Introduction
Ovarian carcinoma is a common gynecological
malignancy and one of the leading causes of cancer-associated death
in women worldwide. In 2018 alone, it accounted for ~295,400 new
cases and ~184,800 deaths globally (1). Annually, there are ~52,100 new cases
and ~22,500 associated deaths in China (2). The majority patients with ovarian
carcinoma are diagnosed with epithelial ovarian carcinoma (EOC),
which accounts for ~90% of ovarian carcinoma cases (3). Most of the patients are diagnosed with
advanced disease due to the non-specific symptoms (4). Breakthroughs in treatment over the past
two decades have improved the prognosis of EOC slightly, but the
5-year overall survival rate is still <30% for patients with
advanced-stage EOC (5). According to
European Society for Medical Oncology and National Comprehensive
Cancer Network (NCCN) guidelines for ovarian carcinoma (6,7), the
standard initial chemotherapeutic treatment for patients with stage
II–IV ovarian carcinoma is based on carboplatin plus paclitaxel
regimens. Those who relapse ≥6 months or <6 months after initial
chemotherapy are termed as platinum-sensitive patients and
platinum-resistant patients, respectively (8). Almost all patients with recurrent
disease eventually develop platinum resistance (9). The available second-line treatment
regimens are liposomal doxorubicin, and weekly paclitaxel,
topotecan, gemcitabine and etoposide, respectively, as single
agents. These second-line treatment regimens have demonstrated an
objective response rate (ORR) ranging from 10–30% and a median
progression-free survival (PFS) time of 3–4 months (10,11). No
appropriate regimens were available for subsequent lines of
treatment (12).
It has been demonstrated that angiogenesis plays an
important role in tumor growth, recurrence and metastasis of EOC
(13). Anti-angiogenic therapy
consists of anti-vascular endothelial growth factor (VEGF)
antibodies and small molecule anti-angiogenesis tyrosine kinase
inhibitors (TKIs) (14).
Bevacizumab, a monoclonal antibody, has been approved for the
therapy of ovarian cancer (15).
Although a previous study showed that the combination of
bevacizumab with chemotherapy in ovarian cancer improves the ORR,
the administration of bevacizumab is ineffective and the prognosis
is clinically unsatisfactory (11).
Pazopanib was the first anti-angiogenic TKI, with positive clinical
significance in a phase II clinical trial, to be recommended by
NCCN guidelines of ovarian cancer (16). Subsequently, sorafenib demonstrated
promising clinical outcomes as a maintenance therapy for patients
with platinum-resistant ovarian cancer (17). These studies suggest the potential
therapeutic significance of anti-angiogenic TKIs in the treatment
of EOC.
Apatinib was the first anti-angiogenic TKI with
demonstrable efficacy and safety in patients with advanced gastric
cancer (18). Additionally, a
previous study supported the effectiveness of apatinib in EOC
(19). Generally, the clinical
application of anti-angiogenic drugs results in a relatively low
ORR. In advanced EOC, the ORR of monotherapy with pazopanib,
sorafenib and apatinib were recorded as 18 (16), 3.4 (20) and 41.4% (21), respectively. Therefore, these
individual differences between TKIs show that the clinical
application of apatinib has a promising effect in patients with
EOC. Unfortunately, no biomarker for patients with EOC who received
apatinib treatment is available to determine prognosis and monitor
disease progression clinically.
An important therapeutic target of apatinib is VEGF
receptor 2 (VEGFR2), which is located on chromosome 4q12 and
contains 30 exons (22). To date,
there are few studies investigating the polymorphism of
VEGFR2 in the Chinese population. rs2071559 is located in
the upstream region of VEGFR2 (23). A previous study indicated that
rs2071559 is significantly associated with a pathological complete
response in patients with advanced breast cancer treated with
capecitabine-based neoadjuvant therapy (24). Furthermore, studies involving
European and American populations have suggested that the
VEGFR2 polymorphism is associated with improved prognosis in
patients with advanced renal cell carcinoma who are treated with
sorafenib. However, the underlying mechanisms for this effect has
not been completely interpreted or understood (25).
Therefore, the present study aimed to explore the
clinical outcomes and safety of apatinib monotherapy in the
treatment of patients with advanced EOC who progressed after
standard regimens, and to analyze the VEGFR2 rs2071559
polymorphism.
Materials and methods
Study design and therapeutic
schedule
The present study was designed as a retrospective
analysis considering patients with ovarian carcinoma receiving
apatinib treatment. Therefore, patients with advanced ovarian
carcinoma that progressed after standard regimens between January
2015 and December 2018 in the Department of Gynecological Oncology
of Beijing Obstetrics and Gynecology Hospital (Beijing, China) were
enrolled in this study. The eligibility criteria included: i)
Histologically or cytologically confirmed diagnosis of epithelial
ovarian carcinoma confirmed by a pathological expert; ii)
pathological stage III or IV according to the International
Federation of Gynecology and Obstetrics (FIGO) staging system
(26); iii) female sex and age ≥18
years; iv) Eastern Cooperative Oncology Group (ECOG) performance
status of 0–2; v) patients treated with apatinib after at least two
failed standard lines of treatment, including patients that had
undergone secondary cytoreductive surgery after relapse; and vi) at
least one measurable target lesion according to Response Evaluation
Criteria in Solid Tumors (RECIST 1.1) (27). The exclusion criteria included: i)
Presence of concomitant tumors or serious diseases; ii) hemoptysis
>50 ml per day; iii) presence of uncontrolled hypertension or
serious presence of protein in the urine; and iv) a diagnosis of
squamous cell skin cancer or in situ cancer of the cervix
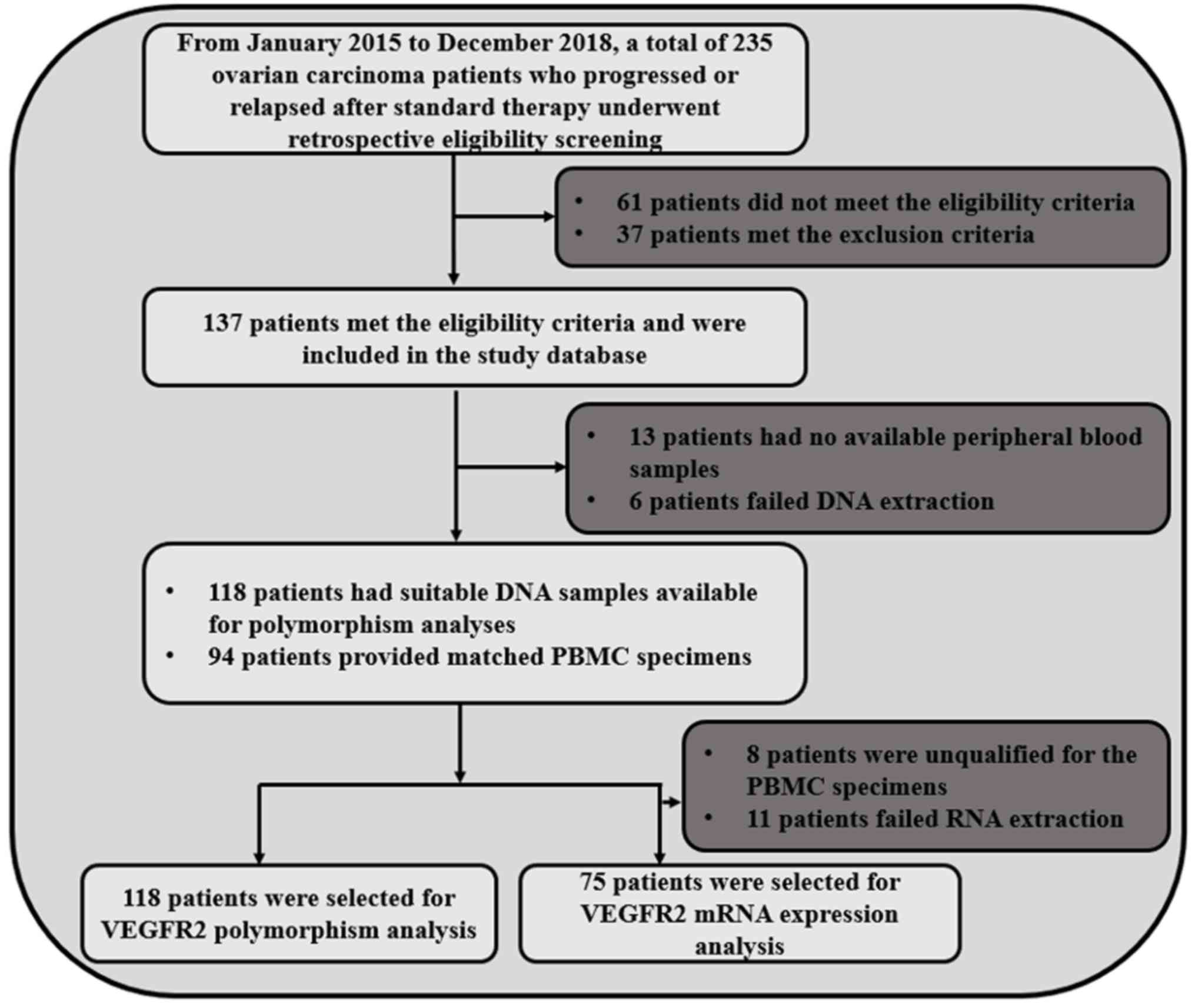
uteri. A flow chart of the study is illustrated in Fig. 1. The primary endpoint of the study
was PFS time, the secondary endpoint was ORR, overall survival (OS)
time and the analysis of VEGFR2 gene polymorphism.
