Introduction
The incidence of bladder urothelial carcinoma (BLCA)
ranks ninth among all malignancies in the global population and
fourth among all malignancies occurring in men (1). The main risk factors of BLCA are
cigarette smoking, exposure to toxic industrial chemicals and
gases, and genetic susceptibility (2). Although standard treatment and
supportive care have improved the overall survival (OS) and quality
of life, the prognosis for patients with BLCA remains poor
(3).
Immune-related mechanisms serve an important role in
BLCA, and immunotherapeutic strategies are considered to be a
promising direction for the treatment of BLCA (4,5).
Immunotherapy seeks to manipulate the patients own immune response
to improve the clinical outcome by promoting immune cells that can
kill target cancer cells (5). The
receptor-ligand pairing of programmed cell death protein-1 (PD-1)
has been identified to be a crucial immune checkpoint; however,
current immunotherapies using anti-PD-1 have only achieved partial
response in patients with advanced BLCA (6,7). In
addition, an increasing number of studies have demonstrated that
patients with bladder cancer with a high level of
tumor-infiltrating lymphocytes exhibit improved survival (8–10).
However, studies on the prognostic value of immune cell subsets in
patients with BLCA have yields completely opposite results
(11). Therefore, there is an urgent
need to elucidate the specific immune phenotypes of tumor-immune
interactions and identify novel immune-associated therapeutic
targets in BLCA.
Epithelial membrane protein 1 (EMP1) is a
protein-coding gene; its expression and significance in human
cancer and its biological effects have been explored in
vitro, demonstrating that EMP1 significantly reduces
cell migration and invasion, and increases apoptosis and caspase-9
expression in carcinoma of the nasopharynx, stomach, breast and
prostate (12–16). By contrast, studies of acute
lymphoblastic leukemia (ALL) have revealed that EMP1 is an
indicator of poor prognosis (17).
Limited information is available about the mechanism
underlying the effects of EMP1. Previous studies have
suggested that EMP1 works primarily by regulating signal
transduction between cells and the extracellular matrix (18). EMP1 may be associated with the
proto-oncogene c-myc (19),
and other studies have revealed that EMP1 is regulated by
the epidermal growth factor receptor (EGFR) (20,21). In
addition, EMP1 is involved in the tight connection between
cells, which may cause the occurrence and development of non-small
cell lung cancer by activating the PI3K/AKT pathway (22). Wang et al (23), demonstrated that EMP family members
and integrins synergistically regulate cell adhesion and migration
in vitro, and integrin-based cell adhesion leads to
autoimmune diseases. Therefore, previous studies have suggested
that EMP1 serves an important role in tumorigenesis and
tumor immunity, but the effects of this gene on the OS of patients
with BLCA and the underlying function of EMP1 in
tumor-immune interactions remain unclear.
The present study aimed to comprehensively analyze
EMP1 expression and its association with the prognosis of
patients with BLCA. In addition, the correlation between
EMP1 and tumor-infiltrating immune cells in the BLCA
microenvironment was determined. The correlation between
EMP1 and the immune cell-specific genes reported in
literature, as well as immunological checkpoint-specific genes were
further studied, and the expression level of EMP1 in
tumor-tissue specimens and adjacent normal tissues of patients with
BLCA were compared.
Materials and methods
Patients and tissue samples
Bladder cancer and adjacent normal tissues were
collected from patients with BLCA at the Peking University Shenzhen
Hospital (Shenzhen, China) between September 2018 and December
2019. The specimens were all collected during bladder cancer
resection, and the distance between tumor tissue and adjacent
normal tissue was >2 cm. BLCA was diagnosed and classified
through pathological examination based on the World Health
Organization classification system (24). Specimens from patients with a history
of preoperative chemotherapy were excluded. The study protocol was
approved by the Ethics Committees for Human Experiments of Peking
University Shenzhen Hospital. All patients signed an informed
consent form before sample collection.
Image processing
The paraffin-embedded tumor sections (5 µm thick)
were stained with H&E or antibodies against EMP1 (cat.
no. ab230445; 1:75; Abcam) according to the routine
immunohistochemical staining method (25). All images shown are wide-field light
microscopy images that were acquired at sufficient resolution.
Acquisition of mRNA data
The gene expression data and corresponding clinical
information were downloaded from TCGA website (https://portal.gdc.cancer.gov) for BLCA, and estimated
as log2(x+1) transformed RSEM normalized counts
(26). BLAC samples comprised
samples of 404 patients with BCLA, including 28 cases with adjacent
non-tumorous tissue as control group. All data were processed using
R-studio software (v3.5.3) (27).
The ‘ESTIMATE’ R package was used to predict the presence of
infiltrating stromal/immune cells in tumor tissues using gene
expression data (28).
Oncomine database analysis
The levels of EMP1 gene expression in various
types of cancer were identified using the Oncomine database
(https://www. oncomine.org). The
threshold was determined according to the following values: P-value
of 0.001 and fold-change of 2.
Kaplan-Meier plotter database
analysis
The Kaplan Meier plotter (http://kmplot.com/analysis/) is capable of assessing
the effect of 54,000 genes on patient survival in 21 types of
cancer (29). The association
between EMP1 expression and survival in patients with BLCA
was analyzed using the Kaplan-Meier plotter. The hazard ratio (HR)
with 95% confidence intervals (CIs) and log-rank P-value were
computed.
Tumor Immune Estimation Resource
(TIMER) database analysis
TIMER (https://cistrome. shinyapps.io/timer/), which is a
comprehensive resource for the systematic analysis of immune
infiltrates across various types of cancer, was used in the present
study to analyze the level of EMP1 expression in BLCA and
the correlation between EMP1 expression and the abundance of
immune infiltrates, including B cells, CD4+ T cells,
CD8+ T cells, neutrophils, macrophages and dendritic
cells via gene modules. The immune cell infiltration score of each
patient in TCGA database was obtained using TIMER, and the patients
were divided into high and low score groups based on the median
value.
Immunological analysis by Tumor immune
system interaction database (TISIDB)
The correlations between the abundance of
tumor-infiltrating lymphocytes and EMP1 expression were
analyzed using TISIDB (http://cis.hku.hk/TISIDB), which is a web portal for
tumor and immune system interactions integrating multiple
heterogeneous data types (30). In
the present study, the enrichment data of 28 immune cells provided
by TISIDB, including activated CD8+ cells (Act CD8),
central memory CD8 cells (Tcm CD8), effector memory CD8 cells (Tem
CD8), activated CD4+ cells (Act CD4), central memory CD4
cells (Tcm CD4), effector memory CD4 cells (Tem CD4), T follicular
helper cells (Tfh), gamma delta T cells (Tgd), type 1 T helper
cells (Th1), type 17 T helper cells (Th17), type 2 T helper cells
(Th2), regulatory T cells (Treg), activated B cells (Act B),
immature B cells (Imm B), memory B cells (Mem B), natural killer
(NK) cells, CD56 bright NK cells (CD56bright), CD56 dim NK cells
(CD56dim), myeloid derived suppressor cells (MDSCs), NK T cells
(NKT), activated dendritic cell (Act DCs), plasmacytoid DCs (pDCs),
immature DCs (iDCs), macrophages, eosinophils, mast cells (Mast),
monocytes and neutrophils, were used to calculate the relationship
with the expression of EMP1 in BLCA.
Gene expression and survival analysis
in Gene Expression Profiling Interactive Analysis (GEPIA)
The online database GEPIA (http://gepia.cancer-pku.cn/index.html) was used to
analyze the differential expression of EMP1 and its
prognostic values.
Co-expression analysis in
cBioPortal
The cBioPortal for Cancer Genomics (https://www.cbioportal.org) is an open-access,
open-source resource for interactive exploration of
multidimensional cancer genomics datasets (31,32) that
was used in the present study to determine the correlations between
EMP1 expression and tumor-infiltrating immune cell markers.
The gene markers of the tumor-infiltrating immune cells included
markers of CD8+ T cells, T cells (general), B cells,
monocytes, tumor associated macrophages (TAMs), M1 macrophages, M2
macrophages, neutrophils, NK cells, DCs, Th1 cells, Th2 cells,
follicular helper T (Tfh) cells, Th17 cells, Tregs and exhausted T
cells. The Spearman rank correlation analysis was used to determine
the correlation coefficient. EMP1 expression was plotted on
the x-axis, and the expression levels of other genes of interest
were represented on the y-axis.
Gene set enrichment analysis
(GSEA)
To identify the potential mechanisms underlying the
effects of EMP1 expression on BLCA prognosis, GSEA was
performed to detect whether an a priori defined set of genes
exhibited statistically significant differential expression between
the high and low EMP1 expression groups. Gene sets with a
P-value <0.05 and false discovery rate (FDR) <0.25 in the
enrichment of MSigDB Collection (c2.cp.kegg.v6.2. symbols) were
considered to be significantly enriched.
Statistical analysis
Survival curves were generated using the GEPIA and
Kaplan-Meier databases. T test and paired t test were implemented
in prism and R software, and the results were displayed in the form
of pictures and tables after sorting out the results. Receiver
operating characteristic (ROC) curve and area under the curve (AUC)
were used to demonstrate the predictive ability of EMP1 for
3- and 5-year OS. The results generated in Oncomine are presented
as P-values, fold-changes and ranks. The results of the
Kaplan-Meier plots and GEPIA are displayed with HR and P or Cox
P-values from a log-rank test. Univariate Cox analysis was
performed to select the potential prognostic factors, and
multivariate Cox analysis was performed to verify the association
between EMP1 expression and survival along with other
clinical features. P<0.05 was considered to indicate a
statistically significant difference.
