Introduction
Primary presacral tumors are relatively rare tumors
occurring in the space between the sacrum and the rectum; in 1985,
the records at the Mayo Clinic in the USA indicate that presacral
lesions occurred in 1 out of 40,000 registrations (1,2). The
surgical boundaries of the presacral tumor include the fascia
propria of the rectum anteriorly and the presacral fascia
posteriorly, while the endopelvic fascia, the bilateral ureter and
iliac vessels may also adhere to the tumor (3). The majority of sacrococcygeal tumors
have a long onset time and relatively insidious clinical symptoms;
pain occurs when the tumor grows and the nerves are compressed by
the tumor (1,2). The main types of primary presacral
tumor in adults are chordoma, schwannoma, paraganglioma,
liposarcoma and chondrosarcoma (4,5).
Due to the complex anatomical location of presacral
tumors, the inconsistent pathological type of the tumors and the
difficulty of surgery, it is extremely difficult for orthopedic
surgeons to diagnose and treat presacral tumors (2,3).
Surgical treatment is the treatment of choice; in the past, open
surgery was mostly adopted, but in recent years, laparoscopic
technology has developed rapidly (2). In previous studies, laparoscopic
resection has been considered a safe, feasible and effective
treatment for retroperitoneal and presacral tumors (6,7).
The da Vinci robotic surgical system was developed
to overcome the limited movement of abdominal endoscopic
instruments in a limited space, the amplification of hand tremors,
the lack of two-dimensional imaging and the movement of
instruments. Surgeons hope to overcome the limitations of
endoscopic abdominal surgery using this innovative product. The da
Vinci surgical system is widely used in rectal, urological and
ovarian cancer types, as it has several advantages (8–10). For
example, it provides 3D vision and visual magnification to improve
the accuracy of tumor resection. In addition, the da Vinci surgical
system consists of 3 or 4 robotic arms that mimic the movements of
a human wrist, providing a high degree of freedom. However, there
are few reports on da Vinci robot-assisted surgical resection of
presacral tumors (11).
The present study summarizes the experience of 12
patients undergoing surgical resection assisted by the da Vinci
surgical system for the treatment of presacral tumors, in the hope
of providing a new alternative for the surgical treatment of these
tumors.
Materials and methods
Ethical approval and consent to
participate
The present study was conducted with the approval of
the Ethics Committee of Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China), and
in line with the Declaration of Helsinki. The surgical procedure
and the collection of tissue specimens were approved by the
aforementioned ethics committee. All patients and legal guardians
were informed of the surgical operation requirements and
regulations on the use of clinical samples, and provided written
informed consent for the collection of human tissue specimens used
in the present study.
Patients
Between March 2016 and April 2019, 12 patients with
presacral tumors were treated using the da Vinci robotic surgery
system. This group included 5 males and 7 females, aged 26–49 years
(median, 43 years). All patients were pathologically diagnosed with
a nerve sheath tumor following surgery. A total of 9 patients with
a presacral tumor attended the Wuhan Union Hospital (Wuhan, China)
due to lower back pain and lower limb numbness, 1 case was
identified in routine health screening and the other 2 cases were
identified using imaging examination due to other diseases.
Preoperative bloodwork, chest x-ray, electrocardiography findings,
blood glucose levels, and liver and kidney function were normal in
all patients. None of the patients had a previous history of
surgery or serious diseases, or contraindications to surgery.
A total of 19 patients with presacral tumors who
were admitted to the Orthopedics Department of Wuhan Union Hospital
from March 2016 to April 2019 were also included. This group
included 7 males and 12 females, aged between 25 and 62 years
(median age, 46 years). These patients received open surgical
treatment and were used as the control group for comparison with
the da Vinci surgery group. The tumor volume of the included
patients was <5×5×5 cm (length × height × width), and patients
with oversized tumors were excluded. Postoperative pathological
analysis of the tumors of these patients revealed they were all
schwannomas.
