Introduction
Cancer stem cells (CSCs), also known as
tumor-initiating cells, are characterized by stem-like properties,
including self-renewal and ability to generate daughter cells.
Cancer initiation, dissemination and recurrence are closely
associated with CSCs (1). CSCs were
first detected in acute myeloid leukemia and have since been
identified in several solid tumors, including gastric cancer
(1). Furthermore, certain
populations of gastric CSCs abilities to self-renewal and undergo
multipotent differentiation have been detected in gastric cancer
(2). Villin+ and
Lgr5+ gastric stem cells have been detected in the
antrum, while Troy+ chief cells have been found in the
corpus (3). Additionally,
Sox2+ gastric stem cells are present in both the antrum
and the corpus (3).
Gastric cancer is the seventh most common cancer and
the third leading cause of cancer-associated mortality worldwide
(4). In 2018, 1,033,701 new gastric
cancer cases and 782,685 mortalities were reported worldwide
(4). Gastric cancer has been
extensively investigated in the biomedical field due to its high
morbidity and mortality rates (4).
It is speculated that gastric carcinogenesis may be associated with
Helicobacter pylori infection, inherited susceptibilities,
and environmental and dietary factors (5,6). In
recent years, the prevailing hypothesis that the occurrence and
progression of gastric cancer is associated with CSCs has been
partially proven (7).
Epithelial cell transforming 2 (ECT2) is a
proto-oncogene gene encoding a guanine nucleotide exchange factor
for the Rho GTPases (8). When
expressed in NIH/3T3 fibroblasts, ECT2 promotes their malignant
transformation (9). Increased ECT2
expression has been detected in several types of human tumor,
including glioma and liver, pancreatic and lung cancer (10–13).
ECT2 upregulation significantly enhances the activity of RhoGPase,
prevents cell apoptosis and induces cancer cell metastasis
(10). Conversely, ECT2
downregulation suppresses activation of the ERK signaling pathway
and impairs the migration of cancer cells (10). However, whether and how ECT2
contributes to gastric cancer malignancy remains elusive.
The present study aimed to investigate the
association between ECT2 expression and the clinicopathological
characteristics of patients with gastric cancer. The expression
levels of ECT2 were investigated using immunohistochemical
analysis, combined with Gene Expression Omnibus database and gene
set enrichment analysis, and it was revealed that gastric tumors
with elevated ECT2 levels exhibited transcriptional traits of CSCs.
In addition, high ECT2 expression predicted poor clinical outcome,
suggesting its use as a novel prognostic indicator for gastric
carcinoma. Further investigation into the role of ECT2 may provide
alternative therapeutic targets for the treatment of gastric
cancer.
Materials and methods
Clinical tissue samples
A total of 130 primary gastric cancer tissues and
108 paired adjacent normal tissues (some paired adjacent normal
tissues were not harvested during the operation due to patients
clinical conditions) were collected from patients who underwent
surgery at the Hospital of Chengdu University of TCM (Chengdu,
China) between March 2012 and December 2015, and retrospectively
analyzed. Paraffin-embedded tissue samples were stored at room
temperature. None of the patients had received anticancer treatment
prior to diagnosis and no additional malignancies were present.
Pathological staging was based on the Union for International
Cancer Control/American Joint Committee on Cancer
Tumor-Node-Metastasis (TNM) Classification (8th edition of 2016)
(14). The present study was
approved by the Institutional Review Board of the Teaching Hospital
of Chengdu University of TCM (Chengdu, China) (approval no.
2018KL-023) and written informed consent was provided by all
patients prior to the study start.
Immunohistochemistry (IHC)
The tissue samples were fixed in 4% paraformaldehyde
>24 h at room temperature, then dehydrated in graded ethanol
series (30, 50, 70, 95 and 100%), and embedded in paraffin. For IHC
analysis, paraffin-embedded samples were cut into 3-µm-thick
sections, dewaxed with xylene at room temperature and rehydrated in
a descending ethanol series (100, 95, 85 and 75%). For antigen
retrieval, sections were heated at 97°C for 20 min. Following a
brief proteolytic digestion with 0.1% trypsin at 37°C for 10 min
and peroxidase blocking with 3% hydrogen peroxide solution at room
temperature for 15 min, the sections were incubated with primary
antibodies against: ECT2 (1:400; cat. no. 07-1364; Sigma-Aldrich,
Merck KGaA), BUB1 (1:200; cat. no. DF6698; Affinity Biosciences)
and E2F transcription factor 7 (E2F7; 1:200; cat. no. DF2444;
Affinity Biosciences) overnight at 4°C. Following the primary
antibody incubation, the sections were incubated with a HRP/Fab
secondary antibody at room temperature for 20 min (freshly prepared
solution from the kit; cat. no. PV-6000-D; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.). Tissue sections were stained with
diaminobenzidine substrate for 5 min and counterstained with
hematoxylin for 20 sec at room temperature. Each slide was analyed
using light microscopy (H-7650; Hitachi, Ltd.). The magnification
used was ×200.
A total of two independent investigators, without
prior knowledge of the clinicopathological data, evaluated the ECT2
staining in a semiquantitative manner. The final immunoreactivity
scores (IRS) were determined according to the sum total of the
percentage of positive cells (0 points, 0–5% positive cells; 1
point, 6–25%; 2 points, 26–50%; 3 points, 51–75% and 4 points,
76–100%), and staining intensity scores (0 points, no staining; 1
point, weak staining; 2 points, moderate staining and 3 points,
strong staining). A final IRS >4 indicated strong positivity,
while scores <4 indicated weak positivity.
ECT2 analysis in the Gene Expression
Omnibus (GEO) database
ECT2 expression was assessed in several independent
gastric cancer clinical datasets (15–18)
available from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). The GSE13861
dataset (15) included a collection
of 65 primary gastric adenocarcinoma and 19 surrounding normal
tissues. The GSE29272 (16) dataset
included a cohort of 134 gastric adenocarcinoma and paired
surrounding normal tissues. The GSE51575 (17) dataset consisted of a cohort of 27
advanced gastric carcinoma and paired surrounding normal tissues.
The GSE65801 (18) dataset consisted
of a cohort of 32 gastric cancer tissues and paired surrounding
noncancerous tissues. The normalization procedures employed for
gene expression intensity data are stated in the individual
datasets and related publications.
Gene set enrichment analysis
(GSEA)
In order to determine how biological processes and
signaling pathways are differentially regulated in gastric cancer
with low or high ECT2 expression, transcriptomic data of gastric
cancer were retrieved from The Cancer Genome Atlas (TCGA) database
and analyzed using GSEA. TCGA gastric cancer cohort, consisting of
407 samples and transcriptional profiles, was downloaded from TCGA
Data Portal (https://tcga-data.nci.nih.gov/docs/publications/tcga).
