Introduction
Recently, immune checkpoint inhibitors (ICIs) have
been used for the treatment of various malignancies, including
non-small-cell lung cancer (NSCLC), renal cell cancer, melanoma,
and other cancers (1–4).
Programmed cell death 1 (PD-1) is a receptor
expressed on activated T cells. It binds to its ligands, programmed
death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2),
expressed in cancer cells, leading to the inhibition of T cell
activation (3,4). The interaction of PD-1 with PD-L1
promotes tumor immune escape, which leads to patients with the
disease having a poor prognosis. Nivolumab, a fully human IgG4 PD-1
ICI antibody, disrupts PD-1-mediated signaling and restores
antitumor immunity (3,4).
In phase II and III studies, nivolumab monotherapy
showed good tolerance and excellent clinical efficacy in patients
with advanced or recurrent NSCLC, which had progressed after
platinum-containing chemotherapy (5–9). In
these studies, nivolumab was associated with an overall response
rate (ORR) of 15–26%, overall survival (OS) of 41–71% at 1 year,
and progression-free survival (PFS) of 19–25% at 1 year
post-treatment (5–9).
Bone is one of the common metastatic sites of
advanced lung cancer. Approximately 30–66% of patients with
advanced NSCLC develop bone metastases during the course of their
disease (10,11).
Bone metastases usually present as lytic, blastic,
or mixed lesions. Lytic or mixed metastatic lesions that have been
successfully treated show osteosclerotic change (OC) on computed
tomography (CT) due to the reparative process known as
re-ossification (12–14). OC has been reported as an indicator
of good therapeutic response to treatment with various anticancer
therapies, such as endocrine therapy, molecular targeted therapy,
bone-modifying agents (BMAs), radiotherapy (RT), and a combination
of these treatments (12–21). Yamashita et al reported OC as
being an indicator of a good therapeutic response in lung cancer
patients with bone metastases treated with gefitinib (15). The authors showed that the OC group
had a significantly higher ORR and improved OS compared to the no
OC (NOC) group. Rong et al reported a median interval of 2
months when OC occurred following chemotherapy in lung cancer
patients with bone metastases (16).
The authors showed that the OC group had significantly higher
3-month disease control rate (DCR) and 1-year PFS than the NOC
group. However, these reports are inadequate, because they do not
take into account the regression of extraskeletal lesions, which
can also be a predictor of treatment as defined in the Response
Evaluation Criteria in Solid Tumors, version 1.1 (RECIST1.1).
In 2004, Hamaoka established the MD Anderson
response classification criteria (MDA criteria), specific for the
assessment of bone metastases (12–14,21). The
MDA criteria divide the responses into 4 categories and can assess
both the regression of extraskeletal lesions and OC. The MDA
criteria allow a greater range of bone lesions to be considered
measurable disease than RECIST1.1 by allowing measurement of
numerous types of bone lesions regardless of soft tissue extension
(12–14). Since their introduction, several
authors reported their usefulness for the assessment of therapeutic
response to chemotherapy and RT in patients with bone metastases
(12–14,21).
Hamaoka reported that the MDA classification is superior to the WHO
classification in differentiating between the responders and
non-responders in breast cancer patients with bone-only metastases
(12). According to the MDA
criteria, there were significant differences in PFS between
patients classified as responders and those classified as
non-responders (P=0.025), but not with the WHO criteria. We
previously reported that the MDA criteria were useful for
assessment of radiological responses of irradiated vertebrae
(21). There was a significant trend
that, with a better response assessed by the MDA criteria, there
were more patients without pain (P=0.021).
Although early prediction of the effects of
chemotherapy for NSCLC patients would help guide clinical practice,
the optimal markers for predicting the outcomes in NSCLC patients
are unknown, particularly in patients with bone metastases after
ICI treatment. To the best of our knowledge, no studies have
focused on the effect of nivolumab in NSCLC patients with bone
metastases. Moreover, no studies have investigated the relationship
between bone response and tumor control with nivolumab
treatment.
Therefore, the clinical outcome of nivolumab
monotherapy was investigated in NSCLC patients with bone
metastases. Furthermore, whether the response of bone metastases
assessed by the MDA criteria can be used as an early imaging
predictor of tumor control in NSCLC patients with bone metastases
was explored.
Patients and methods
Study population
The records of 52 patients who received nivolumab
monotherapy for stage IV or recurrent NSCLC in our institution
between December 2015 (the date nivolumab was approved in Japan)
and March 2017 were retrospectively reviewed. The last follow-up
evaluation of patients was performed in March 2018.
The inclusion criteria were as follows: Patients who
received nivolumab and had previously received platinum-containing
chemotherapy for advanced NSCLC with bone metastases.
Patients were excluded if they had received
immunotherapy or any additional concurrently administered
antineoplastic therapies. Patients were also excluded from the
analysis if they had previously undergone surgery, RT, or other
local interventional therapies to the metastatic bone.
A total of 15 patients (11 men and 4 women) were
included in this study (Table I).
The median age was 67 years (range, 40–75 years) at the time of
receiving nivolumab treatment. Thirteen patients had
adenocarcinoma, and two patients had squamous cell carcinoma. Two
patients had recurrent NSCLC after lung surgery with curative
intent, and 13 patients had stage IV NSCLC at the time of
presentation. All but one patient had metastases to organs other
than bone (93%). The median number of analyzed bone metastases was
2 (1–8). The locations of bone metastases were
vertebral bone (20), ilium
(6), rib (6), scapula (3), and others (6). The median follow-up time of this study
was 12.2 months (1–23).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | Total, n=15 |
|---|
| Sex |
|
|
Male | 11 |
|
Female | 4 |
| Age, years |
|
|
Median | 67 (range,
40-75) |
| Histology |
|
|
Adenocarcinoma | 13 |
|
Squamous cell carcinoma | 2 |
| Metastases |
|
|
Lung | 8 |
|
Liver | 7 |
|
Brain | 3 |
| Adrenal
gland | 1 |
| Pleural
dissemination | 5 |
| Lymph node | 11 |
| Mutation of
epidermal growth | 5 |
Treatment
Patients had received 3 mg/kg nivolumab as an
intravenous infusion every 2 weeks until progressive disease (PD)
or unacceptable toxicity was observed. Prior to nivolumab therapy,
the median number of chemotherapy regimens was 2 (1–9)
(Table II). All patients previously
received platinum-based therapy, and 5 patients previously received
an epidermal growth factor receptor-tyrosine kinase inhibitor. BMAs
were administered to 12 patients. Denosumab was administered to 2
patients, and zoledronic acid was administered to 10 patients.
 | Table II.Prior systemic therapy. |
Table II.
