Introduction
Gliomas, which arise from transformed glial cells,
are the most common, lethal type of primary tumor in the central
nervous system, accounting for 60–70% of primary brain tumors and
80% of all malignant brain tumors (1,2).
Previous studies have reported that glioma has an annual incidence
of ~6/100,000 cases worldwide, with a high recurrence rate due to
its diffuse invasive characteristics and malignant behavior, which
results in a poor prognosis (3–5).
Although neurosurgery combined with radio- and chemotherapy has
contributed to considerable progress in the treatment of the
glioma, the median survival time for patients with glioma remains
at 12–15 months (6,7). Hence, there remains an urgent
requirement to identify novel treatment strategies and effective
therapeutic agents to extend and improve the survival and quality
of life of patients.
Increasing evidence has established that chronic
inflammation influenced almost every aspect of cancer progression
(8–10) and increased the risk of cancer
(11,12), such as the case with
meningitis-associated malignant brain tumors (13). Inflammatory cells and molecules exist
in the tumor microenvironment, which promote tumor development and
progression, such as tumor growth, cell metastasis and even
inflammation (14,15). Cyclooxygenase-2 (COX-2), one of the
pivotal factors in the progress of inflammation, has been causally
associated with the progression of numerous types of human tumor,
including gliomas, lung cancer and breast cancer (9,16,17). In
addition, COX-2 was discovered to be highly expressed in patients
with glioma or glioma specimens (18), and was associated with the degree of
tumor invasiveness and the prognosis of patients (19,20).
Hence, the suppression of COX-2 expression with specific small
molecule inhibitors may represent a potential therapeutic approach
for the suppression of glioma development.
In the past few years, researchers have paid
increasing attention to traditional Chinese medicines, due to their
demonstrated potential to treat various types of cancer with low
toxicity, such as lung cancer, breast cancer and glioma (9,17,21).
Micheliolide (MCL) is a sesquiterpene lactone isolated from
Michelia compressa and Michelia champaca (22). Previous studies have demonstrated
that MCL exerted various therapeutic effects in cancer,
inflammation, immunomodulatory acute myelogenous leukemia and renal
fibrosis (22–26) through numerous signaling pathways,
including PI3K/Akt, NF-kB and MAPK signaling.
Dimethylaminomicheliolide (DMAMCL), the prodrug of MCL, has been
approved by the US Food and Drug Administration for its apparent
efficacy in the treatment of pleomorphic glioblastoma (27,28). In
China, DMAMCL is currently undergoing clinical trials for the
treatment of several advanced or metastatic solid carcinomas,
including gliomas (29). However, as
an active molecule of DMAMCL, the therapeutic potential of MCL in
gliomas and its underlying mechanisms remain unknown.
The findings of the present study suggested that MCL
may potentially exert therapeutic effects against human glioma
through promoting cell apoptosis and suppressing the NF-κB/COX-2
signaling pathway. Thus, the present study highlighted the novel
roles of MCL in the treatment of human gliomas.
Materials and methods
Chemicals and reagents
MCL was purchased from Sigma-Aldrich; Merck KGaA.
Temozolomide (TMZ) was purchased from Sigma-Aldrich; Merck KGaA.
MCL stock solution was dissolved in DMSO and stored at −20°C. Prior
to use, the stock solution was diluted with DMEM medium (Gibco;
Thermo Fisher Scientific, Inc.), and the final concentration of
DMSO was adjusted to <0.1%. The control group was treated with
carrier solvent (0.1% DMSO).
Antibodies and other materials
The following primary antibodies were purchased from
Cell Signaling Technology, Inc.: Anti-cleaved caspase-3 (cat. no.
9664), anti-cleaved caspase-9 (cat. no. 7237), anti-COX-2 (cat. no.
12282), anti-phosphorylated (p)-IκBα (cat. no. 2859), anti-IκBα
(cat. no. 9242) and anti-β-actin (cat. no. 3700). Anti-rabbit IgG,
HRP-linked antibody (cat. no. 7074; 1:5,000) and anti-mouse IgG,
HRP-linked Antibody cat. no. 7076; 1:5,000) secondary antibodies
were also purchased from Cell Signaling Technology, Inc. The
following primary antibodies were purchased from ProteinTech Group,
Inc.: Anti-cytochrome c (cat. no. 66264-1-Ig), anti-Bcl-2
(cat. no. 12789-1-AP), anti-Bax (cat. no. 50599-2-Ig),
anti-Vimentin (cat. no. 10366-1-AP), anti-matrix metalloproteinase
(MMP)-9 (cat. no. 10375-2-AP) and anti-N-cadherin (cat. no.
22018-1-AP). DMEM, FBS and trypsin were purchased from Gibco;
Thermo Fisher Scientific, Inc. Other chemicals (such as NaCl and
KCl) were obtained from Sigma-Aldrich; Merck KGaA.
Cell culture
The human glioma cell line U251MG was obtained from
the American Type Culture Collection. U251MG cells were cultured in
DMEM, supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin, and maintained at 37°C in a humidified atmosphere
with 5% CO2. The authenticity of U251MG cells was
verified via genomic short tandem repeat profiling by Shanghai
Zhongqiao Xizhou Biotechnology Co., Ltd., and the cells were
confirmed to be free of mycoplasma using a Mycoplasma Detection kit
(cat. no. MB000-1591) with UDG PCR Mix and Loading Dye (Excell
Biotech, http://www.excellbio.com/productcenter/info.aspx?itemid=274&Lcid=42).
Cell viability assay
Briefly, 6×103 U251MG cells/well were
seeded into 96-well plates in 100 µl media. Cells were
allowed to adhere overnight and then treated with different
concentrations of MCL (0, 2.5, 5, 10 or 20 µM) for 24 and 48
h at 37°C. Subsequently, 10 µl Cell Counting Kit-8 (CCK-8) buffer
(Dojindo Molecular Laboratories, Inc.) was added into each well and
incubated for 1.5 h at 37°C according to the manufacturer's
protocol. The absorbance at a wavelength of 450 nm was measured
using a microplate reader (PerkinElmer, Inc.).
Colony formation assay
U251MG cells were incubated with 0, 5, 10 or 15
µM MCL for 24 h at 37°C and then digested into single cells
by trypsinization. U251MG cells were seeded into 6-well plates at a
density of 1.5×103 cells/well. Following incubation for
14 days at 37°C with 5% CO2, the colonies were washed
with PBS and fixed with the mixture buffer (methanol: glacial
acetic acid, ddH2O=1:1:8) for 10 min at room
temperature, then stained with 0.1% crystal violet for 30 min at
room temperature. The number of colonies (diameter >1 mm) were
counted using an EVOS XL Core inverted light microscope inverted
light microscope (magnification, ×4; Thermo Fisher Scientific,
Inc.). The colonies were then photographed using HP DeskJet 2132
(Hewlett-Packard Development Company, L.P.).
