Introduction
Pancreatic cancer (PC) is an incurable solid
malignancy that poses a serious threat to human health, worldwide
(1). Despite recent advancements in
the understanding of its molecular pathogenesis, there is still a
lack of effective therapies against this deadly disease (2).
MicroRNAs (miRNAs/miR) are a class of small
non-coding RNAs (~22 nucleotides in length) (3). miRNAs can regulate gene expression by
directly targeting the 3′-untranslated region (3′UTR) of target
gene mRNA (4). Studies have
indicated that miRNAs are aberrantly expressed in serum and cancer
tissues, and they elicit pro- or antitumorigenic functions
(5,6). miRNAs have been shown to serve as
potential diagnostic and therapeutic biomarkers for PC (7). For example, a previous study
demonstrated that miR-23b-3p accelerates the progression of PC,
functioning as an oncogene (8).
However, the molecular mechanisms underlying PC development and
progression are still not fully understood.
Interleukin (IL)-6/Janus kinase(JAK)/STAT3 signaling
axis is a key therapeutic target in PC (9), and contributes to cell invasion in PC
(10). The oncogenic PI3K/Akt, which
is downstream of the JAK/STAT3 pathway, is also a key pathway in
cancer development (11). The
IL-6/JAK2/PI3K signaling pathway can regulate the proliferation and
metastasis of breast cancer cells (12) and was also found to be a potential
therapy strategy for PC (13). PTEN
is a negative regulator of the PI3K/Akt signaling pathway.
Loss-of-function mutations of PTEN have been found in numerous
types of human solid cancer, such as hepatocellular carcinomas,
breast cancer and gastric cancer (14,15).
Evidence has also indicated that knockdown of PTEN could enhance
tumorigenesis and induce tumor development in a variety of
different organs, such as prostate, breast and liver (16–19). On
the other hand, PI3K/Akt signaling has been frequently upregulated
in various types of cancer (20),
plays a key role in tumor cell growth and survival, and has been
associated with poor prognosis in patients with PC (21). In addition, inactivation of PI3K/Akt
signaling has been shown to suppress PC progression (22). In the present study, based on
bioinformatics, miR-23b-3p was predicted to target the 3′UTR of
PTEN mRNA. However, there has been no experimental evidence
demonstrating whether IL-6 induce miR-23b-3p expression and the
role of miR-23b-3p in PC development depends on the JAK/PI3K and
Akt/NF-κB pathways by targeting PTEN.
In the present study, the miR-23b-3p levels in the
serum of patients with PC were examined and the phosphorylation
levels of JAK2, PI3K, Akt and NF-κВ were measured using
immunohistochemical (IHC) staining using PC tissue chip analysis.
The target gene of mR-23b-3p was analyzed using both western blot
analysis and luciferase reporter assays. Furthermore, in
vitro and in vivo studies were performed to determine
whether miR-23b-3p promotes PC cell tumorigenesis and metastasis by
activating the JAK/PI3K and Akt/NF-κB signaling pathways. The
present study might provide novel insights into the potential
application of miR-23b-3p as a predictive marker and therapeutic
target for the prevention and treatment of PC.
Materials and methods
Statement of ethics
The current study was approved by the Institutional
Ethics Committee of Nanjing Medical University. Written informed
consent was provided from all participants included in the study
prior to their enrolment.
Human serum samples and tissue
chips
The diagnosis of PC was based on the National
Comprehensive Cancer Network clinical practice guidelines 2016
(23). The inclusion criteria
comprised of patients who were diagnosed with PC and had not
received chemotherapy or other treatments, while the exclusion
criteria included patients with PC who had infections, immune
system diseases, chronic diseases or other kinds of cancer at the
same time, such as biliary tract or pulmonary infection, rheumatoid
arthritis, uremia and leukemia. The healthy controls were selected
from the Physical Examination Center of Wuxi People's Hospital, and
excluded individuals with any acute or chronic diseases, including
cancer.
Serum samples were collected from 10 healthy
controls and 10 patients with PC at Wuxi People's Hospital from May
2017 to September 2018. The collected clinical data of the 10
patients with PC is showed in Table
I. All the samples were freshly frozen and stored at −80°C
until further use.
 | Table I.Clinical data of the 10 patients with
pancreatic cancer. |
Table I.
