Introduction
Non-Hodgkin's lymphoma (NHL) is a group of malignant
lymphohematopoietic tumors mainly occurring in lymphatic organs,
including lymph nodes, spleen and thymus. In Asia, NHL frequently
displays high grade and aggressive subtypes, which indicates a
relatively poor prognosis (1).
Currently, histopathology is the most important basis for the
diagnosis of NHL (2,3); however, clinicians are usually required
to formulate therapeutic strategies before pathological results are
available. Therefore, it is necessary to identify patients with
poor prognosis and provide them with more comprehensive therapies.
Certain blood biochemical indices, including serum inflammatory
cytokines, tumor burden indicators, and proportion of absolute
lymphocyte to absolute monocyte (4),
are of interest to clinicians. However, some clinicians still
prefer radiological or pathological examinations since they reflect
a more intuitive progress of the disease (5). Whether these non-serological
examinations are superior to blood biochemical indices is
controversial (6,7). Therefore, it is necessary to perform
meticulous comparisons of these indicators.
As the most precise radiographic method to evaluate
tumor metabolic activity, 18F-fludeoxyglucoase (FDG)
positron emission tomography/computed tomography (PET/CT) is widely
applied in tumor staging, grading and therapeutic assessment. The
maximal standardized uptake value (SUVmax) is a descriptive
parameter to indicate the extent of 18F-FDG uptake in
normal and tumor tissue, and usually positively correlated with
tumor malignancy (8–10). The predictive value of SUVmax in
solid tumor has been demonstrated in several studies (11–13),
whereas it remains elusive whether SUVmax is instructional in
hematologic malignancies. Therefore, further studies are required
to determine the role of SUVmax in hematologic malignancies.
Ki67, also known as antigen Ki67 or MKi67, is
associated with cellular proliferation and is only expressed in
proliferating cells. The ratio of Ki67 (Ki67%) in tumor cells prior
to and after chemotherapy is of significance in medication
guidance, particularly for the selection of cell-cycle-specific
chemotherapeutic drugs (14). At
present, no definitive conclusion has been made as to whether Ki67%
has sufficient accuracy in predicting the prognosis of patients
with NHL (15,16). Therefore, further studies are
required.
The present study provided an elaborate assessment
of the relationship among serological factors, SUVmax and Ki67%, as
well as their predictive value regarding the long-term prognosis of
patients with NHL.
Materials and methods
Patients and outcomes
Cases of newly-diagnosed NHL at Shanghai Tongji
Hospital (Shanghai, China) between July 2015 and March 2019 were
collected and evaluated for age, sex, pathological diagnosis,
staging, grading, invasiveness and post-chemotherapy effectiveness.
These patients also underwent necessary serological, radiological
and pathological examinations. The inclusion criteria were defined
as follows: i) The pathological diagnosis was clear and
uncontroversial; ii) the patient had received regular chemotherapy
and followed up for at least 2 months and iii) at least one
radiological or pathological examination was performed. Some
patients lack staging or grading information during
hospitalization, but with complete serological, radiological and
pathological data. For those patients, the data were also
considered analyzable. Following removal of information-deficient
individuals, 120 patients with valid clinical data were included in
the present study. Diagnosis of these patients was performed
according to the World Health Organization classification for
tumors of hematopoietic and lymphoid tissue (17). The International Prognostic Index
(IPI) was calculated before treatment to estimate the prognosis of
patients with NHL (18). The
patients were staged according to Ann Arbor Staging Classification
(19). The patients received regular
chemotherapy [cyclophosphamide, doxorubicin, vincristine and
prednisone (CHOP); or rituximab-CHOP] and were assessed for
effectiveness after four courses of treatment. The criteria of
efficacy were defined as follows: i) Complete remission (CR), all
clinical and radiological lesions had disappeared, lymph nodes and
lumps shrunk to normal size, or the sum of products of greatest
diameters (SPD) was reduced by >75%, tumor markers and
biochemical indicators were in the normal range and stable for ≥4
weeks; ii) partial remission (PR), SPD is reduced by >50%, no
new lesion appeared and maintained for ≥4 weeks; iii) stable
disease, non-PR and non-progressive disease (PD); and iv) PD, the
size of any abnormal lymph node determined prior to treatment
increased by >50% compared with the previous SPD minimum, or a
new lesion appeared during or after treatment. Survival data were
also collected in order to determine prognosis-associated factors.
Patients were followed up every 3 weeks during chemotherapy and
every 3 months after discharge. The last follow-up was in September
2019. Overall survival (OS) time was defined as the interval from
trial admission to death of any cause, and progression-free
survival (PFS) time was defined as the interval from trial
admission to disease progression or death from NHL.
Serological examinations
Serological factors included inflammatory cytokines
and tumor burden indicators. The inflammatory cytokines were
C-reactive protein (CRP), tumor necrosis factor α (TNF-α),
interleukin-6 (IL-6), interleukin-2 receptor (IL-2R) and
interleukin-8 (IL-8), whereas the tumor burden indicators were
lactate dehydrogenase (LDH), ferritin and blood β2-microglobulin
(β2-mg). TNF-α, IL-6, IL-2R and IL-8 were detected using
chemiluminescence with Siemens interleukin test kits (IMMULITE and
IMMULITE 1000 TNF-α/IL-6/IL-2R/IL-8; cat. no. LKNF1, LK6P1, LKIP1
and LK8P, respectively.) provided by Siemens AG. Fasting venous
blood was obtained from patients on the first day of
hospitalization and automatically tested with an Immulete 1000
Analyzer (Siemens AG). β2-mg and ferritin were detected using a
radioimmunoassay. CRP was detected using immunoturbidimetry and LDH
was measured using a creatine kinase assay. The normal ranges for
each indicator were: CRP, <10 mg/l; TNF-α, <8.1 µg/l; IL-6,
<5.9 ng/l; IL-2R, 223–710 U/ml; IL-8, <62 ng/l; LDH, 120–250
U/l; ferritin, 30–400 µg/l (male), 13–150 µg/l (female); β2-mg,
0.7–1.8 mg/l.
Radiological examination
All patients signed informed consent forms before
PET/CT examination. The patients were required to fast for ≥6 h
prior to intravenous infusion of 18F-FDG. Fasting
glucose was measured and a dose of 18F-FDG ranging
between 4.1 and 10.7 mCi was injected into patients depending on
their weight and glucose level. After injection, patients rested
for ≥40 min in a comfortable position and PET/CT scanning was
performed from the skull to the proximal femur. The images were
generated through Siemens Biograph Turepoint 40 PET/CT Imager and
reconstructed after attenuation correction. The images were merged
through Xeleris software. Subsequently, the reconstructed images
were assessed by two experienced nuclear medicine physicians and
SUVmax was calculated after the range of interest was drawn.