GSEA was performed using GSEA software (v2.2.2; www.broadinstitute.org/gsea). The median ECT2
expression level (cut-off value=11.13) was used to dichotomize
samples into low and high expression groups. A total of 1,000
permutations were used to calculate the P-values. All other
parameters were set based on their default values.
Survival analysis
The prognostic value of ECT2 in gastric cancer was
assessed using the Kaplan-Meier (KM) Plotter database (http://kmplot.com/analysis/index.php?p=service&cancer=gastric),
which consists of a pool of gene expression and clinical data
(19). The median time to first
progression (FP) was 18.3 months and the median overall survival
(OS) was 28.9 months. Overall survival time was assessed. The
patient samples were divided into two groups according to the
median gene expression value
(ECT2High/ECT2Low, 437 cases/438 cases). A KM
survival plot was used to compare the two groups. The hazard ratio
(HR) with 95% confidence intervals (CIs) and log rank P-values were
calculated.
Statistical analysis
Data are presented as the mean ± standard error of
the mean from three independent experiments and were analyzed with
SPSS v.22.0 software (IBM, Corp.). Pearsons χ2 test and
Fishers exact test were used to assess the association between ECT2
expression and clinicopathological characteristics of patients with
gastric cancer. Unpaired Students t-test was used to assess the
differences in ECT2, BUB1 and E2F7 expression levels between
gastric cancer and control tissues. Spearmans correlation test was
performed between BUB1 and ECT2 expression levels, and E2F7 and
ECT2 expression levels, respectively. Survival analysis was
performed using the KM method and the log-rank test was used to
assess statistical significance between the curves. Univariate and
multivariate survival analyses were performed using the Cox
proportional hazards regression model. P<0.05 was considered to
indicate a statistically significant difference.
Results
ECT2 expression is upregulated in
human gastric cancer
Following a routine H&E staining, IHC was
performed to detect and grade ECT2 expression in gastric cancer and
paired normal tissue sections. ECT2 positive staining was detected
mainly in the nucleus and cytoplasm of gastric cancer cells. Weak
staining was observed in the adjacent normal tissues. ECT2
expression was identified in 81.5% (106/130) of the gastric
carcinoma tissues and 36.1% (39/108) of the adjacent normal
tissues. The level of ECT2 protein was significantly higher in the
gastric carcinoma tissues compared with the adjacent tissues (IRS,
cancer=3.12±2.00 vs. normal=0.98±1.56; P<0.01; Fig. 1A and B).
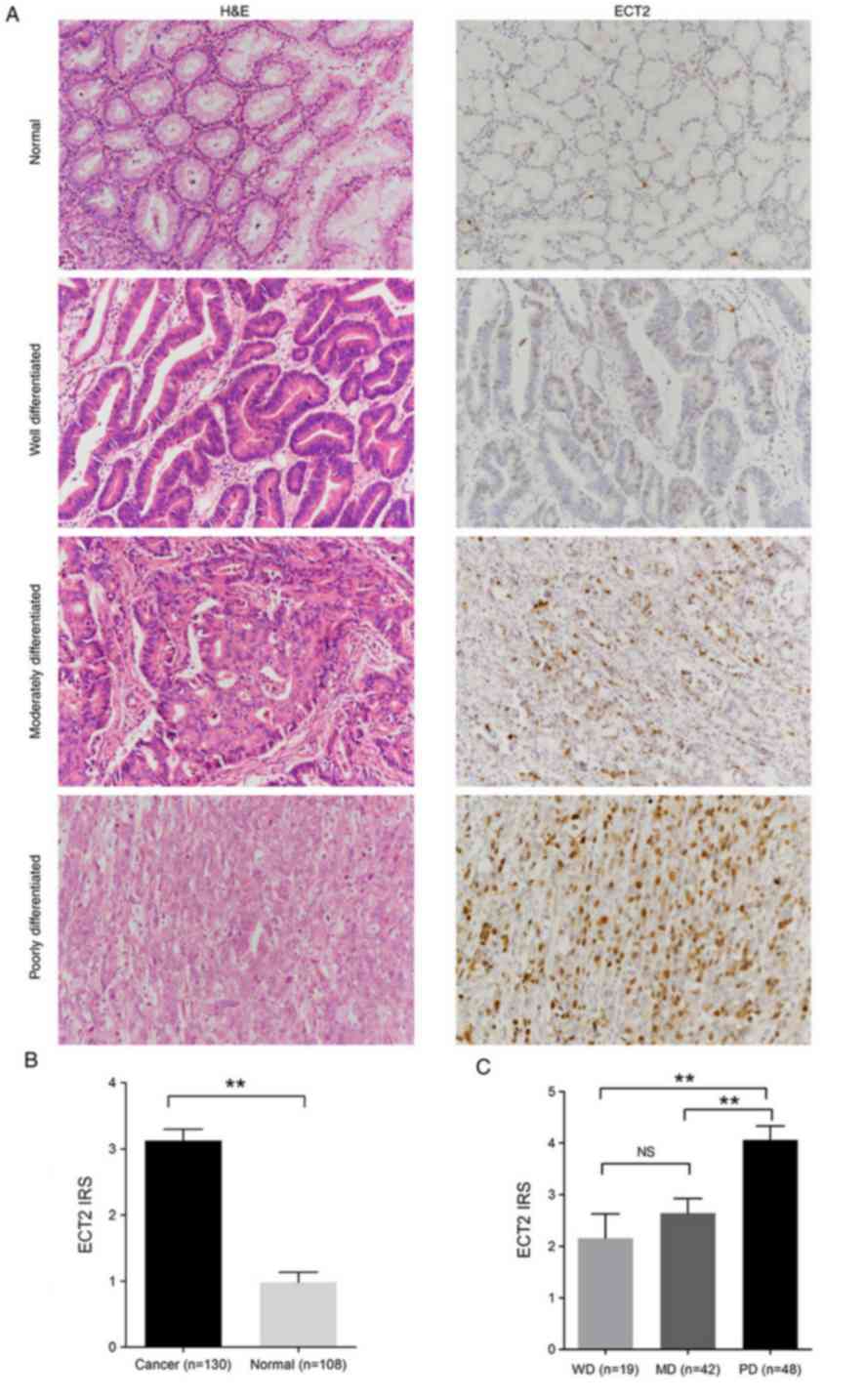 | Figure 1.ECT2 expression is upregulated in
human gastric cancer tissues. (A) Representative images depicting
H&E staining (left panel), and immunohistochemical staining
(right panel) of ECT2 in normal tissues and WD, MD and PD gastric
cancer tissues. Magnification, ×200. (B) ECT2 IRS in gastric cancer
and adjacent normal tissue samples. (C) ECT2 IRS in WD, MD and PD
gastric cancer tissues. Data are presented as the mean ± standard
error of the mean. **P<0.01. ECT2, epithelial cell transforming
2; H&E, hematoxylin and eosin; WD, well differentiated; MD,
moderately differentiated; PD, poorly differentiated; IRS,
immunoreactivity score; NS, no significance. |
Association between
clinicopathological characteristics and ECT2 positivity in gastric
cancer
The clinicopathological significance of ECT2
expression in gastric cancer was investigated. Analysis of the
association between ECT2 expression levels (strong positivity vs.