Prior systemic therapy.
| Prior
chemotherapy | Total, n=15 |
|---|
| Number of prior
chemotherapy |
|
| 1 | 5 |
| 2 | 5 |
| 3 | 1 |
| 5 | 2 |
| 7 | 1 |
| 9 | 1 |
| Type of prior
chemotherapy |
|
|
Platinum-based therapy | 15 |
|
EGFR-TKI | 5 |
Assessment of study outcomes
All patients were assessed by CT (Aquilion, Canon)
at 120 kV and slice thickness of 5 mm from head to pelvis.
Radiographic assessments were performed at the start of nivolumab
therapy, and the first time after treatment at a median of 7 weeks
(3–12 weeks). Two patients were assessed after 3 weeks for sudden
aggravation of clinical symptoms; they were found to have disease
progression on CT. Assessment by CT was done at a median of every 7
weeks (4–9 weeks) thereafter. Two authors (E.N., T.K.) evaluated
the CT images, and any disagreements were resolved by
consensus.
Tumor responses were defined as complete response
(CR), partial response (PR), stable disease (SD), and PD according
to RECIST1.1 (22). Responders were
classified as either CR or PR, and non-responders were classified
as either PD or SD.
For assessing bone lesions, all images were viewed
with routine bone window settings (window level 200 HU, window
width 2,000 HU). Patients were classified depending on their bone
lesions at the start of nivolumab treatment as lytic (L group) in 5
patients, mixed (M group) in 2 patients, blastic (B group) in 1
patient, lytic and mixed (L/M group) in 1 patient, lytic and
blastic (L/B group) in 2 patients, and mixed and blastic (M/B
group) in 4 patients.
Radiological responses of bone metastases were
assessed using the MDA criteria (Table
III) (12–14). According to the MDA criteria by CT,
CR is defined as complete fill-in or sclerosis of lytic lesions for
lytic and mixed lesions or disappearance of the tumor signal for
any lesion. PR is defined as development of a sclerotic rim around
an initially lytic lesion or sclerosis of lesions previously
undetected on CT, or partial fill-in or sclerosis of lytic lesions,
regression of measurable lesion in any lesion, or a decrease in
blastic lesions. PD is defined as an increase in size of any
existing measurable lesions, the appearance of new lesions, or an
increase in blastic/lytic lesions. SD is defined as no change in
blastic/lytic lesions, no change of measurable lesions, or the
appearance of no new lesions. A case of PR in one bone metastasis
and SD in another bone metastasis was considered PR, and a case of
PD in one bone metastasis and SD in another bone metastasis was
considered PD. Responders were classified as either CR or PR, and
non-responders were classified as either PD or SD.
 | Table III.MD Anderson criteria for evaluation
of bone metastases. |
Table III.
MD Anderson criteria for evaluation
of bone metastases.
| Response type | Definition |
|---|
| Complete
response | Complete fill-in or
sclerosis of lytic lesions on CT |
|
| Disappearance of
tumor signal on CT |
|
| Normalization of
osteoblastic lesion on CT |
| Partial
response | Sclerotic rim
around initially lytic lesion or sclerosis of lesions previously
undetected on CT |
|
| Partial fill-in or
sclerosis of lytic lesion on CT |
|
| Regression of
measurable lesion on CT |
|
| Decrease in blastic
lesion on CT |
| Progressive
disease | Increase in size of
any existing measurable lesions on CT |
|
| New lesion on
CT |
|
| Increase in
blastic/lytic lesion on CT |
| Stable disease | No change in
blastic/lytic lesion on CT |
|
| No change in
measurable lesion on CT |
|
| No new lesion on
CT |
Best overall response (BOR), overall response rate
(ORR), disease control rate (DCR), time to response (TTR), and
duration of response (DOR) were investigated by RECIST1.1 and the
MDA criteria (bone metastases). BOR was defined as the best
response designation recorded between the date of the first dose of
nivolumab and the date of initial objectively documented tumor
progression based on RECIST1.1 and the MDA criteria, or the date of
subsequent therapy, whichever occurred first. ORR was calculated as
the proportion of patients with a BOR of CR or PR. DCR was
calculated as the proportion of patients with a BOR of CR or PR or
SD. TTR was defined as the duration of time from first dose of
nivolumab to the date of initial radiographic CR or PR. DOR was
defined as the duration of response, defined as the time from first
confirmed response to the date of initial radiographic progression
for patients with CR or PR.
Progression-free survival (PFS) and overall survival
(OS) were also investigated using the Kaplan-Meier method. PFS was
defined as the time from the first dose of nivolumab to the date of
first documented progression of tumor and bone metastatic site
based on RECIST1.1 and the MDA criteria or death from any cause. OS
was defined as the time from the date of first dose to the date of
death from any cause or last known date alive for patients who were
alive at the time of data analysis.
Patients who neither progressed nor died were
censored on the date of their last tumor assessment. Patients who
died without reported previous progression were considered to have
progressed on the date of their death. Patients who received
subsequent cancer treatment without reported progression were
censored at the date of the last tumor assessment before starting
further treatment.
Statistical analysis
PFS (RECIST1.1 and MDA criteria) and OS were
estimated using the Kaplan-Meier method. The prognostic
significance of the following variables on survival was assessed:
Age, sex, the number of bone metastases, the number of chemotherapy
regimens prior to nivolumab therapy, BMAs, and bone lesions.