Clinical data of the 10 patients with
pancreatic cancer.
| Patient no. | Age, years | Sex | Location, within
the pancreas |
|---|
| 1 | 77 | Female | Head |
| 2 | 61 | Female | Body |
| 3 | 63 | Male | Head |
| 4 | 75 | Female | Head |
| 5 | 45 | Male | Body-tail |
| 6 | 84 | Male | Head-body |
| 7 | 69 | Male | Head |
| 8 | 79 | Female | Body |
| 9 | 72 | Female | Tail |
| 10 | 50 | Male | Body-tail |
The tissue chips, including tumor tissues and
matched adjacent tissues of 36 patients with PC were purchased from
Shanghai Zuocheng Biological Technology Co., Ltd. The clinical data
of the 36 patients with PC was shown in Table II.
 | Table II.Clinical data of the 36 patients with
pancreatic cancer. |
Table II.
Clinical data of the 36 patients with
pancreatic cancer.
| Patient no. | Age, years | Sex | Location, within
the pancreas |
|---|
| 1 | 76 | Male | Head |
| 2 | 33 | Female | Body-tail |
| 3 | 81 | Male | Body-tail |
| 4 | 71 | Female | Head |
| 5 | 69 | Female | Head |
| 6 | 48 | Male | Body-tail |
| 7 | 66 | Male | Head |
| 8 | 67 | Female | Head |
| 9 | 62 | Male | Head |
| 10 | 57 | Female | Head |
| 11 | 58 | Male | Head |
| 12 | 74 | Female | Body-tail |
| 13 | 67 | Male | Head |
| 14 | 55 | Female | Head |
| 15 | 79 | Male | Head |
| 16 | 63 | Male | Head |
| 17 | 51 | Male | Body-tail |
| 18 | 58 | Female | Body-tail |
| 19 | 51 | Male | Head |
| 20 | 62 | Male | Head |
| 21 | 75 | Male | Head |
| 22 | 59 | Male | Head |
| 23 | 52 | Male | Body-tail |
| 24 | 73 | Female | Body-tail |
| 25 | 66 | Male | Body-tail |
| 26 | 69 | Female | Body-tail |
| 27 | 61 | Male | Head |
| 28 | 69 | Female | Head |
| 29 | 38 | Male | Head |
| 30 | 60 | Female | Body-tail |
| 31 | 48 | Male | Body-tail |
| 32 | 59 | Male | Body-tail |
| 33 | 46 | Male | Head |
| 34 | 54 | Male | Head |
| 35 | 54 | Male | Head |
| 36 | 57 | Male | Head |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the serum of patients
using the RNeasy Mini kit (Qiagen GmbH), according to the
manufacturer's instructions. The Bulge-Loop™ miRNA qPCR primer set
for hsa-miR-23b-3p (MQPS0000872-1-100) and U6 small nuclear RNA
(snRNA, MQPS0000002-1-100) were purchased from Guangzhou RiboBio
Co. Ltd. A total of 0.5 µg total RNA was used to synthesize cDNA in
a 20 µl reaction volume at 42°C for 45 min using a PrimeScriptsis
kit (cat. no. RR047A; Takara Bio, Inc.). RT-qPCR was performed in a
20 µl reaction volume using iQ™ SYBR Green Supermix (cat. no.
1708882AP; Bio-Rad Laboratories, Inc.) The thermocycling conditions
were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for
20 sec, 60°C for 30 sec and 70°C for 30 sec.
The 5S ribosomal RNA was used as the internal
control for the serum miR-23b-3p level, which was quantified using
the 2−ΔΔCt method (24).
All RT-qPCR experiments were performed in triplicate.
IHC staining
The tumor and adjacent tumor tissues from patients
with PC were fixed with 40% formaldehyde at room temperature
overnight. Fixed samples were incubated in 70% ethanol at 4°C
overnight, dehydrated in an ascending alcohol series (80, 90 95 and
100%, 30 min for each concentration), cleared in pure xylene and
immersed in liquid paraffin at 60°C for 24 h. Then the samples were
embedded in paraffin and the sections (5-µm) were cut
deparaffinised. The sections were blocked (1X PBS containing 10%
horse serum and 0.01% Triton X-100) with blocking solution at room
temperature for 1 h. The blocking solution was also used for
dilution of antibodies. Following which, they were incubated with
primary antibodies against p-JAK2 (1:1,000; cat. no. abs130652;
Absin Bioscience Inc.) p-Akt (1:100; cat. no. 4060; Cell Signaling
Technology, Inc), p-PI3K (1:100; cat. no. 17366; Cell Signaling
Technology, Inc.), p-NF-κB (1:100; cat. no. 3033T; Cell Signaling
Technology, Inc.) and matrix metalloproteinase-2 (MMP2; 1:100; cat.
