Introduction
Cervical cancer is the second most common type of
gynecological cancer in developing countries, with incidence and
mortality rates only second to breast cancer in China (1). In 2018, there were 106,000 novel cases
of cervical cancer and 33,000 mortalities in China (2). Human papillomavirus (HPV) has been
proven to be the cause of numerous reproductive tract diseases and
is the major cause of cervical cancer (3,4).
According to the risk degree of tumor occurrence associated with
infection with various strains of HPV, these strains may be divided
into high-risk (HR-HPV) and low-risk (LR-HPV) types. In particular,
persistent infection with HR-HPV is the major cause of cervical
cancer and oropharyngeal cancer (5),
while LR-HPV infection mainly leads to genital warts (4).
As HPV has an important role in the natural etiology
of cervical cancer, HR-HPV screening is more sensitive and
cost-effective than other screening methods (6). In recent years, large-scale vaccination
projects have been performed in numerous countries and the
preventive effect of HPV vaccines on HPV-associated diseases has
been confirmed in various studies (7,8). In
2014, Gardasil 9, a vaccine that protects against HPV6, 11, 16, 18,
31, 33, 45, 52 and 58, was approved by the US Food and Drug
Administration and its application was launched. However, Gardasil
9 does not cover all HR-HPV types that may lead to cervical cancer
and the prevalence of HPV types is affected by geography, with a
certain degree of variation in different countries and regions
(9).
In China, although certain studies have been
performed to assess the prevalence and incidence rates of HPV
genotypes in Yunnan, Henan and Qingdao, most of them are based on
small samples (10–12). Xinjiang Province, located in the
northwestern part of China, is relatively undeveloped from an
economical and cultural perspective. There is a large Uygur
population in this region and the HPV distribution reported in
previous studies was only investigated in small numbers of subjects
(13). However, no previous survey
has performed any large-sample screening in this population.
Therefore, it is necessary to investigate the
prevalence and type distribution of HPV in Uyghur females in
Xinjiang Province, northwest China. In the present study, a larger
sample size (12,165) of the population was screened to assess the
prevalence and type distribution of HPV infection in Xinjiang. The
present results will contribute to the evaluation of the potential
cost-effectiveness of HPV screening and vaccination in Xinjiang
province.
Materials and methods
Study population
The present study included females in 12 villages in
Zepu county, Xinjiang Province. A total of 12,165 Uyghur females
attended the Maternal and Child Health Hospital and outpatient
clinics and expressed their willingness to undergo cervical cancer
screening between January 2015 and December 2017. Subjects were
included if they met the following criteria: i) History of sexual
activity; no current pregnancy, menstruation and lactation; ii) no
history of hormone therapy in the past 6 months; iii) no history of
cancer; iv) no history of cervical surgery, radiotherapy or
chemotherapy; and v) no history of treatment for HPV infection.
HPV-DNA testing
A total of 12,165 samples were tested by the HPV
Liquid Bead Microarray (CapitalBio Medical Laboratory Center),
which is able to detect 18 types of HR-HPV (16, 18, 31, 33, 35, 39,
45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82 and 83) and 10 types of
LR-HPV (6, 11, 40, 42, 43, 44, 54, 61, 70 and 81).
Instruments and reagents
A Luminex 200 multi-function flow matrix (Thermo
Fisher Scientific, Inc.), Mastercycler Gradient gene amplification
instrument (Eppendorf) were used in the present study. The DNA
extraction, PCR amplification, bead-coated hybridization, digital
signal processing, biotin universal primers, biotin marking
β-globin PCR primers (Table SI),
and Luminex suspension beads and floating microsphere hybridization
probe were provided by Tellgen Corporation.
Cervical specimen collection
The cervical exfoliated cells of the included women
were collected by the clinician using a specialized cervical brush.
Each cervical specimen was placed in 2.5 ml of a sampling tube with
a special cell preservation solution (Tellgen Corporation). All
samples were delivered to the laboratory at −20°C and tested within
48 h.
DNA extraction
The samples in the preservation solution were
thoroughly mixed before 1 ml of each sample was placed in a
centrifuge tube. The supernatant was collected following
centrifugation at 21,578 × g for 5 min at 4°C, and the nucleic acid
was extracted using a nucleic acid extraction kit after washing
with normal saline. Finally, the sample DNA was dissolved in 50 µl
elution buffer (Tellgen Corporation) and stored at −20°C.
Luminex liquid-phase chip primer
design
The detection of HPV genotypes in tissues by Luminex
liquid-phase chip technology was performed by PCR amplification
using universal primers covering different HPV subtypes; these
primers target the high-protection zone of HPV L1 fragments in
samples. At the same time, to quantify the experimental process and
minimize sampling and instrument errors, an internal control
comprising β-globin PCR primers for the detection of the augmented
β-globin gene was included.
