Introduction
Despite the decline in the rate of non-small cell
lung cancer (NSCLC) in recent years, it remains the leading cause
of cancer mortality worldwide, 1.8 million people are diagnosed
with lung cancer, and 1.6 million individuals died as a result of
the disease in 2017 worldwide (1).
Accurate diagnosis and precise prognostic predictions are critical
for targeted treatment and prolonging the overall survival of
patients with NSCLC (2). However,
commonly used biomarkers, such as carcino-embryonic antigen and
Cyfra21-1, offer low sensitivity and specificity and are not
appropriate for clinical applications (3,4). Novel
molecular or immunohistochemical-based biomarkers may facilitate
identification of cancer at an early stage in the future (5). Therefore, there is a need for the
development of novel markers.
Ring finger protein (RNF)180 is composed of a RING
finger domain, a basic coiled-coil domain, a novel conserved domain
and a C-terminal hydrophobic region (6). RNF180 was first reported in the brain,
kidney, testis and uterus, and it has important biological roles in
the developing lens and brain at the embryonic stage (7). RNF180 is associated with
Helicobacter pylori infection and serves as a biomarker for
atrophic gastritis (8). A previous
report revealed that RNF180 is involved in tumorigenesis in
gastrointestinal cancer (9). Deng
et al (10) demonstrated that
high expression levels of RNF180 inhibit colony formation,
proliferation, migration and invasion. As an anti-oncogene, RNF180
serves key roles in suppressing tumor growth and lymphangiogenesis
(10). In hepatocellular carcinoma,
RNF180 acts as a tumor suppressor during tumorigenesis (11). RNF180 participates in cell growth and
apoptosis, and upregulates not only antiproliferative regulators,
but also proapoptotic mediators (12). Thus, RNF180 is associated with
invasion and metastasis in cancer. However, understanding remains
limited regarding the role of RNF180 in the etiology of NSCLC.
Therefore, the present study aimed to elucidate the clinical
implication of RNF180 expression level and its association with the
survival rate of patients with NSCLC.
Materials and methods
Cell culture
NSCLC cell lines (A549 and HCC827) and a non-tumor
cell line (MRC-5) were obtained from The Cell Bank of Type Culture
Collection of Chinese Academy of Sciences. Cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (HyClone; Cytiva) and 100 U/ml
penicillin-streptomycin (HyClone; Cytiva) at 37°C under a
humidified atmosphere of 5% CO2.
Sample collection
Samples were collected from eligible patients from
First Teaching Hospital of Tianjin University of Traditional
Chinese Medicine (Tianjin, China) with NSCLC between February 2007
and December 2012. The inclusion criteria for the study included i)
Pathologically confirmed patients with NSCLC who underwent radical
surgery; ii) patients with surgical specimens, which were frozen in
−80°C refrigerator; iii) patients diagnosed with
Tumor-Node-Metastasis I–IIIA stage NSCLC (13); and iv) patients who did not receive
treatment prior to radical surgery, such as chemotherapy or
radiation therapy. Following curative surgery, tumor tissue were
frozen in liquid nitrogen immediately and stored at −80°C until
use, all patients were followed up every 3–6 months for 2 years,
then every year until the end of the study or death. The follow-up
was completed in September 2018.
Ethics statement
Written informed consent was obtained from all
participants prior to participation, according to the Helsinki
Declaration. The study protocol [TYLL2018(K) 007] was approved by
the Ethics Committee of Ethics Committee of First Teaching Hospital
of Tianjin University of Traditional Chinese Medicine (Tianjin,
China).
DNA and RNA extraction
RNA and DNA was extracted from cell lines and
tissues using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. RNA extracted
from the cells lines for RT-qPCR and DNA extracted from the tissues
for MSP.
Reverse transcription-quantitative
PCR
In total, 1 µg RNA was synthesized to cDNA using
PrimeScript™ RT Reagent kit (cat. no. DRR0037A; Takara
Biotechnology Co., Ltd.) at 42°C for 30 min and 85°C for 5 min
according to the manufacturer's protocol. qPCR was performed on the
7500 Fast Real Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) Primers designed and utilized for RNF180 and
GAPDH were as follows: RNF180 forward, 5′-TCTGACTTTCCTGATGGACCTG-3′
and reverse, 5′-CCTGAGTATTTACCCTGCTTCTGT-3′ and GAPDH forward,
5′-TGGGTGTGAACCATGAGAAGT-3′ and reverse,
5′-TGAGTCCTTCCACGATACCAA-3′. The PCR cycling conditions for all
sequences were 45 cycles of denaturation at 95°C for 30 sec,
annealing for 30 sec, and extension at 53.5°C for 30 sec, followed
by a final extension at 57.5°C for 10 min. Annealing was performed
at 60.0°C. The relative RNA levels was normalized to the GAPDH
value using the 2−ΔΔCq method (14).