no. 87809; Cell Signaling Technology, Inc.) overnight at 4°C, after
washing three times with Tris-buffered saline (TBS), the sections
were incubated with EnVision-labeled polymer-horseradish peroxidase
(HRP)-conjugated rabbit antibody (1:2,000; cat. no. 7074; Cell
Signaling Technology, Inc.) for 1 h at room temperature and then
visualized using diaminobenzidine. The IHC staining was quantified
using the 3D Histech Quant Center2.1 (3DHISTECH Ltd.) and all
histopathological sections were read, diagnosed and recorded by 2
senior pathologists from Wuxi People's Hospital (Wuxi, China) with
>3 years of combined experience at the same time, when there
were disagreements of interpretation between them the results were
discussed with other pathologists from the aforementioned hospital
and a consensus reached. The positive rate was calculated, as the
number of positive cells/total cells number.
Cell culture, IL-6 treatment and
transfection/infection
The human PANC-1, BXPC-3 and 293T cell lines cells
were purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. Cells were cultured in Dulbecco's
modified Eagle medium (DMEM; cat. no. SH30243.01; Hyclone; GE
Healthcare Life Sciences) supplemented with 10% fetal bovine serum
(FBS; cat. no. 16140071; Gibco; Thermo Fisher Scientific Inc.), 100
U/ml penicillin, and 100 g/ml streptomycin (cat. no. P1400; Beijing
Solarbio Science & Technology Co., Ltd.). PANC-1 cells were
seeded on 6-well plates at a density of 5×105/well in
growth media in a humidified incubator at 37°C and 5%
CO2. After 24 h of culture, the media was replaced with
fresh growth media containing recombinant human IL-6 (cat. no.
PHC0064; Invitrogen; Thermo Fisher Scientific Inc.) at the final
concentration of 10 ng/ml every day (25). After 3 days, cells were centrifuged
at 1,000 × g for 5 min, harvested and total RNA was extracted for
RT-qPCR.
The miR-23b-3p mimic (23b-3pM sense,
5′-AUCACAUUGCCAGGGAUUACC-3′ and antisense,
5′-GGUAAUCCCUGGCAAUGUGAU-3′), non-specific mimic (NSM sense,
5′-UUUGUACUACACAAAAGUACUG-3′ and antisense:
5′-CAGUACUUUUGUGUAGUACAAA-3′) control, miR-23b-3p inhibitor
(23b-3pI, 5′-GGUAAUCCCUGGCAAUGUGAU-3′) and non-specific inhibitor
(NSI, 5′-CAGUACUUUUGUGUAGUACAAA-3′) control were obtained from
Guangzhou RiboBio Co., Ltd. PANC-1 cells (5×105/well)
were seeded into the each well of 6-well plates and cultured in
growth media in a humidified incubator at 37°C and 5%
CO2. After 24 h, the cells reached 70% confluence and
they were transfected with 20 nM 23b-3pM and NSM (control) to
upregulate miR-23b-3p, or 50 nM 23b-3pI and NSI (control) to
downregulate miR-23b-3p, using Lipofectamine® RNAiMAX
reagent (cat. no. 13778075; Invitrogen, Thermo Fisher Scientific,
Inc.). After transfection cells were cultured in a humidified
incubator at 37°C and 5% CO2 for 48 h before the
initiation of further experiments.
miRNA lentivirus can achieve long-term and stable
expression of target gene by integrating foreign gene into host
cell genome, has low immunogenicity and can be used for the animal
experiments (26). miR-23b-3p
lentivirus (Lenti-23b-3p) and negative control lentivirus (NCL)
with fluorescein expression were purchased from Shanghai GeneChem
Co., Ltd. Infectious fluids were including 3.75 ml DMEM+100 µl
HitransGP (cat. no. REVG005; Shanghai Genechem Co., Ltd.) +7.5 µl
Lenti-23b-3p (2.5×109 TU/ml) or NCL (1.0×109
TU/ml) were prepared and the media of the cells was changed to the
2.5 ml infectious fluids. A total of 2×105 BXPC-3 cells
were seeded in a T25 flask and cultured in media, after 24 h when
they reached ~30% confluency the cells were infected with
Lenti-23b-3p. Infected cells were then selected with puromycin
antibiotic (0.5 µg/ml; cat. no. 631305; Clontech Laboratories,
Inc.) treatment for 3 days and the expression of miR-23b-3p was
assessed using RT-qPCR (data not shown). The cells stably
expressing miR-23b-3p were cultured in a humidified incubator at
37°C and 5% CO2. The infected cells were centrifuged at
1,000 × g for 5 min and harvested, then the cells were counted with
a cell counter and resuspended in 0.9% saline (2×107
cells/100 µl) for the following in vivo imaging mouse
experiment.
