Introduction
Gallbladder cancer is the most common malignancy of
the biliary tract, with 219,420 new cases and 165,087 mortalities
cases reported in 2018 worldwide (1,2). At
present, gallbladder resection is the principle treatment option
for patients with gallbladder cancer (3). However, recurrence is common following
complete resection. Furthermore, the recurrence risk is increased
when excision surgery happens in the advanced stage (4,5). It is
therefore essential for patients with gallbladder cancer to be
diagnosed in the earlier stages of the disease (6). However, since patients with gallbladder
cancer have no apparent symptoms in the early stage, they often
miss the optimal treatment opportunity (7). The determination of potential
biomarkers would therefore provide screening opportunities for
patients at high risk of gallbladder cancer.
In order to develop an optimal treatment strategy
that would improve the overall outcome of patients with gallbladder
cancer, it is crucial to better understand the prognostic risk
factors. This strategy may help identifying patients receiving
preventive cholecystectomy who may be at risk of developing
gallbladder cancer, and assisting oncologists to develop
individualized treatment (8).
With the rapid development of microarray and RNA
sequencing technology, a high amount of differentially expressed
genes (DEGs) have been identified in cancer tissues compared with
non-tumor tissues (9,10). A previous study identified 758 long
non-coding RNAs (lncRNAs) and 1,254 mRNAs in gallbladder cancer
tissues compared with adjacent normal tissues (11). However, clinical trials still lack
precise biomarkers for gallbladder cancer (12). The present study identified DEGs in
both gallbladder walls and tumor tissues of gallbladder cancer via
microarray analysis. Microarray analysis has been extensively used
to screen biomarkers of gallbladder cancer. For example, a previous
study demonstrated through microarray analysis that lncRNA GCASPC
can negatively regulate pyruvate carboxylase-dependent cell
proliferation in gallbladder cancer (13). Furthermore, microarray analysis
confirmed that CD44 overexpression is associated with the
progression of gallbladder cancer, confirming its contribution to
poor patient prognosis (14).
However, there is little research on gene expression changes in
gallbladder walls of patients with gallbladder cancer (15). Kyoto Encyclopedia of Genes and
Genomics (KEGG) has been extensively used to better understand the
functions of numerous biological systems, including cells (such as
liver, gastric and gallbladder), organisms (such as heart, kidney
and pancreas) and ecosystems (16,17).
Among all hub genes identified, ubiquitin conjugating enzyme E2T
(UBE2T) was firstly highlighted in a patient with Fanconi anemia
(18). It has been reported that
hypoxia may disrupt the Fanconi anemia pathway and sensitize tumor
cells to chemotherapy by regulating UBE2T (19,20).
Furthermore, UBE2T overexpression is associated with poor prognosis
of patients with various types of cancer, including breast cancer
and multiple myeloma (21,22). In addition, UBE2T is upregulated in
hepatocellular carcinoma and exerts oncogenic activities through
p53 ubiquitination (23). A previous
study demonstrated that UBE2T is present in the nuclei and
cytoplasm of bladder cancer cells, furthermore, silencing UBE2T may
induce cell cycle arrest at the G2/M phase and may
promote cell apoptosis (24).
Furthermore, high UBE2T expression has been reported to promote
osteosarcoma cell proliferation, migration and invasion via
activation of the PI3K/Akt signaling pathway (25). However, the role of UBE2T in the
development of gallbladder cancer remains unknown. The present
study aimed therefore to investigate the expression and clinical
characteristics of UBE2T in patients with gallbladder cancer.
Materials and methods
Patients and specimens for microarray
analysis
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of China Medical
University and written informed consent was provided by all
patients prior to the study. A total of three patients with
gallbladder cancer (2 men and 1 woman; mean age, 61.00) and three
patients with gallbladder adenoma (2 men and 1 woman; mean age,
60.67) as the control groups were recruited from the Department of
Pancreatic and Biliary Surgery of the First Affiliated Hospital of
China Medical University (Shenyang, China) between September 2018
and December 2019. All patients were blindly reviewed by two
independent pathologists at the First Affiliated Hospital of China
Medical University. None of the patients had received radiotherapy
or chemotherapy prior to gallbladder resection. Gallbladder cancer
specimens and wall tissues were obtained from the same patients via
biopsy. The control specimens were collected from adenoma tissues
and gallbladder wall tissues from patients with gallbladder adenoma
by biopsy. Following collection, fresh specimens were instantly
frozen in liquid nitrogen within 15 min and subsequently stored in
RNA Fixer Reagent (Thermo Fisher Scientific, Inc.) at −80°C for
microarray analysis. The clinical characteristics of patients with
gallbladder cancer are presented in Table I.
 | Table I.Clinical characteristics of patients
with gallbladder cancer (n=3). |
Table I.
Clinical characteristics of patients
with gallbladder cancer (n=3).
| Characteristic | Patient, n |
|---|
| Age, years |
|
|
≤60 | 1 |
|
>60 | 2 |
| Sex |
|
|
Male | 2 |
|
Female | 1 |
|
Cholecystolithiasis |
|
|
Absent | 3 |
|
Present | 0 |
| Diabetes |
|
|
Absent | 0 |
|
Present | 3 |
| Jaundice |
|
|
Absent | 3 |
|
Present | 0 |
| Pathological
types |
|
|
Adenocarcinoma | 3 |
|
Adenosquamous carcinoma | 0 |
|
Papillocarcinoma | 0 |
| Degree of
differentiation |
|
|
Poor | 3 |
|
Moderate-well | 0 |
| Resection margin
status |
|
|
Positive | 2 |
|
Negative | 1 |
| T stage |
|
|
Tis-T1a | 0 |
|
T1b-T2b | 0 |
| T3 | 2 |
| T4 | 1 |
| N stage |
|
| N0 | 0 |
| N1 | 1 |
| N2 | 2 |
| Distant
metastasis |
|
|
Absent | 2 |
|
Present | 1 |
RNA isolation, quantification and
quality control
Total RNA was extracted from gallbladder cancer and
adjacent normal tissues using standard methods (RNA Easy; Thermo
Fisher Scientific, Inc.). RNA purity and concentration was detected
using NanoDrop ND-2000 Spectrophotometer (Thermo Fisher Scientific,
Inc.). RNA integrity was determined using Agilent Bioanalyzer 2100
system (Agilent Technologies GmbH).
RNA extraction and microarray
hybridization
Total RNA from tissue samples was analyzed on the
Agilent Bioanalyzer 2100 system (Agilent Technologies GmbH) and
reverse transcribed into cDNA using the PrimeScript™ RT Reagent kit
(cat. no. RR037A; Takara Biotechnology Co., Ltd), according to the
manufacturers' instructions. Briefly, cDNA was synthesized via
first-strand synthesis, and a double-stranded DNA template was
subsequently obtained via second-strand synthesis. cDNA was
purified with purification beads (Beckman Coulter, Inc.) and
fragmented, prior to hybridization with the chip probe. Following
hybridization, the chip was automatically washed and stained
(GeneChip Hybridization Wash and Stain Kit; Affymetrix) using the
GeneChip Fluidics Station 450 instrument, prior to scanning to
obtain the image and the Affymetrix original microarray data.
Differential expression analysis
Microarray data were extracted using Feature
Extraction software [version 10.5.1.1] (26), whereas GeneSpring software (version
12.0; Agilent Technologies, Inc.) was used to normalize the
quantiles of the raw data. DEGs with fold-change (|FC|)>1.5 and
P<0.05 were selected between gallbladder cancer wall tissues and
gallbladder adenoma wall tissues, and DEGs with FC>2.0 and
P<0.05 were selected between gallbladder cancer tissues and
gallbladder adenoma tissues. The DEGs from the two microarray
datasets were overlapped using Bioinformatics & Evolutionary
Genomics (version 1.0; http://bioinformatics.psb.ugent.be/webtools/Venn).
Different FC values were implemented as the two microarray datasets
contained gallbladder tumor walls vs. gallbladder adenoma walls and
gallbladder tumor tissues vs. gallbladder adenoma tissues,
respectively, thus, the gene expression patterns of the two
datasets varied.
Protein-protein interaction (PPI)
network construction and module analysis
A PPI network was constructed using the Search Tool
for Retrieval of Interacting Genes (STRING) database (http://string-db.org), which provides integrated
information of the known and predicted associations for protein
networks (27). An interaction with
a combined score >0.4 was considered to be statistically
significant. Cytoscape software version 3.7.2 (28) was used to visualize the PPI network.
