Introduction
Gastrointestinal (GI) lymphoma is the most common
type of extranodal lymphoma, accounting for 5–20% of all cases
(1,2). Nevertheless, primary GI lymphoma is an
uncommon malignancy that constitutes only 1–4% of GI malignancies
(3). Primary GI lymphoma is likely
to originate within the whole GI tract; however, the most common
site of involvement is the stomach, followed by the small intestine
(4). Diffuse large B-cell lymphoma
(DLBCL) is the major histopathological subtype of primary GI
lymphoma (3). In addition, the
incidence of non-Hodgkin's lymphoma (NHL) increased by ~1-2%
annually in the 1990s (5). The
clinical manifestations of PGI-DLBCL are not obvious, thus
PGI-DLBCL is easily misdiagnosed and can be difficult to detect in
the early stages of the disease (6).
The precise oncogenesis of PGI-DLBCL remains largely unclear.
However, there are associated risk factors. Previous studies have
described that Helicobacter pylori (H. pylori), human
immunodeficiency virus or Epstein-Barr virus infections, as well as
inflammatory bowel disease, celiac disease and autoimmune diseases
may be associated with the oncogenesis of PGI-DLBCL (7,8).
Additionally, genetic changes also serve a crucial role in the
tumorigenesis of PGI-DLBCL. For example, the dysregulation of
microRNAs (miRNAs) may participate in the pathogenesis of DLBCL,
and some specific miRNAs are likely to serve as oncogenes and these
are associated with the prognosis in patients with DLBCL (9). Due to the relative rarity of this
disease, only a small number of studies have been performed in
patients with PGI-DLBCL (7,10). Therefore, disease management in
patients with PGI-DLBCL remains a challenge, and progress should be
made to investigate the pathogenesis and optimal treatment
approaches for PGI-DLBCL.
miRNAs belong to an extensive cluster of short,
endogenous and single-stranded noncoding RNAs (18–22 nucleotides
long), exerting a major role in post-transcriptional gene
expression regulation (11). A
previous study has demonstrated that miRNAs have a modulating
effect upon diverse biological pathways, including cell
proliferation, differentiation, motility, apoptosis and drug
resistance (12). According to
diverse miRNA targets, miRNAs have been demonstrated to be novel
oncogenes or tumor suppressor genes. For example, upregulation of
miR-30a-5p was demonstrated to significantly inhibit cell
proliferation and migration in breast cancer (13,14).
Furthermore, miR-15a and miR-16-1 were frequently downregulated in
chronic lymphocytic leukemia (15).
The dysregulation of miR-155, miR-210, miR-21 expression has been
detected in DLBCL and miR-21 has been revealed to have diagnostic
and prognostic potential in patients with DLBCL (16). The aberrant expression of miR-130a,
located on human chromosome 11, has been found to participate in
cancer pathogenesis and tumor progression (17). Additionally, miR-130a expression
seems to vary in different tumors, including a series of solid and
hematological malignancies. For instance, miR-130a levels are
upregulated in gastric cancer, esophageal carcinoma, adult T cell
leukemia, acute myeloid leukemia and DLBCL, whereas miR-130a levels
are downregulated in hepatocellular carcinoma cells and chronic
lymphocytic leukemia (18–24). Furthermore, accumulating evidence
indicates that aberrant expression of miR-130a has treatment and
prognostic potential in DLBCL (22,25).
However, to the best of our knowledge, the association between
miR-130a expression and clinical outcomes in PGI-DLBCL has not yet
been examined. Therefore, focusing on miR-130a may provide novel
insights into the diagnosis, treatment and prognosis of PGI-DLBCL.
The primary goal of the present study was to investigate the
clinical significance of miR-130a expression in patients with
PGI-DLBCL.
Materials and methods
Study subjects
Tumor tissues from 80 patients with PGI-DLBCL were
collected through surgical resection or endoscopic biopsy at the
Tianjin Medical University Cancer Institute and Hospital (Tianjin,
China) between January 2011 and December 2015. During the same time
period, reactive lymphoid tissue samples were collected from 20
females and 26 males (median age, 56 years; age range 36–68 years)
with the same geographical and ethnic backgrounds to be used as
controls. Each tissue sample was frozen in liquid nitrogen and then
stored at −80°C until further processing. The entire experiment was
approved by Tianjin Medical University Cancer Institute and
Hospital Ethics Committee and each individual signed written
informed consent for participating in the entire study.
Staging and diagnostic procedures
According to the World Health Organization (WHO)
classification system for hematological malignancies (26), each patient had a pathologically
confirmed diagnosis of PGI-DLBCL. Cases with stomach perforation
and intestinal perforation were excluded from the study. Patients
were staged according to the Lugano staging system (27), which was revised from the Ann Arbor
staging system for GI non-Hodgkin lymphoma. The staging system
consisted of the patients' medical history, B symptoms (fever,
night sweats and weight loss), Eastern Cooperative Oncology Group
(ECOG) performance status (score 0–1 defined as a good performance
status in the present study), a medical examination, barium meal
examination or an endoscopy, a biopsy or a gastrectomy, a bone
marrow biopsy, blood routine examination, blood biochemical
profile, abdominal ultrasound and computed tomography (CT) or
positron emission tomography-computed tomography scans of the neck,
thorax, abdomen and pelvis. The ECOG score is an indicator of a
patient's general health status and tolerance to treatment based on
their physical strength. ECOG Physical Fitness rating scale scores
patients from 0–5 points (28). Low
levels of hemoglobin were defined as <120 g/l in males and
<110 g/l in females and high levels of lactate dehydrogenase
(LDH) were defined as >245 U/l. All patients were grouped
according to clinical characteristics, such as age, sex, origin, B
symptoms, ECOG performance status, Lugano staging system (27), pathological type, LDH level,
International Prognostic Index (IPI) score (score 0–2 defined as
low IPI group in the present study) and chemotherapy response. The
IPI scores patients according to poor prognostic factors including
age >60 years, high LDH level, ECOG ≥2, clinical staging of II
or IV and ≥2 extranodal sites (29).
The clinical characteristics and histological features of the
cohort of patients with PGI-DLBCL are shown in Table I.
 | Table I.Clinicopathological features of 80
patients with PGI-DLBCL. |
Table I.
Clinicopathological features of 80
patients with PGI-DLBCL.
|
Characteristics | Number (%) |
|---|
| Age, years |
|
|
≤60 | 45 (56.2) |
|
>60 | 35 (43.8) |
| Sex |
|
|
Male | 39 (48.8) |
|
Female | 41 (51.2) |
| PGI-DLBCL
origin |
|
|
Stomach | 50 (62.5) |
|
Intestinal | 30 (37.5) |
| B symptoms |
|
|
Positive | 26 (32.5) |
|
Negative | 54 (67.5) |
| ECOG performance
status |
|
|
0-1 | 58 (72.5) |
|
2-5 | 22 (27.5) |
| Lugano staging
system |
|
|
I–II | 57 (71.3) |
|
IIE-IV | 23 (28.7) |
| Pathological
type |
|
|
Non-GCB | 59 (73.8) |
|
GCB | 21 (26.2) |
| LDH levels |
|
|
Normal | 49 (61.2) |
|
Elevated | 31 (38.8) |
| IPI score |
|
|
0-2 | 42 (52.5) |
|
3-5 | 38 (47.5) |
| Chemotherapy
response |
|
| Drug
sensitivity | 52 (65.0) |
| Drug
resistance | 28 (35.0) |
| c-MYC
upregulation |
|
|
Negative | 64 (80.0) |
|
Positive | 16 (20.0) |
| BCL-2
upregulation |
|
|
Negative | 52 (65.0) |
|
Positive | 28 (35.0) |
| Ki-67 proliferation
index, % |
|
|
<70 | 33 (41.3) |
|
≥70 | 47 (58.7) |
Treatment
All patients received two treatment methods,
including surgery and chemotherapy. In general, surgery was
intended to remove tumor tissues and obtain pathologic tissues.