Apatinib was administered at the initial dosage of
500 or 750 mg per day, orally with warm water, 30 min after meals,
and for 28 days as one cycle, until disease progression or
intolerable adverse reactions. The precise dose of apatinib was
determined according to the baseline physical conditions of the
patients, namely body surface area, ECOG score and age. The dose of
apatinib was adjusted according to the hematological (neutropenia)
or non-hematological toxicity (hypertension) during the treatment.
The treatment was discontinued when a potentially life-threatening
adverse reaction occurred. The present study was approved by the
Ethics Committee of Beijing Obstetrics and Gynecology Hospital
(Beijing, China). Written informed consent was provided by enrolled
patients or by their relatives.
The clinical outcomes were assessed according to
RECIST 1.1 criteria (27). The
change of target lesions was assessed with computed tomography (CT)
scans after the completion of the first cycle and then after every
two cycles, or more frequently if the clinical symptoms of the
patients deteriorated. The occurrence of adverse reactions during
treatment was evaluated using the Common Terminology Criteria for
Adverse Events version 4.03, in order to register hematological and
non-hematological events that may be drug-related (28). Adverse events classified as grade ≥2
with an incidence ≥10% were recorded and analyzed.
Collection of peripheral blood
specimens and genotyping of VEGFR2 polymorphism
Genomic DNA was extracted from whole venous blood
collected prior to treatment with apatinib during hospitalization
using phenol chloroform methods, according to the standard clinical
procedure. Single nucleotide polymorphisms of VEGFR2, with
the minor allele frequency >10% in the Chinese population, were
identified using the National Center for Biotechnology Information
database (https://www.ncbi.nlm.nih.gov/snp/), and included
rs2071559, rs2305948 and rs11941492. As shown in Table I, of the three polymorphisms analyzed
in the 118 patients with EOC, only rs2071559 was significantly
associated with PFS time. Therefore, the subsequent analysis of
this study was focused on the rs2071559 polymorphism. The rs2071559
polymorphism of VEGFR2 was genotyped through polymerase
chain reaction-restriction fragment length polymorphism (PCR-RFLP)
(Sangon Biotech Co., Ltd.). Initially, the PCR product including
this polymorphism was amplified, using SYBRGreen (Sangon Biotech
Co., Ltd.). The forward primer was 5′-TCACTAGGGCTCTTCGTTGG-3′ and
the reverse primer was 5′-GAAGCGGATACTCAGCCAAG-3′. PCR primers were
synthesized by Sangon Biotech Co., Ltd. PCR reaction conditions
were as follows: 94°C for 5 min; 36 cycles at 94°C for 45 sec,
63.5°C for 45 sec and 72°C for 30 sec; 72°C for 10 min. In total, 2
µl PCR products (size, 271 bp) were digested using the restriction
enzyme BsmI (Thermo Fisher Scientific Inc.). Agarose gel
electrophoresis (1%) was performed on the enzyme digestion
products. The genotypes of the polymorphism were determined by the
size of PCR bands as follows: TT genotype (one 271-bp band); CC
genotype (one 108-bp band and one 163-bp band); and TC genotype
(one 271-bp band, one 108-bp band and one 163-bp band).
 | Table I.Details of the polymorphisms included
in the present study and the preliminary analysis between genotypes
status and PFS. |
Table I.
Details of the polymorphisms included
in the present study and the preliminary analysis between genotypes
status and PFS.
| Polymorphism | Primers
(5′-3′) | Location | MAF | Median PFS time,
months | P-value |
|---|
| rs2071559 | F:
TCACTAGGGCTCTTCGTTGG | Upstream
region | 0.22 | 5.4 vs. 3.1 (TT vs.
TC/CC) | 0.015 |
|
| R:
GAAGCGGATACTCAGCCAAG |
|
|
|
|
| rs2305948 | F:
TTCCAAGACCATAGCTTACCA | Coding region | 0.15 | 4.7 vs. 4.3 (CC vs.
CT/TT) | 0.315 |
|
| R:
AATGTTTACCAAAGCCCAGA |
|
|
|
|
| rs11941492 | F:
TTGAGTTCCAATCTCAGCTTCA | Intron region | 0.28 | 4.5 vs. 4.8 (CC vs.
CT/TT) | 0.553 |
|
| R:
CTGGCCTTGAGAAAATCACC |
|
|
|
|
Collection of peripheral blood
mononuclear cell (PBMC) specimens and analysis of VEGFR2 mRNA
expression
Initially, the PBMC specimens were collected from 94
randomly matched samples in the 118 patients with EOC. However, 8
PBMC specimens were not available and RNA extraction failed in 11
PBMC specimens. Eventually, a total of 75 PBMC specimens were
available for further analysis and preserved in liquid nitrogen.
Total RNA samples were extracted using the TRIzol®
reagent (Bao Biological Engineering Co. Ltd.) according to the
manufacturer's instructions and stored at −80°C for mRNA expression
analysis. In total, 500 ng RNA extracted from each PBMC specimen
was used as a template for reverse transcription-PCR to prepare the
first strand of cDNA using the PrimeScript RT reagent kit (Bao
Biological Engineering Co., Ltd.) according to the manufacturer's
instructions. Relative quantitative analysis of VEGFR2 mRNA
expression was performed on the Roche LightCycler® 480
using a SYBR Premix EX Taq system (Bao Biological Engineering Co.,
Ltd.). The forward primer of VEGFR2 was
5′-ATGCAGAGCAAGGTGCTGC-3′ and the reverse primer was
5′-TTAAACAGGAGGAGAGCTCAGTG-3′. The amplification reaction (20 µl)
contained 10 µl SYBR Premix EX Taq, 0.2 µl each primer (20 µM), 7.6
µl double distilled water (ddH2O) and 2 µl cDNA. PCR reaction
conditions were as follows: 95°C for 5 min; 40 cycles at 95°C for
10 sec; 62°C for 10 sec; and 72°C for 10 sec. The mRNA expression
of VEGFR2 was detected using a comparative Cq method
(2−ΔΔCq) (29). The mRNA
expression of GAPDH was used as an endogenous control. The
forward primer of GAPDH was 5′-GCACCGTCAAGGCTGAGAAC-3′ and
the reverse primer was 5′-TGGTGAAGACGCCAGTGGA-3′.
Statistical analysis
All variables in this study were statistically
analyzed using the statistical analysis software SPSS version 25.0
(IBM Corp.). Hardy-Weinberg equilibrium was measured for the
rs2071559 genotypes using the χ2 test. The significance
of observed differences in proportions was assessed using the
χ2 test, and Fisher's exact test was performed when the
dataset was small. The analysis between continuous variables and
genotype status was performed using the Mann-Whitney U
non-parametric test (between two groups). The primary endpoint was
PFS. The Kaplan-Meier curves were drawn using Stata 14.0 (StataCorp
LP) to compare the differences in PFS and OS times according to
each genotype status. Survival differences were compared using a
log-rank test. PFS was defined as the period from the initial
treatment with apatinib up until disease progression or patient
death, whichever occurred first. OS was defined as the period from
the time of treatment with apatinib to patient death from any
cause. For those without disease progression or death by the end of
the study follow-up, the survival end points were censored at the
date of last follow-up. For the multivariable analysis, a Cox
proportional hazards model was constructed for PFS time, and the
backward selection procedure was used to adjust for potential
confounding covariates. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics of the 118
patients with EOC and genotypes of VEGFR2 rs2071559
polymorphism
The baseline characteristics of the 118 patients
with EOC are shown in Table II. The
median age of the patients was 57 years (range, 41–78 years) and
the ECOG 0 score was reported in 55 patients. According to FIGO
stage criteria, stage III was noted in 92 patients. The most common
histology of EOC was serous, diagnosed in 87 patients. Regarding
the first-line platinum-based chemotherapy response, 34 patients
were identified with platinum-refractory disease, 49 patients with
platinum-resistant disease and 35 patients were platinum-sensitive.