Methylation-specific PCR (MSP)
The following RNF180 primers were used to detect the
methylated or unmethylated alleles of the RNF180 promoter:
Methylated RNF180 forward, 5′-TTTGCGCGGGGTTAAAGTTC and reverse,
5′-CGATACCGATTCGACGAAACG-3′; and unmethylated RNF180 forward,
5′-TGTTTGTTTGTGTGGGGTTAAAGTTT-3′ and reverse,
5′-CAACAACAATACCAATTCAACAAAACA-3′. MSP was performed using Ampli
Taq-Gold (Promega Corporation). The PCR reaction volume was 25 µl,
and the thermocycling conditions were as follows: 97°C initial
denaturation for 5 min, then 25 cycles of denaturation at 95°C for
40 sec, annealing at 60°C for 50 sec and extension for 50 sec at
72°C, then final extension at 72°C for 10 min. PCR amplification
was detected using 2% agarose gel (Jingtianmo Technology
Development Co., Ltd.) under the external light.
Western blotting
Western blotting was performed using Pierce™ Fast
Western Blot kit, ECL Substrate (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Total protein (40 µg,
determined by BCA) was loaded onto 12% gels and separated using
SDS-PAGE and then transferred to PVDF membranes. Membranes were
subsequently blocked with milk or BSA for 60 min at mean room
temperature, and incubated with primary antibodies against RNF180
polyclonal antibody (1:1,000; cat. no. ab127548; Abcam) and GAPDH
(1:1,000; cat. no. Ab8245; Abcam) at 4°C overnight. Subsequently,
the membranes were washed three times with TBS + 0.1% Tween-20 and
incubated with secondary anti-rabbit immunoglobulin antibody
(1:2,000; cat. no. SP-9001; ZhongShan Biotechnology). Finally, the
membranes were washed three times, detected and visualized by an
enhanced chemiluminescence detection system and a Gel Imager system
(both Asia Xingtai Mechanical and Electrical Equipment Company) was
to analyze images and to determine gray values.
Immunohistochemistry analysis
Paraffin blocks (4-µm thick) were fixed in 4%
paraformaldehyde at room temperature for 24 h before used, then
deparaffinized at 70°C for 15 min. Antigen was retrieval was
performed at 95°C for 40 min in 0.01 mol/l sodium citrate buffer
(pH 6.0), and endogenous peroxidase was blocked using 3% hydrogen
peroxide for 30 min at room temperature. Rabbit anti-RNF180
antibody (1:50; cat. no. ab127548) was purchased from Abcam. The
paraffin sections were incubated overnight with primary antibody at
4°C and then treated with peroxidase using a labeled polymer method
at 37°C with Zhongshan peroxidase (OriGene Technologies, Inc.) for
30 min. Antibody binding was visualized using the Avidin Biotin
Complex (ABC) Elite kit and 3,3′-diaminobenzine according to the
manufacturer's instructions (City Key Laboratory of Tianjin Cancer
Center). Sections were then counterstained in hematoxylin for 30
sec at mean room temperature. For general negative controls, the
primary antibody was replaced with PBS. All sections were scored
under a light microscope (Olympus Corporation) and recorded at 100×
or 400× magnification.
Evaluation of immunohistochemical
staining
In order to avoid potential bias, all sections were
assessed blindly by two independent observers; in case of
disagreement, a third independent assessment was involved. Protein
expression levels were analyzed for each patient based on a
randomly selected section. The staining score of each slide was
assessed according to staining intensity and the percentage of
positive cells. Staining intensity was scored as 0–4, where 0 was a
negative value, 1 was weak, 2 was moderate, 3 was strong intensity
pattern and 4 was a very strong intensity pattern. The extent of
staining was scored as follows: 0, 0–10, 1, 11–30; 2, 31–50; 3,
51–75; and 4, >75%, according to the percentage of
positive-staining cells in relation to the total cancer cells. A
final staining score >3 was considered to indicate a positive
expression level.
Statistical analysis
Data are presented as the mean ± standard deviation
and each experiment was repeated three times. Differences between
variables were estimated one-way ANOVA followed by
Student-Newman-Keuls post hoc test. Categorical variables were
analyzed using χ2 test. Survival analysis was performed
with the Kaplan-Meier method and log-rank test. Univariate analysis
of patient variables was performed by log-rank test, and
multivariate Cox regression analysis was performed to identify
prognostic indicators. Statistical calculations were performed
using SPSS software (version 21; IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
mRNA and protein expression level
analysis of RNF180
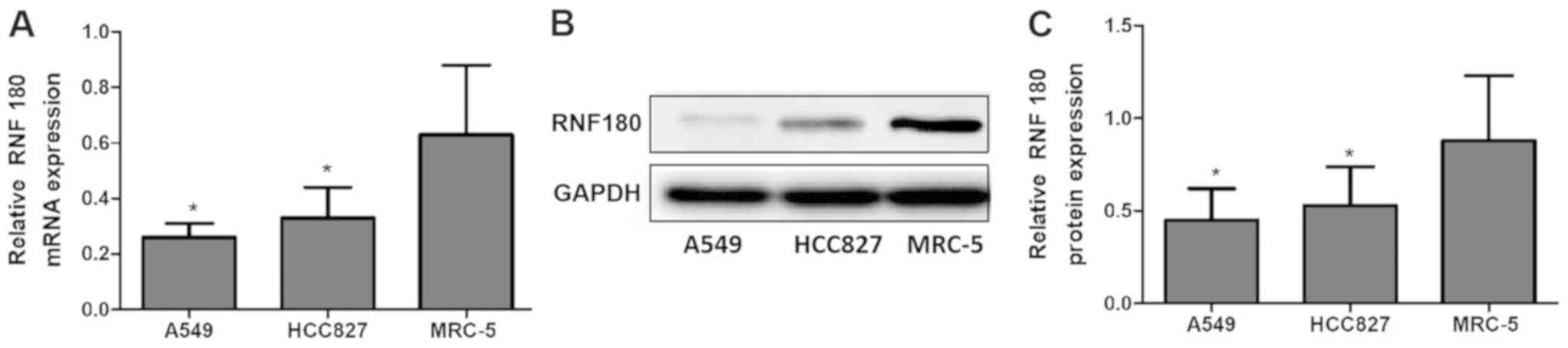
The relative mRNA expression level of RNF180 in the
NSCLC cell lines A549 (0.26±0.05; P=0.003) and HCC827 (0.33±0.11;
P=0.007) were significantly lower compared with the non-tumor cell
line MRC-5 (0.63±0.25). Consistent with mRNA expression levels, the
relative protein expression levels of RNF180 in A549 (0.45±0.17;
P=0.002) and HCC827 (0.53±0.21; P=0.006) were lower compared with
MRC-5 (0.88±0.35; Fig. 1).