Regression of bone metastases according to the MDA criteria was
also assessed as a predictor of OS and PFS (RECIST1.1) in cases of
lytic or mixed bone metastases (14 patients).
To assess risk factors for patients without disease
control (PD) according to RECIST1.1 and non-responders (SD or PD)
according to the MDA criteria, clinical data were assessed,
including the following: Age, sex, the number of bone metastases,
the number of chemotherapy regimens prior to nivolumab therapy,
BMAs, and bone lesions. Risk factors for regression of bone
metastases according to the MDA criteria were also assessed in
patients without disease control (PD) according to RECIST1.1. The
relation between tumor control (RECIST1.1) and bone response (MDA
criteria) and TTS for both was also evaluated to investigate
whether the MDA criteria can predict RECIST1.1.
For univariate analysis, the Mann-Whitney U-test was
used to analyze continuous parameters, and Fisher's exact test was
used for categorical parameters. Multivariate analysis was
performed using multiple logistic regression analysis.
For all analyses, associations were considered
significant if the associated P-value was <0.05. All statistical
analyses were performed with the statistical computing software R
(R Version 3.5.0, R Core Team, Vienna, Austria).
Results
Nivolumab doses and treatment
duration
A median of 4 doses of nivolumab was administered
(1–23 doses), with a median treatment duration of 1.4 months (1–14
months). Fourteen patients discontinued nivolumab due to disease
progression, and one patient discontinued due to adverse events
(grade 2 diarrhea). All patients had PD during nivolumab treatment.
Following the discontinuation of nivolumab, 13 (87%) patients
received subsequent chemotherapy; the median number of chemotherapy
regimens was 1 (1–2). All but one patient had died by the time
of the last follow-up.
Tumor control
With RECIST1.1, BOR was PR in 3 patients (20%), SD
in 3 patients (20%), and PD in 9 patients (60%). The ORR was 20%,
and the DCR was 40%. Median TTR was 3.0 months (2.8–5.6 months),
and median DOR was 5.8 months (1.5–9.3 months), as shown in a
swimmer plot (Fig. 1). As reported
in several studies (5,6,23,24), the
long-term effect of nivolumab (durable response) was observed in
one patient.
 | Figure 1.Swimmer plot. In RECIST1.1, BOR was
PR in three patients (20%), median TTR was 3.0 months (2.8–5.6
months) and median DOR was 5.8 months (1.5–9.3 months) as presented
in the swimmer plot. In the MDA, BOR was CR in one patient and PR
in five patients. Median TTR was 1.4 months (1.4–2.0 months) and
median DOR was 6.2 months (0.7–17.7 months). RECIST1.1, Response
Evaluation Criteria in Solid Tumors, version 1.1; BOR, best overall
response; PR, partial response; TTR, time to response; DOR,
duration of response; MDA, MD Anderson; CR, complete response; OS,
overall survival; SD, stable disease; PD, progressive disease. |
Univariate analysis showed that the number of bone
metastases and the response according to the MDA criteria were the
risk factors for patients without disease control (PD) according to
RECIST1.1 (Table IV). Four of 10
patients (40%) had PD among the patients with <3 bone
metastases. On the other hand, all patients had PD among the
patients with ≥3 bone metastases. All responders according to the
MDA criteria had a DCR of 100% with RECIST1.1, whereas the
non-responders according to the MDA criteria had a DCR of 0% with
RECIST1.1.
 | Table IV.Risk factors for tumor progression
assessed by Response Evaluation Criteria in Solid Tumors, version
1.1. |
Table IV.
Risk factors for tumor progression