weak positivity/absent) and clinicopathological characteristics
demonstrated that strong ECT2 positivity was significantly
associated with advanced TNM stage (P<0.001) and higher pT stage
(deeper tumor invasion; P=0.039). In the 130 gastric cancer cases
assessed, high ECT2 expression was not associated with age, sex,
tumor localization, pN stage and pM stage (all P>0.05; Table I; Table
SI).
 | Table I.Correlation between ECT2 positivity
and clinicopathological characteristics of patients with gastric
cancer (n=130). |
Table I.
Correlation between ECT2 positivity
and clinicopathological characteristics of patients with gastric
cancer (n=130).
|
Characteristics | Total n=130 | ECT2 strong
positivity, n=35 | ECT2 weak
positivity/absent, n=95 | P-value |
|---|
| Age, years |
|
|
| 0.241 |
|
<60 | 67 | 21 | 46 |
|
|
≥60 | 63 | 14 | 49 |
|
| Sex |
|
|
| 0.077 |
|
Male | 93 | 21 | 72 |
|
|
Female | 37 | 14 | 23 |
|
| Tumor
localization |
|
|
| 0.091 |
|
Cardias | 20 | 7 | 13 |
|
|
Body | 40 | 15 | 25 |
|
|
Antrum | 52 | 8 | 44 |
|
|
Whole/Multiple | 18 | 5 | 13 |
|
| Histology |
|
|
| 0.216 |
| ADC,
WD | 19 | 5 | 14 |
|
| ADC,
MD | 42 | 7 | 35 |
|
| ADC,
PD | 48 | 19 | 29 |
|
| Signet
ring cell | 14 | 3 | 11 |
|
|
Mucinous adenocarcinoma | 6 | 1 | 5 |
|
|
Neuroendocrine carcinoma | 1 | 0 | 1 |
|
| TNM stage |
|
|
| <0.001 |
| I +
II | 67 | 8 | 59 |
|
| III +
IV | 63 | 27 | 36 |
|
| pT (Tumor
invasion) |
|
|
| 0.039 |
| T1 +
T2 | 22 | 2 | 20 |
|
| T3 +
T4 | 108 | 33 | 75 |
|
| pN (Lymph node
metastasis) |
|
|
| 0.896 |
| N0 | 42 | 11 | 31 |
|
|
N1-N3 | 88 | 24 | 64 |
|
| pM (Distant
metastasis) |
|
|
| 0.473 |
| M0 | 114 | 29 | 85 |
|
| M1 | 16 | 6 | 10 |
|
A significant association between strong ECT2
positivity and histological patterns of gastric cancer was not
established (Table I; Table SI); however, ECT2 expression was
closely associated with the histological differentiation degree
(Fig. 1C). ECT2 expression escalated
from well differentiated (WD), to moderately differentiated (MD)
and to poorly differentiated (PD) variants (IRS, PD=4.06±1.87 vs.
WD=2.16±2.06 or MD=2.64±1.83; P<0.01; Fig. 1C).
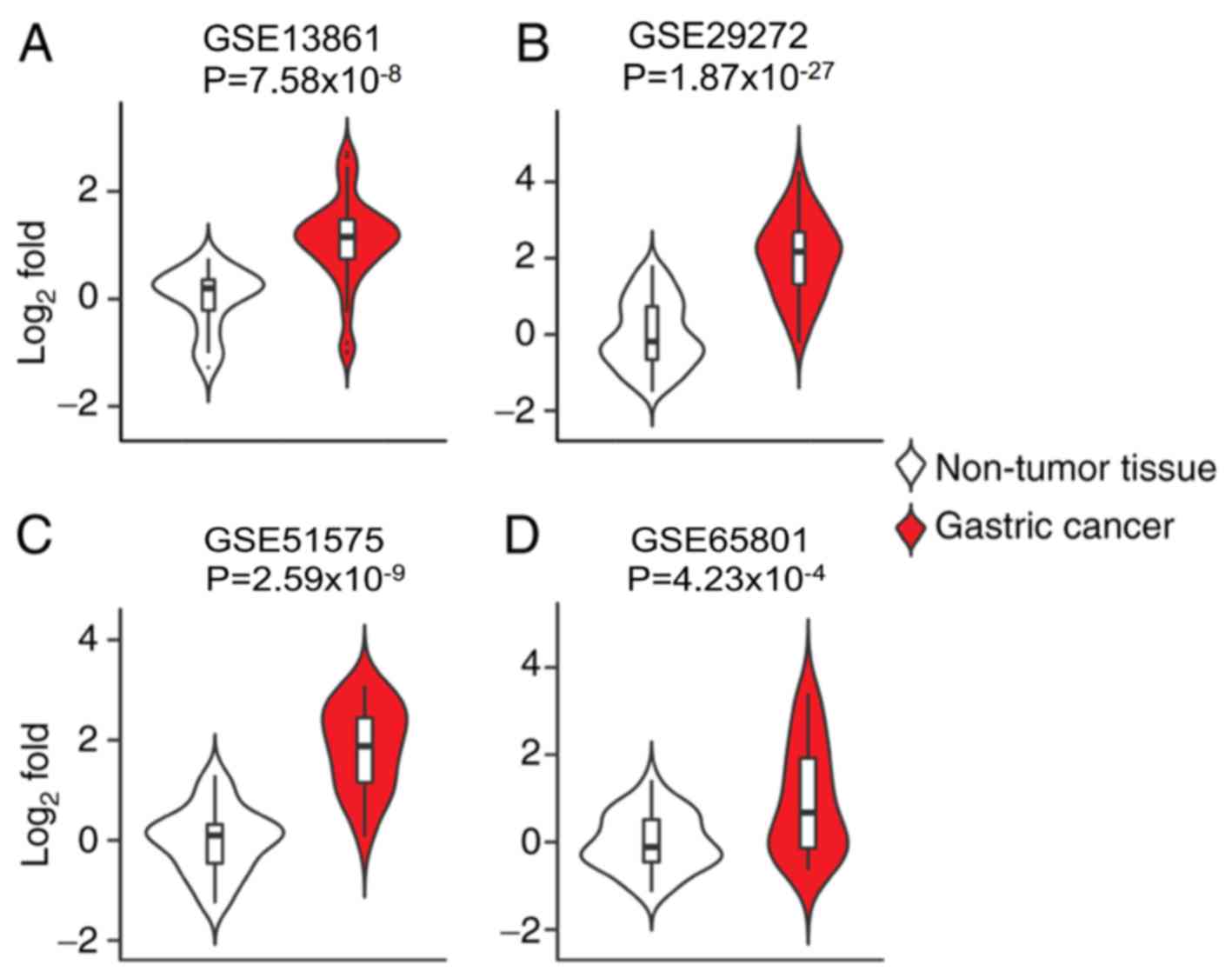
ECT2 mRNA upregulation in public
datasets of gastric cancer
To further investigate the pathological role of ECT2
in the progression of gastric cancer, the ECT2 expression pattern
in gastric cancer samples based on transcriptomic data from the GEO
database was assessed. Consistent with IHC analysis, ECT2 mRNA
expression levels were significantly increased in primary gastric
adenocarcinoma tissues compared with surrounding normal tissues in
the GSE13861 dataset (P<0.0001; Fig.