Patient characteristics
Based on the inclusion criteria, 91 patients with
NSCLC were eligible for the present study. The 5-year survival rate
of the patients with NSCLC was 43.9%. The clinical characteristics
of the patients are presented in Table
I.
 | Table I.Clinicopathological characteristics of
patients with non-small cell lung cancer. |
Table I.
Clinicopathological characteristics of
patients with non-small cell lung cancer.
| Variable | Cases, n |
|---|
| Sex |
|
| Male | 55 |
|
Female | 36 |
| Age, years |
|
| ≤60 | 50 |
|
>60 | 41 |
| Smoking status |
|
| Yes | 37 |
| No | 54 |
| Histological
subtype |
|
|
Squamous | 28 |
|
Adenocarcinoma | 63 |
| Tumor location |
|
|
Peripheral | 66 |
|
Central | 25 |
| T stage |
|
| T1 | 33 |
| T2 | 41 |
| T3 | 17 |
| N stage |
|
| N0 | 40 |
| N1 | 10 |
| N2 | 41 |
Association between RNF180 expression
levels and methylation status of RNF180 promoter
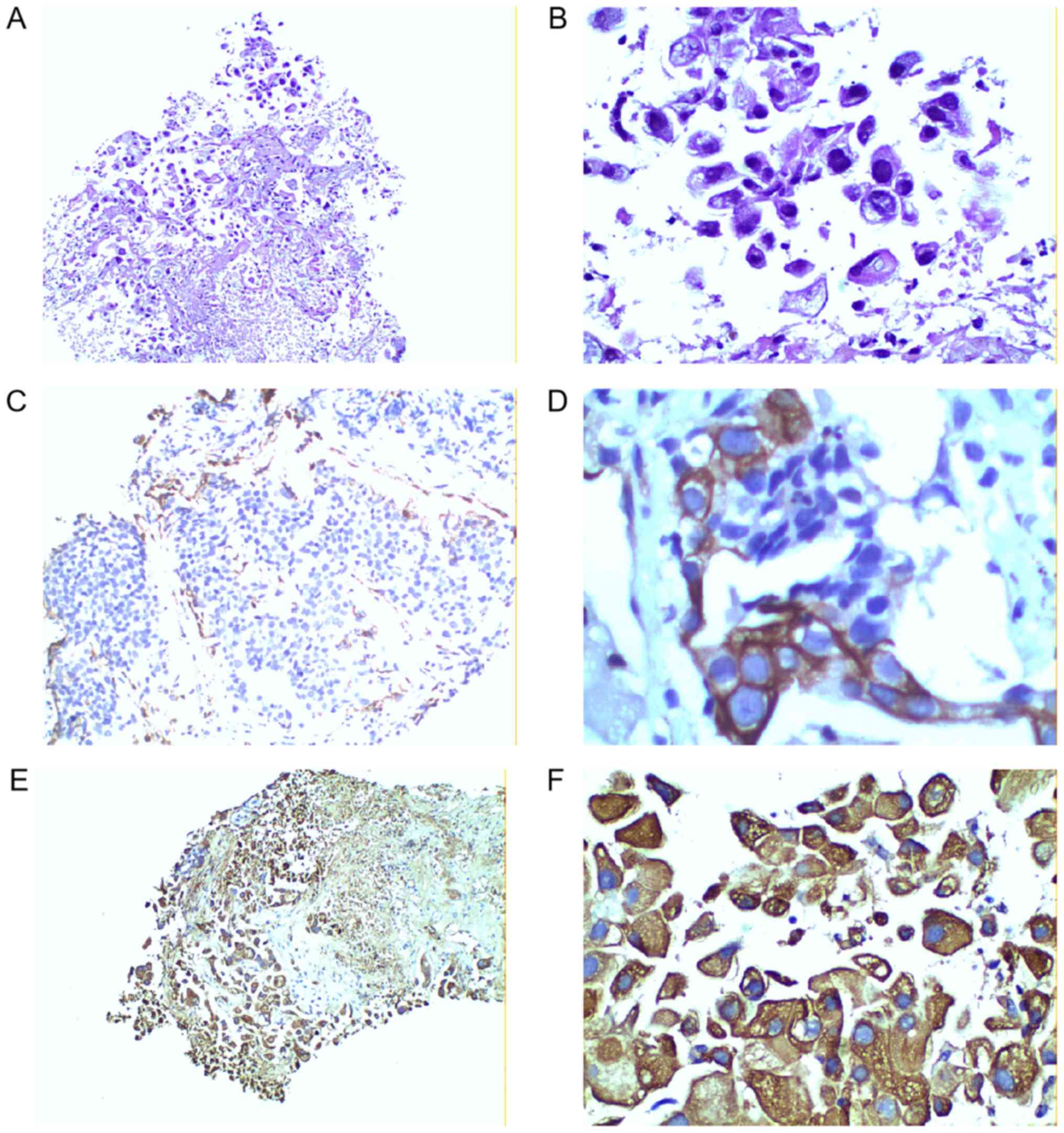
RNF180 expression levels in tissue specimens were
observed in the cytoplasm by immunohistochemical staining, as shown
in the representative images in Fig.