HPV typing and hybridization
assay
Multiple PCR flow hybridization was used to detect
HPV infection in the DNA extracted from the samples, including 18
types of HR-HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58,
59, 66, 68, 73, 82 and 83) and 10 types of LR-HPV (6, 11, 40, 42,
43, 44, 54, 61, 70 and 81). A total of 5 µl of each extracted DNA
sample was used for PCR amplification. PCR was performed at 94°C
for 30 sec, then at 58°C for 30 sec and at 72°C for 30 sec for 5
cycles. Subsequently, PCR was performed at 94°C for 30 sec, then at
55°C for 30 sec and 72°C for 30 sec for 35 cycles, with a final
extension at 72°C for 5 min. PCR products obtained by the above
methods were rapidly hybridized at 94°C for 5 min and at 48°C for
30 min. Analyses of hybridization and its related data were
performed using the Luminex 200 multi-functional streaming lattice
system (Thermo Fisher Scientific, Inc.) and its corresponding
xPONENT® data analysis software (version 3.1; Thermo
Fisher Scientific, Inc.), respectively.
Statistical analysis
Statistical analyses were performed using SPSS
version 22 for Windows (IBM Corp.) for the calculation of the
overall and type-specific prevalence of HPV. All genotypes from
single and multiple infections were computed individually. These
data were stratified by age (≤35, 36–40, 41–45, 46–50, 51–55, 56–60
and ≥61 years). The χ2 test was used to compare the
prevalence between different age groups. P<0.05 (two-sided) was
considered to indicate a statistically significant difference.
Results
Prevalence of HPV infection in
Xinjiang province
A total of 12,217 individuals were initially
considered and total of 12,165 individuals (average age, 44.55±7.41
years) were included in subsequent analyses, after 52 individuals
were excluded from further analysis due to lack of data on the HPV
genotype. The prevalence of HPV infections is presented in Table I. Among the 12,165 individuals, the
overall HPV-positive rate was 9.34% (1,136/12,165). The
HR-HPV-positive rate was 7.41% (901/12,165). Among the HPV-positive
females, 936 were positive for a single HPV type (936/1,136, 82.39%
of HPV infections; 936/12,165, 7.70% of all samples) and 200 were
positive for multiple types (200/1,136, 17.61% of HPV infections;
200/12,165, 1.64% of all samples). In addition, 79.31% (901/1,136)
had HR-HPV infections, 15.32% (174/1,136) had LR-HPV infections and
only 5.37% (61/1,136) had both HR- and LR-HPV infections.
 | Table I.Prevalence of HPV infection in
different age groups. |
Table I.
Prevalence of HPV infection in
different age groups.
| Age group
(years) | Mean age
(years) | Total (n) | Single
infection | Multiple
infection | HR-HPV
infection | LR-HPV
infection | HR & LR-HPV
infection | Total
HPV-positive |
|---|
| ≤35 | 33.91±2.21 | 1050 | 66
(6.29) | 11
(1.05) | 58
(5.52) | 18 (1.71) |
1(0.10) | 77
(7.33) |
| 36-40 | 38.16±1.42 | 2954 | 200 (6.77) | 41
(1.39) | 180 (6.09) | 47 (1.60) | 14(0.47) | 241 (8.16) |
| 41-54 | 43.09±1.41 | 3552 | 252 (7.09) | 51
(1.44) | 238 (6.70) | 49 (1.38) | 16(0.45) | 303 (8.53) |
| 46-50 | 47.80±1.45 | 2215 | 191 (8.62) | 35
(1.58) | 183 (8.26) | 30 (1.35) | 13(0.59) | 226 (10.20) |
| 51-55 | 52.77±1.41 | 1220 | 111 (9.10) | 22
(1.80) | 117 (9.59) | 11 (0.90) |
5(0.41) | 133 (10.90) |
| 56-60 | 57.87±1.45 |
742 | 78 (10.51) | 14
(1.89) | 70
(9.43) | 18 (2.43) |
4(0.53) | 92
(12.40) |
| ≥61 | 63.45±2.32 |
432 | 38
(8.80) | 26
(6.02) | 55 (12.73) | 1 (0.23) |
1(0.23) | 64
(14.81) |
| Total | 44.55±7.41 | 12165 | 936 (7.69) | 200 (1.64) | 901 (7.41) | 174 (1.43) | 61(0.50) | 1136 (9.34) |
Age-specific prevalence of HPV
infection
The age-stratified HPV DNA prevalence across the
different age groups is presented in Table I. The prevalence of HPV infection was
significantly different among the age groups (χ2=41.574,
P<0.001) and was highest in the ≥61-year-old group (14.81%,
64/432). HPV-positive rates increased with age
(χ2=38.880, P<0.001). The HPV-positive rate was
significantly higher in females aged 50 years and above (12.07%,
289/2,394) than in females aged up to 50 years (8.67%, 847/9,771;
χ2=26.307, P<0.001). The prevalence of multiple HPV
infections and frequency of HR-HPV infections were also highest in
the ≥61-year-old group (6.02%, 26/432 and 12.73%, 55/432). The
prevalence of multiple HPV infections and the frequency of HR-HPV
infections also increased with age (χ2=16.485,
P<0.001; χ2=4.619, P=0.032). The increase in HPV
prevalence was most pronounced in the groups aged 56–60 and >60
years. The prevalence of single HPV infections and LR-HPV
infections was highest in the 56–60-year-old group (10.51%, 78/742
and 2.43%, 18/742), but LR-HPV and single HPV infections were
lowest in the ≥61- and ≤35-year-old groups, respectively.