Lesions were diagnosed according to the criteria recommended by the
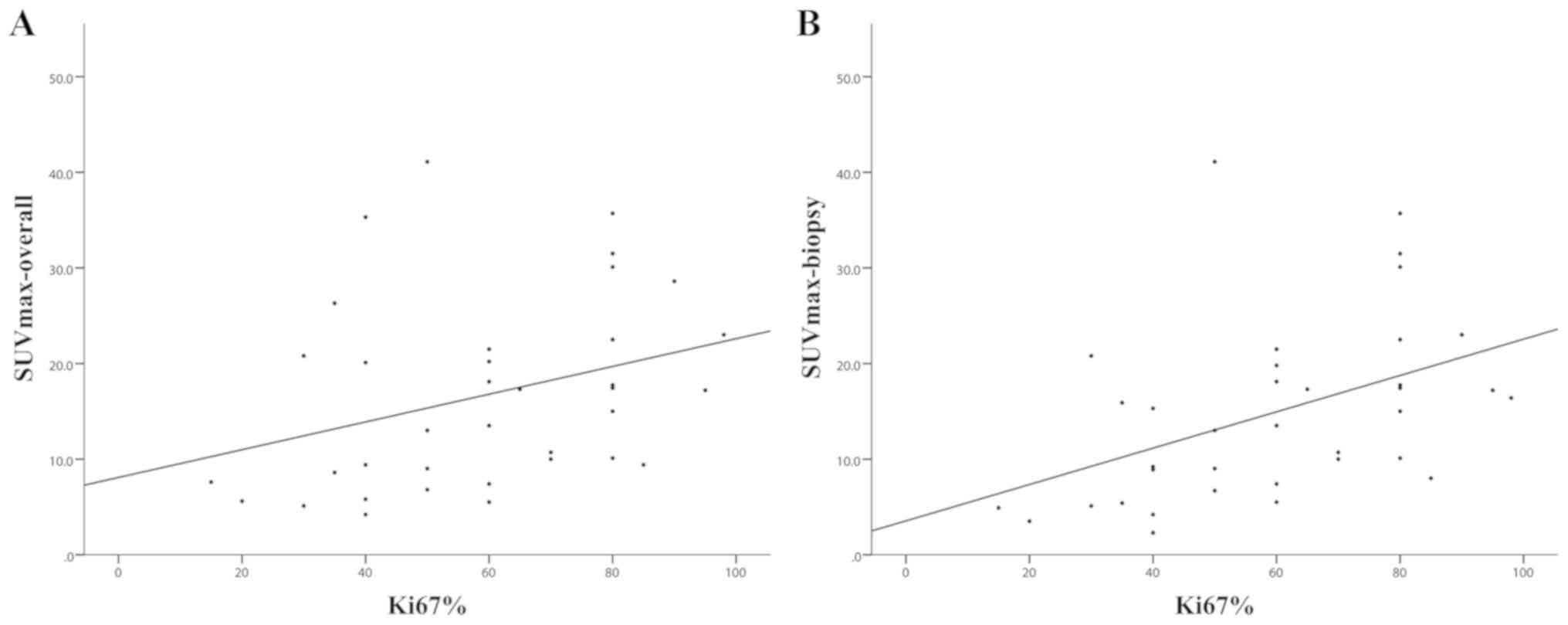
International Group for Imaging Diagnosis of Lymphoma (20). In order to describe the association
between SUVmax and Ki67%, two different types of SUVmax were
calculated, including SUVmax of the whole body (SUVmax-overall) and
SUVmax of the biopsy site (SUVmax-biopsy). Only incipient PET/CT
data were collected, and data after chemotherapy or surgery were
automatically excluded.
Pathological examination
Immunohistochemical staining was performed in order
to determine Ki67%. For pretreatment steps, the specimens were
fixed with 10% neutral formaldehyde at room temperature for 24–48
h, embedded in paraffin wax, cut into 3-µm thick sections and
stained with hematoxylin at room temperature for 5 min and eosin at
room temperature for 1 min. Hematoxylin and eosin were provided by
Solarbio Science & Technology (Beijing) Co., Ltd. For Ki67
immunohistochemical staining, the paraffin sections were stained
using Ki67 antibody (cat. no. GT2094; 1:100; Gene Tech
Biotechnology Co., Ltd.) on an automated immunostainer (cat. no.
GAS95; Gene Tech Biotechnology Co., Ltd.). The antigen was
retrieved by heating with EDTA (pH 9.0) at 100°C for 20 min.
Blocking was performed using 3% H2O2 at 37°C
for 10 min. The primary antigen was incubated at 37°C for 30 min.
EnVision reagent (HRP anti-Rabbit/Mouse, cat. no. GK801030, Gene
Tech Biotechnology Co., Ltd.) was incubated at 37°C for 20 min. The
sections were stained by diaminobenzidine (DAB) at room temperature
for 8 min, and then stained with hematoxylin at room temperature
for 5 min. The nuclei stained with antibody were considered
positive, and the number of Ki67-positive tumor cells in 1,000
tumor cells was calculated by 2 experienced pathologists under a
light microscope at the 200× magnification. Examples of stained
Ki67 sections were shown in Fig.
S7. PBS was used as a negative control and Ki67-positive
sections were regarded as the positive control.
Statistical analysis
All clinical data were analyzed using SPSS v20.0
software (IBM Corp.). Continuous variables are presented as mean ±
SD, categorical variables are presented as n (%). The association
between two different indicators was assessed by Spearman's
non-parametric correlation analysis. For survival analysis,
Kaplan-Meier curves were drawn and the log-rank test was used to
compare the survival between groups. χ2 and P-values
were calculated to show the extent of significance for survival
data. Univariate and multivariate Cox regression analysis was
performed to determine prognosis-associated indicators. The
regression model was obtained with the method of ‘Forward: Wald’
(21). Receiver operating
characteristic (ROC) curves were depicted to identify the optimal
cut-off for indicators, and the area under the curve (AUC) as well
as 95% CI were calculated to determine their predictive value.