Results
Association between EMP1 expression
and clinicopathologic variables in BLCA
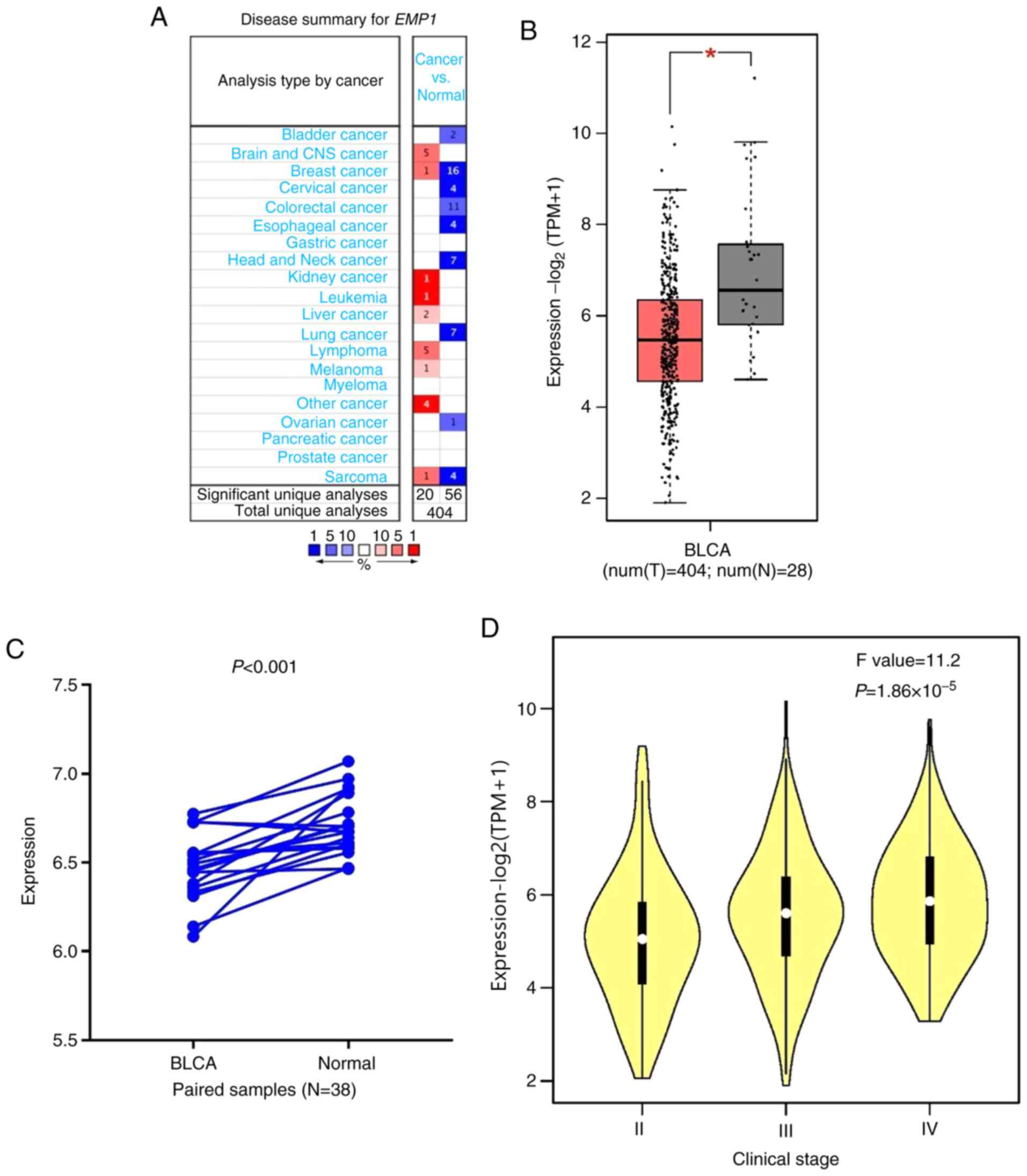
As presented in Fig.
1A, EMP1 expression was upregulated in brain, breast,
kidney and liver cancer, as well as in leukemia, lymphoma, melanoma
and sarcoma compared with that in normal tissues. In addition,
downregulation of EMP1 was observed in bladder, breast,
cervical, colorectal, esophageal, head and neck, lung, ovarian
cancer and sarcoma in a number of data sets. Differential
expression was observed between tumor and normal tissues for
EMP1 in BLCA data from TCGA. The results indicated that
EMP1 was upregulated in BLCA compared with adjacent normal
tissues (P<0.001; Fig. 1B) and
with paired adjacent healthy tissues (P<0.001; Fig. 1C). In addition, the expression of
EMP1 significantly increased with clinical stage
(P<0.001; Fig. 1D). The
expression data of EMP1 are presented in the supplementary
materials (Table SI and Table SII).
Upregulation of EMP1 was significantly
associated with advanced age (≥60 vs. <60, P=0.040),
histological subtype (papillary vs. non-papillary, P=0.043),
pathologic T classification (III–IV vs. I–II, P=0.008), immune
score (high vs. low, P<0.001) and stromal score (high vs. low,
P<0.001; Table I). However, no
significant associations between EMP1 expression and sex,
lymphovascular invasion, tumor recurrence, pathologic M/N
classification and pathologic stage were observed.
 | Table I.Logistic regression of the expression
of EMP1 and the clinicopathological characteristics of patients
with bladder urothelial carcinoma. |
Table I.
Logistic regression of the expression
of EMP1 and the clinicopathological characteristics of patients
with bladder urothelial carcinoma.
| Characteristic | Total | Odds ratio in EMP1
expression | P-value |
|---|
| Age, years (≥60 vs.
<60) | 407 | 1.64
(1.02–2.67) | 0.04a |
| Subtype (papillary
vs. non-papillary) | 402 | 0.65
(0.42–0.98) | 0.04a |
| Sex (male vs.
female) | 407 | 0.80
(0.51–1.23) | 0.30 |
| Lymphovascular
invasion (positive vs. negative) | 273 | 1.29
(0.80–2.09) | 0.30 |
| Recurrence (yes vs.
no) | 371 | 1.23
(0.79–1.89) | 0.36 |
| Pathologic T
classification (III–IV vs. I–II) | 374 | 1.80
(1.17–2.81) |
8.4×10−3a |
| Pathologic M
classification (M+ vs. M0) | 200 | 2.43
(0.66–11.56) | 0.21 |
| Pathologic N
classification (N0 vs. N+) | 367 | 0.90
(0.59–1.38) | 0.64 |
| Pathologic stage
(III–IV vs. I–II) | 405 | 1.496
(0.984–2.283) | 0.06 |
| Stromal score (high
vs. low)b | 408 | 1.65
(0.25–0.56) |
1.91×10−6a |
| Immune score (high
vs. low)b | 408 | 1.54
(0.29–0.64) |
2.89×10−5a |
Survival outcomes and multivariate
analysis
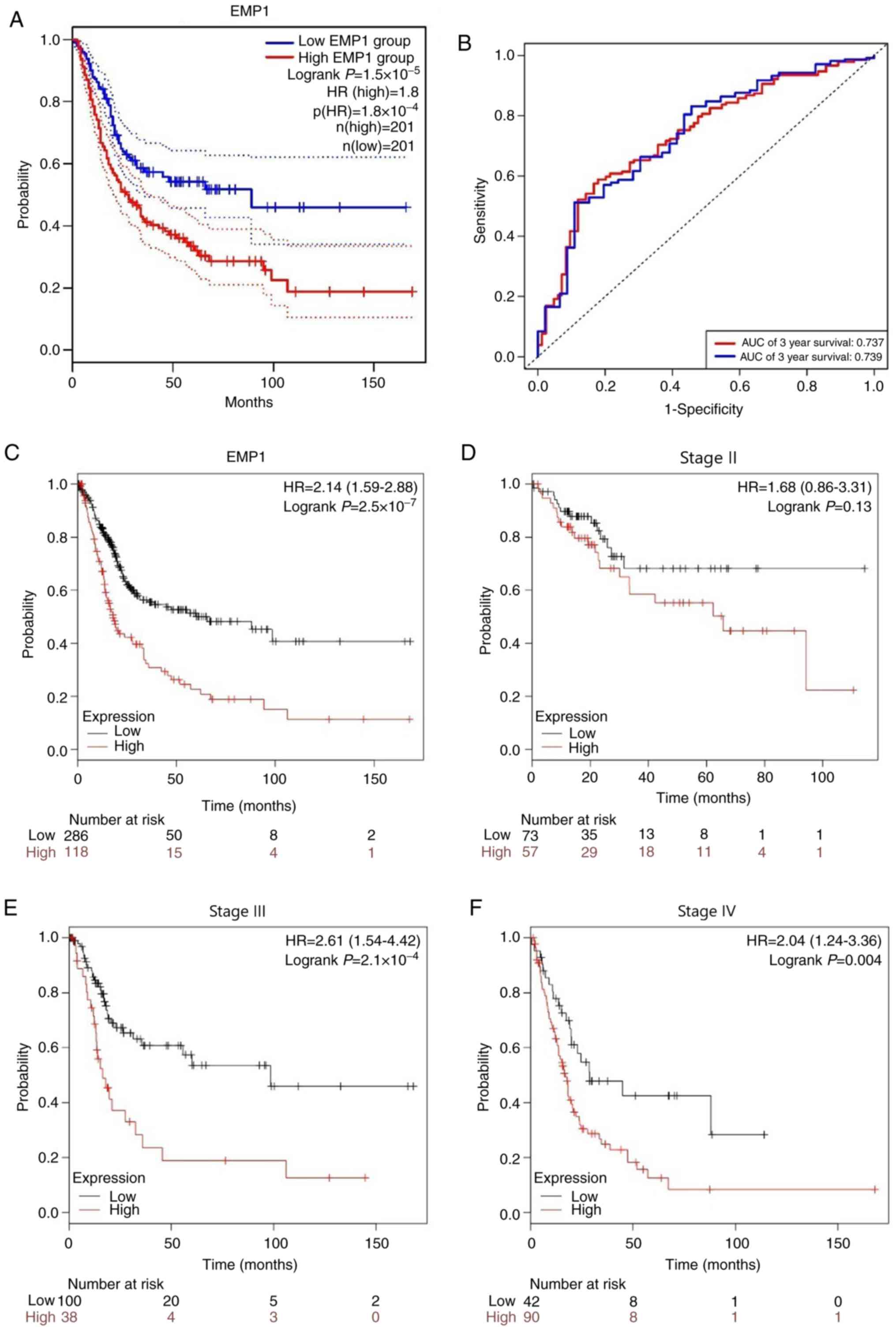
The analysis of BLCA cases in TCGA revealed that the
5-year OS of the high EMP1 expression group was
significantly lower compared with that of the low expression group
(P<0.001; Fig. 2A). Receiver
operating characteristic (ROC) curve analysis demonstrated the
predictive ability of EMP1 for 3- and 5-year OS with the
area under the curve (AUC) of 0.737 and 0.739, respectively
(Fig. 2B). The association between
EMP1 expression and survival outcome was further confirmed
by Kaplan-Meier survival analysis (Fig.
2C). In addition, Fig. 2D-F
demonstrated the relationship between EMP1 and OS in stage
II–IV patients. The results showed that EMP1 overexpression
was significantly associated with the poor prognosis of patients
with stages II and IV.
The univariate analysis revealed that high
EMP1 expression was significantly associated with poor OS
time (HR, 8.93; 95% CI, 3.71–21.47; P<0.001). Other
clinicopathological characteristics associated with worse survival
included age, pathologic stage, CD8+ T cell, macrophage
infiltration, and stomal-score. In the multivariate analysis,
EMP1 remained associated with poor OS (HR, 6.61; 95% CI,
2.39–18.30; P<0.001) in conjunction with advanced age,
pathologic stage, and macrophage infiltration (Table II). The data used for multivariate
Cox regression are presented in the supplementary materials
(Table SIII).
 | Table II.Univariate and multivariate analysis
of overall survival using the Cox proportional hazard regression
model (N=397). |
Table II.