Distribution and genotypes of HPV
infections
The positive rate and the distribution of HPV
infection are listed in Table II.
The most common HR-HPV types were HPV16 (30.28%), HPV31 (10.65%),
HPV68 (9.42%) and HPV52 (7.48%), and the most common LR-HPV types
were HPV54 (10.48%), HPV42 (3.26%), HPV6 (2.99%) and HPV61
(1.94%).
 | Table II.Prevalence of each HPV genotype. |
Table II.
Prevalence of each HPV genotype.
| HPV genotype | Positive samples
(n) | Positive rate in
12,165 total samples (%) | Proportion among
1,136 HPV-positive samples (%) |
|---|
| HR-HPV |
|
|
|
| 16 | 344 | 2.83 | 30.28 |
| 18 | 32 | 0.26 | 2.82 |
| 31 | 121 | 0.99 | 10.65 |
| 33 | 16 | 0.13 | 1.41 |
| 35 | 30 | 0.25 | 2.64 |
| 39 | 60 | 0.49 | 5.28 |
| 45 | 37 | 0.30 | 3.26 |
| 51 | 62 | 0.51 | 5.46 |
| 52 | 85 | 0.70 | 7.48 |
| 53 | 17 | 0.14 | 1.50 |
| 56 | 29 | 0.24 | 2.55 |
| 58 | 49 | 0.40 | 4.31 |
| 59 | 49 | 0.40 | 4.31 |
| 66 | 59 | 0.48 | 5.19 |
| 68 | 107 | 0.88 | 9.42 |
| 73 | 6 | 0.05 | 0.53 |
| 82 | 14 | 0.12 | 1.23 |
| 83 | 10 | 0.08 | 0.88 |
| LR-HPV |
|
|
|
| 6 | 34 | 0.28 | 2.99 |
| 11 | 7 | 0.06 | 0.62 |
| 40 | 10 | 0.08 | 0.88 |
| 42 | 37 | 0.30 | 3.26 |
| 43 | 7 | 0.06 | 0.62 |
| 44 | 5 | 0.04 | 0.44 |
| 54 | 119 | 0.98 | 10.48 |
| 61 | 22 | 0.18 | 1.94 |
| 70 | 4 | 0.03 | 0.35 |
| 81 | 3 | 0.02 | 0.26 |
Among the positive cases, the prevalence of each
HR-HPV type was significantly different between the different age
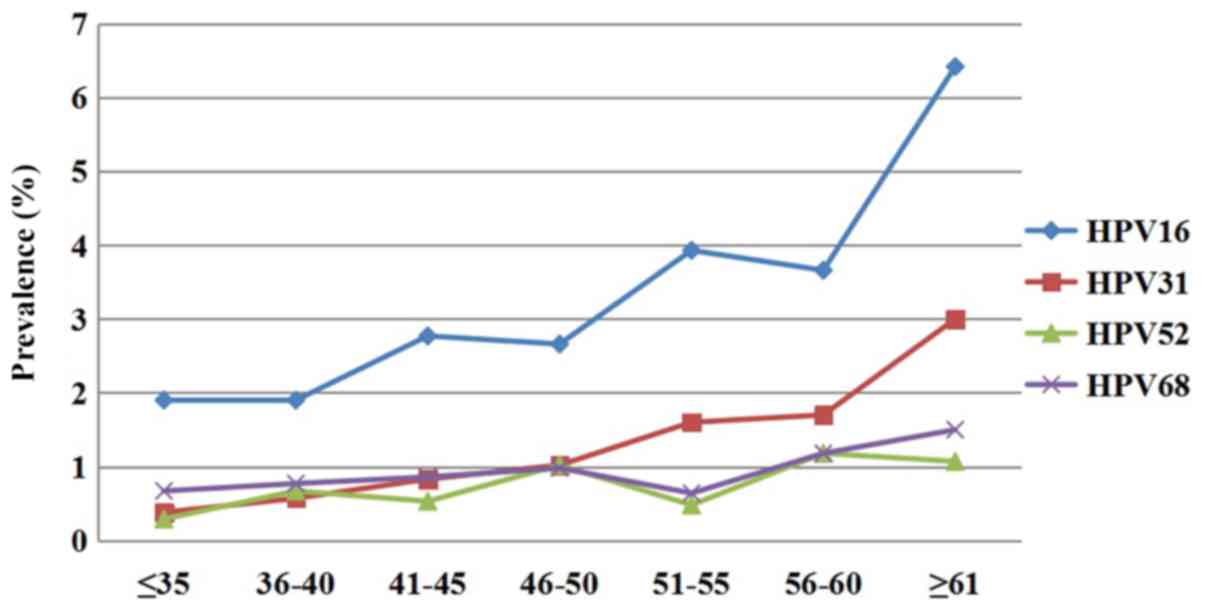
groups (χ2=6.454, P=0.011; Fig. 1). HPV16, 31, 68 and 52 were
consistently the four most common types of HR-HPV in each age
group. The prevalence of HPV16 was the highest in the ≥61-year-old
group, followed by the 51–55- and 56–60-year-old groups. The
prevalence of HPV31 was the highest in the ≥61-year-old group,
followed by the 56–60- and 51–55-year-old groups. However, the
prevalence of HPV52 was highest in the 56–60-year-old group, which
was different from HPV16 and HPV31.