The most significant module in the PPI network was selected by
using the Molecular Complex Detection (MCODE) plug-in (29), within Cytoscape. The criteria for
selection were as follows: MCODE scores >5, degree cut-off=2,
node score cut-off=0.2, Max depth=100 and k-score=2.
Identification of hub genes
The genes in the key module were considered to be
hub genes. Hierarchical clustering analysis of hub genes in the key
module was performed by using GraphPad Prism software (version 7.0;
GraphPad Software, Inc.). A co-expression network of hub genes was
constructed using the cBioPortal online platform (http://www.cbioportal.org). Biological analysis of the
hub genes was performed and visualized using the Biological Network
Gene Ontology tool (BiNGO) plug-in (30), within Cytoscape.
Functional enrichment analysis
KEGG and Gene Oncology (GO) enrichment analyses were
performed using the Database for Annotation, Visualization and
Integrated Discovery (DAVID) (31),
in order to determine the potential biological processes and
signaling pathways the DEGs were involved in. Adjusted P<0.05
was considered to indicate significantly enriched processes or
signaling pathways.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from 30 paired gallbladder
cancer tissues and corresponding adjacent non-cancerous tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Total
RNA was reverse transcribed into cDNA using the PrimeScript RT
Reagent kit (RR037A, Takara), according to the manufacturers'
protocol. qPCR was subsequently performed using the SYBR Green PCR
kit (cat. no. A25742; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for qPCR: One cycle
for 2 min at 50°C (initial denaturation); one cycle for 10 min at
95°C (denaturation); 40 cycles for 15 sec at 95°C (annealing and
elongation) and 40 cycles for 1 min at 60°C (final extension). The
sequences of the primers used were as follows: UBE2T, forward
5′-CAAATATTAGGTGGAGCCAACAC-3′, reverse
5′-TAGATCACCTTGGCAAAGAACC-3′; β-actin, forward
5′-AGAAAATCTGGCACCACACC-3′ and reverse 5′-TAGCACAGCCTGGATAGCAA-3′.
Relative expression level was normalized to the endogenous control
β-actin and was expressed as 2−ΔΔCq (32).
Western blotting
Total protein was extracted from 12 paired
gallbladder cancer tissues and corresponding adjacent normal
tissues using RIPA lysis buffer on ice (Nanjing KeyGen Biotech Co.,
Ltd.). Total protein was quantified using the bicinchoninic acid
assay kit (cat. no. P0009; Beyotime Institute of Biotechnology) and
12 µg protein/lane was separated via SDS-PAGE on a 10% gel, and
subsequently transferred onto a polyvinylidene difluoride membrane
(EMD Millipore). Membranes were blocked with 5% skim milk at room
temperature for 1 h. Subsequently, membranes were incubated with
primary antibodies against UBE2T (1:500; cat. no. 12992; Cell
Signaling Technology, Inc.) and β-actin (1:500; cat. no. 4970; Cell
Signaling Technology, Inc.) overnight at 4°C, followed by
incubation with the HRP-labeled secondary antibody (1:5,000; cat.
no. SA00001-2; ProteinTech Group, Inc.) for 45 min at room
temperature. Enhanced chemiluminescence reagent (Thermo Fisher
Scientific, Inc.) was used to detect the signal on the
membrane.
Immunohistochemistry
A total of 127 cases of formalin-fixed and
paraffin-embedded gallbladder cancer tissues from patients with
gallbladder cancer [40 men (31.5%) and 87 women (68.5%)] were
obtained from the Department of Pancreatic and Biliary Surgery of
the First Affiliated Hospital of China Medical University
(Shenyang, China). All patients provided written informed consent.
Clinicopathological classification and AJCC TNM classification of
the samples were determined according to the criteria from the
American Joint Committee on Cancer (33). Tissues were fixed with 4%
paraformaldehyde overnight at room temperature. Then, tissue
sections were deparaffinized and blocked with 1% bovine serum
albumin (Hyclone, Inc.) in PBS at room temperature for 1 h, prior
to quenching to inhibit endogenous peroxidase activity. The
sections were at 20 µm thickness. Tissue sections were subsequently
incubated with the rabbit anti-human-UBE2T antibody (1:500; cat.
no. 12992; Cell Signaling Technology, Inc.) at 4°C overnight,
followed by incubation with secondary antibody (1:5,000; cat. no.
PV-9001; OriGene Technologies, Inc.) for 2 h at room temperature.
Subsequently, sections were counterstained with ChemMate
Hematoxylin (Dako; Agilent Technologies, Inc.). Sections were
subsequently mounted using neutral gum and observed under an
optical microscope (Olympus Corporation; magnification, ×200).
Sections were blindly assessed by two independent pathologists at
the First Affiliated Hospital of China Medical University
(Shenyang, China) and were semi-quantitatively scored according to
the percentage of positive cells as follows: 1, 2, 3 or 4 for 0–25,
26–50, 51–75 or 76–100% of positively stained cells, respectively.
Furthermore, the sections were stained with Harris hematoxylin for
5 min and Eosin for 2 min at room temperature and scored according
to the cell staining intensity as follows: 0, 1, 2 or 3 for absence
for color, pale yellow color, yellow/brown color and brown color,
respectively. The two scores were multiplied to provide the
following composite scores: low expression (score <6) and high
expression (score ≥6).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 22.0; IBM Corp.) and GraphPad Prism software.
χ2 test was used to determine the correlation between
UBE2T expression and the clinicopathological characteristics of
patients with gallbladder cancer. Survival analysis was performed
using Kaplan-Meier method (34) and
two groups were compared using the log-rank test. Univariate and
multivariate Cox regression analyses were performed. P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of hub genes in tumor
tissues and gallbladder walls of patients with gallbladder
cancer
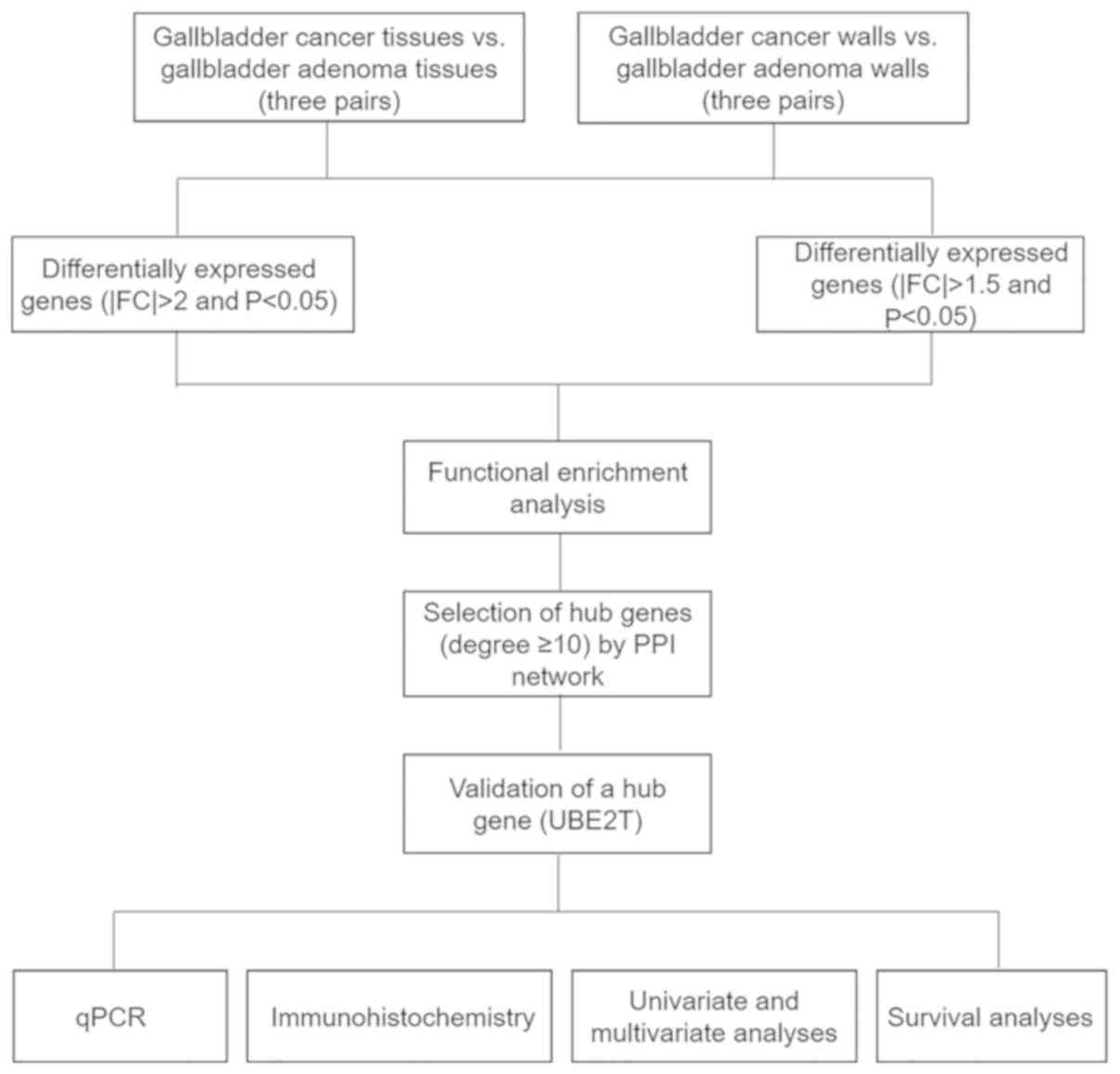
The work flowchart is presented in Fig. 1. Differential expression analysis was
performed for two respective microarray datasets. First, DEGs with
|FC|>1.5 and P<0.05 were selected by comparing three
gallbladder cancer walls with three gallbladder adenoma walls.