None of the patients received treatment prior to the operation,
including chemotherapy, radiotherapy and other treatment. All
patients received a standard-dose CHOP regimen [cyclophosphamide
(750 mg/m2, day 1); doxorubicin (50 mg/m2,
day 1); vincristine (1.4 mg/m2, day 1); and prednisone
(100 mg, days 1–5)] or R-CHOP regimen [rituximab (375
mg/m2, day 0); cyclophosphamide (750 mg/m2,
day 1); doxorubicin (50 mg/m2, day 1); vincristine (1.4
mg/m2, day 1); and prednisone (100 mg, days 1–5)] and
these were administered for 6–8 cycles (21 days each). Resistance
to R-CHOP regimen was defined as patients who had no response to
chemotherapy or disease progression during treatment, or patients
who had relapsed after achieving complete or incomplete response
within 3 months after completing the treatment (22). Patients who were resistant to
chemotherapy continued to receive second-line treatment, including
DHAP (dexamethasone, cisplatin and cytarabine); ESHAP (etoposide,
methylprednisolone, cisplatin and cytarabine); GemOx (gemcitabine
and oxaliplatin); DA-EPOCH (etoposide, doxorubicin, vincristine,
cyclophosphamide and prednisone); ± rituximab. Treatment outcome
was assessed according to the International Working Group response
criteria (30). At the end of every
two cycles of chemotherapy (cycles 2, 4, 6 and 8), the efficacy of
the treatment was re-evaluated. In addition, after treatment,
regular inspections were conducted every 3 months for the first
year, every 6 months for the next year and once a year after five
years. These evaluations included routine blood tests with
biochemical examination, chest and abdomen CT scan,
electrocardiogram and ultrasound examination.
RNA extraction and cDNA
preparation
Total RNA (1–2 µg) was extracted from tumor and
lymphoid tissues with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The concentrations and purity of RNA were
confirmed by NanoDrop 2000 (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.). Subsequently, reverse transcription reactions
were performed at 42°C using a PrimeScript 1st Strand
cDNA synthesis kit (Takara Bio, Inc.) according to the
manufacturer's protocol. The generated cDNA was stored at −20°C
until further use.
Reverse transcription-quantitative PCR
(RT-qPCR)
The generated cDNA was subjected to qPCR
amplification with the CM9600 Sequence Detection System (Bio-Rad
Laboratories, Inc.) and QuantiTect™ SYBR Green RT-PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The cycling
conditions for the PCR reaction included an initialization at 96°C
for 5 min, followed by 38 cycles of 96°C for 30 sec, and a final
extension at 65°C for 45 sec. All measurements were performed in
triplicate. To standardize miR-130a expression, U6 was used as an
internal control. Therefore, the average Cq values of miR-130a
minus the average Cq values of U6 equals the ΔΔCq values, and
2−ΔΔCq indicated the quantitative expression levels of
miR-130a (31). The primer sequences
used in the present study included: miR-130a forward,
5′-TTGCGATTCTGTTTTGTGCT-3′ and reverse, 5′-GTGGGGTCCTCAGTGGG-3′;
and U6 forward, 5′-CTCGCTTCGGCAGCAC-3′ and reverse,
5′-ACGCTTCACGAATTTGC-3′.
Immunohistochemical (IHC) staining and
scoring
For IHC staining, all tissue samples were fixed in
10 % buffered formalin for 24–48 h at room temperature (RT),
embedded in paraffin, and cut into 4-µm-thick sections.
Subsequently, paraffin sections were incubated at 67°C in the oven
for 2 h, followed by dewaxing in xylene for 10 min and hydration
with graded ethanol (concentration, 100, 95, 80, 70 and 50%) for 5
min. To block the activity of endogenous peroxidase, 3% hydrogen
peroxide was added to all sections, followed by incubation for 10
min at RT. After washing with PBS, 10% normal goat serum (Cell
Signaling Technology, Inc.) was added for 20 min at RT as a
blocking agent. Then, the slides were incubated with the following
antibodies: c-MYC (1:200; cat no. ab51154; Abcam), BCL-2 (1:50; cat
no. ZM0010), neprilysin (CD10; 1:100; cat. no. ZM0283;), B-cell
lymphoma 6 protein (BCL-6; 1:100; cat. no. ZM-0011), PWWP
domain-containing DNA repair factor 3A (MUM1; 1:100; cat. no.
ZA-0583) (all purchased from OriGene Technologies, Inc.) and
proliferation marker protein Ki-67 (1:100; cat. no. 12202, Cell
Signaling Technology, Inc.) at 4°C overnight. After washing with
PBS, the secondary antibodies (100 µl neat; cat. no. PV-6000;
OriGene Technologies, Inc.) were applied to the samples for 20 min
at 37°C. Following this, diaminobenzidine was used for staining,
and cell nuclei were counterstained with hematoxylin for 5 min at
RT. The results were identified as positive when ~30% or more of
the sample was stained. The slice was observed under a light
microscope (magnification, ×200) and the results were obtained with
the Image Pro Plus image analysis software version 7.0 (Meyer
Instruments, Inc.). All cases were divided, based on the algorithms
of Muris et al (32) and Hans
et al (33), into germinal
center B-cell-like (GCB) and non-GCB phenotype. The staining
intensity and the percentage of positive cells were recorded.
Staining intensity was scored as follows: 0, no staining; 1+,
>25% of the tumor cells exhibited weak staining; 2+, tumor cells
exhibited moderate staining; and 3+, tumor cells exhibited strong
staining (Fig. 1).