Wound healing assay
A wound healing assay was performed to analyze cell
migration. Briefly, U251MG cells (1.0×105) were seeded
into six-well plates and cultured to 100% confluence. Subsequently,
the complete DMEM medium was replaced with serum-free DMEM medium
and incubated for 6 h at 37°C. The confluent cell monolayer was
then scratched with a sterile 100-µl pipette tip and treated with
0, 5, 10 or 15 µM MCL. The wounds were visualized at 0 h and after
incubation with MCL for 48 h at 37°C using a Leica DM 14000B light
microscope (magnification, ×200).
Transwell invasion assay
The upper surface of Transwell plates were precoated
with Matrigel and incubated for 30 min at 37°C for gelation.
Briefly, 5×104 U251MG cells/well were plated into the
upper chambers of Transwell plates in 400 µl serum-free medium and
the lower chambers were filled with 600 µl DMEM supplemented with
10% FBS; both the upper and lower chambers contained 0, 5, 10 or 15
µM MCL. Following 24 h of incubation at 37°C, the inside surface of
the upper membranes was wiped with cotton swabs to remove
non-invasive cells. The invasive cells in the lower chamber were
fixed with methanol (100%) at room temperature for 10 min and
stained with 0.1% crystal violet at room temperature for 30 min.
Stained cells were visualized using a Leica DM 14000B light
microscope (magnification, ×200) in five randomly selected fields
of view.
Confocal immunofluorescence imaging
(IFI) analysis
The IFI analysis was performed as previously
described (9). Briefly, U251MG cells
(1×105 cells/well) were seeded onto coverslips in 6-well
plates and treated with 0, 5, 10 or 15 µM MCL for 48 h at 37°C.
Subsequently, the cells were stained with MitoTracker™
Red CMXRos (cat. no. M7512; Invitrogen; Thermo Fisher Scientific,
Inc.) for 30 min at 37°C. Cells were then incubated with an
anti-cytochrome c primary antibody (1:200) at 4°C overnight.
Following the primary antibody incubation, the cells were incubated
with an anti-mouse IgG Alexa Fluor 488-conjugated secondary
antibody (1:1,000; cat. no. 4408; Cell Signaling Technology, Inc.)
for 1 h in the dark at room temperature. The nuclei were
counterstained with DAPI for 5 min in the dark at room temperature
and fluorescent slides were visualized using a Leica DM 14000B
confocal microscope (magnification, ×630).
Western blotting
U251MG cells (1×105) were treated with
MCL (0, 5, 10 and 15 µM) for 48 h at 37°C, and then total protein
was extracted by RIPA lysis buffer and quantified using a
bicinchoninic acid assay (Beyotime Institute of Biotechnology).
Protein (30–50 µg) was separated via 10–12% SDS-PAGE. The separated
proteins were subsequently transferred onto a PVDF membrane and
blocked with 5% non-fat dry milk (Beyotime Institute of
Biotechnology) for 1h at room temperature. The membranes were then
incubated at 4°C overnight with the following primary antibodies:
Anti-cleaved caspase-3 (1:1,000), anti-cleaved caspase-9 (1:1,000),
anti-COX-2 (1:1,000), anti-p-IκBα (1:500), anti-IκBα (1:1,000),
anti-β-actin (1:2,000), anti-Bcl-2 (1:2,000), anti-Bax (1:2,000),
anti-Vimentin (1:1,500), anti-MMP-9 (1:800) and anti-N-cadherin
(1:500). Membranes were washed three times with 1X Tris-buffered
saline with 0.1% Tween (Beyotime Institute of Biotechnology) and
incubated with the anti-rabbit IgG (1:5,000; cat. no. 7074; Cell
Signaling Technology, Inc.) or anti-mouse IgG (1:5,000; cat. no.
7076; Cell Signaling Technology, Inc.) secondary antibodies for 2 h
at room temperature. Protein bands were visualized using an
enhanced chemiluminescence reagent (Beyotime Institute of
Biotechnology) and semi-quantification was performed using
ImageQuant TL 7.0 software (GE Healthcare).
Reverse transcription (RT-PCR)
Total RNA was extracted from U251MG cells
(1×105), which were treated with MCL (0, 5, 10 and 15
µM) for 48 h at 37°C, using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The RNA concentration was determined using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.) and total RNA
was reverse transcribed into cDNA using the PrimeScript RT Reagent
kit (cat. no. RR037A; Takara Bio, Inc.), according to the
manufacturer's protocol. The following primer pairs were used for
the PCR: COX-2 forward, 5′-TCACAGGCTTCCATTGACCAG−3′ and reverse,
5′-CCGAGGCTTTTCTACCAGA-3′; and GAPDH forward,
5′-TCTTCGCTTTGTCCTTCGT-3′ and reverse, 5′-TGCTGTAGCCAAATTCGTTG-3′.
The conditions of the PCR were as follows: 94°C for 2 min, 35
cycles at 98°C for 10 sec, 60°C for 30 sec and 68°C for 15 sec, and
68°C for 5 min. Amplification products were analyzed using 1.5%
agarose gel electrophoresis stained with GoldenView™ (Dalian Meilun
Biology Technology Co., Ltd.) and photographed under ultraviolet
light. ImageJ software (National Institutes of Health, version
1.8.0) was used to verify the band intensities as a result of
semi-quantitative RT-PCR (30).
Flow cytometric analysis of
apoptosis
Flow cytometric analysis was performed to analyze
the percentage of sum of early and late apoptotic U251MG cells.
Briefly, U251MG cells (1×105), which were treated with
MCL (0, 5, 10 and 15 µM) for 48 h at 37°C, were stained with
Annexin V-FITC and propidium iodide using the Annexin V-FITC
Apoptosis Detection kit (cat. no. C1062S; Beyotime Institute of
Biotechnology) for 15 min at room temperature, according to the
manufacturer's protocol. Apoptotic cells were subsequently analyzed
by a BD Accuri™ C6 flow cytometer (BD Biosciences) using
the software program BD Accuri™ C6 Plus (version 1.0.227.4).
Statistical analysis
SPSS 17.0 software (SPSS, Inc.) was used for
statistical analysis. All data are presented as the mean ± standard
deviation and each experiment was performed at least three times.