Furthermore, DEGs with |FC|>2 and P<0.05 were selected by
comparing three gallbladder cancer tissues with three gallbladder
adenoma tissues. The two datasets were overlapped to identify DEGs
in both tumor tissues and gallbladder walls of patients with
gallbladder cancer, resulting in the identification of 177 DEGs
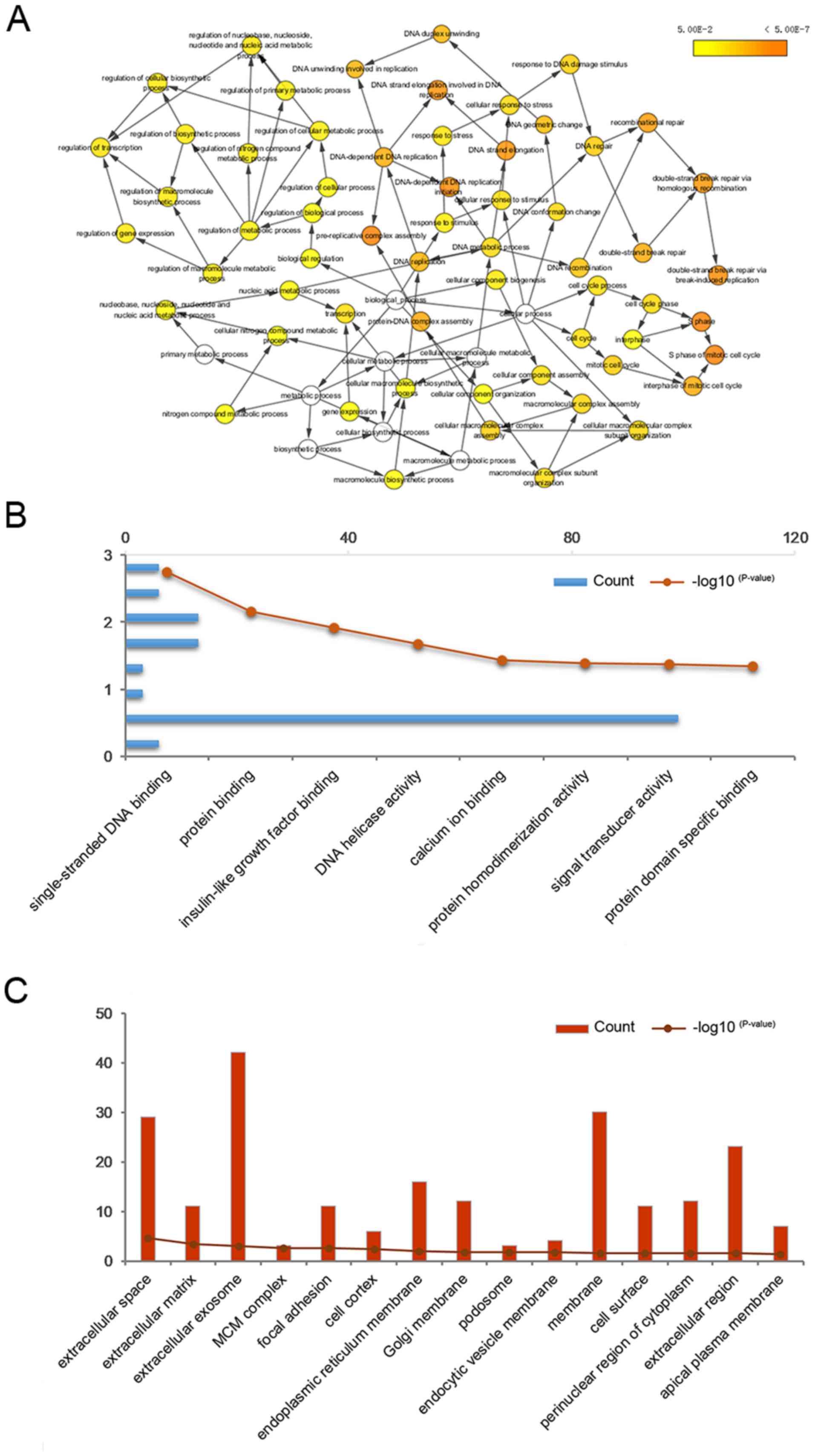
(Fig. 2A). Functional enrichment
analyses were performed to determine the biological processes and
signaling pathways enriched by the DEGs. A network of biological
processes associated with the DEGs was constructed by using BiNGO
(Fig. 3A). Furthermore, results from
GO analysis included molecular function (Fig. 3B), cell component (Fig. 3C) and biological processes (Table II). The results from KEGG pathway
analysis demonstrated that the DEGs were mainly enriched in the
‘TGF-β signaling pathway‘, ‘signaling pathways regulating
pluripotency of stem cells’ and ‘complement and coagulation
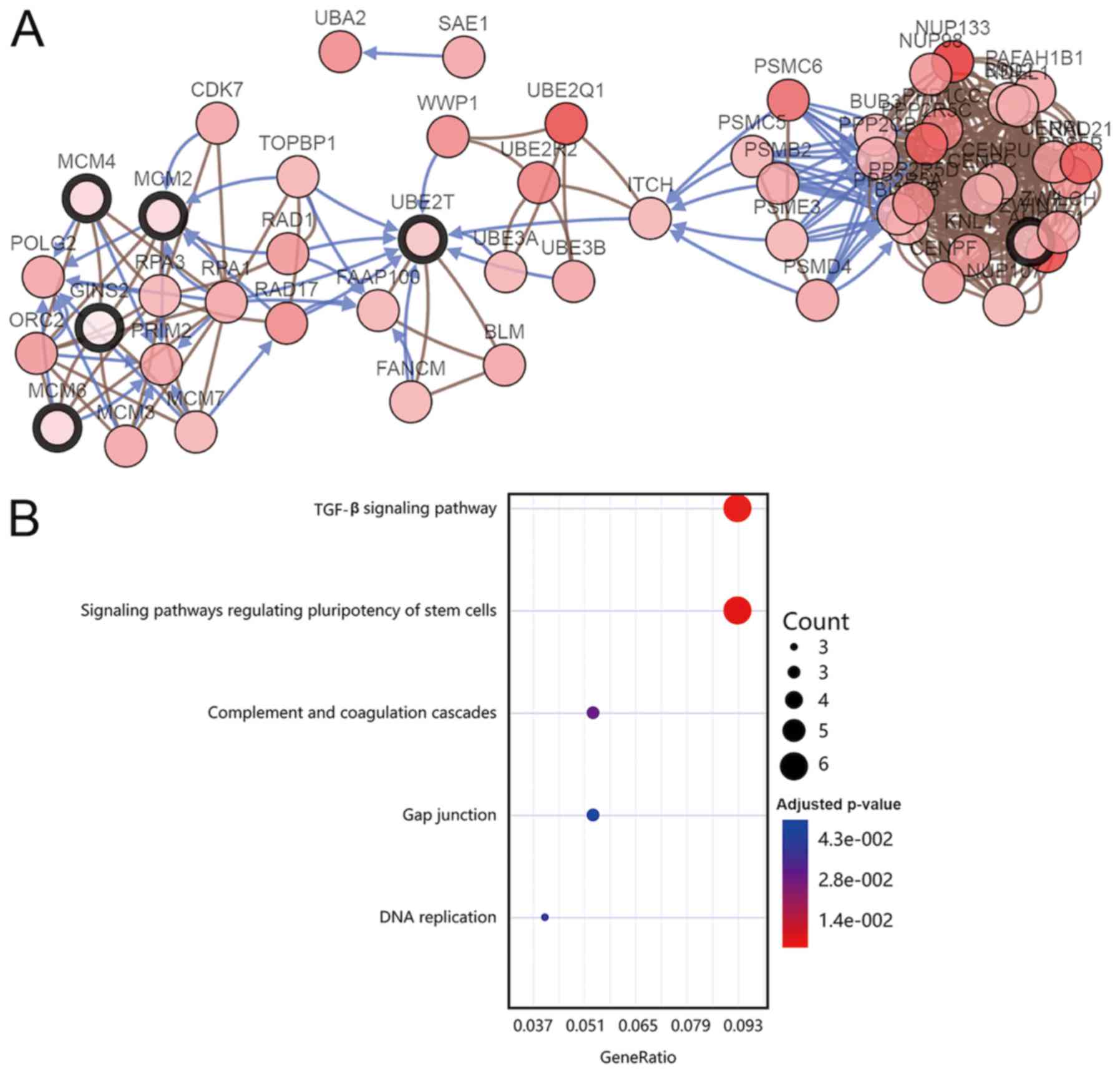
cascades’ (Fig. 4B). Subsequently, a
PPI network of the 177 DEGs was constructed (Fig. 2B) and the key modules were selected
using the MCODE plug-in within Cytoscape (Fig. 2C). A total of 10 nodes were
identified as hub genes (Table
III). Hierarchical clustering analysis was performed to
determine the expression pattern of the 10 hub genes in the two
microarray datasets (Fig. 2D and E).
