Introduction
Multiple myeloma (MM) is a neoplastic plasma cell
disorder characterized by clonal proliferation of malignant plasma
cells in the bone marrow, and usually by the presence of monoclonal
protein in the blood and/or urine of patients. MM is associated
with end-organ damage consisting of anemia, renal insufficiency,
bone lesions and/or hypercalcemia (1). MM is the second most frequent
hematological disease. The incidence rate of MM in Europe was 3.8
in every 100,000 individuals in 2012, and the mortality rate was
2.2 (2). With the application of new
drugs, including proteasome inhibitor, immunomodulatory agent and
anti-CD38 monoclonal antibody, the curative efficacy in patients
with MM has been significantly improved. The median overall
survival (OS) time for the entire cohort was 5.2 years; 4.6 years
for patients in the 2001–2005 group compared with 6.1 years for the
2006–2010 cohort (P=0.002). The improvement was primarily seen
among patients >65 years; the 6-year OS rate improving from
31–56%; P<0.001. Only 10% of patients died during the 1st year
in the latter group, compared with 17% in the earlier cohort
(P<0.01), suggesting improvement in early mortality (3). Chimeric antigen receptors (CARs) are
artificial fusion proteins that incorporate an antigen-recognition
domain and T-cell signaling domains. CAR-T cells (CAR-Ts) represent
a promising novel approach for treating patients with MM, since
they may be able to kill MM cells that are resistant to standard
therapies. MM antigens, including CD138, CD38, signaling
lymphocyte-activating molecule 7 and κ light chain, are under
investigation as CAR targets. Although CAR-T therapies for MM are
currently at an early stage of development, they may improve
treatment of patients with MM (4).
Exosomes are extracellular lipids bilayer vesicles
of 30–100 nm in diameter that contain a variety of proteins and
nucleic acid components. Their density range is 1.13-1.19 g/ml in
sucrose density gradient solution (5). Exosomes are present in almost all body
fluids, including plasma, saliva, milk, cerebrospinal fluid, urine
and semen, and are also present in the tumor microenvironment
(6). It has been reported that
exosomes from tumor cells mediate cell communication in the tumor
microenvironment (7). A previous
study demonstrated that tumor-derived exosomes (TEXs) tend to exert
immune suppression and can block the differentiation of bone marrow
progenitor cells into dendritic cells (DCs) (8). Furthermore, exosomes can carry
transforming growth factor β1 (TGF-β1) and change the response of T
cells to interleukin 2 (IL-2), allowing the transformation of
lymphocytes into regulatory T cells (Tregs) rather than cytotoxic T
cells (9). In addition, TEXs promote
the activation and accumulation of Treg cells (10). Similarly, TEX stimulate the
production of prostaglandin E2, IL-6 and TGF-β by myeloid-derived
suppressor cells, resulting in the formation of a strong
immunosuppressive environment in tumor lesions (11–13).
Previous studies reported that MM-derived exosomes might be
involved in the differentiation and functional regulation of
osteoclasts, promotion of angiogenesis and immune suppression
(14,15). However, the effects of MM-derived
exosomes on the quantity and function of T cells remain
unknown.
Tregs have a crucial role in resolving inflammation
and achieving tissue homeostasis following infection through
multiple mechanisms, including the production of the inhibitory
cytokines IL-10 and TGF-β, the regulation of nutrient and cytokine
availability, and the inhibition of DCs and macrophages maturation
and function (16). Perforin,
granzyme B (GrB) and Fas ligand (FasL) are cytotoxic molecules used
by CD8+ T lymphocytes and natural killer cells to induce apoptosis
of target cells (tumor or infected cells) (17). In the present study, IL-10 and TGF-β
were selected to evaluate the function of Tregs, whereas perforin
and GrB were selected to evaluate the function of CD8+ T cells, in
order to evaluate the effect of myeloma derived exosomes on the
function of T lymphocytes, and to understand the possible mechanism
of the effect of myeloma cells on T lymphocytes.
Materials and methods
Patients
Peripheral blood from patients newly diagnosed with
MM and 45 adult HDs were collected to evaluate the potential effect
of MM cell-derived exosomes on CD4+ T, CD8+ T and Treg cell
function. The present study included 45 patients with MM (19 women
and 26 men; age range, 44–85 years; median age, 68 years) who were
treated at the Department of Hematology of Tianjin Medical
University General Hospital (Tianjin, China) from June 2016 to
December 2017. All HDs and patients with MM provided written
consent prior to the study. This study was performed according to
the Declaration of Helsinki and was approved by the Ethics
Committee of Tianjin Medical University General Hospital.
Magnetic-activated cell sorting
(MACS)
Peripheral blood (5 ml) was collected from HDs and
patients with MM into EDTA-coated tubes. Peripheral blood
mononuclear cells (PBMCs) were isolated by Ficoll-Paque density
gradient centrifugation. The diluted blood was slowly added to the
equal volume Ficoll-Hypaque solution, and then centrifuged at 860 ×
g for 20 min at 4°C. The mononuclear lymphocyte cell layer was
transferred to another centrifuge tube using a sterile pipet (this
will appear as a white, cloudy band between the plasma and the
Ficoll-Hypaque layers). Cells were washed three times with sterile
PBS (the volume of the mononuclear cell layer) and centrifuged at
450 × g for 10 min at 20°C. The supernatant was discarded and the
mononuclear lymphocyte cells were resuspended in sterile PBS for
cell counting. Every 107 PBMCs were re-suspended in 80
µl PBS. Subsequently, CD4+, CD8+ and Treg cells
(CD4+CD25+CD127dim) were isolated from PBMCs by using
human CD4, CD8 and Treg cell MACS kits (cat. nos. 130-095-248,
130-098-194 and 130-094-775, respectively; all from BD
Biosciences), according to the manufacturer's instructions. The
purity of the cell populations obtained was evaluated using a
CytoFLEX flow cytometer (Beckman Coulter, Inc.) (Fig. S1).
Exosome isolation
Firstly, fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences) was placed into polyallomer centrifuge
tubes (Beckman Coulter, Inc.) and ultracentrifuged (Beckman
Coulter, Inc.) for exosome depletion. After ultracentrifugation at
100,000 × g for 18 h at 4°C, exosomes from FBS were at the bottom
of the centrifugal tubes. The supernatant collected as exosome-free
serum was filtered using a 0.22-µm aseptic filter (EMD Millipore)
and subsequently used for cell culture (18). The OPM2 and U266B1 cell lines
purchased from the Cell Center of Chinese Academy of Sciences and
cultured for 48 h in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% exosome-free serum and placed at
37°C in a humidified incubator containing 5% CO2. The
conditioned medium was then collected and centrifuged at 250 × g
for 10 min at 25°C, and the cell-free supernatant was obtained for
exosome separation. The supernatant was divided into several
sterile centrifuge tubes (cat. no. 430791; Corning, Inc.) and
centrifuged at 4°C and 2,000 × g for 30 min to remove cells and
debris. The supernatant was carefully collected and filtered using
a 0.22-µm aseptic filter for sterilization. This filtered cell-free
conditioned medium was transferred to the Pierce protein
concentrator (cat. no. 88532S; Thermo Fisher Scientific, Inc.) and
centrifuged at 4,000 × g for 30 min at 4°C to concentrate the
medium and remove soluble protein (<150 KDa) and small particles
(<15 nm) (19). The concentrated
medium was subsequently incubated with Total Exosome Isolation
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) (volume ratio
of supernatant to reagent was 2:1) at 4°C overnight and centrifuged
at 10,000 × g for 1 h at 4°C. The pellet at the bottom of the
centrifuge tube represented the exosomes, which were resuspended in
PBS or serum-free medium for further co-culturing with T
lymphocytes. The ultracentrifugation method was also used to obtain
exosomes (18) and to compare them
with the exosomes obtained from the aforementioned method, under
transmission electron microscopy.