Plasmid construction and luciferase
reporter assay
The luciferase plasmid was constructed based on
TargetScan (http://www.targetscan.org/). The pmiR-RB-REPORT™vector
(Guangzhou RiboBio Co., Ltd.) was used to clone the wild-type
(NM_000314.6: 896–1686) and mutated 3′-untranslated region (3′-UTR)
of PTEN cDNA. In brief, the 3′-UTR of human PTEN cDNA (791 bp in
length) containing the putative target site of miR-23b-3p
(1608–1615) was amplified from genomic DNA of 293T cell lines using
PCR and cloned into the pmiR-RB-REPORT™ vector downstream of the
Renilla luciferase reporter gene. The binding site-containing
region was amplified using linker primers (h-PTEN-3′UTR forward
[896 (SgfI)], ACTGCGATCGCCAATTGTAACGACTTCTCCATC and
h-PTEN-3′UTR-reverse [1686 (XhoI)], GCGCTCGAGTCAAATGTCAAGGTGAAGGT;
h-PTEN-3′UTR mutant forward, GTAAAGCTTTACACTAGATATTATTAAAAAGGT and
h-PTEN 3′UTR mutant reverse, TAATATCTAGTGTAAAGCTTTACAATAGTAGTT)
containing the XhoI and SgfI restriction sites. The
thermocycling conditions were as follows: 95°C for 2 min followed
by 33 cycles of 95°C for 30 sec, 57°C for 30 sec and 72°C for 30
sec. The mutants were generated with 5 ng template by replacing
seven nucleotides (1608–1615-bp: AATGTGA>TTACACT) in the
miR-23b-3p-binding site using a QuikChange XL Site-Directed
Mutagenesis kit (Agilent Technologies, Inc.). The thermocycling
conditions were as follows: 95°C for 2 min followed by 33 cycles of
95°C for 30 sec, 50°C for 30 sec and 72°C for 30 sec. These
constructed plasmids were amplified and the DNA sequence was
confirmed by first generation sequence analysis (Guangzhou RiboBio
Co., Ltd.).
For the luciferase reporter assay, 293T cells were
seeded into 96-well plates (1.5×104/well) with 90 µl
DMEM in each well and cultured in a humidified incubator at 37°C
and 5% CO2 for 24 h. Prior to transfection, the
following mixtures were prepared: i) miR-23b-3p mimic (20 nM), or
NSM (20 nM) mixed with 5 µl OPTI-MEM (cat. no. 3138013; Gibco;
Thermo Fisher Scientific Inc.) respectively; ii) pmiR-RBREPORT™
luciferase reporter plasmids (50 ng/well) containing either the
wild-type or the mutated PTEN 3′UTR mixed with 0.1 µl P3000
(Lipofectamine™ 3000; cat. no. L3000015; Invitrogen; Thermo Fisher
Scientific Inc.); and iii) 0.225 µl Lipofectamine™ 3000 mixed with
5 µl OPTI-MEM. Mixtures i), ii) and iii) were mixed together (total
volume was about 10 µl) and after 15 min the mixture was added to
the aforementioned 96-well plates. Next, 48 h after cotransfection,
the luciferase reporter assay was performed using a Dual-Luciferase
Reporter Assay System (cat. no. P1041; Promega Corporation). The
luminescence intensity was determined using a Veritas microplate
luminometer (Turner BioSystems; Thermo Fisher Scientific, Inc.).
The luciferase activity was measured with a TD-20/20 luminometer
(Turner Designs, Inc.), using the Dual-Glo Luciferase Assay System
(Promega Corporation). Firefly luciferase activity was used for
normalization of the Renilla luciferase activity. All
experiments were performed in triplicate and repeated at least
three times.