Univariate and multivariate analysis
of overall survival using the Cox proportional hazard regression
model (N=397).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.033 | 1.017–1.049 |
4.67×10−5a | 1.029 | 1.012–1.046 |
5.0×10−4a |
|
Subtypec | 0.650 | 0.454–0.929 | 0.018a | 0.814 | 0.558–1.187 | 0.286 |
| Sex | 0.880 | 0.633–1.224 | 0.449 | 0.862 | 0.611–1.215 | 0.395 |
| Pathologic
stage | 2.240 | 1.538–3.262 |
2.6×10−5a | 1.880 | 1.261–2.802 | 0.002a |
| B cell | 0.0741 | 0.007–0.754 | 0.028 | 0.116 | 0.010–1.269 | 0.078 |
| CD4+ T
cell | 0.286 | 0.042–1.954 | 0.202 | 1.415 | 0.047–42.416 | 0.841 |
| CD8+ T
cell | 5.701 | 1.430–22.730 | 0.014a | 5.243 | 0.407–67.569 | 0.204 |
| Neutrophil | 0.652 | 0.076–5.614 | 0.697 | 0.006 | 0.000–0.737 | 0.037a |
| Macrophage | 18.825 | 4.514–78.518 |
5.62×10−5a | 8.867 | 1.506–52.216 | 0.016a |
| Dendritic | 0.898 | 0.428–1.884 | 0.776 | 1.350 | 0.300–6.039 | 0.696 |
| Stromal
scoreb | 0.728 | 0.530–0.973 | 0.033a | 1.043 | 0.710–1.530 | 0.832 |
| Immune
scoreb | 1.072 | 0.795–1.446 | 0.649 | 1.015 | 0.668–1.542 | 0.944 |
| EMP1 | 8.927 | 3.713–21.466 |
1.01×10−6a | 6.614 | 2.390–18.301 |
2.75×10−4a |
GSEA identifies an EMP1-related
signaling pathway
The most significantly enriched signaling pathways
were selected based on their normalized enrichment score. As
demonstrated in Table III,
pathways such as ‘pathways in cancer’, ‘adherens junction’,
‘neurotrophin signaling pathway’, ‘endometrial cancer and focal
adhesion’ were differentially enriched in the high EMP1
expression phenotype. These signaling pathways may be the
mechanisms involved in EMP1 function.
 | Table III.Gene sets enriched analysis of
upregulated EMP1 in BLCA. |
Table III.
Gene sets enriched analysis of
upregulated EMP1 in BLCA.
| NAME | NES | P-value |
|---|
| KEGG ECM RECEPTOR
INTERACTION | 1.696 | 0.000 |
| KEGG FOCAL
ADHESION | 1.795 | 0.000 |
| KEGG PATHWAYS IN
CANCER | 1.592 | 0.002 |
| KEGG NEUROTROPHIN
SIGNALING PATHWAY | 1.595 | 0.006 |
| KEGG AXON
GUIDANCE | 1.612 | 0.008 |
| KEGG CELL ADHESION
MOLECULES CAMS | 1.547 | 0.008 |
| KEGG GAP
JUNCTION | 1.527 | 0.010 |
| KEGG ENDOMETRIAL
CANCER | 1.625 | 0.017 |
| KEGG HEMATOPOIETIC
CELL LINEAGE | 1.484 | 0.019 |
| KEGG NICOTINATE AND
NICOTINAMIDE METABOLISM | 1.523 | 0.023 |
| KEGG ADHERENS
JUNCTION | 1.753 | 0.024 |
| KEGG GLIOMA | 1.559 | 0.027 |
| KEGG RENAL CELL
CARCINOMA | 1.530 | 0.046 |
| KEGG FC GAMMA R
MEDIATED PHAGOCYTOSIS | 1.536 | 0.046 |
EMP1 expression is associated with the
level of immune infiltration in BLCA
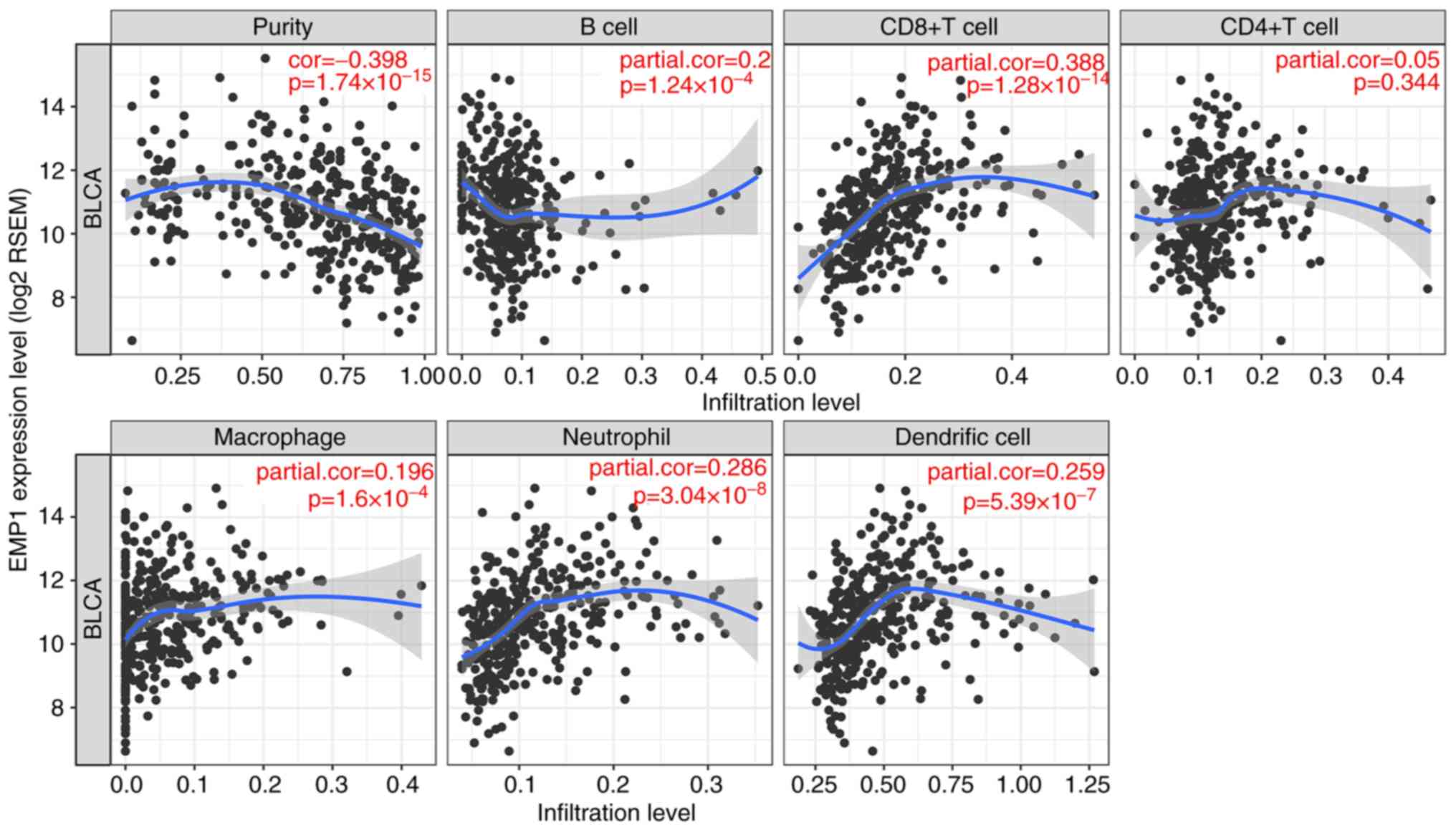
The level of EMP1 expression correlated with
high levels of immune infiltration in five types of immune cells
and tumor purity in the TIMER dataset. EMP1 expression was
significantly correlated with tumor purity and infiltration of B
cells, CD8+ T cells, macrophages, neutrophils and DCs in
BLCA as shown in Fig. 3. These
results suggested that EMP1 may serve a specific role in
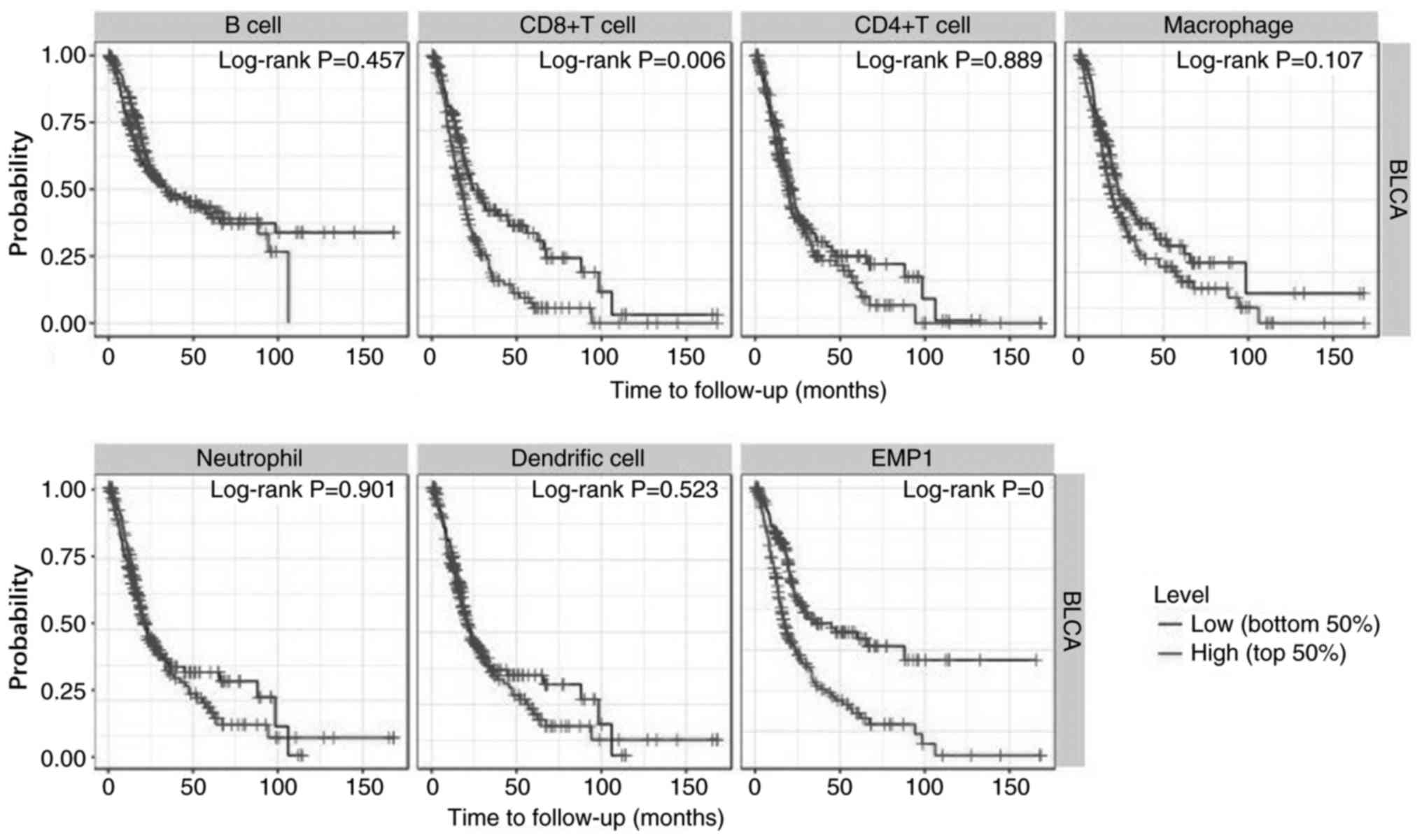
immune infiltration in BLCA. In addition, the survival analysis
from TIMER dataset also showed that high levels of infiltrating
CD8+ T cells were significantly associated with poor OS
in patients with BLCA (P=0.006), and high expression of EMP1
predicted poor OS (P<0.01; Fig.