Transmission electron microscopy
(TEM)
The thin formvar/carbon film coated 200 mesh copper
EM grids (cat. no. G200H-Cu; Electron Microscopy Sciences) were
used for exosomes loading. Purified exosomes were fixed with 1 ml
of 2% paraformaldehyde for 5 min at room temperature. Exosome
suspension (5–7 µl) solution was added to the grids and incubated
for 2 min at room temperature, and the exosome-loading grids were
subsequently stained with ~20 drops of filtered 3% phosphotungstic
acid solution for 3 min at room temperature. The grids were rinsed
with distilled water to remove the excess staining solution and
left to dry at room temperature for 10 min. The exosomes were
observed under a TEM (HT7700; Hitachi, Ltd.) at 80 kV (×20~40 K)
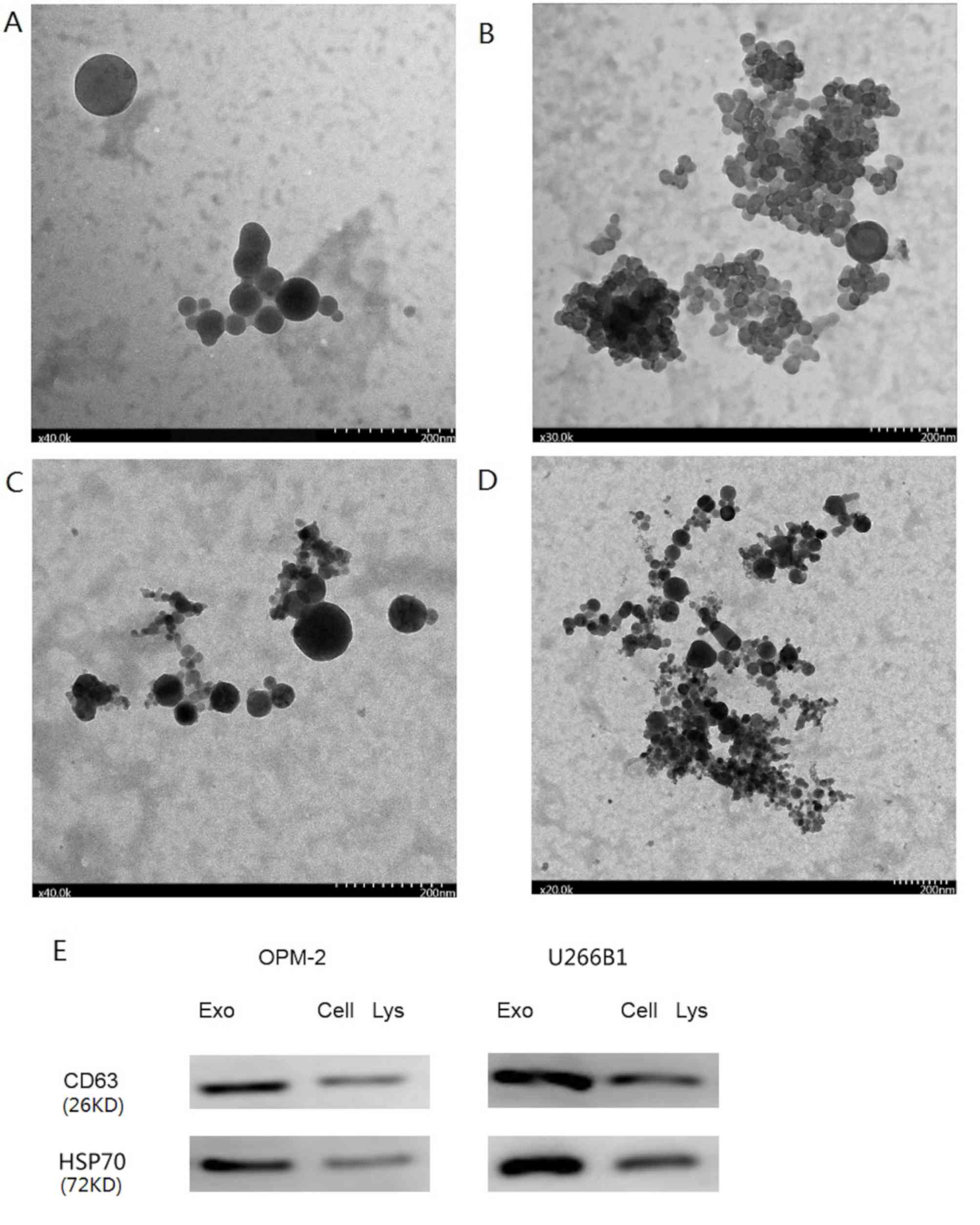
(Fig. 1A-D) (20).
Cell culture with exosomes
CD4+ T, CD8+ T or Treg cells isolated from HDs and
patients with MM were co-cultured with exosomes (100
µg/105 cells) isolated from OPM-2 and U266B1 cells, and
cultured in RPMI-1640 medium with 10% FBS free of exosomes for 48 h
at 37°C in a humidified incubator containing 5% CO2.
Cell apoptosis, cell viability and expression of certain proteins
were next evaluated. CD4+ T cells from 15 HDs were co-cultured with
two different concentrations (50 and 100 µg/105 cells)
of exosomes from OPM2 cells. The control group was treated with
sterile PBS. The early apoptotic rate of CD4+ T cells cultured with
a low and high concentration of exosomes was 11.92±2.59 and
13.42±3.13%, respectively. The early apoptotic rate of CD4+ T cells
in the control group was 9.41±3.00%. These results demonstrated
that the early apoptotic rate of CD4+ T cells significantly
increased in the high exosome concentration group (100
µg/105 cells) compared with the control group (Fig. 2A-C). Thus, this exosome concentration
(100 µg/105 cells) was selected for co-culture of CD4+
T, CD8+ T and Treg cells.
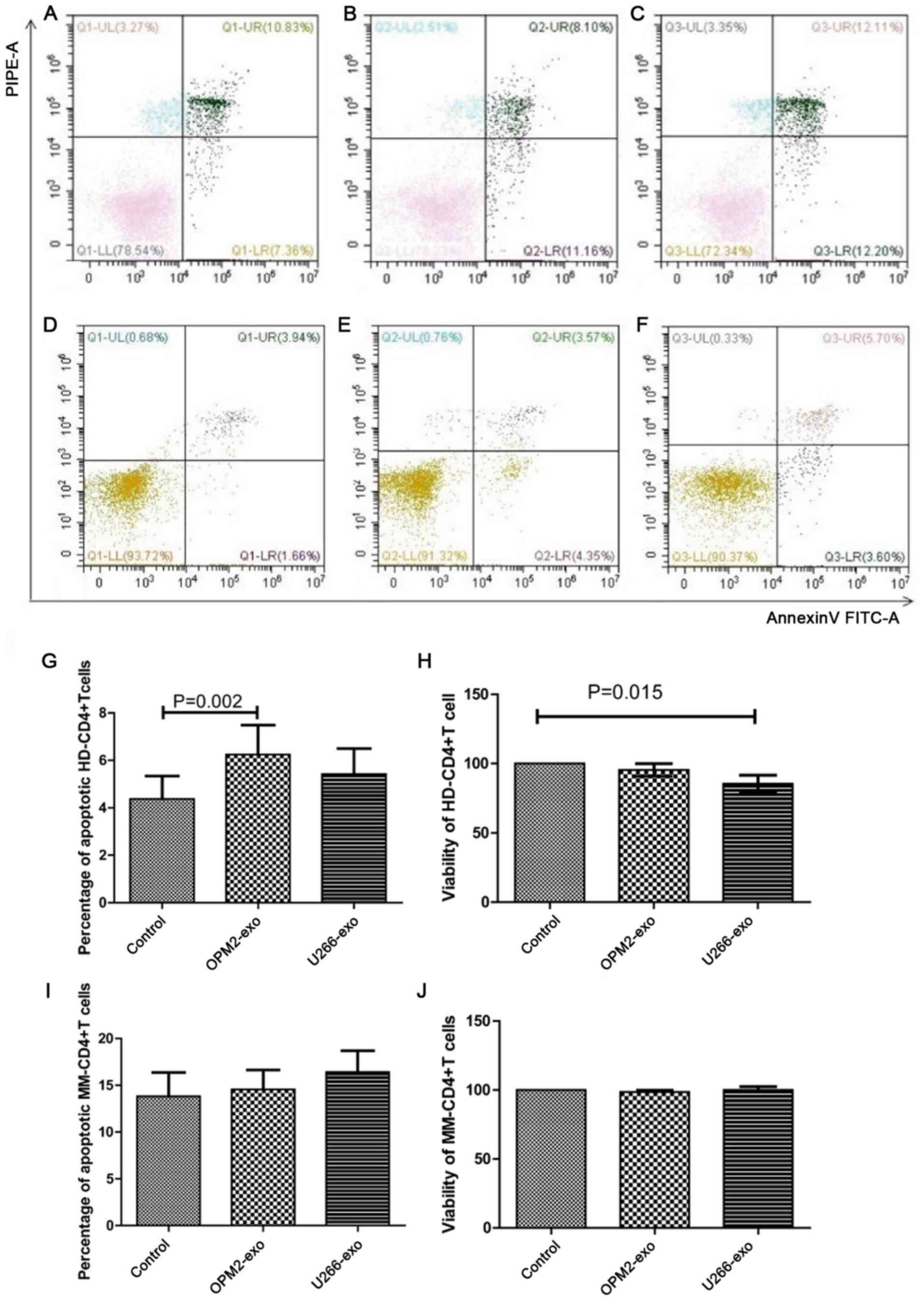 | Figure 2.Effect of MM cell-derived exosomes on
CD4+ T lymphocytes. (A) Apoptotic rate of HD-CD4+ T cells
co-cultured without exosome (control). (B) Apoptotic rate of
HD-CD4+ T cells co-cultured with OPM2-isolated exosomes (50
µg/105 cells). (C) Apoptotic rate of HD-CD4+ T cells
co-cultured with OPM2-isolated exosomes (100 µg/105
cells). (D) Apoptotic rate of HD-CD4+ T cells co-cultured without
exosome (control). (E) Apoptotic rate of HD-CD4+ T cells
co-cultured with OPM2-derived exosomes. (F) Apoptotic rate of
HD-CD4+ T cells co-cultured with U266B1-derived exosomes. (G) The
apoptotic rate of HD-CD4+ T cells was significantly increased in
the OPM2-derived exosomes. (H) The viability of HD-CD4+ T cells was
decreased in the U266B1-derived exosomes compared with that of the
control (n=15). (I) The apoptotic rate of MM-CD4+T cells showed no
statistical significance among the three groups (n=15). (J) The
viability of MM-CD4+ T cells showed no statistical significance
among the three groups (n=15). HD, healthy donor; MM, multiple
myeloma; UL, upper left; UR, upper right; LL, lower left; LR, lower
right; exo, exosome; FITC, fluorescein isothiocyanate; PI,
propidium iodide; PE, phycoerythrin. |
Western blotting
Exosomes, OPM2 and U266B1 cells were lysed using
RIPA buffer (Thermo Fisher Scientific, Inc.) on ice for 30 min.