Youden index (sensitivity + specificity-1) was calculated to find
optimal cutoff for those indicators. Mann-Whitney test was used to
compare the levels of OS- or PFS-predicting indicators between
patients that did or did not achieve CR. P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
Among 120 patients aged between 15 and 93 years, 77
(64.2%) were male and 43 (35.8%) were female, and their respective
average ages were 59±16 and 62±16 years. The age of all patients,
whether grouped by sex or not, was normally distributed. A total of
86 (71.7%) patients were diagnosed with B-cell NHL (B-NHL) and 34
(28.3%) patients were diagnosed with T-cell NHL (T-NHL). Among
them, 104 (86.7%) were diagnosed with invasive NHL, and 16 (13.3%)
were diagnosed with non-invasive NHL. According to Ann Arbor stage
(n=112), most patients were in stage IV (46; 41.1%), and the
numbers of patients in stage I, II and III were 5 (4.5%), 22
(19.6%) and 39 (34.8%), respectively. A total of 55 (49.1%)
patients had B symptoms (fever, night sweat or weight loss),
whereas the other 57 (50.9%) patients did not exhibit B symptoms
(n=112). A total of 44 (41.5%) patients reached CR after four
regular courses of chemotherapy, whereas 62 (58.5%) patients did
not reach CR (n=106). The median follow-up time for all patients
was 14.5 months (range, 0.6–43.6 months). Details of patient
characteristics are provided in Table
I.
 | Table I.Characteristics of 120 patients with
NHL. |
Table I.
Characteristics of 120 patients with
NHL.
| Characteristic | Value |
|---|
| Median age, years
(range) | 61 (15–93) |
| Sex, n (%) |
|
| Male | 77 (64.2) |
|
Female | 43 (35.8) |
| Type, n (%) |
|
|
B-NHL | 86 (71.7) |
|
DLBCL | 62 (51.7) |
| FL | 11 (9.2) |
| MCL | 6 (5.0) |
| MALT | 5 (4.2) |
|
Burkitt | 2 (1.7) |
|
T-NHL | 34 (28.3) |
| AITL | 12 (10.0) |
| PTCL | 10 (8.3) |
| NK/T | 4 (3.3) |
| TLBL | 4 (3.3) |
| ALCL | 4 (3.3) |
| Invasiveness, n
(%) |
|
|
Invasive | 104 (86.7) |
|
Non-invasive | 16 (13.3) |
| Ann Arbor stage, n
(%)a |
|
| I | 5 (4.5) |
| II | 22 (19.6) |
|
III | 39 (34.8) |
| IV | 46 (41.1) |
| B symptoms, n
(%)a |
|
|
Yes | 55 (49.1) |
| No | 57 (50.9) |
| CR after treatment,
n (%)b |
|
|
Yes | 44 (41.5) |
| No | 62 (58.5) |
Correlation analysis
In order to explore the association among different
categories of indicators, correlation analysis was performed. This
unveiled an association between inflammatory cytokines and tumor
burden indicators. LDH was correlated with CRP, IL-6, IL-2R and
TNF-α (P<0.001). β2-mg and ferritin also exhibited a significant
correlation with these inflammatory cytokines (P<0.05). However,
there was no significant correlation identified between IL-8 and
any of the aforementioned tumor burden indicators (P>0.05).
Additionally, LDH, β2-mg, IL-6 and IL-2R were associated with age
(P<0.05; data not shown). Furthermore, LDH, β2-mg, CRP, IL-6,
IL-2R and TNF-α exhibited a significant correlation with stage
(P<0.05; data not shown). By comparison, there was no
significant correlation identified between SUVmax-overall and any
of the serological factors (P>0.05), the same conclusion for
SUVmax-biopsy. A noticeable correlation was identified between
Ki67% and ferritin (Ρ=0.235; P=0.020). The correlation between
SUVmax and Ki67% was also significant, and it appeared that
SUVmax-biopsy exhibited a stronger association with Ki67% (Ρ=0.529;
P<0.001) than SUVmax-overall (Ρ=0.395; P=0.017; Fig. 1; Table
II).
 | Table II.Correlations among serological
factors, SUVmax and Ki67% in 120 patients with NHL. |
Table II.
Correlations among serological
factors, SUVmax and Ki67% in 120 patients with NHL.
|
| CRP | IL-6 | IL-2R | IL-8 | TNF-α | SUV
max-overall | SUV max-biopsy | Ki67% |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Indicators | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value |
|---|
| LDH | 0.507 |
<0.001c | 0.557 |
<0.001c | 0.608 |
<0.001c | 0.076 | 0.483 | 0.638 |
<0.001c | 0.055 | 0.739 | 0.068 | 0.692 | 0.065 | 0.504 |
| β2-mg | 0.522 |
<0.001c | 0.551 |
<0.001c | 0.669 |
<0.001c | 0.045 | 0.688 | 0.660 |
<0.001c | 0.170 | 0.352 | 0.238 | 0.206 | −0.203 | 0.052 |
| Ferritin | 0.506 |
<0.001c | 0.452 |
<0.001c | 0.338 | 0.002b | 0.011 | 0.918 | 0.260 | 0.016a | 0.262 | 0.141 | 0.319 | 0.080 | 0.235 | 0.020a |
| SUVmax-overall | 0.114 | 0.507 | 0.165 | 0.359 | −0.119 | 0.510 | −0.157 | 0.382 | 0.072 | 0.692 |
|
|
|
| 0.395 | 0.017a |
| SUVmax-biopsy | 0.068 | 0.703 | −0.017 | 0.929 | −0.121 | 0.518 | −0.169 | 0.365 | −0.081 | 0.666 |
|
|
|
| 0.529 |
<0.001c |
| Ki67% | −0.031 | 0.761 | 0.076 | 0.500 | −0.074 | −0.511 | 0.148 | 0.187 | −0.183 | 0.103 | 0.395 | 0.017a | 0.529 |
<0.001c |
|
|
Since different subgroups may exhibit different
levels of serological, radiological or pathological indexes, a
subgroup analysis based on T-/B-NHL or invasive/non-invasive NHL
was performed. Compared with when all patients were included, the
association between LDH and TNF-α in patients with T-NHL became
more significant, but most indicators demonstrated an attenuated
correlation (Table SI). For
patients with B-NHL, the correlations were similar to the results
when all patients were included in the study. Notably,
SUXmax-overall was no longer significantly correlated with Ki67%,
whereas the correlation between SUXmax-biopsy and Ki67% retained
its significance (Table SI). When
only patients with invasive NHL were included in the analysis, most
correlations remained similar (Table
SII). Regarding individuals with non-invasive NHL, the sample
size was too small for correlation analysis. Therefore, this was
not performed.
Survival analysis
To estimate the predictive value of different
indicators, patients were automatically stratified into two groups.