Surgical procedure
Preoperatively, the robotic arm, auxiliary channel
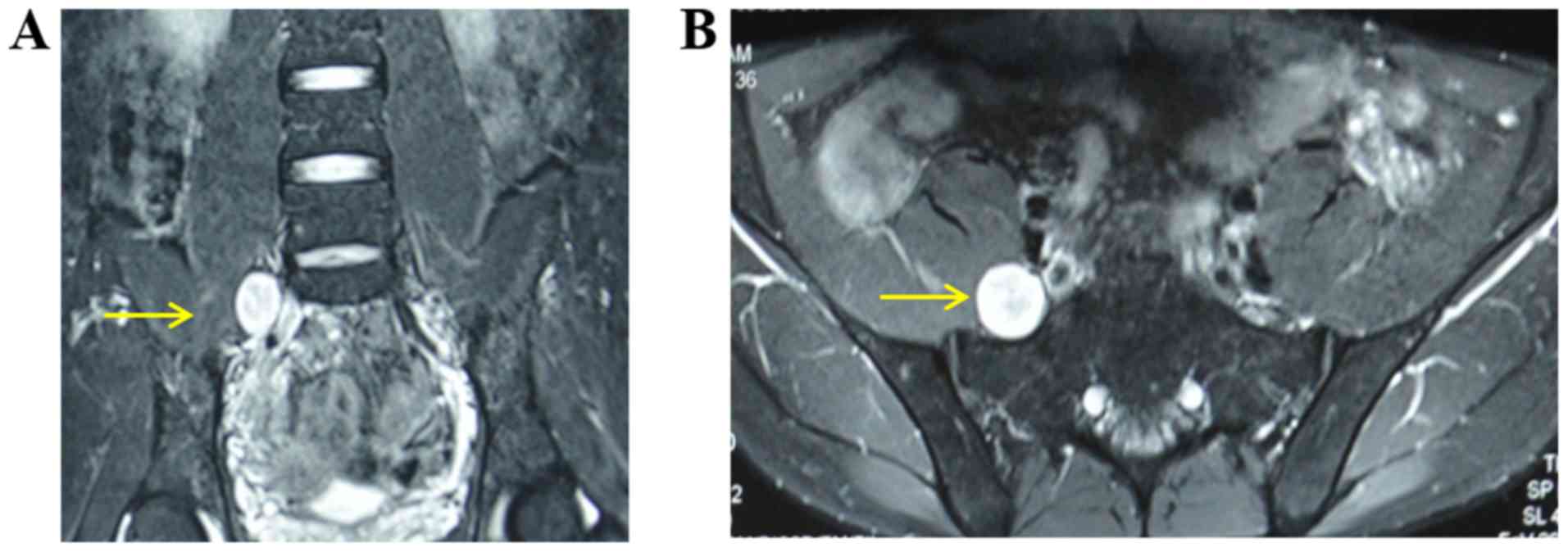
and camera were placed and marked according to the tumor location
and preoperative imaging data (Fig.
1). The patient received general anesthesia and was placed in a
Trendelenburg position (12).
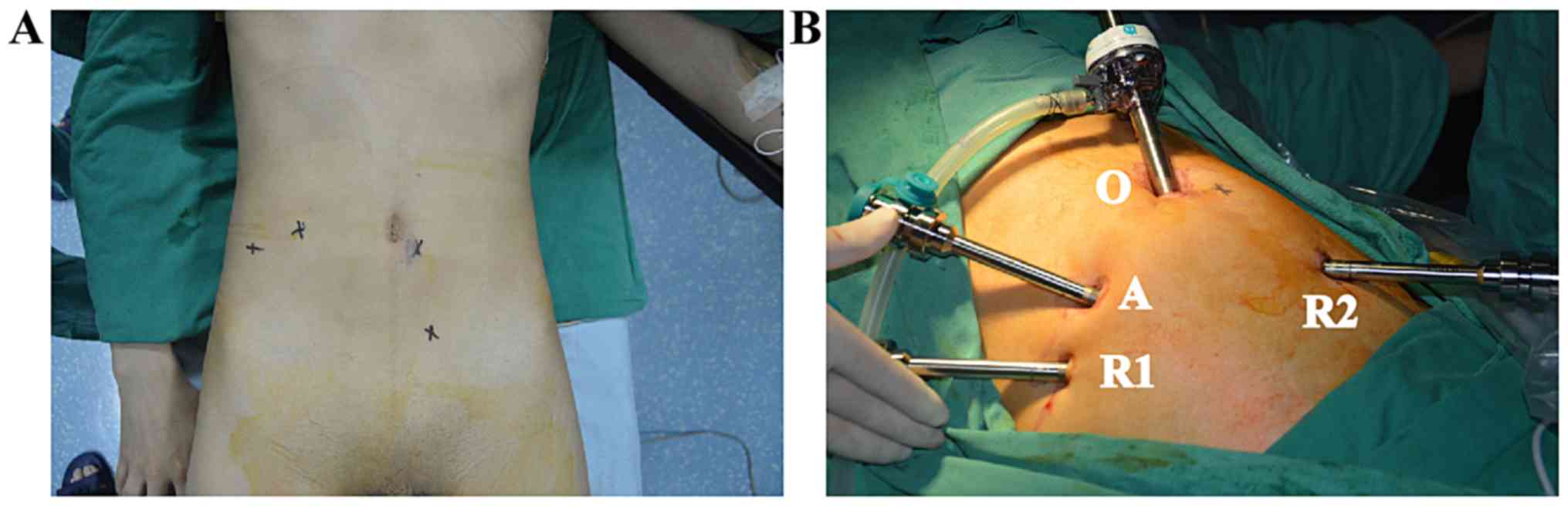
Following routine disinfection, the mechanical arm system was
pushed to a suitable position beside the bed, a small opening ~1-cm
long was made on the umbilicus and a trocar was inserted. To begin
with, the endoscopic imaging system was inserted and connected to
the video screen, and an artificial pneumoperitoneum was formed.
