Introduction
Oral squamous cell carcinoma (OSCC) is the most
common type of cancer in the oral and maxillofacial region and is
estimated to account for ~80% of all oral and maxillofacial
malignancies (1,2). The 5-year survival rate of patients
with OSCC is only 50–60%, which is even lower in patients with
locally advanced lesions (3,4). At present, the recommended treatment
option for patients with locally-advanced resectable OSCC is
radical surgery with postoperative radiation or chemoradiation, a
decision that is dependent on the post-operative pathological
findings (5). Therefore, there is a
demand to improve the clinical outcomes of patients with OSCC.
Induction chemotherapy has been documented to downgrade locally
advanced or aggressive cancers and to increase the likelihood of
primary lesion eradication (6).
Docetaxel, cisplatin and 5-fluorouracil (5-FU; TPF) induction
chemotherapy protocol has been shown to be superior compared that
with only cisplatin and 5-fluorouracil in patients with head and
neck squamous cell carcinoma (HNSCC) (7,8).
Unfortunately, a previous clinical trial conducted, which
investigated the effects of TPF induction chemotherapy in patients
with clinical stages III and IVA OSCC, failed to observe
significant improvements in clinical outcomes (9,10).
However, subgroup analysis revealed that patients with cN2 OSCC and
high cyclin D1 expression benefitted from TPF induction
chemotherapy with respect to clinical outcomes (11).
Functionally, cyclin D1 combines with
cyclin-dependent kinase 4/6 to form a complex to promote
G1-S phase cell cycle progression. This form of
regulation participates in a number of cell processes, including
promotion of cell proliferation, regulation of cell growth,
modulation of mitochondrial activity, inhibition of DNA repair and
acceleration of migration (12,13).
Cyclin D1 has been found to be overexpressed in a large portion of
malignant tumors, including 39–64% primary HNSCCs (14). In addition, previous studies have
shown that cyclin D1 overexpression is a potential biomarker for
predicting the prognosis of HNSCC, where it associates with occult
lymph node metastasis (13,15,16). In
a previous report, patients with OSCC and low cyclin D1 expression
exhibited superior clinical outcomes compared with those in
patients with high cyclin D1 expression (11). This previous study also revealed that
only patients with stage N2 OSCC benefitted from TPF induction
chemotherapy with respect to overall survival (OS) and distant
metastasis-free survival (DMFS) (11). However, the mechanism underlying
responses to TPF chemotherapeutic agents in patients with OSCC and
its association with cyclin D1 overexpression remains poorly
understood. Although it has been previously reported that cyclin D1
overexpression is associated with improved responses to cisplatin
in HNSCC cell lines (17), cyclin D1
overexpression has also been reported to mediate cisplatin,
platamin, neoplatin, cismaplat and cis-diamminedichloridoplatinum
(II) therapy resistance (18–20).
Based on results from a previous study, which
documented survival benefits from TPF induction chemotherapy in
patients with cN2 OSCC and high cyclin D1 expression (9), the present study aimed to determine the
relationship between cyclin D1 expression and responses to TPF
chemotherapy in OSCC cell lines.
Materials and methods
Cell culture
The present study used three OSCC cell lines: HB96
cells, which were previously established in our lab from an in
vitro cellular carcinogenesis model of OSCC (21), CAL27 and HN30 cells. The CAL27 cell
line was purchased from ATCC and the HN30 cell line was a gift from
Professor Li Mao from the University of Maryland Dental School
(Baltimore, MD, USA). All cell lines were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.). All cells were maintained in a 5%
CO2 humidified atmosphere at 37°C.
Patients and samples
From March 2008 to December 2010, 232 patients with
clinical stage III and IVA OSCC (sex, 160 males and 72 females; age
range, 26–75 years; mean age, 55.4±10.0 years) were enrolled into
the present study. The patients participated in a previous phase 3
trial (clinical trial registration no. NCT01542931), which
investigated the potential benefit of TPF induction chemotherapy
prior to standard treatment for locally advanced OSCC. The present
study is a follow-up study of our previous study (11). All the patients were enrolled into
the Department of Oral and Maxillofacial-Head and Neck Oncology at
the Ninth Peoples' Hospital, Shanghai Jiao Tong University School
of Medicine (Shanghai, China). The detailed protocol of the
clinical trial has been previously described (9). Briefly, patients who met the criteria
were randomly assigned to into the experimental group (n=105), who
underwent TPF induction chemotherapy, radical surgery (tumor
resection and neck dissection) and post-operative radiotherapy, or
the control group (n=127), who underwent surgery and post-operative
radiotherapy.
The pretreatment levels of cyclin D1 expression in
the tumor tissues (taken before induction chemotherapy) were
assessed using immunohistochemical staining as previously
described, as well as the representative immunohistochemical images
of cyclin D1 staining (11). Rabbit
monoclonal antibody to cyclin D1 (1:150 dilution; cat. no.
ab134175; Abcam) was used with the Dako Real™ EnVision™ Detection
System, Peroxidase/DAB+, Rabbit/Mouse (cat. no. K5007; Agilent
Technologies, Inc.). Staining for cyclin D1 expression was observed
in the cellular nucleus using light microscopy. Cyclin D1
expression index was calculated on the basis of the proportion of
stained cells using a semi-quantitative scale, described as
follows: i) Negative, ≤10% stained cells; ii) Weakly positive,
<50% of stained cells; and iii) Strong positive, ≥50% of stained
cells. In accordance with previous studies (11,22,23), low
cyclin D1 expression was defined as negative and weakly positive
cyclin D1 expression whereas high cyclin D1 expression was defined
as strong positive cyclin D1 expression. The present study was
approved by the Ethics Committee of the Ninth People's Hospital,
Shanghai Jiao Tong University School of Medicine and written
informed consent was obtained from each patient.