In terms of the tumor differentiation, the majority of patients (58
in total) were found to present with poorly differentiated tumors.
Two lines of previous treatment were reported in 21 patients and ≥3
lines of previous treatment were confirmed in 97 patients. Apatinib
was administered at an initial dosage of 500 and 750 mg in 86 and
32 patients, respectively. However, a dosage reduction had to be
implemented in a total of 48 patients due to hematological or
non-hematological toxicities.
 | Table II.Baseline characteristics of the 118
patients with EOC according to TT (n=72) or TC/CC (n=46) vascular
endothelial growth factor receptor 2 rs2071559 polymorphism
status. |
Table II.
Baseline characteristics of the 118
patients with EOC according to TT (n=72) or TC/CC (n=46) vascular
endothelial growth factor receptor 2 rs2071559 polymorphism
status.
|
|
| 4397T>C
genotypes |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Value | TT | TC/CC | P-value |
|---|
| Median age (range),
years | 57 (41–81) | 57 (41–79) | 58 (44–81) | 0.632 |
| ECOG score, n
(%) |
|
|
| 0.832 |
| 0 | 55 (46.61) | 33 (45.83) | 22 (47.83) |
|
|
1-2 | 63 (53.39) | 39 (54.17) | 24 (52.17) |
|
| FIGO stage, n
(%) |
|
|
| 0.951 |
|
III | 92 (77.97) | 56 (77.78) | 36 (78.26) |
|
| IV | 26 (22.03) | 16 (22.22) | 10 (21.74) |
|
| Histology of EOC, n
(%) |
|
|
| 0.971 |
|
Serous | 87 (73.73) | 53 (73.61) | 34 (73.91) |
|
|
Mixed | 31 (26.27) | 19 (26.39) | 12 (26.09) |
|
| First-line platinum
response, n (%) |
|
|
| 0.793 |
|
Platinum-refractory | 34 (28.81) | 20 (27.78) | 14 (30.43) |
|
|
Platinum-resistant, <6
months | 49 (41.53) | 29 (40.28) | 20 (43.48) |
|
|
Platinum-sensitive, ≥6
months | 35 (29.66) | 23 (31.94) | 12 (26.09) |
|
| Tumor
differentiation, n (%) |
|
|
| 0.949 |
| Well
differentiated | 19 (16.10) | 11 (15.28) | 8 (17.39) |
|
|
Intermediately
differentiated | 41 (34.75) | 25 (34.72) | 16 (34.78) |
|
| Poorly
differentiated | 58 (49.15) | 36 (50.00) | 22 (47.83) |
|
| Lines of previous
treatment regimens, n (%) |
|
|
| 0.927 |
| 2 | 21 (17.80) | 13 (18.06) | 8 (17.39) |
|
| ≥3 | 97 (82.20) | 59 (81.94) | 38 (82.61) |
|
| Initial dosage of
apatinib in mg, n (%) |
|
|
| 0.517 |
|
500 | 86 (72.88) | 54 (75.00) | 32 (69.57) |
|
|
750 | 32 (27.12) | 18 (25.00) | 14 (30.43) |
|
| Dose of reduction,
n (%) |
|
|
| 0.154 |
|
Yes | 48 (40.68) | 33 (45.83) | 15 (32.61) |
|
| No | 70 (59.32) | 39 (54.17) | 31 (67.39) |
|
Of the VEGFR2 polymorphisms analyzed, only
rs2071559 was of clinical significance (Table I). The germline mutation frequency of
rs2071559 among the 118 patients with EOC were as follows: TT
genotype, 72 cases (61.02%); TC genotype, 41 cases (34.75%); CC
genotype, 5 cases (4.23%), and the minor allele frequency of
rs2071559 was 0.22. The distribution of the three genotypes was in
accordance with the Hardy-Weinberg equilibrium (P=0.781). TC and CC
genotypes were merged in the subsequent analysis. As shown in
Table II, patients with TT and
TC/CC genotypes were well balanced, presenting similar baseline
characteristics. No significant differences were observed between
the two groups.
Influence of VEGFR2 rs2071559
polymorphism on the clinical outcomes of the 118 patients with
EOC
All 118 patients with EOC included in this study
were subjected to efficacy evaluation during the treatment with
apatinib, where the best overall response of each patient was
recorded. A complete response was not observed in any of the
patients, whereas a partial response was identified in 46 patients,
stable disease in 29 patients and progression disease in 43
patients. According to RECIST 1.1, the ORR was 38.98% and disease
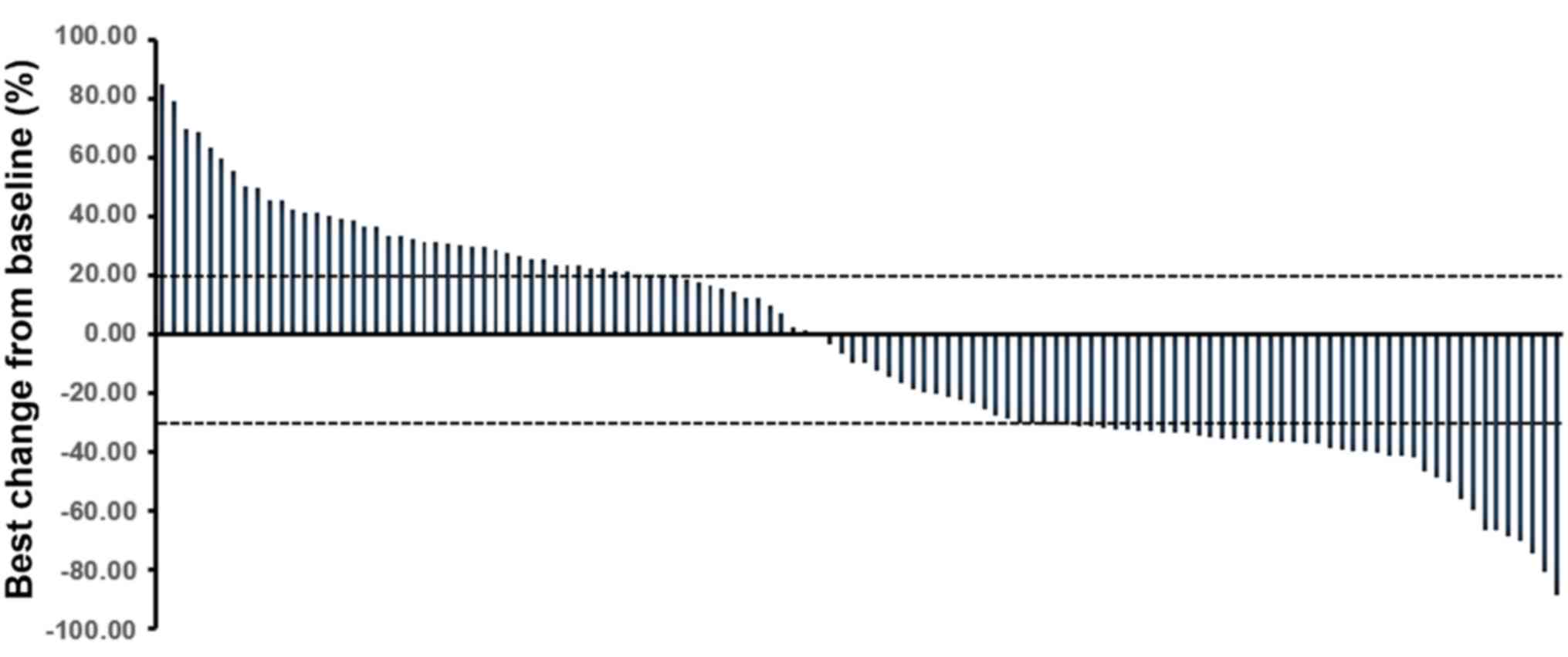
control rate (DCR) was 63.56%. The waterfall plot representing the
best percentage change in the dimensions of the target lesion is
shown in Fig. 2.
