Introduction
Identification of two or more primary lung
carcinomas at presentation is not uncommon, with an incidence
ranging from 5.7–11.5% between 1985–2002 (1–5).
Specifically, a diagnosis of multiple primary lung adenocarcinomas
was observed in up to 8% of 369 patients who underwent pulmonary
resection for adenocarcinoma between 1994–2002 (1,2).
However, as imaging techniques have improved, the incidence of
multiple lung cancer (MLC) has increased. Distinguishing multiple
primary lung cancer (MPLC) from intrapulmonary metastasis (IPM) may
assist in predicting the outcome and appropriate treatment of
patients with this disease (6).
However, classifying multiple lung nodules as MPLC or IPM remains
challenging using histological typing alone.
Previously, novel diagnostic criteria have been
developed to determine whether intrapulmonary polynodules are
primary tumors or IPM (7). According
to the most recent 8th edition American Joint Committee on Cancer
staging manual (AJCC staging manual) (8); i) two or more distinct and
histologically different masses were considered MPLC; ii) multiple
ground-glass or part-solid nodules, histologically of with lepidic
growth pattern were considered MPLC and iii) multiple tumor nodules
with the same histological type and/or with same molecular profile
were considered IPM (6,7). Genomic alterations were commonly
assessed using fluorescence in-situ hybridization and NGS (6,9). These
criteria have emphasized the need for a combination of diagnostic
approaches, including clinical, histopathological and molecular
diagnoses. However, to the best of our knowledge, there is still no
universally accepted standard diagnostic method for patients with
MLC. Previously, studies have reported that the use of next
generation sequencing (NGS) may be a promising tool for patient
diagnosis based on the hypothesis that clonally related (IPM) and
independent tumors (MPLC) exert different patterns of mutational
concordance (10–12). In theory, the simultaneous
identification of multiple tumor driver genes should enable
improved distinction between MPLC and IPM (13,14).
Xiao et al (14), utilized
NGS to investigate non-small cell lung cancer as this cancer type
often demonstrates genetic heterogeneity. In their cohort, 1 of the
6 patients with similar comprehensive histological assessment
results and EGFR mutation type was identified as having different
gene mutation types via NGS, revealing that the patient had
synchronous multiple primary lung adenocarcinomas (MPLA). In
addition, in a study by Li et al (12), 20 paired tumors (Each patient had two
tumors considered as a pair) obtained from 20 patients with
synchronous multifocal lung adenocarcinomas were analyzed using
NGS. The results revealed no discordance of mutational status in
all tumor pairs diagnosed as intrapulmonary metastasis via
histological examination, whereas the discordance rate was as high
as 61.5% (8 out of 13) in tumor pairs diagnosed as equivocal or
multiple primary cancer tumors. Li et al (12), hypothesized that the mutational
status of all multifocal tumors may aid the diagnosis and selection
of the most effective treatment strategies. Donfrancesco et
al (9), demonstrated that
pathological criteria were less accurate compared with molecular
criteria when staging MLC. However, pathological criteria can be
used in conjunction with molecular analysis, although this method
is not ideal as NGS is not available everywhere (9). In addition, a diagnostic lineage test
based on genomic rearrangements from mate-pair sequencing has been
previously applied for distinguishing independent primary from
metastatic lung cancer (14,15). Therefore, the screening and function
of these diagnostic markers requires further study. The aim of the
present study was to probe and analyze genetic differences between
patients with MPLC and IPM using NGS. The results may help identify
novel biomarkers for the classification of MLC.
Materials and methods
Patients
A total of 17 patients were diagnosed with MLC
through surgical resection at the Beijing Chao Yang Hospital
affiliated to Capital Medical University (Beijing, China) between
January 2017 and December 2018. The median age of the patients was
58 years (age range, 45–81 years), 4 men and 13 women were
included. All these patients were diagnosed as primary lung
carcinoma by pathologists with complete clinical data, including
preoperative examination, postoperative treatment and follow-up
data. Recurrent cases of lung cancer, incomplete clinical data and
lack of postoperative treatment and follow-up records were
excluded. Two experienced pathologists reviewed all cases
independently, blindly and simultaneously. Lobectomy and wedge
resections were performed for seven patients, wedge resections for
six patients and lobectomy resections for four patients. Of the MLC
cases, 7 were adenocarcinoma (ADC) and adenocarcinoma in
situ (AIS), 2 were ADC and squamous cell carcinoma (SCC), 6
were ADC and ADC, and 2 cases were diagnosed with multiple AIS
(Table I). A total of 35 resected
pulmonary nodules and 17 matched normal tissue samples were
obtained from the 17 patients. A total of 16/17 patients each had 2
nodules, one patient had 3 nodules, one normal lung tissue sample
was selected from each patient as the control for NGS. The present
study was approved by the Medical Ethics Committee of Beijing
Chaoyang Hospital, Capital Medical University (Beijing, China)
(approval no. 2018-Scientific-311). A patient who came from Beijing
signed the informed written consent as a representative. The
remaining 16 patients were from other provinces of China, thus
these patients were contacted via telephone to obtain verbal
informed consent before participation in the present study.
 | Table I.Genetic alterations and predominant
patterns of each tumor obtained in the present study. |
Table I.
Genetic alterations and predominant
patterns of each tumor obtained in the present study.
| Patient no. | Tumor location | Histological
type | Predominant
histological pattern | Gene
alterations | Final
diagnosis |
|---|
| 1 | RLL | AIS | Lepidic growth
pattern | NRAS p.Q61L; BRAF
p.N581S | MPLC |
|
| RLL | ADC | Acinar | BRAF p.V600E |
|
| 2 | RUL | AIS | Lepidic growth
pattern | EGFR p.L858R | MPLC |
|
| RML | ADC | Acinar | EGFR p.L858R; TP53
p.V272L |
|
| 3 | LUL | AIS | Lepidic growth
pattern | EPHA3 p.K889N; MET
p.K1132T; TP53 p.V172F; MET c.2888-24_2888-8del; RB1 p.G449E | MPLC |
|
| LUL | ADC | Lepidic 70%, Acinar
30% | RB1 p.G449E; MET
c.2888-24_2888-8del; MET p.D1010Y EPHA3 p.K889N; TP53 p.V172F |
|
|
| LUL | ADC | Lepidic 75%, Acinar
10%, Papillary 15% | APC p.E538K; EGFR
p.L861Q |
|
| 4 | LUL | ADC | Lepidic | EGFR
p.E746_A750del | MPLC |
|
| LUL | AIS | Lepidic growth
pattern | EGFR p.L858R |
|
| 5 | LLL | AIS | Lepidic growth
pattern | EGFR p.L858R; FGFR3
cn_del | MPLC |
|
| LLL | ADC | Lepidic | EGFR p.L858R; ERBB2
cn_amp |
|
| 6 | RLL | AIS | Lepidic growth
pattern | EGFR p.L858R | MPLC |
|
| RUL | ADC | Acinar | TP53 p.E287; EGFR
p.L858R |
|
| 7 | RLL | AIS | Lepidic growth
pattern | EGFR p.L858R | MPLC |
|
| RUL | ADC | Acinar | FGF3 p.N158=;
SMARCA4 cn_amp; PIK3CA p.E545K EGFR p.L747_P753delinsS; MYC
p.E432K |
|
| 8 | LLL | ADC | Acinar | CCNE1 p.V352I; TP53
c.994-1G>A; SMAD4 p.R380_G386delinsS; EGFR cn_amp; EGFR p.L858R;
PIK3R1 NA; FGFR1 NA | IPM |
|
| LUL | ADC | Acinar | BRINP3 p.S470=;