The two robotic arms and auxiliary channels were implanted under
video monitoring. The intraoperative trocar placement should meet
the ‘20-10-5’ principle, whereby the distance between the lens
point and the surgical target center is 10–20 cm, the instrument
arm trocar is 8–10 cm away from the optimal position of the lens
arm trocar, the line between the two points are at an angle of
l5-30 to the horizontal position, the distance between instrument
arm trocar and auxiliary hole trocar is >5 cm, and the lens arm,
center column of the patient cart and patient surgical target are
in a straight line (Fig. 2).
After the channel was established, only one
instrument nurse and assistant were left next to the operating
table, and the surgeon operated the surgical robot system on the
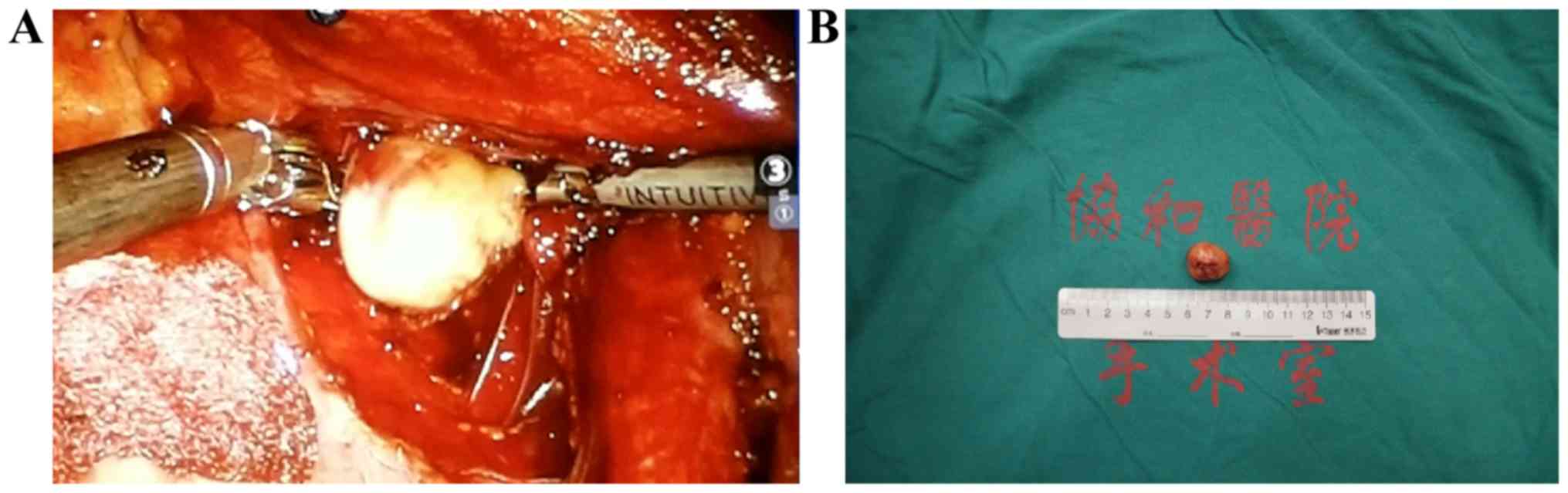
table. Following observation of the overall structure of the pelvic
cavity, the retroperitoneum was cut open to identify the ureter and
iliac vessels. The mechanical arm was operated on the workbench to
separate the ureter and iliac vessels, and the assistant on the
platform assisted with pulling and retracting to expose the tumor
(Fig. 3). If bleeding occurred
during the operation, this was stopped by electrocoagulation using
the mechanical arm. The blood vessels clearly visible under the
microscope were dissociated first and cut off once a blood vessel
clip had been used to clamp the blood vessels via the auxiliary
channel. Following complete tumor resection, the stump was closed
and removed by auxiliary channels, which can be expanded when a
tumor is large. The wound was rinsed, the bleeding carefully
stopped, and the camera and mechanical arm removed. Drainage was
applied and the incision was sutured. All tissue specimens were
fixed in 10% buffered-formalin for 24 h at 25°C, embedded in
paraffin and cut into 4-µm-thick sections. Hematoxylin and eosin
(H&E) staining was performed for 20 min at 37°C and then
observed using an Olympus BX51 light microscope (magnification,
×100; Olympus Corporation). Immunohistochemical staining was
performed to aid the diagnosis of some patients to detect S100
(monoclonal mouse anti-human antibody; 1:500; cat. no. 5529; Cell
Signaling Technology, Inc.) and vimentin (monoclonal rabbit
antibody; 1:500; cat. no. 5741; Cell Signaling Technology, Inc.).