The co-expression network of the 10 hub genes was constructed using
cBioPortal (Fig. 4A).
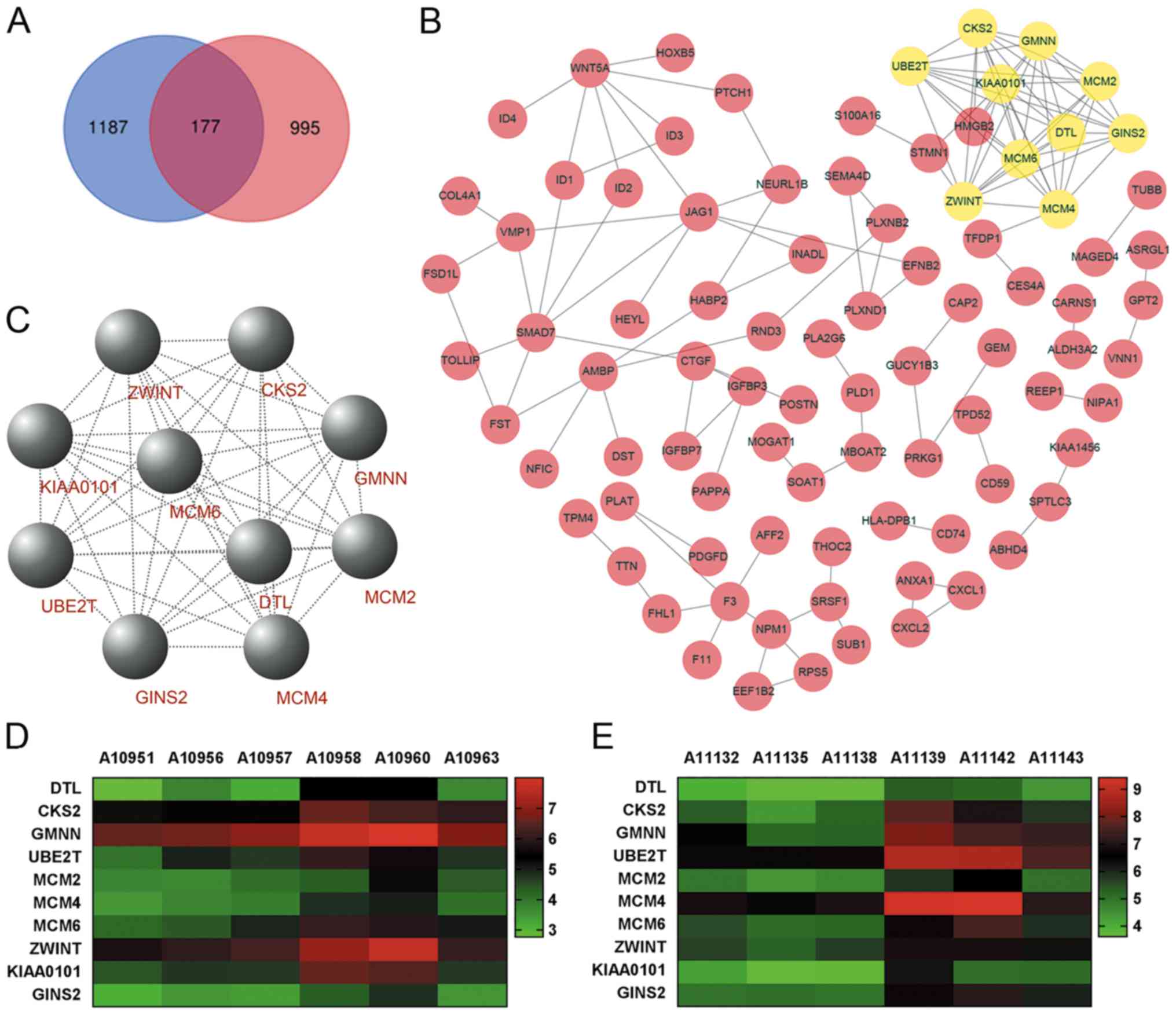 | Figure 2.PPI network and the key modules of
DEGs. (A) Intersection between three pairs of DEGs with FC>1.5
and P<0.05 (gallbladder tumor walls vs. gallbladder adenoma
walls), and three pairs of DEGs with |FC|>2 and P<0.05
(gallbladder tumor tissues vs. gallbladder adenoma tissues). For
microarray analysis, gallbladder adenoma was used as the control.
Blue represents DEGs in gallbladder tumor walls and pink represents
DEGs in gallbladder adenoma walls. (B) PPI network of 177 DEGs was
constructed using Cytoscape software. In the PPI network, each node
represents a protein and each edge represents a PPI. (C) DEGs with
degrees ≥10 were selected from the PPI network as the hub genes for
the key modules. Hierarchical clustering analysis of the hub genes
in the datasets, (D) three pairs of gallbladder tumor walls vs.
gallbladder adenoma walls and (E) three pairs of gallbladder tumor
tissues vs. gallbladder adenoma tissues. A10951, A10956 and A10957;
gallbladder adenoma wall samples; A10958, A10960 and A10963,
gallbladder tumor wall samples; A11132, A11135 and A11138;
gallbladder adenoma tissue samples; A11139, A11142 and A11143,
gallbladder tumor tissue samples. PPI, protein-protein interaction;
DEG, differentially expressed gene; FC, fold-change. |
 | Table II.Biological processes associated with
the 177 differentially expressed genes. |
Table II.
Biological processes associated with
the 177 differentially expressed genes.