Protein quantification was performed using the Pierce BCA Protein
Analysis kit (Thermo Fisher Scientific, Inc.), according to the
manufacturers' instructions. Protein lysates from exosomes, OPM2
and U266B1 cells were boiled at 100°C for 5 min. Equal amount of
protein (30 µg) was loaded and separated by 10% SDS-PAGE for 30 min
and transferred onto a nitrocellulose (0.45 µm) membrane for 110
min (Bio-Rad Laboratories, Inc.). Membranes were blocked with 5%
non-fat skimmed milk for 1 h at room temperature and incubated with
monoclonal antibodies against CD63, which is a specific (although
not the only one) marker of exosomes (18) (1:1,000; cat. no. ab59479; Abcam) and
heat shot protein 70 (HSP70; 1:1,000; cat. no. 4872; Cell Signaling
Technology, Inc.) at 4°C overnight. The HRP-conjugated goat
anti-Rabbit IgG (1:10,000; cat. no. 7074S; Cell Signaling
Technology, Inc.) was added as secondary antibody for 1 h at room
temperature. Membranes were washed three times with TBS containing
Tween-20 (0.05%), and bands were detected using an enhanced
chemiluminescence substrate (GE Healthcare Bio-Sciences).
Flow cytometry
For the cell apoptosis assay, 1×105 CD4+
T, CD8+ T or Treg cells were seeded in a 24-well plate and
co-cultured with exosomes (100 µg/105 cells) for 48 h.
Apoptosis was evaluated by staining the cells with FITC Annexin V
Apoptosis Detection kit (cat. no. 556547; BD Biosciences),
according to the manufacturer's instructions using a CytoFLEX flow
cytometer (Beckman Coulter, Inc.). Unlabeled cells were used as
controls.
The gating strategy of flow cytometry was performed
as follows: First, unstained cells were detected, cells were
displayed on the forward scatter (FSC)-side scatter (SSC) scatter
plot and the target cell population was circled by gates. Then, the
two-parameter logFL1 (FL1, flow1, FITC)-logFL2 (FL2, flow2, PE) was
established and the cells within gates in the scatter plots were
analyzed. It was ensured that >98% of cells were located in the
center of the lower left quadrant and that this region was
negative. Secondly, only Annexin V-FITC-labeled cells were detected
and FL1-FL2 scatter plots were checked to ensure that there were no
particles in the upper left and right quadrants. Appearance of
particles in the upper quadrant was indicative of fluorescence
leakage, which was adjusted by increasing the compensation of FL1
leakage to FL2 fluorescence. If this regulation did not effectively
remove the positive signal of FL2, the voltage of FL2 was lowered.
Using this method, cells labeled only with propidium iodide (PI)
were detected and compensation was adjusted if necessary. Thirdly,
Annexin V and PI-labeled cells were analyzed by flow cytometry. The
right lower and upper quadrants were considered as early and late
apoptotic cells, respectively, and the sum corresponded to the
apoptotic rate (Fig. S2).
CD8+ T cells were permeabilized using BD
Cytofix/Cytoperm™ reagent (BD Biosciences), and added 5 µl
perforin-PE (cat. no. 130-096-578; Miltenyi) or Granzyme B-PE (cat.
no. 130-101-351; Miltenyi), incubated at room temperature for 15
min in the dark, and then washed with PBS for flow cytometric
detection of intracellular expression of perforin and Granzyme B.
Isoform-matched isotypes were used as controls for extra and
intracellular staining. Cells were immediately analyzed using
Beckman CytoFLEX flow cytometer and CytExpert software (version
2.1; Beckman Coulter, Inc.). Quantification of GrB and perforin was
performed according to the percentage of positive cells.
Cell viability
The sorted CD4+ T, CD8+ T and Treg cells were seeded
into 96 well plates (2×104 cells/100 µl per well), and
co-cultured with OPM2/U266-exosomes (100 µg/105 cells,
10 µl/well) or PBS (10 µl/well as control) for 48 h at 37°C. After
cell incubation, 10 µl CCK-8 (cat. no. EC020; Engreen Biosystem Co.
Ltd.) reagent was added to the wells and incubated for 4 h at 37°C
in a humidified incubator containing 5% CO2. Cell
viability was analyzed at a wavelength of 450 nm, using a
microplate reader (GEN5; BioTek Instruments, Inc.).
ELISA
Following Treg cell (1×105 cells/500 µl
per well into a 24 well plate) incubation with exosomes (100
µg/105 cells) for 48 h at 37°C, the cell culture was
collected and centrifuged at 710 × g for 10 min at room
temperature. The supernatant was separated to assess IL-10 and
TGF-β [Hangzhou MultiSciences (Lianke) Biotech, Co., Ltd.]
concentration according to the manufacturer's instructions with a
colorimetric reader (GEN5; BioTek Instruments, Inc.). The minimum
detectable cytokine levels for IL-10 and TGF-β were 0.59 and 3.36
pg/ml, respectively.
Statistical analysis
The results were analyzed with GraphPad Prism 6.0
software (GraphPad Software, Inc.) and SPSS 20.0 software (SPSS
Inc.). Data are expressed as the mean ± standard error of the mean.
ANOVA of randomized block design was used for comparison of
apoptosis rate and viability of HD/MM-CD4+ T, CD8+ T and Treg
cells, level of perforin, Granzyme B, IL-10 and TGF-β among the
three groups (OPM2 exosome, U266B1 exosome and control groups),
with Dunnett's post hoc test. Interpolation to the standard curve
of the measurements was obtained through linear regression using
GraphPad Prism software version 6 (GraphPad Software, Inc.)
(21). All experiments were
performed in triplicate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of MM cell-derived exosomes on
CD4+ T lymphocytes
The results from the TEM analysis demonstrated that
MM cell-derived exosomes had intact continuous membranes and a
diameter of 30–150 nm (Fig. 1). The
central area was darker with a circled bright area around it, which
is a typical morphological characteristic of exosomes (22,23). The
exosomes separated from conditioned medium of the OPM2 cell line by
ultracentrifugation were uniform in size. Fig. 1A demonstrates that the exosomes
isolated by ultracentrifugation had intact continuous membranes and
a diameter of 30–150 nm. Fig. 1B
presents exosomes isolated by ultracentrifugation in clusters. The
exosomes isolated using the Total Exosome Isolation reagent kit
were similar in morphology to the exosomes isolated by
ultracentrifugation. The diameter of exosomes isolated using the
Total Exosome Isolation reagent kit was 30–150 nm, as presented in
Fig. 1C. A large number of clustered
exosomes isolated using the Total Exosome Isolation reagent kit is
presented in Fig. 1D. The results
from western blotting demonstrated that exosomes extracted from the
two MM cell lines were positive for CD63 and HSP70 staining, which
are specific markers of exosomes. In addition, CD63 and HSP70
protein expression in the exosomes of the OPM2 and U266B1 cell
lines was higher compared with that of OPM2 and U266B1 cell lysates
(Fig. 1E). The TEM results
demonstrated that the exosomes extracted using the Exosome
Isolation reagent kit were similar in morphology to the exosomes
extracted via ultracentrifugation.