Cyclin D1 RNA interference
In total, two sets of small interfering (si)RNA
oligonucleotides for cyclin D1 and a negative control
oligonucleotide were designed and synthesized by Sangon Biotech
Co., Ltd. Their sequences are as follows: SiRNA1 sense,
5′-CCCGCAGAUUUCAUUGAAdtdt-3′ and antisense,
5′-UUCAAUGAAAUCGUGCGGGdtdt-3′; siRNA2 sense,
5′-GUAUACUGCUCUAUUCCAAdtdt-3′ and antisense,
5′-UUGGAAUAGAGCAGUAAUCdtdt-3′ and siRNA-NC sense,
5′-UUCUCCGAACGUGUCACGUdtdt-3′ and antisense,
5′-ACGUGACACGUUCGGAGAAdtdt-3′. The siRNAs (100 nM) were transiently
transfected into HB96 and CAL27 cells using the
Lipofectamine® 3000 transfection reagent, according to
the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). Western blotting was applied to measure the expression
levels of cyclin D1 (Fig. S1). The
time interval between transfection and subsequent experiments was
24 h.
Cyclin D1 gene transfection
The lentiviral overexpression vector
pLVX-puro-hcyclin D1 (cat. no. V109020035) and the empty pLVX puro
(cat. no. V109050901) plasmids were obtained from Shanghai Qihe
Biotechnology Co., Ltd. The plasmids (0.28 µg/ml) were transfected
into 293T cells (cultured in DMEM supplemented with 10% FBS in a 5%
CO2 humidified atmosphere at 37°C), and after ~7 days
the supernatant containing the lentiviral particles was collected
and filtered through a 4-µm filter. CAL27 and HN30 cells were then
treated with the vector supernatant (5×107 TU/ml) and
screened with puromycin (Life Technologies; Thermo Fisher
Scientific, Inc.), which was added to the medium at a final
concentration of 1 µg/ml. Western blotting was applied to measure
the expression levels of cyclin D1 (Fig. S1).
Western blotting assay
Total protein was extracted from collected cells
(HB96, CAL27 or HN30) at 80% confluency and lysed in ice cold 2X
lysis buffer containing 125 mM Tris-HCl (pH 6.8), 5% w/v SDS and
24.75% glycerol, as previously described (24). All procedures were performed on ice.
Total protein concentration was determined using the Bradford assay
according to the manufacturer's protocol (Pierce; Thermo Fisher
Scientific, Inc.) Extracted proteins (15 µg/lane) were separated
using 10–12% SDS-PAGE and then transferred electrophoretically onto
0.22-µm PVDF membranes (EMD Millipore) using a wet transfer system
(Bio-Rad Laboratories, Inc.). The membranes were blocked with
blocking buffer containing 5% dry skimmed milk in TBS with 0.02%
Tween-20 at room temperature for 1 h and incubated overnight with
primary antibodies at 4°C before being incubated with anti-mouse
(1:5,000; cat. no. 7076; Cell Signaling Technology, Inc.) or
anti-rabbit (1:5,000; cat. no. 7704; Cell Signaling Technology,
Inc.) IgG secondary antibodies conjugated to horseradish peroxidase
at room temperature for 1 h for chemiluminescent detection
(LumiBest ECL substrate solution kit; cat. no. sb-wb011; Share-Bio,
Inc.). Finally, the PVDF membranes were scanned and analyzed using
an enhanced chemiluminescence detection system (Amersham™ Imager
600; GE Healthcare). β-actin (1:10,000; cat. no. 1226; Cell
Signaling Technology, Inc.) was used as a loading control. The
primary antibodies used were as follows: Rabbit anti-cyclin D1
monoclonal antibody (1:500; cat. no. ab16663; Abcam); rabbit
anti-cleaved fragment of human PARP (Asp214; 1:500; cat. no. 5625P;
Cell Signaling Technology, Inc.) and cleaved fragment of caspase-3
(Asp175, 1:500; cat. no. 9664P; Cell Signaling Technology,
Inc.).
Cytotoxicity assay and
chemotherapeutic agents
Transfected cells (2×103 per well) were
seeded into 96-well plates and cultured in 100 µl medium without
glutamine and penicillin-streptomycin at 37°C for 8–12 h before
being exposed to 2X, 3X or 10X drug gradient concentrations
(depending on the respective IC50 for each cell line,
which was calculated according to the cell viability after
treatment with different drug gradient concentrations) of
docetaxel, cisplatin or 5-FU at 37°C for 72 h. The supernatant of
each well was then removed before 100 µl Cell Counting Kit-8
(CCK-8) solution was added according to the manufacturer's protocol
(Dojindo Molecular Technologies, Inc.), consisting of fresh medium
with 10% CCK-8 solution. Subsequently, the 96-well plates were
incubated at 37°C for an additional 2 h. Absorbance values were
then read at 450 nm, which was used to calculate cell viability.
This experiment was performed in triplicate.
Statistical analysis
Data analysis was performed using SPSS18.0 for
Windows (SPSS, Inc.). Following initial treatment, patients were
monitored every 3 months for the first 2 years, every 6 months in
the subsequent 3–5 years and once a year thereafter until death or
censoring of data. OS was calculated from the date of random
assignment to the date of death. Disease-free survival (DFS),
locoregional recurrence-free survival (LRFS) and DMFS were
calculated from the date of random assignment to recurrence,
locoregional recurrence and distant metastasis, respectively, or
death from any causes. Survival analysis was conducted using the
Kaplan-Meier method and compared using log-rank test.