CCNE1 p.V352I; TP53 c.994-1G>A; SMAD4 p.R380_G386delinsS; EGFR
cn_amp; EGFR p.L858R |
| 9 | RLL | ADC | Acinar | EMSY p.T288R; EGFR
p.L858R; TP53 p.S241C; CDKN2A p.Y44fs | IPM |
|
| RUL | ADC | Acinar | EMSY p.T288R; TP53
p.S241C; EGFR p.L858R; EGFR cn_amp |
|
| 10 | RUL | ADC | Lepidic 50%, Acinar
40%, Papillary 10% | EGFR p.L858R; TP53
p.R249S | IPM |
|
| RML | ADC | Lepidic 70%, Acinar
30% | EGFR p.L858R; TP53
p.R249S |
|
| 11 | LLL | ADC | Acinar | TP53 p.E224D; EGFR
p.E746_T751delinsA; EGFR cn_amp | IPM |
|
| LUL | ADC | Lepidic 70%,
Acinar30% | TP53 p.E224D; IL7R
p.C349*; EGFR p.E746_T751delinsA; EGFR cn_amp |
|
| 12 | RLL | AIS | Lepidic growth
pattern | ERBB2
p.Y772_A775dup | MPLC |
|
| RUL | AIS | Lepidic growth
pattern | KRAS p.G12D |
|
| 13 | RUL | AIS | Lepidic growth
pattern | EGFR p.L858R | MPLC |
|
| RUL | AIS | Lepidic growth
pattern | EGFR p.L858R |
|
| 14 | RLL | SCC | Middle-low
differentiation | TP53 c.97-11C>G;
CHEK1 p.S251L; EPHA5 p.L53=; FGFR1 p.L614M; APC p.E1544 | MPLC |
|
| RUL | ADC | Lepidic 70%, Acinar
30% | KRAS p.G13D; STK11
p.E130; EPHA3 p.N866K |
|
| 15 | RUL | ADC | Lepidic | Negative | MPLC |
|
| RML | ADC | Lepidic | EGFR
p.A767_V769dup |
|
| 16 | RUL | ADC | Lepidic | EGFR p.L861Q; MSH2
p.Q264E; EGFR p.G719A | MPLC |
|
| RLL | ADC | Acinar | EGFR
p.E746_A750del; PIK3CA p.H1047L |
|
| 17 | RUL | ADC | Acinar | BRAF p.V600E | MPLC |
|
| RUL | SCC | Middle-low
differentiation | RET c.1264-1G>T;
FGF19 cn_amp; FGF4 cn_amp; FGF3 cn_amp; CCND1 cn_amp; PIK3CA
cn_amp; SOX2 cn_amp; FBXW7 p.R393=; TP53 p.R273L; PMS2 p.M136V;
PMS2 p.G132=; CDKN2A p.R22P; CARD11 p.E1096=; TRIM58 p.P297= |
|
Targeted DNA sequencing of resected
pulmonary nodules
All commercial kits were used according to the
manufacturer's protocols. Total DNA was extracted from
formalin-fixed paraffin-embedded (FFPE) tissue samples (including
35 cancer tissue samples and 17 matched normal tissue samples),
using the QIAamp DNA FFPE Tissue kit (buffer ATL, buffer AL, buffer
AW1, buffer AW2, buffer ATE and proteinase K; Qiagen, Inc.). DNA
concentration was subsequently measured using a Qubit dsDNA assay
(Thermo Fisher Scientific, Inc.). The quality of genomic DNA was
assessed and samples with an A260/A280 ratio of 1.8–2.0 were
selected for subsequent analysis. DNA was profiled using a Lung
Plasma panel (Guangzhou Burning Rock Medical Laboratory Co., Ltd.),
which included 168 cancer-associated genes. The concentration of
DNA within samples was measured using a Qubit dsDNA assay (Thermo
Fisher Scientific, Inc.), the results of which determined that all
samples contained >40 ng DNA. Subsequently, 200–400 bp fragments
were selected for analysis using the Agencourt AMPure XP kit
(Beckman Coulter, Inc.), and the sequencing libraries were prepared
using the NEBNext Ultra II DNA Library Prep kit for Illumina (cat.
no. E7645), according to the manufacturer's protocols. A
bioanalyzer high-sensitivity DNA assay (Qubit 2.0; Thermo Fisher
Scientific, Inc.) was performed to assess the quality and size of
DNA samples. Available indexed samples were sequenced using a MiSeq
system (Illumina, Inc.) with paired end reads.
Sequencing analysis
Sequencing data were mapped to the human genome
(hg19) using the BWA aligner version 0.7.10 (http://bio-bwa.sourceforge.net). PCR duplicate reads
were removed prior to the detection of base substitution. Local
alignment optimization and variant calling were performed using
GATK version 3.2–2 (Broad Institute, Inc.). DNA translocation
analysis was performed using Tophat2 (Center for Computational
Biology, Johns Hopkins University and the Genome Sciences
Department) and Factera version 1.4.3 (https://factera.stanford.edu). Insert size
distribution and the library complexity of each sample was
determined to assess levels of DNA degradation. Different mutation
calling thresholds were applied to samples with differing levels of
DNA quality to avoid false-positive mutation calls due to DNA
damage. Gene variants were filtered using the VarScan (Genome
Institute, Washington University, USA) filter pipeline. Gene
variants loci with a sequencing depth <100 were filtered out. At
least two and five supporting reads were required for
insertions/deletions in FFPE tissue samples, respectively, while
eight supporting reads were required for single number variations
(SNVs) in the samples, and selected exons and introns of 168 genes
were captured. Single nucleotide variants and indels were annotated
using dbNSFP (version 30a; http://varianttools.sourceforge.net/Annotation/dbNSFP),
Catalogue of Somatic Mutations (version 69; http://cancer.sanger.ac.uk/cosmic) and dbSNP (snp138)
databases (ftp.ncbi.nih.gov/snp). Variants with a global minor
allele frequency >1.0% included in the 1000Genome Project (Phase
3; 1000genomes.org/data) were considered to be common single
nucleotide polymorphisms and were removed.
Integrative Genomics Viewer (Broad Institute, Inc.)
(16) was used to visualize variants
aligned against the reference genome to confirm the accuracy of
variant calls by checking for possible strand biases and sequencing
errors. Gene-level copy number variation was assessed using a
statistic after normalizing read depth at each region by total read
number and region size, and correcting for GC-bias using the LOESS
algorithm (https://www.weisang.com/en/documentation/loessandlowessalgorithm).
Tumor cellularity assessment
Tumor cellularity was assessed by two pathologists
in patients with lung cancer. NGS testing was not conducted with
specimens that contained <5% tumor cells and could not be
macro-dissected. Therefore, based on the study by Li et al
(17), a standard of 10% tumor cells
was set. All samples were categorized into three groups based on
the estimated percentage of tumor cells: Group 1, 6–19% tumor
cellularity; group 2, 20–30% tumor cellularity; and group 3,
>30% tumor cellularity. There were 11 specimens in group 1, 4
specimens in group 2 and 20 specimens in group 3.
The heterogeneity scores (HSs) of EGFR and tumor
suppressor protein 53 (TP53) were calculated as described
previously (17,18). A HS of <1 indicated that mutations
were present in a subpopulation of tumor cells; a score of 1
suggested that mutations were present in all tumor cells; and a
score of >1 indicated that copy-number variations may exist in
tumor cells (12).
Identification of tumor suppressor
gene mutations
Whether a tumor had a tumor suppressor gene mutation
or not was determined by the mutation status of TP53,
phosphatidylinositol-3 kinase catalytic subunit α (PIK3CA),
retinoblastoma 1 (RB1) or serine/threonine kinase 11 (STK11).
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 (SPSS Inc.). Continuous variable homogeneity was
analyzed using a two-way independent sample t-tests for normally
distributed data and a non-parametric rank sum test for
non-normally distributed data (Z test was used for comparing EGFR
mutation abundance between ADC group and AIS group, and
Mann-Whitney U test was used for comparing EGFR mutation abundance
between MPLC and IPM). ANOVA was used to test for differences among
≥2 groups followed by Tukey's post hoc test. A Pearson
χ2 test and Fisher's exact test were used to analyze the
observed differences in frequencies. Multivariate logistic
regression analysis was used to evaluate the relative risk of IPM
when compared with MPLC, irrespective of cause, and was expressed
as the odds ratio (OR) with 95% confidence intervals (CIs).