CD4+ T cells from 15 HDs were co-cultured with two
different concentrations (50 and 100 µg/105 cells) of
exosomes from OPM2 cells. The control group was treated with
sterile PBS. The early apoptotic rate of CD4+ T cells cultured with
a low and high concentration of exosomes was 11.92±2.59 and
13.42±3.13%, respectively. The early apoptotic rate of CD4+ T cells
in the control group was 9.41±3.00%. These results demonstrated
that the early apoptotic rate of CD4+ T cells significantly
increased in the high exosome concentration group (100
µg/105 cells) compared with the control group (Fig. 2A-C). Thus, this exosome concentration
(100 µg/105 cells) was selected for co-culture of CD4+
T, CD8+ T and Treg cells.
CD4+ T cells from 15 HDs and 15 patients with MM
were collected and co-cultured with exosomes from the OPM2 or
U266B1 cell lines for 48 h. The apoptotic rate of HD-CD4+ T cells
in the OPM2 (6.24±1.24%) and U266B1 (5.42±1.07%) groups was higher
than that in the control group (4.37±0.96%). The result of
comparing these three groups revealed a statistically significant
difference (P=0.004). The apoptotic rate of HD-CD4+ T cells in OMP2
group was significantly higher than the control group (P=0.002)
(Fig. 2D-G). The cell viability of
HD-CD4+ T cells in the OPM2 (95.34±4.58%) and U266B1 (85.45±6.09%)
groups was decreased compared with that of the control group
(100%), which was statistically significant (P=0.024). The
viability of CD4+ T cells in the U266B1 group was inhibited
(P=0.015; Fig. 2H). The apoptotic
rate of MM-CD4+ T cells in the OPM2 (14.56±4.65%) and U266B1
(16.42±5.08%) groups was increased compared with that of the
control group (13.83±5.69%), although this was not significant
(P>0.05; Fig. 2I). In addition,
the viability of MM-CD4+ T cells in the OPM2 group (98.61±2.80%),
U266B1 group (100.02±5.48%) and the control group (100%) showed no
statistical significance (P>0.05; Fig. 2J).
Effect of MM cell-derived exosomes on
CD8+ T lymphocytes
CD8+ T cells from 15 HDs and 15 patients with MM
were co-cultured with exosomes from OPM2 or U266B1 cells for 48 h.
The apoptotic rate of HD-CD8+ T cells in the OPM2 (9.97±1.28%) and
U266B1 (11.00±1.75%) groups was significantly lower than that in
the control group (16.12±2.95%; both P<0.001; Fig. 3A-C and G). The viability of HD-CD8+ T
cells in the OPM2 (112.63±3.88%) and U266B1 (111.70±3.62%) groups
was significantly increased compared with that of the control group
(P=0.030 and 0.045, respectively; Fig.
3H). The apoptotic rate of MM-CD8+ T cells in the OPM2
(14.40±4.86%) and U266B1 (14.39±4.36%) groups showed no
statistically significant difference (17.37±4.05%; P>0.05;
Fig. 3D-F presents the apoptotic
rates of MM-CD8+ T cells in the control, OPM2 and U266B1 groups,
respectively. Fig. 3I demonstrates
the statistical analysis of the apoptotic rates of MM-CD8+ T cells
among the three groups). The viability of MM-CD8+ T cells in the
OPM2 (101.58±10.82%) and U266B1 (100.31±10.08%) groups was not
different from the cell viability in the control group (P>0.05;
Fig. 3J).
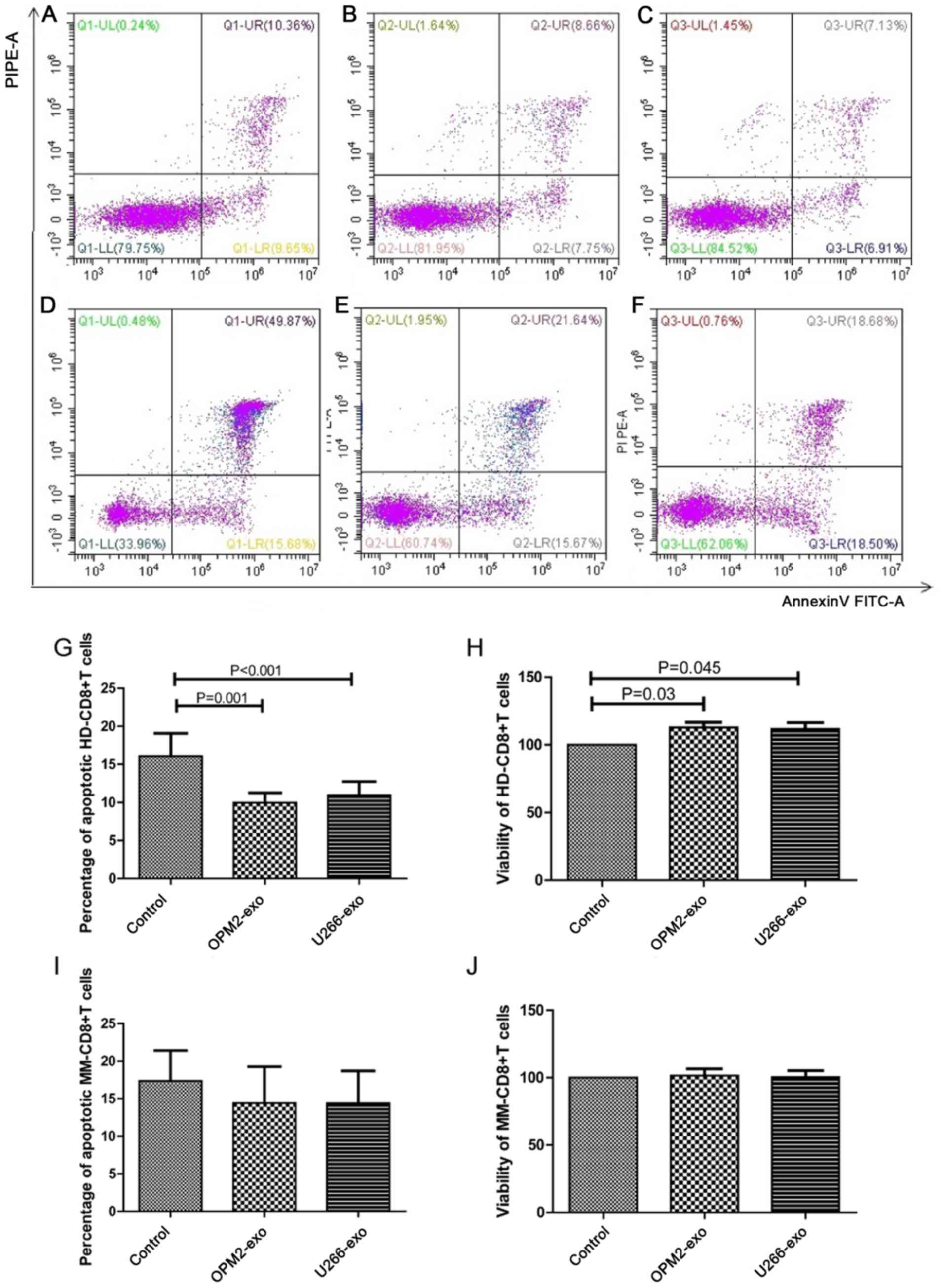 | Figure 3.Effect of MM cell-derived exosomes on
CD8+ T lymphocytes. Assessment of apoptosis by flow cytometry of
HD-CD8+ T cells co-cultured with (A) Apoptotic rate of HD-CD8+ T
cells co-cultured without exosome (control). (B) Apoptotic rate of
HD-CD8+ T cells co-cultured with OPM2-derived exosomes. (C)
Apoptotic rate of HD-CD8+ T cells co-cultured with U266B1-derived
exosomes. (D) Apoptotic rate of MM-CD8+ T cells co-cultured without
exosome (control). (E) Apoptotic rate of MM-CD8+ T cells
co-cultured with OPM2-derived exosomes. (F) Apoptotic rate of
MM-CD8+ T cells co-cultured with U266B1-derived exosomes. (G) The
apoptotic rate of HD-CD8+ T cells co-cultured with OPM2- and
U266B1-derived exosomes was significantly decreased compared with
the control group (n=15). (H) The viability of HD-CD8+ T cells
co-cultured with OPM2- and U266B1-derived exosomes was
significantly increased compared with the control group (n=15). (I)
The apoptotic rate of MM-CD8+T cells showed no statistical
significance among the three groups (n=15). (J) The viability of
MM-CD8+ T cells showed no statistical significance among the three
groups (n=15). HD, healthy donor; MM, multiple myeloma; UL, upper
left; UR, upper right; LL, lower left; LR, lower right; exo,
exosome; FITC, fluorescein isothiocyanate; PI, propidium iodide;
PE, phycoerythrin. |
CD8+ T cells from 15 HDs and 15 patients with MM
were co-cultured with exosomes from OPM2 or U266B1 cells for 48 h.