The last follow-up time of this study was June 2019.
The median follow-up time of all patients from the time of
enrollment to the last follow-up was 14.50 months (range, 1–36
months). The median PFS time of the 118 patients with EOC included
in this study was 4.65 months [95% confidence interval (CI),
3.45–5.80]. Additionally, the median PFS time according to
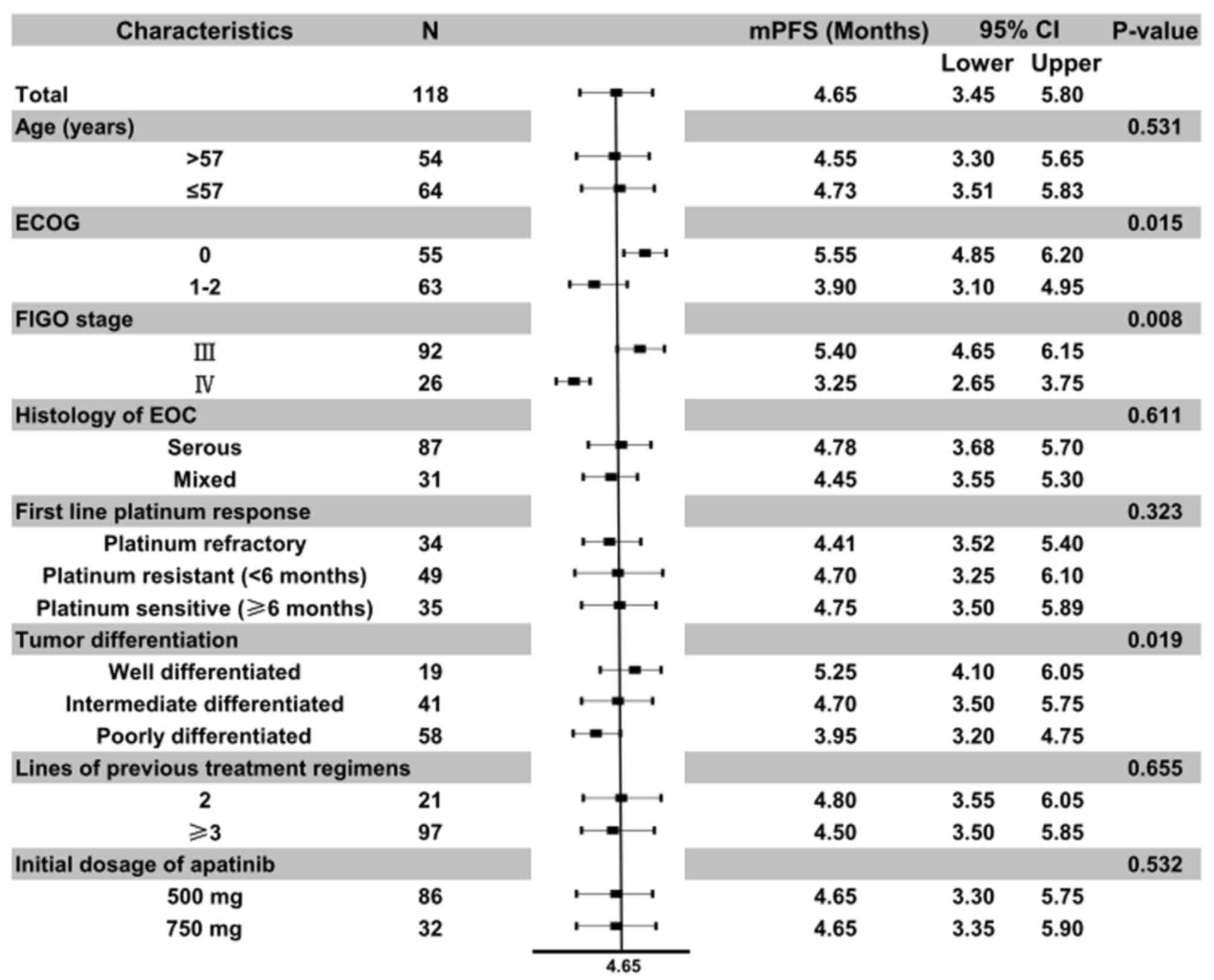
different baseline characteristic was further analyzed (Fig. 3). Univariate analysis according to
baseline characteristics suggested that ECOG score (P=0.015), FIGO
stage (P=0.008) and tumor differentiation (P=0.019) were
significantly associated with PFS time. In addition, the results
indicated that the median PFS time of patients with an ECOG score
of 0 was significantly longer compared with that of patients with a
score of 1–2 (5.55 vs. 3.90 months), the median PFS time of
patients with FIGO stage III was significantly higher compared with
that of patients with stage IV (5.40 vs. 3.25 months) and the
median PFS time of patients with well and intermediate
differentiation was significantly superior compared with that of
patients with poor differentiation (5.25 and 4.70 vs. 3.95
months).
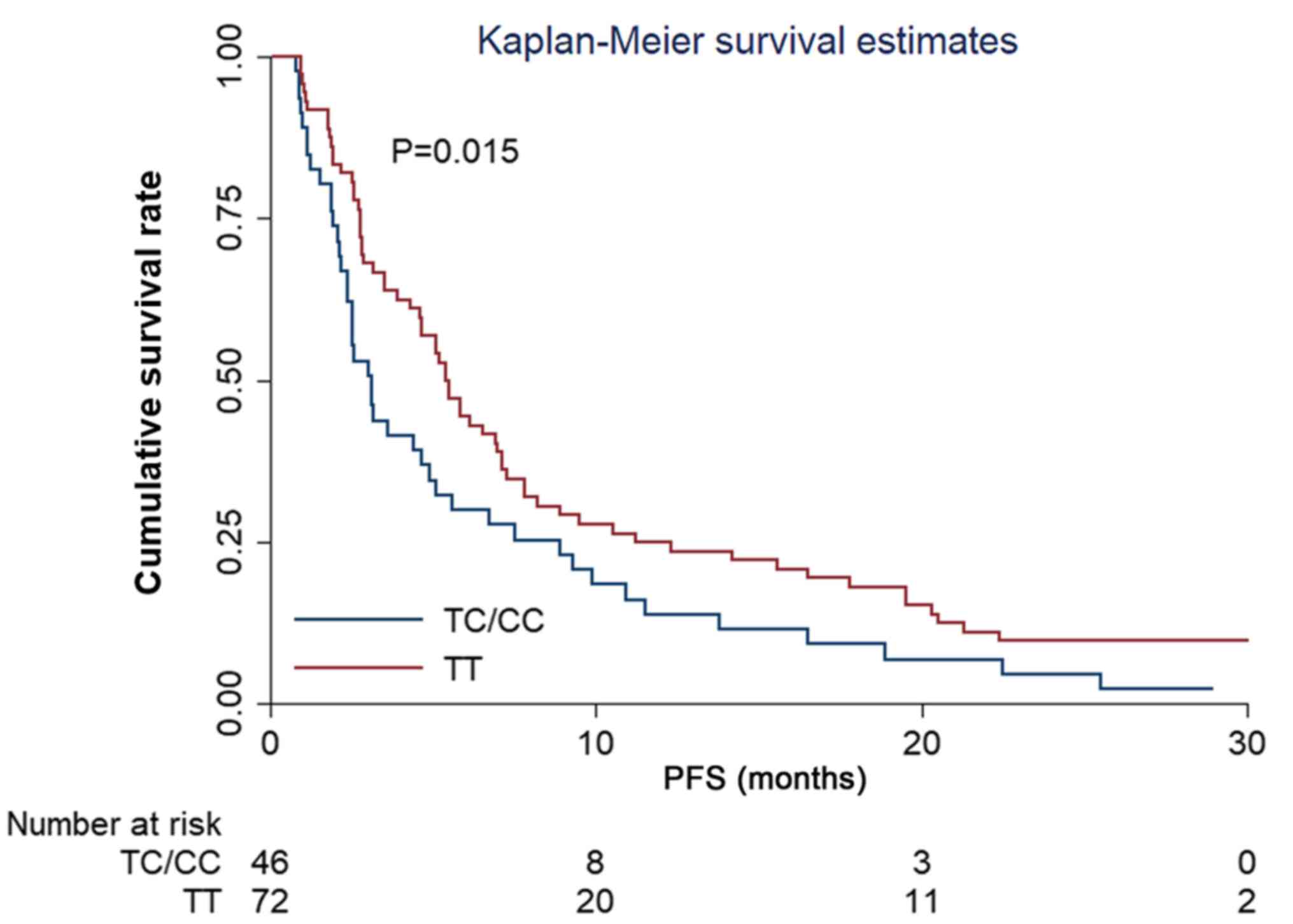
The analysis of the effect of the VEGFR2
rs2071559 polymorphism in terms of the PFS, showed that the median
PFS time between patients with TT and TC/CC genotypes was
significantly different, being 5.40 and 3.10 months, respectively
(χ2=6.50) (Fig. 4).