Statistical significances between different groups were determined
using a one-way ANOVA, followed by a Tukey's post hoc test for
multiple comparisons, and paired Student's t-test for two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MCL inhibits viability and alters cell
morphology in human glioma cells and TMZ inhibits viability in
human glioma cells
To investigate the tumor suppressive effect of MCL,
(3aS)-3aβ,4,5,7,8,9,9aβ,9bα-Octahydro-9β-hydroxy-6,9-dimethyl-3-methyleneazuleno[4,5-b]furan-2(3H)-one
(Fig. 1A), the effects of MCL on
cell viability in human glioma U251MG cells were determined using a
CCK-8 assay. MCL inhibited the cell viability of U251MG cells in
both a time- and dose-dependent manner (Fig. 1B), and the IC50 for 48 h was
12.586±1.632 µM. In order to further investigate the effects of MCL
on cell phenotype, mRNA and protein, we choose 5, 10, 15 µM MCL for
the next experiment. The effect of MCL on cell morphology was also
investigated in U251MG cells; compared with the 0 µM MCL group, MCL
treatment (10 and 15 µM) induced cell wrinkles, reduced cytoplasm
and reduced the formation of filopodia (Fig. 1C). To investigate the anti-tumor
ability of TMZ, the cell viability of human glioma U251MG cells was
determined using a CCK-8 assay. As shown in Fig. 1D, cell viability of U251MG cells was
reduced in a dose-dependent manner with TMZ treatment, and the IC50
for 48 h was 445.823±59.625 µM. The results indicated that MCL may
exhibit marked antitumor effects in human glioma.
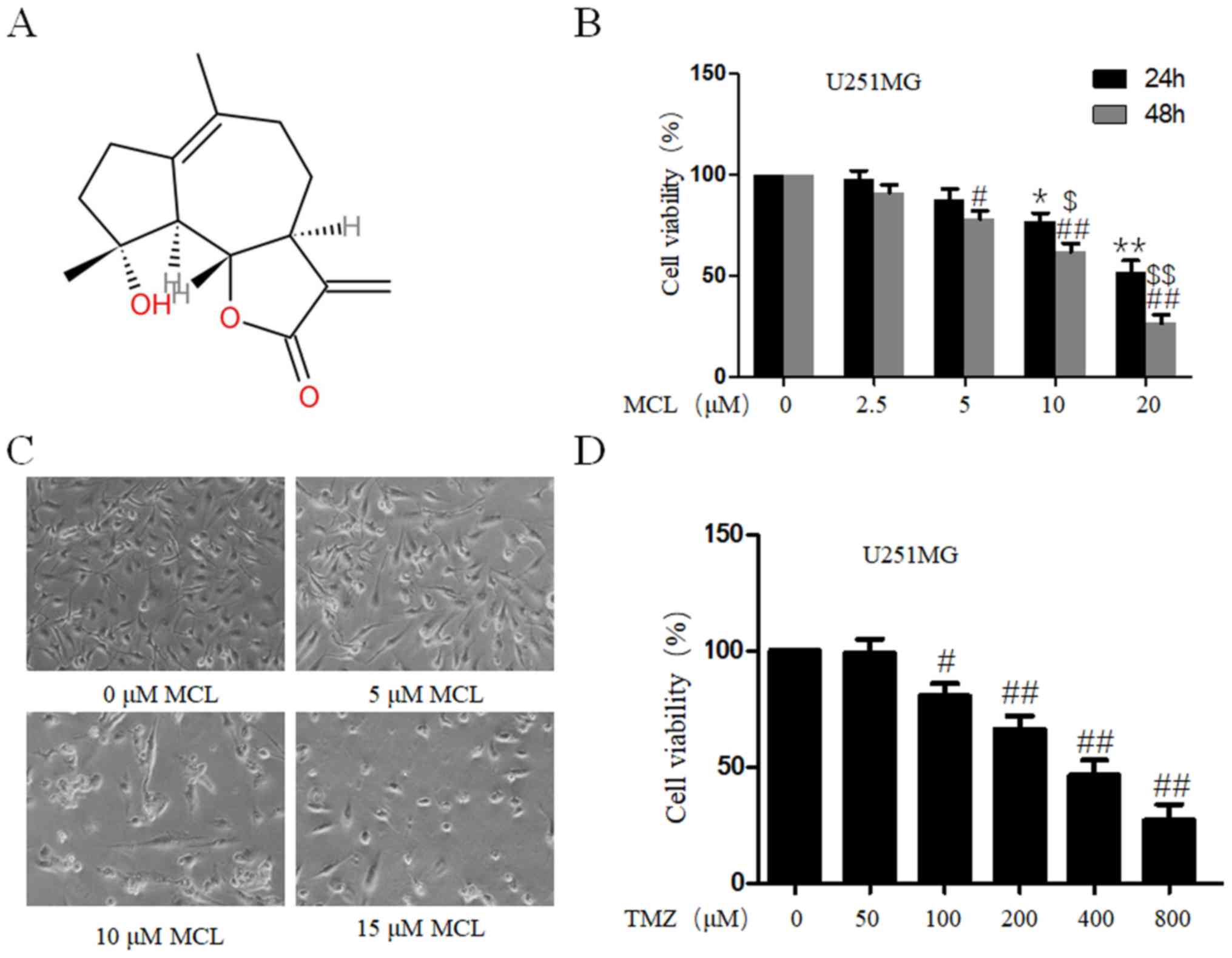 | Figure 1.Effects of MCL on cell viability and
morphology. (A) Chemical structure of MCL. (B) U251MG cells were
treated with MCL at the indicated doses (0, 2.5, 5, 10 or 20
µM). Following treatment for 24 and 48 h, cell viability was
determined using a Cell Counting Kit-8 assay. Cells treated with
vehicle control (DMSO) were used as the reference group (viability
set at 100%). (C) Changes in cell morphology of U251MG cells
treated with MCL (0, 5, 10 and 15 µM) were observed. Cells
were photographed using a microscope fitted with a digital camera
(magnification, ×100). (D) U251MG cells were treated with TMZ at
the indicated doses (0, 50, 100, 200, 400 or 800 µM).
Following treatment for 48 h, cell viability was determined using
the Cell Counting Kit-8 assay. Cells treated with vehicle control
(DMSO) were used as the reference group (viability set at 100%).