2. Fig. 2A and B represent
negative expression, C and D represent moderate positive
expression, and E and F represent strong positive expression. The
present results demonstrated that 27 patients were positive for
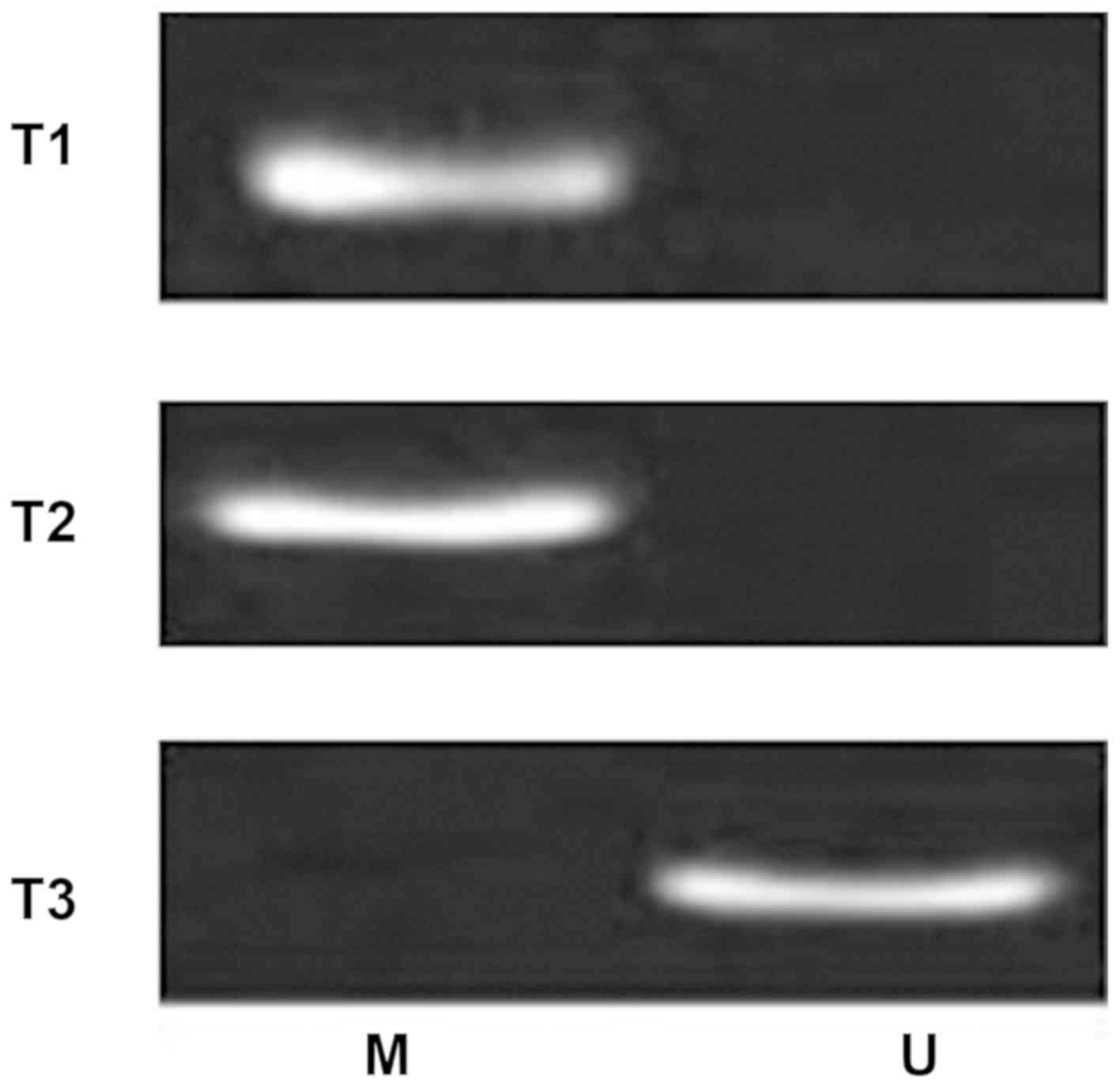
RNF180, whereas 64 patients were RNF180-negative. Furthermore, MSP
analysis detected the methylation status of the RNF180 promoter
(Fig. 3), showing randomly selected
different patients with lung cancer. T1 and T2 were for patients
with lung cancer with promoter methylation, while T3 was for lung
cancer without promoter methylation). A total of 60 patients
exhibited a methylated RNF180 promoter in NSCLC tissues, and the
other 31 patients exhibited an unmethylated RNF180 promoter.