Progression-free survival (PFS) time was analyzed using the
Kaplan-Meier method and survival curves were compared using a
log-rank or Renyi test, as appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
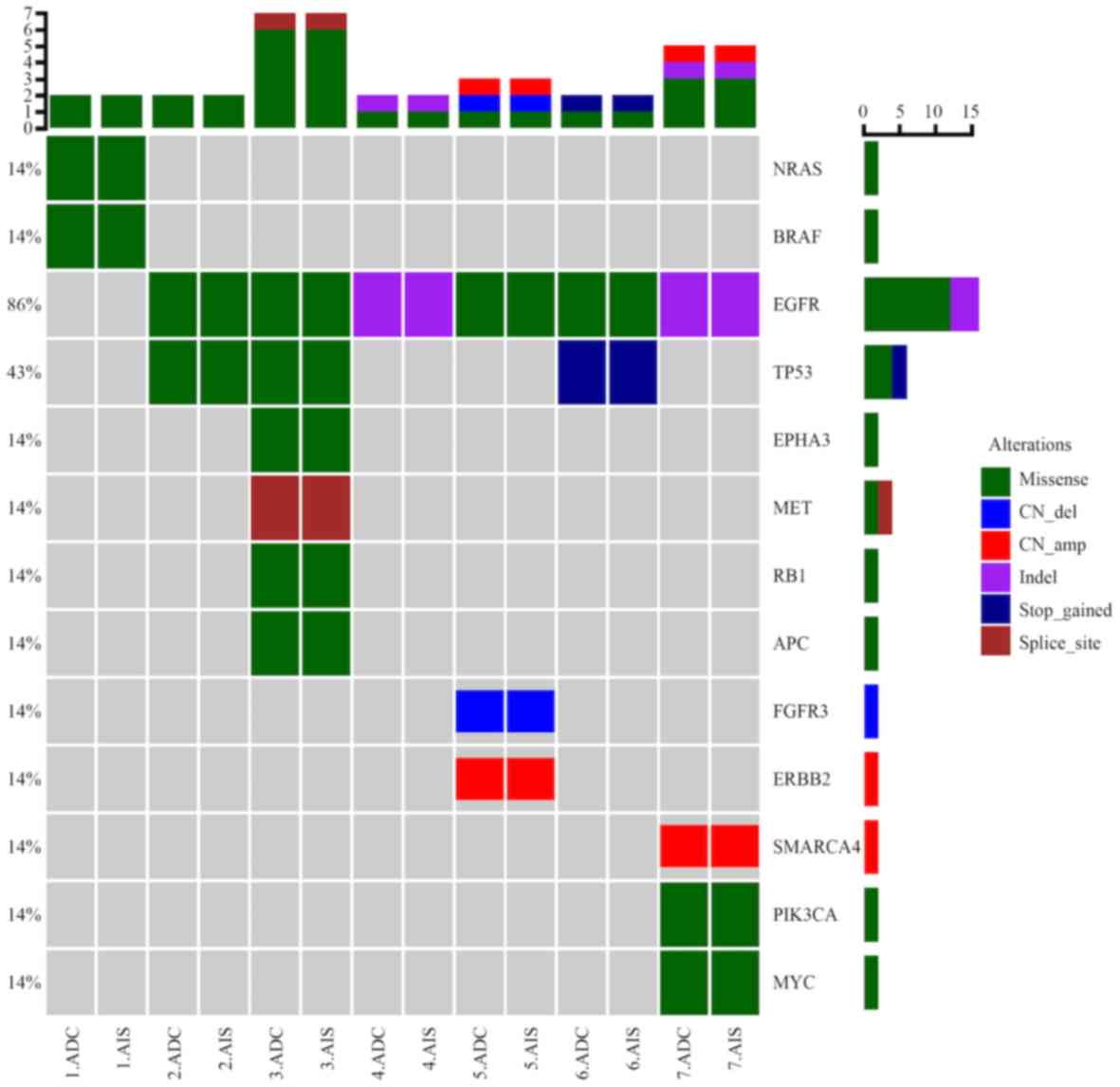
Alterations of genes
Alterations of the EGFR gene were identified in 13
of the 17 patients enrolled (76.5%), presenting in 24 of the
lesions obtained. Among the ADC lesions, nine exhibited exon 21
L858R, two presented with exon 21 L861Q and five lesions exhibited
an exon 19 deletion missense mutation. Additionally, copy number
amplification was identified in five ADC lesions. The results also
revealed EGFR gene exon 21 L858R alterations in seven AIS lesions.
Anaplastic lymphoma kinase (ALK) fusion was not identified in any
sample. Kirsten rat sarcoma (KRAS) mutations were identified in 2
lesions of 2 patients and a neuroblastoma rat sarcoma (NRAS)
mutation was detected in another patient. The rate of KRAS
detection was (2/17, 11.76%). No positive mutations were detected
in normal lung tissue in 17 patients. The detailed genetic changes
of each case are presented in Table
I.
Patients with ADC and AIS
A total of seven lesions in nine patients with AIS
and 17 lesions in 12 patients with ADC were found to exhibit EGFR
mutations. The results of the non-parametric rank sum test
demonstrated that the mean abundance of EGFR mutations in the ADC
group was higher compared with that in the AIS group (Z=−2.845,
P=0.004; data not shown). Analysis of EGFR mutation location in
patients with AIS and ADC (EGFR non-L858R and EGFR L858R) revealed
that the rate of EGFR L858R mutations in AIS was significantly
higher compared with that in ADC (χ2=4.941, P=0.026;
data not shown). There were no significant differences in the
mutation status of any other core driver gene between patients with
AIS and ADC (χ2=0.688, P=0.407).
The mutation status of certain tumor suppressor
genes, including TP53, PIK3CA, RB1 and STK11 were statistically
different in the ADC group compared with the AIS group
(χ2=7.506, P=0.006; data not shown). Of the nine cases
of AIS, 7 were ADC and AIS, and 2 cases were diagnosed with
multiple AIS (Table I). In patients
with AIS, only one tumor exhibited a tumor suppressor gene
mutation, while 10 did not. In patients with ADC, 13 tumors
exhibited a tumor suppressor gene mutation and nine did not. The
positive rate of mutations in the tumor suppressor genes was
assessed, demonstrating that there was a significantly higher
positive rate of tumor suppressor gene mutations in ADC compared
with AIS (χ2=7.506, P=0.006; data not shown).
EGFR status and tumor suppressor gene mutation
analysis in patients with AIS and ADC revealed that no AIS tumor
exhibited simultaneous mutations: Eight exhibited one mutation,
while three did not exhibit a mutation. In ADC lesions, 11 tumors
exhibited simultaneous mutations, eight tumors exhibited one
mutation and three did not present any mutations. Following
statistical analysis, a significant difference was identified
(χ2=8.250, P=0.016; likelihood ratio,
χ2=11.511, P=0.003; data not shown), indicating that
there were more concurrent EGFR and tumor suppressor gene mutations
in ADC lesions compared with AIS lesions.
The results of mean EGFR local mutation abundance
analysis revealed that abundance was significantly higher in ADC
lesions compared with simultaneous AIS lesions (t=4.598, P=0.001;
data not shown). However, no significant differences were
identified in the positioning of EGFR mutations, the mutation
abundance of other core driver genes, mutations in tumor suppressor
genes and EGFR mutations accompanied with mutations in tumor
suppressor genes (Fig. 1).
MPLC diagnosis
MPLC was diagnosed using histological subtyping and
gene alteration analysis. Of the two cases of simultaneous SCC and
ADC, it was demonstrated that each exhibited different histological
types and different driver gene mutation spectra, which was
indicative of multi primary lung cancer. Co-occurrence of AIS and
ADC in seven cases and co-occurrence of AIS in 2 cases were
diagnosed as MPLC (Table I),
according to the AJCC staging manual (7).
Histological subtypes and genetic alteration maps
were compared among 6 patients with multiple ADC lesions. Among
them, two tumors from patient 16 exhibited different histological
subtypes; one tumor exhibited acinar features and one tumor
exhibited lepidic features. Both tumors possessed EGFR mutations at
different positions (the former: EGFR p.L861Q and EGFR p.G719A, the
latter: EGFR p.E746_A750del). In addition, the two tumors from
patient 15 exhibited similar histological subtypes (lepidic), but
one did not present with any genetic changes, while the other
exhibited EGFR driver gene mutations (Table I). As driver gene mutations were
different in the 2 aforementioned patients, the diagnosis of MPLC
was supported.
The other 4 patients with multiple ADC lesions
(patients 8–11) exhibited a similar driver gene mutation spectrum.
EGFR and TP53 were detected in the two tumors of patient number 10;
however, the abundance of each mutation differed. Among the four
patients with multiple ADC lesions, three exhibited similar
histological subtypes, (the histological pattern of all four tumors
in patient numbers 8 and 9 was acinar, in patient number 10, the
lepidic structure accounted for 50%, acinar 40% and papillary 10%
in one tumor, the lepidic structure accounted for 70% and acinar
30% in another tumor), while one exhibited different histological
subtype (in patient number 11, one tumor was dominated by acinar
structure, while in another tumor, lepidic structure and acinar
structure accounted for 70 and 30%, respectively; Table I). Combined with the results of NGS,
these data supported the diagnosis of IPM (Table I).
The results revealed there was ~8% (1/13) of
discordance of mutational status in all tumor pairs diagnosed as
MPLC via histological examination, whereas the discordance rate was
25% (1/4) in tumor pairs diagnosed as intrapulmonary metastasis.
However, there was no statistically significant difference
(P=0.347; Table SI).