For serological indicators (CRP, TNF-α, IL-6, IL-2R, IL-8, LDH,
ferritin and β2-mg), the patients were divided into groups
according to the normal range of each indicator, with a normal
group and an elevated group; For SUVmax and Ki67%, patients were
divided into groups according to the median value. Based on the
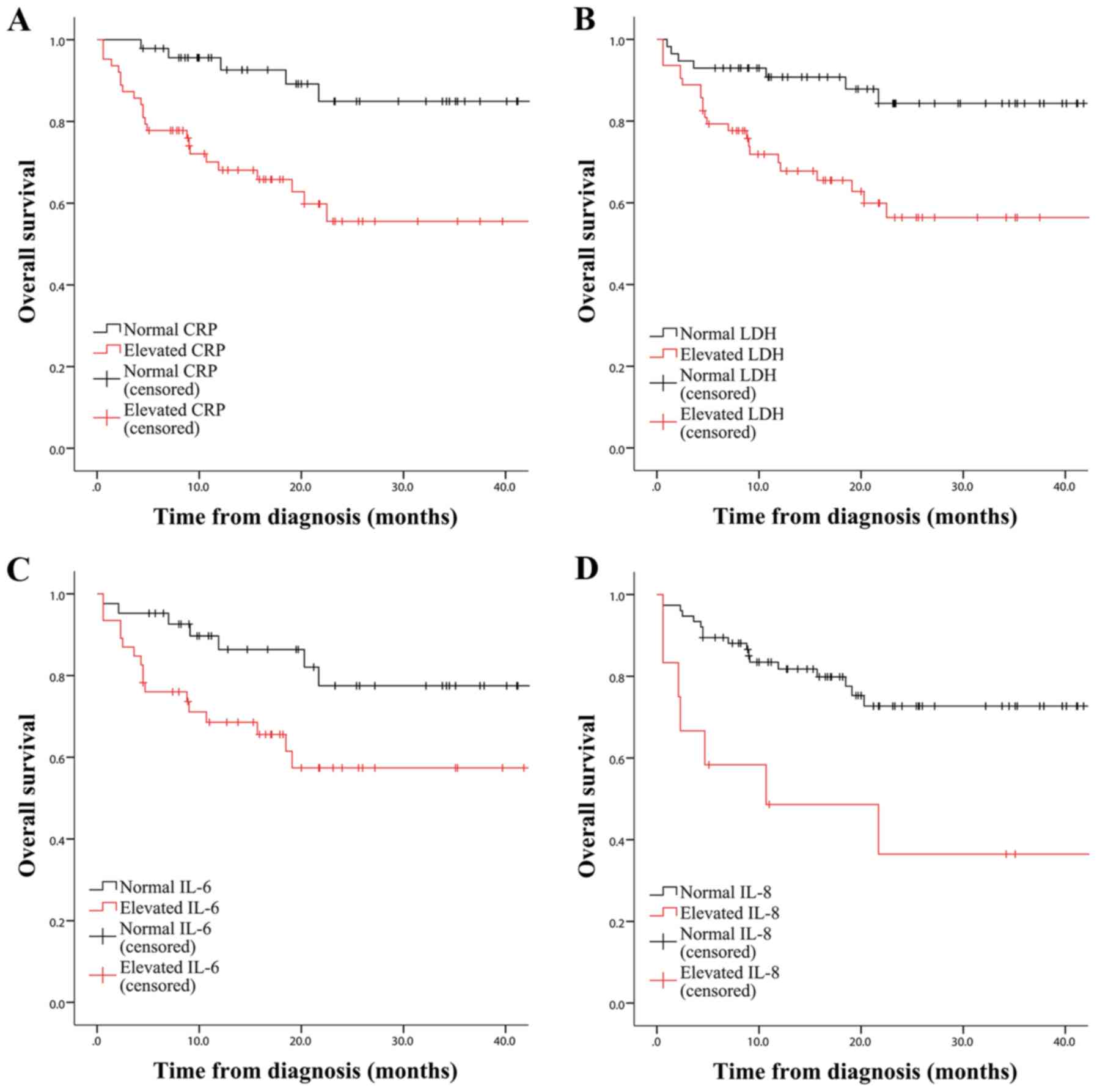
Kaplan-Meier survival curves, it became apparent that CRP had the
greatest capacity to predict OS of patients with NHL
(χ2=10.124, P<0.001). LDH, IL-6 and IL-8 were
also prognostic factors (χ2=9.325, P=0.002;
χ2=4.968, P=0.026; and χ2
=8.507, P=0.004, respectively, Fig.
2), whereas β2-mg, ferritin, IL-2R, TNF-α, SUVmax and Ki67%
were not statistically significant in OS prediction (data not
shown). The subgroup analysis further demonstrated the significance
of IL-8 in OS estimation of patients with T-NHL (P=0.038; Fig. S1). Furthermore, CRP, LDH and IL-6
had the potential to predict OS of patients diagnosed with B-NHL
(P<0.01; Fig. S2). For
aggressive NHL, CRP, LDH and IL-8 were observed to be significant
indicators (P<0.05; Fig. S3),
whereas no analysis was performed for non-aggressive NHL due to
insufficient sample size.