4).
Association between the abundance of
tumor-infiltrating lymphocytes and EMP1 expression
The results obtained from TISIDB demonstrated that
EMP1 expression was strongly associated with the abundance
of Tcm CD8 and neutrophil cells (both correlation coefficients
>0.5 and P<0.05), and moderately related with the abundance
of Mem B, Act CD4, Tcm CD4, Tem CD8, Act DC, pDC, iDC, NK, NKT,
eosinophil, Tfh, Tgd, Th1, Th2, Treg, macrophage, mast and MDSC
(all correlation coefficients between 0.3–0.5 and all P<0.05)
(Fig. 5).
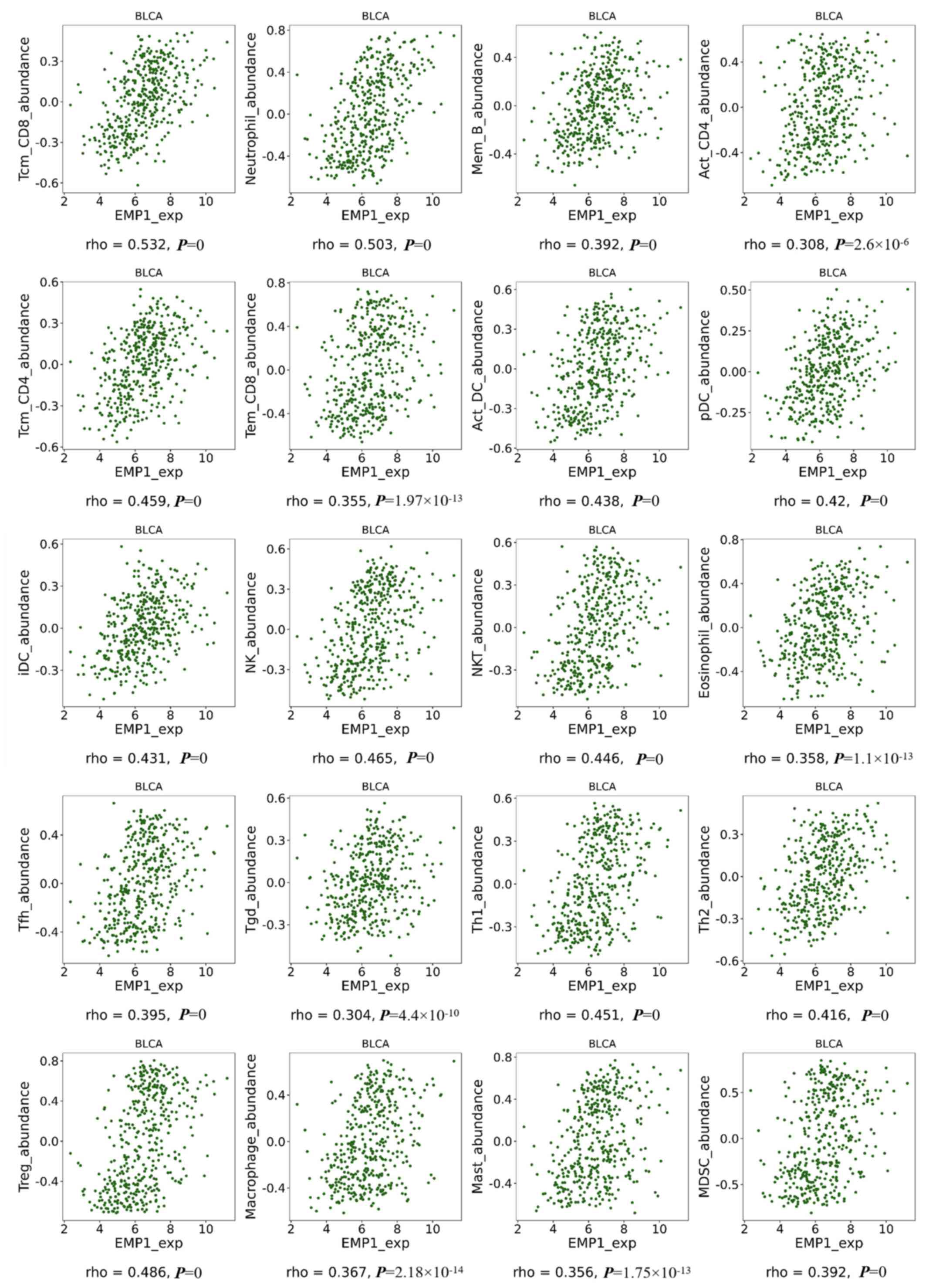 | Figure 5.Correlations between EMP1 expression
levels and lymphocyte abundance in BLCA using Tumor immune system
interaction database. BLCA, bladder urothelial carcinoma; EMP1,
epithelial membrane protein 1; Tcm CD8, central memory CD8 cells;
Mem B, memory B cells; Act CD4, activated CD4+ cells;
Tcm CD4, central memory CD4 cells; Tem CD8, effector memory CD8
cells; Act DCs, activated dendritic cell; pDCs, plasmacytoid DCs;
iDCs, immature DCs; NK, natural killer cells; NKT, NK T cells; Tfh,
T follicular helper cells; Tgd, gamma delta; Th1, type 1 T helper
cells; Th2, type 2 T helper cells; Treg, regulatory T cells; MDSCs,
myeloid derived suppressor cells; Mast, mast cells. |
Correlation between EMP1 expression
and immune markers
The correlations between EMP1 expression and
immune marker genes of the different immune cells, including
CD8+ T cells, T cells (general), B cells, monocytes,
TAMs, M1 and M2 macrophages, neutrophils, NK cells and DCs are
presented in Table IV. The results
revealed that the level of EMP1 expression was significantly
correlated with immune markers of various immune cells. The
expression levels of the majority of marker sets of monocytes, TAMs
and M2 macrophages exhibited a significant correlation with
EMP1 expression. In particular, chemokine (C-C motif) ligand
(CCL)-2, CD68, interleukin 10 (IL10) of TAMs,
prostaglandin-endoperoxide synthase 2 (PTGS2), interferon
regulatory factor 5 (IRF5) of the M1 phenotype,
CD163, V-set and immunoglobulin domain containing 4
(VSIG4), and membrane spanning 4-domains A4A (MS4A4A)
of the M2 phenotype significantly correlated with EMP1
expression in BLCA (P<0.05), suggesting that EMP1 may
regulate macrophage polarization. High EMP1 expression is
was associated with a high level of DC infiltration in BLCA; DC
markers, such as Major Histocompatibility Complex, Class II, DP
Beta 1 (HLA-DPB1) and Integrin Subunit Alpha X
(ITGAX) also exhibited a significant correlation with EMP1
expression. In addition, for Tregs, a moderate positive correlation
was observed between forkhead box P3 (FOXP3), C-C Motif
Chemokine Receptor 8 (CCR8) and EMP1 in BLCA. Therefore,
these results further confirmed that EMP1 was associated
with infiltrating immune cells in BLCA, which suggested that
EMP1 may serve a crucial role in immune enhancement in the
BLCA microenvironment.
 | Table IV.Correlation analysis between EMP1 and
the genes and markers of immune cells in cBioportal. |
Table IV.
Correlation analysis between EMP1 and
the genes and markers of immune cells in cBioportal.