Furthermore, a Cox regression model was constructed. Results of the
multivariate analysis are shown in Table III. After adjustment for other
confounding factors, a significant difference was observed for the
influence of TC/CC genotype of rs2071559 on PFS time. Results
indicated that the rs2071559 polymorphism was an independent factor
for PFS time [hazard ratio (HR), 1.69; P=0.021]. Additionally, ECOG
high score (HR, 1.41; P=0.018), FIGO IV stage (HR, 1.81; P=0.011)
and tumor poor differentiation (HR, 1.28; P=0.046) were also
independent factors for PFS.
 | Table III.Multivariate Cox regression analysis
of progression-free survival according to baseline characteristics
and vascular endothelial growth factor receptor 2 rs2071559
polymorphism. |
Table III.
Multivariate Cox regression analysis
of progression-free survival according to baseline characteristics
and vascular endothelial growth factor receptor 2 rs2071559
polymorphism.
|
Characteristics | HR (95% CI) | df | P-value |
|---|
| ECOG score |
| 1 | 0.018 |
| 0 | 1.00a |
|
|
|
1-2 | 1.41
(1.21–1.68) |
|
|
| FIGO stage |
|
|
|
|
III | 1.00a |
|
|
| IV | 1.81
(1.38–2.36) | 1 | 0.011 |
| Tumor
differentiation |
| 1 | 0.046 |
| Well
and intermediately differentiated | 1.00a |
|
|
| Poorly
differentiated | 1.28
(1.02–1.51) |
|
|
| rs2071559
genotype |
| 1 | 0.021 |
| TT
genotype | 1.00a |
|
|
| TC/CC
genotype | 1.69
(1.21–1.95) |
|
|
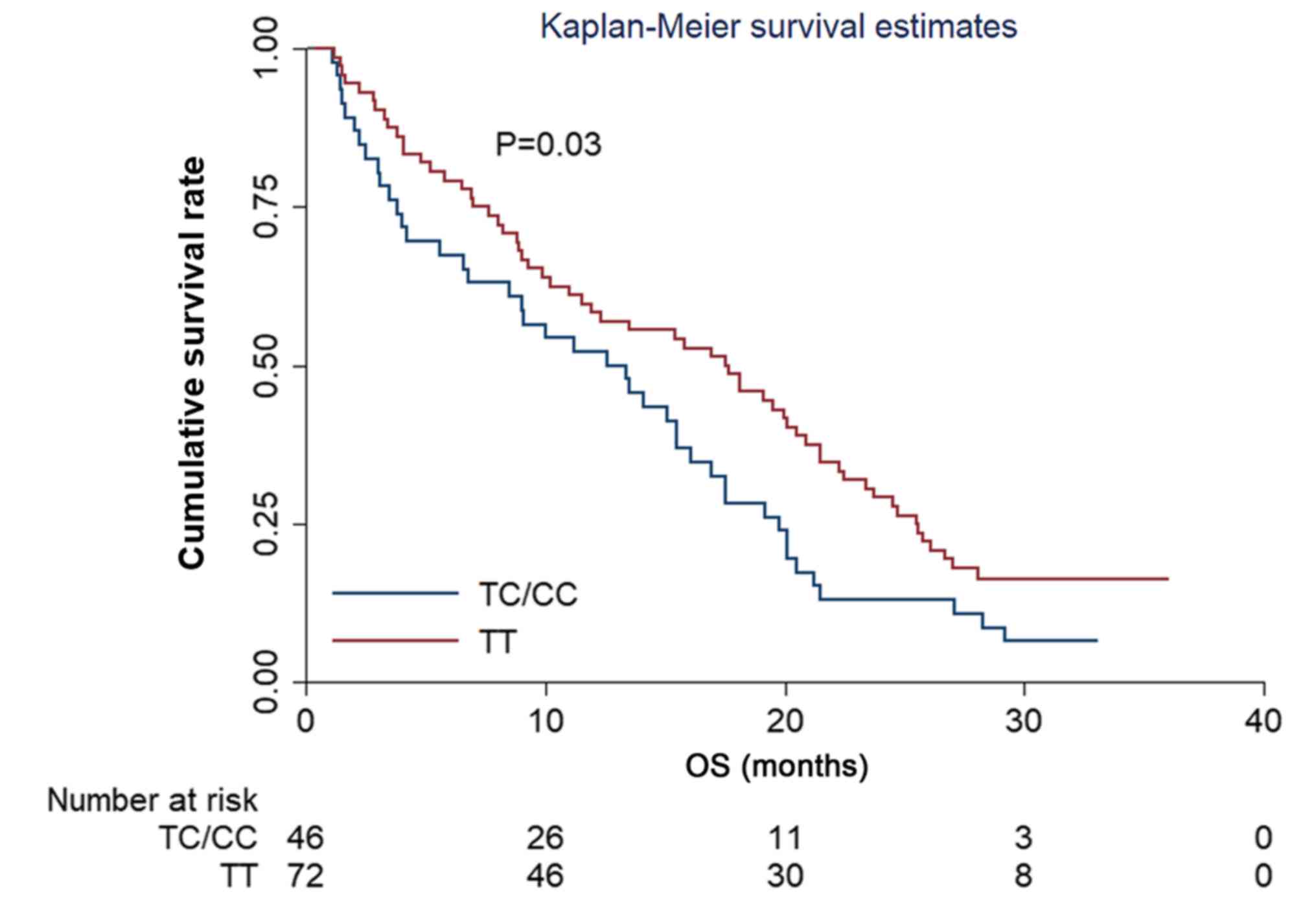
The evaluation of OS in the present study showed
that the median OS time of the 118 patients with EOC was 15.10
months (95% CI, 13.10–17.25). Regarding the effect of the
VEGFR2 rs2071559 polymorphism on OS, the results showed that
the median OS time between patients with TT and TC/CC genotypes was
significantly different, with an OS of time 12.60 and 17.50 months,
respectively (χ2=4.50) (Fig.
5).
Association between VEGFR2 rs2071559
polymorphism and the safety of patients with EOC
Adverse reactions classified as grade ≥2 with an
incidence ≥10% were analyzed. The most common treatment-related
adverse reactions were hand-foot syndrome, hypertension,
nausea/vomiting, proteinuria, fatigue and diarrhea, with an
incidence of 40.68, 25.42, 18.64, 13.56, 11.86 and 10.17%,
respectively (Table IV). The
evaluation of the effect of the rs2071559 polymorphism on the
incidence of adverse events grade ≥2 showed no significant
difference between the patients with the TT and TC/CC
genotypes.
 | Table IV.Analysis between vascular endothelial
growth factor receptor 2 rs2071559 genotype status and adverse
reactions classified as grade ≥2. |
Table IV.
Analysis between vascular endothelial
growth factor receptor 2 rs2071559 genotype status and adverse
reactions classified as grade ≥2.
