoperative recurrence, which was consistent with
previous reports (49,50). Therefore, maximum radical resection
of the tumor and reduction of residual cancer tissue are essential
to improve the prognosis of HGSOC. In addition, the expression
level of MBD2 in HCC is higher compared with that in normal tissues
(51). MBD2 is an independent
prognostic factor that affects the overall survival and
disease-free survival of patients and is considered to be a
potential clinical prognostic marker (17). Notably, the current study also
identified MBD2 as an independent prognostic factor for
relapse-free survival of HGSOC.
To the best of our knowledge, the present study is
the first to demonstrate that high expression levels of MBD2 are
associated with platinum resistance and poor prognosis in patients
with HGSOC. The results suggested that MBD2 is a promising target
for cancer treatment. Sequence-specific antisense MBD2 inhibitors
suppress the anchorage-independent growth of human tumor cell lines
in vitro and the growth of human tumor xenografts in
vivo (52). The newly discovered
MBD2 inhibitor KCC-08 combined with the retinoic acid receptor
agonist isoretinoic acid can significantly reduce the growth and
survival of cancer cells (53).
However, the present study had some limitations, such as a small
sample size and the nature of the study as a single-center study.
In conclusion, patients with HGSOC with high MBD2 expression levels
are more likely to develop platinum resistance and have a poor
prognosis; thus MBD2 is a potential biomarker for HGSOC.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Natural
Science Foundation of Zhejiang Province (grant no. LY19H160003) and
the Medical Health Science and Technology Project of Zhejiang
Province (grant no. 2018KY294).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WG wrote the paper and performed the experiments. MN
and ZC participated in the data collection and analysis. ZZ
conceived and designed the study. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhejiang Cancer Hospital (approval no. IRB-2015-175) and written
informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colombo PE, Fabbro M, Theillet C, Bibeau
F, Rouanet P and Ray-Coquard I: Sensitivity and resistance to
treatment in the primary management of epithelial ovarian cancer.
Crit Rev Oncol Hematol. 89:207–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lheureux S, Braunstein M and Oza AM:
Epithelial ovarian cancer: Evolution of management in the era of
precision medicine. CA Cancer J Clin. 69:280–304. 2019.PubMed/NCBI
|
|
5
|
Wallace S, Kumar A, Mc Gree M, Weaver A,
Mariani A, Langstraat C, Dowdy S, Bakkum-Gamez J and Cliby W:
Efforts at maximal cytoreduction improve survival in ovarian cancer
patients, even when complete gross resection is not feasible.
Gynecol Oncol. 145:21–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li YC, Wang Y, Li DD, Zhang Y, Zhao TC and
Li CF: Procaine is a specific DNA methylation inhibitor with
anti-tumor effect for human gastric cancer. J Cell Biochem.
119:2440–2449. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parry L and Clarke AR: The roles of the
methyl-CpG binding proteins in cancer. Genes Cancer. 2:618–630.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Defossez PA and Stancheva I: Biological
functions of methyl-CpG-binding proteins. Prog Mol Biol Transl Sci.
101:377–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stirzaker C, Song JZ, Ng W, Du Q,
Armstrong NJ, Locke WJ, Statham AL, French H, Pidsley R,
Valdes-Mora F, et al: Methyl-CpG-binding protein MBD2 plays a key
role in maintenance and spread of DNA methylation at CpG islands
and shores in cancer. Oncogene. 36:1328–1338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du Q, Luu PL, Stirzaker C and Clark SJ:
Methyl-CpG-binding domain proteins: Readers of the epigenome.
Epigenomics. 7:1051–1073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai AY and Wade PA: Cancer biology and
NuRD: A multifaceted chromatin remodelling complex. Nat Rev Cancer.
11:588–596. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramirez J, Dege C, Kutateladze TG and
Hagman J: MBD2 and multiple domains of CHD4 are required for
transcriptional repression by Mi-2/NuRD complexes. Mol Cell Biol.
32:5078–5088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai Y, Geutjes EJ, de Lint K, Roepman P,
Bruurs L, Yu LR, Wang W, van Blijswijk J, Mohammad H, de Rink I, et
al: The NuRD complex cooperates with DNMTs to maintain silencing of
key colorectal tumor suppressor genes. Oncogene. 33:2157–2168.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan CP and Nakielny S: Control of the DNA
methylation system component MBD2 by protein arginine methylation.