| Cell type | Gene | Spearman's
correlation | P-value |
|---|
| CD8+ T
cell | CD8A | 0.214 |
1.30×10−5a |
|
| CD8B | 0.092 | 0.060 |
|
| CD80 | 0.375 |
4.48×10−15a |
| T cell
(general) | CD3D | 0.160 | 0.001a |
|
| CD3E | 0.240 |
9.70×10−7a |
|
| CD2 | 0.207 |
2.42×10−5a |
| B cell | CD19 | 0.104 | 0.036a |
|
| CD79A | 0.177 |
3.26×10−4a |
| Monocyte | CD86 | 0.434 |
3.88×10−20a |
|
| CD115 | 0.417 |
4.11×10−17a |
| TAM | CCL2 | 0.342 |
1.24×10−12a |
|
| CD68 | 0.366 |
2.38×10−14a |
|
| IL10 | 0.394 |
1.25×10−16a |
| M1 Macrophage | NOS2 | 0.131 | 0.008a |
|
| IRF5 | −0.199 |
5.37×10−5a |
|
| PTGS2 | 0.296 |
1.10×10−9a |
| M2 Macrophage | CD163 | 0.442 |
6.27×10−21a |
|
| VSIG4 | 0.436 |
2.22×10−20a |
|
| MS4A4A | 0.403 |
2.85×10−18a |
| Neutrophil | ITGAM | 0.381 |
1.51×10−15a |
|
| CCR7 | −0.141 | 0.004a |
| Natural killer
cell | KIR2DL1 | 0.200 |
4.87×10−5a |
|
| KIR2DL3 | 0.223 |
5.35×10−6a |
|
| KIR2DL4 | 0.268 |
3.92×10−8a |
|
| KIR3DL1 | 0.141 | 0.004* |
|
| KIR3DL2 | 0.170 |
5.83×10−4a |
|
| KIR3DL3 | 0.067 | 0.179 |
|
| KIR2DS4 | 0.149 | 0.003a |
| Dendritic cell |
HLA-DPB1 | 0.279 |
9.81×10−9a |
|
|
HLA-DQB1 | 0.240 |
9.04×10−7a |
|
| HLA-DRA | 0.270 |
3.06×10−8a |
|
|
HLA-DPA1 | 0.277 |
1.35×10−8a |
|
| CD1C | 0.262 |
8.34×10−8a |
|
| ITGAX | 0.392 |
1.99×10−16a |
|
| NRP1 | 0.455 |
3.15×10−22a |
| Th1 | TBX21 | 0.210 |
1.98×10−5a |
|
| STAT4 | 0.364 |
2.94×10−14a |
|
| STAT1 | 0.282 |
6.46×10−9a |
|
| IFN-g | 0.161 | 0.001a |
|
| TNF-a | 0.153 | 0.002a |
| Th2 | GATA3 | −0.497 |
7.77×10−27a |
|
| STAT6 | −0.173 |
4.48×10−4a |
|
| IL13 | 0.181 |
2.38×10−4a |
| Tfh | BCL6 | −0.144 | 0.004a |
|
| IL21 | 0.041 | 0.409 |
| Th17 | STAT3 | 0.431 |
7.67×10−20a |
|
| IL17A | −0.071 | 0.153 |
| Treg | FOXP3 | 0.254 |
1.91×10−7a |
|
| CCR8 | 0.274 |
1.80×10−8a |
|
| TGFb | 0.314 |
8.82×10−11a |
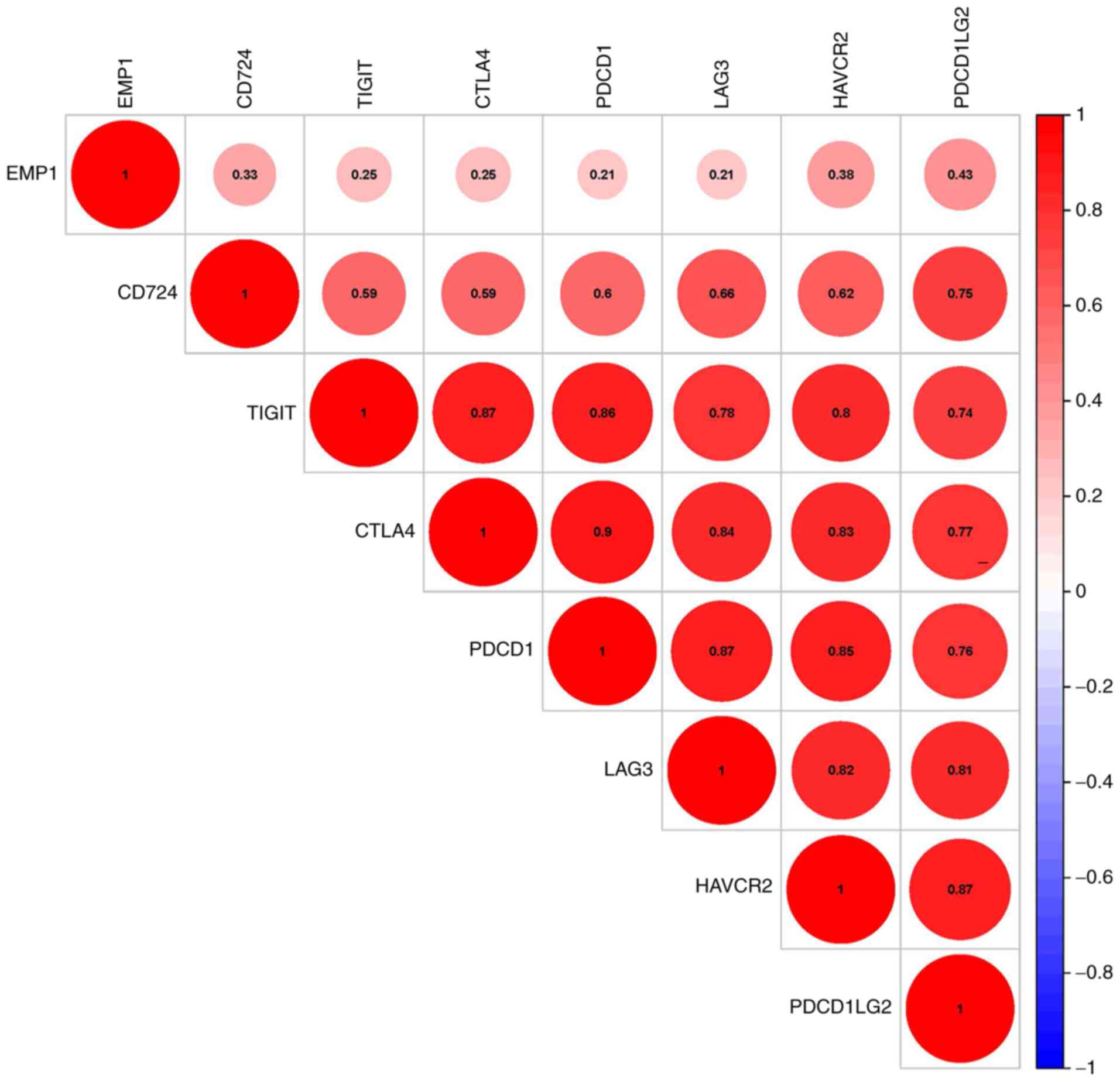
EMP1 expression and immune checkpoint
correlation analysis
The correlations between EMP1 and the
specific genes of the immune checkpoints that have been reported in
the literature were further assessed. Programmed cell death 1
ligand 1 (CD274), programmed cell death 1 ligand 2
(PDCD1LG2), hepatitis A virus cellular receptor 2
(HAVCR2), cytotoxic T-lymphocyte associated protein 4
(CTLA4), lymphocyte-activating 3 (LAG3), programmed
cell death 1 (PDCD1) and T cell immunoreceptor with Ig and
ITIM domains (TIGIT) were selected for analysis as they have
been previously reported to be immunological checkpoint-specific
genes. EMP1 was significantly associated with the expression
of these genes (P<0.05; Fig.
6).
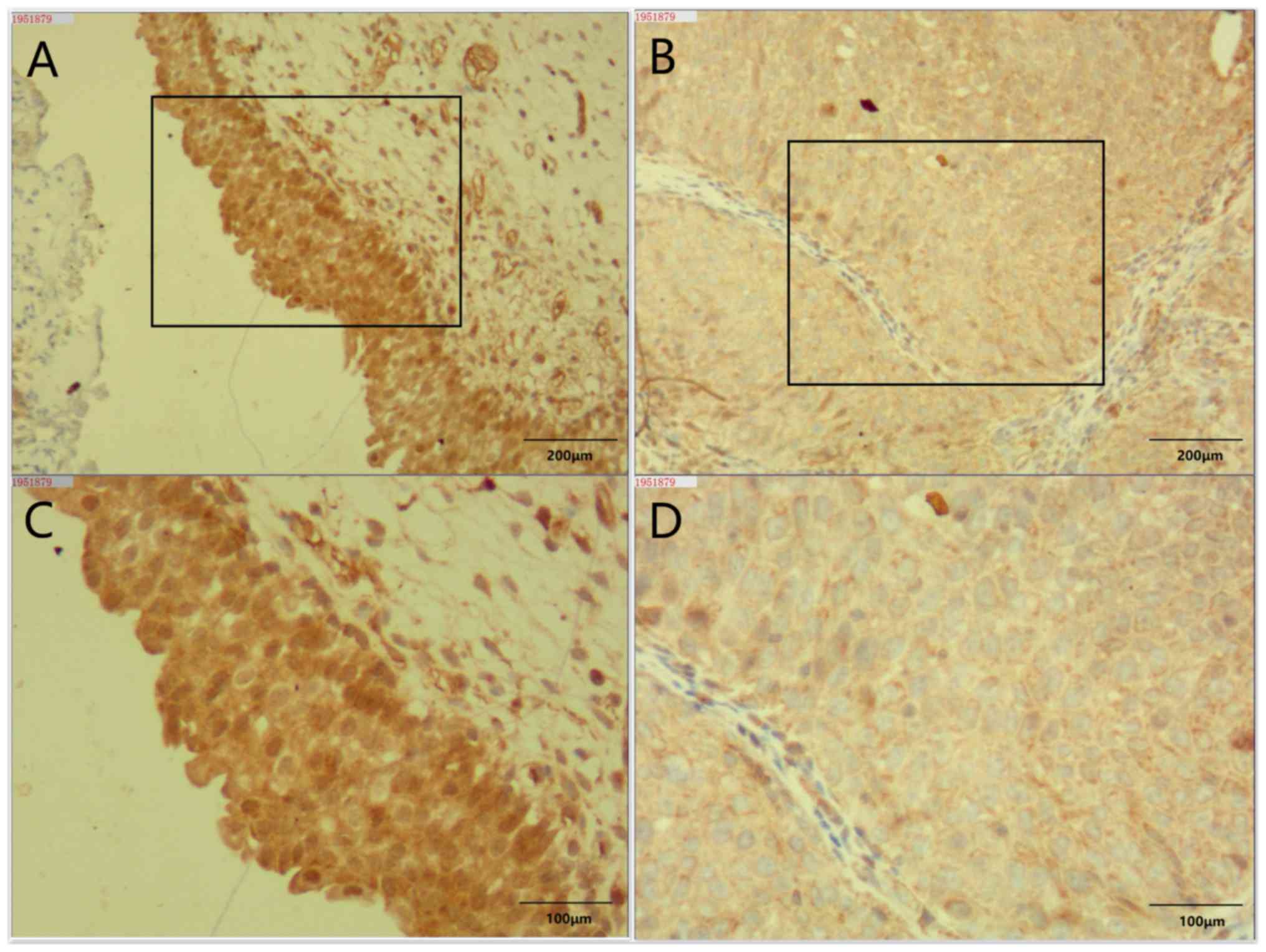
Expression of EMP1 in BLCA specimens
and adjacent normal tissue
Bladder cancer specimens from eight patients with
pathologically confirmed BLCA were analyzed in the present study.
The patients were all male, and the age range was 66–82 years.
Immunohistochemical staining of BLCA tissue specimens and adjacent
normal tissues revealed that EMP1 was strongly stained in
adjacent normal tissues, but was the staining intensity was lower
in tumor tissues (Fig. 7).
Discussion
The present study examined the levels of EMP1
expression and the systematic prognostic landscape in BLCA using
independent datasets in the Oncomine and TCGA databases.