assessed by Response Evaluation Criteria in Solid Tumors, version
1.1.
|
| Number of
patients |
|
|---|
|
|
|
|
|---|
| Covariates | Patients with
CR/PR/SD | Patients with
PD | P-value |
|---|
| Age, years |
|
|
|
|
<65 | 3 | 3 |
|
|
≥65 | 3 | 6 | 0.62 |
| Sex |
|
|
|
|
Male | 5 | 6 |
|
|
Female | 1 | 3 | 0.60 |
| Number of prior
chemotherapy |
|
|
|
|
<3 | 5 | 5 |
|
| ≥3 | 1 | 4 | 0.58 |
| Number of bone
metastases |
|
|
|
|
<3 | 6 | 4 |
|
| ≥3 | 0 | 5 | 0.04 |
| Bone modifying
agent |
|
|
|
|
Yes | 4 | 8 |
|
| No | 2 | 1 | 0.52 |
| Bone lesion |
|
|
|
| Lytic
only | 4 | 1 |
|
|
Others | 2 | 8 | 0.09 |
| MDA criteria |
|
|
|
|
Responder | 6 | 0 |
|
|
Non-responder | 0 | 9 | <0.001 |
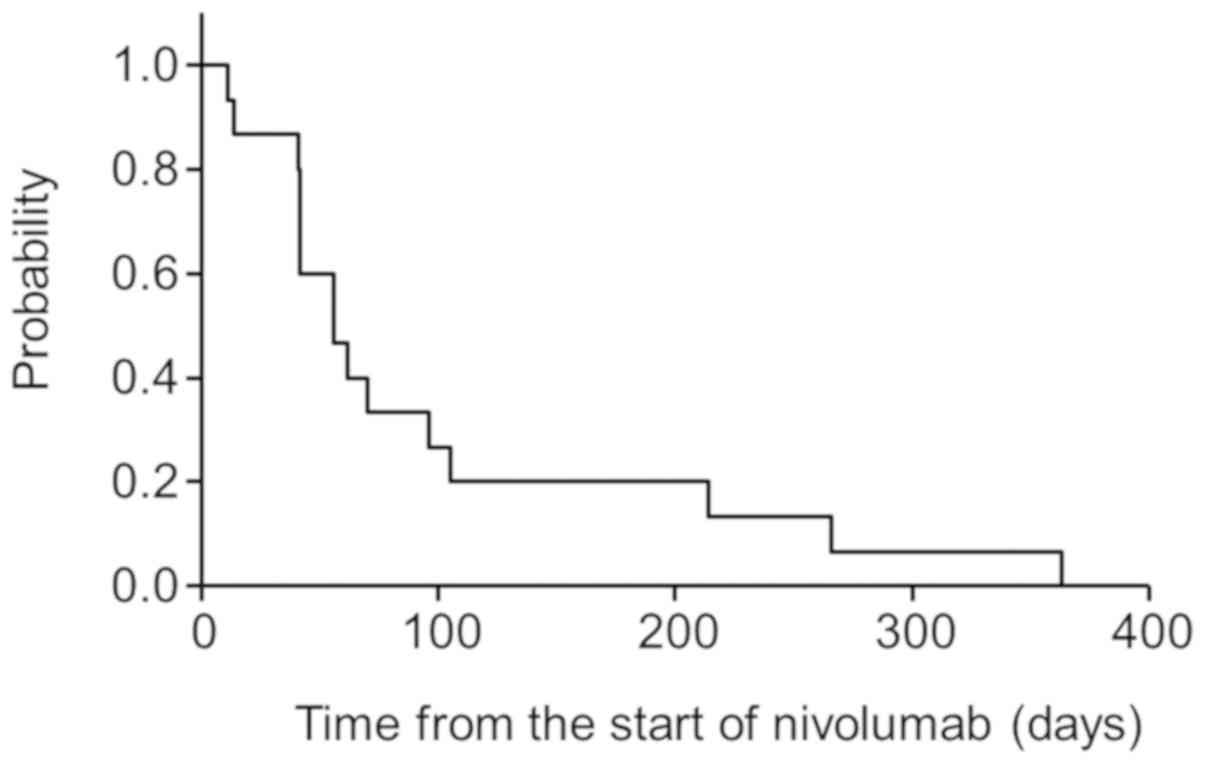
Median PFS was 1.9 months (0.4–12.1 months), with
PFS of 33 and 20% at 3 and 6 months after the start of nivolumab
treatment, respectively (Fig. 2).
Univariate analysis showed that being a non-responder according to
the MDA criteria was the only risk factor for PFS. PFS was 83 and
50% at 3 and 6 months after the start of nivolumab treatment,
respectively, in patients who were responders according to the MDA
criteria within 3 months. However, all patients who were
non-responders according to the MDA criteria converted to PD within
3 months, which was significant (P<0.01) (Fig. 3).
Assessment of bone response by MDA
criteria
Following nivolumab treatment, bone response was
achieved in 8 lytic lesions in 6 patients (40%). No change was seen
in mixed and blastic lesions. There were 2 patients with PR in one
bone lesion and SD in another bone lesion, which were considered
PR. There was 1 patient with PD in one bone lesion and SD in
another bone lesion, which was considered PD. In the remaining 12
patients, the same response was seen in all bone metastatic
sites.
According to the MDA criteria, BOR was CR in 1
patient in group L and PR in 5 patients (3 patients in group L, 1
patient in group L/M, and 1 patient in group L/B) (Table V). The ORR was 40%. The median TTR
was 1.4 months (1.4–2.0 months), which was the first-time
assessment after the start of nivolumab therapy. The initial bone
response was OC in 5 patients with a median TTR of 1.5 months
(1.4–2.0 months) and regression of soft tissue extension in 1
patient with TTR of 1.4 months. Median DOR was 6.2 months (0.7–17.7
months), with 2 patients progression-free at the time of analysis.
SD was achieved in 8 patients (1 patient in group L, 2 patients in
group M, 1 patient in group B, 1 patient in group L/B, and 3
patients in group M/B). There was 1 patient with PD (one patient in
group M/B received RT for progression of an extraskeletal lesion of
the right ilium, which was considered PD).
 | Table V.Bone lesions and response according
to the MD Anderson response criteria. |
Table V.
Bone lesions and response according
to the MD Anderson response criteria.
| Bone lesions | CR | PR | SD | PD |
|---|
| Lytic | 1 | 3 | 1 |
|
| Mixed |
|
| 2 |
|
| Blastic |
|
| 1 |
|
| Lytic/Mixed |
| 1 |
|
|
| Lytic/Blastic |
| 1 | 1 |
|
| Mixed/Blastic |
|
| 3 | 1 |
Univariate analysis showed that the number of bone
metastases was the only risk factor for non-responders (Table VI). Four of 10 patients (40%) were
non-responders among the patients with <3 bone metastases. On
the other hand, all patients were non-responders among the patients
with ≥3 bone metastases.
 | Table VI.Risk factors for non-responders of
bone metastases assessed according to the MD Anderson response
criteria. |
Table VI.
Risk factors for non-responders of
bone metastases assessed according to the MD Anderson response
criteria.