The correlation between the methylation status of
RNF180 promoter and RNF180 expression levels was also investigated.
The methylation status of the RNF180 promoter was significantly
associated with RNF180 expression levels (χ2=22.528;
P<0.001; Table II). However, 8
RNF180-positive patients with methylated DNA and 12 RNF180-negative
patients with unmethylated DNA were identified.
 | Table II.Association between methylation status
of RNF180 promoter and RNF180 expression level. |
Table II.
Association between methylation status
of RNF180 promoter and RNF180 expression level.
|
| Methylation status of
RNF180 promoter |
|
|
|---|
|
|
|
|
|
|---|
| RNF180 expression
level | Methylated | Unmethylated | χ2
value | P-value |
|---|
| Negative | 52 | 12 | 22.528 | <0.001 |
| Positive | 8 | 19 |
|
|
Association between RNF180 expression
levels and clinical parameters
The associations between RNF180 expression level and
various parameters, such as sex, age, smoking status, histological
subtype, tumor location, T stage and N stage, were analyzed.
According to univariate analysis, T stage (P<0.001) and N stage
(P=0.043) were significantly associated with RNF180 expression
(demonstrated by χ2 test). Following multivariate
analysis, only T stage was identified to be independently
associated with RNF180 expression level (P=0.003; Table III).
 | Table III.Association between RNF180 expression
level and variables of patients with non-small cell lung
cancer. |
Table III.
Association between RNF180 expression
level and variables of patients with non-small cell lung
cancer.
|
| RNF180 expression
level | P-value |
|---|
|
|
|
|
|---|
| Variable | Negative, n | Positive, n | Univariate
analysis | Multivariate
analysis |
|---|
| Sex |
|
| 0.277 |
|
| Male | 41 | 14 |
|
|
|
Female | 23 | 13 |
|
|
| Age, years |
|
| 0.318 |
|
|
≤60 | 33 | 17 |
|
|
|
>60 | 31 | 10 |
|
|
| Smoking status |
|
| 0.063 |
|
|
Yes | 30 | 7 |
|
|
| No | 34 | 20 |
|
|
| Histological
subtype |
|
| 0.400 |
|
|
Squamous | 18 | 10 |
|
|
|
Adenocarcinoma | 46 | 17 | 0.214 |
|
| Tumor location |
|
|
|
|
|
Peripheral | 44 | 22 |
|
|
|
Central | 20 | 5 |
|
|
| T stage |
|
| <0.001 | 0.003 |
| T1 | 15 | 18 |
|
|
| T2 | 35 | 6 |
|
|
| T3 | 14 | 3 | 0.043 | 0.075 |
| N stage |
|
|
|
|