χ2 test was performed to compare the
differences between the low and high cyclin D1 expression groups
based on the different baseline factors. Bonferroni test was
performed following Kruskal-Wallis test for the comparison of
non-parametric data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cyclin D1 expression and treatment
outcomes
Among the 232 patients with OSCC, no significant
differences could be found between the high and low cyclin D1
expression groups in terms of gender, age, primary tumor site, T
stage, N stage, pathologic grade, tobacco use or alcohol
consumption (Table SI). In
accordance with IHC staining, patients with low cyclin D1
expression were defined as negative and weak positive cyclin D1
expression (<50% of stained cells), whilst high cyclin D1
expression was defined as strong positive cyclin D1 expression
(≥50% of stained cells). During the follow-up period (first
quartile, 55 months; median, 67 months; third quartile, 75 months),
patients with low cyclin D1 expression exhibited significantly
superior long-term clinical outcomes compared with those in
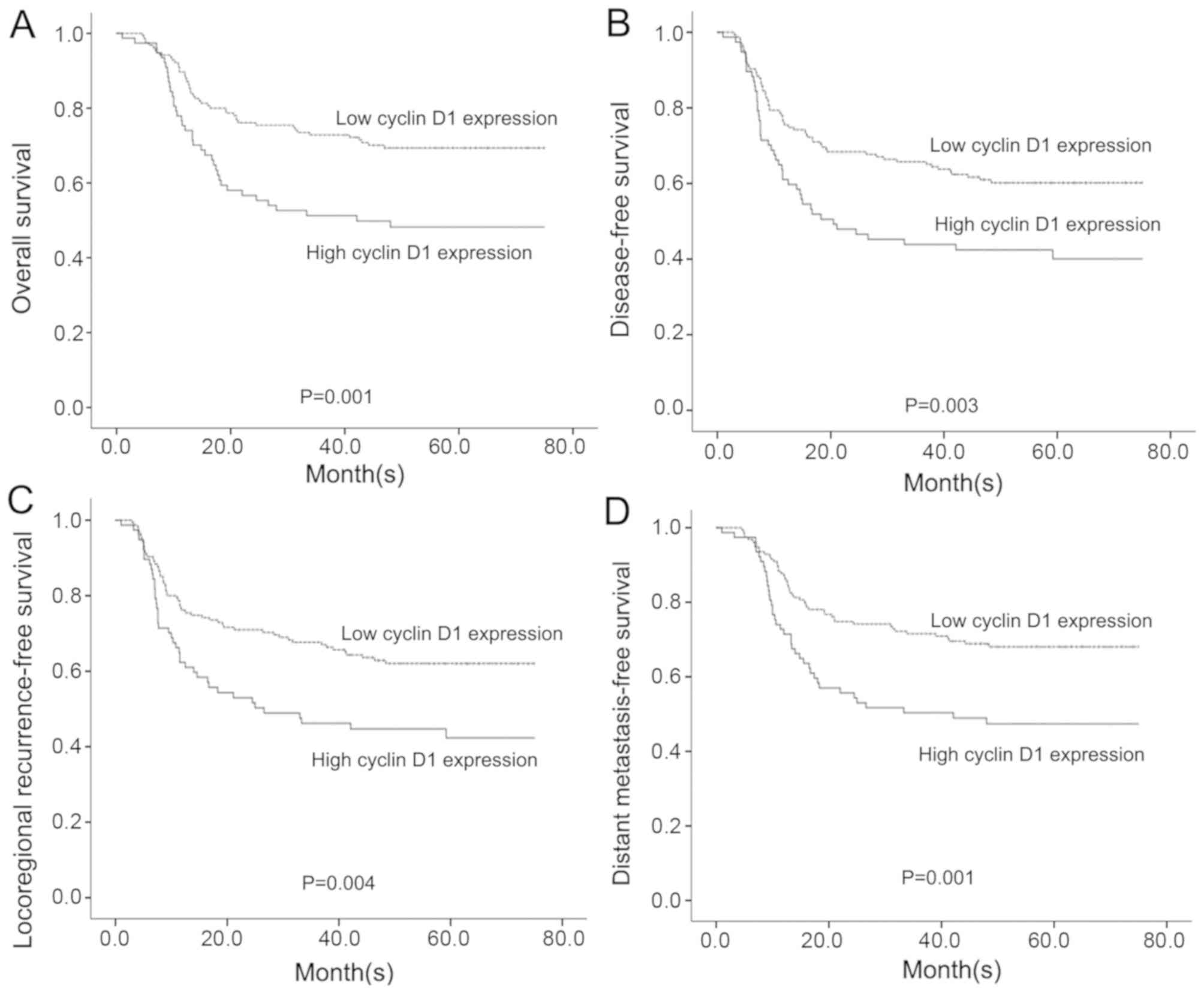
patients with high cyclin D1 expression (Fig. 1; Table
SII) with respect to OS (P=0.001), DFS (P=0.003), LRFS
(P=0.004) and DMFS (P=0.001).
Subgroup analysis was subsequently performed to
identify patients with different levels of cyclin D1 expression who
may benefit from TPF induction chemotherapy with respect to
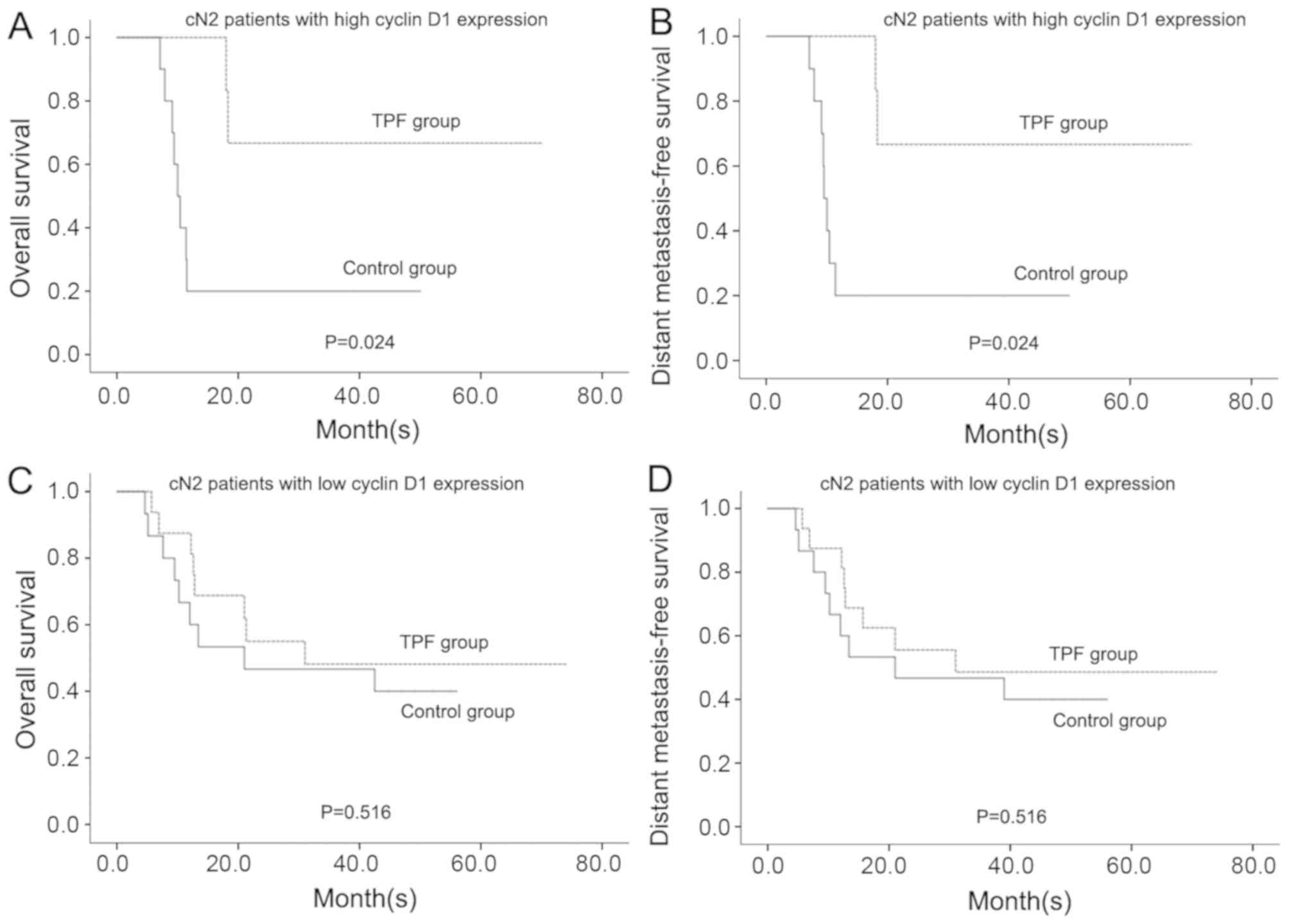
long-term prognosis. Only patients with cN2 OSCC and high cyclin D1
expression, who were at high risk for distant metastasis and death,
were found to be able to benefit from TPF induction chemotherapy.