Mol Cell Biol. 26:7224–7235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita H, Fujii R, Aratani S, Amano T,
Fukamizu A and Nakajima T: Antithetic effects of MBD2a on gene
regulation. Mol Cell Biol. 23:2645–2657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu D, Hunter SB, Vertino PM and Van Meir
EG: Overexpression of MBD2 in glioblastoma maintains epigenetic
silencing and inhibits the antiangiogenic function of the tumor
suppressor gene BAI1. Cancer Res. 71:5859–5870. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan ZX, Zhang XY, Chen SR and Li CZ:
Upregulated exosomal miR-221/222 promotes cervical cancer via
repressing methyl-CpG-binding domain protein 2. Eur Rev Med
Pharmacol Sci. 23:3645–3653. 2019.PubMed/NCBI
|
|
18
|
Izquierdo-Torres E, Hernández-Oliveras A,
Meneses-Morales I, Rodríguez G, Fuentes-García G and
Zarain-Herzberg Á: Resveratrol up-regulates ATP2A3 gene expression
in breast cancer cell lines through epigenetic mechanisms. Int J
Biochem Cell Biol. 113:37–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pontes TB, Chen ES, Gigek CO, Calcagno DQ,
Wisnieski F, Leal MF, Demachki S, Assumpção PP, Artigiani R,
Lourenço LG, et al: Reduced mRNA expression levels of MBD2 and MBD3
in gastric carcinogenesis. Tumour Biol. 35:3447–3453. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
May S, Owen H, Phesse TJ, Greenow KR,
Jones GR, Blackwood A, Cook PC, Towers C, Gallimore AM, Williams
GT, et al: Mbd2 enables tumourigenesis within the intestine while
preventing tumour-promoting inflammation. J Pathol. 245:270–282.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu W, Wang N, Lu M, Du XJ and Xing BC:
MBD2 as a novel marker associated with poor survival of patients
with hepatocellular carcinoma after hepatic resection. Mol Med Rep.
14:1617–1623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mian OY, Wang SZ, Zhu SZ, Gnanapragasam
MN, Graham L, Bear HD and Ginder GD: Methyl-binding domain protein
2-dependent proliferation and survival of breast cancer cells. Mol
Cancer Res. 9:1152–1162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan K, Xie K, Fox J, Zeng H, Gao H, Huang
C and Wu M: Decreased levels of miR-224 and the passenger strand of
miR-221 increase MBD2, suppressing maspin and promoting colorectal
tumor growth and metastasis in mice. Gastroenterology. 145:853–864
e9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thul PJ and Lindskog C: The human protein
atlas: A spatial map of the human proteome. Protein Sci.
27:233–244. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thul PJ, Åkesson L, Wiking M, Mahdessian
D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM,
et al: A subcellular map of the human proteome. Science.
356:eaal33212017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeppernick F and Meinhold-Heerlein I: The
new FIGO staging system for ovarian, fallopian tube, and primary
peritoneal cancer. Arch Gynecol Obstet. 290:839–842. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang M, Liu T, Xia B, Yang C, Hou S, Xie
W and Lou G: Platelet-derived growth factor D is a prognostic
biomarker and is associated with platinum resistance in epithelial
ovarian cancer. Int J Gynecol Cancer. 28:323–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Devailly G, Grandin M, Perriaud L, Mathot
P, Delcros JG, Bidet Y, Morel AP, Bignon JY, Puisieux A, Mehlen P
and Dante R: Dynamics of MBD2 deposition across methylated DNA
regions during malignant transformation of human mammary epithelial
cells. Nucleic Acids Res. 43:5838–5854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wood KH and Zhou Z: Emerging molecular and
biological functions of MBD2, a reader of DNA methylation. Front
Genet. 7:932016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mahmood N and Rabbani SA: DNA methylation
readers and cancer: Mechanistic and therapeutic applications. Front
Oncol. 9:4892019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le Guezennec X, Vermeulen M, Brinkman AB,
Hoeijmakers WA, Cohen A, Lasonder E and Stunnenberg HG: MBD2/NuRD
and MBD3/NuRD, two distinct complexes with different biochemical
and functional properties. Mol Cell Biol. 26:843–851. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Magdinier F and Wolffe AP: Selective
association of the methyl-CpG binding protein MBD2 with the silent
p14/p16 locus in human neoplasia. Proc Natl Acad Sci USA.
98:4990–4995. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng L, Tang Y, Chen X, Zhao L, Liu S, Ma
Y, Wang N, Zhou K, Zhou J and Zhou M: Deletion of MBD2 inhibits
proliferation of chronic myeloid leukaemia blast phase cells.