Staging
The stage of all 17 patients was confirmed according
to lung cancer staging criteria by AJCC staging manual and NGS
sequencing results. The tumor pairs from 13 MPLC patients were
staged separately. Different histological types for separate tumor
(T), nodal metastases (N) and distant metastases (M) for each
tumor, so the two cases of simultaneous SCC and ADC were separate
T, N and M for each tumor (data not shown). Co-occurrence of AIS
and ADC in seven cases and co-occurrence of AIS in 2 cases were
diagnosed as MPLC, the pathological (p)T stage of the largest tumor
or main lesion was defined as the highest pT stage (data not
shown). IPM usually represented by multiple tumor nodules of the
same histological type and/or molecular profile. A separate tumor
nodule in the same lobe is staged as T3, in the ipsilateral lobe as
T4. A total of four patients with IPM were respectively staged as
pathological T4, two tumors of each patient were both on the same
side (left or right lung), but not in the same lung lobe, so the
four patients with IPM were considered pathological T4 stage
(Table II). Because there were only
a few cases with TNM stage II B, stage III and IV (data not shown),
in order to perform a more reasonable statistical analysis, these
were combined into stage ≥IIB, the rest were stage <IIB
(including Tis, I and IIA; data not shown).
 | Table II.Clinicopathological characteristics
of patients with MPLC and IPM. |
Table II.
Clinicopathological characteristics
of patients with MPLC and IPM.
| Clinicopathological
characteristic | MPLC (n=13) | IPM (n=4) | Test value | P-value |
|---|
| Age, mean ±
standard deviation (min, max) | 61.96±8.97 (47,
81) | 55.75±13.89 (49,
76) | t=1.16 | 0.27 |
| Mean tumor size, cm
(min, max) |
|
| t=−0.41 | 0.68 |
|
ADC | 1.79 (0.5,
4.0) | 2.50 (1.0,
5.0) |
|
|
|
AIS | 0.88 (0.5,
1.7) |
|
|
|
| Sex |
|
|
χ2=2.04 | 0.15 |
|
Female | 11 | 2 |
|
|
|
Male | 2 | 2 |
|
|
| Smoker |
|
|
χ2=1.12 | 0.29 |
|
Yes | 3 | 0 |
|
|
| No | 10 | 4 |
|
|
| Stage |
|
|
χ2=5.86 | 0.02 |
|
<IIB | 9 | 0 |
|
|
|
≥IIB | 4 | 4 |
|
|
Comparison of patient characteristics
between MPLC and IPM groups
Clinicopathological characteristics of patients with
MPLC and IPM are listed in Table
II, there were no statistical differences in age, sex, tumor
size and smoking status between the two groups (all P>0.05),
while the tumor TNM stage was significantly higher in the IPM group
compared with the MPLC group (P<0.05). Genetic changes of
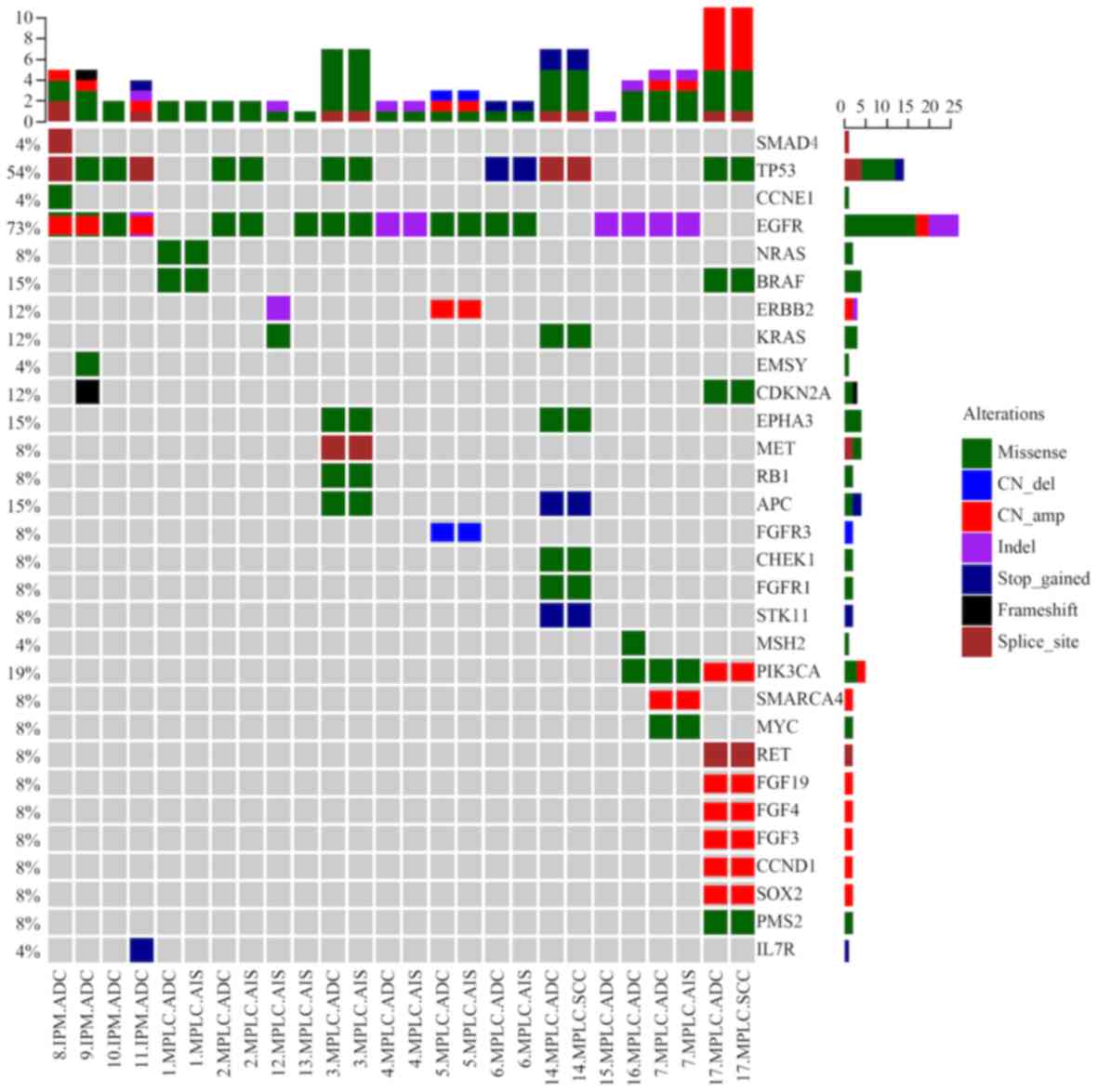
patients with MPLC and IPM are presented in Fig. 2 and the specific comparisons are
listed in Table III. There were 27
tumors in the MPLC group, among which 16 had EGFR mutations. In the
present study, significant differences in EGFR mutation abundance
were detected when comparing patients with MPLC and IPM, the mean
abundance of EGFR mutations was significantly higher in the IPM
group compared with the MPLC group (P<0.05). Furthermore, the
incidences of EGFR driver mutations, involvement of tumor
suppressor genes, EGFR accompanied with tumor suppressor gene
mutations were significantly higher in the IPM group compared with
the MPLC group (all P<0.05).
 | Table III.Genetic changes of patients with MPLC
and IPM. |
Table III.
Genetic changes of patients with MPLC
and IPM.