These patients benefited from TPF induction chemotherapy with
respect to OS (P=0.024) and DMFS (P=0.024) whereas the patients
with cN2 OSCC with low cyclin D1 expression did not benefit from
TPF induction chemotherapy (Fig.
2).
Upregulation of cyclin D1 expression
enhances sensitivity to docetaxel, cisplatin and 5-FU in OSCC
cells
To support the clinical findings of patients with
cN2 OSCC benefiting from TPF induction chemotherapy in
vitro, the association between cyclin D1 expression and
sensitivity to the TPF chemotherapeutic agents was analyzed in OSCC
cell lines, especially the CAL27 cell line, which was originally
established from a patient with cN2 oral tongue squamous cell
carcinoma (25). CCK-8 assay was
used to determine cell viability of OSCC cell lines after the down-
or upregulation of cyclin D1 expression following treatment with
chemotherapeutic agents docetaxel, cisplatin or 5-FU for 72 h at
different concentrations, which was dependent on their respective
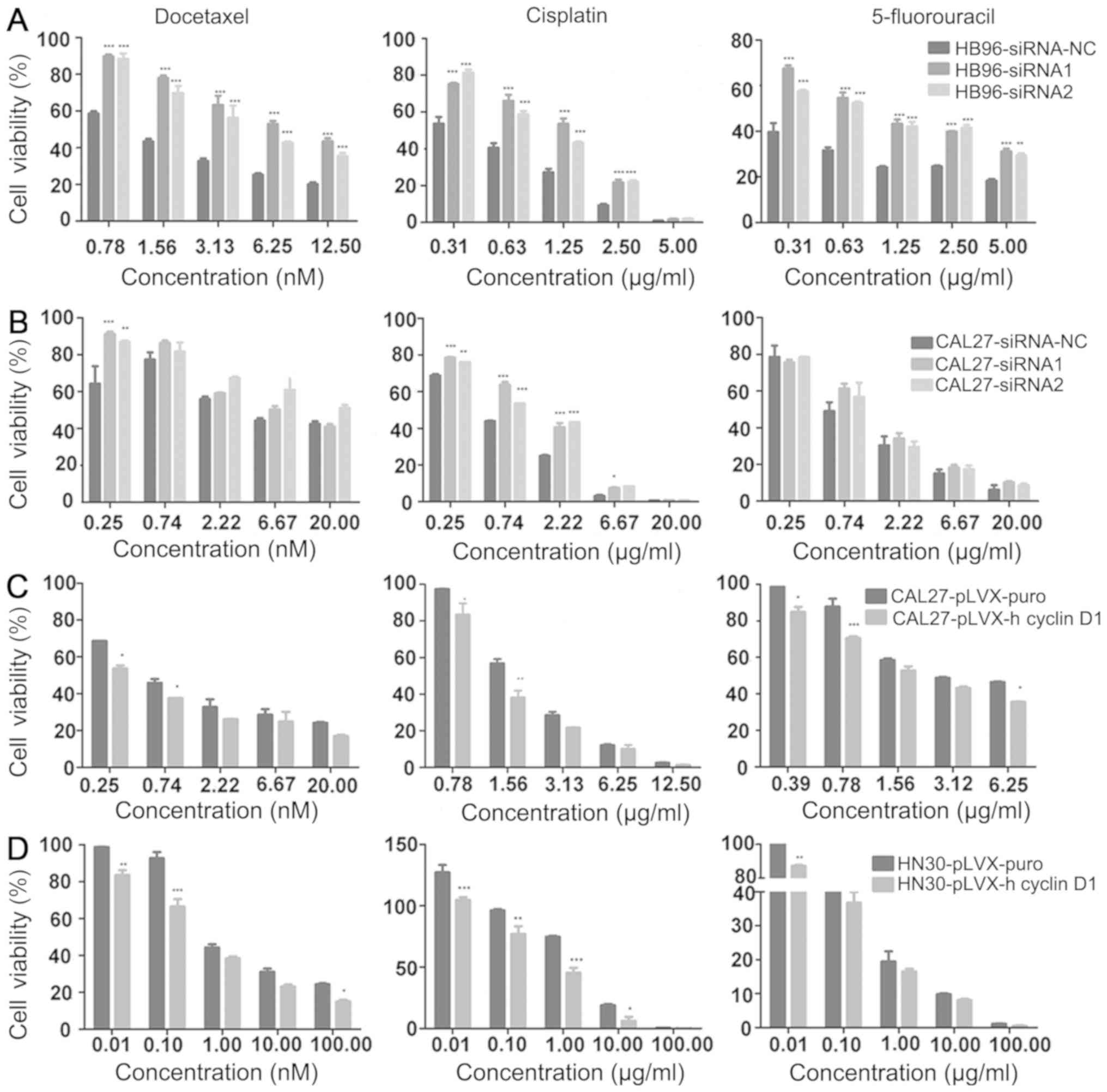
IC50 values for each cell line (Table SIII). Following the downregulation
of cyclin D1 expression in HB96 cells, sensitivity to docetaxel,
cisplatin or 5-FU was found to be significantly reduced compared
with cells transfected with the siRNA-NC, resulting in increased
cell viability at all doses in the siRNA groups (Fig. 3A). Following the downregulation of
cyclin D1 expression in CAL27 cells, a significant reduction in the
sensitivity to docetaxel and cisplatin was observed at low doses
(0.25 nM for docetaxel and <2.5 µg/ml for cisplatin), but not at
high doses, and no reduction in the sensitivity to 5-FU was
observed at all doses (Fig. 3B).