| Term | Count | P-value |
|---|
| Negative regulation
of osteoblast differentiation | 5 |
4.580×10−4 |
| Cellular response
to transforming growth factor beta stimulus | 5 |
1.097×10−3 |
| Negative regulation
of sequence-specific DNA binding transcription factor activity | 5 |
2.333×10−3 |
| DNA
replication | 7 |
3.76×10−3 |
| DNA unwinding
involved in DNA replication | 3 |
3.675×10−3 |
| Positive regulation
of cell proliferation | 12 |
4.225×10−3 |
| Regulation of cell
growth | 5 |
6.561×10−3 |
| Negative regulation
of fat cell differentiation | 4 |
6.926×10−3 |
| Negative regulation
of transcription, DNA-templated | 12 |
6.973×10−3 |
| Brain
development | 7 |
8.699×10−3 |
| Organ
morphogenesis | 5 |
1.064×10−2 |
| Cell adhesion | 11 |
1.072×10−2 |
| Positive regulation
of fibroblast proliferation | 4 |
1.380×10−2 |
| Negative regulation
of neuron differentiation | 4 |
1.522×10−2 |
| Negative regulation
of transcription from RNA polymerase II promoter | 14 |
1.685×10−2 |
| Positive regulation
of neutrophil chemotaxis | 3 |
1.754×10−2 |
| Positive regulation
of T cell proliferation | 4 |
1.829×10−2 |
| Response to
wounding | 4 |
2.081×10−2 |
| Cell
proliferation | 9 |
2.116×10−2 |
| Positive regulation
of endothelial cell proliferation | 4 |
2.638×10−2 |
| Negative regulation
of apoptotic process | 10 |
2.641×10−2 |
| Epithelial cell
differentiation | 4 |
2.737×10−2 |
| Positive regulation
of cell migration | 6 |
2.873×10−2 |
| Ventricular septum
morphogenesis | 3 |
2.955×10−2 |
| Actin cytoskeleton
organization | 5 |
3.310×10−2 |
| Regulation of
angiogenesis | 3 |
3.344×10−2 |
| DNA replication
initiation | 3 |
3.546×10−2 |
| Diacylglycerol
biosynthetic process | 2 |
3.665×10−2 |
| Semaphorin-plexin
signaling pathway | 3 |
3.752×10−2 |
| Positive regulation
of catalytic activity | 4 |
3.966×10−2 |
| Signal
transduction | 18 |
4.163×10−2 |
| Proteolysis | 10 |
4.397×10−2 |
| Lipid catabolic
process | 4 |
4.471×10−2 |
 | Table III.Ten hub genes in tumor tissues and
gallbladder walls of gallbladder cancer. |
Table III.
Ten hub genes in tumor tissues and
gallbladder walls of gallbladder cancer.
| Gene symbol | Full name |
|---|
| DTL | Denticleless E3
ubiquitin protein ligase homolog (Drosophila) |
| CKS2 | CDC28 protein
kinase regulatory subunit 2 |
| GMNN | Geminin, DNA
replication inhibitor |
| UBE2T | Ubiquitin
conjugating enzyme E2T |
| MCM2 | Minichromosome
maintenance complex component 2 |
| MCM4 | Minichromosome
maintenance complex component 4 |
| MCM6 | Minichromosome
maintenance complex component 6 |
| ZWINT | ZW10 interacting
kinetochore protein |
| KIAA0101 | KIAA0101 |
| GINS2 | GINS complex
subunit 2 (Psf2 homolog) |
UBE2T is upregulated in gallbladder
cancer tissues
Functional enrichment analysis indicated that UBE2T
is involved in several biological processes and pathways that are
closely associated with gallbladder cancer, such as the TGF-β
signaling pathway (35).
Subsequently, UBE2T was selected for further analysis in the
present study, and UBE2T expression in gallbladder cancer tissues
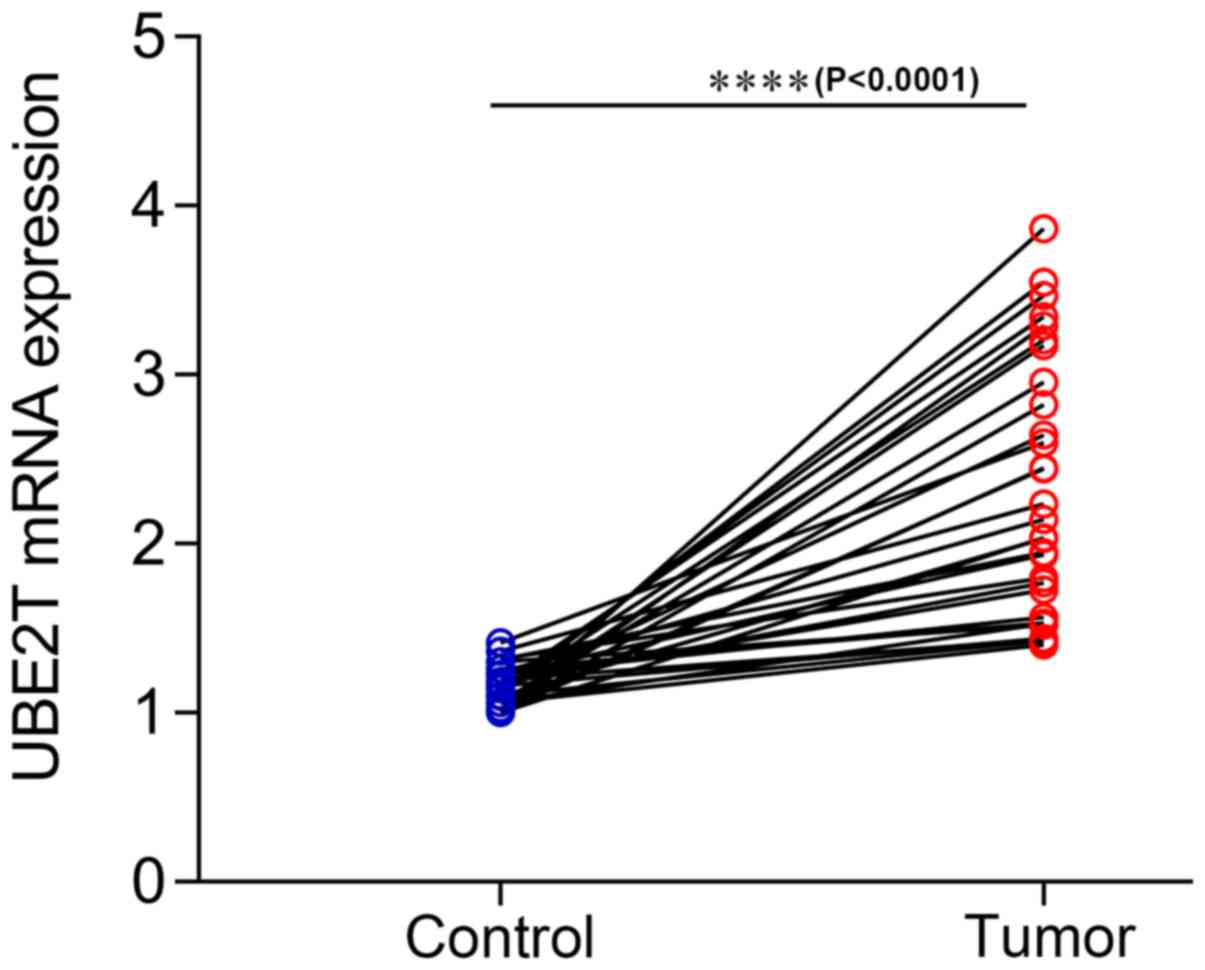
was assessed. The results from RT-qPCR on 30 paired gallbladder
cancer tissues and corresponding adjacent noncancerous tissues
demonstrated that UBE2T was upregulated in gallbladder cancer
tissues compared with adjacent noncancerous tissues (P<0.0001;
Fig. 5). Furthermore, the results
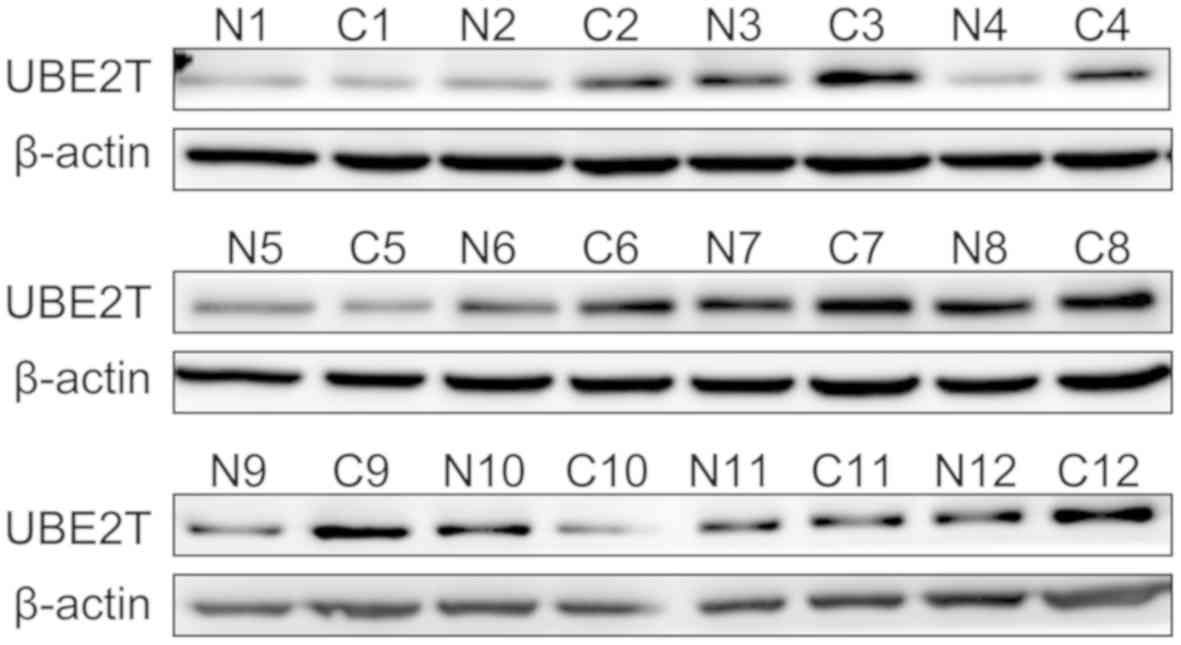
from western blotting demonstrated that UBE2T was highly expressed
in 12 paired gallbladder cancer tissues compared with corresponding
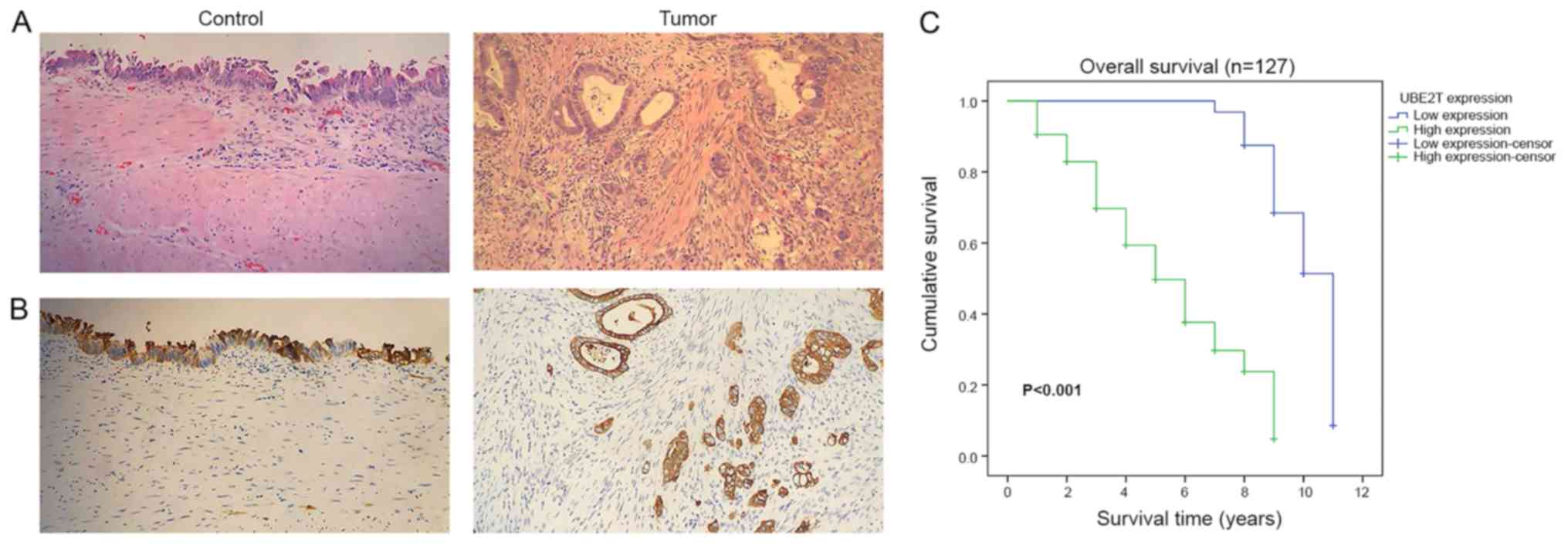
adjacent noncancerous tissues at the protein level (Fig. 6). The results from IHC indicated that