|
| Number of
patients |
|
|---|
|
|
|
|
|---|
| Covariates | Patients with
CR/PR | Patients with
SD/PD | P-value |
|---|
| Age, years |
|
|
|
|
<65 | 3 | 3 |
|
|
≥65 | 3 | 6 | 0.62 |
| Sex |
|
|
|
|
Male | 5 | 6 |
|
|
Female | 1 | 3 | 0.60 |
| Number of prior
chemotherapy |
|
|
|
|
<3 | 5 | 5 |
|
| ≥3 | 1 | 4 | 0.58 |
| Number of bone
metastases |
|
|
|
|
<3 | 6 | 4 |
|
| ≥3 | 0 | 5 | 0.04 |
| Bone modifying
agent |
|
|
|
|
Yes | 4 | 8 |
|
| No | 2 | 1 | 0.52 |
| Bone lesion |
|
|
|
| Lytic
only | 4 | 1 |
|
|
Others | 2 | 8 | 0.09 |
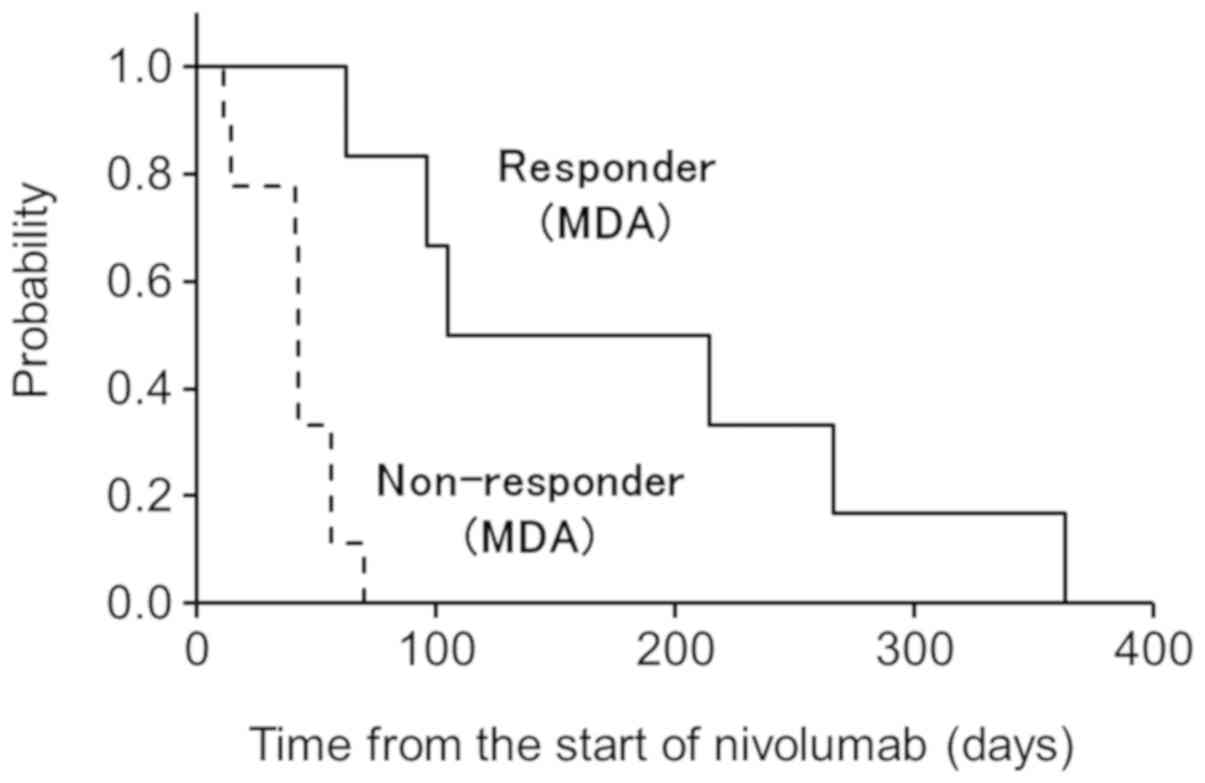
Median PFS was 7.3 months (1.4–19 months), with PFS
of 86 and 72% at 3 and 6 months after the start of nivolumab
therapy, respectively (Fig. 4).
Univariate analysis showed that no factors were correlated with
PFS.
Relation between tumor control
(RECIST1.1) and bone response (MDA criteria)
Responders according to RECIST1.1 had significantly
more responders according to the MDA criteria than non-responders
according to RECIST1.1 (P<0.05). Although all responders (3
patients) according to RECIST1.1 were responders according to the
MDA criteria (Table VII), in
non-responders according to RECIST1.1, there were 3 responders and
9 non-responders according to the MDA criteria. In these 3 patients
who were responders according to both RECIST1.1 and the MDA
criteria, TTR occurred earlier according to the MDA criteria
(1.4–2.0 months) than according to RECIST1.1 (2.8–3.0 months) in
all patients.
 | Table VII.Relation of tumor control and bone
response. |
Table VII.
Relation of tumor control and bone
response.
|
| MDA criteria |
|---|
|
|
|
|---|
| RECIST1.1 | CR | PR | SD | PD |
|---|
| CR |
|
|
|
|
| PR | 1 | 2 |
|
|
| SD |
| 3 |
|
|
| PD |
|
| 8 | 1 |
OS
At the time of analysis, 14 patients (93%) had died.
OS was 80 and 44% at 0.5 years and 1 year after the start of
nivolumab therapy, respectively (Fig.
5). Univariate analysis showed that no factor was correlated
with OS.
Case 1
A case of durable response of whole lesions and CR
of bone metastases by nivolumab treatment is shown in Fig. 6. The patient was 72 years old when he
initially presented with lung adenocarcinoma and metastases to the
lung, lymph node, and bone (lytic type bone lesion in third rib and
T11 vertebra). He was initially treated with carboplatin,
paclitaxel, and bevacizumab. Due to disease progression, he was
switched to second-line therapy with nivolumab [original lesion
(Fig. 6A), lung metastases (Fig. 6B) and left third rib metastases
(Fig. 6C)].