2A). Similar trends were observed in comparisons between paired
samples of gastric carcinoma and adjacent normal tissues in the
GSE29272 dataset (P<0.0001; Fig.
2B), advanced gastric carcinoma and adjacent normal tissues in
the GSE51575 dataset (P<0.0001; Fig.
2C), and gastric cancer tissues and adjacent normal tissues in
the GSE65801 dataset (P<0.0001; Fig.
2D). Collectively, these results indicate that ECT2
upregulation may play an important role in the malignant
progression of human gastric cancer.
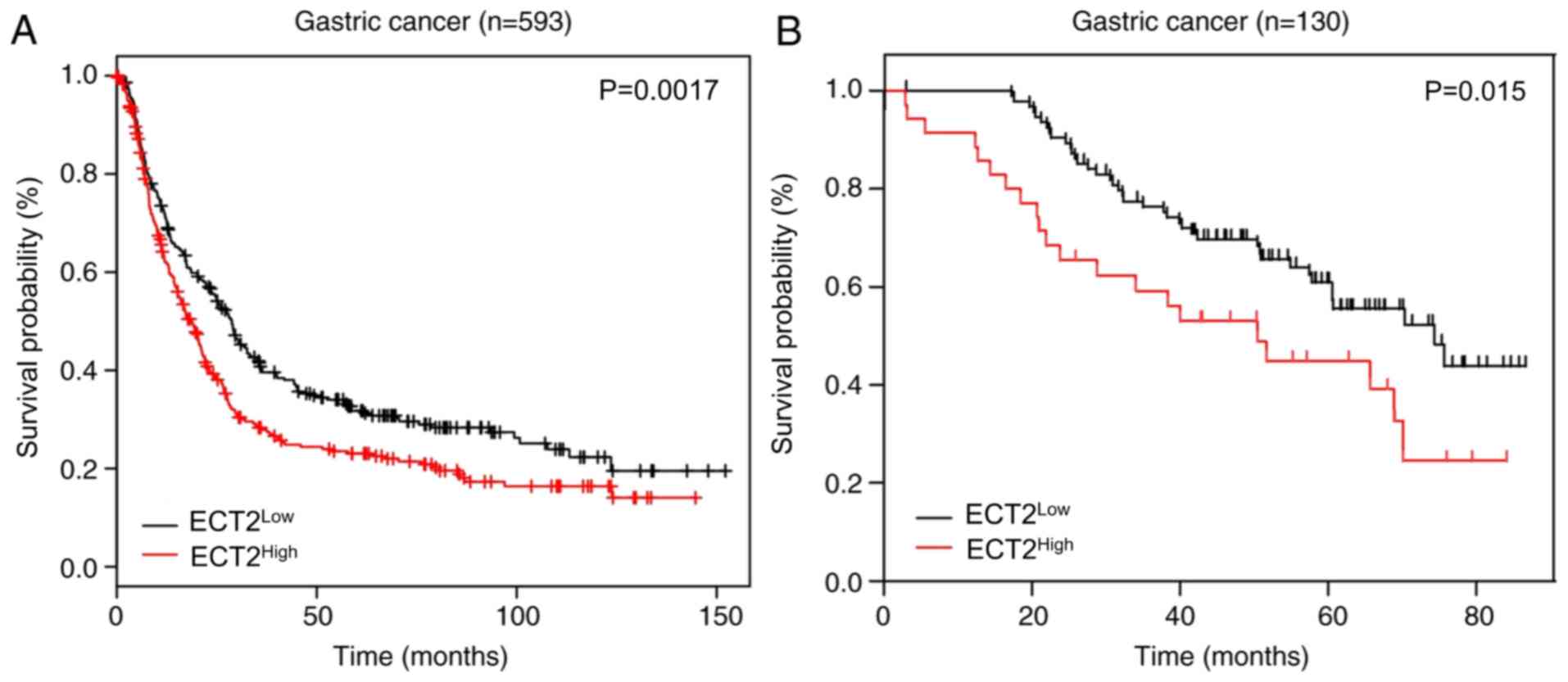
ECT2 upregulation predicts poor
clinical outcome
The association between ECT2 expression and the
prognosis of patients with gastric cancer was assessed using the KM
plot database. High ECT2 expression was significantly associated
with a shorter survival time in patients with gastric cancer,
stratified according to ECT2 expression levels. The median survival
time for ECT2High patients was 16 months, which was
significantly shorter than the 26 months observed for
ECT2Low patients (P=0.0017; Fig. 3A). Survival analysis using KM curves
was performed to verify these results. The results demonstrated
that patients with gastric cancer, with low ECT2 expression
exhibited a significantly longer overall survival time than those
with high ECT2 expression (P=0.015; median OS, 74.360 vs. 50.430
months; Fig. 3B).
To determine whether ECT2 is an independent
prognostic factor for the survival of patients with gastric cancer,
univariate and multivariate Cox regression analyses were performed.
As presented in Table II, the
univariate analysis suggested that ECT2 was significantly
associated with overall survival time in patients with gastric
cancer [P=0.017; HR (95% CI), 1.905 (1.122-3.233)]. Tumor location
(body, whole and multiple), histology (PD of adenocarcinoma, signet
ring cell, mucinous adenocarcinoma) and TNM stage were all
associated with overall survival time in patients with gastric
cancer (all P<0.05). Multivariate analysis further demonstrated
that high ECT2 expression was a significant independent prognostic
marker for patients with gastric cancer [P=0.001; HR (95% CI),
3.105 (1.567-6.153)]. Taken together, these results suggest that
ECT2 may serve as a prognostic biomarker for patients with gastric
cancer.
 | Table II.Prognostic factors associated with
overall survival as determined by univariate and multivariate
analyses. |
Table II.