| Mutation
characteristic | MPLC (n=27) | IPM (n=8) | Test value | P-value |
|---|
| EGFR mutation
abundance, mean ± standard deviation | 0.111±0.075 | 0.496±0.278 | U=14 | 0.002 |
| EGFR mutation
site |
|
|
χ2=0.375 | 0.540 |
|
L858R | 10 | 6 |
|
|
|
Non-L858R | 6 | 2 |
|
|
| Driver
mutations |
|
|
χ2=4.753 | 0.029 |
| EGFR
driver mutations | 16 | 8 |
|
|
|
Non-EGFR driver mutations | 11 | 0 |
|
|
| Involvement of
tumor suppressor genes |
|
|
χ2=12.315 | <0.01 |
|
Yes | 8 | 8 |
|
|
| No | 19 | 0 |
|
|
| EGFR accompanied
with tumor suppressor gene mutations |
|
|
χ2=22.626 | <0.01 |
| Both
mutations simultaneously | 3 | 8 |
|
|
| One of
the mutations | 18 | 0 |
|
|
|
None | 6 | 0 |
|
|
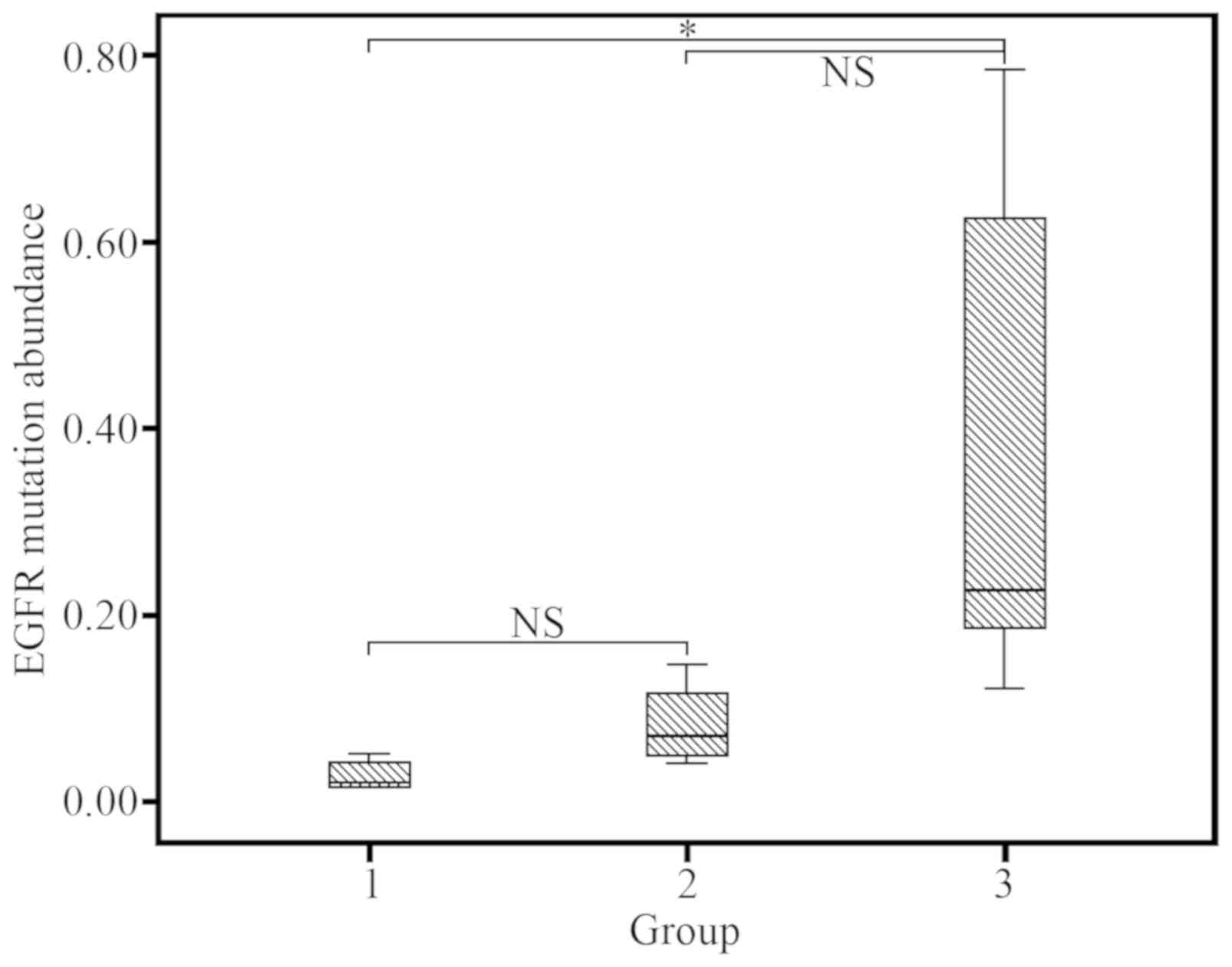
Tumor cellularity
EGFR mutation rates were high in each of the three
groups (group 1, 7/11 64%; group 2, 3/4; 75%); group 3, 14/20,
70%), despite results not being statistically different
(P>0.05). There was no significant difference between group 1
vs. 2 (P=0.904), and group 2 vs. group 3 (P=0.056); however, a
significant difference was observed between group 1 vs. 3 (P=0.006;
Fig. 3).
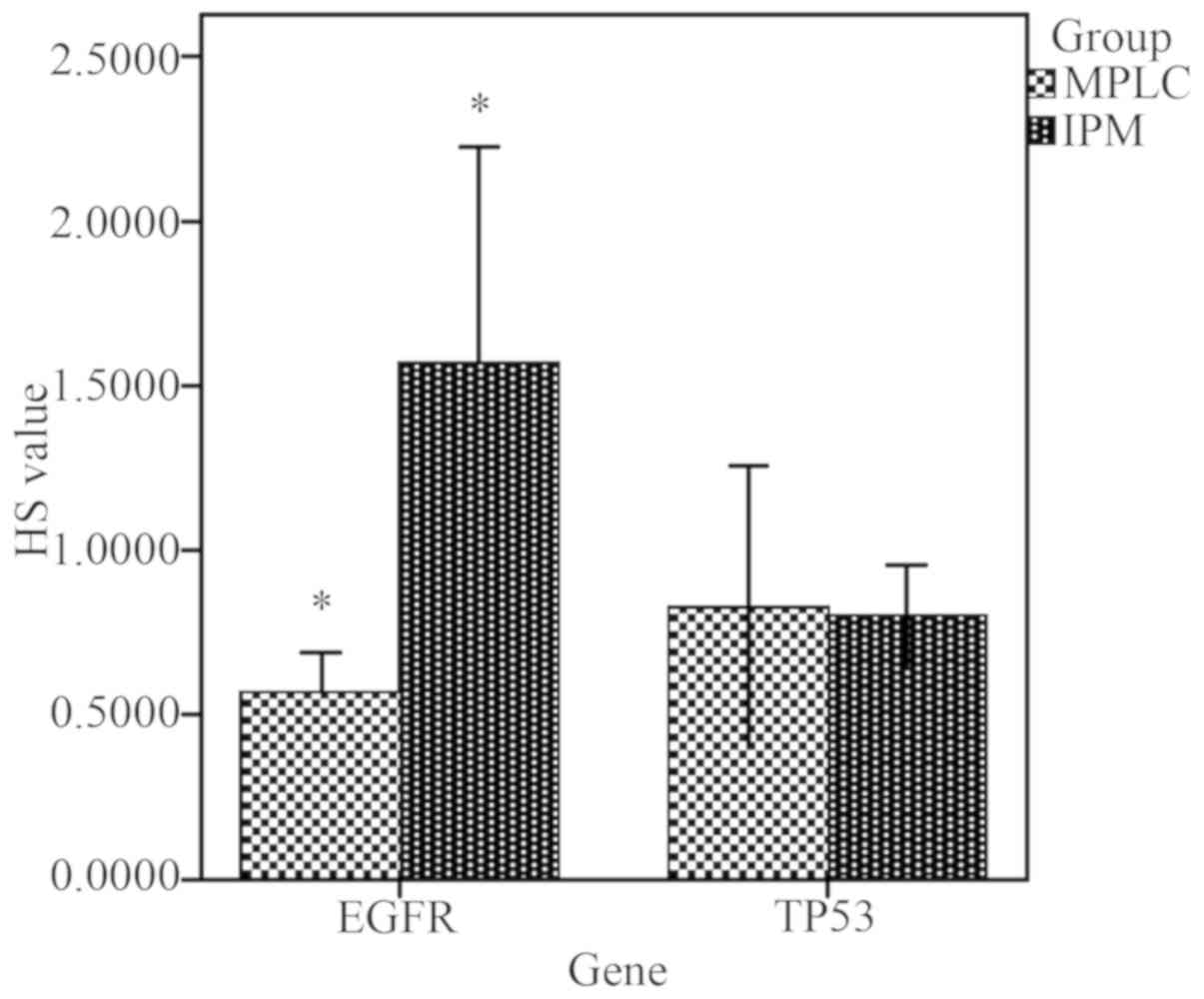
HS calculation and comparison
Among the patients with IPM, the mean EGFR HS value
was significantly higher compared with patients with MPLC (t=3.502;
P=0.009; EGFR HS >1 in IPM), indicating that copy number
variations may exist in tumor cells. However, there was no
statistical difference in the mean HS value of TP53 between the two
groups (t=0.151, P=0.883; HS <1 in both groups; Fig. 4).
Identification of PFS risk factors in
patients with MPLC and IPM
Univariate regression analysis revealed that EGFR
mutation frequency, EGFR HS, TP53 mutation frequency, TP53 HS and
sex were all independent risk factors of IPM (Table SII). Furthermore, multivariate
dichotomous logistic regression analysis demonstrated that EGFR HS
(OR, 760; P=0.024; 95% CI, 2.443–236,548) and the male sex (OR,
355; P=0.048; 95% CI, 1.053–120,267) were risk factors of IPM
(Table SIII). Therefore, a higher
EGFR HS value and the male sex were associated with an increased
risk of IPM.