Cancer Biol Ther. 19:676–686. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alvarado S, Wyglinski J, Suderman M,
Andrews SA and Szyf M: Methylated DNA binding domain protein 2
(MBD2) coordinately silences gene expression through activation of
the microRNA hsa-mir-496 promoter in breast cancer cell line. PLoS
One. 8:e740092013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He M, Fan J, Jiang R, Tang WX and Wang ZW:
Expression of DNMTs and MBD2 in GIST. Biomed Rep. 1:223–227. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pei YF, Xu XN, Wang ZF, Wang FW, Wu WD,
Geng JF and Liu XQ: Methyl-CpG binding domain protein 2 inhibits
the malignant characteristic of lung adenocarcinoma through the
epigenetic modulation of 10 to 11 translocation 1 and miR-200s. Am
J Pathol. 189:1065–1076. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berns EM and Bowtell DD: The changing view
of high-grade serous ovarian cancer. Cancer Res. 72:2701–2704.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang RL, Gu F, Kirma NB, Ruan J, Chen CL,
Wang HC, Liao YP, Chang CC, Yu MH, Pilrose JM, et al: Comprehensive
methylome analysis of ovarian tumors reveals hedgehog signaling
pathway regulators as prognostic DNA methylation biomarkers.
Epigenetics. 8:624–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu W, Jin C, Lou X, Han X, Li L, He Y,
Zhang H, Ma K, Zhu J, Cheng L and Lin B: Global analysis of DNA
methylation by Methyl-Capture sequencing reveals epigenetic control
of cisplatin resistance in ovarian cancer cell. PLoS One.
6:e294502011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kehoe S, Hook J, Nankivell M, Jayson GC,
Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M,
et al: Primary chemotherapy versus primary surgery for newly
diagnosed advanced ovarian cancer (CHORUS): An open-label,
randomised, controlled, non-inferiority trial. Lancet. 386:249–257.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsuyoshi H, Orisaka M, Fujita Y,
Asare-Werehene M, Tsang BK and Yoshida Y: Prognostic impact of
dynamin related protein 1 (Drp1) in epithelial ovarian cancer. BMC
Cancer. 20:4672020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mirza MR, Monk BJ, Herrstedt J, Oza AM,
Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I,
et al: Niraparib maintenance therapy in platinum-sensitive,
recurrent ovarian cancer. N Engl J Med. 375:2154–2164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ducie J, Dao F, Considine M, Olvera N,
Shaw PA, Kurman RJ, Shih IM, Soslow RA, Cope L and Levine DA:
Molecular analysis of high-grade serous ovarian carcinoma with and
without associated serous tubal intra-epithelial carcinoma. Nat
Commun. 8:9902017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Joueidi Y, Dion L, Bendifallah S, Mimoun
C, Bricou A, Nyangoh Timoh K, Collinet P, Touboul C, Ouldamer L,
Azaïs H, et al: Management and survival of elderly and very elderly
patients with ovarian cancer: An age-stratified study of 1123 women
from the FRANCOGYN group. J Clin Med. 9:14512020. View Article : Google Scholar
|
|
47
|
Scott LJ: Niraparib: First global
approval. Drugs. 77:1029–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lorusso D, Scambia G, Pignata S, Sorio R,
Amadio G, Lepori S, Mosconi A, Pisano C, Mangili G, Maltese G, et
al: Prospective phase II trial of trabectedin in BRCA-mutated
and/or BRCAness phenotype recurrent ovarian cancer patients: The
MITO 15 trial. Ann Oncol. 27:487–493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fan XM, Zhang J, Niu SH, Li KX and Song
CZ: Secondary cytoreductive surgery in recurrent epithelial ovarian
cancer: A prognostic analysis with 103 cases. Int J Surg. 38:61–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
du Bois A, Reuss A, Pujade-Lauraine E,
Harter P, Ray-Coquard I and Pfisterer J: Role of surgical outcome
as prognostic factor in advanced epithelial ovarian cancer: A
combined exploratory analysis of 3 prospectively randomized phase 3
multicenter trials: By the arbeitsgemeinschaft gynaekologische
onkologie studiengruppe ovarialkarzinom (AGO-OVAR) and the groupe
d'Investigateurs nationaux pour les etudes des cancers de l'Ovaire
(GINECO). Cancer. 115:1234–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
E C, Li C, Li H and Yang J: Silencing of a
novel lncRNA LOC105369748 suppresses the progression of
hepatocellular carcinoma by sponging miR-5095 from MBD2. J Cell
Physiol. 234:18504–18512. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Campbell PM, Bovenzi V and Szyf M:
Methylated DNA-binding protein 2 antisense inhibitors suppress
tumourigenesis of human cancer cell lines in vitro and in vivo.
Carcinogenesis. 25:499–507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Giovinazzo H, Reichert ZR, Bergman A, Lin
X, Wyhs N, Esopi D, Vaghasia A, Liu J, Jain Y, Bhamidipati A, et
al: Abstract 5881: Novel inhibitors of the epigenetic reader
protein MBD2. Cancer Res. 78 (Suppl 13):S58812018.
|