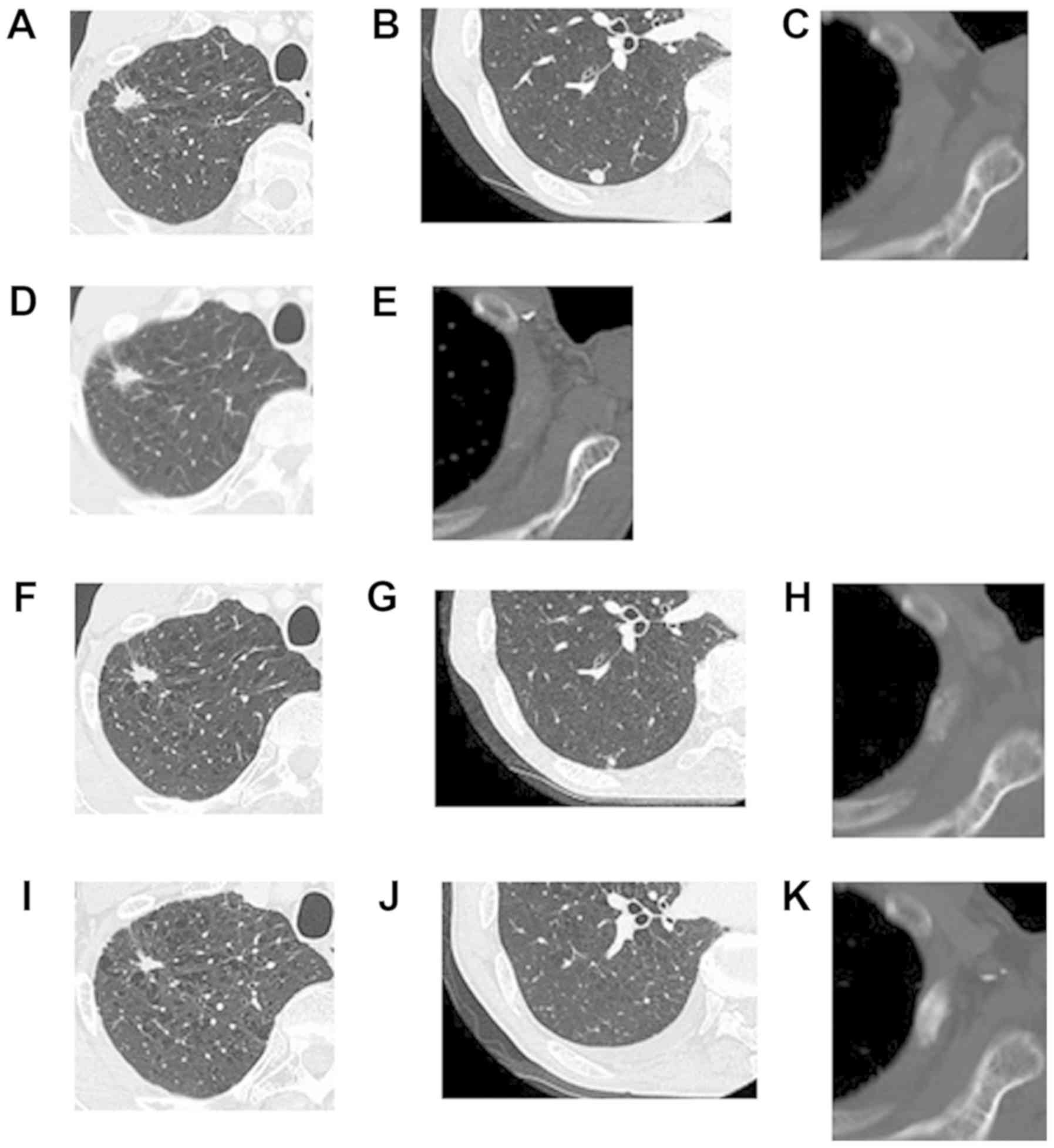 | Figure 6.A case of durable response of whole
lesions and CR of bone metastases by nivolumab treatment is shown.
Due to disease progression, the patient was switched to second-line
therapy with nivolumab. (A) Original lesion, (B) lung metastases
and (C) left third rib metastases. After three doses of nivolumab
(1.4 months), (D) CT showed a stable appearance of the right
original malignancy along with (E) a decrease in the size of the
lung metastases and regression of soft tissue extension and OC of
the left third rib metastases compared with the patient's baseline
CT, which was considered SD according to RECIST1.1 and PR according
to the MDA criteria. At 3 months after nivolumab treatment, (F)
decreases in size of the original malignancy and (G) lung
metastases were observed, which was considered PR according to
RECIST1.1. (H) At 5 months after nivolumab treatment, complete
sclerotic fill-in of the lytic lesions was demonstrated, which was
considered CR according to the MDA criteria. At 8 months after
nivolumab treatment, (I) a significant decrease in size of the
original lesion, (J) disappearance of the lung metastases and (K)
further OC of the left third rib metastases were noted. CR,
complete response; OC, osteosclerotic change; SD, stable disease;
PR, partial response. |
After three doses of nivolumab (1.4 months), CT
showed a stable appearance of the right original malignancy
(Fig. 6D) along with decreases in
size of the lung metastases and regression of soft tissue extension
and OC of the left third rib metastases (Fig. 6E), compared with his baseline CT,
which was considered SD according to RECIST1.1 and PR according to
the MDA criteria. However, the nivolumab therapy was discontinued
due to treatment-related grade 2 diarrhea, which could not be
resolved with corticosteroid treatment. Although the patient
received no further chemotherapy, each lesion showed decreases in
size. Approximately 3 months after nivolumab treatment, decreases
in size of the original malignancy (Fig.
6F) and lung metastases (Fig.
6G) were seen, which was considered PR according to RECIST1.1.
Five months after nivolumab treatment, complete sclerotic fill-in
of lytic lesions was demonstrated, which was considered CR
according to the MDA criteria (Fig.
6H). Eight months after nivolumab treatment, a significant
decrease in size of the original lesion (Fig. 6I), disappearance of the lung
metastases (Fig. 6J), and further OC
of the left third rib metastases (Fig.
6K) were noted. One year after nivolumab treatment, although
these lesions were not changed, a new bone lesion was detected on
bone scintigraphy, which was considered PD. The patient was
subsequently treated with docetaxel and ramucirumab.
Discussion
The present study is the first to investigate the
clinical results of nivolumab monotherapy in NSCLC patients with
bone metastases, and it found that nivolumab monotherapy resulted
in OS of 44% at 1-year post-treatment. The patient population was
highly refractory, with almost one-third having received three or
more systemic treatments previously. However, this result is
consistent with previous studies reporting 1-year OS of 41–71%.
In the present study, ORR according to RECIST1.1 was
20%, which is consistent with previous studies reporting an ORR of
15–26%. In numerous previous reports, molecular markers for
prediction of response to nivolumab treatment were investigated, as
the positive expression of PD-L1 and high tumor mutation burden
were associated with significantly higher ORR than the others
(5,6,25,26). The
present univariate analysis showed that the number of bone
metastases and bone response assessed by the MDA criteria were the
risk factors for patients with PD. Four of 10 patients (40%) had PD
among the patients with <3 bone metastases. On the other hand,
all patients had PD among the patients with ≥3 bone metastases.