After the CAL27 and HB96 cells with cyclin D1 expression knocked
down were treated with docetaxel, cisplatin and 5-FU altogether,
increased cell viability was observed but the difference was not
significant (Fig. S2). By contrast,
when cyclin D1 was overexpressed in CAL27 and HN30 cells (this was
not performed in HB96 cells since they already had high cyclin D1
expression), significantly increased sensitivity to these agents
was found compared with cells transfected with the empty vector,
with decreased cell viability, especially at low doses (Fig. 3C and D).
Upregulation of cyclin D1 expression
enhances sensitivity to docetaxel, cisplatin and 5-FU via
caspase-3-dependent apoptosis in OSCC cells
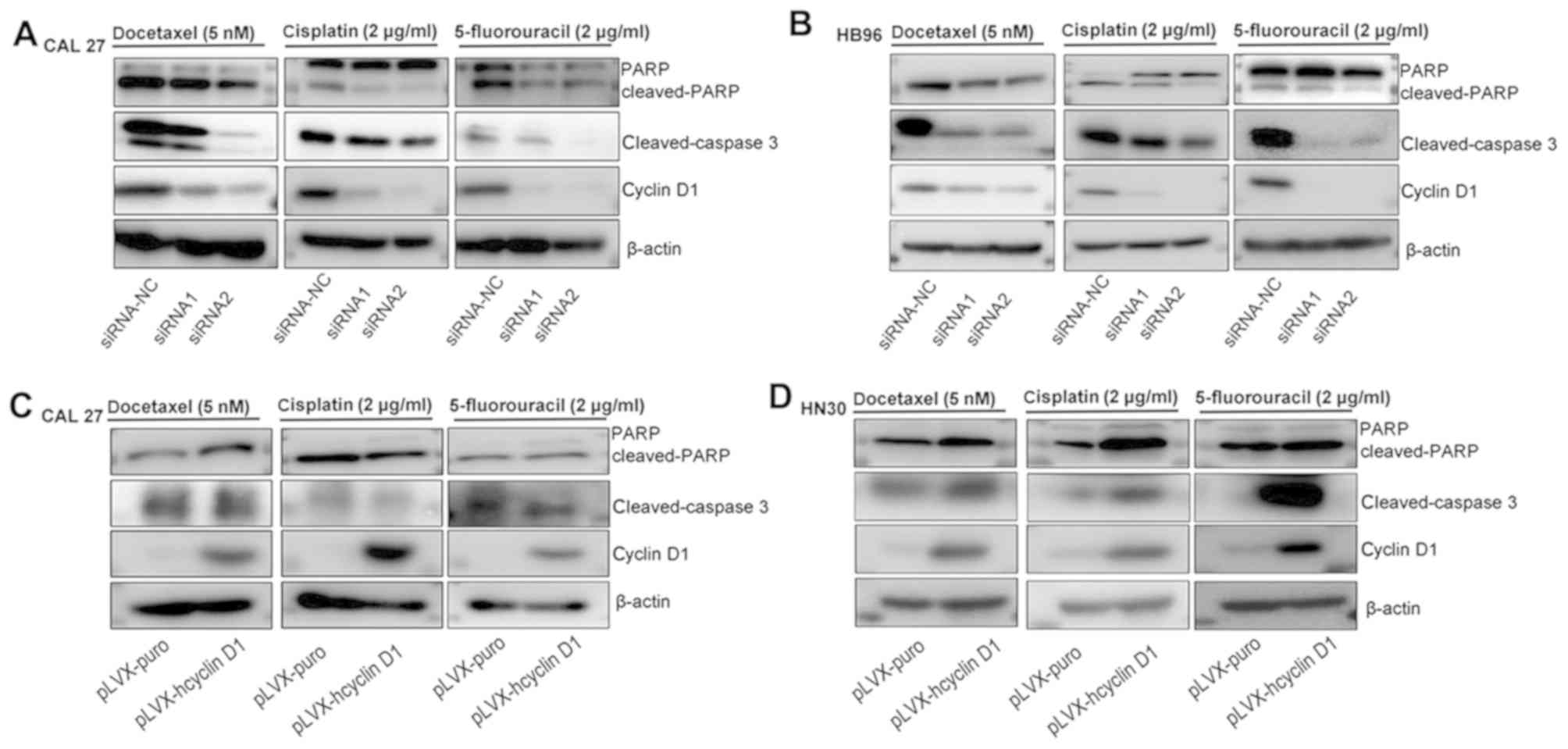
Apoptotic protein levels (caspase-3 and PARP) were
next measured in CAL27, HN30 and HB96 cells that were treated
docetaxel, cisplatin and 5-FU. After CAL27 and HB96 cells
transfected with cyclin D1 expression knocked down were treated
with docetaxel, cisplatin and 5-FU for 72 h, the levels of cleaved
caspase-3 and PARP levels were found to be reduced compared with
cells transfected with siRNA-NC (Fig. 4A
and B). By contrast, after the HN30 cells overexpressing cyclin
D1 were treated with docetaxel, cisplatin and 5-FU for 72 h,
cleaved caspase-3 and PARP levels were increased compared with
cells transfected with the empty plasmid; however, in CAL27 cells
overexpressing cyclin D1 treated with the three agents, an increase
in cleaved PARP was observed in cells treated with docetaxel and
5-FU, but not in those treated with cisplatin, and no differences
in cleaved caspase-3 with the three agents were observed (Fig. 4C and D).