UBE2T was mostly expressed in the cytoplasm (Fig. 7A and B). As presented in Table IV, among the 127 gallbladder cancer
cases, UBE2T was downregulated in 32 cases (25.2%) and upregulated
in 95 cases (74.8%). Taken together, these results demonstrated
that UBE2T expression was upregulated in gallbladder cancer.
 | Table IV.Association between UBE2T expression
and the clinicopathological characteristics of patients with
gallbladder cancer (n=127). |
Table IV.
Association between UBE2T expression
and the clinicopathological characteristics of patients with
gallbladder cancer (n=127).
|
|
| UBE2T
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases, n (%) | Low (n=32) | High (n=95) | P-value |
|---|
| Age, years |
|
<50 | 4 (3.1) | 0 | 4 | 0.238 |
|
≥50 | 123 (96.9) | 32 | 91 |
|
| Sex |
|
Male | 40 (31.5) | 9 | 31 | 0.635 |
|
Female | 87 (68.5) | 23 | 64 |
|
| Clinical stage |
| I | 2 (1.6) | 2 | 0 |
<0.001b |
| II | 87 (68.5) | 30 | 57 |
|
|
III | 23 (18.1) | 0 | 23 |
|
| V | 15 (11.8) | 0 | 15 |
|
| T
classification |
| T1 | 2 (1.6) | 2 | 0 |
<0.001b |
| T2 | 88 (69.3) | 30 | 58 |
|
| T3 | 32 (25.2) | 0 | 32 |
|
| T4 | 5 (3.9) | 0 | 5 |
|
| N
classification |
| N0 | 91 (71.7) | 32 | 59 |
<0.001b |
| N1 | 28 (22.0) | 0 | 28 |
|
| N2 | 8 (6.3) | 0 | 8 |
|
| M
classification |
| M0 | 113 (89.0) | 32 | 81 | 0.021a |
| M1 | 14 (11.0) | 0 | 14 |
|
| Histologic
grade |
| I | 24 (18.9) | 9 | 15 | 0.3 |
| II | 60 (47.2) | 13 | 47 |
|
|
III | 43 (33.9) | 10 | 33 |
|
High UBE2T expression is associated
with poor prognosis of patients with gallbladder cancer
The results from Kaplan-Meier survival curves
demonstrated that the overall survival time of patients with high
UBE2T expression was significantly shorter than those with low
UBE2T expression (P<0.001; Fig.
7C). The results indicated that upregulated UBE2T may be
associated with poor prognosis of patients with gallbladder
cancer.
UBE2T may serve as an independent risk
factor of gallbladder cancer
The association between UBE2T expression and the
clinicopathological characteristics of patients with gallbladder
cancer was assessed (Table IV). The
results demonstrated that upregulated UBE2T was significantly
associated with clinical stage (P<0.001), T classification
(P<0.001), N classification (P<0.001) and M classification
(P=0.021). However, no significant association was observed between
UBE2T expression and age (P=0.238), sex (P=0.635) and histologic
grade (P=0.3). These results were consistent with Spearman's
correlation analysis (Table V).
Furthermore, whether UBE2T expression may be considered a a risk
factor for gallbladder cancer was determined via univariate and
multivariate Cox regression analyses. The results demonstrated that
high UBE2T expression was significantly associated with an
increased risk of gallbladder cancer (P<0.001; hazard ratio,
6.453; 95% confidence interval, 2.985–13.952; Table VI). Taken together, these results
suggested that UBE2T expression may be considered as an independent
risk factor for patients with gallbladder cancer.
 | Table V.Spearman's correlation between UBE2T
expression and the clinicopathological characteristics of patients
with gallbladder cancer (n=127). |
Table V.
Spearman's correlation between UBE2T
expression and the clinicopathological characteristics of patients
with gallbladder cancer (n=127).
|
| UBE2T
expression |
|---|
|
|
|
|---|
| Characteristic | Spearman's
correlation | P-value |
|---|
| Age | −0.105 | 0.242 |
| Sex | −0.042 | 0.638 |
| Clinical stage | 0.402 |
<0.001b |
| T
classification | 0.398 |
<0.001b |
| N
classification | 0.362 |
<0.001b |
| M
classification | 0.204 | 0.021a |
| Grade | 0.09 | 0.316 |
 | Table VI.Univariate and multivariate analyses
of prognostic parameters in patients with gallbladder cancer. |
Table VI.