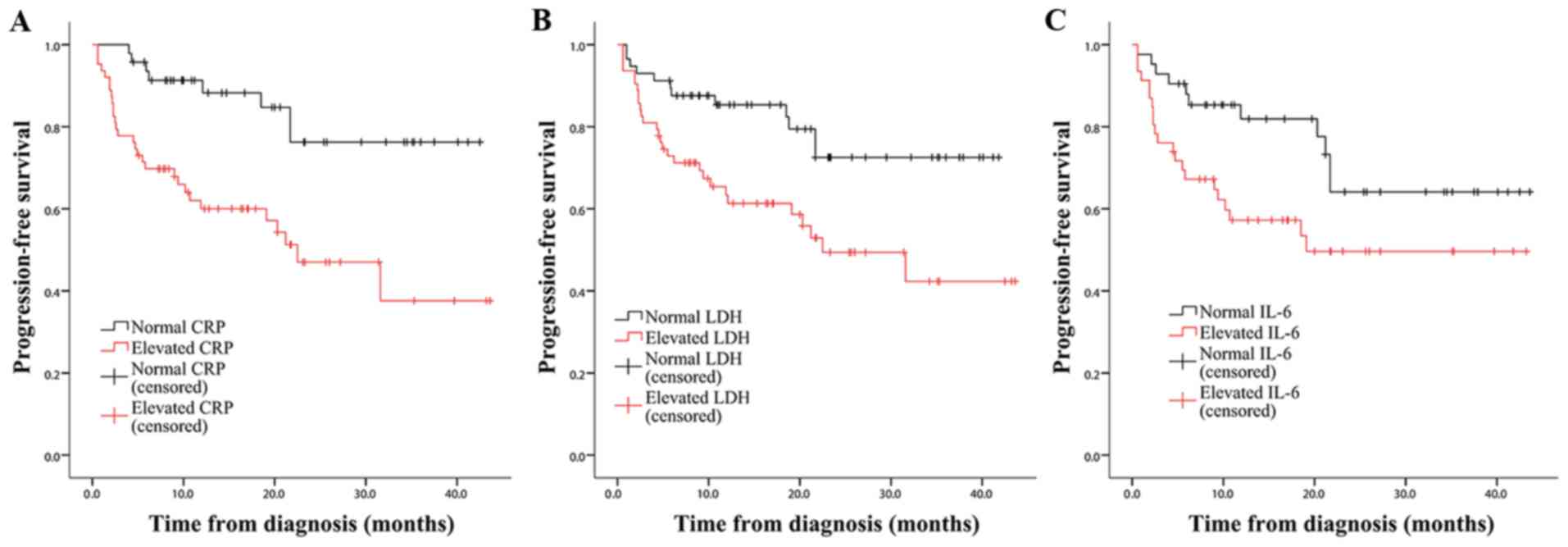
For PFS, a similar result highlighting the
predictive value of CRP (χ2 =11.463; P<0.001),
LDH (χ2=7.689; P=0.006) and IL-6
(χ2=4.366; P=0.037) was observed (Fig. 3). The subgroup analysis revealed that
SUVmax-biopsy was a significant prognostic factor for patients with
T-NHL (χ2=5.266; P=0.022; Fig. S4). However, none of the other
indicators were statistically significant in patients with T-NHL,
probably due to limited sample size and heterogeneity of data. By
contrast, CRP, LDH and IL-6 had the ability to predict PFS of
patients diagnosed with B-NHL, and IL-6 appeared to distinguish PFS
of patients with B-NHL more remarkably compared with when all
patients were included (χ2=7.327; P=0.007;
Fig. S5). For aggressive NHL, only
CRP was useful for PFS prediction (χ2=9.632;
P=0.002; Fig. S6), and patients
with non-aggressive NHL were automatically excluded due to small
sample size.
Univariate and multivariate
analysis
Since multiple variables were included in the
present study, a univariate analysis followed by a multivariate
analysis was performed to determine their predictive value. In Cox
regression analysis, multiple variables, including age, sex,
aggressiveness, type, stage, B symptoms, IPI score, SUVmax, Ki67%
and all serological factors were set as independent variables. To
perform univariate analysis, each indicator was added to the
variable list individually. When entry was set at 0.05 and removal
at 0.10, with the method of ‘Forward: Wald’, significant
indicators, including age (P=0.003), type (P=0.019), stage
(P=0.006), B symptoms (P=0.004), IPI score (P<0.001), LDH
(P=0.001), β2-mg (P<0.001), ferritin (P=0.015), CRP (P<0.001)
and IL-2R (P=0.019), were obtained for OS estimation (data not
shown). Subsequently, multivariate analysis was performed with the
same method and the aforementioned indicators. A regression model
containing LDH (P=0.007), β2-mg (P=0.002), CRP (P=0.012) and IL-8
(P=0.001) was obtained (Table
III), indicating that these indicators were independent
prognostic factors.
 | Table III.Cox regression analysis for overall
survival estimation of 120 patients with non-Hodgkin's
lymphoma. |
Table III.
Cox regression analysis for overall
survival estimation of 120 patients with non-Hodgkin's
lymphoma.
| Variables | Β | SE | Wald | P-value | RR (95% CI) |
|---|
| LDH | 0.001 | 0.000 | 7.169 | 0.007 | 1.001
(1.000–1.002) |
| β2-mg | 0.403 | 0.131 | 9.421 | 0.002 | 1.496
(1.157–1.934) |
| CRP | 0.024 | 0.010 | 6.358 | 0.012 | 1.024
(1.005–1.044) |
| IL-8 | 0.037 | 0.011 | 11.025 | 0.001 | 1.037
(1.015–1.060) |
For PFS estimation, univariate analysis revealed age
(P=0.011), type (P=0.004), stage (P=0.004), B symptoms (P=0.001),
IPI score (P<0.001), LDH (P=0.001), β2-mg (P<0.001), ferritin
(P=0.015) and CRP (P<0.001) as significant indicators (data not
shown), and the multivariate analysis demonstrated that LDH
(P=0.001), β2-mg (P=0.003), CRP (P=0.003) and IL-8 (P=0.042) were
significant prognostic indicators (Table IV).
 | Table IV.Cox regression analysis for
progression-free survival estimation of 120 patients with
non-Hodgkin's lymphoma. |
Table IV.
Cox regression analysis for
progression-free survival estimation of 120 patients with
non-Hodgkin's lymphoma.