Data are presented as the mean ± standard deviation of three times
independent experiments. *P<0.05 and **P<0.01 vs. control
group at 24h; #P<0.05 and ##P<0.01 vs.
control group at 48 h; $P<0.05 and
$$P<0.01 vs. 24 h group. MCL, micheliolide; TMZ,
Temozolomide; DMSO, dimethyl sulfoxide. |
MCL inhibits clonogenesis, and the
migratory and invasive ability of U251MG cells
The influence of MCL on the clonogenic ability of
U251MG cells was evaluated. Consistent with the inhibition over
cell viability, MCL treatment significantly inhibited the colony
formation ability in a dose-dependent manner compared with the
control group (Fig. 2A and B). The
inhibitory effects of MCL treatment on tumor cell migration and
invasion in U251MG cells were subsequently investigated using wound
healing and Transwell Matrigel assays, respectively. The migratory
ability of U251MG cells was markedly suppressed in a dose-dependent
manner following the treatment of 5, 10 or 15 µM compared with the
control group (Fig. 2C). The
Transwell Matrigel assay also revealed similar results following
MCL treatment (Fig. 2D).
Furthermore, to analyze the underlying mechanisms of MCL on cell
migration, the relative protein expression levels of MMP-9,
N-Cadherin and Vimentin were analyzed. The expression levels of
these proteins were downregulated a dose-dependent manner following
MCL treatment compared with the control group (Fig. 2E and F). The results suggested that
MCL not only inhibited cell viability, but also inhibited cell
migration and invasion.
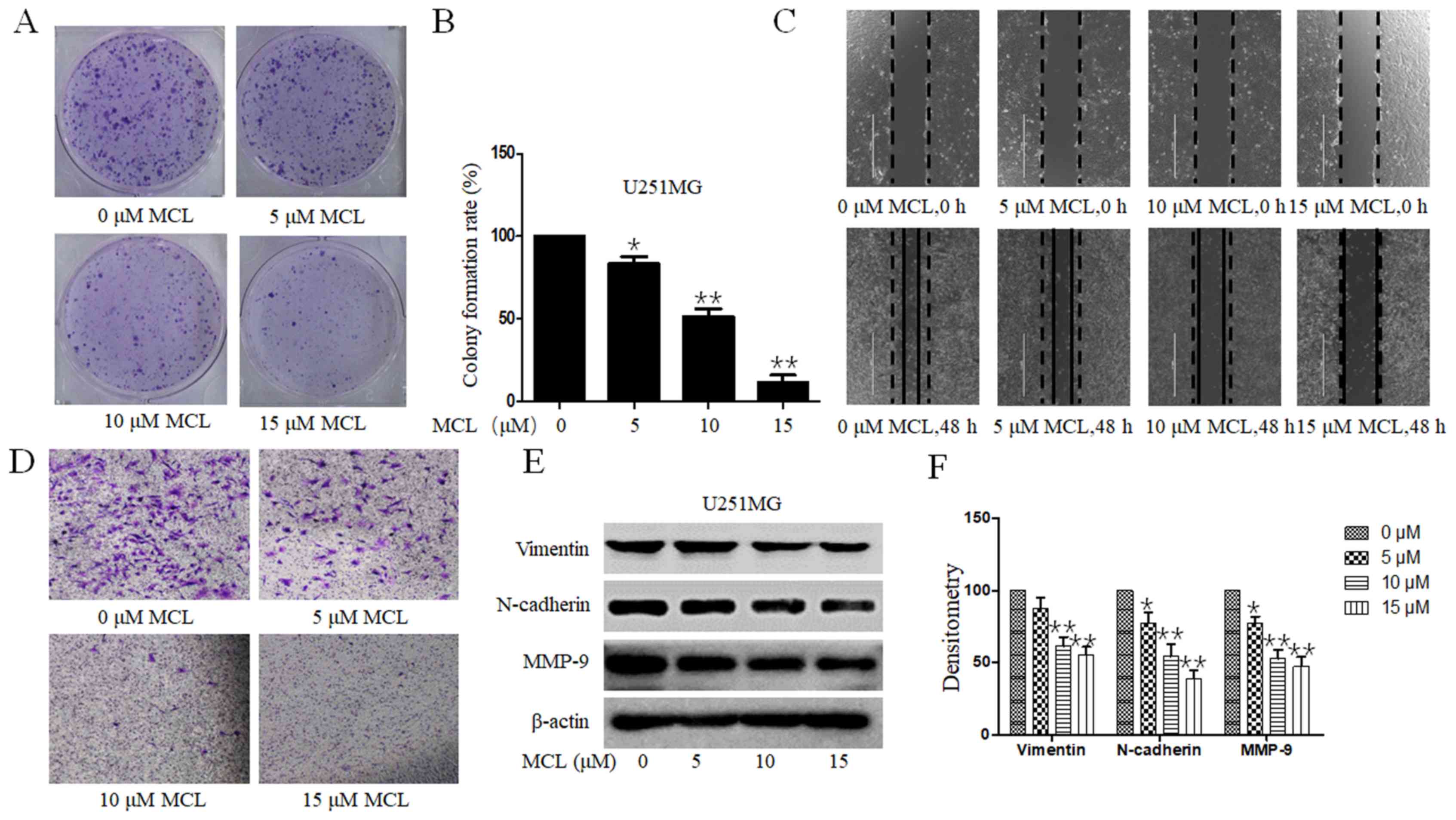 | Figure 2.Effects of MCL on colony formation,
cell migration and invasion. (A) U251MG cells were treated with MCL
(0, 5, 10 or 15 µM) for 24 h. Following culture for 2 weeks,
the colony formation of cells was (A) imaged using HP DeskJet 2132
(magnification, 1X) and the (B) colony formation rate was
calculated. (C) Effects of MCL on cell migration were analyzed
using a wound healing assay. Following 48 h of treatment with MCL
(0, 5, 10 or 15 µM), the wound gap was visualized under a
microscope. Black dotted line and solid line represent the wound
edge of 0 and 48 h, respectively. White solid line represents the
scale bars (1,000 µm). (D) Effects of MCL on cell invasion
were analyzed using a Transwell Matrigel assay. Following treatment
for 24 h, the invasive cells were visualized using a microscope
(magnification, ×200). (E) Expression levels of levels of MMP-9,
N-cadherin and Vimentin were analyzed using western blotting. (F)
Quantitative analysis of proteins. Data are presented as the mean ±
standard deviation of three times independent experiments.