Univariate and multivariate analyses
of prognostic parameters in patients with gallbladder cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Clinical stage |
<0.001a | 3.784 | 2.508–5.711 | 0.162 | 3.958 | 0.576–27.176 |
| T
classification |
<0.001a | 3.454 | 2.261–5.278 | 0.397 | 0.495 | 0.097–2.522 |
| N
classification |
<0.001a | 4.266 | 2.735–6.654 | 0.208 | 1.917 | 0.696–5.279 |
| M
classification |
<0.001a | 5.663 | 2.322–13.811 | 0.342 | 0.368 | 0.047–2.892 |
| UBE2T
expression |
<0.001a | 7.831 | 3.724–16.466 |
<0.001a | 6.453 | 2.985–13.952 |
Discussion
Late diagnosis and poor prognosis are the main
issues in the effective treatment of gallbladder cancer (36). Surgical resection remains the
principle curable treatment option (37). However, >1/3 patients experience
locoregional and distant recurrence following gallbladder cancer
resection (5). It is therefore
crucial to determine potential diagnostic or prognostic
biomarkers.
Microarray technology is commonly used to assess
gene expression changes in gallbladder cancer, which was proven
useful in identifying novel biomarkers (38). In the present study, 177 genes were
differentially expressed in gallbladder wall tissues and tumor
tissues of patients with gallbladder cancer. To the best of our
knowledge, not much is known about DEGs in gallbladder cancer
walls. Unlike other studies, the present study used microarray
analysis to identify DEGs for gallbladder cancer and gallbladder
adenoma, whereas a precancerous lesion of gallbladder cancer was
used as the control (35,39–41).
To the best of our knowledge, there is currently no
research on the PPIs of gallbladder cancer. Subsequently, a PPI
network of 177 DEGs was constructed in the present study by using
the STRING database. A total of 10 genes with degree ≥10 were
selected as the hub genes. The results from KEGG analysis
demonstrated that the 10 hub genes were enriched in the following
pathways: ‘TGF-β signaling pathway’, ‘signaling pathways regulating
pluripotency of stem cells’ and ‘complement and coagulation
cascades’. These pathways have been associated with the development
and progression of gallbladder cancer (42–44). For
example, the crosstalk between the TGF-β signaling pathway and
lncRNAs plays a key role in cancer. It has been reported that
several members of the TGF-β signaling pathway are targeted by
certain lncRNAs (such as HOXD-AS1 and UCA1), and that the
production of numerous lncRNAs is induced by TGF-β treatment in
different types of cancer, such as gastric cancer (45). Furthermore, focal adhesion (focal
adhesion proteins such as, vinculin, talin, zyxin, FAK, and
paxillin) activation mediates cell migration and metastasis in
different types of cancer, including gallbladder cancer (46). In the present study, hub genes were
significantly enriched in stem cell-related pathways. As a critical
characteristic of cancer stem cells, pluripotency contributes to
self-renewal and chemoresistance (47). Therefore, these 10 hub genes may be
involved in the development of gallbladder cancer.
The present study demonstrated that UBE2T was
upregulated in gallbladder tumor and walls of patients with
gallbladder cancer. The results from RT-qPCR and western blotting
confirmed that UBE2T was upregulated in 30 pairs of gallbladder
cancer tissues compared with adjacent normal tissues. Furthermore,
results from IHC demonstrated that UBE2T was mainly expressed in
the cytoplasm of gallbladder cancer cells.
Previous studies demonstrated that UBE2T serves a
key role in protein ubiquitination, which is an essential
post-translational modification that regulates several biological
processes, including inflammation, immune response, cell
differentiation and cell proliferation (48–51). The
drug vulnerability of cancer cells is dependent on protein
participation in ubiquitination and degradation, which allowed the
development of therapeutic agents based on druggable genomic
modifications (52,53). Therefore, UBE2T may be considered as
a potential drug target for patients with gallbladder cancer.
Numerous studies demonstrated that UBE2T is overexpressed in
various types of cancer, including multiple myeloma (20), hepatocellular carcinoma (54), bladder cancer (24) and osteosarcoma (25), suggesting that it may be an
attractive drug target. The results from the present study
indicated that UBE2T may be considered as a prognostic factor for
patients with gallbladder cancer. Furthermore, the Kaplan-Meier
survival curves analysis demonstrated that high UBE2T expression
was associated with worse prognosis compared with low UBE2T
expression group. T stage independently affects prognosis of
gallbladder cancer (55,56). The present study demonstrated that
UBE2T overexpression was associated with certain
clinicopathological characteristics of patients with gallbladder
cancer, including clinical stage, T classification, N
classification and M classification. In addition, results from
univariate and multivariate Cox regression analyses indicated that
high UBE2T expression may be considered as an independent risk
factor for patients with gallbladder cancer. Taken together, the
findings from the present study suggested that upregulated UBE2T
expression may have a prognostic value for patients with
gallbladder cancer.
In summary, the present study confirmed that UBE2T
expression was upregulated in gallbladder tumor tissues and
gallbladder walls of patients with gallbladder cancer compared with
patients with gallbladder adenoma. Furthermore, high UBE2T
expression was demonstrated to be associated with certain
clinicopathological characteristics of patients with gallbladder
cancer, including the AJCC TNM classification and clinical stage.
Furthermore, high UBE2T expression was associated with poor patient
prognosis. Univariate and multivariate Cox regression analyses
indicated that UBE2T may serve as an independent prognostic
biomarker for patients with gallbladder cancer. The present study
is not without limitations. First, the patient population was
heterogeneous. Thus, UBE2T should be validated in a larger cohort
of patents with gallbladder cancer. Secondly, the present study
only assessed the expression of UBE2T in gallbladder cancer
tissues, thus lacking functional in vivo and in vitro
studies on UBE2T. Prospective studies will therefore aim to further
investigate the underlying mechanism of UBE2T in gallbladder
cancer.
Acknowledgements
Not applicable.
Funding
This work was funded by Natural Science Foundation
of Liaoning Province (2020-BS-283).
Availability of data and materials
The datasets analyzed during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
CLG conceived and designed the present study. XZ, TL
performed most of the experiments, analyzed the data and drafted
the initial manuscript. XN, LJC acquired the data and helped draft
the initial manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of China Medical
University and written informed consent was provided by all
patients prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PPI
|
protein-protein interaction
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomics
|
|
GO
|
Gene Oncology
|
|
FC
|
fold-change
|
|
STRING
|
Search Tool for Retrieval of
Interacting Genes
|
|
MCODE
|
Molecular Complex Detection
|
|
BiNGO
|
Biological Network Gene Ontology
tool
|
|
UBE2T
|
ubiquitin conjugating enzyme E2T
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ethun CG, Postlewait LM, Le N, Pawlik TM,
Buettner S, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, et
al: Association of optimal time interval to Re-resection for
incidental gallbladder cancer with overall survival: A
multi-institution analysis from the US extrahepatic biliary
malignancy consortium. JAMA Surg. 152:143–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopez-Aguiar AG, Ethun CG, McInnis MR,
Pawlik TM, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC,
Krasnick BA, et al: Association of perioperative transfusion with
survival and recurrence after resection of gallbladder cancer: A
10-institution study from the US extrahepatic biliary malignancy
consortium. J Surg Oncol. 117:1638–1647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Margonis GA, Gani F, Buettner S, Amini N,
Sasaki K, Andreatos N, Ethun CG, Poultsides G, Tran T, Idrees K, et
al: Rates and patterns of recurrence after curative intent
resection for gallbladder cancer: A multi-institution analysis from
the US extra-hepatic biliary malignancy consortium. HPB (Oxford).
18:872–878. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
7
|
Baiu I and Visser B: Gallbladder Cancer.
JAMA. 320:12942018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barreto SG, Dutt A and Chaudhary A: A
genetic model for gallbladder carcinogenesis and its dissemination.
Ann Oncol. 25:1086–1097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valle JW, Lamarca A, Goyal L, Barriuso J
and Zhu AX: New horizons for precision medicine in biliary tract
cancers. Cancer Discov. 7:943–962. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Renfro LA, An MW and Mandrekar SJ:
Precision oncology: A new era of cancer clinical trials. Cancer
Lett. 387:121–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J and Liu H, Shen X, Wang Y, Zhang D,
Shen S, Suo T, Pan H, Ming Y, Ding K and Liu H: Long non-coding RNA
expression profiles in gallbladder carcinoma identified using
microarray analysis. Oncol Lett. 13:3508–3516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sicklick JK, Fanta PT, Shimabukuro K and
Kurzrock R: Genomics of gallbladder cancer: The case for
biomarker-driven clinical trial design. Cancer Metastasis Rev.
35:263–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma MZ, Zhang Y, Weng MZ, Wang SH, Hu Y,
Hou ZY, Qin YY, Gong W, Zhang YJ, Kong X, et al: Long Noncoding RNA
GCASPC, a Target of miR-17-3p, negatively regulates pyruvate
carboxylase-dependent cell proliferation in gallbladder cancer.