Differential levels of EMP1 expression between cancer and
normal tissues were observed. Consistent prognostic associations of
EMP1 expression in BLCA were identified, in which high
levels of EMP1 expression were associated with a poor OS
rate, and further analysis using the Kaplan-Meier plotter revealed
that EMP1 overexpression was associated with poor BLCA
prognosis in stages III to IV. The results of the TIMER database
analysis demonstrated significant positive correlations between the
levels of EMP1 expression and the infiltration of
CD8+ T cells, macrophages, neutrophils and DCs in BLCA;
however, EMP1 expression was negatively correlated with the
infiltration of B cells, as well as the degree of tumor purity.
Further analysis revealed that the upregulation of EMP1 was
significantly positively associated with an abundance of
macrophages, but negatively associated with the levels of Tregs,
plasma and CD4-naïve T cells. The co-expression analysis of
EMP1 and the previously reported immunolabeled genes also
yielded consistent results. The expression level of EMP1 in
BLCA tissues was further validated using independent specimens.
These results suggested that EMP1 may be a prognostic
biomarker, as well as an important factor for the recruitment and
regulation of infiltrating immune cells in BLCA.
EMP1 was selected as the research object
mainly based on the following considerations: Through the analysis
of multiple databases, a significant difference was observed in the
expression of EMP1 between bladder cancer and paired normal
tissues, and only one study suggested that EMP1 was
expressed at low levels in bladder cancer (33). However, it was necessary to further
study this gene as its function in bladder cancer was not clear.
EMP1 belongs to the peripheral myelin protein 22
(PMP22) family and has high homology among the family
members (34,35). PMP22 is considered to serve an
important role in the immune response (36–38).
Thus, it was speculated that EMP1 may exert a similar
function.
Alteration of EMP1 has been implicated in
different types of human cancer. Cancer cell lines transfected with
EMP1 in vitro, including PC-3 prostate cancer, SW-480 colon
cancer and MCF-7 breast cancer have been demonstrated to exhibit a
high rate of apoptosis and a poor survival rate (14,15). The
EMP1 gene expression has also been demonstrated to be
downregulated in laryngeal, esophageal, head and neck, sclerosing
gastric and prostate cancer, as well as in uterine fibroids
compared with that in normal tissues (39–43). Sun
et al (12), have
demonstrated that as a tumor suppressor gene, high expression of
EMP1 can improve the 5-year survival rate of patients with
nasopharyngeal cancer. This result was also confirmed in gastric
cancer (13), and EMP1 was
reported to be associated with nodal metastasis in oral squamous
cell cancer (44). EMP1
overexpression has also been established to be associated with poor
OS in pediatric leukemia (17).
However, some studies have yielded different
results. Lai et al (45),
demonstrated that EMP1 expression levels were higher in
non-small-cell lung cancer compared with those in the benign
control group. When a recombinant adenovirus overexpressing
EMP1 was constructed and virus-infected PC9 cells were
transplanted into nude mice, the growth of the transplanted tumors
could be observed. Another study by Zhang et al (46), demonstrated that EMP1 was
upregulated in human gliomas, and that EMP1 expression was
significantly increased in patients with World Health Organization
tumor grade III–IV compared with grade I–II.
The results of the present study demonstrated that
EMP1 expression was downregulated in BLCA compared with that
in normal tissues, but patients with low EMP1 expression
exhibited an improved OS rate compared with those in the high
expression group. These conflicting results may be caused by the
function of EMP1. Previous studies have demonstrated that
EMP1 serves an important role in cell differentiation
(35,47,48) and
proliferation (34,49); thus, EMP1 promotes cell
differentiation, whereas tumor cells are characterized by
de-differentiation. Low degree of tumor cell differentiation leads
to low expression of EMP1. In addition, EMP1 is a
direct or indirect target gene of the classical proto-oncogene
c-myc, which serves a role in promoting cell proliferation
(34). The results of the present
study also demonstrated that in patients with high EMP1
expression, the prognosis of BLCA was poor. This result was
contrary to that of Peter et al (33), whose findings suggested that low
expression of EMP1 was associated with an increased risk of
urothelial cancer-specific mortality. These differences may be due
to the histological differences among the tumors, due to the
differences in the internal and external environments of the tumor
cells, and even due to the differences in the methods of data
collection and analysis.
There is relatively little information about the
signaling pathways associated with EMP1-mediated biological
processes. Silencing experiments in the T-precursor ALL and B-ALL
cell lines have indicated that EMP1 may signal through the
Src kinase family (17). Wang et
al (50), transfected
EMP1 into the esophageal cancer cell line EC9706 and
reported that EMP1 inhibited the proliferation of esophageal
cancer cells, arrest the tumor cells in the S phase of the cell
cycle or prolong the G1 phase. However, other study suggested that
the EMP1 gene serves a role in promoting cell proliferation
as a target gene of the proto-oncogene c-myc (19). EMP1 is highly expressed with
c-myc in active embryonic stem cells, but the expression
gradually decreases as the embryo differentiates and matures
(51). Currently, the mechanism of
EMP1 in cell proliferation and apoptosis is not clear, but
it is worth affirming that EMP1 exerts its effects mainly by
regulating signal transduction between cells or between cells and
the extracellular matrix (52).
Ramnarain et al (21), have
demonstrated that mutation in the EGFR gene leads to the activation
of a series of downstream signals, including EMP1, as a
result of which patients harboring the mutated EGFR are more likely
to develop glioblastoma compared with those with wild-type EGFR.
Durgan et al (53), have
suggested that EMP1, as an important transcriptional target
in the Ras/mitogen-activated protein kinase pathway of bronchial
epithelial cells, participates in the tight junction between cells
and serves an important role in tracheal morphogenesis; its
deletion may be associated to lung tumors. Lai et al
(45), have reported that high
expression of EMP1 leads to the occurrence and development
of non-small-cell lung cancer by activating the PI3K/AKT
pathway.
A number of previous studies have stated that
members of the EMP family affect the integrin heterodimer
repertoire on the plasma membrane, and modulation of the expression
or localization of EMP proteins may alter the surface repertoire of
molecules (54–56). The surface molecular repertoire,
including major histocompatibility complex 1 proteins, integrins
and other immunoglobulin superfamily members such as CD54 and
glycosylphosphatidyl-inositol-linked proteins may be altered as the
expression of EMP2 changes (57). These results suggest that members of
the EMP family may influence the development of cancer cells via
the tumor immune microenvironment and ultimately affect the
prognosis of patients. However, there is lack of research on the
association between EMP1 and different immune cell
infiltration in BLCA.
Based on previous studies, the present study
further analyzed the correlation between EMP1 and the
infiltration of various immune cells.
The results of the present study suggested that
macrophage infiltration was an independent prognostic factor in
BLCA, which was consistent with previous studies that have
demonstrated significant associations between tumor-associated
macrophage infiltration and shorter survival of patients with
bladder cancer (10,58,59). A
number of studies have reported that a high neutrophil-lymphocyte
ratio is a negative predictor of bladder cancer (60–63). In
addition, neutrophil infiltration is significantly associated with
poor prognosis of bladder cancer (59,64).
High CD4+ T-cell density has been identified to be
associated with poor prognosis in patients with bladder cancer
(65,66), and a high level of mature
tumor-infiltrating DCs predicts progression to muscle invasion in
bladder cancer (58). In the present
study, EMP1 expression was positively correlated with
macrophage, neutrophil, CD4+ cell and DC infiltration,
and negatively correlated with B lymphocyte infiltration,
indicating that EMP1 expression may be a negative regulator
of tumor immunity. These results were consistent with previous
studies of immune cell infiltration.
The correlation between EMP1 expression and
the enrichment of neutrophils and macrophages was further confirmed
in the TISIDB in the present study. EMP1 also exhibited a
significant positive correlation with the enrichment of immune
cells such as CD4, CD8, DCs, NK and NKT cells, which suggested that
EMP1 may aggravate the prognosis of patients by affecting
the level of infiltration of specific immune cells. In addition,
Treg and MDSC cells are considered to be suppressors of antitumor
immune responses, and their enrichment is associated with poor
patient outcomes in cancer (8,67–70).
However, the results of B cell enrichment in TIMER and TISIBD in
the present study were not consistent, reflecting the different
algorithms used for the two immune scores. Although the mechanism
of EMP1 in tumor immunology is not fully understood, the
correlations between EMP1 expression and immune cell
infiltration implicated the role of EMP1 in regulating tumor
immunology in BLCA.
Recent studies have provided possible mechanisms
that may explain the association between EMP1 expression and
inflammation. Wang et al (71), have demonstrated that low microRNA-31
expression in mesenchymal stem cells in patients with psoriasis
causes an increase in the expression of EMP1, which in turn
facilitates T lymphocyte activation. A study by Pan et al
(72) has indicated that EMP1
is activated by zinc finger protein 750 and regulates signaling
pathways associated with proliferation and inflammation in CAL-27
cells.
To further elucidate the possible mechanism of
EMP1 expression in immunity, the present study speculated
its possible function by assessing co-expression with previously
reported gene markers. Co-expression of CCL-2, CD68, IL10 of
TAMs, PTGS2, IRF5 of M1 phenotype, CD163, VSIG4 and
MS4A4A of the M2 phenotype with EMP1 suggested that
EMP1 may regulate macrophage polarization. DC markers (e.g.,
HLA-DPB1, HLA-DRA, BDCA-1, and ITGAX) also exhibited
a significant correlation with EMP1 expression, indicating
an association between DC penetration and EMP1. Together, DC
and T cells can secrete IL-12 and IL-18 to activate T cell
proliferation, induce CTL production, and trigger a Th1-type immune
response, which are conducive to tumor clearance (73). Treg markers (FOXP3, CCR8,
TGFB1) were significantly co-expressed with EMP1.
FOXP3 serves an essential role in maintaining homeostasis of
the immune system by facilitating the acquisition of full
suppressive function and stability of the Treg lineage, and by
directly modulating the expansion and function of conventional T
cells (74). These results indicated
that EMP1 expression may serve a complex role in the immune
regulation network.
Checkpoint inhibitors are monoclonal antibodies
that block inhibitory checkpoint antigens and repress the
stimulation of T cells, exhibiting anticancer effects (75,76).
Upon chronic stimulation by tumor antigens, tumor-infiltrating T
cells lose their effector functions and their ability to kill tumor
cells, accompanied by a progressive increase in the diversity and
number of inhibitory receptors expressed on them, including
CD274, PDCD1LG2, HAVCR2, CTLA4, LAG3, PDCD1 and TIGIT
(75–82). Therefore, genes that were associated
with immune checkpoints were selected for analysis in the present
study.
The results of the present study revealed
significant co-expression between EMP1 and the genes
reported to be associated with immune checkpoints, suggesting that
EMP1 may serve a role in BLCA by affecting these immune
checkpoints. Previous results have suggested a positive correlation
between EMP1 and Treg, which inhibits the immune response of
other immune cells. These results suggest that EMP1 may
restore the function of immune cells by inhibiting immune
checkpoints. Blocking antibodies against EMP1 may be a
promising treatment strategy for patients with BLCA.