Antigen retrieval was performed using 0.01 mol/l citrate buffer at
98°C for 10 min, endogenous peroxidase activity was blocked using
0.3% hydrogen peroxide in methanol for 15 min at room temperature
and non-specific binding was blocked using 10% normal goat serum
(Wuhan Servicebio Technology Co., Ltd.) at 37°C for 30 min.
Subsequently, overnight incubation at 4°C was performed using the
aforementioned primary antibodies against S100 and vimentin,
followed by a 30-min incubation at 37°C with a horseradish
peroxidase-labeled anti-rabbit IgG antibody (1:500; cat. no. NL004;
R&D Systems, Inc.) and a horseradish peroxidase-labeled goat
anti-mouse IgG antibody (1:500; cat. no. GB23301; Wuhan Servicebio
Technology Co., Ltd.). Postoperative pathology was confirmed by two
independent pathologists.
Outcome assessment
The perioperative outcomes, surgical duration,
visual analog scale (VAS) score pre-operatively and at 1 week, 1
and 3 months postoperatively, blood loss, time to liquid intake,
hospitalization time and complications of the 12 patients were
calculated. Data on postoperative outcomes, a functional assessment
(motor and sensory function), tumor recurrence and metastasis
(radiography and MRI) were examined every 3 months for the first 2
years and every 6 months in the third year.
Results
The characteristics of the 12 included patients are
presented in Table I. All 12
patients underwent successful surgery and had stable intraoperative
vital signs. Postoperatively, the patients were extubated and
returned to the ward. All tumors were completely excised, and the
postoperative pathological results all revealed schwannomas.
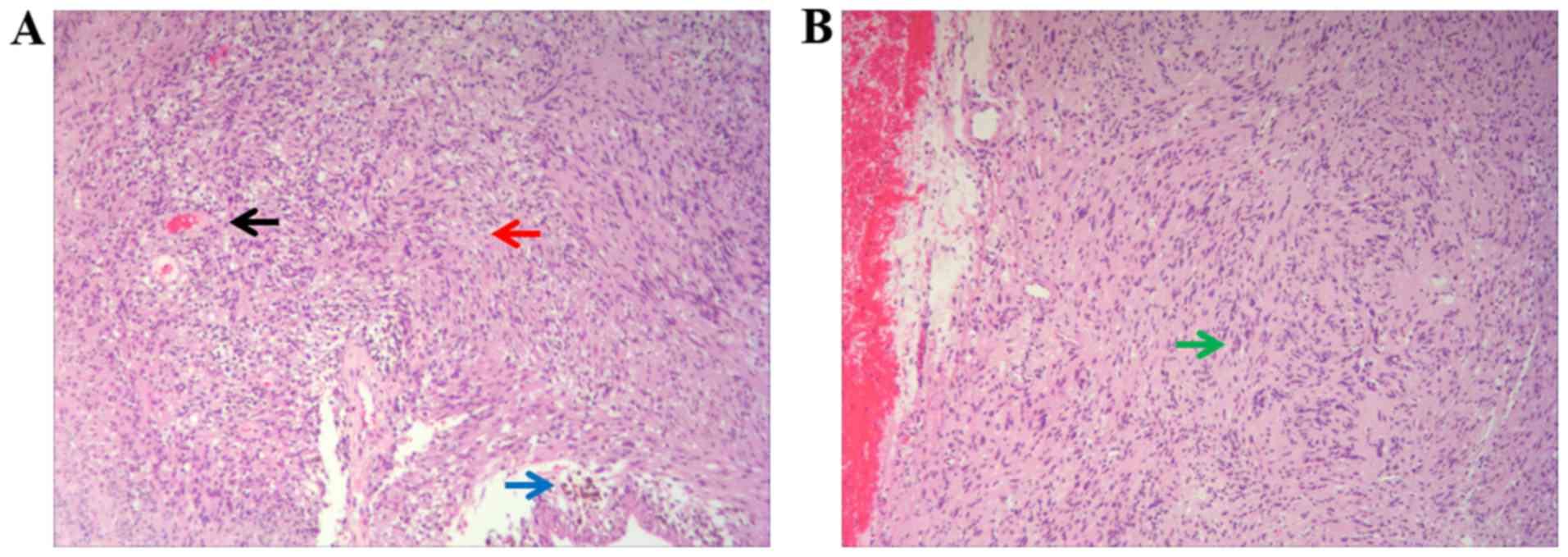
H&E staining was observed under the microscope, revealing that
the spindle cells were arranged in fascicles with red cytoplasm and
uniform nucleus size, and there were small thick-walled blood
vessels in the stroma (Fig. 4A). A
typical palisading pattern was also displayed (Fig. 4B). The tumor was completely removed
in all 12 patients, the surgical duration ranged between 76 and 245
min (mean, 106.08 min), and the intraoperative blood loss was
76–145 ml (mean, 101.67 ml). The average preoperative VAS score of
the patients was 3.25, and the average VAS score at 1 week, 1 and 3
months postoperatively was 1.08, 0.42 and 0.08, respectively. All
patients were out of bed on the second day after surgery, and the
postoperative drainage was 10–50 ml (mean, 33.50 ml). The drainage
tube was removed on the first day after the operation and the
patient returned to bed activities. Normal daily activities were