In addition to the 15 healthy donors, 15 patients with myeloma were
also randomly selected for assessment. The expression of perforin
and GrB after cell permeabilization was assessed by flow cytometry.
The level of perforin in HD-CD8+ T cells was lower in the OPM2
(10.82±2.53%) and U266B1 (9.34±2.36%) groups compared with that in
the control group (12.76±3.31%) (P=0.038). The level of perforin in
HD-CD8+ T cells in the U266B1 group was inhibited, showing
statistical significance (P=0.024), but the level of perforin
showed no statistical significance between the OPM2 group and the
control group (P>0.05; Fig. 4A-C
present the perforin levels of HD-CD8+ T cells in the control, OPM2
and U266B1 groups, respectively. Fig.
4G demonstrates the statistical analysis of perforin levels of
HD-CD8+ T cells among the three groups). The results demonstrated
that U266B1-derived exosomes had a more significant effect on the
perforin secretion of HD-CD8+ T cells. The level of GrB in HD-CD8+
T cells in the OPM2 (31.87±6.10%) and U266B1 (32.99±7.08%) groups
was not significantly different from that of the control group
(28.74±6.21%; P>0.05; Fig. 4D-F
showed scatter diagram of level of GrB). The level of perforin in
MM-CD8+ T cells in the OPM2 (6.48±1.06%) and U266B1 (5.63±1.15%)
groups was significantly lower than that in the control group
(10.55±2.50%; P=0.008). The difference between the perforin level
in the OPM2 and U266B1 groups was statistically significant
(P=0.017 and P=0.006, respectively; Fig.
4H). There was no statistical significance in the level of GrB
in MM-CD8+ T in OPM2 group (34.48%±9.98%), U266B1 group
(41.49±9.17%) and the control group (34.91±8.14%) (P>0.05).
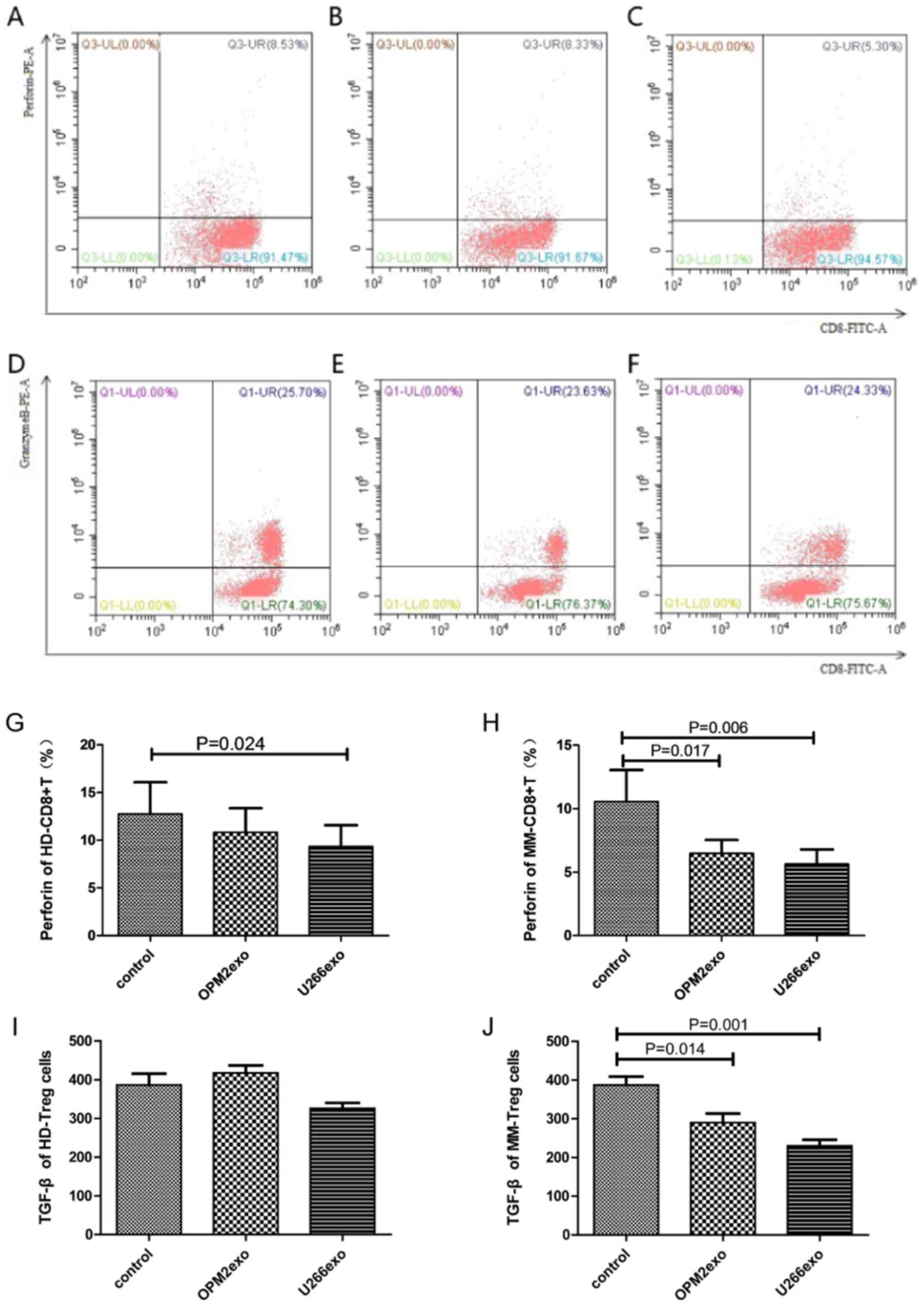 | Figure 4.Effect of MM cell-derived exosomes on
the function of CD8 and Treg cells. (A) Perforin levels of HD-CD8+
T cells co-cultured without exosome (control). (B) Perforin levels
of HD-CD8+ T cells co-cultured with OPM2-derived exosomes. (C)
Perforin levels of HD-CD8+ T cells co-cultured with U266B1-derived
exosomes. (D) Granzyme B levels of HD-CD8+ T cells co-cultured
without exosome (control). (E) Granzyme B levels of HD-CD8+ T cells
co-cultured with OPM2-derived exosomes. (F) Granzyme B levels of
HD-CD8+ T cells co-cultured with U266B1-derived exosomes. (G) The
levels of perforin secreted by HD-CD8+ T cells was decreased in the
U266B1-derived exosome group compared with the control group. (H)
The levels of perforin secreted by MM-CD8+T cells was significantly
decreased both in the OPM2 and U266B1-derived exosome groups
compared with the control group. (I) The levels of TGF-β in the
supernatant of HD-Treg cells showed no statistical significance and
(J) the levels of TGF-β secreted by MM-Tregs was decreased in the
OPM2 and U266B1-derived exosome groups compared with that of the
control (n=15). HD, healthy donor; IL, interleukin; MM, multiple
myeloma; TGF-β, transforming growth factor β; UL, upper left; UR,
upper right; LL, lower left; LR, lower right; exo, exosome; FITC,
fluorescein isothiocyanate; PE, phycoerythrin. |
Effect of MM cell-derived exosomes on
Tregs
Treg cells from 15 HDs and 15 patients with MM were
co-cultured with exosomes from OPM2 or U266B1 cells for 48 h. The
apoptotic rate of HD-Treg cells in OPM2 group (15.33±3.87%) and
U266 group (11.71±2.71%) was lower than that in the control group
(19.61±3.50%). The apoptotic rate of HD-Treg cells in U266 group
was significantly lower than that of the control group (P=0.003;
Fig. 5A-C present the apoptotic
rates of HD-Treg cells in the control, OPM2 and U266B1 groups,
respectively; Fig. 5G demonstrated
the statistical analysis of apoptotic rates of HD-Treg among the
three groups). The viability of HD-Treg cells in the OPM2 group
(139.54±23.24) and U266B1 group (173.48±34.99%) was higher than the
control group (100%). But the viability of HD-Treg cells only in
U266B1 group was significantly higher than the control group
(P=0.037; Fig. 5H). The apoptotic
rate of MM-Treg cells in the U266B1 group (37.29±6.54%) was
significantly increased compared with that of the control group
(29.95±6.68%; P=0.011). However, there was no significant
difference between the apoptotic rate of MM-Treg cells in the OPM2
group (30.91±7.25%) and the control group (P>0.05; Fig. 5D-F present the apoptotic rates of
MM-Treg cells in the control, OPM2 and U266B1 groups, respectively;
Fig. 5I demonstrates the statistical
analysis of apoptotic rates of MM-Treg among the three groups). The
viability of MM-Treg cells in the OPM2 (82.29±12.16%) and U266B1
(85.09±13.50%) groups was decreased compared with that of the
control group (P=0.013). The viability of MM-Treg cells in the OPM2
group and U266B1 group was significantly lower compared with that
of the control group (P=0.012 and P=0.027, respectively; Fig. 5J).