|
|
| rs2071559 genotype
status, n (%) |
|
|---|
|
|
|
|
|
|---|
| Adverse
reactions | Total, n (%) | TT (n=72) | TC/CC (n=46) | P-value |
|---|
| Hand-foot
syndrome | 48 (40.68) | 30 (41.67) | 18 (39.13) | 0.784 |
| Hypertension | 30 (25.42) | 19 (26.39) | 11 (23.91) | 0.763 |
|
Nausea/vomiting | 22 (18.64) | 14 (19.44) | 8 (17.39) | 0.780 |
| Proteinuria | 16 (13.56) | 10 (13.89) | 6 (13.04) | 0.896 |
| Fatigue | 14 (11.86) | 9 (12.50) | 5 (10.87) | 0.789 |
| Diarrhea | 12 (10.17) | 8 (11.11) | 4 (8.70) | 0.672 |
Association between rs2071559
polymorphism and the mRNA expression of VEGFR2
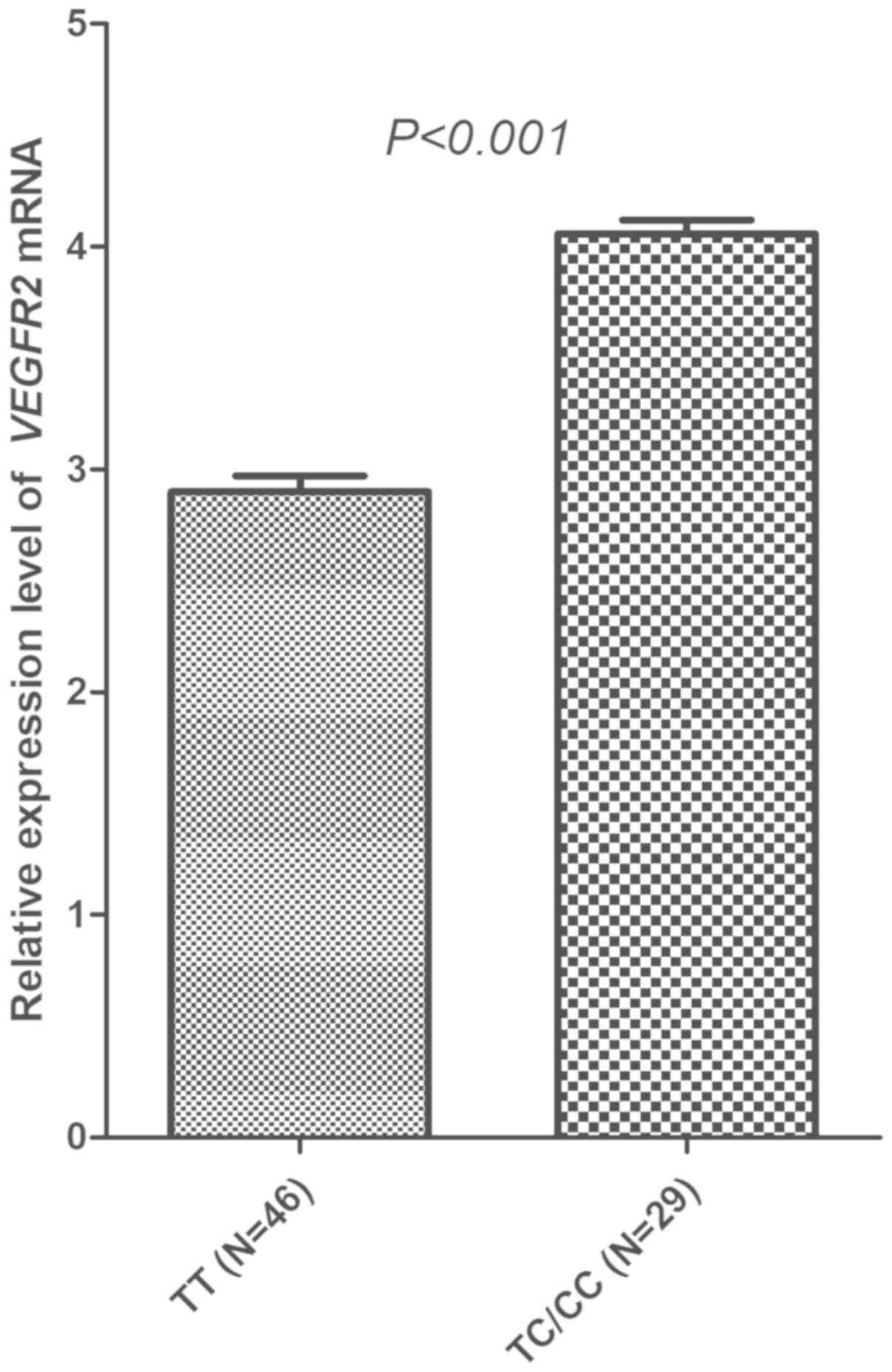
Across the 75 specimens included, the prevalence of
the VEGFR2 rs2071559 polymorphism was as follows: TT
genotype, 46 patients (61.33%); TC genotype, 26 patients (34.67%);
and CC genotype, 3 cases (4.00%). The prevalence was comparable
with the genotype frequency observed among the 118 patients with
EOC. The distribution of the three genotypes was in accordance with
the Hardy-Weinberg equilibrium (P=0.776). Similarly, CC and TC
genotypes were merged in the subsequent analysis, and patients with
the TC/CC genotype showed a significantly higher relative
expression of VEGFR2 mRNA in PBMC specimens compared with
those with the TT genotype (4.06±0.331 vs. 2.90±0.480; Fig. 6).
Discussion
The present retrospective study provides evidence
regarding the clinical outcomes and safety of apatinib in the
treatment for patients with EOC who progressed after standard
regimens. Polymorphism analysis indicated that patients with the
TC/CC genotype of the rs2071559 polymorphism were associated with
worse PFS and OS times. Furthermore, the results suggested that the
mRNA expression levels of VEGFR2 were significantly
different according to rs2071559 genotype status. Therefore, the
clinical outcomes of patients with EOC receiving apatinib therapy
may be influenced by the VEGFR2 rs2071559 polymorphism
through the mediation of VEGFR2 mRNA expression.
As an anti-angiogenic TKI, apatinib has been
demonstrated to be effective in gastric cancer, colorectal cancer,
lung cancer, soft tissue sarcoma and gynecological tumors (30–33). In
the present study, the clinical outcomes of 118 patients with EOC
receiving apatinib treatment were evaluated. The ORR was 38.98%,
the DCR was 63.56% and the median PFS time was 4.65 months, all
values that were lower than those reported in the phase II clinical
trial of apatinib in recurrent EOC by Miao et al (21), where ORR was 41.4%, DCR was 68.9% and
median PFS time was 5.10 months. It was hypothesized that the
reason for these discrepancies maybe attributed to the
retrospective design of the present study, with insufficient
patient management compared with clinical trials, which was also
observed in the other retrospective study, and the clinical
outcomes in this retrospective study were inferior to that in
clinical trials (34). Furthermore,
patients with an ECOG score of 2 were included in the present
study, in contrast to the phase II clinical trial, where these
patients were excluded. However, the clinical significance of ECOG
score has been confirmed in previous studies, suggesting that the
higher the score, the worse the prognosis (35,36). In
the present study, the results of Cox's regression analysis
indicated that patients with a score of 1–2 were associated with a
worse prognosis. Notably, the present median OS time was 15.10
months, which was slightly longer compared with that reported in
the phase II clinical trial with a median OS time of 14.50 months
(21). It is possible that the
reason for this difference may be the licensing of the PARP
inhibitor olaparib in 2018. Patients with EOC in China had the
opportunity to be treated with olaparib in the subsequent lines of
treatment, which was effective and offered an extended survival
benefit for the patients (37).
Although apatinib was licensed in China ~5 years
ago, only a few studies have investigated potential predictive
biomarkers for the treatment with apatinib. Results from a previous
study suggested that the occurrence of proteinuria or hand-foot
syndrome during treatment with apatinib could predict superior
clinical outcomes (38,39). However, polymorphism studies that
could indicate a potential benefit for the use of apatinib are
still limited Regarding EOC in particular, one study investigated
the effect of polymorphisms in the susceptibility of EOC (40). Previous studies have also indicated
that germline polymorphisms may play a role in the development of
ovarian cancer (41,42). To the best of our knowledge, the
present study was the first to research the effect of the presence
of a polymorphism in Chinese patients with EOC receiving apatinib
treatment. The result of polymorphism analysis was partly
consistent with that of a previous study by Scartozzi et al
(43). In the aforementioned study,
a total of 148 patients with advanced hepatocellular cancer treated
with sorafenib were enrolled and analyzed. Although, the prevalence
of the rs2071559 polymorphism was greater compared with that in the
present study (0.49 vs. 0.22), the clinical outcomes showed that
the prognosis of patients with the TC/CC genotype were worse, which
was consistent with the findings of the present study.
Additionally, the results of a recent study by Sullivan et
al (44) were also in line with
those of the present study. In the aforementioned study, patients
with advanced non-small cell lung cancer (NSCLC) treated with
platinum-based chemotherapy were enrolled, and the polymorphisms of
VEGF-A, VEGFR1 and VEGFR2 were investigated. The
results of multivariate analysis suggested that VEGF-A
rs2010963 and VEGFR2 rs2071559 were significantly associated
with prognosis. Specifically, patients with the TT genotype of
rs2071559 were associated with an improved prognosis, which was
consistent with the results of the present study, where the TT
genotype was associated with improved PFS and OS times. A recent
phase III clinical trial that investigated the effect of adjuvant
sunitinib in patients with high-risk renal cell carcinoma (45) suggested that the median disease-free
survival time was longer in patients with the TT genotype, which is
consistent with the effects observed for the TT genotype in the
present study. In conclusion, the VEGFR2 rs2071559
polymorphism might be of clinical significance as a biomarker for
the prediction of patients with EOC who might show an improved
response towards apatinib treatment.