*P<0.05 and **P<0.01 vs. 0 µM MCL, micheliolide; MMP-9,
matrix metalloproteinase 9. |
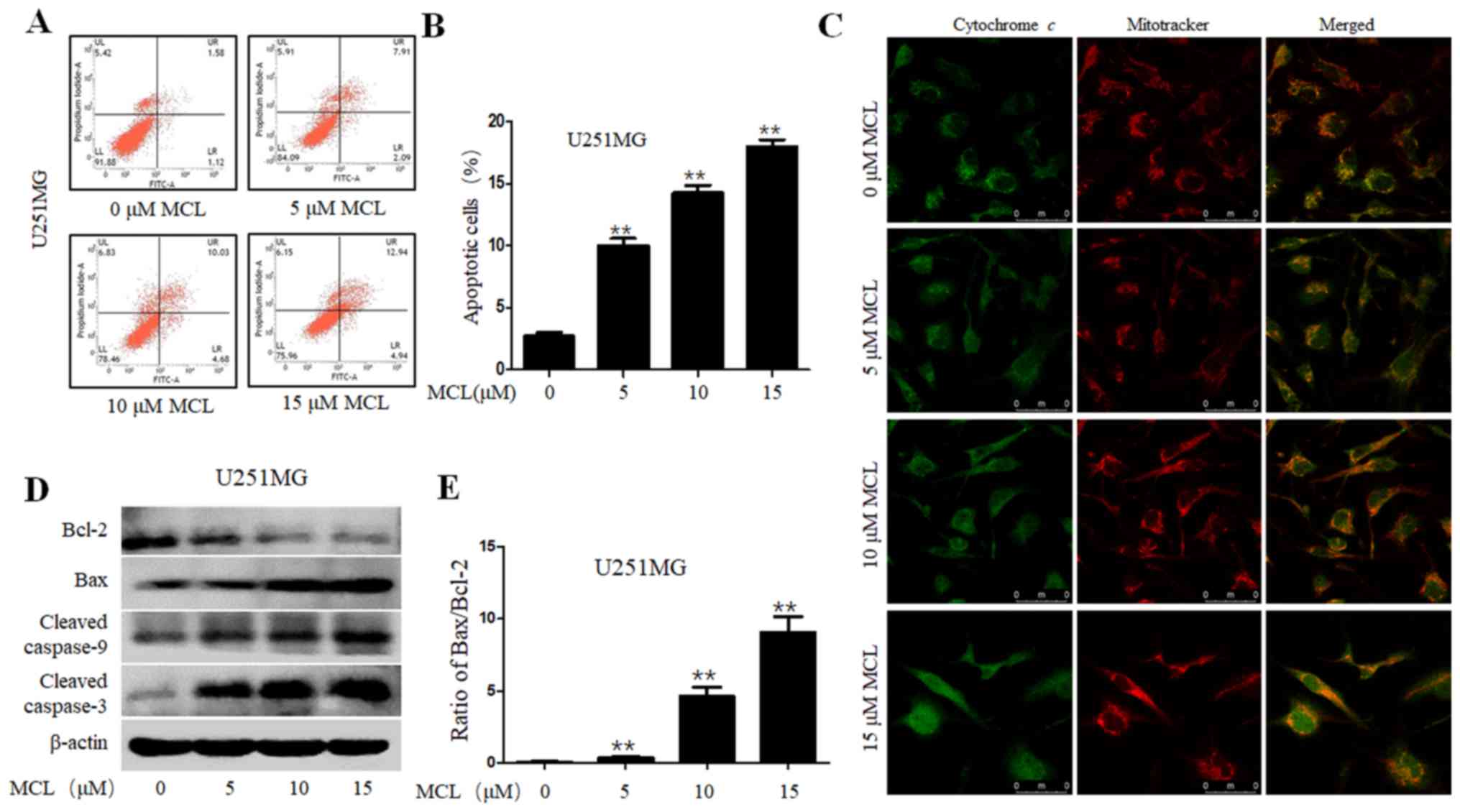
MCL promotes apoptosis through
modulating cytochrome c and caspase signaling
Apoptosis induction has been identified as a
therapeutic target for all types of cancer treatments (31). Thus, the present study aimed to
determine whether the inhibition over cell viability induced by MCL
was related to the activation of the apoptotic pathway. The results
revealed that MCL significantly induced apoptosis in a
dose-dependent manner, from 2.7% in the control group to 17.9% in
the 15 µM MCL group (Fig. 3A and B).
Previous studies have reported that cytochrome c released
into the cytoplasm from the mitochondrial intermembrane activated
cell apoptosis (32). Thus, IFI
analysis was performed to determine whether MCL could promote
cytochrome c release in U251MG cells, by detecting the
co-localization of cytochrome c and mitochondria. The IFI
results revealed that MCL treatment triggered cytochrome c
release from the inter-mitochondrial space into the cytosol
(Fig. 3C). Furthermore, to determine
the mechanisms underlying MCL-induced cell apoptosis, the
expression levels of cell apoptosis-related proteins, such as
caspase-3, caspase-9, Bax and Bcl-2, were also analyzed using
western blotting in U251MG cells. Compared with the control group,
MCL treatment upregulated the protein expression levels of cleaved
caspase-3 and −9 and Bax, while downregulating Bcl-2 protein
expression levels, and the Bax/Bcl-2 ratio was also upregulated in
a dose-dependent manner (Fig. 3D and
E). These results indicated that MCL may promote cell apoptosis
through triggering cytochrome c release into the cytoplasm
and facilitating the activation of multiple caspase cascades.