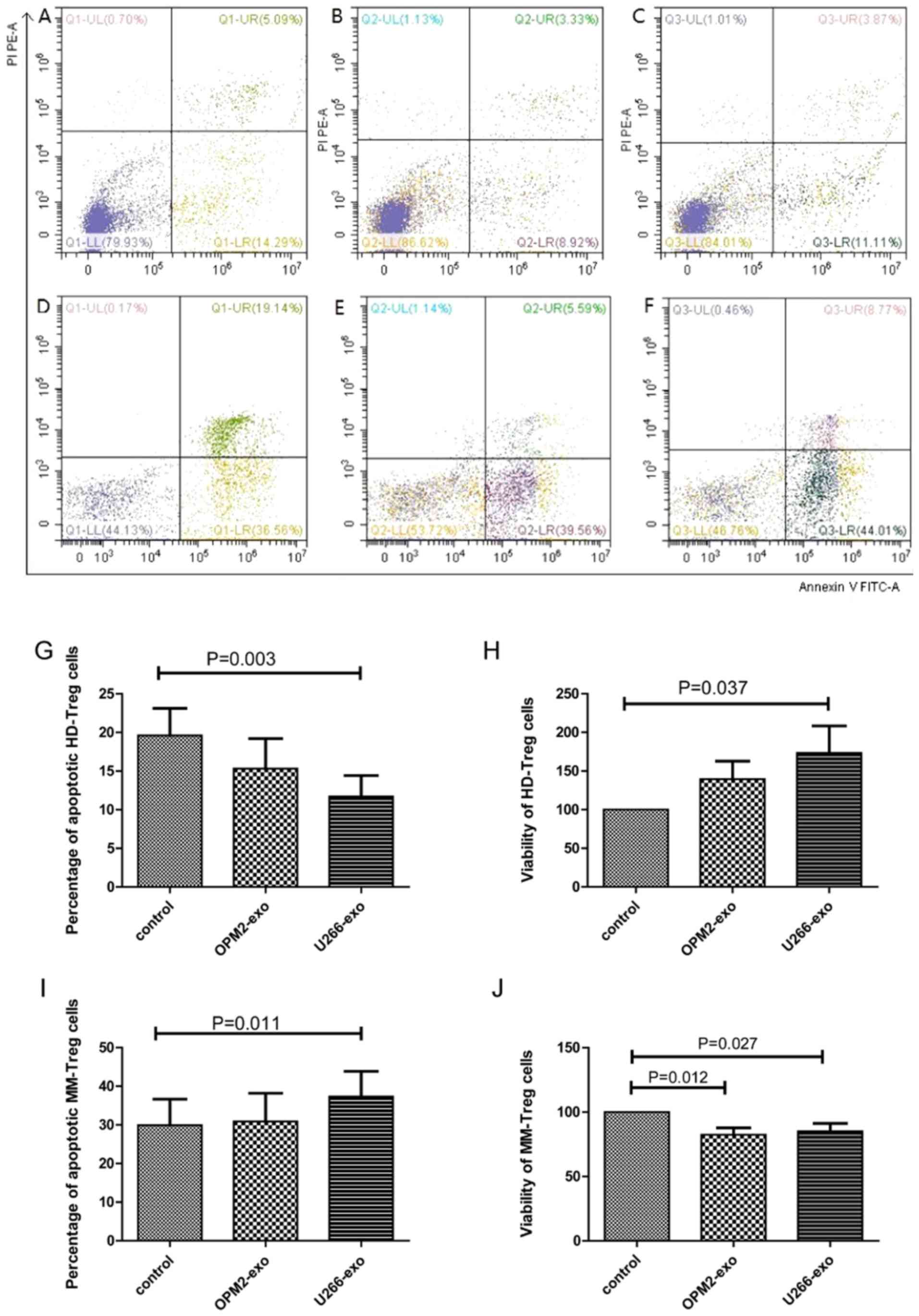 | Figure 5.Effect of MM cell-derived exosomes on
Tregs. (A) Apoptotic rate of HD-Treg cells co-cultured without
exosome (control). (B) Apoptotic rate of HD-Treg cells co-cultured
with OPM2-derived exosomes. (C) Apoptotic rate of HD-Treg cells
co-cultured with U266B1-derived exosomes. (D) Apoptotic rate of
MM-Treg cells co-cultured without exosome (control). (E) Apoptotic
rate of MM-Treg cells co-cultured with OPM2-derived exosomes. (F)
Apoptotic rate of MM-Treg cells co-cultured with U266B1-derived
exosomes. (G) The apoptotic rate of HD-Tregs was significantly
decreased in the U266B1-derived exosome group compared with that of
the control. (H) The viability of HD-Tregs was significantly
increased in the U266B1-derived exosome group. (I) The apoptotic
rate of MM-Tregs was significantly increased in the U266B1-derived
exosome group compared with that of the control. (J) The viability
of MM-Tregs was decreased in the OPM2- and U266B1-derived exosome
groups (n=15). HD, healthy donor; MM, multiple myeloma; Treg,
regulatory T cell; UL, upper left; UR, upper right; LL, lower left;
LR, lower right; exo, exosome; FITC, Fluorescein isothiocyanate;
PI, Propidium iodide; PE, Phycoerythrin. |
Treg cells from 15 HDs and 15 patients with MM were
co-cultured with exosomes from OPM2 or U266B1 cells for 48 h, and
the IL-10 and TGF-β levels in the supernatant were detected by
ELISA. The level of IL-10 in the supernatant of HD-Treg cells in
the OPM2 (18.53±8.08 pg/ml) and U266B1 (13.51±7.83 pg/ml) groups
was increased compared with the control group (8.49±4.02 pg/ml).
The difference in IL-10 levels between HD-Treg cells in the OPM2
and the control group was significant (P=0.043). There was no
significant difference between the IL-10 level in the supernatant
of HD-Treg cells in the OPM2 group and the IL-10 level in the
supernatant of HD-Treg cells in the U266B1 group (P>0.05). The
IL-10 level in the supernatant of MM-Treg cells in the OPM2
(3.29±1.84 pg/ml) and U266B1 (2.60±1.72 pg/ml) groups was increased
compared with that of the control group (1.39±1.02 pg/ml), although
this was not significant (P>0.05).
The level of TGF-β in the supernatant of HD-Treg
cells in OPM2 (417.57±19.33 pg/ml), U266B1 (325.96±14.32 pg/ml) and
control group (386.60±28.89 pg/ml) were significantly different
(P=0.033), but the difference between the OPM2, U266B1 and control
groups was not significant (P>0.05; Fig. 4I). The level of TGF-β in the
supernatant of MM-Treg cells in the OPM2 (290.29±23.21 pg/ml),
U266B1 (325.96±14.32 pg/ml) and the control group (387.49±21.60
pg/ml) were statistically significant (P=0.001). The level of TGF-β
in the supernatant of MM-Treg cells in the OPM2 and U266B1 group
were significantly lower than the control group (P=0.014 and
P=0.001, respectively; Fig. 4J).
Discussion
A new evolutionarily conserved system of
intercellular communication used by single-cell and multicellular
organisms has been identified. This system involves information
transfer between cells via extracellular vesicles (EVs). Mammalian
cells spontaneously release EVs of various sizes (30-5,000 nm) into
all body fluids. The current EV nomenclature is based on size and
markers present on membranes. It comprises small EVs or exosomes
(30–150 nm in diameter), intermediate-sized EVs or microvesicles
(200-1,000 nm in diameter) and apoptotic bodies (1,000-5,000 nm in
diameter) (24). In the present
study, and according to the scale on the images (Fig. 1), the vesicles isolated were 30–150
nm in diameter, suggesting that they were exosomes. The results
from western blotting demonstrated the presence of CD63 and HSP70,
confirming that these EVs were exosomes.