| Variables | Β | SE | Wald | P-value | RR (95% CI) |
|---|
| LDH | 0.001 | 0.000 | 11.683 | 0.001 | 1.001
(1.000–1.002) |
| β2-mg | 0.211 | 0.070 | 9.110 | 0.003 | 1.235
(1.077–1.416) |
| CRP | 0.018 | 0.006 | 8.796 | 0.003 | 1.018
(1.006–1.030) |
| IL-8 | 0.012 | 0.006 | 4.142 | 0.042 | 1.012
(1.000–1.023) |
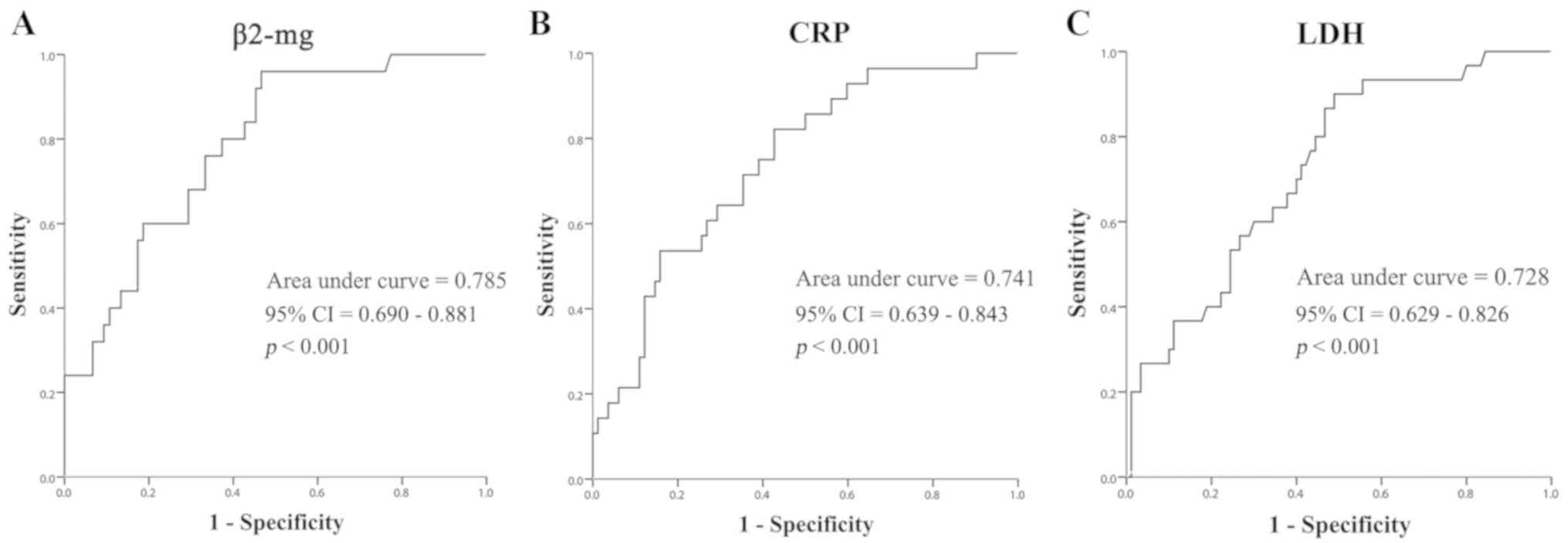
ROC curves
The predictive accuracy of these significant
prognostic factors was further evaluated using ROC curves. This
revealed that β2-mg had the highest potential to predict OS of
patients with NHL (AUC, 0.785; 95% CI, 0.690–0.881; P<0.001;
Fig. 4). CRP (AUC, 0.741; 95% CI,
0.639–0.843; P<0.001) and LDH (AUC, 0.728; 95% CI, 0.629–0.826;
P<0.001) were also indicated to be prognostic factors for OS. As
for IL-8, ROC curves did not indicate any significant differences
(P=0.145; data not shown), and this was therefore not further
discussed. By calculating the Youden Index (sensitivity +
specificity-1), the optimal cut-off was determined for these
prominent indicators. The most suitable cut-off for β2-mg with the
optimal effectiveness of prediction was 2.89 mg/l (sensitivity,
0.960; specificity, 0.467), The best cut-off value for LDH to
predict OS was 215 U/l (sensitivity, 0.900; specificity, 0.489).
For CRP, the Youden Index reached the maximum when the cut-off was
set at 12.01 mg/l (sensitivity, 0.821; specificity, 0.427) (data
not shown).
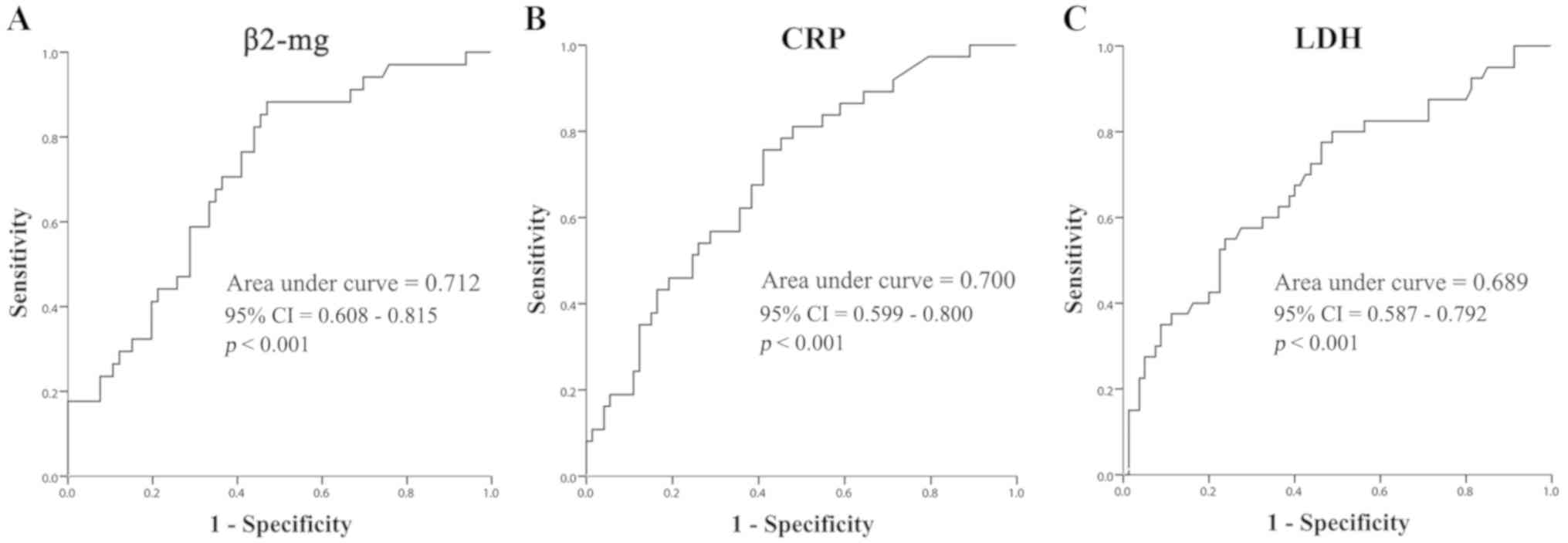
As for PFS, a similar result demonstrating the
predictive potential of β2-mg (AUC, 0.712; 95% CI, 0.608–0.815;
P<0.001), CRP (AUC, 0.700; 95% CI, 0.599–0.800; P<0.001) and
LDH (AUC, 0.689; 95% CI, 0.587–0.792; P<0.001) was observed
(Fig. 5), and the optimal cutoff
points were set as 2.78 mg/l (sensitivity, 0.882; specificity,
0.530), 12.01 mg/l (sensitivity, 0.757; specificity, 0.589) and 224
U/l (sensitivity, 0.775; specificity, 0.538), respectively.