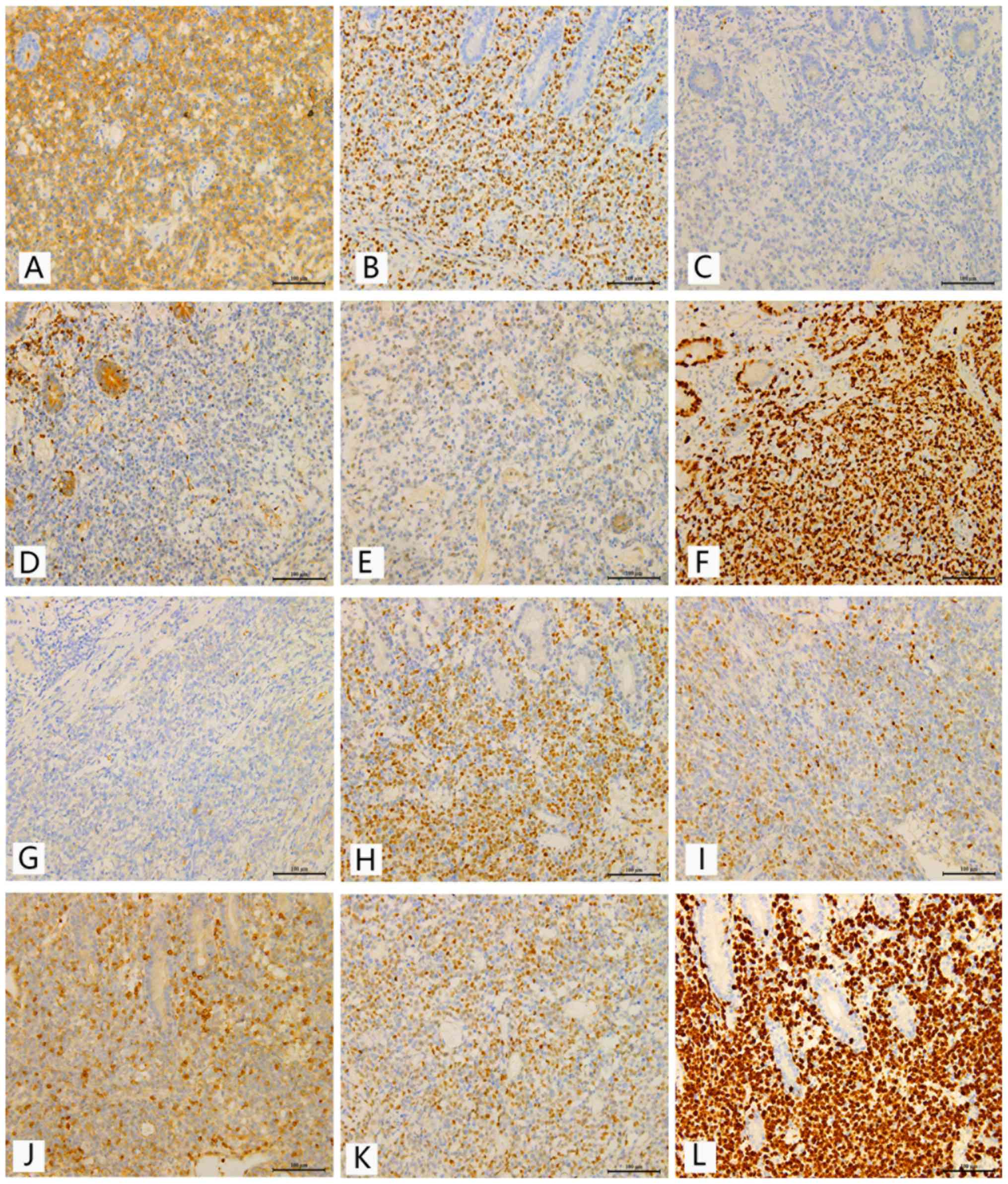 | Figure 1.Representative immunohistochemical
analyses of CD10, BCL-6, MUM-1, BCL-2, c-MYC and Ki-67 in samples
from patients with primary gastrointestinal diffuse large B-cell
lymphoma. Scale bar, 100 µm. Immunostaining for (A) CD10, (B)
BCL-6, (C) MUM-1, (D) BCL-2, (E) c-MYC and (F) Ki-67 in the GCB
subgroup. Immunostaining for (G) CD10, (H) BCL-6, (I) MUM-1, (J)
BCL-2, (K) c-MYC and (L) Ki-67 in the non-GCB subgroup. GCB,
germinal center B-cell like; BCL-6, B-cell lymphoma 6; MUM-1,
multiple myeloma antigen 1; BCL-2, B-cell lymphoma 2. |
Statistical analysis
Each sample was run in triplicate. All statistical
calculations were performed with the IBM SPSS Statistics software
(version 20.0; IBM Corp.). The GraphPad Prism software package
(version 6.01; GraphPad Software, Inc.) was used to generate all
presented graphics. Data were presented as mean ± SD. The miR-130a
levels in patients with PGI-DLBCL and control individuals were
analyzed using the non-parametric Mann-Whitney U test. Group
comparisons were made using the χ2 test. Receiver
operating characteristic (ROC) curves and the area under the ROC
curve (AUC) were determined to evaluate the feasibility of using
miRNA levels for the diagnosis of PGI-DLBCL. The survival curve was
constructed by Kaplan-Meier analysis and log-rank tests were used
to analyze the differences in survival curves. Overall survival
(OS) referred to the period from the date of diagnosis until death
or final follow-up (in March 2019). Progression-free survival (PFS)
referred to the time between the date of diagnosis and the
observation of treatment failure, clinical recurrence of the
disease, death or last follow-up. Significant parameters identified
in univariate analysis (P<0.05) were incorporated into
multivariate Cox regression analysis to determine independent
prognostic factors. The minimal sample size was estimated by PASS.
An AUC value of miR-130a in DLBCL reported in a prior study was
~0.7 (22). The minimal sample size
was calculated with an AUC0 value of 0.5, AUC1 of 0.7 and a
case/control ratio of 1. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics and
immunophenotypic features in PGI-DLBCL
A total of 80 patients, including 39 men and 41
women with a median age of 53 years, and 46 healthy subjects were
recruited in the present study. The majority of patients (56.2%)
were ≤60 years old, 31 patients (38.8%) presented with elevated LDH
levels and 26 patients (32.5%) exhibited positive B symptoms.
According to Zubrod-ECOG-WHO (namely ECOG score) and the Lugano
staging system, 58 patients (72.5%) presented a good performance
status (0–1), whereas 23 patients (28.7%) presented with stage
IIE/IV (advanced stage) PGI-DLBCL at the time of diagnosis. BCL-6,
CD10 and MUM1 staining were performed for all cases, and 21
patients (26.2%) were confirmed with GCB, and 59 patients (73.8%)
with non-GCB. Additionally, low IPI scores (0–2) were identified in
42 patients (52.5%), and chemotherapy drug resistance was observed
in 28 patients (35.0%). Table I
summarizes the baseline clinicopathological features of patients
with PGI-DLBCL.
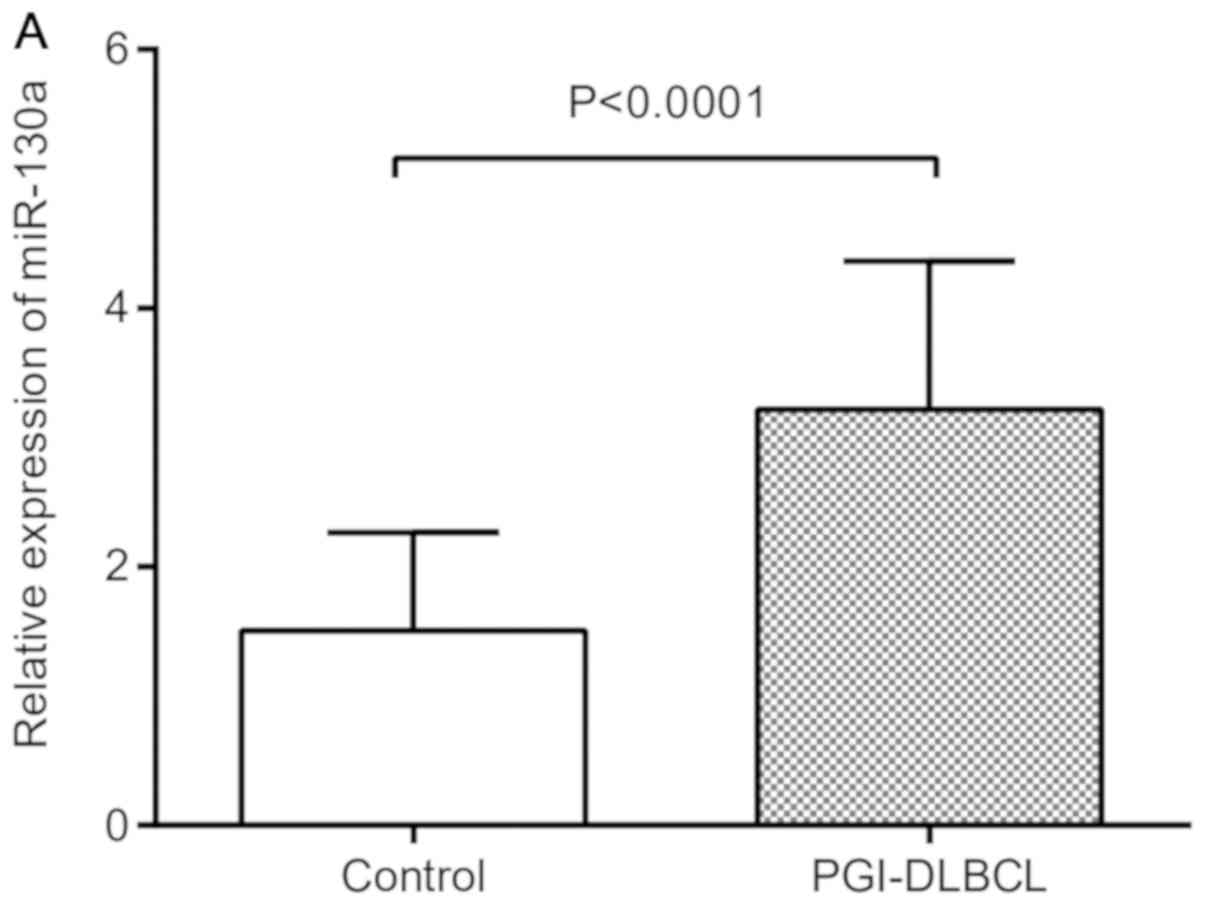
To examine the difference in miR-130a expression
between the case group and the control group, the recruited 80
patients and 46 controls were used to perform a case-control study.