resumed on the second day after the operation. Postoperative bowel
movements and food intake were restored in the patients at 24–72 h
postoperatively. The postoperative hospital stay was 3–5 days
(mean, 3.92 days). No patients died perioperatively. No cases of
intestinal or ureteral injuries, adhesion obstruction, intestinal
obstruction, incision infection or pulmonary infection occurred
during the hospitalization. Of the patients, 2 reported wound pain
that was relieved 2 days later following oral anti-inflammatory and
analgesic drug administration. Follow-up continued for 16–41
months. During the follow-up period, a B-ultrasound or CT
examination was performed; no cases of tumor recurrence or
intestinal obstruction occurred.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
|
|
|
|
|
|
| Postoperative
VAS |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Sex | Age, years | Tumor size,
cma | Surgical duration,
min | Preoperative VAS | 1 week | 1 month | 3 month | Intraoperative blood
loss, ml | Postoperative
drainage, ml | Pathological
diagnosis | Hospitalization,
days | Tumor location |
|---|
| 1 | Male | 26 | 3.2×2.1×1.7 | 116 | 4 | 2 | 1 | 0 | 76 | 17 | Schwannoma | 3 | S1 |
| 2 | Female | 49 | 3.8×2.5×2.6 | 102 | 3 | 2 | 1 | 0 | 92 | 28 | Schwannoma | 4 | S1-2 |
| 3 | Female | 30 | 4.2×3.5×2.9 | 90 | 4 | 1 | 1 | 0 | 131 | 45 | Schwannoma | 5 | S2-4 |
| 4 | Female | 43 | 2.1×2.4×2.0 | 76 | 2 | 0 | 0 | 0 | 106 | 10 | Schwannoma | 4 | L5 |
| 5 | Male | 41 | 4.1×2.3×1.6 | 89 | 3 | 1 | 1 | 0 | 95 | 40 | Schwannoma | 4 | S1 |
| 6 | Female | 35 | 4.3×3.6×3.1 | 94 | 4 | 1 | 0 | 0 | 98 | 36 | Schwannoma | 3 | S1-2 |
| 7 | Female | 47 | 3.5×3.3×2.8 | 85 | 2 | 0 | 0 | 0 | 87 | 42 | Schwannoma | 4 | L5 |
| 8 | Male | 32 | 3.6×2.7×3.2 | 96 | 2 | 1 | 0 | 0 | 102 | 35 | Schwannoma | 4 | S3 |
| 9 | Female | 36 | 4.0×3.6×2.8 | 103 | 1 | 0 | 0 | 0 | 94 | 25 | Schwannoma | 5 | L5-S1 |
| 10 | Male | 29 | 4.6×3.6×3.4 | 92 | 5 | 2 | 0 | 0 | 108 | 45 | Schwannoma | 3 | S2-3 |
| 11 | Male | 42 | 3.7×3.3×4.1 | 85 | 3 | 1 | 0 | 0 | 86 | 29 | Schwannoma | 4 | S1 |
| 12 | Male | 46 | L5, 1.1×0.8×1.2;
S3, 4.3×3.2×3.4 | 245 | 6 | 2 | 1 | 1 | 145 | 50 | Schwannoma | 4 | L5, S3 |
In the traditional open surgery control group, the
results revealed that the surgical incision was 6–8 cm long, the
average surgical duration was 160.24 min, the average
intraoperative hemorrhage was 152 ml, the postoperative drainage
volume was 93 ml and the hospital stay was 9 days; in addition,
postoperative femoral nerve injury occurred in 1 patient during the
open surgery (data not shown).
