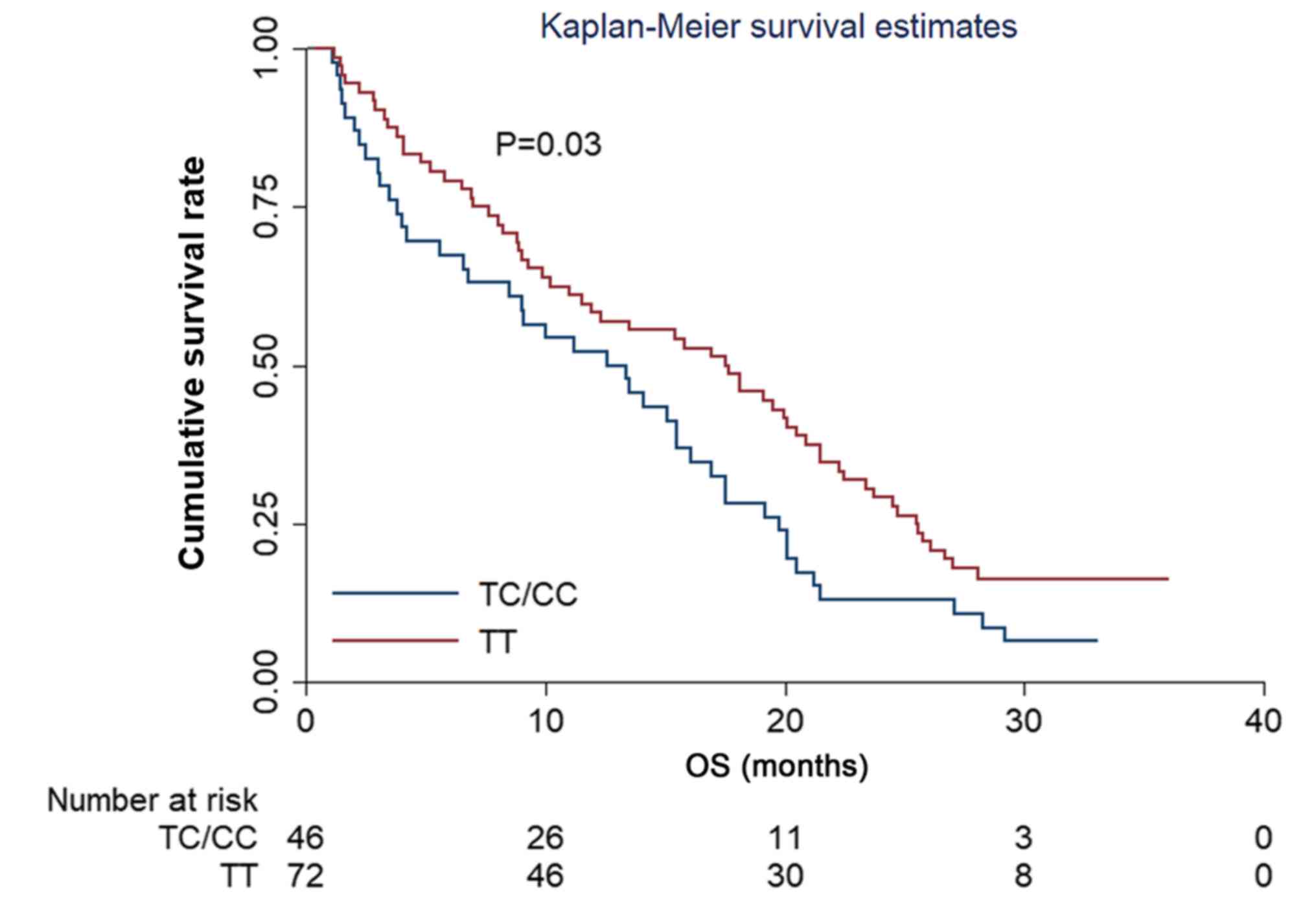
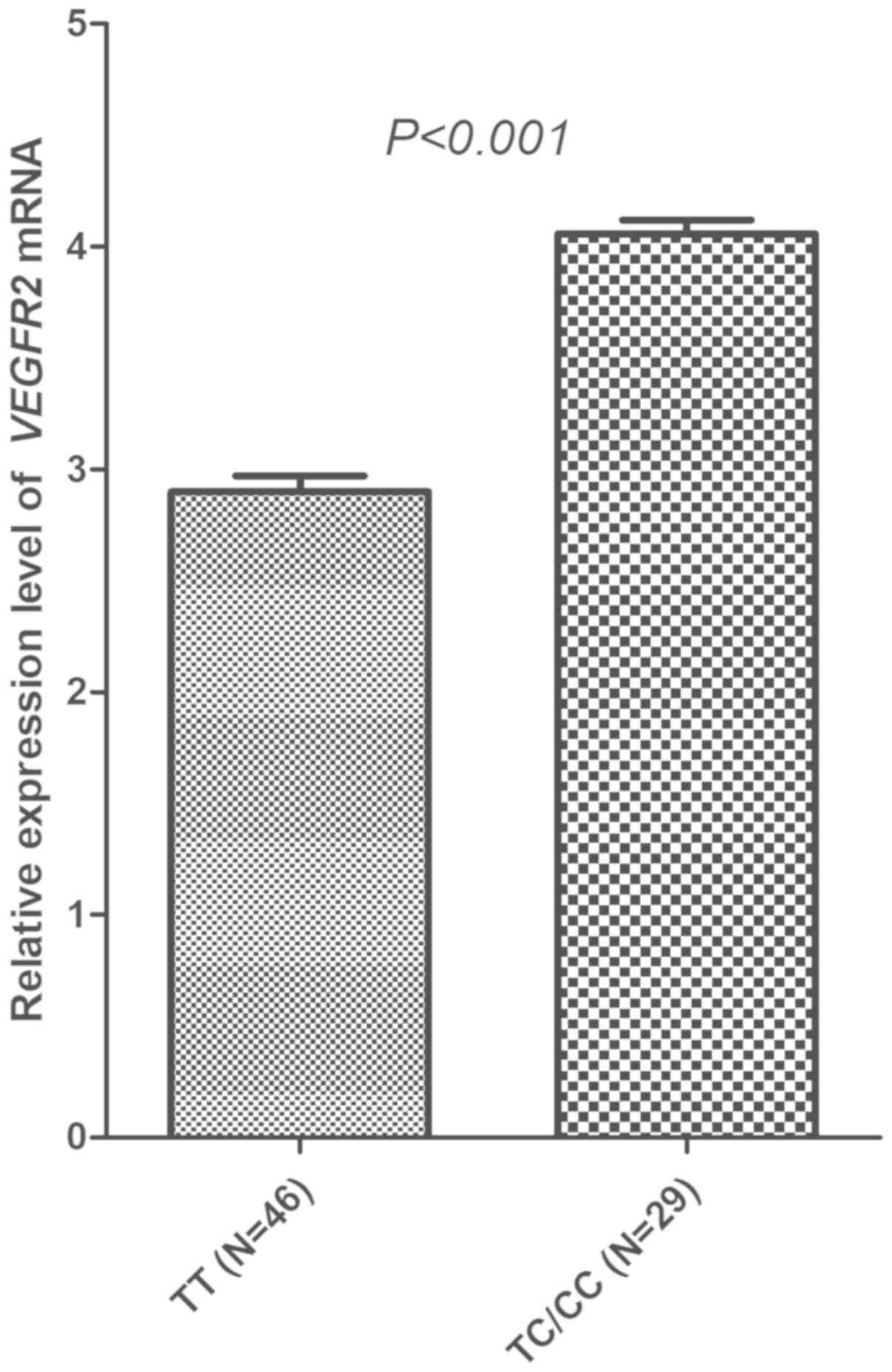
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li X, Zhang Y, Chai X, Zhou S, Zhang H, He
J, Zhou R, Cai L, Chen L and Tao G: Overexpression of MEF2D
contributes to oncogenic malignancy and chemotherapeutic resistance
in ovarian carcinoma. Am J Cancer Res. 9:887–905. 2019.PubMed/NCBI
|
|
4
|
Mari R, Mamessier E, Lambaudie E,
Provansal M, Birnbaum D, Bertucci F and Sabatier R: Liquid biopsies
for ovarian carcinoma: How blood tests may improve the clinical
management of a deadly disease. Cancers (Basel). 11:7742019.
View Article : Google Scholar
|
|
5
|
Liu CL, Pan HW, Torng PL, Fan MH and Mao
TL: SRPX and HMCN1 regulate cancer-associated fibroblasts to
promote the invasiveness of ovarian carcinoma. Oncol Rep.