The results demonstrated that the expression levels of miR-130a in
tumor tissues from patients with PGI-DLBCL were markedly increased
compared with those of the controls (Fig. 2A). ROC curves were used to evaluate
the sensitivity and specificity of miR-130a expression levels in
discriminating between normal and tumor tissues. When the optimal
cut-off value of miR-130a was 3.21 and the AUC was 0.874, the
optimum sensitivity and specificity were obtained (60 and 78%,
respectively) in the present study (Fig.
2B). According to this cut-off value, 32 patients were included
in the low-miR-130a expression group (expression Cq value <3.21)
and 48 patients were included in the high-miR-130a expression group
(expression Cq value ≥3.21).
Association between miR-130a
expression and the clinical features of PGI-DLBCL
Clinical information of patients with PGI-DLBCL,
including age, sex, origin, B symptoms, ECOG score, staging,
pathological type, LDH level, IPI score and chemotherapy response,
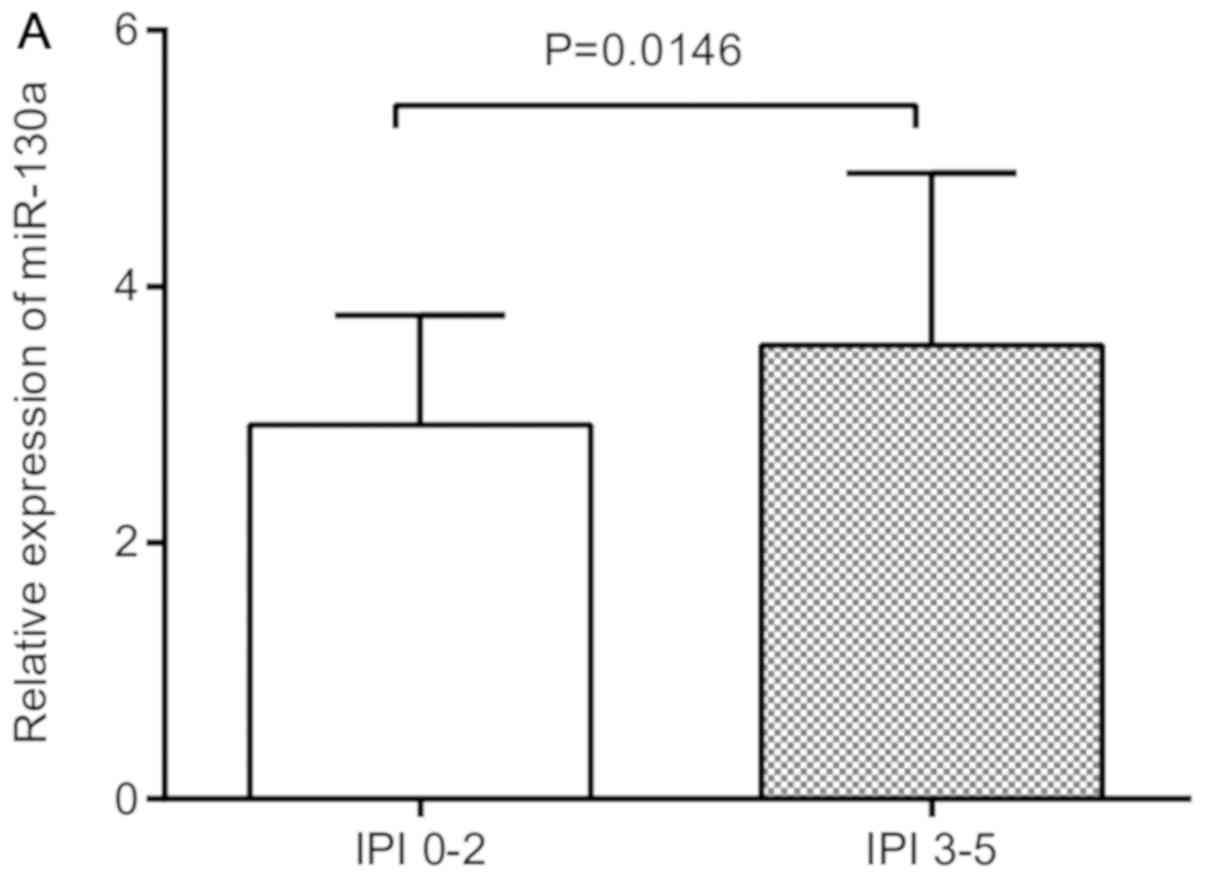
was collected. Increased levels of miR-130a were closely associated
with high IPI score (P=0.01; Table
II) and drug resistance (P=0.044; Table II), indicating the difference was
statistically significant. Nevertheless, no statistical differences
were observed in other subgroups based on age, sex, origin, B
symptoms, ECOG score, staging, pathological type and LDH level in
patients with PGI-DLBCL, between the high and low expression of
miR-139a groups. When investigating the association between the
expression levels of miR-130a and high IPI score (3–5) and drug
resistance, the relative expression of miR-130a was found to be
significantly higher in IPI score (3–5) and drug
resistance groups compared with their respective controls, IPI
score (0–2) and drug sensitivity (Fig.
3A and B).
 | Table II.Association between clinical
characteristics and miR-130a expression in patients with
PGI-DLBCL. |
Table II.
Association between clinical
characteristics and miR-130a expression in patients with
PGI-DLBCL.