With regard to the safety analysis, results from a
previous study indicated that hypertension is usually the most
common adverse reaction in patients with cancer receiving apatinib
treatment (46). However, the
present study reported that hand-foot syndrome was the most common
adverse reaction, with an incidence >40%, which was consistent
with the results observed in patients with EOC who received
sorafenib therapy (47). It
hypothesized that this effect might be attributed to a difference
in sex. A previous study suggested that the incidence of hand-foot
syndrome and other overall toxicity in women was higher than that
in men (48). Additionally, the
other adverse reactions classified as grade ≥2 with an incidence
≥10% included hypertension, nausea/vomiting, proteinuria, fatigue
and diarrhea, which were similar to the adverse reactions observed
in another previous study for apatinib treatment (49). In the present study, the association
analysis between genotype status and adverse reactions failed to
show any significant difference, which suggested that the
polymorphism was not associated with apatinib disposition for
specific adverse reactions.
Notably, mRNA expression analysis indicated that the
VEGFR2 mRNA expression levels were significantly different
according to rs2071559 polymorphism status. VEGFR2 is the receptor
with the strongest binding affinity to VEGF-A and has an important
role in signal transmission. In addition, the expression levels of
VEGFR2 show a crucial role in the process of angiogenesis
(50). Moreover, previous studies
also suggested that higher expression levels of VEGFR2 in
tumor cells were associated with a higher likelihood for tumor
cells to relapse and metastasize in gastric cancer and epithelial
cancer (51,52). In addition, higher expression levels
of VEGFR2 have been associated with worse PFS and OS times
in patients with NSCLC (53) and
cervical cancer (54), which is
consistent with the preliminary results of the present study.
There are a number of limitations to the present
study. Firstly, the sample size was small. Secondly, the study was
designed as a retrospective analysis, so some bias (no control
group and randomization) might be unavoidable. Thirdly, the present
study did not perform cell- and animal-based experiments. Lastly,
the results of the present study need to be validated in clinical
trials. However, the prognostic value of the VEGFR2
rs2071559 polymorphism was fully evaluated, and the determination
of VEGFR2 mRNA expression levels might indicate the
polymorphism and prognosis of patients with EOC. Therefore, the
present study was of clinical significance for the evaluation of
prognosis in patients with EOC who received apatinib treatment.
Acknowledgements
The authors would like to thank Professor Todd
Knepper from the America Moffitt Cancer Center for editing and
polishing the English language of the original manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY, YYG, WD and YG conceived and designed the study.
ZY, XDH, QZ and MW collected the characteristics of the patients
and performed the experiments. ZY and HLK analyzed the data. YYG,
MW and YG contributed with reagents and analysis tools. ZY, YYG, WD
and YG wrote the paper. All authors read and approved the final
manuscript and agreed to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work was appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Beijing Obstetrics and Gynecology Hospital, Capital Medical
University (Beijing, China) (approval no. 2017-KY-01105). Written
informed consent was obtained from all patients or their family
members. Only in circumstances where the patients had passed away
or the patient was unaware of their diagnosis of EOC, was written
informed consent obtained from their relatives.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li X, Zhang Y, Chai X, Zhou S, Zhang H, He
J, Zhou R, Cai L, Chen L and Tao G: Overexpression of MEF2D
contributes to oncogenic malignancy and chemotherapeutic resistance
in ovarian carcinoma. Am J Cancer Res. 9:887–905. 2019.PubMed/NCBI
|
|
4
|
Mari R, Mamessier E, Lambaudie E,
Provansal M, Birnbaum D, Bertucci F and Sabatier R: Liquid biopsies
for ovarian carcinoma: How blood tests may improve the clinical
management of a deadly disease. Cancers (Basel). 11:7742019.
View Article : Google Scholar
|
|
5
|
Liu CL, Pan HW, Torng PL, Fan MH and Mao
TL: SRPX and HMCN1 regulate cancer-associated fibroblasts to
promote the invasiveness of ovarian carcinoma. Oncol Rep.
42:2706–2715. 2019.PubMed/NCBI
|
|
6
|
Morgan RJ Jr, Armstrong DK, Alvarez RD,
Bakkum-Gamez JN, Behbakht K, Chen LM, Copeland L, Crispens MA,
DeRosa M, Dorigo O, et al: Ovarian cancer, version 1.2016, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
14:1134–1163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ledermann JA, Raja FA, Fotopoulou C,
Gonzalez-Martin A, Colombo N and Sessa C; ESMO Guidelines Working
Group, : Newly diagnosed and relapsed epithelial ovarian carcinoma:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 29 (Suppl 4):iv2592018. View Article : Google Scholar
|
|
8
|
Canaz E, Grabowski JP, Richter R, Braicu
EI, Chekerov R and Sehouli J: Survival and prognostic factors in
patients with recurrent low-grade epithelial ovarian cancer: An
analysis of five prospective phase II/III trials of NOGGO metadata
base. Gynecol Oncol. 154:539–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Champer M, Huang Y, Hou JY, Tergas AI,
Burke WM, Hillyer GC, Ananth CV, Neugut AI, Hershman DL and Wright
JD: Adherence to treatment recommendations and outcomes for women
with ovarian cancer at first recurrence. Gynecol Oncol. 148:19–27.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corrado G, Salutari V, Palluzzi E,
Distefano MG, Scambia G and Ferrandina G: Optimizing treatment in
recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther.
17:1147–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monk BJ, Randall LM and Grisham RN: The
evolving landscape of chemotherapy in newly diagnosed advanced
epithelial ovarian cancer. Am Soc Clin Oncol Educ Book.
39:e141–e151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin B, Song X, Yang D, Bai D, Yao Y and Lu
N: Anlotinib inhibits angiogenesis via suppressing the activation
of VEGFR2, PDGFRβ and FGFR1. Gene. 654:77–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Nicum S, Reichardt P, Croitoru K,
Illek B, Schmidinger M, Rogers C, Whalen C and Jayson GC:
Assessment and management of diarrhea following VEGF receptor TKI
treatment in patients with ovarian cancer. Gynecol Oncol.
150:173–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burger RA, Sill MW, Monk BJ, Greer BE and
Sorosky JI: Phase II trial of bevacizumab in persistent or
recurrent epithelial ovarian cancer or primary peritoneal cancer: A
gynecologic oncology group study. J Clin Oncol. 25:5165–5171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedlander M, Hancock KC, Rischin D,
Messing MJ, Stringer CA, Matthys GM, Ma B, Hodge JP and Lager JJ: A
Phase II, open-label study evaluating pazopanib in patients with
recurrent ovarian cancer. Gynecol Oncol. 119:32–37. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chekerov R, Hilpert F, Mahner S, El-Balat
A, Harter P, De Gregorio N, Fridrich C, Markmann S, Potenberg J,
Lorenz R, et al: Sorafenib plus topotecan versus placebo plus
topotecan for platinum-resistant ovarian cancer (TRIAS): A
multicentre, randomised, double-blind, placebo-controlled, phase 2
trial. Lancet Oncol. 19:1247–1258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao D, Hou H and Zhang X: Progress in the
treatment of solid tumors with apatinib: A systematic review. Onco
Targets Ther. 11:4137–4147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matei D, Sill MW, Lankes HA, DeGeest K,
Bristow RE, Mutch D, Yamada SD, Cohn D, Calvert V, Farley J, et al:
Activity of sorafenib in recurrent ovarian cancer and primary
peritoneal carcinomatosis: A gynecologic oncology group trial. J
Clin Oncol. 29:69–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miao M, Deng G, Luo S, Zhou J, Chen L,
Yang J, He J, Li J, Yao J, Tan S and Tang J: A phase II study of
apatinib in patients with recurrent epithelial ovarian cancer.