| N0 | 25 | 15 |
|
|
| N1 | 5 | 5 |
|
|
| N2 | 34 | 7 |
|
|
Survival analysis of patients with
NSCLC
Univariate analysis revealed significant
associations between overall survival and the methylation status of
the RNF180 promoter, RNF180 expression level, T stage and N stage
(P<0.05; Table IV). However, no
association with sex, age, smoking status, histological subtype or
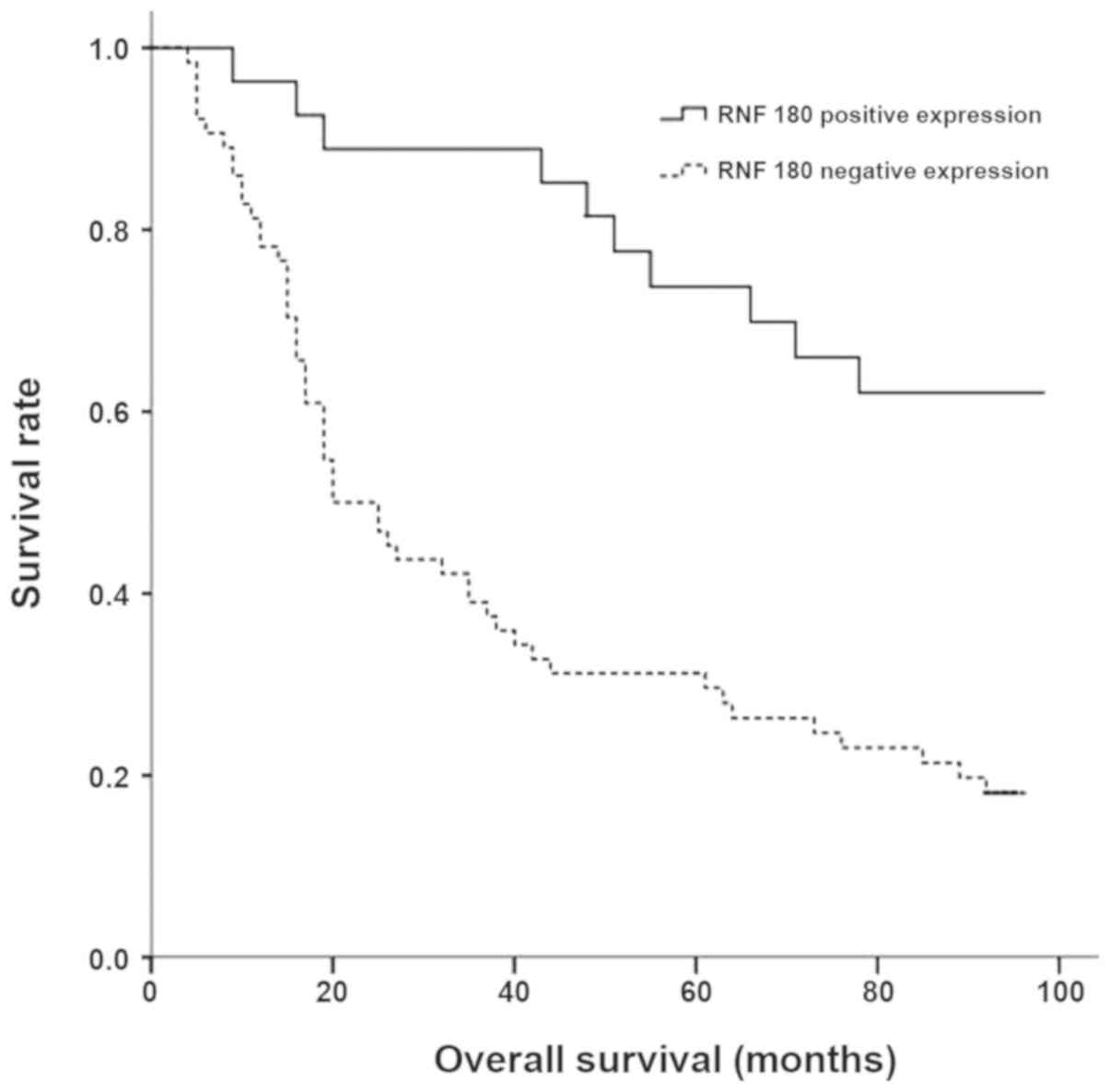
tumor location was detected (P>0.05). Patients with negative
RNF180 expression exhibited a significantly shorter overall
survival compared with patients with positive RNF180 expression
(Fig. 4).
 | Table IV.Survival analysis of patients with
non-small cell lung cancer. |
Table IV.
Survival analysis of patients with
non-small cell lung cancer.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | 5-year survival
rate, % | χ2
value | P-value | Hazard ratio | P-value |
|---|
| Sex |
| 0.048 | 0.826 |
|
|
|
Male | 45.5 |
|
|
|
|
|
Female | 41.7 |
|
|
|
|
| Age, years |
| 0.131 | 0.717 |
|
|
|
≤60 | 39.8 |
|
|
|
|
|
>60 | 48.8 |
|
|
|
|
| Smoking status |
| 1.766 | 0.184 |
|
|
|
Yes | 35.1 |
|
|
|
|
| No | 49.9 |
|
|
|
|
| Histological
subtype |
| 1.013 | 0.314 |
|
|
|
Squamous | 50.0 |
|
|
|
|
|
Adenocarcinoma | 41.2 |
|
|
|
|
| Tumor location |
| 1.935 | 0.164 |
|
|
|
Peripheral | 48.4 |
|
|
|
|
|
Central | 32.0 |
|
|
|
|
| T stage | 6.255 | 0.044 | 1.269 | 0.226 |
|
| T1 | 54.5 |
|
|
|
|
| T2 | 41.5 |
|
|
|
|
| T3 | 29.4 |
|
|
|
|
| N stage |
| 23.003 | <0.001 | 1.670 | <0.001 |
| N0 | 70.0 |
|
|
|
|
| N1 | 62.5 |
|
|
|
|
| N2 | 19.5 |
|
|
|
|
| RNF180 expression
level |
| 17.027 | <0.001 | 2.254 | 0.033 |
|
Negative | 31.3 |
|
|
|
|
|
Positive | 73.7 |
|
|
|
|
| Methylation status
of RNF180 promoter |
| 13.791 | <0.001 | 1.988 | 0.035 |
|
Methylated | 30.0 |
|
|
|
|
|
Unmethylated | 70.8 |
|
|
|
|
The variables that were significant in univariate
analysis were included in the multivariate Cox proportional hazard
analysis to adjust for the effects of covariates. According to this
analysis, the methylation status of the RNF180 promoter [hazard
ratio (HR), 1.988; P=0.035], RNF180 promoter expression levels (HR,
2.254; P=0.033) and N stage (HR, 1.670; P<0.001) were identified
as independent factors of overall survival (Table IV).