Suppression of antitumor immune responses by
tumor-derived soluble factors, including inhibitory cytokines, has
long been recognized as a mechanism contributing to tumor
progression (25). Although the
existence of cross-talk between tumor and recipient immune cells is
widely accepted (5–7), the underlying mechanisms remain
unclear. The present study investigated the effect of MM
cell-derived exosomes on the apoptosis, viability, and function of
T cells from HDs and patients with MM. The results demonstrated the
immune-suppression effect of TEX on T cells and the abnormal immune
function of T cells in patients with MM.
In the present study, MM-derived exosomes promoted
the apoptosis and inhibited the viability of CD4+ T cells in HDs.
These findings had been previously described. Furthermore, Ludwig
et al (26) reported that
exosomes of patients with active disease (AD) were significantly
more effective than exosomes of patients with no evident disease
(NED) in inducing apoptosis of CD8+ T cells, suppression of CD4+ T
cell proliferation and upregulation of regulatory T cell (Treg)
suppressor functions.
In the present study, MM-derived exosomes promoted
proliferation, inhibited apoptosis and decreased perforin
expression in CD8+ T cells from HDs. These results suggested that,
although the quantity of CD8+ T cells increased, their function
decreased, which further confirmed the inhibitory effect of
exosomes on CD8+ T cells from HDs. The ability of TEXs to induce
CD8+ T cell apoptosis is due to the presence of the
membrane-associated form of FasL and major histocompatibility
complex (MHC) class I molecules on TEXs (27,28).
TEXs with the highest content of FasL and MHC class I molecules can
most actively induce T cell apoptosis, which can be partially
blocked by anti-Fas or anti-MHC class I antibodies, and completely
blocked in the presence of both antibodies (27). Although the role of FasL carried by
TEXs in the apoptosis induction of activated Fas+ CD8+ T cells has
been described, the role of MHC class I molecules remains unclear.
In the peripheral blood of patients with cancer, almost all CD8+
lymphocytes express CD95 at their surface (29), and a number of them express
programmed cell death 1 (PD-1) (30). Because TEXs in the serum of these
patients carry FasL and/or programmed cell death-ligand 1 (PD-L1),
the corresponding death pathways (Fas/FasL or PD-1/PD-L1,
respectively) may be responsible for the spontaneous apoptosis of
CD8+ T cells observed in vivo (31).
The present study did not evaluate the secretion of
cytokines from CD4+ T cells, including interferon γ (IFN-γ) and
IL-4. Ye et al (32) had
studied the effect of TEXs on cytokines secreted by CD4+ T cells
and reported that, under exosome stimulation, the secretion of
IL-1β, IL-6 and IL-10 from CD4+ T cells is increased, which is not
the case for IL-4. However, only IL-6 increased secretion is
significant. In addition, the secretion of tumor necrosis factor α
(TNFα), IL-12, granulocyte-macrophage colony-stimulating factor,
INF-γ, IL-2 and IL-17 from CD4+ T cells under TEXs stimulation is
decreased; however, only the secretion of IL-12, IL-17, IL-2 and
IFN-γ is significantly decreased. Since CD4+ T cells can be
subdivided into different cell subsets, including Th1/Th2, Treg and
CD17+ T, the present study focused on Treg of CD4+ T cells, and
only IL-10 and TGF-β secretion was evaluated.
MM-derived exosomes inhibited the apoptosis and
promoted the proliferation of Treg cells from HDs. It was reported
that plasma exosomes from patients with nasopharyngeal carcinoma
could partially enhance the immune-suppression function of normal
Treg cells (33). In addition, TEXs
could promote the generation and function of Tregs. When
co-incubated with exosomes purified from supernatants of tumor
cells, CD4+ CD25- T cells are converted into Tregs, which display
elevated expression of IL-10, TGF-β and CTLA4 (33). Muller et al (13) co-cultured T lymphocytes with TEXs or
dendritic cells-derived exosomes (DEXs) and detected the expression
level of immune response-associated genes. The results demonstrated
that, in activated T cells, TEXs and DEXs increase the mRNA
expression of numerous genes, and that Tregs are more sensitive to
the effect of co-culture with TEXs and DEXs than CD4+ T and CD8+ T
subsets. Furthermore, in Tregs co-cultured with TEXs or DEXs, the
CD39 gene, which regulates the adenosine pathway, is overexpressed,
leading to increased production of adenosine. TEX also induces the
upregulation of CD69 in CD4+ T, resulting in the loss of function
of CD4+ T cells (13).
The effect of TEXs on Tregs is different from their
effect on CD4+ T cells. The change of 23 immune response-associated
mRNA expression level in activated Tregs is higher than that in
other T cells, suggesting that activated Tregs may be more
sensitive to TEX-mediated effects than other T cells. After
incubated with TEXs, CD4+ T cells were activated, and the gene
profiles demonstrated that the expression of immunosuppressive
genes, including cyclooxygenase-2 (COX-2), cytotoxic
T-lymphocyte-associated protein 4, Fas, FasL and TGF-β, was
decreased compared with before incubation, which was associated
with the decrease in mean of intensity fluorescence (MIF) of CD69
on the cell surface. TEXs may therefore inhibit the activation of
CD4+ T cells by promoting the translation of inhibitory proteins.
TEXs can upregulate the expression of immunosuppressive genes in
activated Tregs and promote their rapid transformation into
inhibitory proteins, including TGF-β, IL-10, COX-2, CD39, CD73 and
adenosine (13). These findings
suggest that TEXs could mediate the differential regulation of CD4+
T and Treg gene expression and cell function, and confirm the
results from the present study demonstrating that MM-derived
exosomes promoted the apoptosis and inhibited the proliferation of
CD4+ T cells from HDs, and inhibited the apoptosis and promoted the
proliferation and IL-10 expression of Tregs from HDs.
The ratio of CD4+/CD8+ T cells in the blood of
patients with MM decreased compared with healthy donors (34). A previous study reported Treg cells
measured by FOXP3 expression are significantly decreased in the
blood of patients with monoclonal gammopathy of undetermined
significance (MGUS) and MM compared with healthy donors, despite
their elevated number of CD25+/CD4+ cells (35). This group suggested that CD25+ T
cells from patients with MGUS or MM fail to inhibit the
proliferation of PBMCs treated with anti-CD3, suggesting that MM
Tregs are dysfunctional. However, the results remain conflicting,
and Treg frequency is alternatively described as increased
(36–38) or decreased (35,39) in
patients with MM. Notably, patients with higher percentage of Treg
in peripheral blood have a decreased overall survival (median
overall survival 21 months vs. not reached, P=0.013) (38).
Since TEXs contain tumor antigens, they may be used
as vaccines for immunotherapy of cancer (31,40,41). For
example, exosomes released from MM cells that are designed to
express TNF-α (EXOTNF-α) can induce a more efficient
tumor antigen-specific CD8+ T cell response and inhibit tumor
growth than exosomes designed to express IL-2 (EXOIL-2)
or INF-γ (EXOINF-γ) in mice (42).
Although the role of MM-derived exosomes on T cell
immunity is not comprehensive, evidence is accumulating to
demonstrate that MM-derived exosomes are involved in the immune
abnormalities of Treg cells and the formation of bone marrow
immunosuppressive microenvironment. Therefore, further studies are
required to clarify the regulatory mechanism of exosomes on Treg,
so as to provide theoretical basis for alleviating the
immunosuppression of bone marrow microenvironment and improving the
prognosis of patients with MM.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Tianjin Key
Projects of Health and Family Planning Commission (grant no.
15KG150) and the Anticancer Major Special Project of Tianjin (grant
no. 12ZCDZSY18000).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
RF designed the present study. QS and LD performed
most of the experiments, analyzed the data, drew the figures and
drafted the initial manuscript. JC, FJ and SY helped with ELISA,
western blotting and the flow cytometry. HL and ZL analysed and
interpreted the data, and RF checked the manuscript and figures.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Medical University General Hospital (Tianjin,
China; approval no. IRB2020-WZ-064). All healthy donors and
patients with multiple myeloma provided written informed consent
prior to the study.
Patient consent for publication
Written informed consent was obtained from the
patients for the publication of this report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MM
|
multiple myeloma
|
|
HD
|
healthy donor
|
|
Treg
|
regulatory T cell
|
|
DC
|
dendritic cell
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
MHC
|
major histocompatibility complex
|
|
TEX
|
tumor-derived exosome
|
|
GrB
|
granzyme B
|
|
DEX
|
dendritic cell-derived exosome
|
|
IL
|
interleukin
|
|
TGF-β
|
transforming growth factor β
|
|
HSP 70
|
heat shot protein 70
|
|
EV
|
extracellular vesicle
|
References
|
1
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International Myeloma Working Group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar SK, Dispenzieri A, Lacy MQ, Gertz
MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N, et
al: Continued improvement in survival in multiple myeloma: Changes
in early mortality and outcomes in older patients. Leukemia.