Prognostic factors associated with
overall survival as determined by univariate and multivariate
analyses.
| Variables | HR (95% CI) | P-value |
|---|
| Univariate
analysis |
|
ECT2 | 1.905
(1.122-3.233) | 0.017 |
| Tumor
localization (Body) | 4.842
(1.436-16.328) | 0.011 |
| Tumor
localization (Whole/Multiple) | 14.106
(4.057-49.039) | <0.001 |
|
Histology (ADC, PD) | 8.274
(2.538-26.971) | <0.001 |
|
Histology (signet ring
cell) | 5.511
(1.403-21.644) | 0.014 |
|
Histology (mucinous
adenocarcinoma) | 5.319
(1.066-26.531) | 0.042 |
| TNM
stage | 4.992
(2.797-8.909) | <0.001 |
|
pT stage | 18.481
(2.557-133.551) | 0.004 |
|
pN stage | 2.846
(1.510-5.367) | 0.001 |
|
pM stage | 3.013
(1.612-5.632) | 0.001 |
| Multivariate
analysis |
|
ECT2 | 3.105
(1.567-6.153) | 0.001 |
| Tumor
localization (Whole/Multiple) | 13.301
(3.468-51.015) | <0.001 |
|
Histology (ADC, PD) | 4.109
(1.246-13.550) | 0.020 |
|
Histology (Mucinous
adenocarcinoma) | 6.186