However, age, the number of chemotherapy regimens prior to
nivolumab therapy, and the number of bone lesions were not
significantly related. Bone is one of the most common sites
affected by metastatic cancer (1,2). The
previous studies reported the existence of complex crosstalk among
cancer cells, immune cells, and the bone microenvironment (27,28).
Although these molecular factors were not investigated, a greater
number of bone metastases could indicate a more advanced stage of
NSCLC, which would lead to a poor response.
In the present study, the median TTR of the cancer
was 3.0 months, which is consistent with previous studies reporting
TTR of 2.1–3.3 months (5–8). The median DOR was 5.8 months. Of note,
as reported in several studies (23,24), the
long-term effect of nivolumab (durable response) was observed in
some patients. Of the three patients who achieved an objective
response, two (13%) were progression-free for 9 and 12 months,
respectively. Moreover, every lesion showed a decrease in size in
one patient, although the patient received no further chemotherapy
after the discontinuation of nivolumab, as shown in Fig. 6.
The present study is the first to investigate the
effect of nivolumab therapy on metastatic bone lesions. Following
nivolumab treatment, OC was noted in 8 lytic lesions in 6 patients
on CT (40%), of which partial sclerosis was seen in 5 patients
(33%), and complete sclerosis was seen in 1 patient (7%). Four of
10 patients (40%) were non-responders among the patients with <3
bone metastases. On the other hand, all patients were
non-responders among the patients with ≥3 bone metastases. A
greater number of bone metastases could suggest a more advanced
status of NSCLC, which would lead to the poor response.
Recently, several studies have indicated that OC is
a marker of favorable response in various cancers, particularly in
patients receiving chemotherapy and bone-targeted therapies
(12–18). Some of these studies also showed that
OC could be a marker of favorable response after chemotherapy and
gefitinib in lung cancer (15,16).
Rong et al reported a median interval of 2
months when OC occurred after chemotherapy in NSCLC patients with
bone metastases (16). They showed
that OC was a significant independent predictor of PFS on
multivariate analysis, and patients with OC within 3 months had
significantly higher 1-year PFS rates than patients with NOC
(P<0.001). The authors also showed that patients in the OC group
had a significantly higher 3-month DCR than those in the NOC group
(P<0.001). The authors concluded that early OC within 3 months
after the start of chemotherapy can predict the response to
chemotherapy in NSCLC patients with bone metastases. Yamashita
et al also reported OC as an indicator of good therapeutic
response in lung cancer patients treated with gefitinib (15). Although all patients with OC were
responders (RECIST1.1), only 40% were responders among the patients
without OC, which was significant (P<0.001). Moreover, there was
a significant difference in the survival rate between the two
groups (P<0.01), since the median survival of patients without
OC was 170 days, while none of the patients with OC died within 170
days.
However, OC, as well as the regression of soft
tissue extension, should be investigated in the assessment of bone
metastases. Therefore, the MDA criteria were used in the present
study. A significant relationwas demonstrated between the bone
response and the tumor response in NSCLC patients with bone
metastases who received nivolumab monotherapy. Response according
to RECIST1.1 was significantly correlated with the response
according to the MDA criteria (P<0.05). All responders (3
patients) according to RECIST1.1 were responders according to the
MDA criteria. Importantly, the median time for bone response
detected by CT was as early as 1.4 months (range 1.4–2 months). TTR
according to the MDA criteria occurred earlier than that according
to RECIST1.1 in all patients who were responders according to both
RECIST1.1 and the MDA criteria.
Furthermore, bone response within 2 months was
indicative of better PFS according to RECIST1.1 in NSCLC patients
with bone metastases. In patients with bone response within 2
months, PFS was 83 and 50% at 3 and 6 months after the start of
nivolumab therapy, respectively. On the other hand, all patients
were PD according to RECIST1.1 within 3 months were non-responder
patients according to the MDA criteria.
Although early prediction of the effects of
chemotherapy in NSCLC patients would help guide clinical practice,
the optimal markers for predicting the outcomes of bone metastases
in NSCLC patients have not yet been reported.
The present data suggest that, for patients who have
received nivolumab monotherapy, being a responder according to the
MDA criteria is indicative of better tumor responses than being a
non-responder according to the MDA criteria, which could make
predictions earlier than the previous research that assessed only
OC in patients receiving chemotherapy (16) and gefitinib (15).
Moreover, these data could provide clinicians with a
new means of evaluating the prognosis of NSCLC patients with bone
metastases by monitoring bone metastases during nivolumab
monotherapy. Considering that NSCLC with bone metastases is highly
malignant, early prediction of the efficiency of chemotherapy
should help improve patient outcomes. Thus, bone response assessed
by the MDA criteria performed within 2 months of nivolumab
monotherapy is useful for the early prediction of response and
prognosis in NSCLC patients with bone metastases.
There were several limitations in this study. First,
because this was a retrospective study, there could have been
selection and verification biases; the patients had received
various numbers of regimens of chemotherapy. Second, as we excluded
patients from the analysis if they had previously undergone
surgery, RT, or other local interventional therapies to the
metastatic bone, the present study included a small sample size of
only 15 patients. Third, median follow-up time of this study was
12.2 months, which was relatively short. These limitations were
seen in other studies and is common in studies of NSCLC patients
with bone metastases who have a relatively short survival (15,16). A
prospective study with a larger number of cases may be necessary to
verify our hypothesis.
In conclusion, nivolumab monotherapy is effective
for NSCLC patients with bone metastases. Bone response correlated
significantly with tumor control. Early bone response to nivolumab
monotherapy according to the MDA criteria can be useful for the
early prediction of response and prognosis in NSCLC patients with
bone metastases. Future studies with larger cohorts are needed to
verify the results of the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EN, SS, TF and TT organized the study, and collected
and analyzed data. RN, TKu and TO also analyzed data. YS, TKo, NN
and DH treated the patients presented in this manuscript, and
collected and analyzed data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All patients were informed about the effect and
adverse events of nivolumab, the use of radiological examination,
and other information, and patient consent was obtained. The
present study was approved by the Ethics Review Board of Shikoku
Cancer Center (approval no. 2017-26) and conducted in accordance
with the World Medical Association Declaration of Helsinki.