Discussion
In the present study it was found that patients with
cN2 OSCC and high cyclin D1 expression conferred long-term survival
benefits from TPF induction chemotherapy compared with those who
received standard treatment. By contrast, patients with cN2 OSCC
and low cyclin D1 expression did not benefit from TPF induction
chemotherapy compared with those who received standard treatment.
In vitro studies subsequently confirmed that OSCC cells
overexpressing cyclin D1 were more sensitive to chemotherapeutic
agents docetaxel, cisplatin and 5-FU via the caspase-3-dependent
pathway.
Cyclin D1 serves an oncogenic role in the majority
of malignant tumors, with previously documented roles including the
inhibition of DNA repair, enhancements in cell proliferation and
migration (26,27). Patients with cancer harboring high
cyclin D1 expression have been reported to exhibit inferior
clinical outcomes compared with those with low cyclin D1
expression, including breast cancer, pancreatic adenocarcinoma,
colorectal carcinoma and OSCC (28,29). To
determine the optimal treatment protocol with which to improve the
clinical outcomes of patients with OSCC and high cyclin D1
expression, patients from a previous randomized trial (9) involving TPF induction chemotherapy in
OSCC were chosen for the measurement of cyclin D1 expression in the
pretreatment samples. Only patients with cN2 OSCC and high cyclin
D1 expression benefitted from TPF induction chemotherapy compared
with those who received standard treatment, whilst patients in
other subgroups did not benefit from TPF induction chemotherapy. Of
note, patients with cN2 OSCC have a relatively higher risk of
distant metastasis compared with patients with cN0 and cN1 OSCC
(9). OSCC cells with high cyclin D1
expression tend to be more aggressive compared with those with low
cyclin D1 expression, which was demonstrated in previous studies,
where cyclin D1 overexpression increased oral cancer cell migration
and cell motility (13,30). Therefore, patients with cN2 OSCC and
high cyclin D1 expression may have a high risk for distant
metastasis. Induction chemotherapy has been previously shown to
benefit patients with HNSCC with respect to DMFS (31,32). As
demonstrated by results in the present study, patients with cN2
OSCC and high cyclin D1 expression exhibited higher DMFS after
being treated with TPF induction chemotherapy compared with
standard treatment, which translated into improvements in OS.
To explain the clinical benefit from TPF induction
chemotherapy in Patients with cN2 OSCC, in vitro experiments
on the sensitivity to TPF chemotherapeutic agents was performed in
OSCC cell lines. The CAL27 cell line was originally derived from a
patient with cN2 oral tongue squamous cell carcinoma (25). Sensitivity to the chemotherapeutic
agents docetaxel, cisplatin and 5-FU in OSCC cells following cyclin
D1 overexpression was found to be increased compared with cells
transfected with empty plasmids. By contrast, sensitivity to these
chemotherapeutic agents was decreased in OSCC cells following
cyclin D1 knockdown compared with cells transfected with siRNA-NC.
These findings suggest that the OSCC cells overexpressing cyclin D1
are more sensitive to docetaxel, cisplatin and 5-FU. The anticancer
activity of cisplatin is to combine with DNA to form adducts by
cross-linking, in turn inhibiting DNA replication and
transcription, blocking G2 phase entry or
S/G2 phase progression (33). By contrast, 5-FU inhibits the
synthesis of pyrimidine by inhibiting thymidylate synthase, leading
to the depletion of intracellular dTTP library (34). Docetaxel inhibits microtubule
depolymerization to arrest the cell cycle at G2/M phase
and induce apoptosis (35). These
molecular basis promotes the clinical application combining cyclin
D1 overexpression with TPF induction chemotherapy. Although
controversies remain regarding the association between cyclin D1
expression and responses to induction chemotherapy, the present
study demonstrated a positive association between cyclin D1
overexpression and sensitivity to TPF induction chemotherapy in
patients with cN2 OSCC. Akervall et al (17) previously studied 23 SCC cell lines
and demonstrated that cyclin D1 overexpression is associated with
favorable responses to cisplatin, which is in agreement with
results from the present study. In addition, Perisanidis et
al (36) analyzed the influence
of cyclin D1 overexpression on the effectiveness of induction
chemoradiotherapy with mitomycin and 5-FU, which found no
differences in responses among patients with different cyclin D1
expression levels. However, in their cohort of patients, only seven
patients were reported to be at the pathological N2 stage (36). Therefore, it was difficult to predict
the clinical value of induction chemoradiotherapy compared with
standard treatment in patients with cN2 OSCC.