Discussion
Protein ubiquitylation is initiated by enzymatic
cascades that involve E3 ubiquitin ligases (15). RNF180 is an E3 ubiquitin ligase that
serves a key role in the function of the ubiquitin-proteasome
system by determining the specificity and timing of ubiquitination
and subsequent degradation of its substrates (16). RNF180 is either downregulated or
absent in different types of cancer, and is regarded as a
suppressor gene. Cheung et al (9) revealed that RNF180 is silenced in the
majority of gastric cancer cell lines and is significantly
downregulated in gastric cancer compared with normal tissue.
Results from a previous study suggest mRNA expression levels of
RNF180 in non-tumor tissue are ~3.60-fold higher compared with in
cancer tissue (17). However, little
is understood regarding the expression levels of RNF180 in NSCLC.
In the present study, significantly decreased levels of RNF180 mRNA
and protein were observed in lung cancer cell lines compared with
normal control cell lines. The results of the present study suggest
that the low expression level of RNF180 is modulated by methylation
of the RNF180 promoter region in the etiology of NSCLC. The
promoter region of the RNF180 gene is composed of abundant CpG
islands, which are primarily located in −202/+372 promoter region
of RNF180 (17). DNA methylation
regulates transcription and acts on CpG islands (18–20).
However, unmethylated DNA results in either loss or low levels of
gene expression. The present study identified 8 patients with
methylated RNF180 that exhibited a high expression level and 12
patients with unmethylated RNF180 that exhibited a lower level of
expression. It was hypothesized that methylation of only core CpG
islands may change the expression levels of the RNF180 gene. The
present results were consistent with a previous report (10). Immunohistochemical staining detected
negative RNF180 expression level in 70.3% of cases, and 18.75% of
cases contained unmethylated CpG sites. It was hypothesized that
the abnormal expression levels of RNF180 in NSCLC may result from
DNA promoter methylation.
A previous study suggested that the methylation of
RNF180 promoter is associated with lymph node metastasis by
downregulating RNF180-mediated expression levels of hepatocyte
growth factor, matrix metalloproteinase (MMP)-2, MMP-14 and
vascular endothelial growth factor C/D (10). Similarly, a previous study had also
demonstrated that negative RNF180 expression levels are observed
more frequently in patients with advanced TNM stages (7). Therefore, low RNF180 expression level
is associated with poor biological behavior of cancer. Similarly,
the present study identified that a negative RNF180 expression
level was associated with advanced T stage and increased N stage.
These results suggested that RNF180 expression levels were
associated with the invasion and metastasis of NSCLC.
There have been limited studies investigating the
prognostic potential of RNF180 expression levels in patients with
NSCLC. In a previous study, Xie et al (21) reported patients who possess ≤7
hypermethylated CpG sites of the RNF180 DNA promoter exhibit
improved overall survival. A previous study identified methylated
gene Dishevelled-associated antagonist of β-catenin 1 expression
levels as an independent predictor of patient survival (22). A previous study had demonstrated that
the number of methylated CpG sites can provide distinct survival
discrimination of patients (23).
However, a cheaper and simpler method to predict the prognosis of
patients with NSCLC is required. Assessing methylated CpG site
counts and concrete CpG islands by bisulfite genomic sequencing
method is expensive and laborious and is not widely used in
clinical applications (24). In the
present study, the methylation status of the RNF180 promoter and
RNF180 expression levels were detected by MSP and
immunohistochemistry; and their potential prognostic value for
patients with NSCLC was assessed using Cox regression analysis. The
present study demonstrated that the methylation status of the
RNF180 promoter was an independent factor of the overall survival.
However, RNF180 expression level had a higher HR value compared
with the methylation status of RNF180 promoter, and hence it was
speculated that RNF180 expression levels are more accurate in
predicting prognosis. This result indicated that RNF180 expression
level detected by immunohistochemistry was a simpler optimal
predictor of prognosis for patients with NSCLC compared with
methylation status.
In conclusion, the present study demonstrated that a
low level of methylated RNF180 promoter is associated with low
RNF180 expression level, which is associated with poor biological
behavior. Furthermore, the level of RNF180 can be used as a marker
for the clinical prediction of the prognosis of NSCLC.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Scientific
Research Project of Tianjin Municipal Education Commission (grant
no. 2018KJ015).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors were responsible for the conception and
design of the present study. HGL and PYY were responsible for the
provision of the study materials and the collection and assembly of
the data. HGL, PYY, XJL and YJJ performed the data analysis and
interpretation and contributed to writing of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants prior to participation, according to the Helsinki
Declaration. The study protocol [TYLL2018(K) 007] was approved by
the Ethics Committee of Ethics Committee of First Teaching Hospital
of Tianjin University of Traditional Chinese Medicine (Tianjin,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miranda-Filho A, Piñeros M and Bray F: The
descriptive epidemiology of lung cancer and tobacco control: A
global overview 2018. Salud Publica Mex. 61:219–229. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Zhu H, Sun L, Xu W and Wang X:
Prognostic value of site-specific metastases in lung cancer: A
population based study. J Cancer. 10:3079–3086. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao W, Yu H, Han Z, Gao N, Xue J and Wang
Y: Clinical significance of joint detection of serum CEA, SCCA, and
bFGF in the diagnosis of lung cancer. Int J Clin Exp Pathol.