(1.049-36.481) | 0.044 |
| pT
stage | 12.216
(1.445-103.281) | 0.022 |
| pN
stage | 3.967
(1.935-8.132) | <0.001 |
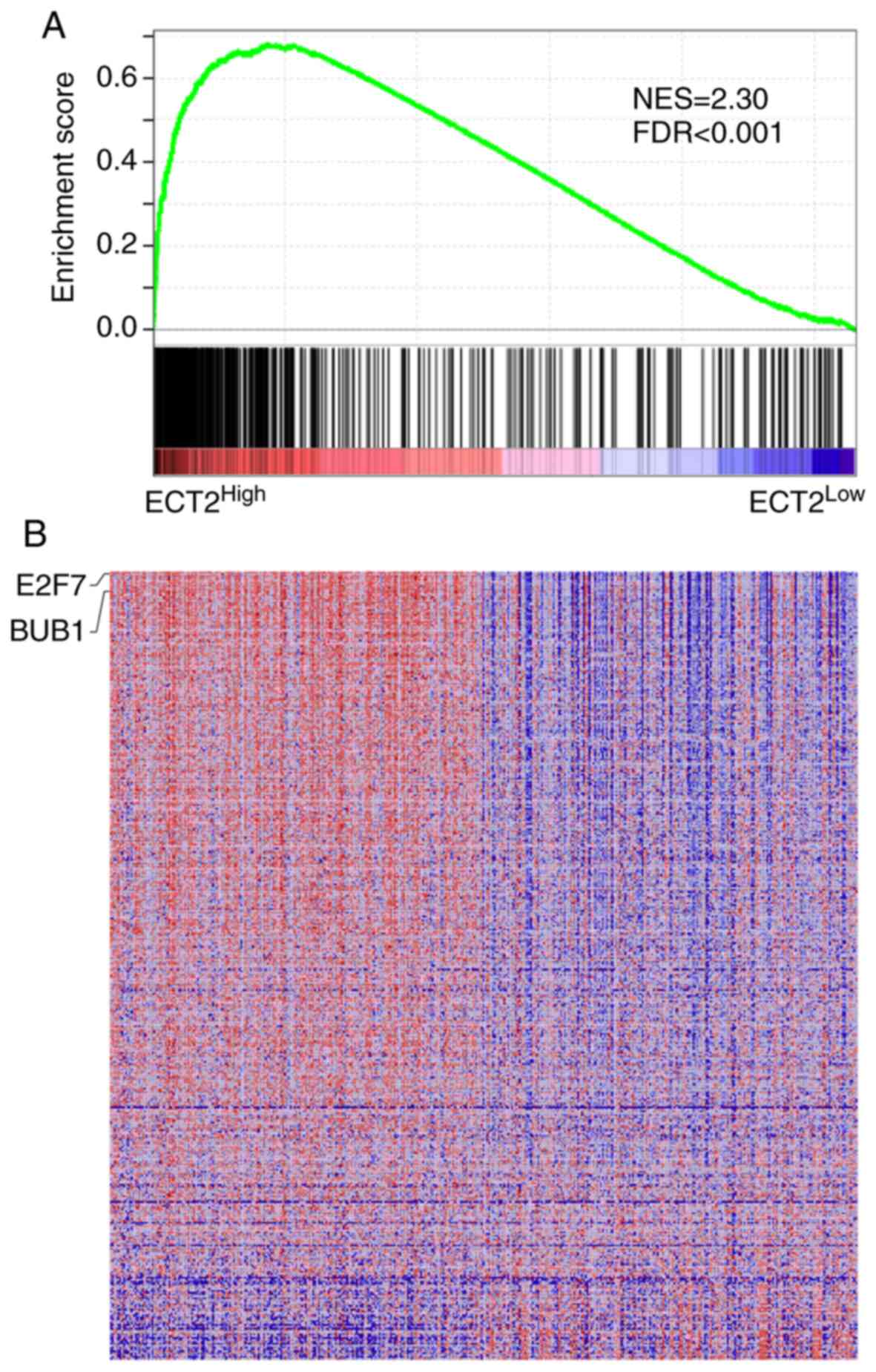
Gastric tumors with higher ECT2
expression levels possess transcriptional traits of CSCs
To determine the ECT2-associated cellular processes
and signaling pathways in gastric cancer, GSEA was performed using
transcriptome data from TCGA. GSEA demonstrated highly significant
enrichment of breast-cancer-progenitor-related genes in gastric
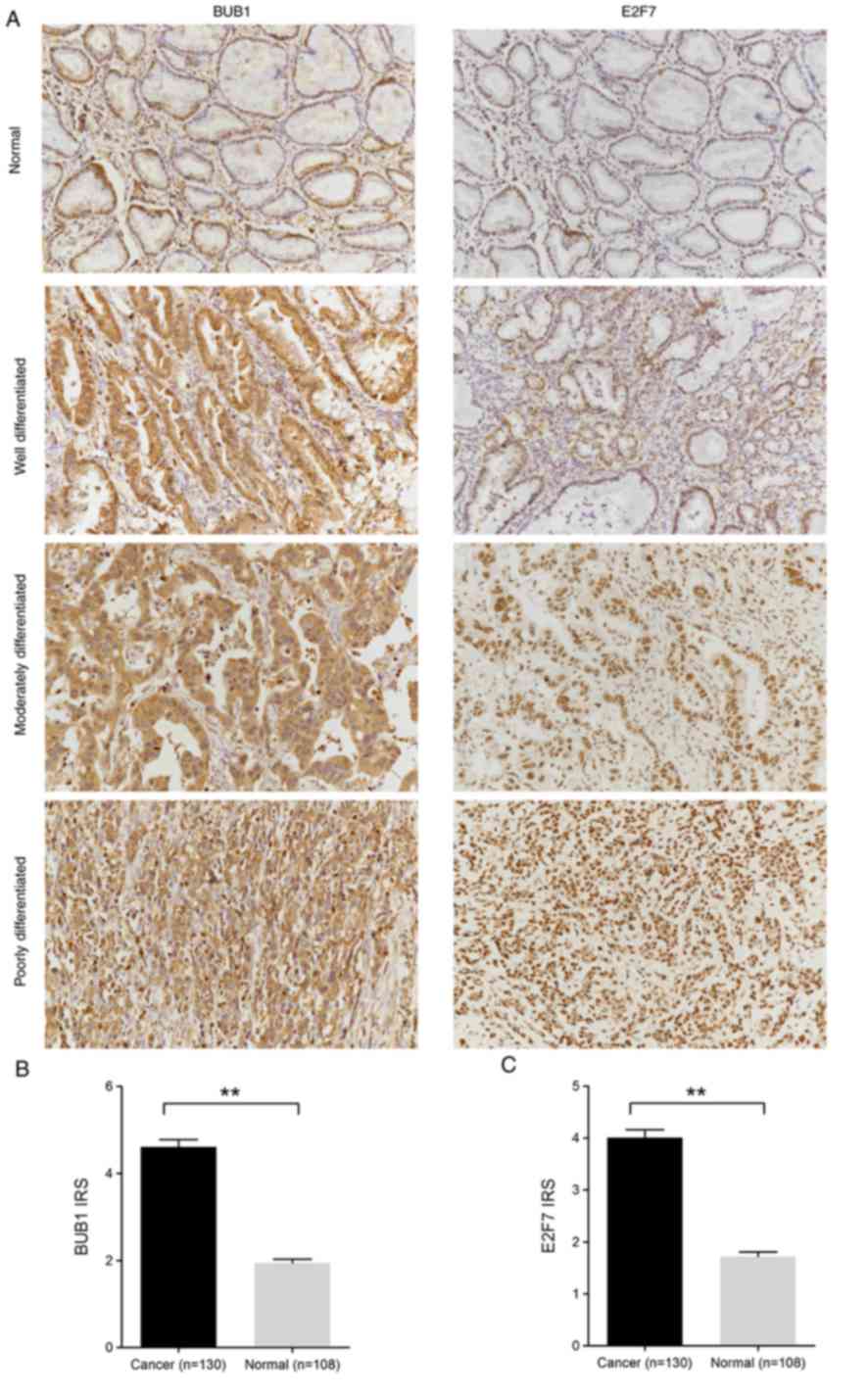
cancer samples with higher ECT2 expression (Fig. 4A). Notably, BUB1 mitotic checkpoint
serine/threonine kinase and E2F7, two genes previously reported to
account for CSC functionality (20–22),
were strongly enriched in ECT2High gastric cancer
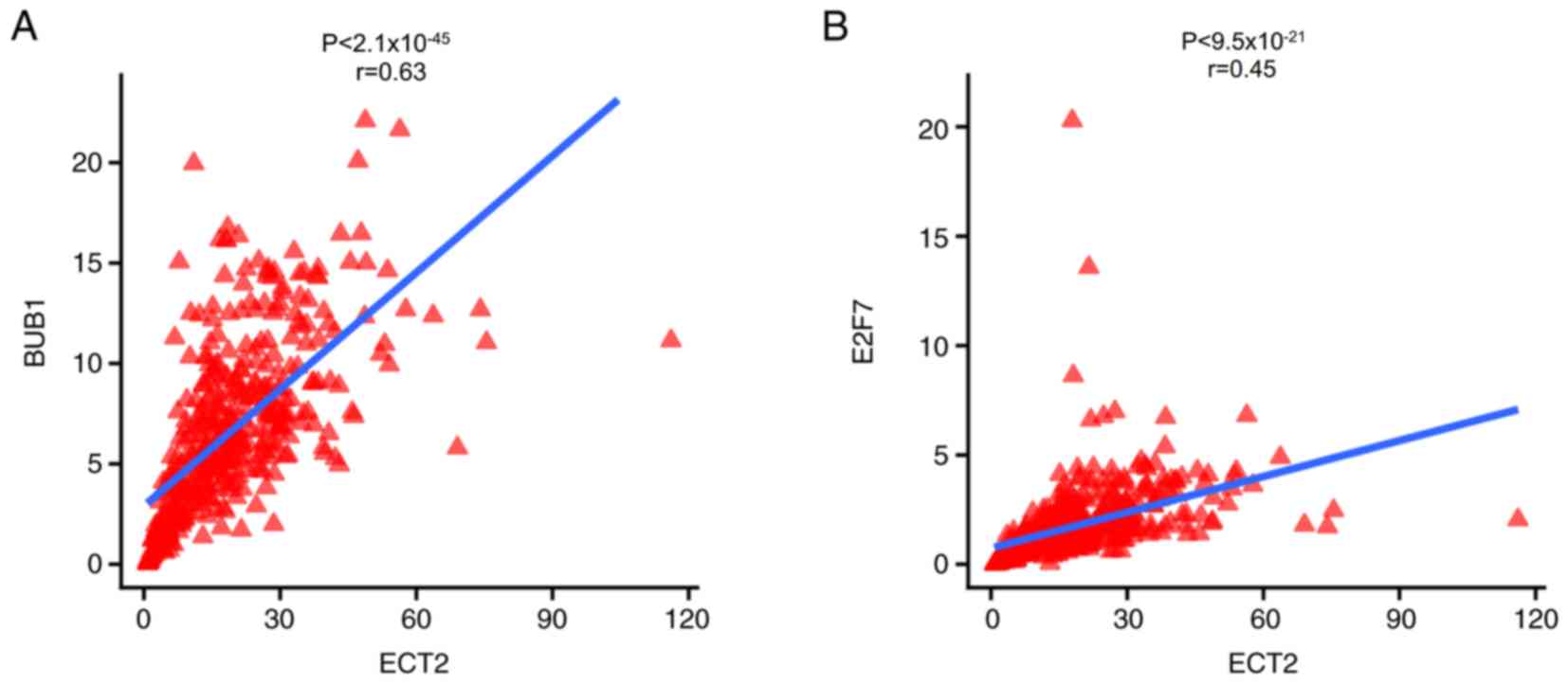
samples (Fig. 4B). A significant
correlation was identified between ECT2 and BUB1 mRNA expression
levels (r=0.63; P<0.0001; Fig.