To elucidate the potential mechanism underlying the
increased sensitivity to the chemotherapeutic agents docetaxel,
cisplatin and 5-FU in OSCC cells following cyclin D1
overexpression, cleaved caspase-3 and PARP protein levels were
measured. In OSCC cells overexpressing cyclin D1 overexpression,
increased cleaved caspase-3 and PARP levels were detected after the
cells were treated with docetaxel, cisplatin and 5-FU, suggesting
increased apoptosis. In OSCC cells following cyclin D1 knockdown,
decreased cleaved caspase-3 and PARP levels were detected after
treatment with these chemotherapeutic agents. Therefore, the
increased sensitivity to docetaxel, cisplatin and 5-FU in OSCC
cells following cyclin D1 overexpression may be due to activation
of the caspase-3 pathway. Cyclin D1 overexpression has been
reported to correlate with increased sensitivity to the
chemotherapeutic agents fenretinide and bortezomib by activating
apoptosis in breast cancer, rhabdoid tumors and lymphomas (37–39).
Therefore, in some types of cancers overexpressing cyclin D1,
chemotherapeutic agents may exert their effects by activating
apoptosis. In addition, the possibility of using chemotherapeutic
agents or molecules targeting cyclin D1 have also been studied to
treat patients with OSCC and cyclin D1 overexpression (13). However, the detailed mechanism of
targeting cyclin D1 remains poorly understood and warrants further
investigation.
The limitation of the present study is that the
sensitivity experiments in OSCC cells and cyclin D1 intervention
were performed using each of the three chemotherapeutic drugs
alone, instead of combined treatment. Although many combinations
with different concentrations have been attempted, differences in
cell viability among the control, single agent and three agents
altogether were not satisfactory. The possible reason is that these
three agents all operate via different molecular mechanisms in OSCC
cells. When added together into the OSCC cells following cyclin D1
manipulation, the mechanism became too complex to be elucidated
fully, which requires further investigation. Another limitation of
the present study is that data obtained using the OSCC cell lines
for the in vitro experiments could not be translated into
the patients with OSCC treated with the chemotherapeutic agents.
Concentrations of chemotherapeutic agents used for the OSCC cells
did not correspond to the concentrations obtained in the serum
samples of patients with OSCC.
In conclusion, the present study demonstrated that
patients with cN2 OSCC and high cyclin D1 expression exhibited
long-term survival benefits from TPF induction chemotherapy
compared with those who received standard treatment. In addition,
OSCC cells overexpressing cyclin D1 were found to be more sensitive
to TPF chemotherapeutic agents via the caspase-3-dependent
pathway.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Li Mao
(University of Maryland Dental School, Baltimore, MD, USA) for
providing the HN30 cell line as a gift.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81972525 and
81672660), The Shuguang Program of the Shanghai Municipal Education
Commission (grant no. 17SG18), The Shanghai Municipal Commission of
Health and Family Planning (grant no. 2018BR41) and The Program of
Shanghai Academic/Technology Research Leader (grant no.
19XD1422300).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and ZZ were responsible for the study design,
interpretation of the data and revision of the manuscript. YH and
WS were responsible for data acquisition, analysis of the work
presented and the preparation of the manuscript. TZ, YL, DZ, LW, JL
and CZ participated in the clinical management of patients and
laboratory experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Ninth People's Hospital of Shanghai Jiao Tong
University School of Medicine (Shanghai, China; approval nos.
2008-12 and 2014-41) and written informed consent was obtained from
all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kademani D: Oral cancer. Mayo Clin Proc.
82:878–887. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petersen PE: The world oral health report
2003: Continuous improvement of oral health in the 21st century-the
approach of WHO global oral programme. Community Dent Oral
Epidemiol. 31:3–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chinn SB and Myers JN: Oral cavity
carcinoma: Current management, controversies, and future
directions. J Clin Oncol. 33:3269–3276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines®) Head and Neck Cancers. Version 2. 2020.
|
|
6
|
Zhong LP and Zhang ZY: Neoadjuvant versus
induction chemotherapy: More than semantics reply. J Clin Oncol.
31:2972–2973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Posner RM, Hershock MD, Blajman RC,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vermorken BJ, Remenar E, Van HerpenC,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong LP, Zhang CP, Ren GX, Guo W, William
WN Jr, Sun J, Zhu HG, Tu WY, Li J, CaI YL, et al: Randomized phase
III trial of induction chemotherapy with docetaxel, cisplatin, and
fluorouracil followed by surgery versus up-front surgery in locally
advanced resectable oral squamous cell carcinoma. J Clin Oncol.
31:744–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong LP, Zhang CP, Ren GX, Guo W, William
WN Jr, Sun J, Hong CS, Zhu HG, Tu WY, Li J, et al: Long-Term
results of a randomized phase III trial of TPF induction
chemotherapy followed by surgery and radiotherapy in locally
advanced oral squamous cell carcinoma. Oncotarget. 6:18707–18714.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong LP, Zhu DW, William WN Jr, Liu Y, Ma
J, Yang CZ, Yang X, Wang LZ, Li J, Myers JN, et al: Elevated cyclin
D1 expression is predictive for a benefit from TPF induction
chemotherapy in oral squamous cell carcinoma patients with advanced
nodal disease. Mol Cancer Ther. 12:1112–1121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sherr CJ, Beach D and Shapiro GI:
Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discov.
6:353–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramos-García P, Gil-Montoya JA, Scully C,
Ayén A, González-Ruiz L, Navarro-Triviño FJ and González-Moles MA:
An update on the implications of cyclin D1 in oral carcinogenesis.
Oral Dis. 23:897–912. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hardisson D: Molecular pathogenesis of
head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol.
260:502–508. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Capaccio P, Pruneri G, Carboni N, Pagliari
AV, Quatela M, Cesana BM and Pignataro L: Cyclin D1 expression is
predictive of occult metastases in head and neck cancer patients
with clinically negative cervical lymph nodes. Head Neck.
22:234–240. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang SF, Cheng SD, Chuang WY, Chen IH,
Liao CT, Wang HM and Hsieh LL: Cyclin D1 overexpression and poor
clinical outcomes in Taiwanese oral cavity squamous cell carcinoma.