42:2706–2715. 2019.PubMed/NCBI
|
|
6
|
Morgan RJ Jr, Armstrong DK, Alvarez RD,
Bakkum-Gamez JN, Behbakht K, Chen LM, Copeland L, Crispens MA,
DeRosa M, Dorigo O, et al: Ovarian cancer, version 1.2016, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
14:1134–1163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ledermann JA, Raja FA, Fotopoulou C,
Gonzalez-Martin A, Colombo N and Sessa C; ESMO Guidelines Working
Group, : Newly diagnosed and relapsed epithelial ovarian carcinoma:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 29 (Suppl 4):iv2592018. View Article : Google Scholar
|
|
8
|
Canaz E, Grabowski JP, Richter R, Braicu
EI, Chekerov R and Sehouli J: Survival and prognostic factors in
patients with recurrent low-grade epithelial ovarian cancer: An
analysis of five prospective phase II/III trials of NOGGO metadata
base. Gynecol Oncol. 154:539–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Champer M, Huang Y, Hou JY, Tergas AI,
Burke WM, Hillyer GC, Ananth CV, Neugut AI, Hershman DL and Wright
JD: Adherence to treatment recommendations and outcomes for women
with ovarian cancer at first recurrence. Gynecol Oncol. 148:19–27.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corrado G, Salutari V, Palluzzi E,
Distefano MG, Scambia G and Ferrandina G: Optimizing treatment in
recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther.
17:1147–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monk BJ, Randall LM and Grisham RN: The
evolving landscape of chemotherapy in newly diagnosed advanced
epithelial ovarian cancer. Am Soc Clin Oncol Educ Book.
39:e141–e151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin B, Song X, Yang D, Bai D, Yao Y and Lu
N: Anlotinib inhibits angiogenesis via suppressing the activation
of VEGFR2, PDGFRβ and FGFR1. Gene. 654:77–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Nicum S, Reichardt P, Croitoru K,
Illek B, Schmidinger M, Rogers C, Whalen C and Jayson GC:
Assessment and management of diarrhea following VEGF receptor TKI
treatment in patients with ovarian cancer. Gynecol Oncol.
150:173–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burger RA, Sill MW, Monk BJ, Greer BE and
Sorosky JI: Phase II trial of bevacizumab in persistent or
recurrent epithelial ovarian cancer or primary peritoneal cancer: A
gynecologic oncology group study. J Clin Oncol. 25:5165–5171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedlander M, Hancock KC, Rischin D,
Messing MJ, Stringer CA, Matthys GM, Ma B, Hodge JP and Lager JJ: A
Phase II, open-label study evaluating pazopanib in patients with
recurrent ovarian cancer. Gynecol Oncol. 119:32–37. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chekerov R, Hilpert F, Mahner S, El-Balat
A, Harter P, De Gregorio N, Fridrich C, Markmann S, Potenberg J,
Lorenz R, et al: Sorafenib plus topotecan versus placebo plus
topotecan for platinum-resistant ovarian cancer (TRIAS): A
multicentre, randomised, double-blind, placebo-controlled, phase 2
trial. Lancet Oncol. 19:1247–1258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao D, Hou H and Zhang X: Progress in the
treatment of solid tumors with apatinib: A systematic review. Onco
Targets Ther. 11:4137–4147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matei D, Sill MW, Lankes HA, DeGeest K,
Bristow RE, Mutch D, Yamada SD, Cohn D, Calvert V, Farley J, et al:
Activity of sorafenib in recurrent ovarian cancer and primary
peritoneal carcinomatosis: A gynecologic oncology group trial. J
Clin Oncol. 29:69–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miao M, Deng G, Luo S, Zhou J, Chen L,
Yang J, He J, Li J, Yao J, Tan S and Tang J: A phase II study of
apatinib in patients with recurrent epithelial ovarian cancer.