5A), and between ECT2 and E2F7 mRNA expression levels in
gastric cancer tissues (r=0.45; P<0.0001; Fig. 5B).
BUB1 and E2F7 IHC staining was performed in gastric
cancer samples to verify these findings. The results demonstrated
that BUB1 protein expression was significantly higher in gastric
cancer tissues compared with adjacent normal tissues (IRS,
cancer=4.61±1.89 vs. normal=1.94±0.89; P<0.01; Fig. 6A and B). Similarly, E2F7 protein
expression was significantly higher in gastric cancer tissues
compared with adjacent normal tissues (IRS, cancer=4.01±1.75 vs.
normal=1.72±0.86; P<0.01; Fig. 6A and
C). Collectively, these results suggest that gastric tumors
with high ECT2 expression levels may possess transcriptional traits
of CSCs.
Discussion
Gastric cancer remains the third leading cause of
cancer-associated mortality worldwide (4). CSCs are implicated in different types
of cancer, including gastric cancer (1). The identification of gastric CSCs has
improved understanding of the molecular and cellular etiology of
gastric cancer, and may aid the development of effective
treatments. Experimentally, CSCs are characterized by their
capacity for tumor propagation (2).
CSCs are resistant to chemotherapy and radiotherapy and possess a
quiescent nature (23). Thus, these
cells play an important role in cancer recurrence (24). As a result, identification of
specific gastric CSCs and the detection of their expression level
will lead to the development of novel methods for the diagnosis and
treatment of gastric cancer, which can further improve the survival
rate of patients with the disease.
The results of the present study demonstrated that
ECT2 expression was upregulated in gastric cancer tissues compared
with adjacent normal tissues. This result was further verified
based on the transcriptomic data from several independent clinical
datasets. Consistent with the results of IHC analysis, ECT2 mRNA
expression levels were significantly increased in gastric cancer
tissues compared with adjacent normal tissues, suggesting that ECT2
upregulation may serve an important role in the malignant
progression of human gastric cancer.
The present study also investigated the biological
implications of ECT2 upregulation using GSEA. BUB1 and E2F7
upregulation have previously been demonstrated to play important
roles in essential cellular processes, such as cell proliferation
(25–27). It is speculated that BUB1 and E2F7
may be associated with transcriptional features of CSCs (20–22). A
previous study revealed that BUB1 depletion using shRNAs reduces
cancer stem cell potential of the MDA-MB-231 breast cancer cell
line, resulting in inhibited formation of xenografts in
immunocompromised mice (20). In
addition, overexpression of E2F7 significantly enhanced the
spheroid formation and growth rate of HepG2 and Huh7 cells
(hepatocellular carcinoma cell lines), and also decreased their
apoptosis (28). GSEA analysis
indicated that ECT2 expression was notably associated with the
transcriptional program of CSCs, with co-staining of BUB1 and E2F7
in gastric cancer tissues confirmed by IHC analysis.
The present study is not without limitations. First,
only IHC analysis was performed to determine ECT2 protein
expression, additional methods such as western blotting should be
considered in future studies. However, IHC can simultaneously
evaluate tissue expression localization, as well as morphology in
cancer tissues, and thus is the preferred approach in analysis of
clinical samples. Furthermore, future studies will focus on in
vitro experiments to better understand the molecular mechanisms
underlying ECT2 function in gastric cancer.
Carcinoembryonic antigen and cancer antigen 19-9
serve as the standard biomarkers for the diagnosis of gastric
cancer; however, their use in clinical practice is limited due to
low diagnostic sensitivity (29).
Although emerging candidate biomarkers, such as microRNA and DNA
methylation products have been extensively studied, several
challenges hinder their application in a clinical setting (30). The results of the present study
demonstrated that strong ECT2 positivity was significantly
associated with advanced TNM stage and deeper tumor invasion.
Furthermore, high ECT2 expression levels were associated with a
shorter overall survival time. Thus, ECT2 expression may serve as
an independent prognostic marker for the overall survival time of
patients with gastric cancer. Similar results were reported by
previous studies that demonstrated the prognostic value of ECT2 for
gastric cancer (31,32). Taken together, the results of the
present study suggest that upregulation of ECT2 predicts
unfavorable clinical outcomes of patients with gastric cancer.
Thus, ECT2 may serve as a potential prognostic marker and
therapeutic target for the management of gastric cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81803183, 81804066,
81904178 and 81873073), the Key Scientific Research Foundation of
Department of Science and Technology of Sichuan Province (grant no.
19ZX0161Z090116002), the Project of Sichuan Provincial
Administration of TCM (grant no. 2018QN022), the Science and
Technology Developmental Foundation of the Hospital of Chengdu
University of TCM (grant nos. 19TS03, 19LW05 and 19LW06), the
‘Xing-lin Scholars’ Project of Chengdu University of Traditional
Chinese Medicine (grant no. QNXZ2019017) and ‘Hundred Talents
Program’ of the Hospital of Chengdu University of Traditional
Chinese Medicine (grant nos. 20-Q03, 20-Q05 and 20-Q18).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors contributions
JHZ and SZC conceived and designed the present
study. TLY and YG collected and prepared the clinical samples. XC
and YW performed H&E staining and confirmed pathological
diagnosis. JHZ and DYG performed IHC and clinicopathological
characteristics analyses. SZC acquired, interpreted and analyzed
the GEO and GSEA data. DYG and SZC drafted the initial manuscript
and critically revised it for important intellectual content. All
authors have read and approved the final manuscript to be
published.
Ethics approval and consent to
participate
This retrospective study was approved by the
Institutional Review Board of the Teaching Hospital of Chengdu
University of TCM (Chengdu, China) (approval no. 2018KL-023) and
written informed consent was provided by all patients prior to the
study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shackleton M, Quintana E, Fearon ER and
Morrison SJ: Heterogeneity in cancer: Cancer stem cells versus
clonal evolution. Cell. 138:822–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCracken KW, Catá EM, Crawford CM,
Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence
JR, Zavros Y, et al: Modelling human development and disease in
pluripotent stem-cell-derived gastric organoids. Nature.