|
|
| miR-130a expression
levels, n |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | No. of patients
(%) | Low (n=32) | High (n=48) | P-value |
|---|
| Age, years |
|
|
|
|
|
≤60 | 45 (56.2) | 20 | 25 | 0.358 |
|
>60 | 35 (43.8) | 12 | 23 |
|
| Sex |
|
|
|
|
|
Male | 39 (48.8) | 14 | 25 | 0.465 |
|
Female | 41 (51.2) | 18 | 23 |
|
| PGIDLBCL
origin |
|
|
|
|
|
Stomach | 50 (62.5) | 21 | 29 | 0.637 |
|
Intestinal | 30 (37.5) | 11 | 19 |
|
| B symptoms |
|
|
|
|
|
Positive | 26 (32.5) | 9 | 17 | 0.495 |
|
Negative | 54 (67.5) | 23 | 31 |
|
| ECOG performance
status |
|
|
|
|
|
0-1 | 58 (72.5) | 21 | 37 | 0.261 |
|
2-5 | 22 (27.5) | 11 | 11 |
|
| Lugano staging
system |
|
|
|
|
|
I–II | 57 (71.3) | 21 | 36 | 0.364 |
|
IIE-IV | 23 (28.7) | 11 | 12 |
|
| Pathological
type |
|
|
|
|
|
Non-GCB | 59 (73.8) | 21 | 38 | 0.177 |
|
GCB | 21 (26.2) | 11 | 10 |
|
| LDH |
|
|
|
|
|
Normal | 49 (61.2) | 23 | 26 | 0.111 |
|
Elevated | 31 (38.8) | 9 | 22 |
|
| IPI score |
|
|
|
|
|
0-2 | 42 (52.5) | 22 | 20 | 0.017 |
|
3-5 | 38 (47.5) | 10 | 28 |
|
| Chemotherapy
response |
|
|
|
|
| Drug
sensitivity | 52 (65.0) | 25 | 27 | 0.044 |
| Drug
resistance | 28 (35.0) | 7 | 21 |
|
IHC findings
As shown in Table
III, there were 28 cases (35.0%) with BCL-2 positive staining,
16 cases (20.0%) with c-MYC overexpression and 47 cases (58.7%)
with high Ki-67 positive staining. The co-expression of BCL-2 and
c-MYC proteins in DLBCL is known as ‘double-expression’ lymphoma
(34,35), and this was observed in 12 patients
in the present study. Additionally, the overexpression of BCL-2 and
c-MYC were associated with increased expression levels of miR-130a
(Fig. 3C and D), and co-expression
of BCL-2 and c-MYC showed a significant association with higher
miR-130a levels (Fig. 3E).
 | Table III.Association between IHC markers and
miR-130a expression in primary gastrointestinal diffuse large
B-cell lymphoma. |
Table III.
Association between IHC markers and
miR-130a expression in primary gastrointestinal diffuse large
B-cell lymphoma.
|
|
| miR-130a expression
levels |
|
|---|
|
|
|
|
|
|---|
| IHC markers | No. of patients
(%) | Low (n=32) | High (n=48) | P-value |
|---|
| c-MYC
upregulation |
|
|
|
|
|
Negative | 64 (80.0) | 30 | 34 | 0.012 |
|
Positive | 16 (20.0) | 2 | 14 |
|
| BCL-2
upregulation |
|
|
|
|
|
Negative | 52 (65.0) | 28 | 24 | 0.001 |
|
Positive | 28 (35.0) | 4 | 24 |
|
| Ki-67 proliferation
index |
|
|
|
|
|
<70% | 33 (41.3) | 17 | 16 | 0.078 |
|
≥70% | 47 (58.7) | 15 | 32 |
|
Survival and prognostic analysis
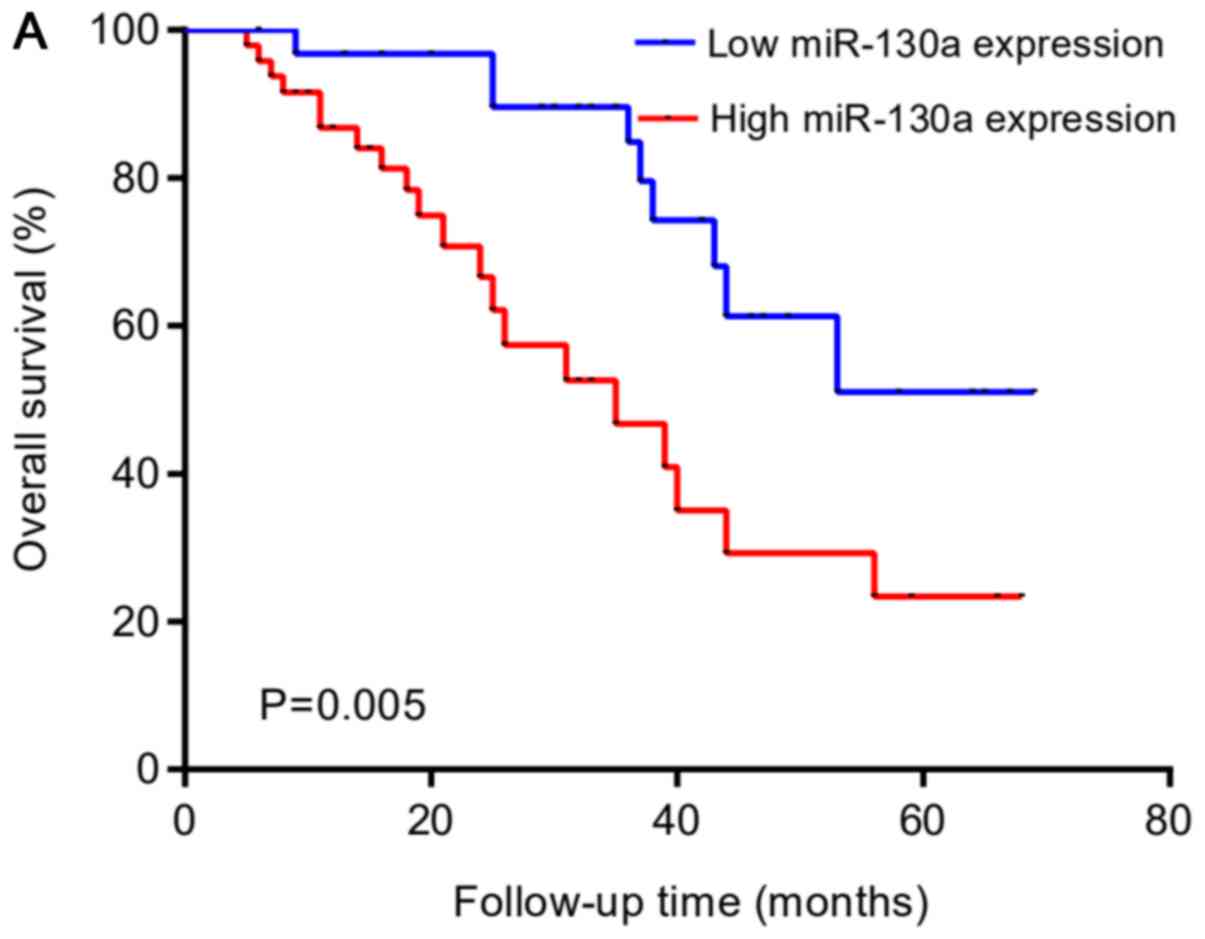
Upregulation of miR-130a was associated with shorter
OS rate and PFS rate in patients with PGI-DLBCL (Fig. 4A and B). As shown in Table IV, the univariate analysis suggested
overexpression of miR-130a and high IPI score were associated with
unfavorable patient outcomes. All other analyzed parameters
exhibited no prognostic significance. Multivariate survival
analysis indicated that miR-130a expression was an independent
negative prognostic factor in PGI-DLBCL (Table V).
 | Table IV.Univariate analysis of the
significance of different prognostic variables for primary
gastrointestinal diffuse large B cell lymphoma. |
Table IV.
Univariate analysis of the
significance of different prognostic variables for primary
gastrointestinal diffuse large B cell lymphoma.
|
| Overall
survival | Progression-free
survival |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≤60 vs. >60
years) | 1.110
(0.521-2.362) | 0.787 | 1.030
(0.483-2.196) | 0.940 |
| Sex (male vs.
female) | 0.847
(0.406-1.769) | 0.659 | 0.770
(0.369-1.606) | 0.485 |
| Origin (stomach vs.
intestinal) | 1.555
(0.746-3.243) | 0.239 | 1.605
(0.772-3.334) | 0.205 |
| B symptoms
(positive vs. negative) | 0.704
(0.311-1.593) | 0.400 | 0.733
(0.324-1.659) | 0.455 |
| ECOG (0–1 vs.