Discussion
Presacral tumors, also known as retrorectal tumors,
are tumors occurring in the space of the sacrum and rectum. There
is loose connective tissue in the anterior sacral space that
contains various residual tissues of fetal embryos. The embryonic
development process is extremely complex, with diverse tissue
structures that are prone to tumorigenesis during the development
process (3,4). Although schwannomas grow slowly and
most patients have no pain or other clinical symptoms, it has been
suggested that surgery may not be necessary for asymptomatic benign
schwannomas. Choudry et al (13) followed up 8 cases of retroperitoneal
schwannomas confirmed by biopsy but not operated upon, and followed
them up for 13 to 63 months. After this time, imaging examination
showed no change in tumor size. In the case of benign
retroperitoneal schwannomas, malignancy rarely occurs; however,
they are also capable of local destruction and surgical removal is
required (1). Most orthopedic
surgeons still believe that the mass effect of the presacral
schwannomas should be included in the surgical indications
(4). Schwannomas arise from nerve
tissue, and it is important to prevent the injury of important
nerves during surgery. Surgical resection of schwannomas is
different from other benign tumors in that the tumor must be
completely removed without damaging the nerve fibers (2). Therefore, the majority of scholars
believe that the best treatment for primary retroperitoneal
schwannoma is total resection of the tumor without serious or
disabling complications, which is also consistent with the basic
principles of tumor treatment (3–5).
According to the literature, most of the asymptomatic patients or
those with mildly painful symptoms are between 20 and 50 years old,
and the majority are women (2,4). In the
absence of symptoms, the probability of local tumor recurrence or
distant metastasis is relatively low, and complete resection of the
tumor is an ideal treatment. The lack of invasive growth exhibited
by these tumors and their thick capsular lining make presacral
schwannomas amenable to complete resection (14).
At present, the standard treatment strategy for
presacral tumors is complete surgical resection, which can reduce
the symptoms of the tumor pressing on the surrounding organs, such
as constipation and frequent urination, as well as pain in the
waist and leg caused by the tumor pressing on the nerve root.
According to a previous report, tumors in ~40% of patients are
resected using the anterior surgical approach, tumors in 35% of
patients are resected using the posterior surgical approach, and
the tumors in the remaining patients are resected using an anterior
combined with posterior surgical approach (15). The choice of surgical approach also
depends on the experience of the orthopedic surgeon and the tumor
size, location and morphology. In recent years, with the
development of laparoscopic technology, there have been several
cases of laparoscopic resection of presacral tumors internationally
(14–16). The advantages of laparoscopic surgery
are adequate exposure, minor trauma and quick recovery; compared
with laparotomy, dissection provides a clearer surgical field, and
the intraoperative blood loss is significantly reduced. However,
laparoscopic surgery also has its limitations, such as poor
flexibility of the instrument limiting the range of motion of the
operator, narrow surgical vision, and the need for the operator to
concentrate for a long time, which can cause fatigue (17,18).
The da Vinci robotic surgery system has the
advantage of being minimally invasive, which makes up for its
shortcomings and limitations. Since it was approved by the United
States Food and Drug Administration in 2005, it has been widely
used abroad, particularly in urological surgery and gynecological
surgery (8–10). Robot-assisted surgical systems
provide surgeons with greater dexterity and accuracy, and can
reduce damage to the internal abdominal organs. The robotic surgery
system has the following characteristics (8,9,18): i) The use of a 3D high-definition
image that is magnified 10–15 times enables the clear
identification of the anatomical structure, improving surgical
accuracy; ii) the instrument arm mimics the movements of the
surgeon with 7 degrees of freedom, making it more flexible and
accurate; iii) the controller filters tremors automatically and is
more stable than a manual tool; iv) the surgeon adopts a sitting
posture, which is conducive to the completion of a long, complex
surgery; and v) the incidence of perioperative complications is
decreased due to minor trauma, rapid recovery and a short hospital
stay. Pacchiarotti et al (19) reported two cases of schwannomas, one
in the posterior superior mediastinal sulcus and the other in the
inferior thoracic sulcus, the removal of the schwannomas in these
patients by conventional surgery is technically challenging.
Robotic thoracoscopic surgery was used to completely remove the
tumors, which suggests that simple anterior endoscopic surgery at
extreme locations is safe and effective. Garzon-Muvdi et al
(20) found that the use of a nerve
stimulator and da Vinci's bipolar cauterizer were good choices for
laparoscopic-assisted surgical resection of a presacral mass.
During the surgery, the nerve adjacent to the tumor can be
monitored and stimulated, and the surgeon can obtain the best
surgical resection while understanding its anatomical structure and
preserving the nerve function.
The present study also found certain advantages of
robotic surgery in the surgical resection of presacral tumors.