Gynecol Oncol. 148:286–290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Wu M, Zhang B, Xiong X, Wang H and
Zhou X: Analysis of genetic polymorphisms for age-related macular
degeneration (AMD) in Chinese Tujia ethnic minority group. BMC Med
Genet. 20:252019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song ZZ, Zhao LF, Zuo J, Fan ZS, Wang L
and Wang YD: Clinical outcomes and safety of apatinib mesylate in
the treatment of advanced non-squamous non-small cell lung cancer
in patients who progressed after standard therapy and analysis of
the KDR gene polymorphism. Onco Targets Ther. 13:603–613. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Babyshkina N, Zavyalova M, Tarabanovskaya
N, Dronova T, Krakhmal N, Slonimskaya E, Kzhyshkowska J, Choynzonov
E and Cherdyntseva N: Predictive value of vascular endothelial
growth factor receptor type 2 in triple-negative breast cancer
patients treated with neoadjuvant chemotherapy. Mol Cell Biochem.
444:197–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Escudier B, Rini BI, Motzer RJ, Tarazi J,
Kim S, Huang X, Rosbrook B, English PA, Loomis AK and Williams JA:
Genotype correlations with blood pressure and efficacy from a
randomized phase III trial of second-line axitinib versus sorafenib
in metastatic renal cell carcinoma. Clin Genitourin Cancer.
13:328–337.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mert I, Kumar A, Torres D, Huang Y, McGree
ME, Weaver AL and Cliby WA: Should mucosal bowel invasion in
ovarian cancer be assigned to FIGO stage IV disease? Gynecol Oncol.
153:238–241. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miller TP, Fisher BT, Getz KD, Sack L,
Razzaghi H, Seif AE, Bagatell R, Adamson PC and Aplenc R:
Unintended consequences of evolution of the common terminology
criteria for adverse events. Pediatr Blood Cancer. 66:e277472019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian Z, Gu Z, Wang X, Liu Z, Yao W, Wang
J, Zhang P, Cai Q and Ge H: Efficacy and safety of apatinib in
treatment of osteosarcoma after failed standard multimodal therapy:
An observational study. Medicine (Baltimore). 98:e156502019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Qiu T, Zhu Y, Sun J, Li P, Wang B,
Lin P, Cai X, Han X, Zhao F, et al: A single-arm, phase II study of
apatinib in refractory metastatic colorectal cancer. Oncologist.
24:883–e407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao Z, Li F, Zhang C, Zhu L, Shi Y, Zhao
G, Bai X, Hassan S, Liu X, Li T, et al: Phase II trial of VEGFR2
inhibitor apatinib for metastatic sarcoma: Focus on efficacy and
safety. Exp Mol Med. 51:1–11. 2019. View Article : Google Scholar
|
|
33
|
Qiu H, Li J, Liu Q, Tang M and Wang Y:
Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor
growth in cervical cancer and synergizes with paclitaxel. Cell
Cycle. 17:1235–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yilmaz E, Sahin N, Koleli I, Melekoglu R,
Tanrikut E, Faydali S, Karaer A and Coskun EI: Retrospective
analysis of borderline ovarian tumors: Outcomes at a single center.
Acta Clin Croat. 58:29–36. 2019.PubMed/NCBI
|
|
35
|
Agemi Y, Shimokawa T, Sasaki J, Miyazaki
K, Misumi Y, Sato A, Aida S, Ishii M, Nakamura Y, Naoki K and
Okamoto H: Prospective evaluation of the G8 screening tool for
prognostication of survival in elderly patients with lung cancer: A
single-institution study. PLoS One. 14:e02104992019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu JY, Zhu BR, Wang YD and Sun X: The
efficacy and safety of Apatinib mesylate in the treatment of
metastatic osteosarcoma patients who progressed after standard
therapy and the VEGFR2 gene polymorphism analysis. Int J Clin
Oncol. 25:1195–1205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boussios S, Karihtala P, Moschetta M,
Karathanasi A, Sadauskaite A, Rassy E and Pavlidis N: Combined
strategies with poly (ADP-Ribose) polymerase (PARP) inhibitors for
the treatment of ovarian cancer: A literature review. Diagnostics
(Basel). 9:872019. View Article : Google Scholar
|
|
38
|
Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai
Y, Wang Z, Yang Y, Sun G, Wang L, et al: Early presence of
anti-angiogenesis-related adverse events as a potential biomarker
of antitumor efficacy in metastatic gastric cancer patients treated
with apatinib: A cohort study. J Hematol Oncol. 10:1532017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang SC, Huang W, Zhang YM, Zhang HT and
Xie WP: Hypertension as a predictive biomarker in patients with
advanced non-small-cell lung cancer treated with apatinib. Onco
Targets Ther. 12:985–992. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lawrenson K, Song F, Hazelett DJ, Kar SP,
Tyrer J, Phelan CM, Corona RI, Rodríguez-Malavé NI, Seo JH, Adler
E, et al: Genome-wide association studies identify susceptibility
loci for epithelial ovarian cancer in east Asian women. Gynecol
Oncol. 153:343–355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Narod SA: Personalised medicine and
population health: Breast and ovarian cancer. Hum Genet.
137:769–778. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jin Y: Association between EPHX1
polymorphism rs1051740 and the risk of ovarian cancer: A
meta-analysis. Artif Cells Nanomed Biotechnol. 47:2338–2342. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scartozzi M, Faloppi L, Svegliati Baroni
G, Loretelli C, Piscaglia F, Iavarone M, Toniutto P, Fava G, De
Minicis S, Mandolesi A, et al: VEGF and VEGFR genotyping in the
prediction of clinical outcome for HCC patients receiving
sorafenib: The ALICE-1 study. Int J Cancer. 135:1247–1256. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sullivan I, Riera P, Andrés M, Altés A,
Majem M, Blanco R, Capdevila L, Barba A, Barnadas A and Salazar J:
Prognostic effect of VEGF gene variants in metastatic
non-small-cell lung cancer patients. Angiogenesis. 22:433–440.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
George DJ, Martini JF, Staehler M, Motzer
RJ, Magheli A, Donskov F, Escudier B, Li S, Casey M, Valota O, et
al: Phase III trial of adjuvant sunitinib in patients with
high-risk renal cell carcinoma: Exploratory pharmacogenomic
analysis. Clin Cancer Res. 25:1165–1173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu Y, Feng B, Mei L, Sun R, Guo C and Zhu
J: Clinical efficacy of TACE combined with apatinib in the
treatment of advanced hepatocellular carcinoma. J BUON. 24:608–614.
2019.PubMed/NCBI
|
|
47
|
Bodnar L, Górnas M and Szczylik C:
Sorafenib as a third line therapy in patients with epithelial
ovarian cancer or primary peritoneal cancer: A phase II study.
Gynecol Oncol. 123:33–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mueller F, Büchel B, Köberle D, Schürch S,
Pfister B, Krähenbühl S, Froehlich TK, Largiader CR and Joerger M:
Gender-specific elimination of continuous-infusional 5-fluorouracil
in patients with gastrointestinal malignancies: Results from a
prospective population pharmacokinetic study. Cancer Chemother
Pharmacol. 71:361–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lan CY, Wang Y, Xiong Y, Li JD, Shen JX,
Li YF, Zheng M, Zhang YN, Feng YL, Liu Q, et al: Apatinib combined
with oral etoposide in patients with platinum-resistant or
platinum-refractory ovarian cancer (AEROC): A phase 2, single-arm,
prospective study. Lancet Oncol. 19:1239–1246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cho HD, Moon KD, Park KH, Lee YS and Seo
KI: Effects of auriculasin on vascular endothelial growth factor
(VEGF)-induced angiogenesis via regulation of VEGF receptor 2
signaling pathways in vitro and in vivo. Food Chem Toxicol.
121:612–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu Y, Xu Q, Zuo Y, Liu L, Liu S, Chen L,
Wang K, Lei Y, Zhao X and Li Y: Isoprenaline/β2-AR activates
Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells
to promote tumor angiogenesis. BMC Cancer. 17:8752017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jinesh GG, Manyam GC, Mmeje CO, Baggerly
KA and Kamat AM: Surface PD-L1, E-cadherin, CD24, and VEGFR2 as
markers of epithelial cancer stem cells associated with rapid
tumorigenesis. Sci Rep. 7:96022017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ding M, Liu L, Hu C, Liu Y, Qiao Y and
Jiang X: Expression of VEGFR2 and NRP-1 in non-small cell lung
cancer and their clinical significance. Chin J Cancer Res.
26:669–677. 2014.PubMed/NCBI
|
|
54
|
Dang YZ, Zhang Y, Li JP, Hu J, Li WW, Li
P, Wei LC and Shi M: High VEGFR1/2 expression levels are predictors
of poor survival in patients with cervical cancer. Medicine
(Baltimore). 96:e57722017. View Article : Google Scholar : PubMed/NCBI
|