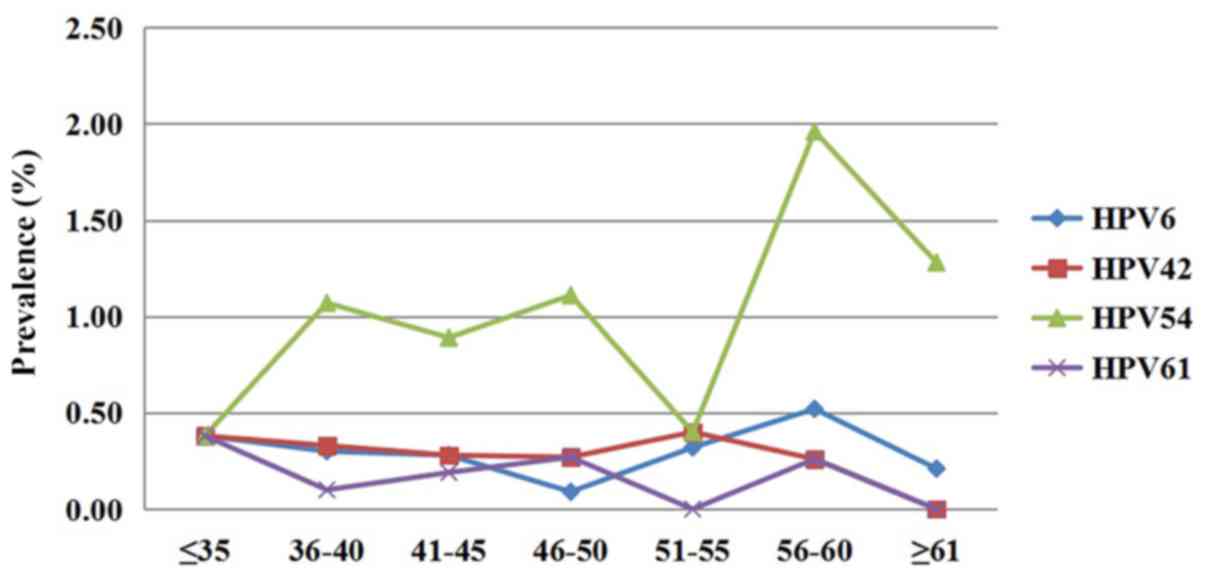
The prevalence of each type of LR-HPV was also
significantly different between the different age groups
(χ2=12.991, P<0.001). The most common types were
HPV54, 6, 42 and 61 in each age group. HPV54 was the most common
type in all age groups except for the 51–55-year-old group.
However, there was only a slight difference in the prevalence of
the other three HPV types in each age group (Fig. 2).
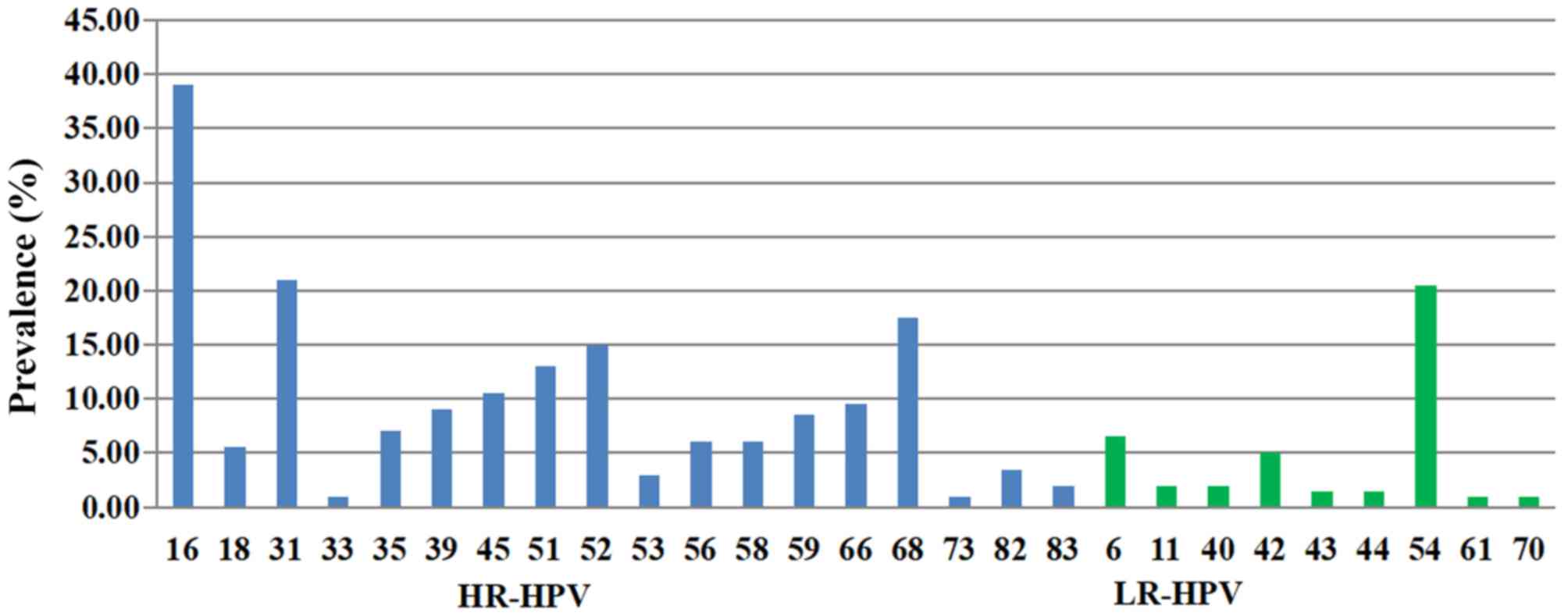
Among the 200 cases positive for multiple HPV types,
three genotypes exhibited comparatively higher positive rates:
HPV16 (39.00%), HPV31 (21.00%) and HPV54 (20.50%) (Fig. 3). Among the 200 individuals infected
with multiple HPV types, 169 had dual infections (169/200, 84.50%),
26 had triple infections (26/200, 13.00%) and 5 were infected with
four or more types of HPV (5/200, 2.50%). The most common
combinations of genotypes were HPV16 + HPV54 (n=30, 15.00%), HPV16
+ HPV31 (n=16, 8.00%) and HPV16 + HPV 68 (n=12, 6.00%) (Table SII).
Discussion
HPV-DNA detection is of great importance for the
screening and prevention of cervical cancer. However, cervical
cancer screening started comparatively late in China and there is
still a huge gap between China and certain developed countries in
terms of screening, diagnosis and treatment (14). The distribution data of HPV genotypes
in different regions are still not sufficiently comprehensive,
particularly in remote areas in western China, and the molecular
epidemiological data still require to be further improved to
provide a scientific basis for the prevention, treatment and
elimination of HPV infection-associated diseases.
In the present study, 28 common oncogenic HPV types
were detected in the Xinjiang Uyghur Autonomous Region of China,
each with a distinct distribution pattern. There were certain
differences in the distribution of HPV infection rates and subtypes
in different regions and studying these differences will be helpful
in the prevention and treatment of HPV infection and the
development of HPV vaccines.
The present study suggested that the total HPV
infection rate in the female screening population was 9.34%. The