Robotic surgery has the spatial advantage of exposing the posterior
tumor, and the robotic arm can pull the rectum and sacrum to
increase clearance and better reveal the iliac vein, former sacral
venous plexus and pelvic plexus, notably reducing intraoperative
bleeding and damage to the surrounding tissues. Jun et al
(21) reported the case of a young
female patient with a large presacral schwannoma (originating from
the right S2 nerve). The da Vinci surgical robotic system provides
better visualization during deep pelvic surgery and provides
two-wrist instrumental control, which is sufficient for the
operator to successfully detach the tumor from the surrounding
sensitive structures. Nerve stimulation during surgery can
differentiate important nerves and avoid causing nerve damage. The
patient suffered no complications, lost <75 ml of blood during
the operation, was hospitalized for 3 days and returned to normal
work within 2 weeks after the operation. The study suggested that
for certain patients with presacral tumors, surgical resection
assisted by da Vinci surgical robot has a greater advantage. In the
present study, all tumors were completely excised, and the
postoperative pathological results indicated all neurilemmomas.
Intraoperative blood loss was 76–145 ml (mean, 101.67 ml) and
postoperative drainage was 10–50 ml (mean, 33.50 ml). The drainage
tube was removed on the first day after the operation and the
patient returned to bed activities. Normal daily activities were
resumed on the second day after the operation. The data of patients
with retroperitoneal schwannoma previously resected by open surgery
in the Orthopedics of Departments of Wuhan Union Hospital were
compared and analyzed. In the traditional open surgery group, the
length of the incision was longer, the operation time was
prolonged, the average intraoperative bleeding and postoperative
drainage volume were increased, while the hospital stay was
shortened compared with in the da Vinci surgery group. In addition,
postoperative femoral nerve injury occurred in 1 patient during the
open surgery, while no complications were found in all the patients
undergoing da Vinci surgery. The results of the present study are
consistent with those reported in the literature (11); en bloc robot-assisted resection of
presacral nerve sheath tumors was associated with limited procedure
duration, minor blood loss and satisfying intra- and post-operative
outcomes. da Vinci surgery can be used as a safe surgical treatment
for retroperitoneal schwannomas. However, traditional open surgery
should be performed for patients with large tumors, which may not
be malignant, or for patients whose economic conditions do not
allow for the use of the robotic surgery system.
The da Vinci robot system consists of a doctor's
console, a video system and a bedside arm tower. The console doctor
can simultaneously operate the robotic arm to achieve
electrocoagulation and perform other motions. The assistant on the
operating table can achieve traction, suction and other auxiliary
motions through the auxiliary channel. Therefore, a complicated
operation can be performed by only one anesthesiologist, one
instrument nurse, one doctor on the operating table and one doctor
on the console, which can greatly save manpower and material
resources. However, there are still some shortcomings of the whole
operation system. To begin with, the da Vinci robotic surgery
system is a mechanical operation device. In the whole operation
process, there is visual perception but no tactile perception, and
the operation process largely depends on the surgeon's anatomical
knowledge and surgical skills. Additionally, although the
flexibility of the operating system is greatly increased and it can
largely improve the operation scale, the flexible operation
requirements present a big challenge, probably as the requirements
cause system overload and the lack of sensory feedback can lead to
a mechanical arm-induced injury. Furthermore, the price of the
whole surgical system is high, as are the maintenance and wear
costs, which places a high economic burden on hospitals and
patients. Finally, the da Vinci robotic surgery system can
currently only be used in soft tissue systems, meaning that it
cannot be used for orthopedic surgeries.
In summary, compared with traditional open surgery
or laparoscopic surgery, the da Vinci robotic surgery system has
unique advantages for presacral tumor surgery. However, the present
study still has certain limitations. The main limitation is that
the study is retrospective and the sample size included is
relatively small. Therefore, a multi-center prospective study with
a large sample size to will be performed in the future to compare
and analyze the clinical effects of robot-assisted da Vinci surgery
versus open surgery or thoracoscopic surgery in presacral tumor
surgery. The da Vinci robotic surgery system is becoming more
widely used in China, and its clinical value is being recognized by
more surgeons. Although this system still has some shortcomings,
the continuous progress of technology and the increasing
familiarity of clinicians will bring benefits to more patients with
different diseases in the near future.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81904231), the China
Postdoctoral Science Foundation (grant no. 2020M672369) and the
PostDoctoral Innovation Practice Post in Hubei Province (grant no.
34).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FP, ZZ, QW and BW performed the experiments and
wrote the manuscript. FP and DS made substantial contributions to
conception and design of the study. ZZ and BW were responsible for
the design of the experiments. ZC and KC analyzed the experimental
data. ZC, KC, JL and ZS assisted with the statistical analysis. ZS
and JL critically revised the manuscript, provided final approval
of the version to be published and made substantial contributions
to conception and design. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted with the approval of
the Ethics Committee of Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China), and
in line with the Declaration of Helsinki. All patients and legal
guardians provided written informed consent for the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao XH, Zhang W, Fu CG, Liu LJ, Yu ED and
Meng RG: Local recurrence after intended curative excision of
presacral lesions: Causes and preventions. World J Surg.