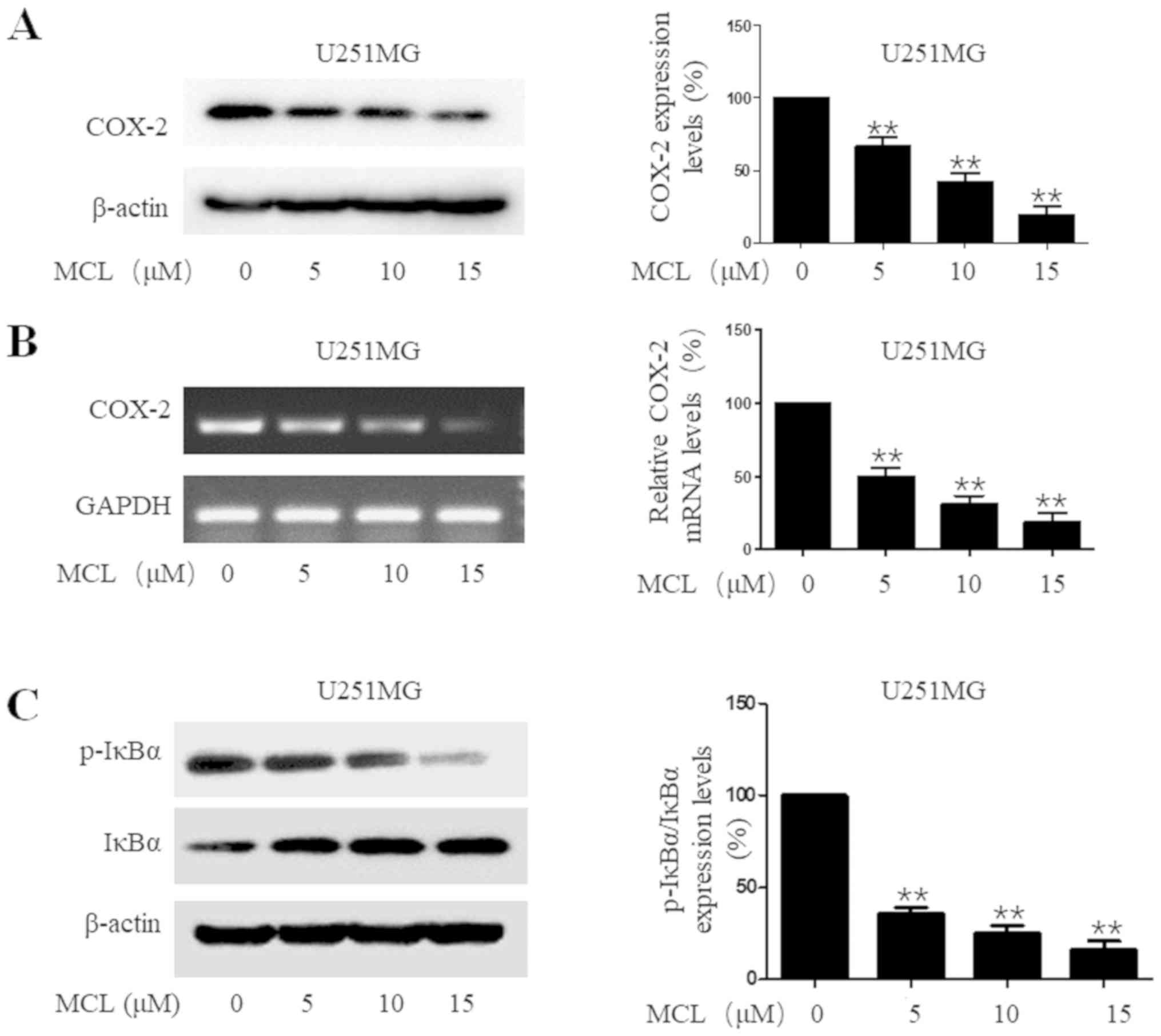
MCL downregulates COX-2 expression
levels and NF-κB activity
COX-2 is often induced by inflammatory signals and
has been identified to promote tumor development (33). Accumulating evidence has indicated
that COX-2 expression levels are markedly upregulated in a high
percentage of gliomas (33,34). Thus, the effects of MCL on COX-2
expression levels were investigated. MCL treatment significantly
downregulated COX-2 expression levels at both the protein and mRNA
level in a dose-dependent manner in U251MG cells compared with the
control group (Fig. 4A and B). Since
COX-2 expression levels were discovered to be tightly controlled by
the transcription factor NF-κB in numerous types of cancer
(35,36), the effect of MCL on NF-κB activity
was also investigated, through determining the degradation of IκBα,
an essential event for the activation of NF-κB signaling (37), which occurs through its
phosphorylation. The results demonstrated that MCL treatment
significantly downregulated the ratio of p-IκBα/IκBα protein
expression levels compared with the control group. Meanwhile, on an
individual basis, MCL treatment downregulated the expression levels
of p-IκBα, while upregulating the expression levels of IκBα
(Fig. 4C). Based on these results,
it was suggested that MCL may suppress the degradation of IκBα and
inhibit the activation of the NF-κB signaling pathway.
Overall, these findings supported the hypothesis
that MCL may exhibit a strong inhibitory effect on NF-κB/COX-2
signaling and suppress the proliferation of human glioma
carcinoma.
Discussion
In the past few years, Chinese medical herbs have
received increasing attention due to their long history of clinical
application, low toxicity and therapeutic potential in various
types of disease, including numerous types of cancer, such as lung
cancer, breast cancer and glioma (9,17,21). MCL
is an extract isolated from Michelia compressa and
Michelia champaca, which has been discovered to possess
various physiological and pharmacological properties, such as
anti-inflammatory and anticancer activities via various signaling
pathways, such as PI3K/Akt, NF-kB and MAPK signaling (22–26).
However, to the best of our knowledge, its detailed mechanisms of
action have not been fully elucidated.
The results of the present study revealed that MCL
exerted antitumor effects in human glioma. Previous research has
reported that MCL exhibited significant anticancer effects through
activating the cytochrome c/caspase-dependent apoptotic
pathway, thereby downregulating the expression levels of COX-2 by
inactivating NF-κB signaling (38,39). To
the best of our knowledge, the current study was the first report
demonstrating the therapeutic effect of MCL on COX-2 expression
levels and its underlying mechanism of action in glioma.
In the present study, MCL inhibited cell viability
in human glioma cells at dose of 5, 10 and 15 µM, demonstrating a
48 h half maximal inhibitory concentration (IC50) value
of 12.586±1.632 µM; however, although 12.586±1.632 µM MCL inhibited
the survival of tumor cells, to study the antitumor mechanism of
MCL, only survival cells were collected and used in western
blotting and RT-PCR experiments. Thus, the present study used MCL
at concentrations of 5, 10 or 15 µM, and these concentrations did
not affect the results. The results of the present study suggested
that MCL may inhibit the migration and invasion of U251MG cells
through downregulating the expression levels of N-cadherin,
Vimentin and MMP-9. To observe the effects of MCL treatment on cell
morphology, the cell density was required to be low; however, for
the wound healing assay, U251MG cells were cultured to 100%
confluence as a high cell density was required. This provides
reasoning for why the cell morphology was different for these two
assays following the treatment with 15 µM MCL.
Inflammation is involved in multiple different
stages of tumor development, including the initiation, promotion
and migration (40,41). In previous studies, the expression
levels of COX-2 were reported to be significantly overexpressed in
patients with glioma, positively correlated with glioma malignancy
and negatively correlated with prognosis (19,20). In
the current study, MCL significantly downregulated COX-2 expression
levels, alongside inhibiting U251MG cell viability and migration,
while inducing cell apoptosis. COX-2 expression levels are strictly
transcriptionally controlled by several regulatory elements,
including NF-κB, which serves important roles in COX-2 promoter
activity (42). However, in the
canonical pathway, inactive NF-κB residues are sequestered in the
cytoplasm through association with inhibitory proteins such as IκB
(37). IκB degradation is a crucial
step for NF-κB activation; IkBs are rapidly phosphorylated by the
active IκB kinase complex and further ubiquitinated by the
proteasome (43). Consequently,
activated NF-κB proteins (p50/p65 heterodimers) translocate from
the cytoplasm to the nucleus, where they bind to the promoters of
target genes and regulate their expression, such as COX-2 (9). Thus, inhibiting NF-κB activation may be
a beneficial treatment strategy for human gliomas. In the present
study, the results demonstrated that MCL treatment inhibited the
phosphorylation of IκBα, suggesting attenuation of the NF-κB
signaling pathway, thereby downregulating COX-2 expression levels,
as observed.