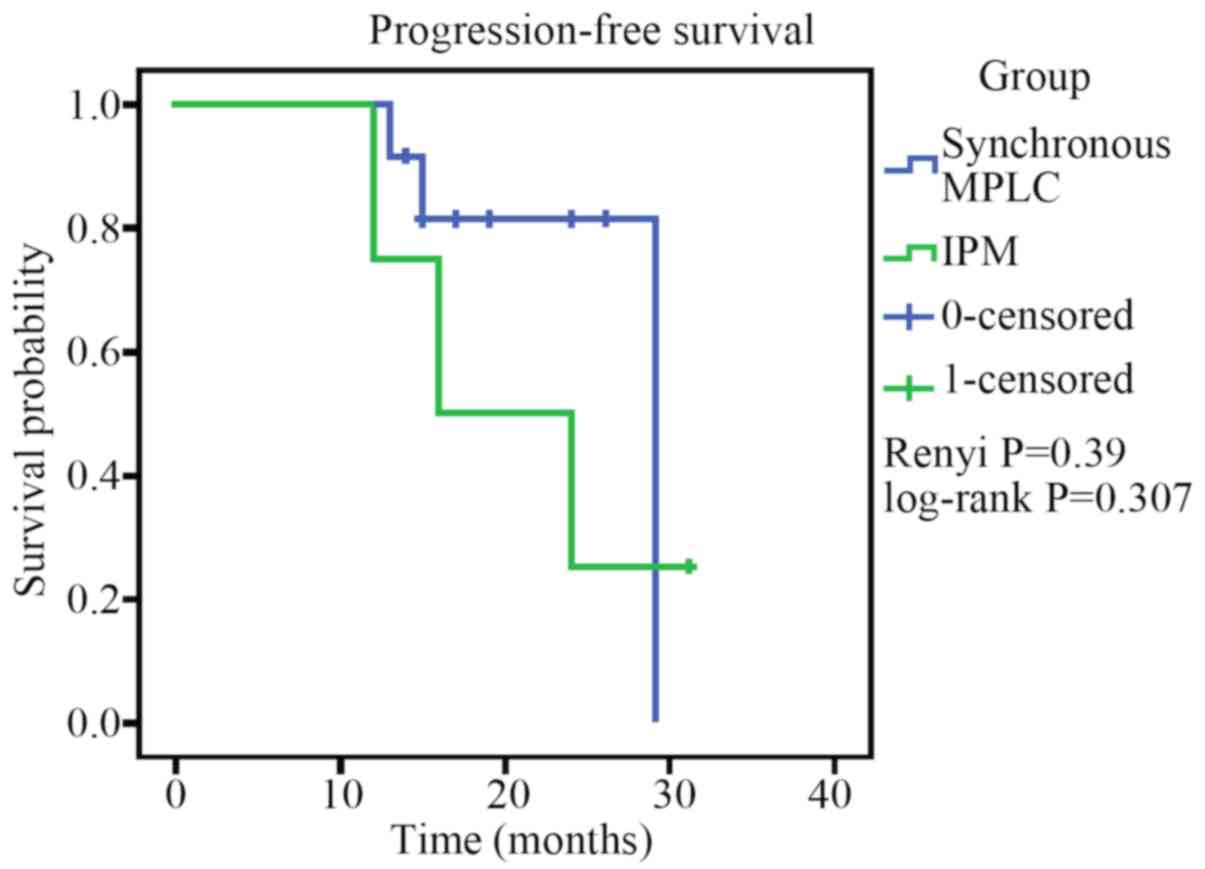
Kaplan-Meier PFS analysis of the 13 patients with
MPLC and the 4 patients with IPM are presented in Fig. 5. The postoperative median PFS time of
the 13 patients with MPLC was 29 months and was 16 months for
patients with IPM. There was no significant difference between the
PFS of patients with MPLC and IPM (Renyi P=0.39; log-rank
P=0.307).
Discussion
Comprehensive histological assessment has provided a
suitable and reliable method for the identification of MPLCs
(19); however, certain patients
with ≥2 lung adenocarcinomas exhibit similar pathological subtypes
(17). It is therefore difficult to
distinguish MLPC from IPM using histology alone. It is now
generally accepted that diagnostic criteria should be based on
clinical, histopathological and molecular data combined (6,11).
Lung cancer may result from the accumulation of
mutations in a branched evolutionary model similar to that of a
growing tree (20–24). However, the comprehensive genomic
landscape of synchronous multifocal lung adenocarcinomas, primarily
those of pre-invasive and invasive lung cancer, have not been
extensively compared. Additionally, to the best of our knowledge,
correlations between specific clinicopathological characteristics,
genes alterations and the prognosis of patients with MPLC or IPM
are yet to be performed.
In the present study, four patients were diagnosed
with IPM, three of which exhibited similar histological subtypes
and genetic changes in each lesion, another one patient exhibited
different histological subtypes but similar genetics changes. These
patients were diagnosed with IPM for two reasons. Firstly, the two
tumors had similar mutations in the main driver gene and thus were
classified as IPM, based on the NGS test results and in combination
with the literature (6,10–12).
Some studies report that the use of NGS appears promising in
addressing this challenge in distinguishing MLPC from IPM, based on
the hypothesis that clonally related (IPM) and independent tumors
(MPLC) exert different patterns of mutational concordance (10–12).
Secondly, one patient had adenocarcinoma with two lesions with
different histological subtypes. Some tumors had morphological
changes after metastasis, and histological subtype changes. Changes
in the morphological subtype of lung adenocarcinoma with distant
metastases are common in clinical practice. Donfrancesco et
al (9) demonstrated that
architectural patterns cannot be used to differentiate multiple
primary tumors from IPM, the lepidic architecture may be noteworthy
to distinguish separate tumor nodules (25) because of the poor repeatability of
the results of the lepidic architecture between multiple observers.
However, additional studies are required to support this
conclusion.
The present results demonstrated that there was ~8%
(1/13) discordance of mutational status in all tumor pairs
diagnosed as MPLC via histological examination, whereas the
discordance rate was 25% (1/4) in tumor pairs diagnosed as IPM.
However, this difference was not significantly different (P=0.347;
Table SI). It has been reported
that discrepancy between clinical and molecular classification of
originally presumed cases of multiple primary lung cancer tumors
range between 18 and 30% in different study cohorts (26,27).
Therefore, histological characteristics used in combination with
genetic alteration analysis may be an effective method to diagnose
MPLC and IPM. It was hypothesized that the mutational status of all
multifocal tumors may aid the diagnosis and selection of the most
effective treatment strategies.
In the present study, EGFR gene alterations were
identified in 13/17 patients (76.5%), which included 24 lesions.
Xiao et al (14) observed
EGFR mutations in 64 tumors obtained from 35/37 patients (94.6%)
with synchronous MPLAs. Further studies have revealed higher EGFR
mutation frequencies compared with reported rates of single lung
adenocarcinoma mutations, ranging between 11 and 51.4% (28–32). In
the present study, significant differences in EGFR mutation
abundance were detected when comparing patients with MPLC and IPM
(Table III). High rates of EGFR
mutations and EGFR abundance may be a result of gene alterations in
patients with IPM, therefore aiding the differentiation between
MPLC and IPM. However, a larger sample size is required to confirm
this.
In the present study, all patients with IPM
exhibited EGFR driver mutations, accompanied with a tumor
suppressor gene mutation, which included TP53, PIK3CA, STK11 or RB1
mutations. A recent study that analyzed 17,664 patients with lung
cancer identified 2–3 concomitant driver mutations in ~1% of cases
(33). In another large database
study, the occurrence of EGFR co-mutations with other cancer driver
mutations was ~10% (34). The
results of the present study revealed that the co-mutation rate of
EGFR and tumor suppressor genes in the IPM group was markedly
higher compared with those presented in other studies with single
ADC (29,33). These results may partly explain the
mechanism of intrapulmonary metastasis, in which tumor suppressor
gene mutations may exert a promotive effect. Furthermore,
co-mutations may potentially impair the efficacy of tyrosine kinase
inhibitors (TKIs). For example, it was demonstrated that patients
with EGFR/PIK3CA co-mutations had a less favorable PFS time during
TKI therapy compared with patients with EGFR mutations alone
(33,35). Therefore, prognosis should be
determined to analyze the implications of these alterations. The
present study speculated that the genetic features of the IPM group
may be associated with poor prognosis and TKI resistance. Despite
genetic alterations requiring further confirmation, detecting
genetic alterations may assist in the identification of MPLC or IPM
and the subsequent application of therapy. No ALK gene alterations
were detected in the present study, which is not congruent with
previous results where concurrent EGFR mutations and ALK
rearrangements in lung ADC were frequently observed (36). The lack of ALK rearrangements in the
present study may have been characteristic of MLC, or perhaps the
small cohort size affected the results. This was consistent with
the results of Saab et al (1), who performed NGS and ALK FISH analyses
on 52 lung adenocarcinomas tumors from 18 patients, none of the
tumors harbored ALK gene rearrangements. In the present study, KRAS
mutations were identified in 2 lesions of 2 patients and a NRAS
mutation was detected in another patient. The rate (2/17, 11.76%)
of KRAS detection was lower in the current study compared with that
reported in Asian and Western populations (4–24 and 25%,
respectively) (37). The present
results may therefore be associated with regional divergence and
MLC characteristics.