516:400–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao Y, Feng F and Zhou YN: Stem cells in
gastric cancer. World J Gastroenterol. 21:112–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maeda M, Yamashita S, Shimazu T, Iida N,
Takeshima H, Nakajima T, Oda I, Nanjo S, Kusano C, Mori A, et al:
Novel epigenetic markers for gastric cancer risk stratification in
individuals after Helicobacter pylori eradication. Gastric Cancer.
21:745–755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yaghoobi M, Bijarchi R and Narod SA:
Family history and the risk of gastric cancer. Br J Cancer.
102:237–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuo J, Kimura S, Yamamura A, Koh CP,
Hossain MZ, Heng DL, Kohu K, Voon DC, Hiai H, Unno M, et al:
Identification of stem cells in the epithelium of the stomach
corpus and antrum of mice. Gastroenterology. 152:218–231.e14. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tatsumoto T, Xie X, Blumenthal R, Okamoto
I and Miki T: Human ECT2 is an exchange factor for Rho GTPases,
phosphorylated in G2/M phases, and involved in cytokinesis. J Cell
Biol. 147:921–928. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan AM, McGovern ES, Catalano G, Fleming
TP and Miki T: Expression cDNA cloning of a novel oncogene with
sequence similarity to regulators of small GTP-binding proteins.
Oncogene. 9:1057–1063. 1994.PubMed/NCBI
|
|
10
|
Chen J, Xia H, Zhang X, Karthik S, Pratap
SV, Ooi LL, Hong W and Hui KM: ECT2 regulates the Rho/ERK
signalling axis to promote early recurrence in human hepatocellular
carcinoma. J Hepatol. 62:1287–1295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu B, Yang H, Taher L, Denz A, Grützmann
R, Pilarsky C and Weber GF: Identification of prognostic biomarkers
by combined mRNA and miRNA expression microarray analysis in
pancreatic cancer. Transl Oncol. 11:700–714. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou S, Wang P, Su X, Chen J, Chen H, Yang
H, Fang A, Xie L, Yao Y and Yang J: High ECT2 expression is an
independent prognostic factor for poor overall survival and
recurrence-free survival in non-small cell lung adenocarcinoma.
PLoS One. 12:e01873562017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sano M, Genkai N, Yajima N, Tsuchiya N,
Homma J, Tanaka R, Miki T and Yamanaka R: Expression level of ECT2
proto-oncogene correlates with prognosis in glioma patients. Oncol
Rep. 16:1093–1098. 2006.PubMed/NCBI
|
|
14
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch
CM, Winchester DP, Asare EA, Madera M, Gress DM and Meyer LR: AJCC
Cancer Staging Manual. 8th. Springer; New York, NY: 2016
|
|
15
|
Cho JY, Lim JY, Cheong JH, Park YY, Yoon
SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, et al: Gene expression
signature-based prognostic risk score in gastric cancer. Clin
Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang G, Hu N, Yang HH, Wang L, Su H, Wang
C, Clifford R, Dawsey EM, Li JM, Ding T, et al: Comparison of
global gene expression of gastric cardia and noncardia cancers from
a high-risk population in china. PLoS One. 8:e638262013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SY, Park C, Kim HJ, Park J, Hwang J,
Kim JI, Choi MG, Kim S, Kim KM and Kang MS: Deregulation of immune
response genes in patients with Epstein-Barr virus-associated
gastric cancer and outcomes. Gastroenterology. 148:137–147.e9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Yu B, Li J, Su L, Yan M, Zhang J, Li
C, Zhu Z and Liu B: Characterization of differentially expressed
genes involved in pathways associated with gastric cancer. PLoS
One. 10:e01250132015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han JY, Han YK, Park GY, Kim SD and Lee
CG, Jo WS and Lee CG: Bub1 is required for maintaining cancer stem
cells in breast cancer cell lines. Sci Rep. 5:159932015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Venere M, Miller TE and Rich JN: Mitotic
control of cancer stem cells. Cancer Discov. 3:141–144. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mariani SA, Minieri V, De Dominici M,
Iacobucci I, Peterson LF and Calabretta B: CDKN2A-independent role
of BMI1 in promoting growth and survival of Ph+ acute lymphoblastic
leukemia. Leukemia. 30:1682–1690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hur W and Yoon SK: Molecular pathogenesis
of radiation-induced cell toxicity in stem cells. Int J Mol Sci.
18:27492017. View Article : Google Scholar
|
|
24
|
Moore N and Lyle S: Quiescent,
slow-cycling stem cell populations in cancer: A review of the
evidence and discussion of significance. J Oncol. 2011:3960762011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gjoerup OV, Wu J, Chandler-Militello D,
Williams GL, Zhao J, Schaffhausen B, Jat PS and Roberts TM:
Surveillance mechanism linking Bub1 loss to the p53 pathway. Proc
Natl Acad Sci USA. 104:8334–8339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu B, Xu T, Liu H, Min Q, Wang S and Song
Q: miR-490-5p suppresses cell proliferation and invasion by
targeting BUB1 in hepatocellular carcinoma cells. Pharmacology.
100:269–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitxelena J, Apraiz A, Vallejo-Rodríguez
J, Malumbres M and Zubiaga AM: E2F7 regulates transcription and
maturation of multiple microRNAs to restrain cell proliferation.
Nucleic Acids Res. 44:5557–5570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma YS, Lv ZW, Yu F, Chang ZY, Cong XL,
Zhong XM, Lu GX, Zhu J and Fu D: MicroRNA-302a/d inhibits the
self-renewal capability and cell cycle entry of liver cancer stem
cells by targeting the E2F7/AKT axis. J Exp Clin Cancer Res.
37:2522018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan YQ, Ruan YY, Peng JB, Han QY, Zhang X,
Lin A and Yan WH: Diagnostic significance of soluble human
leukocyte antigen-G for gastric cancer. Hum Immunol. 77:317–324.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toiyama Y, Okugawa Y and Goel A: DNA
methylation and microRNA biomarkers for noninvasive detection of
gastric and colorectal cancer. Biochem Biophys Res Commun.
455:43–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin Y, Yu Y, Shao Q, Ma Y, Zhang R, Yao H
and Xu Y: Up-regulation of ECT2 is associated with poor prognosis
in gastric cancer patients. Int J Clin Exp Pathol. 7:8724–8731.
2014.PubMed/NCBI
|
|
32
|
Wang HB, Yan HC and Liu Y: Clinical
significance of ECT2 expression in tissue and serum of gastric
cancer patients. Clin Transl Oncol. 18:735–742. 2016. View Article : Google Scholar : PubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBI
|