Over the past decade, temozolomide (TMZ) has been
commonly used as an effective imidazotetrazine agent against glioma
(44,45). The present study investigated the
inhibitory effects of TMZ on cell proliferation in human glioma
U251MG cells; following 48 h, the IC50 value of TMZ was
445.823 µM, while the IC50 value of MCL in the present
study was 12.586 µM. Furthermore, the antitumor activity was
increased ~35-fold following MCL treatment. These findings
indicated that MCL may be an effective and alternative antitumor
agent for the treatment of human glioma carcinomas.
In conclusion, the results of the present study
revealed the potential of MCL to effectively inhibit the biological
properties of human gliomas in a number of ways, that is inhibition
of invasion/migration and the NF-κB signaling pathway. In addition,
the results discovered that the anticancer properties of MCL may be
mediated, at least in part, via inhibition of the NF-κB/COX-2
signaling pathway. Thus, the present findings provided preclinical
evidence for the development of MCL as a potential agent for the
treatment of human glioma carcinomas.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health
Commission of Hubei Province Science Research Project (grant nos.
WJ2019H544 and WJ2019H557) and Three Gorges University Hubei Key
Laboratory of Tumor Microenvironment and Immunotherapy (grant no.
2019KZL07).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JZ and FS conceived and designed the experiments.
DF, ML, YL, XJZ, HS and XZ performed the experiments. DF, ML and YL
conducted the statistical analysis. DF, ML and XJZ drafted the
initial manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McNeill KA: Epidemiology of brain tumors.
Neurol Clin. 34:981–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu J, Cao X, Pang D, Luo Q, Zou Y, Feng B,
Li L, Chen Z and Huang C: Tumor grade related expression of
neuroglobin is negatively regulated by PPARgamma and confers
antioxidant activity in glioma progression. Redox Biol. 12:682–689.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sabo B: Primary malignant brain tumours,
psychosocial distress and the intimate partner experience: What do
we know? Can J Neurosci Nurs. 36:9–15. 2014.PubMed/NCBI
|
|
4
|
Vannini E, Maltese F, Olimpico F, Fabbri
A, Costa M, Caleo M and Baroncelli L: Progression of motor deficits
in glioma-bearing mice: Impact of CNF1 therapy at symptomatic
stages. Oncotarget. 8:23539–23550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chi G, Xu D, Zhang B and Yang F: Matrine
induces apoptosis and autophagy of glioma cell line U251 by
regulation of circRNA-104075/BCL-9. Chem Biol Interact.
308:198–205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krex D, Klink B, Hartmann C, von Deimling
A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger
G, et al: Long-term survival with glioblastoma multiforme. Brain.
130:2596–2606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tso JL, Yang S, Menjivar JC, Yamada K,
Zhang Y, Hong I, Bui Y, Stream A, McBride WH, Liau LM, et al: Bone
morphogenetic protein 7 sensitizes O6-methylguanine
methyltransferase expressing-glioblastoma stem cells to clinically
relevant dose of temozolomide. Mol Cancer. 14:1892015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacDonald N: Chronic inflammatory states:
Their relationship to cancer prognosis and symptoms. J R Coll
Physicians Edinb. 41:246–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Zhao J, Yu Z, Shrestha S, Song J,
Liu W, Lan W, Xing J, Liu S, Chen C, et al: Epoxymicheliolide, a
novelguaiane-type sesquiterpene lactone, inhibits NF-κB/COX-2
signaling pathways by targeting leucine 281 and leucine 25 in IKKβ
in renal cell carcinoma. Int J Oncol. 53:987–1000. 2018.PubMed/NCBI
|
|
10
|
Murata M: Inflammation and cancer. Environ
Health Prev Med. 23:502018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Q, Li G, Li R, Shen J, He Q, Deng L,
Zhang C and Zhang J: IL-6 promotion of glioblastoma cell invasion
and angiogenesis in U251 and T98G cell lines. J Neurooncol.
100:165–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Madan E, Dikshit B, Gowda SH, Srivastava
C, Sarkar C, Chattopadhyay P, Sinha S and Chosdol K: FAT1 is a
novel upstream regulator of HIF1alpha and invasion of high grade
glioma. Int J Cancer. 139:2570–2582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindholm J: A century of pituitary
surgery: Schloffer's legacy. Neurosurgery. 61:865–868. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwitalla S, Ziegler PK, Horst D, Becker
V, Kerle I, Begus-Nahrmann Y, Lechel A, Rudolph KL, Langer R,
Slotta-Huspenina J, et al: Loss of p53 in enterocytes generates an
inflammatory microenvironment enabling invasion and lymph node
metastasis of carcinogen-induced colorectal tumors. Cancer Cell.
23:93–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Yu Z, Wang C, Cheng W, Tian X, Huo
X, Wang Y, Sun C, Feng L, Xing J, et al: Alantolactone, a natural
sesquiterpene lactone, has potent antitumor activity against
glioblastoma by targeting IKKbeta kinase activity and interrupting
NF-κB/COX-2-mediated signaling cascades. J Exp Clin Cancer Res.