overall prevalence of HPV in different parts of China ranges from
7.23% (Chaozhou, South China) to 36.55% (Harbin, Northeast China)
(15–17). The present results were similar to
those for Ningxia, Shanxi, Qinghai and northwest Gansu (7.67-9.03%)
(18), but lower than those of a
previous study on a Xinjiang population (10.96%) (13). The difference may be mainly due to
the fact that the present study, which was based on a screening
population, had a much larger sample size. With the increasing
awareness of cervical cancer, numerous asymptomatic individuals
participate in HPV screening and the prevalence of HPV determined
in the present study is likely to be more reflective of the actual
prevalence. In Asian countries, the prevalence of HPV varies from
1.60 to 14.26% in females without any cervical abnormalities
(19). Furthermore, the prevalence
of HPV may be affected by the type of recruitment (outpatient-based
or screening-based), examination techniques and time interval for
HPV-DNA test (20).
From the present results, an age-associated HPV
infection prevalence curve was produced. The prevalence curve,
whether representing total infections with HPV, multiple strains of
HPV or HR-HPV, exhibited only one maximum, which was in females
aged 61 years and above. Other studies have reported a bimodal age
distribution, with a peak in females aged ≤20 years and a second
peak in females aged >60 years (21–24). As
the subjects who were screened for cervical cancer in this area
were all >30 years old, it was not possible to determine the HPV
infection rate in young females, which may be the reason why the
peak previously reported in younger females was not observed. In
fact, the peak in younger females may be attributed to the fact
that young females may have multiple sexual partners and also have
less immunity to HPV infection (25). More attention should be paid to the
second peak of HPV infection in females aged >60 years. The high
rate of HPV infection in menopausal females may be due to the
physiological and immune disorders caused by the persistence of the
virus or the reactivation of latent HPV (26,27).