28:1122–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mikkilineni L and Kochenderfer JN:
Chimeric antigen receptor T-cell therapies for multiple myeloma.
Blood. 130:2594–2602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Yuan X, Shi H, Wu L, Qian H and
Xu W: Exosomes in cancer: Small particle, big player. J Hematol
Oncol. 8:832015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu S, Cao H, Shen B and Feng J:
Tumor-derived exosomes in cancer progression and treatment failure.
Oncotarget. 6:37151–37168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Milane L, Singh A, Mattheolabakis G,
Suresh M and Amiji MM: Exosome mediated communication within the
tumor microenvironment. J Control Release. 219:278–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L,
Li C, Cong Y, Kimberly R, Grizzle WE, et al: Tumor exosomes inhibit
differentiation of bone marrow dendritic cells. J Immunol.
178:6867–6875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clayton A, Mitchell JP, Court J, Mason MD
and Tabi Z: Human tumor-derived exosomes selectively impair
lymphocyte responses to interleukin-2. Cancer Res. 67:7458–7466.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szajnik M, Czystowska M, Szczepanski MJ,
Mandapathil M and Whiteside TL: Tumor-derived microvesicles induce,
expand and up-regulate biological activities of human regulatory T
cells (Treg). PLoS One. 5:e114692010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chalmin F, Ladoire S, Mignot G, Vincent J,
Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau
D, et al: Membrane-associated Hsp72 from tumor-derived exosomes
mediates STAT3-dependent immune suppressive function of mouse and
human myeloid-derived suppressor cells. J Clin Invest. 120:457–471.
2010.PubMed/NCBI
|
|
12
|
Xiang X, Poliakov A, Liu C, Liu Y, Deng
ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, et al: Induction of
myeloid-derived suppressor cells by tumor exosomes. Int J Cancer.
124:2621–2633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muller L, Mitsuhashi M, Simms P, Gooding
WE and Whiteside TL: Tumor-derived exosomes regulate expression of
immune function related genes in human T cell subsets. Sci Rep.
6:202542016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, De Veirman K, Faict S, Frassanito
MA, Ribatti D, Vacca A and Menu E: Multiple myeloma exosomes
establish a favourable bone marrow microenvironment with enhanced
angiogenesis and immunosuppression. J Pathol. 239:162–173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raimondi L, De Luca A, Amodio N, Manno M,
Raccosta S, Taverna S, Bellavia D, Naselli F, Fontana S, Schillaci
O, et al: Involvement of multiple myeloma cell-derived exosomes in
osteoclast differentiation. Oncotarget. 6:13772–13789. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vignali DAA, Collison LW and Workman CJ:
How regulatory T cells work. Nat Rev Immunol. 8:523–532. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andersen MH, Schrama D, Thor Straten P and
Becker JC: Cytotoxic T cells. J Invest Dermatol. 126:32–41. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Théry C, Amigorena S, Raposo G and Clayton
A: Isolation and characterization of exosomes from cell culture
supernatants and biological fluids. Curr Protoc Cell Biol Chapter.
3:Unit 3.22. 2006.
|
|
19
|
Wang J, Hendrix A, Hernot S, Lemaire M, De
Bruyne E, Van Valckenborgh E, Lahoutte T, De Wever O, Vanderkerken
K and Menu E: Bone marrow stromal cell-derived exosomes as
communicators in drug resistance in multiple myeloma cells. Blood.
124:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung MK and Mun JY: Sample preparation and
imaging of exosomes by transmission electron microscopy. J Vis Exp.
131:564822018.
|
|
21
|
Quispe EÁ, Li XM and Yi H: Comparison and
relationship of thyroid hormones, IL-6, IL-10 and albumin as
mortality predictors in Case-mix critically ill patients. Cytokine.
81:94–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lobb RJ, Becker M, Wen SW, Wong CS,
Wiegmans AP, Leimgruber A and Möller A: Optimized exosome isolation
protocol for cell culture supernatant and human plasma. J Extracell
Vesicles. 4:270312015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vojtech L, Woo S, Hughes S, Levy C,
Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R,
Tewari M and Hladik F: Exosomes in human semen carry a distinctive
repertoire of small non-coding RNAs with potential regulatory
functions. Nucleic Acids Res. 42:7290–7304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boyiadzis M and Whiteside TL: The emerging
roles of tumor-derived exosomes in hematological malignancies.
Leukemia. 31:1259–1268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Whiteside TL: Immune responses to
malignancies. J Allergy Clin Immunol. 125 (Suppl):S272–S283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ludwig S, Floros T, Theodoraki MN, Hong
CS, Jackson EK, Lang S and Whiteside TL: Suppression of lymphocyte
functions by plasma exosomes correlates with disease activity in
patients with head and neck cancer. Clin Cancer Res. 23:4843–4854.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JW, Wieckowski E, Taylor DD, Reichert
TE, Watkins S and Whiteside TL: Fas ligand-positive membranous
vesicles isolated from sera of patients with oral cancer induce
apoptosis of activated T lymphocytes. Clin Cancer Res.
11:1010–1020. 2005.PubMed/NCBI
|
|
28
|
Wieckowski EU, Visus C, Szajnik M,
Szczepanski MJ, Storkus WJ and Whiteside TL: Tumor-derived
microvesicles promote regulatory T cell expansion and induce
apoptosis in tumor-reactive activated CD8+T lymphocytes. J Immunol.
183:3720–3730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JW, Tsukishiro T, Johnson JT and
Whiteside TL: Expression of pro- and antiapoptotic proteins in
circulating CD8+ T cells of patients with squamous cell carcinoma
of the head and neck. Clin Cancer Res. 10:5101–5110. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tran TH, Mattheolabakis G, Aldawsari H and
Amiji M: Exosomes as nanocarriers for immunotherapy of cancer and
inflammatory diseases. Clin Immunol. 160:46–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS,
Zhang XS, Cui J, Zeng YX and Li J: Tumor-derived exosomes promote
tumor progression and T-cell dysfunction through the regulation of
enriched exosomal microRNAs in human nasopharyngeal carcinoma.
Oncotarget. 5:5439–5452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Gu Y and Cao X: The exosomes in
tumor immunity. Oncoimmunology. 4:e10274722015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mills KH and Cawley JC: Abnormal
monoclonal antibody defined helper/suppressor T-cell subpopulations
in multiple myeloma: Relationship to treatment and clinical stage.
Br J Haematol. 53:271–275. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Prabhala RH, Neri P, Bae JE, Tassone P,
Shammas MA, Allam CK, Daley JF, Chauhan D, Blanchard E, Thatte HS,
et al: Dysfunctional T regulatory cells in multiple myeloma. Blood.
107:301–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bryant C, Suen H, Brown R, Yang S,
Favaloro J, Aklilu E, Gibson J, Ho PJ, Iland H, Fromm P, et al:
Long-term survival in multiple myeloma is associated with a
distinct immunological profile, which includes proliferative
cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood
Cancer J. 3:e1482013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beyer M, Kochanek M, Giese T, Endl E,
Weihrauch MR, Knolle PA, Classen S and Schultze JL: In vivo
peripheral expansion of naive CD4+CD25 high FoxP3+ regulatory T
cells in patients with multiple myeloma. Blood. 107:3940–3949.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Giannopoulos K, Kaminska W, Hus I and
Dmoszynska A: The frequency of T regulatory cells modulates the
survival of multiple myeloma patients: Detailed characterisation of
immune status in multiple myeloma. Br J Cancer. 106:546–552. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng P, Yan R, Dai X, Xie X, Wen H and
Yang S: The alteration and clinical significance of
Th1/Th2/Th17/Treg cells in patients with multiple myeloma.
Inflammation. 38:705–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andre F, Schartz NE, Chaput N, Flament C,
Raposo G, Amigorena S, Angevin E and Zitvogel L: Tumor-derived
exosomes: A new source of tumor rejection antigens. Vaccine. 20
(Suppl 4):A28–A31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Natasha G, Gundogan B, Tan A, Farhatnia Y,
Wu W, Rajadas J and Seifalian AM: Exosomes as immunotheranostic
nanoparticles. Clin Ther. 36:820–829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie Y, Bai O, Zhang H, Li W and Xiang J:
Tumor necrosis factor gene-engineered J558 tumor cell-released
exosomes stimulate tumor antigen P1A-specifc CD8+ CTL
responses and antitumor immunity. Cancer Biother Radiopharm.
25:21–28. 2010. View Article : Google Scholar : PubMed/NCBI
|