Patient consent for publication
The patient described in the case report provided
written informed consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Darvin P, Toor SM, Sasidharan Nair V and
Elkord E: Immune checkpoint inhibitors: Recent progress and
potential biomarkers. Exp Mol Med. 50:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
LaFleur MW, Muroyama Y, Drake CG and
Sharpe AH: Inhibitors of the PD-1 pathway in tumor therapy. J
Immunol. 200:375–383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang T, Xie J, Arai S, Wang L, Shi X, Shi
N, Ma F, Chen S, Huang L, Yang L, et al: The efficacy and safety of
anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory
cancers: A meta-analysis. Oncotarget. 7:73068–73079. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hida T, Nishio M, Nogami N, Ohe Y,
Nokihara H, Sakai H, Satouchi M, Nakagawa K, Takenoyama M, Isobe H,
et al: Efficacy and safety of nivolumab in Japanese patients with
advanced or recurrent squamous non-small cell lung cancer. Cancer
Sci. 108:1000–1006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishio M, Hida T, Atagi S, Sakai H,
Nakagawa K, Takahashi T, Nogami N, Saka H, Takenoyama M, Maemondo
M, et al: Multicentre phase II study of nivolumab in Japanese
patients with advanced or recurrent non-squamous non-small cell
lung cancer. ESMO Open. 1:e0001082017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brodowicz T, O'Byrne K and Manegold C:
Bone matters in lung cancer. Ann Oncol. 23:2215–2222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsuya A, Kurata T, Tamura K and Fukuoka M:
Skeletal metastases in non-small cell lung cancer: A retrospective
study. Lung Cancer. 57:229–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamaoka T, Costelloe CM, Madewell JE, Liu
P, Berry DA, Islam R, Theriault RL, Hortobagyi GN and Ueno NT:
Tumour response interpretation with new tumour response criteria vs
the World Health Organisation criteria in patients with bone-only
metastatic breast cancer. Br J Cancer. 102:651–657. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Costelloe CM, Chuang HH, Madewell JE and
Ueno NT: Cancer response criteria and bone metastases: RECIST 1.1,
MDA and PERCIST. J Cancer. 1:80–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashi N, Costelloe CM, Hamaoka T, Wei C,
Niikura N, Theriault RL, Hortobagyi GN, Madewell JE and Ueno NT: A
prospective study of bone tumor response assessment in metastatic
breast cancer. Clin Breast Cancer. 13:24–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamashita Y, Aoki T, Hanagiri T, Yoshii C,
Mukae H, Uramoto H and Korogi Y: Osteosclerotic lesions in patients
treated with gefitinib for lung adenocarcinomas: A sign of
favorable therapeutic response. Skeletal Radiol. 41:409–414. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rong D, Mao Y, Yang Q, Xu S, Zhao Q and
Zhang R: Early osteosclerotic changes predict chemotherapy response
in non-small-cell lung cancer patients with bone metastases. Eur
Radiol. 28:4362–4369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang CY, Simeone FJ, Torriani M and
Bredella MA: Quantitative contrast-enhanced CT attenuation
evaluation of osseous metastases following chemotherapy. Skeletal
Radiol. 46:1385–1395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amir E, Whyne C, Freedman OC, Fralick M,
Kumar R, Hardisty M and Clemons M: Radiological changes following
second-line zoledronic acid treatment in breast cancer patients
with bone metastases. Clin Exp Metastasis. 26:479–484. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vassiliou V, Kalogeropoulou C,
Christopoulos C, Solomou E, Leotsinides M and Kardamakis D:
Combination ibandronate and radiotherapy for the treatment of bone
metastases: Clinical evaluation and radiologic assessment. Int J
Radiat Oncol Biol Phys. 67:264–272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quattrocchi CC, Piciucchi S, Sammarra M,
Santini D, Vincenzi B, Tonini G, Grasso RF and Zobel BB: Bone
metastases in breast cancer: Higher prevalence of osteosclerotic
lesions. Radiol Med. 112:1049–1059. 2007.(In Italian). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakata E, Sugihara S, Kataoka M, Yamashita
N, Furumatsu T, Takigawa T, Tetsunaga T and Ozaki T: Early response
assessment of re-ossification after palliative conventional
radiotherapy for vertebral bone metastases. J Orthop Sci.
24:332–336. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gettinger S, Horn L, Jackman D, Spigel D,
Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC,
et al: Five-year follow-up of nivolumab in previously treated
advanced non-small-cell lung cancer: Results from the CA209-003
study. J Clin Oncol. 36:1675–1684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nadal E, Massuti B, Dómine M,
García-Campelo R, Cobo M and Felip E: Immunotherapy with checkpoint
inhibitors in non-small cell lung cancer: Insights from long-term
survivors. Cancer Immunol Immunother. 68:341–352. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blons H, Garinet S, Laurent-Puig P and
Oudart JB: Molecular markers and prediction of response to
immunotherapy in non-small cell lung cancer, an update. J Thorac
Dis. 11 (Suppl 1):S25–S36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prelaj A, Tay R, Ferrara R, Chaput N,
Besse B and Califano R: Predictive biomarkers of response for
immune checkpoint inhibitors in non-small-cell lung cancer. Eur J
Cancer. 106:144–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoneda T and Hiraga T: Crosstalk between
cancer cells and bone microenvironment in bone metastasis. Biochem
Biophys Res Commun. 328:679–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sterling JA, Edwards JR, Martin TJ and
Mundy GR: Advances in the biology of bone metastasis: How the
skeleton affects tumor behavior. Bone. 48:6–15. 2011. View Article : Google Scholar : PubMed/NCBI
|