CR prediction of OS or PFS
indicators
Further investigations were performed to identify
whether these serological indicators predicting OS or PFS could
predict CR as well. Mann-Whitney U tests demonstrated that patients
who reached CR after four rounds of chemotherapy exhibited
significantly lower levels of CRP and β2-mg at the beginning of
treatment compared with those patients who did not achieve CR
(P<0.001; data not shown), indicating that CRP and β2-mg were
prognostic factors for survival and CR evaluation. Among the other
indicators in survival analysis, IL-6 was also a prognostic factor
in CR assessment (P<0.001; data not shown), providing evidence
that serological factors have a higher potential to predict CR
after four rounds of chemotherapy compared with SUVmax and
Ki67%.
Discussion
With the advantage of intuitively displaying size,
location and metabolic activity of malignancies, PET/CT has been
widely applied for precise staging and therapeutic evaluation. A
higher SUV is indicative of a larger quantity of vigorous and
invasive tumor tissue, and a corresponding poor prognosis (8–10).
Several studies have demonstrated this relationship in NHL
(22,23). In the present study, only a limited
significance for SUVmax-biopsy to predict PFS of patients with
T-NHL was observed. This may be due to heterogeneity of data, and
may not represent the actual situation. A more detailed subgroup
analysis based on individual diseases, such as DLBCL, is required
for future studies in order to reduce bias. Furthermore, some
articles (24,25) have suggested substitution of SUVmax
with a more responsive indicator, the alteration of SUVmax
(∆SUVmax), to evaluate interdependency. However, since repeated
PET/CT examination may cause a large financial burden and
additional exposure to radiation, not all patients consent to
frequent follow-up examinations and clinical data may be difficult
to collect. One solution is to calculate SUVmax of the biopsy site
(SUVmax-biopsy) instead of the entire body (26). This has been demonstrated in the
present study and certain other studies, and a more significant
correlation between SUVmax-biopsy and Ki67% was observed.
Ki67% is considered to be one of the most useful
pathological indicators to reflect tumor proliferation (27). However, in the present study, it was
not a significant prognostic factor for NHL. One primary reason is
that the definition of Ki67% is largely subjective and
technology-dependent. At times, pathologists tend to overestimate
the grade when the percentage of Ki67-positive tumor cells is high.
Additionally, the location of the biopsy site affects judgement,
and low-quality samples may increase measurement errors and reduce
the efficacy of correlation assessment. Previous studies do not
provide affirmative answers regarding whether Ki67% has promising
predictive power as opposed to other mainstream predictors
(6,28). Perhaps a combination of Ki67% with
other pathological indicators will provide a more suitable option.
However, it is certain that the association between SUVmax and
Ki67% is close, which indicates that the metabolic activity of the
tumor is associated with its proliferative activity, and this has
been demonstrated in multiple studies (10,29).
The present study indicated that traditional
serological factors exhibited a higher predictive value than SUVmax
and Ki67%. In the survival analysis, CRP was demonstrated to be the
best predictor for both OS and PFS. Additionally, LDH and IL-6 had
a marked practicality in the estimation of OS and PFS. By
performing Cox regression analysis, it was demonstrated that CRP,
LDH and β2-mg were fully independent predictors of OS and PFS, and
their ROC curves indicated high sensitivity and specificity.
Furthermore, CRP, β2-mg and IL-6 were significant predictors of CR
after four courses of chemotherapy. This is instructional because
clinicians may regard them as key indicators for NHL prognosis and
formulate individualized therapies based on their detection level.
However, the present study also had some limitations. First, the
sample size was relatively small. An increased sample size and
extended observation of patient outcome are required in future
studies. Second, the number of cases exhibited a great variation
among different subgroups. As a result, certain subgroups were too
small to be analyzed. Finally, PET data may not be sufficiently
adequate to draw firm conclusions. However, our study have
demonstrated that serological factors are superior to SUVmax and
Ki67%, which are of clinical importance and have a guidance
value.
In conclusion, the present study provided
comparisons of serological, radiological and pathological
indicators and their ability to predict survival and treatment
outcomes for patients with NHL. Overall, it is recommended to use
β2-mg, CRP and LDH for estimating OS and PFS as the first choice,
and taking CRP, β2-mg and IL-6 into account for CR prediction. CRP
and β2-mg can be used for estimating survival as well as CR. By
contrast, SUVmax and Ki67% did not exhibit sufficient predictive
power and should not be recommended. The present study may serve as
a reference for clinicians to formulate therapeutic regimens for
patients diagnosed with NHL by referring to indicators with high
predictive value.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Bing Xiu
(Department of Hematology, Shanghai Tongji Hospital, Shanghai
Tongji University School of Medicine), for her guidance in writing
this article, and her support for statistical analysis and
polishing the language of the manuscript. The authors would also
like to thank Dr Yu Zeng, Dr Bing Li, Miss Yihan Liu and Miss You
Wu (Department of Hematology, Shanghai Tongji Hospital, Shanghai
Tongji University School of Medicine), who joined in this project
and provided reasonable suggestions and necessary support in
writing the manuscript.
Funding
This article was supported by grants from the
National Natural Science Foundation (grant nos. 81600156 and
81401882), the clinical research cultivation project of Shanghai
Tongji Hospital (ITJ(QN)1907) and the Key Project of Natural
Science Foundation of China (grant no. 81830004).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
JL collected PET/CT data and was a major contributor
in writing the manuscript. YZ provided all necessary pathological
data including supplementary immunohistochemical photographs. YW
was the main collector of serological data and contributed to
writing the manuscript. BX revised the manuscript and provided
assistance for statistical analysis. BL and XL collected basic
information of enrolled patients and collected survival data from
patient follow-up. BX, JF, WZ and AL contributed to the conception
and design of this study as well as acquisition of funding. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were ethically approved by The
Ethics Committee of Shanghai Tongji Hospital (approval no.
KYSB-2016-19), and written informed consent was provided by each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Müller AM, Ihorst G, Mertelsmann R and
Engelhardt M: Epidemiology of non-Hodgkin's lymphoma (NHL): Trends,
geographic distribution, and etiology. Ann Hematol. 84:1–12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Basharat S, Iqtidar BH, Naeem S, Batool Z,
Ali N and Ahmad Z: Clinicopathological and immunohistochemical
profile of patients with Non-hodgkin's lymphoma. RMJ. 44:472–476.
2019.
|
|
3
|
Iyer VK: Pediatric lymphoma diagnosis:
Role of FNAC, biopsy, immunohistochemistry and molecular
diagnostics. Indian J Pediatr. 80:756–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Binder M, O'Byrne MM, Maurer MJ, Ansell S,
Feldman AL, Cerhan J, Novak A, Porrata LF, Markovic S, Link BK and
Witzig TE: Associations between elevated pre-treatment serum
cytokines and peripheral blood cellular markers of
immunosuppression in patients with lymphoma. Am J Hematol.