Therefore, HPV detection through cervical cancer screening programs
holds clinical value for perimenopausal females. HPV-preventive
vaccines yield more clinical benefits when vaccinated in early life
(8). Thus, it is speculated that
adolescent girls aged 13–15 years could benefit more than older
women of HPV-infection status would from an HPV vaccination program
in China (28).
The most common HR-HPV subtypes among females
worldwide are HPV16, 18, 52, 31, 58, 33 and 51, although their
prevalence varies by region (29).
In the present study, the four most prevalent HR-HPV types were
HPV16, 31, 68 and 52. Similar to the results of previous studies
(15,30–32),
HPV16 was the most common HR-HPV. However, the present results were
different in that HPV31 and HPV52 were more common in the present
cohort than HPV18. Another noteworthy point is that HPV68 was not
common in Europe, North America and Australia (29,33,34), but
HPV68 was the third most prevalent strain in the present study,
uncovering the unique distribution of HR-HPV in Uyghur females in
Xinjiang. This suggests the regional diversity and dynamic changes
characteristic of HPV infection and that HPV infection
distributions may exhibit inhomogeneities among different
ethnicities and regions. The samples of the present study were
obtained from the Kashgar in southern Xinjiang, which has a large
Uyghur population, and the sample size was relatively large; hence,
the results obtained should be representative to a certain extent.
Of note, Gardasil 9, which is a 9-valent HPV vaccine, was approved
for use in China in 2018. Compared with the 2-valent and 4-valent
HPV vaccines, the 9-valent HPV vaccine offers protection against
>90% of cervical cancers (35),
thereby being able to prevent cervical cancer. The 9-valent vaccine
targets high-risk HPV16, −18, −52, −58, −31, −33 and −45, as well
as low-risk HPV6 and −11. Due to the distribution of HPV genotypes
exhibiting obvious regional differences, elucidation of the
distribution of HR-HPV types in a specific region, as was performed
in the present study, is conducive to targeted HPV vaccine
development and clinical application.
Certain studies have indicated that infection with
multiple HPV types has a significant impact on the duration of
type-specific episodes and the development of cervical cancer. Fife
et al (36) reported a
correlation between the high incidence of cervical cancer and
acquiring infection with multiple types of HPV. In addition,
Torttier et al (37)
indicated that co-infection increases the duration of infection.
Perrons et al (38) reported
that co-infection with HPV68 and −16 increased the risk of
high-grade squamous intraepithelial lesions and cervical cancer
compared to that with HPV16 alone or HPV68 alone. Due to its impact
on the occurrence of cervical cancer, the prevalence of multiple
HPV infections is of great significance in the prognosis of
patients with persistent infection. The present study indicated
that the proportion of multiple infections in HPV-positive females
was 17.61%, which was lower than that in Beijing (27.73%) and
Shanxi (24.31%) (21). The most
common genotype combinations in the present study population were
HPV16 + HPV54 and HPV16 + HPV31, which are different from those
determined by Zhao et al (39), namely HPV16 + HPV52 and HPV52 +
HPV58.
There are several limitations to the present study.
First, the study lacked information on the behavior of the enrolled
individuals. Certain studies have indicated that behavioral factors
may increase the risk of HPV infection and cervical cancer, but the
lack of such information in the present study makes it difficult to
analyze the effect of these potential exposure variables on the
development of cervical cancer (40). In addition, the present study only
focused on the distribution data of HPV genotypes in Xinjiang
province. As no cytological data were collected from enrolled
individuals; hence, the cytological classification of the cervical
lesions was unknown, which may affect the baseline prevalence. In
addition, due to the lack of samples from females below the age of
30 years, these results may not fully reflect the overall
population of Uyghur females in Xinjiang. Furthermore, certain
studies have reported that HPV infection may cause not only
cervical cancer but oropharyngeal cancer and head and neck cancer
(5), suggesting the requirement to
focus on the association between HPV infection and oropharyngeal
cancer and head and neck cancer in Uyghur females in future
studies. In addition, the obtainment of data on HPV infection in
Uyghur males was beyond the scope of the present study, while this
may be considered as a potential direction for future research.
In conclusion, the present study revealed the
distribution of different HPV types and the prevalence of HPV
infection by genotypes with vaccines available for >12,000
Uyghur females in a screening population from Xinjiang province,
northwest China. This information will help evaluate the potential
cost-effectiveness of HPV screening and vaccination in Xinjiang. In
addition, the present results have important implications for the
development and application of HPV vaccines, suggesting that more
HPV types (such as HPV68 and 54) should be considered for inclusion
in the next generation of HPV vaccines.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Plan Project of Xinjiang Production and Construction
Corps (grant no. 2013BB015).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XY and MJ were involved in the study conception and
design. XY, XH and YH collected and assembled the data. KL, XH and
MZ were involved in data analysis and interpretation. XY drafted
the initial manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics review
committee of the Medical College of Shihezi University (Shihezi,
China; approval no. 2015-058-01). Participating subjects provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim K, Zang R, Choi SC, Ryu SY and Kim JW:
Current status of gynecological cancer in China. J Gynecol Onco.