35:2134–2142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolpert A, Beer-Gabel M, Lifschitz O and
Zbar AP: The management of presacral masses in the adult. Tech
Coloproctol. 6:43–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shenoy S: Diagnosis and management of
presacral (retrorectal) tumors. J Gastrointest Cancer. 49:373–378.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dziki Ł, Włodarczyk M,
Sobolewska-Włodarczyk A, Saliński A, Salińska M, Tchórzewski M, Mik
M, Trzciński R and Dziki A: Presacral tumors: Diagnosis and
treatment-a challenge for a surgeon. Arch Med Sci. 15:722–729.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saxena D, Pandey A, Bugalia RP, Kumar M,
Kadam R, Agarwal V, Goyal A, Kankaria J and Jenaw RK: Management of
presacral tumors: Our experience with posterior approach. Int J
Surg Case Rep. 12:37402015. View Article : Google Scholar
|
|
6
|
Ohsawa M, Miguchi M, Yoshimitsu M, Oishi
K, Kohashi T, Hihara J, Mukaida H, Kaneko M, Egi H, Ohdan H and
Hirabayashi N: Laparoscopic excision of a retroperitoneal
schwannoma: A case report. Asian J Endosc Surg. 12:192–196. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jan H, Kapoor T and Ghai V: A stepwise
approach to laparoscopic enucleation and excision of
retroperitoneal cysts. J Minim Invasive Gynecol. 26:367–368. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atallah S, Parra-Davila E, Melani AGF,
Romagnolo LG, Larach SW and Marescaux J: Robotic-assisted
stereotactic real-time navigation: Initial clinical experience and
feasibility for rectal cancer surgery. Tech Coloproctol. 23:53–63.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al Tinawi B, Jessop M and Salkini MW:
Utilizing da Vinci® surgical system to treat challenging
urinary stones. Urol Ann. 11:304–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoo JG, Kim WJ and Lee KH: Single-site
robot-assisted laparoscopic staging surgery for presumed clinically
early-stage ovarian cancer. J Minim Invasive Gynecol. 25:380–381.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin J, Wu H, Tu J, Zou C, Huang G, Xie X,
He Y and Shen J: Robot-assisted sacral tumor resection: A
preliminary study. BMC Musculoskelet Disord. 19:1862018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu H, Wang S and Shao Z: Robot-assisted
excision of multiple retroperitoneal schwannomas: A case and
literature review. Int J Clin Exp Med. 12:2833–2837. 2019.
|
|
13
|
Choudry HA, Nikfarjam M, Liang JJ, Kimchi
ET, Conter R, Gusani NJ and Staveley-O'Carroll KF: Diagnosis and
management of retroperitoneal ancient schwannomas. World J Surg
Oncol. 7:122009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Emohare O, Stapleton M and Mendez A: A
minimally invasive pericoccygeal approach to resection of a large
presacral schwannoma: Case report. J Neurosurg Spine. 23:81–85.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pennington Z, Reinshagen C, Ahmed AK,
Barber S, Goodwin ML, Gokaslan Z and Sciubba DM: Management of
presacral schwannomas-a 10-year multi-institutional series. Ann
Transl Med. 7:2282019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Näf F, Choschzick M and Melcher GA:
Atypical case of a painful presacral tumor. Am J Case Rep.
16:760–762. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye SP, Shi J, Liu DN, Jiang QG, Lei X, Qiu
H and Li TY: Robotic-assisted versus conventional
laparoscopic-assisted total gastrectomy with D2 lymphadenectomy for
advanced gastric cancer: Short-term outcomes at a mono-institution.
BMC Surg. 19:862019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bo T, Chuan L, Hongchang L, Chao Z,
Huaxing L and Peiwu Y: Robotic versus laparoscopic rectal resection
surgery: Short-term outcomes and complications: A retrospective
comparative study. Surg Oncol. 29:71–77. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pacchiarotti G, Wang MY, Kolcun JPG, Chang
KH, Al Maaieh M, Reis VS and Nguyen DM: Robotic paravertebral
schwannoma resection at extreme locations of the thoracic cavity.
Neurosurg Focus. 42:E172017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garzon-Muvdi T, Belzberg A, Allaf ME and
Wolinsky JP: Intraoperative Nerve monitoring in robotic-assisted
resection of presacral ganglioneuroma: Operative technique. Oper
Neurosurg (Hagerstown). 16:103–110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jun C, Sukumaran M and Wolinsky JP:
Robot-assisted resection of pre-sacral schwannoma. Neurosurg Focus.
45 (VideoSuppl1):V12018. View Article : Google Scholar : PubMed/NCBI
|