92:752–758. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ansell SM and Armitage J: Non-Hodgkin
lymphoma: Diagnosis and treatment. Mayo Clin Proc. 80:1087–1097.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Küçükzeybek BB, Bener S, Çallı AO, Paksoy
TD and Payzin B: Prognostic significance of Bcl-2 and p53 protein
expressions and Ki67 proliferative index in diffuse large B-cell
lymphoma. Turk J Haematol. 30:275–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fayad L, Keating MJ, Reuben JM, O'Brien S,
Lee BN, Lerner S and Kurzrock R: Interleukin-6 and interleukin-10
levels in chronic lymphocytic leukemia: Correlation with phenotypic
characteristics and outcome. Blood. 97:256–263. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng G, Servaes S, Alavi A and Zhuang H:
FDG PET and PET/CT in the management of pediatric lymphoma
patients. PET Clin. 3:621–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watabe T, Tatsumi M, Watabe H, Isohashi K,
Kato H, Yanagawa M, Shimosegawa E and Hatazawa J: Intratumoral
heterogeneity of F-18 FDG uptake differentiates between
gastrointestinal stromal tumors and abdominal malignant lymphomas
on PET/CT. Ann Nucl Med. 26:222–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Novelli S, Briones J, Flotats A and Sierra
J: PET/CT Assessment of follicular lymphoma and high Grade B cell
lymphoma-good correlation with clinical and histological features
at diagnosis. Adv Clin Exp Med. 24:325–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berghmans T, Dusart M, Paesmans M,
Hossein-Foucher C, Buvat I, Castaigne C, Scherpereel A, Mascaux C,
Moreau M, Roelandts M, et al: Primary tumor standardized uptake
value (SUVmax) measured on fluorodeoxyglucose positron emission
tomography (FDG-PET) is of prognostic value for survival in
non-small cell lung cancer (NSCLC): A systematic review and
meta-analysis (MA) by the European lung cancer working party for
the IASLC lung cancer staging project. J Thorac Oncol. 3:6–12.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diao W, Tian F and Jia Z: The prognostic
value of SUV (max) measuring on primary lesion and ALN by (18)
F-FDG PET or PET/CT in patients with breast cancer. Eur J Radiol.
105:1–7. 2018. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
13
|
Hu SL, Yang ZY, Zhou ZR, Yu XJ, Ping B and
Zhang YJ: Role of SUV(max) obtained by 18F-FDG PET/CT in patients
with a solitary pancreatic lesion: Predicting malignant potential
and proliferation. Nucl Med Commun. 34:533–539. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tokuda E, Horimoto Y, Arakawa A, Himuro T,
Senuma K, Nakai K and Saito M: Differences in Ki67 expressions
between pre- and post-neoadjuvant chemotherapy specimens might
predict early recurrence of breast cancer. Hum Pathol. 63:40–45.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaudio F, Giordano A, Perrone T, Pastore
D, Curci P, Delia M, Napoli A, de Risi C, Spina A, Ricco R, et al:
High Ki67 index and bulky disease remain significant adverse
prognostic factors in patients with diffuse large B cell lymphoma
before and after the introduction of rituximab. Acta Haematol.
126:44–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uzurov-Dinić V, Savic A, Lazarević T,
Cemerikić-Martinović V, Agić D and Popović S: Prognostic factors in
patients with diffuse large B-cell lymphoma. Med Pregl. 62:171–176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Swerdlow SH: WHO Classification of Tumours
of Haematopoietic and Lymphoid. 2008.
|
|
18
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project: A predictive model for aggressive
Non-Hodgkin's-Lymphoma. N Engl J Med. 329:987–994. 1993.
|
|
19
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the Committee on Hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
20
|
Juweid ME, Stroobants S, Hoekstra OS,
Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L,
Scheidhauer K, Buck A, et al: Use of positron emission tomography
for response assessment of lymphoma: Consensus of the Imaging
Subcommittee of International Harmonization Project in Lymphoma. J
Clin Oncol. 25:571–578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gigliotti E: Discovering statistics using
SPSS, Second edition. JAN. 58:3032007. View Article : Google Scholar
|
|
22
|
Lee H, Kim SK, Kim YI, Kim TS, Kang SH,
Park WS, Yun T and Eom HS: Early determination of prognosis by
interim 3′-deoxy-3′-18F-fluorothymidine PET in patients with
non-Hodgkin lymphoma. J Nucl Med. 55:216–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ying Z, Wang X, Song Y, Zheng W, Wang X,
Xie Y, Lin N, Tu M, Ping L, Liu W, et al: Prognostic value of
interim (18)F-FDG PET/CT in diffuse large B-cell lymphoma. Chin J
Cancer Res. 25:95–101. 2013.PubMed/NCBI
|
|
24
|
Wen SY, Hua JZ, Li QW, Yan X, Xiang C and
Jian-Hua S: Prognostic significance of interim F-18-F-FDG PET/CT
SUV reduction associated with Ki67 in patients with diffuse large
B-cell lymphoma. Nuclear Sci Techniques. 25:1–7, 2014. 2014.
|
|
25
|
Casasnovas RO, Meignan M,
Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, Vera P,
Bologna S, Brière J, Jais JP, et al: SUVmax reduction improves
early prognosis value of interim positron emission tomography scans
in diffuse large B-cell lymphoma. Blood. 118:37–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang B, Malysz J, Douglas-Nikitin V,
Zekman R, Wong RH, Jaiyesimi I and Wong CY: Correlating metabolic
activity with cellular proliferation in follicular lymphomas. Mol
Imaging Biol. 11:296–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pătraşcu AM, Rotaru I, Olar I, Pătraşcu Ş,
Ghiluşi MC, NeamŢu SD, Nacea JG and Gluhovschi A: The prognostic
role of Bcl-2, Ki67, c-MYC and p53 in diffuse large B-cell
lymphoma. Rom J Morphol Embryol. 58:8372017.PubMed/NCBI
|
|
29
|
Albano D, Bertoli M, Ferro P, Fallanca F,
Gianolli L, Picchio M, Giubbini R and Bertagna F: 18F-FDG PET/CT in
gastric MALT lymphoma: A bicentric experience. Eur J Nucl Med Mol
Imaging. 44:589–597. 2017. View Article : Google Scholar : PubMed/NCBI
|