36:932017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng Y, Wang Y, Tang N, Sun D, Lan Y, Yu
Z, Zhao X, Feng L, Zhang B, Jin L, et al: Andrographolide inhibits
breast cancer through suppressing COX-2 expression and angiogenesis
via inactivation of p300 signaling and VEGF pathway. J Exp Clin
Cancer Res. 37:2482018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perdiki M, Korkolopoulou P, Thymara I,
Agrogiannis G, Piperi C, Boviatsis E, Kotsiakis X, Angelidakis D,
Diamantopoulou K, Thomas-Tsagli E and Patsouris E: Cyclooxygenase-2
expression in astrocytomas. Relationship with microvascular
parameters, angiogenic factors expression and survival. Mol Cell
Biochem. 295:75–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Festa-Vasconcellos JS, Piranda DN, Amaral
LM, Indio-do-Brasil V, Koifman S and Vianna-Jorge R: Polymorphisms
in cycloxygenase-2 gene and breast cancer prognosis: Association
between PTGS2 haplotypes and histopathological features. Breast
Cancer Res Treat. 132:251–258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen G, Li X, Yang J, Li J, Wang X, He J
and Huang Z: Prognostic significance of cyclooxygenase-2 expression
in patients with hepatocellular carcinoma: A meta-analysis. Arch
Med Sci. 5:1110–1117. 2016. View Article : Google Scholar
|
|
21
|
Liu X, Zhao P, Wang X, Wang L, Zhu Y, Song
Y and Gao W: Celastrol mediates autophagy and apoptosis via the
ROS/JNK and Akt/mTOR signaling pathways in glioma cells. J Exp Clin
Cancer Res. 38:1842019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Viennois E, Xiao B, Ayyadurai S, Wang L,
Wang PG, Zhang Q, Chen Y and Merlin D: Micheliolide, a new
sesquiterpene lactone that inhibits intestinal inflammation and
colitis-associated cancer. Lab Invest. 94:950–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin X, Jiang X, Jiang X, Wang Y, Miao Z,
He W, Yang G, Lv Z, Yu Y and Zheng Y: Micheliolide inhibits
LPS-induced inflammatory response and protects mice from LPS
challenge. Sci Rep. 6:232402016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalantary-Charvadeh A, Sanajou D,
Hemmati-Dinarvand M, Marandi Y, Khojastehfard M, Hajipour H,
Mesgari-Abbasi M, Roshangar L and Nazari Soltan Ahmad S:
Micheliolide protects against doxorubicin-induced cardiotoxicity in
mice by regulating PI3K/Akt/NF-kB signaling pathway. Cardiovasc
Toxicol. 19:297–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Li S, Guo J, Li Q, Long J, Ma C,
Ding Y, Yan C, Li L, Wu Z, et al: Natural product micheliolide
(MCL) irreversibly activates pyruvate kinase M2 and suppresses
leukemia. J Med Chem. 61:4155–4164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng F, Li H, Li S, Wang Y, Liu W, Gong W,
Yin B, Chen S, Zhang Y, Luo C, et al: Micheliolide ameliorates
renal fibrosis by suppressing the Mtdh/BMP/MAPK pathway. Lab
Invest. 99:1092–1106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xi XN, Liu N, Wang QQ, Wu HT, He HB, Wang
LL, Zhang TJ, Sun L, Yin Z, Chen Y and Lu YX: Pharmacokinetics,
tissue distribution and excretion of ACT001 in Sprague-Dawley rats
and metabolism of ACT001. J Chromatogr B Analyt Technol Biomed Life
Sci. 1104:29–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Q, Lu Y, Ding Y, Zhai J, Ji Q, Ma W,
Yang M, Fan H, Long J, Tong Z, et al: Guaianolide sesquiterpene
lactones, a source to discover agents that selectively inhibit
acute myelogenous leukemia stem and progenitor cells. J Med Chem.
55:8757–8769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhang J, Yang Y, Liu Q, Xu G,
Zhang R and Pang Q: ROS generation and autophagosome accumulation
contribute to the DMAMCL-induced inhibition of glioma cell
proliferation by regulating the ROS/MAPK signaling pathway and
suppressing the Akt/mTOR signaling pathway. Onco Targets Ther.
12:1867–1880. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nanes BA: Slide Set: Reproducible image
analysis and batch processing with ImageJ. BioTechniques.
59:269–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu S, Luo C, Li F, Hameed NUF, Jin Q and
Zhang J: Silencing expression of PHF14 in glioblastoma promotes
apoptosis, mitigates proliferation and invasiveness via Wnt signal
pathway. Cancer Cell Int. 19:3142019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amin RM, Elfeky SA, Verwanger T and
Krammer B: Fluorescence-based CdTe nanosensor for sensitive
detection of cytochrome C. Biosens Bioelectron. 98:415–420. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang F, Chu J and Wang F: Expression and
clinical significance of cyclooxygenase 2 and survivin in human
gliomas. Oncol Lett. 14:1303–1308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu K, Wang L and Shu HK: COX-2
overexpression increases malignant potential of human glioma cells
through Id1. Oncotarget. 5:1241–1252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Annabi B, Laflamme C, Sina A, Lachambre MP
and Beliveau R: A MT1-MMP/NF-kappaB signaling axis as a checkpoint
controller of COX-2 expression in CD133+U87 glioblastoma cells. J
Neuroinflammation. 6:82009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ye Y, Shan Y, Bao C, Hu Y and Wang L:
Ginsenoside Rg1 protects against hind-limb ischemia reperfusion
induced lung injury via NF-κB/COX-2 signaling pathway. Int
Immunopharmacol. 60:96–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhong J, Gong W, Chen J, Qing Y, Wu S, Li
H, Huang C, Chen Y, Wang Y, Xu Z, et al: Micheliolide alleviates
hepatic steatosis in db/db mice by inhibiting inflammation and
promoting autophagy via PPAR-γ-mediated NF-κB and AMPK/mTOR
signaling. Int Immunopharmacol. 59:197–208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jia Y, Zhou L, Tian C, Shi Y, Wang C and
Tong Z: Dynamin-related protein 1 is involved in
micheliolide-induced breast cancer cell death. Onco Targets Ther.
8:3371–3381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tao Y, Yuan D, Pang H, Wu H, Liu D, Jin N,
Wu N, Qiu J and Cao Y: Dynamic impact of in fl ammation-based
indices in colorectal cancer patients receiving FOLFOX-based
chemotherapy. Cancer Manag Res. 11:2817–2829. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang XP, Zhang DX, Teng M, Zhang Q, Zhang
JP and Huang YS: Downregulation of CD9 in keratinocyte contributes
to cell migration via upregulation of matrix metalloproteinase-9.
PLoS One. 8:e778062013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zandi E, Rothwarf DM, Delhase M, Hayakawa
M and Karin M: The IkappaB kinase complex (IKK) contains two kinase
subunits, IKKalpha and IKKbeta, necessary for IkappaB
phosphorylation and NF-kappaB activation. Cell. 91:243–252. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong T, Li C, Wang X, Dian L, Zhang X, Li
L, Chen S, Cao R, Li L, Huang N, et al: Ainsliadimer A selectively
inhibits IKKα/β by covalently binding a conserved cysteine. Nat
Commun. 6:65222015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang L, Su J, Jia X and Ren H: Treating
malignant glioma in Chinese patients: Update on temozolomide. Onco
Targets Ther. 7:235–244. 2014.PubMed/NCBI
|
|
45
|
Daniel P, Sabri S, Chaddad A, Meehan B,
Jean-Claude B, Rak J and Abdulkarim BS: Temozolomide induced
hypermutation in glioma: Evolutionary mechanisms and therapeutic
opportunities. Front Oncol. 9:412019. View Article : Google Scholar : PubMed/NCBI
|