Gynecol Oncol. 148:286–290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Wu M, Zhang B, Xiong X, Wang H and
Zhou X: Analysis of genetic polymorphisms for age-related macular
degeneration (AMD) in Chinese Tujia ethnic minority group. BMC Med
Genet. 20:252019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song ZZ, Zhao LF, Zuo J, Fan ZS, Wang L
and Wang YD: Clinical outcomes and safety of apatinib mesylate in
the treatment of advanced non-squamous non-small cell lung cancer
in patients who progressed after standard therapy and analysis of
the KDR gene polymorphism. Onco Targets Ther. 13:603–613. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Babyshkina N, Zavyalova M, Tarabanovskaya
N, Dronova T, Krakhmal N, Slonimskaya E, Kzhyshkowska J, Choynzonov
E and Cherdyntseva N: Predictive value of vascular endothelial
growth factor receptor type 2 in triple-negative breast cancer
patients treated with neoadjuvant chemotherapy. Mol Cell Biochem.
444:197–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Escudier B, Rini BI, Motzer RJ, Tarazi J,
Kim S, Huang X, Rosbrook B, English PA, Loomis AK and Williams JA:
Genotype correlations with blood pressure and efficacy from a
randomized phase III trial of second-line axitinib versus sorafenib
in metastatic renal cell carcinoma. Clin Genitourin Cancer.
13:328–337.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mert I, Kumar A, Torres D, Huang Y, McGree
ME, Weaver AL and Cliby WA: Should mucosal bowel invasion in
ovarian cancer be assigned to FIGO stage IV disease? Gynecol Oncol.
153:238–241. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miller TP, Fisher BT, Getz KD, Sack L,
Razzaghi H, Seif AE, Bagatell R, Adamson PC and Aplenc R:
Unintended consequences of evolution of the common terminology
criteria for adverse events. Pediatr Blood Cancer. 66:e277472019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian Z, Gu Z, Wang X, Liu Z, Yao W, Wang
J, Zhang P, Cai Q and Ge H: Efficacy and safety of apatinib in
treatment of osteosarcoma after failed standard multimodal therapy:
An observational study. Medicine (Baltimore). 98:e156502019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Qiu T, Zhu Y, Sun J, Li P, Wang B,
Lin P, Cai X, Han X, Zhao F, et al: A single-arm, phase II study of
apatinib in refractory metastatic colorectal cancer. Oncologist.
24:883–e407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao Z, Li F, Zhang C, Zhu L, Shi Y, Zhao
G, Bai X, Hassan S, Liu X, Li T, et al: Phase II trial of VEGFR2
inhibitor apatinib for metastatic sarcoma: Focus on efficacy and
safety. Exp Mol Med. 51:1–11. 2019. View Article : Google Scholar
|
|
33
|
Qiu H, Li J, Liu Q, Tang M and Wang Y:
Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor
growth in cervical cancer and synergizes with paclitaxel. Cell
Cycle. 17:1235–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yilmaz E, Sahin N, Koleli I, Melekoglu R,
Tanrikut E, Faydali S, Karaer A and Coskun EI: Retrospective
analysis of borderline ovarian tumors: Outcomes at a single center.
Acta Clin Croat. 58:29–36. 2019.PubMed/NCBI
|
|
35
|
Agemi Y, Shimokawa T, Sasaki J, Miyazaki
K, Misumi Y, Sato A, Aida S, Ishii M, Nakamura Y, Naoki K and
Okamoto H: Prospective evaluation of the G8 screening tool for
prognostication of survival in elderly patients with lung cancer: A
single-institution study. PLoS One. 14:e02104992019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu JY, Zhu BR, Wang YD and Sun X: The
efficacy and safety of Apatinib mesylate in the treatment of
metastatic osteosarcoma patients who progressed after standard
therapy and the VEGFR2 gene polymorphism analysis. Int J Clin
Oncol. 25:1195–1205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boussios S, Karihtala P, Moschetta M,
Karathanasi A, Sadauskaite A, Rassy E and Pavlidis N: Combined
strategies with poly (ADP-Ribose) polymerase (PARP) inhibitors for
the treatment of ovarian cancer: A literature review. Diagnostics
(Basel). 9:872019. View Article : Google Scholar
|
|
38
|
Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai
Y, Wang Z, Yang Y, Sun G, Wang L, et al: Early presence of
anti-angiogenesis-related adverse events as a potential biomarker
of antitumor efficacy in metastatic gastric cancer patients treated
with apatinib: A cohort study. J Hematol Oncol. 10:1532017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang SC, Huang W, Zhang YM, Zhang HT and
Xie WP: Hypertension as a predictive biomarker in patients with
advanced non-small-cell lung cancer treated with apatinib. Onco
Targets Ther. 12:985–992. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lawrenson K, Song F, Hazelett DJ, Kar SP,
Tyrer J, Phelan CM, Corona RI, Rodríguez-Malavé NI, Seo JH, Adler
E, et al: Genome-wide association studies identify susceptibility
loci for epithelial ovarian cancer in east Asian women. Gynecol
Oncol. 153:343–355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Narod SA: Personalised medicine and
population health: Breast and ovarian cancer. Hum Genet.