Hu et al (20), demonstrated genomic evolution from
atypical adenomatous hyperplasia to AIS, minimally invasive
adenocarcinoma and ADC, and suggested that the neoplastic
transformation of lung preneoplasia may be predominantly associated
with the survival of best adapted subclones in a specific
microenvironment. Therefore, in the present study, the differences
and similarities of genetic alterations in patients with AIS and
ADC were analyzed. The results of the non-parametric rank sum test
revealed that the mean EGFR mutation abundance was higher in ADC
group compared with the AIS group (Z=−2.845, P=0.004). In addition,
EGFR mutation location analysis in patients with AIS and ADC (EGFR
non-L858R and L858R) demonstrated that the mutation detection rate
of EGFR L858R in AIS was significantly higher compared with ADC.
The mutation analysis of various tumor suppressor genes, including
TP53, PIK3CA, RB1 and STK11, revealed a significant difference
between the ADC group and the AIS group (χ2=7.506,
P=0.006). Furthermore, a higher proportion of tumor suppressor gene
mutations were observed in the ADC group compared with the AIS
group. Concurrent EGFR and tumor suppressor gene mutations were
significantly increased in the ADC group compared with the AIS
group. Based on these results, the present study hypothesized that
the mean EGFR mutation abundance, mutations of tumor suppressor
genes and each mutation combined may serve an important role in the
development of AIS into ADC. This may help elucidate the mechanisms
underlying lung adenocarcinoma development and identify more
effective targets for disease intervention.
The results of the present study revealed that the
mean EGFR mutation abundance in ADC was significantly higher
compared with AIS. This further indicates the importance of testing
for genetic alterations. The current diagnosis of AIS and ADC is
based on morphological assessment, which may not fully reflect the
underlying pathology of these lesions. In future studies, more
definitive endpoints, such as postsurgical recurrence and overall
survival, should be integrated with molecular markers to improve
the definition of indeterminate pulmonary nodule molecular subtypes
and to assess their prognostic value (20). The present study recommends that
patients with AIS should be genetically tested.
Previously, it was recommended that the proportion
of tumor cells detected by NGS was 10% (17). The tumor cellularity of the majority
of cases in the present study was >10%, and only five specimens
of tumor cellularity were 6–10%. EGFR, KRAS and TP53 mutations were
also detected in patients in the present study. The results
indicated that samples with >6% tumor cellularity may be
screened using NGS. However, there are various limitations to the
present study. Firstly, although tumor cell content was assessed
independently by two pathologists to obtain accurate data, the
estimation of tumor cellularity can be subjective. Secondly,
caution should be taken in analyzing tumor cellularity,
particularly when small sample sizes are used. Thirdly, there were
instances where tumor cellularity was <10%, and whether this
affected detection was not determined. In addition, the majority of
cases of MLC in the present study were lung ADC, and only two cases
were SCC. This may be because ADC accounts for the vast majority of
cases of lung cancer in China (38).
Therefore, the results of the present study do not apply to
patients with SCC. Future studies should be performed with a larger
sample size to further support the conclusions of the present study
and incorporate patients with SCC.
Intratumor heterogeneity is at least partially
responsible for the discordance in mutation status between
different sites of a tumor, and this heterogenicity may be a
challenge for the application of targeted therapies (39). In studies by Li et al
(17) and Normanno et al
(18), HS values were used to assess
intratumor mutational heterogeneity. The results of the current
study indicated that intratumor heterogeneity existed in patients
with MPLC and IPM. The mean HS value of EGFR in patients with IPM
was higher compared with MPLC. Additionally, metastasis in patients
with an EGFR HS >1 indicated that copy number variation may
exist in the metastatic cells of IPM. No significant differences
were identified in the TP53 HS value between the MPLC and IPM, with
each exhibiting values <1, suggesting that mutations were
present in a subpopulation of tumor cells. These results support
the notion that EGFR mutations are more frequent in patients with
IPM (17). Regression analysis
revealed that a higher EGFR HS value and the male sex were
associated with a greater risk of IPM, thus providing a potentially
novel diagnostic marker to distinguish IPM from patients with
MPLC.
In conclusion, the detection of genetic alterations
using NGS may assist the identification of MPLC or IPM.
Additionally, samples with >6% tumor cellularity may be screened
using NGS. Histological characteristics combined with genetic
alterations may be an effective method to identify MPLC and IPM.
MLC may have its own unique molecular characteristics, such as a
higher rate and abundance of EGFR mutations, EGFR driver and tumor
suppressor gene mutations combined, a lack of ALK mutations and a
low rate RAS mutations, all of which may aid the identification of
MPLC from IPM. The present study hypothesizes that mean EGFR
mutation abundance, mutations of tumor suppressor genes and
mutations of each combined may serve an important role in the
development of AIS into ADC. This may help elucidate the mechanism
of lung adenocarcinoma development to identify more effective
measures of therapeutic intervention. Furthermore, the present
study recommends that patients with AIS should be genetically
tested.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81602138) and The
Beijing Hospitals Authority Youth Program (grant no.
QML20180304).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
MJ and JL designed the current study. XC, JL, YW
collected the data, MJ and JL were responsible of pathological
study. YW, YG, YL and HZ performed the experiments preparation. YW
followed up with the patients and prepared the figures and tables.
XJ, YG, YL were responsible for the molecular study. XC, JL and XJ
analyzed and interpreted the data. XC analyzed the statistics and
drafted the initial manuscript. XC, JL and XJ revised the
manuscript. All authors gave final approval for publication. MJ
took full responsibility for the work as a whole, including the
study design, access to data, and the decision to submit and
publish the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Beijing Chaoyang Hospital, Capital Medical University
(Beijing, China; approval no. 2018-Scientific-311). A patient who
came from Beijing signed the informed written consent as a
representative. The remaining 16 patients were from other provinces
of China, thus these patients were contacted via telephone to
obtain verbal informed consent before participation in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saab J, Zia H, Mathew S, Kluk M, Narula N
and Fernandes H: Utility of genomic analysis in differentiating
synchronous and metachronous lung adenocarcinomas from primary
adenocarcinomas with intrapulmonary metastasis. Transl Oncol.
10:442–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakata M, Sawada S, Yamashita M, Saeki H,
Kurita A, Takashima S and Tanemoto K: Surgical treatments for
multiple primary adenocarcinoma of the lung. Ann Thorac Surg.
78:1194–1199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pairolero PC, Williams DE, Bergstralh EJ,
Piehler JM, Bernatz PE and Payne WS: Postsurgical stage I
bronchogenic carcinoma: Morbid implications of recurrent disease.
Ann Thorac Surg. 38:331–338. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martini N, Bains MS, Burt ME, Zakowski MF,
McCormack P, Rusch VW and Ginsberg RJ: Incidence of local
recurrence and second primary tumors in resected stage I lung
cancer. J Thorac Cardiovasc Surg. 109:120–129. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aziz TM, Saad RA, Glasser J, Jilaihawi AN
and Prakash D: The management of second primary lung cancers. A
single centre experience in 15 years. Eur J Cardiothorac Surg.