The present study had certain limitations. Further
research, including deep sequencing, is needed to elucidate the
full spectrum of variability and any functional variants of
EMP1 in BLCA. It is also necessary to further explore the
specific molecular mechanism of EMP1 in bladder cancer
cells.
In conclusion, the results of the present study
demonstrated that variations in the EMP1 expression levels
were associated with the prognosis of patients with BLCA. High
EMP1 expression was associated with a poor OS rate. In
addition, these results revealed that the extent of immune cell
infiltration and the diversity of immune marker expression were
associated with EMP1 expression in BLCA. Therefore, the
results of the present study may provide insights into the
potential function of EMP1 in tumor immunology and its
potential as a cancer biomarker.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
No applicable.
Funding
The current study was supported by the Shenzhen
Healthcare Research Project (grant no. SZLY2018022).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors contributions
HY designed the research and reviewed the
manuscript. BL analyzed the data and prepared the original draft.
TZ and XY performed statistical calculation and experiments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Peking university Shenzhen hospital (Shenzhen, China). Signed
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global Cancer Statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S, et al: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cumberbatch MGK, Jubber I, Black PC,
Esperto F, Figueroa JD, Kamat AM, Kiemeney L, Lotan Y, Pang K,
Silverman DT, et al: Epidemiology of bladder cancer: A systematic
review and contemporary update of risk factors in 2018. Eur Urol.
74:784–795. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Obara W, Eto M, Mimata H, Kohri K,
Mitsuhata N, Miura I, Shuin T, Miki T, Koie T, Fujimoto H, et al: A
phase I/II study of cancer peptide vaccine S-288310 in patients
with advanced urothelial carcinoma of the bladder. Ann Oncol.
28:798–803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lavoie JM, Bidnur S, Black PC and Eigl BJ:
Expanding immunotherapy options for bladder cancer: commentary on:
Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. Urology. 106:1–2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamilou Z, Lavaud P and Loriot Y:
Atezolizumab in urothelial bladder carcinoma. Future Oncol.
14:331–341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamat AM, Bellmunt J, Galsky MD, Konety
BR, Lamm DL, Langham D, Lee CT, Milowsky MI, ODonnell MA, ODonnell
PH, et al: Society for Immunotherapy of Cancer consensus statement
on immunotherapy for the treatment of bladder carcinoma. J
Immunother Cancer. 5:682017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu YN, Zhang H, Zhang L, Cai TT, Huang
DJ, He J, Ni HH, Zhou FJ, Zhang XS and Li J: Sphingosine 1
phosphate receptor-1 (S1P1) promotes tumor-associated regulatory T
cell expansion: Leading to poor survival in bladder cancer. Cell
Death Dis. 10:502019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mukherjee N, Ji N, Hurez V, Curiel TJ,
Montgomery MO, Braun AJ, Nicolas M, Aguilera M, Kaushik D, Liu Q,
et al: Intratumoral CD56bright natural killer cells are associated
with improved survival in bladder cancer. Oncotarget.
9:36492–36502. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sjödahl G, Lövgren K, Lauss M, Chebil G,
Patschan O, Gudjonsson S, Månsson W, Fernö M, Leandersson K,
Lindgren D, et al: Infiltration of CD3+ and CD68+ cells in bladder
cancer is subtype specific and affects the outcome of patients with
muscle-invasive tumors. Urol Oncol. 32:791–797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang B, Xie S, Bi J, Liu Z, Zeng H, Huang
H, Xue M, He Z, Yang M, Yu H, et al: Elevated pre-existing
lymphocytic infiltrates in tumour stroma predict poor prognosis in
resectable urothelial carcinoma of the bladder. Histopathology.
75:354–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun GG, Lu YF, Fu ZZ, Cheng YJ and Hu WN:
EMP1 inhibits nasopharyngeal cancer cell growth and metastasis
through induction apoptosis and angiogenesis. Tumour Biol.
35:3185–3193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun G, Zhao G, Lu Y, Wang Y and Yang C:
Association of EMP1 with gastric carcinoma invasion, survival and
prognosis. Int J Oncol. 45:1091–1098. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun GG, Wang YD, Cui DW, Cheng YJ and Hu
WN: Epithelial membrane protein 1 negatively regulates cell growth
and metastasis in colorectal carcinoma. World J Gastroenterol.
20:4001–4010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun GG, Wang YD, Lu YF and Hu WN: EMP1, a
member of a new family of antiproliferative genes in breast
carcinoma. Tumour Biol. 35:3347–3354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun GG, Wang YD, Cui DW, Cheng YJ and Hu
WN: EMP1 regulates caspase-9 and VEGFC expression and suppresses
prostate cancer cell proliferation and invasion. Tumour Biol.
35:3455–3462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ariës IM, Jerchel IS, van den Dungen RE,
van den Berk LC, Boer JM, Horstmann MA, Escherich G, Pieters R and
den Boer ML: EMP1, a novel poor prognostic factor in pediatric
leukemia regulates prednisolone resistance, cell proliferation,
migration and adhesion. Leukemia. 28:1828–1837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jain A, Tindell CA, Laux I, Hunter JB,
Curran J, Galkin A, Afar DE, Aronson N, Shak S, Natale RB, et al:
Epithelial membrane protein-1 is a biomarker of gefitinib
resistance. Proc Natl Acad Sci USA. 102:11858–11863. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ben-Porath I and Benvenisty N:
Characterization of a tumor-associated gene, a member of a novel
family of genes encoding membrane glycoproteins. Gene. 183:69–75.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YQ, Xue T, Wang L, Xu ZC, Xi ZQ, Yuan
J, Wang XF, Chen YM, Zhang M and Yao L: Up-regulation of epithelial
membrane protein-1 in the temporal neocortex of patients with
intractable epilepsy. Neurochem Res. 34:1594–1602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramnarain DB, Park S, Lee DY, Hatanpaa KJ,
Scoggin SO, Otu H, Libermann TA, Raisanen JM, Ashfaq R, Wong ET, et
al: Differential gene expression analysis reveals generation of an
autocrine loop by a mutant epidermal growth factor receptor in
glioma cells. Cancer Res. 66:867–874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Marco C, Laudanna C, Rinaldo N,
Oliveira DM, Ravo M, Weisz A, Ceccarelli M, Caira E, Rizzuto A,
Zoppoli P, et al: Specific gene expression signatures induced by
the multiple oncogenic alterations that occur within the
PTEN/PI3K/AKT pathway in lung cancer. PLoS One. 12:e01788652017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YW, Li WM, Wu WJ, Chai CY, Chang TY,
Sun Y, Cheng CJ, Shiue YL, Su SJ, Cheng HL, et al: Epithelial
membrane protein 2 is a prognostic indictor for patients with
urothelial carcinoma of the upper urinary tract. Am J Pathol.
183:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Humphrey PA, Moch H, Cubilla AL, Ulbright
TM and Reuter VE: The 2016 WHO Classification of Tumours of the
Urinary System and Male Genital Organs-Part B: Prostate and bladder
tumours. Eur Urol. 70:106–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Demirag GG, Kefeli M, Kemal Y and Yucel I:
Epithelial membrane protein 1 expression in ovarian serous tumors.
Oncol Lett. 11:2140–2144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li B and Dewey CN: RSEM: Accurate
transcript quantification from RNA-Seq data with or without a
reference genome. BMC Bioinformatics. 12:3232011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Team R: Core. A language and environment
for statistical computing. R Foundation for Statistical Computing.
2014.
|
|
28
|
Yoshihara K, Shahmoradgoli M, Martínez E,
Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW,
Levine DA, et al: Inferring tumour purity and stromal and immune
cell admixture from expression data. Nat Commun. 4:26122013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ru B, Wong CN, Tong Y, Zhong JY, Zhong
SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, et al: TISIDB: An
integrated repository portal for tumor-immune system interactions.
Bioinformatics. 35:4200–4202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peter S, Borkowska E, Drayton RM, Rakhit
CP, Noon A, Chen W and Catoo JW: Identification of differentially
expressed long noncoding RNAs in bladder cancer. Clin Cancer Res.
20:5311–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ben-Porath I, Kozak CA and Benvenisty N:
Chromosomal mapping of Tmp (Emp1), Xmp (Emp2), and Ymp (Emp3),
genes encoding membrane proteins related to Pmp22. Genomics.
49:443–447. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Medvedev A, Ruzanov P, Marvin KW
and Jetten AM: cDNA cloning, genomic structure, and chromosome
mapping of the human epithelial membrane protein CL-20 gene (EMP1),
a member of the PMP22 family. Genomics. 41:40–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahmat Amin MKB, Shimizu A and Ogita H: The
pivotal roles of the epithelial membrane protein family in cancer
invasiveness and metastasis. Cancers (Basel). 11:112019. View Article : Google Scholar
|
|
37
|
Da Y and Jia J: Study of antibodies to
PMP22, IL-6 and TNF-alpha concentrations in serum in a CMTX1
family. Neurosci Lett. 424:73–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gabriel CM, Gregson NA and Hughes RA:
Anti-PMP22 antibodies in patients with inflammatory neuropathy. J
Neuroimmunol. 104:139–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kornberg LJ, Villaret D, Popp M, Lui L,
McLaren R, Brown H, Cohen D, Yun J and McFadden M: Gene expression
profiling in squamous cell carcinoma of the oral cavity shows
abnormalities in several signaling pathways. Laryngoscope.
115:690–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuriakose MA, Chen WT, He ZM, Sikora AG,
Zhang P, Zhang ZY, Qiu WL, Hsu DF, McMunn-Coffran C, Brown SM, et
al: Selection and validation of differentially expressed genes in
head and neck cancer. Cell Mol Life Sci. 61:1372–1383. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hippo Y, Yashiro M, Ishii M, Taniguchi H,
Tsutsumi S, Hirakawa K, Kodama T and Aburatani H: Differential gene
expression profiles of scirrhous gastric cancer cells with high
metastatic potential to peritoneum or lymph nodes. Cancer Res.
61:889–895. 2001.PubMed/NCBI
|
|
42
|
Wei Q, Li M, Fu X, Tang R, Na Y, Jiang M
and Li Y: Global analysis of differentially expressed genes in
androgen-independent prostate cancer. Prostate Cancer Prostatic
Dis. 10:167–174. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arslan AA, Gold LI, Mittal K, Suen TC,
Belitskaya-Levy I, Tang MS and Toniolo P: Gene expression studies
provide clues to the pathogenesis of uterine leiomyoma: New
evidence and a systematic review. Hum Reprod. 20:852–863. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang J, Cao W, Xu Q and Chen WT: The
expression of EMP1 is downregulated in oral squamous cell carcinoma
and possibly associated with tumour metastasis. J Clin Pathol.