20:72–76. 2009. View Article : Google Scholar
|
|
2
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:E191–E203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Forman D, Martel CD, Lacey CJ,
Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J,
Bray F, Plummer M and Franceschi S: Global burden of human
papillomavirus and related diseases. Vaccine. 30 (Suppl 5):F12–F23.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jayaraj R, Kumarasamy C, Royam MM,
Sabarimurugan S and Baxi S: Prognostic implications of pathologic
lymph nodes in HPV-positive oropharyngeal cancers: Clinical
validity and strategies for routine clinical practice. Oral Oncol.
92:99–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao FH, Lin MJ, Chen F, Hu SY, Zhang R,
Belinson JL, Sellors JW, Franceschi S, Qiao YL and Castle PE;
Cervical Cancer Screening Group in China, : Performance of
high-risk human papillomavirus DNA testing as a primary screen for
cervical cancer: A pooled analysis of individual patient data from
17 population-based studies from China. Lancet Oncol. 11:1160–1171.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei L, Xie X, Liu J, Zhao Y, Chen W, Zhao
C, Wang S, Liao X, Shou Q, Qiu Y, Qiao Y and Saah AJ: Efficacy of
quadrivalent human papillomavirus vaccine against persistent
infection and genital disease in Chinese women: A randomized,
placebo-controlled trial with 78-month follow-up. Vaccine.
37:3617–3624. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giuliano AR, Joura EA, Garland SM, Huh WK,
Iversen OE, Kjaer SK, Ferenczy A, Kurman RJ, Ronnett BM, Stoler MH,
et al: Nine-valent HPV vaccine efficacy against related diseases
and definitive therapy: Comparison with historic placebo
population. Gynecol Oncol. 154:110–117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang L, Tian X, Peng D, Zhang L, Xie F,
Bi C, Wang R, Wang J and Qi D: HPV prevalence and genotype
distribution among women in Shandong Province, China: Analysis of
94,489 HPV genotyping results from Shandong's largest independent
pathology laboratory. PLoS One. 14:e02103112019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XC, Sun LQ, Ma L, Li HX, Wang XL,
Wang X, Yun T, Meng NL and Lv D: Prevalence and genotype
distribution of human papillomavirus among women from Henan, China.
Asian Pac J Cancer Prev. 15:7333–7336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bi Q, Zhang L, Zhao Z, Mu X, Zhang M and
Wang P: Human papillomavirus prevalence and genotypes distribution
among female outpatients in Qingdao, East China. J Med Virol.
87:2114–2121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Liu F, Cheng S, Shi L, Yan Z, Yang
J, Shi L, Yao Y and Ma Y: Prevalence of HPV infection among 28,457
Chinese women in Yunnan Province, southwest China. Sci Rep.
6:210392016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Tang DD, Wang K, Wang J, Zhang Z,
Chen Y, Zhang X and Ma C: HPV genotype prevalence and distribution
during 2009–2018 in Xinjiang, China: Baseline surveys prior to mass
HPV vaccination. Bmc Womens Health. 19:902019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rong-Min W, Jing-Jing P and Zhi-Xue Y: The
Interpretation of 2012 ASCCP Management of Abnormal Cervical Cancer
Screening Tests and Cancer Precursors(II). Journal of International
Obstetrics and Gynecology. 42:2015.PubMed/NCBI
|
|
15
|
Li J, Huang R, Schmidt JE and Qiao YL:
Epidemiological features of Human Papillomavirus (HPV) infection
among women living in Mainland China. Asian Pac J Cancer Prev.
14:4015–4023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Xie LX, Qing ZR, Li LJ, Luo ZY,
Lin M, Zhang SM, Chen WZ, Lin BZ, Lin QL, et al: Epidemiologic
characterization of human papillomavirus infection in rural
chaozhou, eastern guangdong province of China. PLoS One.
7:e321492012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun B, He J, Chen X, He M, He Z, Wang Y,
Shang Q, Yu L and Wei L: Prevalence and genotype distribution of
human papillomavirus infection in Harbin, Northeast China. Arch
Virol. 159:1027–1032. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao BW, Yu JL, He H, Li ST and Zhao YL:
Meta-analysis on the Relationship between HPV Infection and
Esophageal Cancer in Chinese Population. J Capital Medical
University. 97:148–150. 2010.
|
|
19
|
Clifford GM, Gallus S, Herrero R, Muñoz N,
Snijders PJ, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E,
et al: Worldwide distribution of human papillomavirus types in
cytologically normal women in the International Agency for Research
on Cancer HPV prevalence surveys: A pooled analysis. Lancet.
366:991–998. 2016. View Article : Google Scholar
|
|
20
|
Uusküla A, Kals M, Kosenkranius L, McNutt
LA and DeHovitz JJ: Population-based type-specific prevalence of
high-risk human papillomavirus infection in Estonia. BMC Infect
Dis. 10:632010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu EQ, Liu B, Cui JF, Chen W, Wang JB, Lu
L, Niyazi M, Zhao C, Ren SD, Li CQ, et al: Prevalence of
type-specific human papillomavirus and pap results in Chinese
women: A multi-center, population-based cross-sectional study.