21:527–533. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schneider F and Dacic S: Histopathologic
and molecular approach to staging of multiple lung nodules. Transl
Lung Cancer Res. 6:540–549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Detterbeck FC, Nicholson AG, Franklin WA,
Marom EM, Travis WD, Girard N, Arenberg DA, Bolejack V, Donington
JS, Mazzone PJ, et al: The IASLC lung cancer staging project:
Summary of proposals for revisions of the classification of lung
cancers with multiple pulmonary sites of involvement in the
forthcoming eighth edition of the TNM classification. J Thorac
Oncol. 11:639–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amin MB, Edge SB, Greene FL, et al: AJCC
cancer staging manual. 8th. New York: Springer; 2017, View Article : Google Scholar
|
|
9
|
Donfrancesco E, Yvorel V, Casteillo F,
Stachowicz ML, Patoir A, Tiffet O, Péoc'h M and Forest F:
Histopathological and molecular study for synchronous lung
adenocarcinoma staging. Virchows Arch. 476:835–842. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel SB, Kadi W, Walts AE, Marchevsky AM,
Pao A, Aguiluz A, Mudalige T, Liu Z, Deng N and Lopategui J:
Next-generation sequencing: A novel approach to distinguish
multifocal primary lung adenocarcinomas from intrapulmonary
metastases. J Mol Diagn. 19:870–880. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider F, Derrick V, Davison JM,
Strollo D, Incharoen P and Dacic S: Morphological and molecular
approach to synchronous non-small cell lung carcinomas: Impact on
staging. Mod Pathol. 29:735–742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li W, Qiu T, Ling Y, Gao S and Ying J:
Subjecting appropriate lung adenocarcinoma samples to
next-generation sequencing-based molecular testing: Challenges and
possible solutions. Mol Oncol. 12:677–689. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Zhang J, Li L, Yin G, Zhang J,
Zheng S, Cheung H, Wu N, Lu N, Mao X, et al: Genomic heterogeneity
of multiple synchronous lung cancer. Nat Commun. 7:132002016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao F, Zhang ZR, Wang XW, Liu DR, Guo YQ,
Shi B, Song ZY and Liang CY: Applying comprehensive histologic
assessment and genetic testing to synchronous multifocal lung
adenocarcinomas and further survival analysis. Chin Med J (Engl).
132:227–231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy SJ, Aubry MC, Harris FR, Halling
GC, Johnson SH, Terra S, Drucker TM, Asiedu MK, Kipp BR, Yi ES, et
al: Identification of independent primary tumors and intrapulmonary
metastases using DNA rearrangements in non-small-cell lung cancer.
J Clin Oncol. 32:4050–4058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robinson JT, Thorvaldsdóttir H, Wenger AM,
Zehir A and Mesirov JP: Variant review with the integrative
genomics viewer. Cancer Res. 77:e31–e34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Qiu T, Guo L and Ying JM: Major
challenges related to tumor biological characteristics in accurate
mutation detection of colorectal cancer by next-generation
sequencing. Cancer Lett. 410:92–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Normanno N, Rachiglio AM, Lambiase M,
Martinelli E, Fenizia F, Esposito C, Roma C, Troiani T, Rizzi D,
Tatangelo F, et al: Heterogeneity of KRAS, NRAS, BRAF and PIK3CA
mutations in metastatic colorectal cancer and potential effects on
therapy in the CAPRI GOIM trial. Ann Oncol. 26:1710–1714. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng H, Lei BF, Peng PJ, Lin YJ and Wang
XJ: Histologic lung cancer subtype differentiates synchronous
multiple primary lung adenocarcinomas from intrapulmonary
metastases. J Surg Res. 211:215–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu X, Fujimoto J, Ying L, Fukuoka J,
Ashizawa K, Sun W, Reuben A, Chow CW, McGranahan N, Chen R, et al:
Multi-region exome sequencing reveals genomic evolution from
preneoplasia to lung adenocarcinoma. Nat Commun. 10:29782019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nik-Zainal S, Van Loo P, Wedge DC,
Alexandrov LB, Greenman CD, Lau KW, Raine K, Jones D, Marshall J,
Ramakrishna M, et al: The life history of 21 breast cancers. Cell.
149:994–1007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shah SP, Morin RD, Khattra J, Prentice L,
Pugh T, Burleigh A, Delaney A, Gelmon K, Guliany R, Senz J, et al:
Mutational evolution in a lobular breast tumour profiled at single
nucleotide resolution. Nature. 461:809–813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gerlinger M, Rowan AJ, Horswell S, Math M,
Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N,
Stewart A, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anderson K, Lutz C, van Delft FW, Bateman
CM, Guo Y, Colman SM, Kempski H, Moorman AV, Titley I, Swansbury J,
et al: Genetic variegation of clonal architecture and propagating
cells in leukaemia. Nature. 469:356–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thunnissen E, Beasley MB, Borczuk AC,
Brambilla E, Chirieac LR, Dacic S, Flieder D, Gazdar A, Geisinger
K, Hasleton P, et al: Reproducibility of histopathological subtypes
and invasion in pulmonary adenocarcinoma. An international
interobserver study. Mod Pathol. 25:1574–1583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Girard N, Deshpande C, Lau C, Finley D,
Rusch V, Pao W and Travis WD: Comprehensive histologic assessment
helps to differentiate multiple lung primary nonsmall cell
carcinomas from metastases. Am J Surg Pathol. 33:1752–1764. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Girard N, Ostrovnaya I, Lau C, Park B,
Ladanyi M, Finley D, Deshpande C, Rusch V, Orlow I, Travis WD, et
al: Genomic and mutational profiling to assess clonal relationships
between multiple non-small cell lung cancers. Clin Cancer Res.
15:5184–5190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
La Fleur L, Falk-Sörqvist E, Smeds P,
Berglund A, Sundström M, Mattsson JS, Brandén E, Koyi H, Isaksson
J, Brunnström H, et al: Mutation patterns in a population-based
non-small cell lung cancer cohort and prognostic impact of
concomitant mutations in KRAS and TP53 or STK11. Lung Cancer.
130:50–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jakobsen JN, Santoni-Rugiu E, Grauslund M,
Melchior L and Sørensen JB: Concomitant driver mutations in
advanced EGFR-mutated non-small-cell lung cancer and their impact
on erlotinib treatment. Oncotarget. 40:26195–26208. 2018.
View Article : Google Scholar
|
|
30
|
Skov BG, Høgdall E, Clementsen P, Krasnik
M, Larsen KR, Sørensen JB, Skov T and Mellemgaard A: The prevalence
of EGFR mutations in non-small cell lung cancer in an unselected
Caucasian population. APMIS. 123:108–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi Y, Li J, Zhang S, Wang M, Yang S, Li
N, Wu G, Liu W, Liao G, Cai K, et al: Molecular epidemiology of
EGFR mutations in asian patients with advanced non-small-cell lung
cancer of adenocarcinoma histology-mainland China subset analysis
of the PIONEER study. PLoS One. 10:e01435152015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
Prospective, molecular epidemiology study of EGFR mutations in
asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guibert N, Barlesi F, Descourt R, Lena H,
Besse B, Beau-Faller M, Mosser J, Pichon E, Merlio JP, Ouafik L, et
al: Characteristics and outcomes of patients with lung cancer
harboring multiple molecular alterations: Results from the IFCT
study biomarkers France. J Thorac Oncol. 12:963–973. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tetsu O, Hangauer MJ, Phuchareon J, Eisele
DW and McCormick F: Drug resistance to EGFR inhibitors in lung
cancer. Chemotherapy. 61:223–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eng J, Woo KM, Sima CS, Plodkowski A,
Hellmann MD, Chaft JE, Kris MG, Arcila ME, Ladanyi M and Drilon A:
Impact of concurrent PIK3CA mutations on response to EGFR tyrosine
kinase inhibition in EGFR-Mutant lung cancers and on prognosis in
oncogene-driven lung adenocarcinomas. J Thorac Oncol. 10:1713–1719.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ulivi P, Chiadini E, Dazzi C, Dubini A,
Costantini M, Medri L, Puccetti M, Capelli L, Calistri D, Verlicchi
A, et al: Nonsquamous, non-small-cell lung cancer patients who
carry a double mutation of EGFR, EML4-ALK or KRAS: Frequency,
clinical-pathological characteristics, and response to therapy.
Clin Lung Cancer. 17:384–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Riely GJ, Kris MG, Rosenbaum D, Marks J,
Li A, Chitale DA, Nafa K, Riedel ER, Hsu M, Pao W, Miller VA and
Ladanyi M: Frequency and distinctive spectrum of KRAS mutations in
never smokers with lung adenocarcinoma. Clin Cancer Res.
14:5731–5734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McIntyre A and Ganti AK: Lung cancer-A
global perspective. J Surg Oncol. 115:550–554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang L, Huang J, Morehouse C, Zhu W,
Korolevich S, Sui D, Ge X, Lehmann K, Liu Z, Kiefer C, et al: Low
frequency KRAS mutations in colorectal cancer patients and the
presence of multiple mutations in oncogenic drivers in non-small
cell lung cancer patients. Cancer Genet. 206:330–339. 2013.
View Article : Google Scholar : PubMed/NCBI
|