64:25–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lai S, Wang G, Cao X, Li Z, Hu J and Wang
J: EMP-1 promotes tumorigenesis of NSCLC through PI3K/AKT pathway.
J Huazhong Univ Sci Technolog Med Sci. 32:834–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Ht: Lu YcandHe J. Expression of
epithelial membrane protein 1 in human gliomas and its clinical
implications. Zhongguo Zhongliu Shengwu Zhiliao Zazhi. 14:466–470.
2007.
|
|
47
|
Turashvili G, Bouchal J, Baumforth K, Wei
W, Dziechciarkova M, Ehrmann J, Klein J, Fridman E, Skarda J,
Srovnal J, et al: Novel markers for differentiation of lobular and
ductal invasive breast carcinomas by laser microdissection and
microarray analysis. BMC Cancer. 7:552007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mackay A, Jones C, Dexter T, Silva RL,
Bulmer K, Jones A, Simpson P, Harris RA, Jat PS, Neville AM, et al:
cDNA microarray analysis of genes associated with ERBB2 (HER2/neu)
overexpression in human mammary luminal epithelial cells. Oncogene.
22:2680–2688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lobsiger CS, Magyar JP, Taylor V, Wulf P,
Welcher AA, Program AE and Suter U: Identification and
characterization of a cDNA and the structural gene encoding the
mouse epithelial membrane protein-1. Genomics. 36:379–387. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang HT, Kong JP, Ding F, Wang XQ, Wang
MR, Liu LX, Wu M and Liu ZH: Analysis of gene expression profile
induced by EMP-1 in esophageal cancer cells using cDNA Microarray.
World J Gastroenterol. 9:392–398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ruegg CL, Wu HY, Fagnoni FF, Engleman EG
and Laus R: B4B, a novel growth-arrest gene, is expressed by a
subset of progenitor/pre-B lymphocytes negative for cytoplasmic
mu-chain. J Immunol. 157:72–80. 1996.PubMed/NCBI
|
|
52
|
Wang YW, Cheng HL, Ding YR, Chou LH and
Chow NH: EMP1, EMP 2, and EMP3 as novel therapeutic targets in
human cancer. Biochim Biophys Acta Rev Cancer. 1868:199–211. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Durgan J, Tao G, Walters MS, Florey O,
Schmidt A, Arbelaez V, Rosen N, Crystal RG and Hall A: SOS1 and Ras
regulate epithelial tight junction formation in the human airway
through EMP1. EMBO Rep. 16:87–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Morales SA, Telander DG, Mareninov S, Nagy
A, Wadehra M, Braun J and Gordon LK: Anti-EMP2 diabody blocks
epithelial membrane protein 2 (EMP2) and FAK mediated collagen gel
contraction in ARPE-19 cells. Exp Eye Res. 102:10–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wadehra M, Forbes A, Pushkarna N,
Goodglick L, Gordon LK, Williams CJ and Braun J: Epithelial
membrane protein-2 regulates surface expression of alphavbeta3
integrin in the endometrium. Dev Biol. 287:336–345. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Morales SA, Mareninov S, Wadehra M, Zhang
L, Goodglick L, Braun J and Gordon LK: FAK activation and the role
of epithelial membrane protein 2 (EMP2) in collagen gel
contraction. Invest Ophthalmol Vis Sci. 50:462–469. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wadehra M, Goodglick L and Braun J: The
tetraspan protein EMP2 modulates the surface expression of
caveolins and glycosylphosphatidyl inositol-linked proteins. Mol
Biol Cell. 15:2073–2083. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ayari C, LaRue H, Hovington H, Caron A,
Bergeron A, Têtu B, Fradet V and Fradet Y: High level of mature
tumor-infiltrating dendritic cells predicts progression to muscle
invasion in bladder cancer. Hum Pathol. 44:1630–1637. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pichler R, Fritz J, Zavadil C, Schäfer G,
Culig Z and Brunner A: Tumor-infiltrating immune cell
subpopulations influence the oncologic outcome after intravesical
Bacillus Calmette-Guérin therapy in bladder cancer. Oncotarget.
7:39916–39930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kawahara T, Furuya K, Nakamura M, Sakamaki
K, Osaka K, Ito H, Ito Y, Izumi K, Ohtake S, Miyoshi Y, et al:
Neutrophil-to-lymphocyte ratio is a prognostic marker in bladder
cancer patients after radical cystectomy. BMC Cancer. 16:1852016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Peng D, Gong YQ, Hao H, He ZS, Li XS,
Zhang CJ and Zhou LQ: Preoperative prognostic nutritional index is
a significant predictor of survival with bladder cancer after
radical cystectomy: A retrospective study. BMC Cancer. 17:3912017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hamilton-Reeves JM, Bechtel MD, Hand LK,
Schleper A, Yankee TM, Chalise P, Lee EK, Mirza M, Wyre H, Griffin
J, et al: Effects of immunonutrition for cystectomy on immune
response and infection rates: A pilot randomized controlled
clinical trial. Eur Urol. 69:389–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Demirer Z and Uslu AU: Predictive value of
neutrophil-lymphocyte ratio in non-muscle-invasive bladder cancer.
Urol Oncol. 34:1–2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou L, Xu L, Chen L, Fu Q, Liu Z, Chang
Y, Lin Z and Xu J: Tumor-infiltrating neutrophils predict benefit
from adjuvant chemotherapy in patients with muscle invasive bladder
cancer. OncoImmunology. 6:e12932112017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang Q, Hao C, Cheng G, Wang L, Wang X,
Li C, Qiu J and Ding K: High CD4+ T cell density is associated with
poor prognosis in patients with non-muscle-invasive bladder cancer.
Int J Clin Exp Pathol. 8:11510–11516. 2015.PubMed/NCBI
|
|
66
|
Pfannstiel C, Strissel PL, Chiappinelli
KB, Sikic D, Wach S, Wirtz RM, Wullweber A, Taubert H, Breyer J,
Otto W, et al BRIDGE Consortium Germany; BRIDGE Consortium Germany;
BRIDGE Consortium Germany; BRIDGE Consortium Germany, : The Tumor
Immune Microenvironment Drives a Prognostic Relevance That
Correlates with Bladder Cancer Subtypes. Cancer Immunol Res.
7:923–938. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wu K, Tan MY, Jiang JT, Mu XY, Wang JR,
Zhou WJ, Wang X, Li MQ, He YY and Liu ZH: Cisplatin inhibits the
progression of bladder cancer by selectively depleting G-MDSCs: A
novel chemoimmunomodulating strategy. Clin Immunol. 193:60–69.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Smith SG, Baltz JL, Koppolu BP,
Ravindranathan S, Nguyen K and Zaharoff DA: Immunological
mechanisms of intravesical chitosan/interleukin-12 immunotherapy
against murine bladder cancer. OncoImmunology. 6:e12590502016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Horn T, Laus J, Seitz AK, Maurer T, Schmid
SC, Wolf P, Haller B, Winkler M, Retz M, Nawroth R, et al: The
prognostic effect of tumour-infiltrating lymphocytic subpopulations
in bladder cancer. World J Urol. 34:181–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Winerdal ME, Krantz D, Hartana CA,
Zirakzadeh AA, Linton L, Bergman EA, Rosenblatt R, Vasko J,
Alamdari F, Hansson J, et al: Urinary bladder cancer tregs suppress
MMP2 and potentially regulate invasiveness. Cancer Immunol Res.
6:528–538. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Q, Chang W, Yang X, Cheng Y, Zhao X,
Zhou L, Li J, Li J and Zhang K: Levels of miR-31 and its target
genes in dermal mesenchymal cells of patients with psoriasis. Int J
Dermatol. 58:198–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pan L, Yang H, Tang W, Xu C, Chen S, Meng
Z, Li K and Chen H: Pathway-focused PCR array profiling of CAL-27
cell with over-expressed ZNF750. Oncotarget. 9:566–575. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang Y, Chaudhri G, Jackson RJ and
Karupiah G: IL-12p40 and IL-18 play pivotal roles in orchestrating
the cell-mediated immune response to a poxvirus infection. J
Immunol. 183:3324–3331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kehrmann J, Effenberg L, Wilk C, Schoemer
D, Ngo Thi Phuong N, Adamczyk A, Pastille E, Scholtysik R,
Klein-Hitpass L, Klopfleisch R, et al: Depletion of Foxp3+
regulatory T cells is accompanied by an increase in the relative
abundance of Firmicutes in the murine gut microbiome. Immunology.
159:344–353. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sperk M, Domselaar RV and Neogi U: Immune
checkpoints as the immune system regulators and potential
biomarkers in HIV-1 infection. Int J Mol Sci. 19:192018. View Article : Google Scholar
|
|
76
|
Chan AW, Zhang Z, Chong CC, Tin EK, Chow C
and Wong N: Genomic landscape of lymphoepithelioma-like
hepatocellular carcinoma. J Pathol. 249:166–172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yoshikawa T, Nakatsugawa M, Suzuki S,
Shirakawa H, Nobuoka D, Sakemura N, Motomura Y, Tanaka Y, Hayashi S
and Nakatsura T: HLA-A2-restricted glypican-3 peptide-specific CTL
clones induced by peptide vaccine show high avidity and
antigen-specific killing activity against tumor cells. Cancer Sci.
102:918–925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Germeau C, Ma W, Schiavetti F, Lurquin C,
Henry E, Vigneron N, Brasseur F, Lethé B, De Plaen E, Velu T, et
al: High frequency of antitumor T cells in the blood of melanoma
patients before and after vaccination with tumor antigens. J Exp
Med. 201:241–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Takashima Y, Kawaguchi A, Sato R, Yoshida
K, Hayano A, Homma J, Fukai J, Iwadate Y, Kajiwara K, Ishizawa S,
et al: Differential expression of individual transcript variants of
PD-1 and PD-L2 genes on Th-1/Th-2 status is guaranteed for
prognosis prediction in PCNSL. Sci Rep. 9:100042019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhou G, Sprengers D, Boor PPC, Doukas M,
Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J,
Gaspersz M, et al: Antibodies against immune checkpoint molecules
restore functions of tumor-infiltrating T cells in hepatocellular
carcinomas. Gastroenterology. 153:1107–1119e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kim JY, Lee E, Park K, Park WY, Jung HH,
Ahn JS, Im YH and Park YH: Immune signature of metastatic breast
cancer: Identifying predictive markers of immunotherapy response.
Oncotarget. 8:47400–47411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Flecken T, Schmidt N, Hild S, Gostick E,
Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, et
al: Immunodominance and functional alterations of tumor-associated
antigen-specific CD8+ T-cell responses in hepatocellular carcinoma.
Hepatology. 59:1415–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|