8:9506–9511. 2015.PubMed/NCBI
|
|
4
|
Fu L, Wang R, Yin L, Shang X, Zhang R and
Zhang P: CYFRA21-1 tests in the diagnosis of non-small cell lung
cancer: A meta-analysis. Int J Biol Markers. 34:251–261. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qin K, Chen Y, Long H, Chen J, Wang D,
Chen L and Liang Z: The biomarkers and potential pathogenesis of
lung cancer related cerebral hemorrhage. Medicine (Baltimore).
98:e156932019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogawa M, Mizugishi K, Ishiguro A, Koyabu
Y, Imai Y, Takahashi R, Mikoshiba K and Aruga J: Rines/RNF180, a
novel RING finger gene-encoded product, is a membrane-bound
ubiquitin ligase. Genes Cells. 13:397–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Zhang X, Sun B, Lu H, Wang D,
Yuan X and Huang Z: Detection of aberrant promoter methylation of
RNF180, DAPK1 and SFRP2 in plasma DNA of patients with gastric
cancer. Oncol Lett. 8:1745–1750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han F, Sun LP, Liu S, Xu Q, Liang QY,
Zhang Z, Cao HC, Yu J, Fan DM, Nie YZ, et al: Promoter methylation
of RNF180 is associated with H. pylori infection and serves
as a marker for gastric cancer and atrophic gastritis. Oncotarget.
7:24800–24809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheung KF, Lam CN, Wu K, Ng EK, Chong WW,
Cheng AS, To KF, Fan D, Sung JJ and Yu J: Characterization of the
gene structure, functional significance, and clinical application
of RNF180, a novel gene in gastric cancer. Cancer. 118:947–959.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng J, Liang H, Zhang R, Hou Y, Liu Y,
Ying G, Pan Y and Hao X: Clinical and experimental role of ring
finger protein 180 on lymph node metastasis and survival in gastric
cancer. Br J Surg. 103:407–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Udali S, Guarini P, Ruzzenente A,
Ferrarini A, Guglielmi A, Lotto V, Tononi P, Pattini P, Moruzzi S,
Campagnaro T, et al: DNA methylation and gene expression profiles
show novel regulatory pathways in hepatocellular carcinoma. Clin
Epigenetics. 7:432015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han F, Liu S, Jing J, Li H, Yuan Y and Sun
LP: Identification of high-frequency methylation sites in RNF180
promoter region affecting expression and their relationship with
prognosis of gastric cancer. Cancer Manag Res. 12:3389–3399. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akhurst T: Staging of non-small-cell lung
cancer. PET Clin. 13:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ottis P, Toure M, Cromm PM, Ko E,
Gustafson JL and Crews CM: Assessing different E3 ligases for small
molecule induced protein ubiquitination and degradation. ACS Chem
Biol. 12:2570–2578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ning Y, Hui N, Qing B, Zhuo Y, Sun W, Du
Y, Liu S, Liu K and Zhou J: ZCCHC10 suppresses lung cancer
progression and cisplatin resistance by attenuating MDM2-mediated
p53 ubiquitination and degradation. Cell Death Dis. 10:4142019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng J, Guo J, Guo X, Hou Y, Xie X, Sun C,
Zhang R, Yu X and Liang H: Mediation of the malignant biological
characteristics of gastric cancer cells by the methylated CpG
islands in RNF180 DNA promoter. Oncotarget. 7:43461–43474. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Potabattula R, Dittrich M, Schorsch M,
Hahn T, Haaf T and El Hajj N: Male obesity effects on sperm and
next-generation cord blood DNA methylation. PLoS One.
14:e02186152019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang ZH, Dang YQ and Ji G: Role of
epigenetics in transformation of inflammation into colorectal
cancer. World J Gastroenterol. 25:2863–2877. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sutton LP, Jeffreys SA, Phillips JL,
Taberlay PC, Holloway AF, Ambrose M, Joo JE, Young A, Berry R,
Skala M, et al: DNA methylation changes following DNA damage in
prostate cancer cells. Epigenetics. 14:989–1002. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie XM, Deng JY, Hou YC, Cui JL, Wu WP,
Ying GG, Dong QP, Hao XS and Liang H: Evaluating the clinical
feasibility: The direct bisulfite genomic sequencing for
examination of methylated status of E3 ubiquitin ligase RNF180 DNA
promoter to predict the survival of gastric cancer. Cancer Biomark.
15:259–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng J, Liang H, Zhang R, Ying G, Xie X,
Yu J, Fan D and Hao X: Methylated CpG site count of dapper homolog
1 (DACT1) promoter prediction the poor survival of gastric cancer.
Am J Cancer Res. 4:518–527. 2014.PubMed/NCBI
|
|
23
|
Deng J, Liang H, Ying G, Li H, Xie X, Yu
J, Fan D and Hao X: Methylation of ras association domain protein
10 (RASSF10) promoter negative association with the survival of
gastric cancer. Am J Cancer Res. 4:916–923. 2014.PubMed/NCBI
|
|
24
|
Paun O, Verhoeven KJF and Richards CL:
Opportunities and limitations of reduced representation bisulfite
sequencing in plant ecological epigenomics. New Phytol.
221:738–742. 2019. View Article : Google Scholar : PubMed/NCBI
|