Cancer Res. 76:5361–5371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He Y, Xue C, Yu Y, Chen J, Chen X, Ren F,
Ren Z, Cui G and Sun R: CD44 is overexpressed and correlated with
tumor progression in gallbladder cancer. Cancer Manag Res.
10:3857–3865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Xu M, Cai Z, Yuan W, Cui W and Li
MD: Identification of LIFR, PIK3R1, and MMP12 as
novel prognostic signatures in gallbladder cancer using
network-based module analysis. Front Oncol. 9:3252019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Machida YJ, Machida Y, Chen Y, Gurtan AM,
Kupfer GM, D'Andrea AD and Dutta A: UBE2T is the E2 in the Fanconi
anemia pathway and undergoes negative autoregulation. Mol Cell.
23:589–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramaekers CH, van den Beucken T, Meng A,
Kassam S, Thoms J, Bristow RG and Wouters BG: Hypoxia disrupts the
Fanconi anemia pathway and sensitizes cells to chemotherapy through
regulation of UBE2T. Radiother Oncol. 101:190–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Zhang Y, Yang Z, Liu X, Yang P,
Wang J, Hu K, He X, Zhang X and Jing H: High expression of UBE2T
predicts poor prognosis and survival in multiple myeloma. Cancer
Gene Ther. 26:347–355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mamrak NE, Shimamura A and Howlett NG:
Recent discoveries in the molecular pathogenesis of the inherited
bone marrow failure syndrome Fanconi anemia. Blood Rev. 31:93–99.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perez-Peña J, Corrales-Sánchez V, Amir E,
Pandiella A and Ocana A: Ubiquitin-conjugating enzyme E2T (UBE2T)
and denticleless protein homolog (DTL) are linked to poor outcome
in breast and lung cancers. Sci Rep. 7:175302017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu LP, Yang M, Peng QZ, Li MY, Zhang YS,
Guo YH, Chen Y and Bao SY: UBE2T promotes hepatocellular carcinoma
cell growth via ubiquitination of p53. Biochem Biophys Res Commun.
493:20–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong YQ, Peng D, Ning XH, Yang XY, Li XS,
Zhou LQ and Guo YL: UBE2T silencing suppresses proliferation and
induces cell cycle arrest and apoptosis in bladder cancer cells.
Oncol Lett. 12:4485–4492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Leng H, Chen H, Wang L, Jiang N,
Huo X and Yu B: Knockdown of UBE2T inhibits osteosarcoma cell
proliferation, migration, and invasion by suppressing the PI3K/Akt
signaling pathway. Oncol Res. 24:361–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zahurak M, Parmigiani G, Yu W, Scharpf RB,
Berman D, Schaeffer E, Shabbeer S and Cope L: Pre-processing
Agilent microarray data. BMC Bioinformatics. 8:1422007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41((Database issue)): D808–D815. 2013.PubMed/NCBI
|
|
28
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bandettini WP, Kellman P, Mancini C,
Booker OJ, Vasu S, Leung SW, Wilson JR, Shanbhag SM, Chen MY and
Arai AE: MultiContrast Delayed Enhancement (MCODE) improves
detection of subendocardial myocardial infarction by late
gadolinium enhancement cardiovascular magnetic resonance: A
clinical validation study. J Cardiovasc Magn Reson. 14:832012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maere S, Heymans K and Kuiper M: BiNGO: A
Cytoscape plugin to assess overrepresentation of gene ontology
categories in biological networks. Bioinformatics. 21:3448–3449.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID gene functional classification tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miller RG Jr: What price Kaplan-Meier?
Biometrics. 39:1077–1081. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiu Y, Luo X, Kan T, Zhang Y, Yu W, Wei Y,
Shen N, Yi B and Jiang X: TGF-β upregulates miR-182 expression to
promote gallbladder cancer metastasis by targeting CADM1. Mol
Biosyst. 10:679–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharma A, Sharma KL, Gupta A, Yadav A and
Kumar A: Gallbladder cancer epidemiology, pathogenesis and
molecular genetics: Recent update. World J Gastroenterol.
23:3978–3998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ethun CG, Postlewait LM, Le N, Pawlik TM,
Buettner S, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, et
al: A novel pathology-based preoperative risk score to predict
locoregional residual and distant disease and survival for
incidental gallbladder cancer: A 10-institution study from the U.S.
Extrahepatic biliary malignancy consortium. Ann Surg Oncol.
24:1343–1350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen H, He M, Lin R, Zhan M, Xu S, Huang
X, Xu C, Chen W, Yao Y, Mohan M and Wang J: PLEK2 promotes
gallbladder cancer invasion and metastasis through EGFR/CCL2
pathway. J Exp Clin Cancer Res. 38:2472019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qin Y, Zhou Y, Ge A, Chang L, Shi H, Fu Y
and Luo Q: Overexpression of SNORA21 suppresses tumorgenesis of
gallbladder cancer in vitro and in vivo. Biomed Pharmacother.
118:1092662019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang L, Gao Q, Wu X, Feng F and Xu K: Long
noncoding RNA HEGBC promotes tumorigenesis and metastasis of
gallbladder cancer via forming a positive feedback loop with
IL-11/STAT3 signaling pathway. J Exp Clin Cancer Res. 37:1862018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Niu J, Li Z and Li F: Overexpressed
microRNA-136 works as a cancer suppressor in gallbladder cancer
through suppression of JNK signaling pathway via inhibition of
MAP2K4. Am J Physiol Gastrointest Liver Physiol. 317:G670–G681.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Manohar R, Li Y, Fohrer H, Guzik L, Stolz
DB, Chandran UR, LaFramboise WA and Lagasse E: Identification of a
candidate stem cell in human gallbladder. Stem Cell Res.
14:258–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kong L, Wu Q, Zhao L, Ye J, Li N and Yang
H: Identification of messenger and long noncoding RNAs associated
with gallbladder cancer via gene expression profile analysis. J
Cell Biochem. 120:19377–19387. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang J, Shao N, Ding X, Tan B, Song Q,
Wang N, Jia Y, Ling H and Cheng Y: Crosstalk between transforming
growth factor-β signaling pathway and long non-coding RNAs in
cancer. Cancer Lett. 370:296–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Banning A, Babuke T, Kurrle N, Meister M,
Ruonala MO and Tikkanen R: Flotillins Regulate Focal Adhesions by
Interacting with α-Actinin and by Influencing the Activation of
Focal Adhesion Kinase. Cells. 7:282018. View Article : Google Scholar
|
|
47
|
Sharif T, Martell E, Dai C, Kennedy BE,
Murphy P, Clements DR, Kim Y, Lee PW and Gujar SA: Autophagic
homeostasis is required for the pluripotency of cancer stem cells.
Autophagy. 13:264–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang L, Guo W, Zhang S and Wang G:
Ubiquitination-proteasome system: A new player in the pathogenesis
of psoriasis and clinical implications. J Dermatol Sci. 89:219–225.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qiu J, Sheedlo MJ, Yu K, Tan Y, Nakayasu
ES, Das C, Liu X and Luo Z-Q: Ubiquitination independent of E1 and
E2 enzymes by bacterial effectors. Nature. 533:120–124. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kao SH, Wu HT and Wu KJ: Ubiquitination by
HUWE1 in tumorigenesis and beyond. J Biomed Sci. 25:672018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Alpi AF, Chaugule V and Walden H:
Mechanism and disease association of E2-conjugating enzymes:
Lessons from UBE2T and UBE2L3. Biochem J. 473:3401–3419. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pagliarini R, Shao W and Sellers WR:
Oncogene addiction: Pathways of therapeutic response, resistance,
and road maps toward a cure. EMBO Rep. 16:280–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Garraway LA, Verweij J and Ballman KV:
Precision oncology: An overview. J Clin Oncol. 31:1803–1805. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tao Y, Li R, Shen C, Li J, Zhang Q, Ma Z,
Wang F and Wang Z: SENP1 is a crucial promotor for hepatocellular
carcinoma through deSUMOylation of UBE2T. Aging (Albany NY).
12:1563–1576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aloia TA, Jarufe N, Javle M, Maithel SK,
Roa JC, Adsay V, Coimbra FJ and Jarnagin WR: Gallbladder cancer:
Expert consensus statement. HPB (Oxford). 17:681–690. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Goetze TO and Paolucci V: Adequate extent
in radical re-resection of incidental gallbladder carcinoma:
Analysis of the German Registry. Surg Endosc. 24:2156–2164. 2010.
View Article : Google Scholar : PubMed/NCBI
|