2–4) | 1.569
(0.747-3.299) | 0.234 | 1.630
(0.773-3.440) | 0.199 |
| Lugano stage (I–II
vs. IIE-IV) | 0.877
(0.386-1.994) | 0.754 | 0.854
(0.376-1.936) | 0.705 |
| Pathological type
(non-GCB vs. GCB) | 0.588
(0.249-1.391) | 0.227 | 0.657
(0.279-1.548) | 0.337 |
| LDH (normal vs.
elevated) | 1.835
(0.884-3.810) | 0.103 | 1.983
(0.954-4.123) | 0.067 |
| IPI (0–2 vs.
3–5) | 2.170
(1.034-4.551) | 0.040 | 2.231
(1.059-4.701) | 0.035 |
| Chemotherapy
response (drug sensitivity vs. drug resistance) | 0.719
(0.317-1.631) | 0.430 | 0.830
(0.367-1.878) | 0.654 |
| c-MYC (positive vs.
negative) | 1.469
(0.552-3.904) | 0.441 | 1.793
(0.659-4.876) | 0.253 |
| BCL-2 (positive vs.
negative) | 1.289
(0.607-2.740) | 0.509 | 1.461
(0.687-3.108) | 0.324 |
| BCL-2/c-MYC
co-expression (BCL-2+/c-MYC+ vs. others) | 1.111
(0.449-2.751) | 0.820 | 1.218
(0.494-3.001) | 0.669 |
| Ki-67 index (<70
vs. ≥70%) | 1.398
(0.659-2.966) | 0.383 | 1.346
(0.635-2.854) | 0.439 |
| miR-130a expression
(low vs. high) | 2.998
(1.347-6.673) | 0.007 | 3.325
(1.488-7.429) | 0.003 |
 | Table V.Multivariate analysis of the
significance of independent prognostic variables for primary
gastrointestinal diffuse large B cell lymphoma. |
Table V.
Multivariate analysis of the
significance of independent prognostic variables for primary
gastrointestinal diffuse large B cell lymphoma.
|
| Overall
survival | Progression-free
survival |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| IPI (0–2 vs.
3–5) | 1.479
(0.654-3.342) | 0.347 | 1.379
(0.596-3.188) | 0.453 |
| miR-130a expression
(low vs. high) | 2.516
(1.046-6.052) | 0.039 | 2.828
(1.143-6.994) | 0.024 |
Discussion
Primary GI lymphoma is a malignant tumor which
gradually infiltrates the alimentary tract. The IPI score is used
to predict the prognosis of patients with DLBCL aggressive lymphoma
and includes five clinical parameters (age, ECOG score, clinical
staging, LDH levels and the number of sites of extranodal invasion)
(7). However, the IPI evaluation
system only contains some clinical features, dismissing the
molecular biology of cancer. Therefore, novel biomarkers should be
explored to improve the assessment of the prognosis of PGI-DLBCL.
Increasing evidence indicates that miRNAs are closely associated
with the pathogenesis and prognostic significance of diverse types
of cancer, including DLBCL. For example, miR-155 is overexpressed
in DLBCL cells and has emerged as a negative prognostic marker
(36). In addition, reduced miR-34a
expression in DLBCL acts as a tumor suppressor (37,38).
Interestingly, previous studies have demonstrated that miR-130a
serves a vital role in the tumorigenesis of numerous hematological
malignancies, including chronic lymphocytic leukemia (24), acute myeloid leukemia (21) and adult T cell leukemia (20). However, miR-130a expression in
PGI-DLBCL still requires further research.
In the present study, miR-130a expression in the
tumor tissues from patients with PGI-DLBCL and control subjects was
evaluated by RT-qPCR. The results revealed significantly higher
miR-130a expression in PGI-DLBCL tissues compared with the control
tissues, suggesting that miR-130a may be a potential diagnostic
factor of PGI-DLBCL. In addition, the data indicated that miR-130a
expression was associated with high IPI score and chemotherapy
resistance. Several studies have indicated that miR-130a has a
modulating effect on chemotherapy drug resistance in diverse types
of cancer, including ovarian cancer (39), lung cancer (40), liver cancer (41) and DLBCL (42). Furthermore, Yuan et al
(22) reported that higher levels of
miR-130a and miR-125b in patients with DLBCL treated with R-CHOP
had an adverse effect on disease remission and chemosensitivity.
Similar results have been reported in other cancer types, including
hematologic malignancies, where miR-130a has the potential to
regulate drug susceptibility by activating the Wnt/β-catenin and
PI3K/AKT/mTOR signaling pathways (17,41,42). As
aforementioned, in the present study, miR-130a was likely to be
associated with chemotherapy drug resistance in PGI-DLBCL. However,
the potential mechanism remains to be elucidated, as there was no
adequate evidence showing that miR-130a induces drug resistance by
activating a particular signaling pathway in PGI-DLBCL.
BCL-2 and c-MYC serve crucial roles in the
pathogenesis of DLBCL (43,44). The constitutive activity of NF-κB
results in the upregulation of numerous NF-κB target genes,
including MYC and BCL-2 (45,46).
Furthermore, some miRNAs may have an effect on NF-κB expression,
whereas NF-κB can transcriptionally modulate miR-130a expression
(47,48). In addition, the elevated expression
of c-MYC might control miR-130a upregulation, which could be
regulated by product c-MYC (namely the c-MYC protein encoded by
exons 2 and 3), forming complex regulatory loops (49–51). In
the present study, data demonstrated that BCL-2 positive and c-MYC
positive were associated with high expression levels of miR-130a,
which, considering the aforementioned observations, suggests that
miR-130a might be involved in regulating BCL-2 and c-MYC
expression. Previous studies have reported that patients with
BCL-2+, c-MYC+ or
BCL-2+/c-MYC+ have a worse prognosis compared
with patients with BCL-2−, c-MYC− or others
(including BCL-2−/c-MYC+,
BCL-2+/c-MYC− and
BCL-2−/c-MYC−) in patients with DLBCL or
PGI-DLBCL (7,34,43,45,52).
However, in the present study, data suggested that BCL-2 and c-MYC
expression or co-expression of both, along with high Ki-67 index
were not associated with prognosis in PGI-DLBCL. Therefore,
additional investigations are required to elucidate the association
between BCL-2, c-MYC and their co-expression and the prognosis of
patients with PGI-DLBCL.
The association between miR-130a expression and the
prognosis of various types of cancer has been investigated.
Increased miR-130a expression in gastric cancer is associated with
inferior OS (53), and increased
miR-130a expression in non-small cell lung cancer is associated
with an unfavorable prognosis (54).
In the present study, patients with high miR-130a levels had poor
outcomes compared with those with low miR-130a levels.
Additionally, multivariate analyses adjusting for known factors
indicated that miR-130a was an independent prognostic factor for OS
and PFS rates. This result is consistent with a previous study
where miR-130a and miR-125b were identified as potential novel
biomarkers for assessing treatment response and predicting survival
of patients with DLBCL (22).
Nevertheless, the mechanism of the potential relationship between
increased miR-130a expression and poor prognosis remains unclear.
However, previous studies have revealed that aberrant miR-130a
expression increases DNA methylation resulting in promoter
silencing, and the overexpression of DNA methyltransferase 1 is
associated with adverse prognosis in patients with PGI-DLBCL
(24,55,56).
Overall, these results indicated that miR-130a overexpression could
be a promising predictor of poor prognosis for PGI-DLBCL. In the
future, miR-130a expression might become a useful clinical tool to
predict patient survival and guide therapeutic strategies.