137:769–778. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jin Y: Association between EPHX1
polymorphism rs1051740 and the risk of ovarian cancer: A
meta-analysis. Artif Cells Nanomed Biotechnol. 47:2338–2342. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scartozzi M, Faloppi L, Svegliati Baroni
G, Loretelli C, Piscaglia F, Iavarone M, Toniutto P, Fava G, De
Minicis S, Mandolesi A, et al: VEGF and VEGFR genotyping in the
prediction of clinical outcome for HCC patients receiving
sorafenib: The ALICE-1 study. Int J Cancer. 135:1247–1256. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sullivan I, Riera P, Andrés M, Altés A,
Majem M, Blanco R, Capdevila L, Barba A, Barnadas A and Salazar J:
Prognostic effect of VEGF gene variants in metastatic
non-small-cell lung cancer patients. Angiogenesis. 22:433–440.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
George DJ, Martini JF, Staehler M, Motzer
RJ, Magheli A, Donskov F, Escudier B, Li S, Casey M, Valota O, et
al: Phase III trial of adjuvant sunitinib in patients with
high-risk renal cell carcinoma: Exploratory pharmacogenomic
analysis. Clin Cancer Res. 25:1165–1173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu Y, Feng B, Mei L, Sun R, Guo C and Zhu
J: Clinical efficacy of TACE combined with apatinib in the
treatment of advanced hepatocellular carcinoma. J BUON. 24:608–614.
2019.PubMed/NCBI
|
|
47
|
Bodnar L, Górnas M and Szczylik C:
Sorafenib as a third line therapy in patients with epithelial
ovarian cancer or primary peritoneal cancer: A phase II study.
Gynecol Oncol. 123:33–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mueller F, Büchel B, Köberle D, Schürch S,
Pfister B, Krähenbühl S, Froehlich TK, Largiader CR and Joerger M:
Gender-specific elimination of continuous-infusional 5-fluorouracil
in patients with gastrointestinal malignancies: Results from a
prospective population pharmacokinetic study. Cancer Chemother
Pharmacol. 71:361–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lan CY, Wang Y, Xiong Y, Li JD, Shen JX,
Li YF, Zheng M, Zhang YN, Feng YL, Liu Q, et al: Apatinib combined
with oral etoposide in patients with platinum-resistant or
platinum-refractory ovarian cancer (AEROC): A phase 2, single-arm,
prospective study. Lancet Oncol. 19:1239–1246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cho HD, Moon KD, Park KH, Lee YS and Seo
KI: Effects of auriculasin on vascular endothelial growth factor
(VEGF)-induced angiogenesis via regulation of VEGF receptor 2
signaling pathways in vitro and in vivo. Food Chem Toxicol.
121:612–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu Y, Xu Q, Zuo Y, Liu L, Liu S, Chen L,
Wang K, Lei Y, Zhao X and Li Y: Isoprenaline/β2-AR activates
Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells
to promote tumor angiogenesis. BMC Cancer. 17:8752017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jinesh GG, Manyam GC, Mmeje CO, Baggerly
KA and Kamat AM: Surface PD-L1, E-cadherin, CD24, and VEGFR2 as
markers of epithelial cancer stem cells associated with rapid
tumorigenesis. Sci Rep. 7:96022017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ding M, Liu L, Hu C, Liu Y, Qiao Y and
Jiang X: Expression of VEGFR2 and NRP-1 in non-small cell lung
cancer and their clinical significance. Chin J Cancer Res.
26:669–677. 2014.PubMed/NCBI
|
|
54
|
Dang YZ, Zhang Y, Li JP, Hu J, Li WW, Li
P, Wei LC and Shi M: High VEGFR1/2 expression levels are predictors
of poor survival in patients with cervical cancer. Medicine
(Baltimore). 96:e57722017. View Article : Google Scholar : PubMed/NCBI
|