Cancer Causes Control. 24:795–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jing L, Zhong X, Zhong Z, Huang W, Liu Y,
Yang G, Zhang X, Zou J, Jing C and Wei X: Prevalence of human
papillomavirus infection in Guangdong Province, China: A
population-based survey of 78,355 women. Sex Transm Dis.
41:732–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang R, Guo XL, Wisman GB, Schuuring E,
Wang WF, Zeng ZY, Zhu H and Wu SW: Nationwide prevalence of human
papillomavirus infection and viral genotype distribution in 37
cities in China. BMC Infect Dis. 15:2572015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang CH, Garvilles RG and Chen CY:
Characterization of human papillomavirus infection in north Taiwan.
J Med Virol. 82:1416–1423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torres-Poveda K, Ruiz-Fraga I,
Madrid-Marina V, Chavez M and Richardson V: High risk HPV infection
prevalence and associated cofactors: A population-based study in
female ISSSTE beneficiaries attending the HPV screening and early
detection of cervical cancer program. Bmc Cancer. 19:12052019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Althoff KN, Paul P, Burke AE, Viscidi R,
Sangaramoorthy M and Gravitt PE: Correlates of cervicovaginal human
papillomavirus detection in perimenopausal women. J Womens Health
(Larchmt). 18:1341–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang LN, Castle PE, Zhao FH, Jeronimo J,
Chen F, Bansil P, Li J, Chen W, Zhang X and Qiao YL: A prospective
study of age trends of high-risk human papillomavirus infection in
rural China. Bmc Infect Dis. 14:962014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen R and Wong E: The feasibility of
universal HPV vaccination program in Shenzhen of China: A health
policy analysis. Bmc Public Health. 19:7812019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Sanjosé S, Diaz M, Castellsagué X,
Clifford G, Bruni L, Muñoz N and Bosch FX: Worldwide prevalence and
genotype distribution of cervical human papillomavirus DNA in women
with normal cytology: A meta-analysis. Lancet Infect Dis.
7:453–459. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun LL, Jin Q, Li H, Zhou XR, Song ZQ,
Cheng XM, Tao T, Liang B, Xu L, Wang YR, et al: Population-based
study on the prevalence of and risk factors for human
papillomavirus infection in Qujing of Yunnan province, Southwest
China. Virol J. 9:1532012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Argyri E, Papaspyridakos S, Tsimplaki E,
Michala L, Myriokefalitaki E, Papassideri I, Daskalopoulou D,
Tsiaoussi I, Magiakos G and Panotopoulou E: A cross sectional study
of HPV type prevalence according to age and cytology. Bmc Infect
Dis. 13:532013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang Y, Brassard P, Severini A, Mao Y, Li
YA, Laroche J, Chatwood S, Corriveau A, Kandola K, Hanley B, et al:
The prevalence of human papillomavirus and its impact on
cervical;dysplasia in Northern Canada. Infect Agent Cancer.
8:252013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bouvard V, Baan R, Straif K, Grosse Y,
Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C,
Galichet L, et al: A review of human carcinogens-Part B: Biological
agents. Lancet Oncol. 10:321–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshikawa H: Progress and challenges on
HPV vaccination. Uirusu. 59:243–248. 2009.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huh WK, Joura EA, Giuliano AR, Iversen OE,
de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN,
Hirschberg AL, et al: Final efficacy, immunogenicity, and safety
analyses of a nine-valent human papillomavirus vaccine in women
aged 16–26 years: A randomised, double-blind trial. Lancet.
390:2143–2159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fife KH, Cramer HM, Schroeder JM and Brown
DR: Detection of multiple human papillomavirus types in the lower
genital tract correlates with cervical dysplasia. J Med Virol.
64:550–559. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Trottier H, Mahmud S, Prado JC, Sobrinho
JS, Costa MC, Rohan TE, Villa LL and Franco EL: Type-specific
duration of human papillomavirus infection: Implications for human
papillomavirus screening and vaccination. J Infect Dis.
197:1436–1447. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Perrons C, Jelley R, Kleter B, Quint W and
Brink N: Detection of persistent high risk human papillomavirus
infections with hybrid capture II and SPF10/LiPA. J Clin Virol.
32:278–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao R, Zhang WY, Wu MH, Zhang SW, Pan J,
Zhu L, Zhang YP, Li H, Gu YS and Liu XZ: Human papillomavirus
infection in Beijing, People's Republic of China: A
population-based study. Br J Cancer. 101:1635–1640. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lai CH, Chao A, Chang CJ, Huang CC, Wang
LC, Hsueh S, Lin CT, Wu TI, Jao MS and Chou HH: Age factor and
implication of human papillomavirus type-specific prevalence in
women with normal cervical cytology. Epidemiol Infect. 140:466–473.
2012. View Article : Google Scholar : PubMed/NCBI
|