There are a number of limitations to the present
study. Firstly, there was a limited number of samples used, which
is partly due to the relative rarity of PGI-DLBCL. However, the
minimal sample size computed by PASS software was 41 patients and
41 controls, which indicated that the used sample size in the
present study was suitable for preliminary exploration. In
addition, the present findings may provide a basis for further
experiments to validate the expression and role of miR-130a in a
larger cohort of patients with PGI-DLBCL. Secondly, the association
between drug resistance and the overexpression of miR-130a only
showed a significance of 0.044. However, there is evidence showing
that miR-130a has an impact on the resistance to chemotherapeutic
drugs in various types of cancer, including DLBCL (22). Therefore, the low significance
described in the present study might be partially attributed to the
relatively limited sample size. Therefore, larger sample size
studies are required to validate the results. Finally, miR-130a
expression levels were assessed by RT-qPCR which is a relative
quantification approach with reduced accuracy compared with digital
PCR (57,58) which is an absolute quantitative
technique for nucleic acids. Therefore, digital PCR may be more
adequate for further studies and validation of the present
findings.
In conclusion, the present data suggest that
miR-130a levels can distinguish patients with PGI-DLBCL from
healthy individuals, which might be a potential diagnostic marker
for PGI-DLBCL. Furthermore, high miR-130a expression may represent
a potential novel biomarker for the prognosis of PGI-DLBCL. This
finding might be used in daily clinical work and provide novel
therapeutic options for patients with PGI-DLBCL.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by The
National Natural Science Foundation of China (grant nos. 81100337
and 81470283).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC performed the experiments, analyzed the data and
wrote the paper. YK and XYW performed the experiments. PG, TD and
QZ contributed to patient sample collection and literature
retrieval. YW, YY, XFW, ZZ, HY, XL, LL and LQ participated in
gathering clinical information and acquisition of data. HYZ
conceived the experiments and acquired the funding. LC, ZZQ, HLZ
and HFZ designed the experiments and supervised the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Tianjin Medical
University Cancer Institute and Hospital Ethics Committee. Written
informed consent was obtained from each patient included in the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Freeman C, Berg JW and Cutler SJ:
Occurrence and prognosis of extranodal lymphomas. Cancer.
29:252–260. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berglund M, Hedström G, Amini RM, Enblad G
and Thunberg U: High expression of microRNA-200c predicts poor
clinical outcome in diffuse large B-cell lymphoma. Oncol Rep.
29:720–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papaxoinis G, Papageorgiou S, Rontogianni
D, Kaloutsi V, Fountzilas G, Pavlidis N, Dimopoulos M, Tsatalas C,
Xiros N and Economopoulos T: Primary gastrointestinal non-Hodgkin's
lymphoma: A clinicopathologic study of 128 cases in Greece. A
Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma.
47:2140–2146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herrmann R, Panahon AM, Barcos MP, Walsh D
and Stutzman L: Gastrointestinal involvement in non-Hodgkin's
lymphoma. Cancer. 46:215–222. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Müller AM, Ihorst G, Mertelsmann R and
Engelhardt M: Epidemiology of non-Hodgkin's lymphoma (NHL): Trends,
geographic distribution, and etiology. Ann Hematol. 84:1–12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malipatel R, Patil M, Pritilata Rout P,
Correa M and Devarbhavi H: Primary gastric lymphoma:
Clinicopathological profile. Euroasian J Hepatogastroenterol.
8:6–10. 2018.PubMed/NCBI
|
|
7
|
Xia B, Zhang L, Guo SQ, Li XW, Qu FL, Zhao
HF, Zhang LY, Sun BC, You J and Zhang YZ: Coexpression of MYC and
BCL-2 predicts prognosis in primary gastrointestinal diffuse large
B-cell lymphoma. World J Gastroenterol. 21:2433–2442. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng JC, Zhong L and Ran ZH: Primary
lymphomas in the gastrointestinal tract. J Dig Dis. 16:169–176.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao H, Zhang L, Guo S, Yuan T, Xia B, Qu
F, Zhang L and Zhang Y: Downregulated expression of Dicer1 predicts
inferior survival in primary gastrointestinal diffuse large B-cell
lymphoma treated with CHOP-like regimen and rituximab. Med Oncol.
31:2062014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Treiber T, Treiber N and Meister G:
Regulation of microRNA biogenesis and function. Thromb Haemost.
107:605–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Lu P, Wang DD, Yang SJ, Wu Y, Shen
HY, Zhong SL, Zhao JH and Tang JH: The role of miRNAs in drug
resistance and prognosis of breast cancer formalin-fixed
paraffin-embedded tissues. Gene. 595:221–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang D, Qiu C, Zhang H, Wang J, Cui Q and
Yin Y: Human microRNA oncogenes and tumor suppressors show
significantly different biological patterns: from functions to
targets. PLoS One. 5:e130672010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong J, Wei B, Ye Q and Liu W:
MiR-30a-5p/UBE3C axis regulates breast cancer cell proliferation
and migration. Biochem Biophys Res Commun. 516:1013–1018. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lawrie CH, Gal S, Dunlop HM, Pushkaran B,
Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J,
Wainscoat JS, et al: Detection of elevated levels of
tumour-associated microRNAs in serum of patients with diffuse large
B-cell lymphoma. Br J Haematol. 141:672–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang HD, Jiang LH, Sun DW, Li J and Ji
ZL: The role of miR-130a in cancer. Breast Cancer. 24:521–527.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang H, Yu WW, Wang LL and Peng Y:
miR-130a acts as a potential diagnostic biomarker and promotes
gastric cancer migration, invasion and proliferation by targeting
RUNX3. Oncol Rep. 34:1153–1161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu SG, Qin XG, Zhao BS, Qi B, Yao WJ,
Wang TY, Li HC and Wu XN: Differential expression of miRNAs in
esophageal cancer tissue. Oncol Lett. 5:1639–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishihara K, Sasaki D, Tsuruda K, Inokuchi
N, Nagai K, Hasegawa H, Yanagihara K and Kamihira S: Impact of
miR-155 and miR-126 as novel biomarkers on the assessment of
disease progression and prognosis in adult T-cell leukemia. Cancer
Epidemiol. 36:560–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding C, Chen SN, Macleod RAF, Drexler HG,
Nagel S, Wu DP, Sun AN and Dai HP: MiR-130a is aberrantly
overexpressed in adult acute myeloid leukemia with t(8;21) and its
suppression induces AML cell death. Ups J Med Sci. 123:19–27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan WX, Gui YX, Na WN, Chao J and Yang X:
Circulating microRNA-125b and microRNA-130a expression profiles
predict chemoresistance to R-CHOP in diffuse large B-cell lymphoma
patients. Oncol Lett. 11:423–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Huang P, Qiu J, Liao Y, Hong J and
Yuan Y: MicroRNA-130a is down-regulated in hepatocellular carcinoma
and associates with poor prognosis. Med Oncol. 31:2302014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kovaleva V, Mora R, Park YJ, Plass C,
Chiramel AI, Bartenschlager R, Döhner H, Stilgenbauer S, Pscherer
A, Lichter P and Seiffert M: miRNA-130a targets ATG2B and DICER1 to
inhibit autophagy and trigger killing of chronic lymphocytic
leukemia cells. Cancer Res. 72:1763–1772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Borges NM, do Vale Elias M, Fook-Alves VL,
Andrade TA, de Conti ML, Macedo MP, Begnami MD, Campos AH, Etto LY,
Bortoluzzo AB, et al: Angiomirs expression profiling in diffuse
large B-Cell lymphoma. Oncotarget. 7:4806–4816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomonaga M: Outline and direction of
revised WHO classification of tumors of haematopoietic and lymphoid
tissues. Rinsho Ketsueki. 50:1401–1406. 2009.PubMed/NCBI
|
|
27
|
Rohatiner A, d'Amore F, Coiffier B,
Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem
P, Shipp M, et al: Report on a workshop convened to discuss the
pathological and staging classifications of gastrointestinal tract
lymphoma. Ann. Oncol. 5:397–400. 1994.
|
|
28
|
Sok M, Zavrl M, Greif B and Srpčič M:
Objective assessment of WHO/ECOG performance status. Support Care
Cancer. 27:3793–3798. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghimire P, Wu GY and Zhu L: Primary
gastrointestinal lymphoma. World J. Gastroenterol. 17:697–707.