World J Surg Oncol. 10:402012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akervall J, Kurnit DM, Adams M, Zhu S,
Fisher SG, Bradford CR and Carey TE: Overexpression of cyclin D1
correlates with sensitivity to cisplatin in squamous cell carcinoma
cell lines of the head and neck. Acta Otolaryngol. 124:851–857.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Warenius HM, Seabra LA and Maw P:
Sensitivity to cis-diamminedichloroplatinum in human cancer cells
is related to expression of cyclin D1 but not c-raf-1 protein. Int
J Cancer. 67:224–231. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakashima T and Clayman GL: Antisense
inhibition of cyclin D1 in human head and neck squamous cell
carcinoma. Arch Otolaryngol Head Neck Surg. 126:957–961. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kothari V and Mulherkar R: Inhibition of
cyclin D1 by shRNA is associated with enhanced sensitivity to
conventional therapies for head and neck squamous cell carcinoma.
Anticancer Res. 32:121–128. 2012.PubMed/NCBI
|
|
21
|
Zhong LP, Pan HY, Zhou XJ, Ye DX, Zhang L,
Yang X, Chen WT and Zhang ZY: Characteristics of a cancerous cell
line, HIOEC-B(a)P-96, induced by benzo(a)pyrene from human
immortalized oral epithelial cell line. Arch Oral Biol. 53:443–452.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mineta H, Miura K, Takebayashi S, Ueda Y,
Misawa K, Harada H, Wennerberg J and Dictor M: Cyclin D1
overexpression correlates with poor prognosis in patients with
tongue squamous cell carcinoma. Oral Oncol. 36:194–198. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bova RJ, Quinn DI, Nankervis JS, Cole IE,
Sheridan BF, Jensen MJ, Morgan GJ, Hughes CJ and Sutherland RL:
Cyclin D1 and p16INK4A expression predict reduced survival in
carcinoma of the anterior tongue. Clin Cancer Res. 5:2810–2819.
1999.PubMed/NCBI
|
|
24
|
Zhong LP, Wei KJ, Yang X, Pan HY, Wang LZ
and Zhang ZY: Overexpression of galectin-1 is positively correlated
with pathologic differentiation grade in oral squamous cell
carcinoma. J Cancer Res Clin Oncol. 136:1527–1535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gioanni J, Fischel JL, Lambert JC, Demard
F, Mazeau C, Zanghellini E, Ettore F, Formento P, Chauvel P and
Lalanne CM: Two new human tumor cell lines derived from squamous
cell carcinomas of the tongue: Establishment, characterization and
response to cytotoxic treatment. Eur J Cancer Clin Oncol.
24:1445–1455. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dozier C, Mazzolini L, Cénac C, Froment C,
Burlet-Schiltz O, Besson A and Manenti S: CyclinD-CDK4/6 complexes
phosphorylate CDC25A and regulate its stability. Oncogene.
36:3781–3788. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong Z, Yeow WS, Zou C, Wassell R, Wang
C, Pestell RG, Quong JN and Quong AA: Cyclin D1/cyclin-dependent
kinase 4 interacts with filamin A and affects the migration and
invasion potential of breast cancer cells. Cancer Res.
70:2105–2114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qie S and Diehl JA: Cyclin D1, cancer
progression, and opportunities in cancer treatment. J Mol Med
(Berl). 94:1313–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sales KU, Giudice FS, Castilho RM, Salles
FT, Squarize CH, Abrahao AC and Pinto DS Jr: Cyclin D1-induced
proliferation is independent of beta-catenin in head and neck
cancer. Oral Dis. 20:e42–e48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fusté NP, Fernández-Hernández R, Cemeli T,
Mirantes C, Pedraza N, Rafel M, Torres-Rosell J, Colomina N,
Ferrezuelo F, Dolcet X and Garí E: Cytoplasmic cyclin D1 regulates
cell invasion and metastasis through the phosphorylation of
paxillin. Nat Commun. 7:115812016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma J, Liu Y, Huang XL, Zhang ZY, Myers JN,
Neskey DM and Zhong LP: Induction chemotherapy decreases the rate
of distant metastasis in patients with head and neck squamous cell
carcinoma but does not improve survival or locoregional control: A
meta-analysis. Oral Oncol. 48:1076–1084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pignon JP, le Maître A, Maillard E and
Bourhis J; MACH-NC Collaborative Group, : Meta-Analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jamieson ER and Lippard SJ: Structure,
recognition, and processing of cisplatin-DNA adducts. Chem Rev.
99:2467–2498. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Longley DB, Harkin DP and Johnson PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garcia P, Braguer D, Carles G, el Khyari
S, Barra Y, de Ines C, Barasoain I and Briand C: Comparative
effects of taxol and Taxotere on two different human carcinoma cell
lines. Cancer Chemother Pharmacol. 34:335–343. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perisanidis C, Perisanidis B, Wrba F,
Brandstetter A, El Gazzar S, Papadogeorgakis N, Seemann R, Ewers R,
Kyzas PA and Filipits M: Evaluation of immunohistochemical
expression of p53, p21, p27, cyclin D1, and Ki67 in oral and
oropharyngeal squamous cell carcinoma. J Oral Pathol Med. 41:40–46.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pirkmaier A, Yuen K, Hendley J, O'Connell
MJ and Germain D: Cyclin d1 overexpression sensitizes breast cancer
cells to fenretinide. Clin Cancer Res. 9:1877–1884. 2003.PubMed/NCBI
|
|
38
|
Cowan AJ, Frayo SL, Press OW,
Palanca-Wessels MC, Pagel JM, Green DJ and Gopal AK: Bortezomib and
fenretinide induce synergistic cytotoxicity in mantle cell lymphoma
through apoptosis, cell-cycle dysregulation, and IκBα kinase
downregulation. Anticancer Drugs. 26:974–983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smith ME, Das BC and Kalpana GV: In vitro
activities of novel 4-HPR derivatives on a panel of rhabdoid and
other tumor cell lines. Cancer Cell Int. 11:342011. View Article : Google Scholar : PubMed/NCBI
|