2011.
|
|
30
|
Chen F, Liu S, Zhou Y, Shen H and Zuo X:
Mad2 overexpression is associated with high cell proliferation and
reduced disease-free survival in primary gastrointestinal diffuse
large B-cell lymphoma. Hematology. 21:399–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muris JJ, Meijer CJ, Vos W, van Krieken
JH, Jiwa NM, Ossenkoppele GJ and Oudejans JJ: Immunohistochemical
profiling based on Bcl-2, CD10 and MUM1 expression improves risk
stratification in patients with primary nodal diffuse large B cell
lymphoma. J Pathol. 208:714–723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horn H, Ziepert M, Becher C, Barth TF,
Bernd HW, Feller AC, Klapper W, Hummel M, Stein H, Hansmann ML, et
al: MYC status in concert with BCL2 and BCL6 expression predicts
outcome in diffuse large B-cell lymphoma. Blood. 121:2253–2263.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Juarez-Salcedo LM, Sokol L, Chavez JC and
Dalia S: Primary gastric lymphoma, epidemiology, clinical
diagnosis, and treatment. Cancer Control. 25:1–12. 2018. View Article : Google Scholar
|
|
36
|
Ahmadvand M, Eskandari M, Pashaiefar H,
Yaghmaie M, Manoochehrabadi S, Khakpour G, Sheikhsaran F and
Montazer Zohour M: Over expression of circulating miR-155 predicts
prognosis in diffuse large B-cell lymphoma. Leuk Res. 70:45–48.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Craig VJ, Tzankov A, Flori M, Schmid CA,
Bader AG and Muller A: Systemic microRNA-34a delivery induces
apoptosis and abrogates growth of diffuse large B-cell lymphoma in
vivo. Leukemia. 26:2421–2424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Craig VJ, Cogliatti SB, Imig J, Renner C,
Neuenschwander S, Rehrauer H, Schlapbach R, Dirnhofer S, Tzankov A
and Müller A: Myc-mediated repression of microRNA-34a promotes
high-grade transformation of B-cell lymphoma by dysregulation of
FoxP1. Blood. 117:6227–6236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang F, Miao L, Mei Y and Wu M: Retinoic
acid-induced HOXA5 expression is co-regulated by HuR and miR-130a.
Cell Signal. 25:1476–1485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou YM, Liu J and Sun W: MiR-130a
overcomes gefitinib resistance by targeting met in non-small cell
lung cancer cell lines. Asian Pac. J Cancer Prev. 15:1391–1406.
2014.
|
|
41
|
Xu N, Shen C, Luo Y, Xia L, Xue F, Xia Q
and Zhang J: Upregulated miR-130a increases drug resistance by
regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell.
Biochem Biophys Res Commun. 425:468–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu X and Li Z: New insights into MicroRNAs
involves in drug resistance in diffuse large B cell lymphoma. Am J
Transl Res. 7:2536–2542. 2015.PubMed/NCBI
|
|
43
|
Kawamoto K, Miyoshi H, Yoshida N, Nakamura
N, Ohshima K, Sone H and Takizawa J: MYC translocation and/or BCL 2
protein expression are associated with poor prognosis in diffuse
large B-cell lymphoma. Cancer Sci. 107:853–861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Plati J, Bucur O and Khosravi-Far R:
Apoptotic cell signaling in cancer progression and therapy. Integr
Biol. 3:279–96. 2011. View Article : Google Scholar
|
|
45
|
Hu S, Xu-Monette ZY, Tzankov A, Green T,
Wu L, Balasubramanyam A, Liu WM, Visco C, Li Y, Miranda RN, et al:
MYC/BCL2 protein coexpression contributes to the inferior survival
of activated B-cell subtype of diffuse large B-cell lymphoma and
demonstrates high-risk gene expression signatures: A report from
The International DLBCL Rituximab-CHOP consortium program. Blood.
121:4021–4031. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lim KH, Yang Y and Staudt LM: Pathogenetic
importance and therapeutic implications of NF-kB in lymphoid
malignancies. Immunol Rev. 246:359–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Feng Y, Zhou S, Li G, Hu C, Zou W, Zhang H
and Sun L: Nuclear factor-κB-dependent microRNA-130a upregulation
promotes cervical cancer cell growth by targeting phosphatase and
tensin homolog. Arch Biochem Biophys. 598:57–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Y, Zhang X, Tang W, Lin Z, Xu L, Dong
R, Li Y, Li J, Zhang Z, Li X, et al: miR-130a upregulates mTOR
pathway by targeting TSC1 and is transactivated by NF-κB in
high-grade serous ovarian carcinoma. Cell Death Differ.
24:2089–2100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Filip D and Mraz M: The role of MYC in the
transformation and aggressiveness of ‘indolent’ B-cell
malignancies. Leuk. Lymphoma. 61:510–524. 2019. View Article : Google Scholar
|
|
50
|
Zhu J, Zheng X and Yang X: Diagnostic and
mechanistic values of microRNA-130a and microRNA-203 in patients
with papillary thyroid carcinoma. J Cell Biochem. 121:3657–3666.
2020. View Article : Google Scholar
|
|
51
|
Nguyen L, Papenhausen P and Shao H: The
Role of c-MYC in B-cell lymphomas: Diagnostic and molecular
aspects. Genes (Basel). 8:1162017. View Article : Google Scholar
|
|
52
|
Johnson NA, Slack GW, Savage KJ, Connors
JM, Ben-Neriah S, Rogic S, Scott DW, Tan KL, Steidl C, Sehn LH, et
al: Concurrent expression of MYC and BCL2 in diffuse large B-cell
lymphoma treated with rituximab plus cyclophosphamide, doxorubicin,
vincristine, and prednisone. J Clin Oncol. 30:3452–3459. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee SH, Jung YD, Choi YS and Lee YM:
Targeting of RUNX3 by miR-130a and miR-495 cooperatively increases
cell proliferation and tumor angiogenesis in gastric cancer cells.
Oncotarget. 6:33269–33278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang XC, Tian LL, Wu HL, Jiang XY, Du LQ,
Zhang H, Wang YY, Wu HY, Li DG, She Y, et al: Expression of
miRNA-130a in nonsmall cell lung cancer. Am J Med Sci. 340:385–388.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao H, Zhang LE, Guo S, Yuan T, Xia B,
Zhang L and Zhang Y: Overexpression of DNA methyltransferase 1 as a
negative independent prognostic factor in primary gastrointestinal
diffuse large B-cell lymphoma treated with CHOP-like regimen and
rituximab. Oncol Lett. 9:2307–2312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pallasch CP, Patz M, Park YJ, Hagist S,
Eggle D, Claus R, Debey-Pascher S, Schulz A, Frenzel LP, Claasen J,
et al: miRNA deregulation by epigenetic silencing disrupts
suppression of the oncogene PLAG1 in chronic lymphocytic leukemia.
Blood. 114:3255–3264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kanagal-Shamanna R: Digital PCR:
Principles and Applications. Methods Mol Biol. 1392:43–50. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cao L, Cui X, Hu J, Li Z, Choi JR, Yang Q,
Lin M, Ying Hui L and Xu F: Advances in digital polymerase chain
reaction (dPCR) and its emerging biomedical applications. Biosens
Bioelectron. 90:459–474. 2017. View Article : Google Scholar : PubMed/NCBI
|