Western blot analysis
Cells were centrifuged at 1,000 × g for 5 min at
4°C, harvested and then lysed with radioimmunoprecipitation assay
buffer (Pierce; Thermo Fisher Scientific, Inc.). Cell lysates were
cleared by centrifugation with the speed of 12,000 × g for 15 min
at 4°C, and the protein concentration was determined using the
bicinchoninic acid assay. Proteins were separated using 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
The membranes were blocked with 5% skimmed in TBS-Tween-20 (TBST),
followed by overnight incubation at 4°C with rabbit anti-human JAK2
(1:1,000; cat. no. abs131625; Absin Bioscience Inc.) and p-JAK2
(1:1,000; cat. no. abs130652; Absin Bioscience Inc.), Akt (1:1,000;
cat. no. 9272; Cell Signaling Technology, Inc.), p-Akt (1:2,000;
cat. no. 4060; Cell Signaling Technology, Inc.), PI3K (1:1,000;
cat. no. 4255; Cell Signaling Technology, Inc.), p-PI3K (1:1,000;
cat. no. 17366; Cell Signaling Technology, Inc.), MMP2 (1:1,000;
cat. no. 87809; Cell Signaling Technology, Inc.), NF-κВ (1:1,000;
cat. no. 8242T; Cell Signaling Technology, Inc.), p-NF-κВ (1:1,000;
cat. no. 3033T; Cell Signaling Technology, Inc.), PTEN (1:1,000;
cat. no. 9559; Cell Signaling Technology, Inc.), p-PTEN (1:1,000;
cat. no. 9551T; Cell Signaling Technology, Inc.) and β-actin
(1:1,000; cat. no. 4967; Cell Signaling Technology, Inc.) primary
antibodies. The membranes were then washed with TBST three times,
followed by incubation with HRP-conjugated goat anti-rabbit IgG
(1:2,000; cat. no. 7074; Cell Signaling Technology, Inc.) for 1 h
at room temperature. The protein bands on the membrane were
visualized using the SuperSignal West Pico Chemiluminescent
Substrate (Pierce; Thermo Fisher Scientific, Inc.) and quantified
using Image-Pro Plus software version 6.0.0.260 (Media Cybernetics,
Inc.).
Subcutaneous tumor xenograft
model
Female nude mice (6–8-weeks-old) were purchased from
Changzhou Kaiwen Laboratory Animal Co., Ltd. The mice were
maintained under specific pathogen-free conditions and housed in
ventilated cages with free access to food and water. The mice were
allowed to acclimatize for one week prior to the start of the
experiment. The animal procedures were performed in accordance with
the guidelines of the Nanjing Medical University Laboratory Animal
Center.
PANC-1 cells were cultured in 10% FBS-containing
DMEM and then harvested by centrifugation with the speed of 1,000 ×
g for 5 min at room temperature. A total of ~4×107 cells
were resuspended in 100 ml saline and inoculated subcutaneously on
the right flank of mice. At 40 days following inoculation, the
negative control agomir (NCA), miR-23b-3p agomir (23b-3pA), or
miR-23b-3p antagomir (23b-3pAnt) were injected every 3 days into
the tumor, and the tumor width and length were measured every 5
days, the volume was calculated using the formula: Volume =
width2 × length × 0.5 (27). The mice were anesthetized with 2%
isoflurane prior to euthanasia using cervical dislocation to
confirm the death, and the tumors were harvested for measurement of
their diameter and for histopathological investigation, 10 days
later. Mice injected with 0.9% saline solution only into the right
flank were used as the normal group. A total of 3 mice were used in
each experimental group and the experiment was repeated three
times.
Imaging of metastatic role of
miR-23b-3p upregulation in vivo
A total of 100 µl of 2×107 BXPC-3 cells
(overexpressing the miR-23b-3p lentivirus) were injected in the
caudal vein of each nude mice, the cells infected with negative
control lentivirus were used as the control (NCL). After 52 days,
D-Luciferin (15 mg/ml) was subsequently injected intraperitoneally
at a dose of 10 ml/g. After 15 min, animals were anesthetized with
40 mg/kg sodium pentobarbital by intraperitoneal injection. After
several minutes, the animals were placed in an in vivo
imaging system (PerkinElmer, Inc.) for scanning. The data were
collected and analyzed using the Living Image version 4.5 software
(Xenogen Corporation) according to the manufacturer's instructions.
Quantification of luminescence signals was achieved using the
Region of Interest (ROI) function within the same Living Image
software, for each mouse, an ROI area was drawn with the red circle
and total flux within the ROI was considered as the signal
intensity.
Statistical analysis
All experiments were repeated in triplicate and all
data were presented as the mean ± standard error of the mean.
GraphPad Prism software (version 5; GraphPad Software, Inc.) was
used to perform statistical analyses. Differences between groups
were analyzed using one-way analysis of variance followed by
Bonferroni's post hoc test. The paired datasets (adjacent normal
and tumor tissues) were analyzed using Wilcoxon signed-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Serum miR-23b-3p levels are
upregulated and JAK2/Akt/NF-κВ signaling is activated in patients
with PC
To investigate the possible role of miR-23b-3p in PC
development, the levels of miR-23b-3p in the serum of patients with
PC were examined using RT-qPCR. Results showed that the serum
miR-23b-3p levels were significantly upregulated in patients with
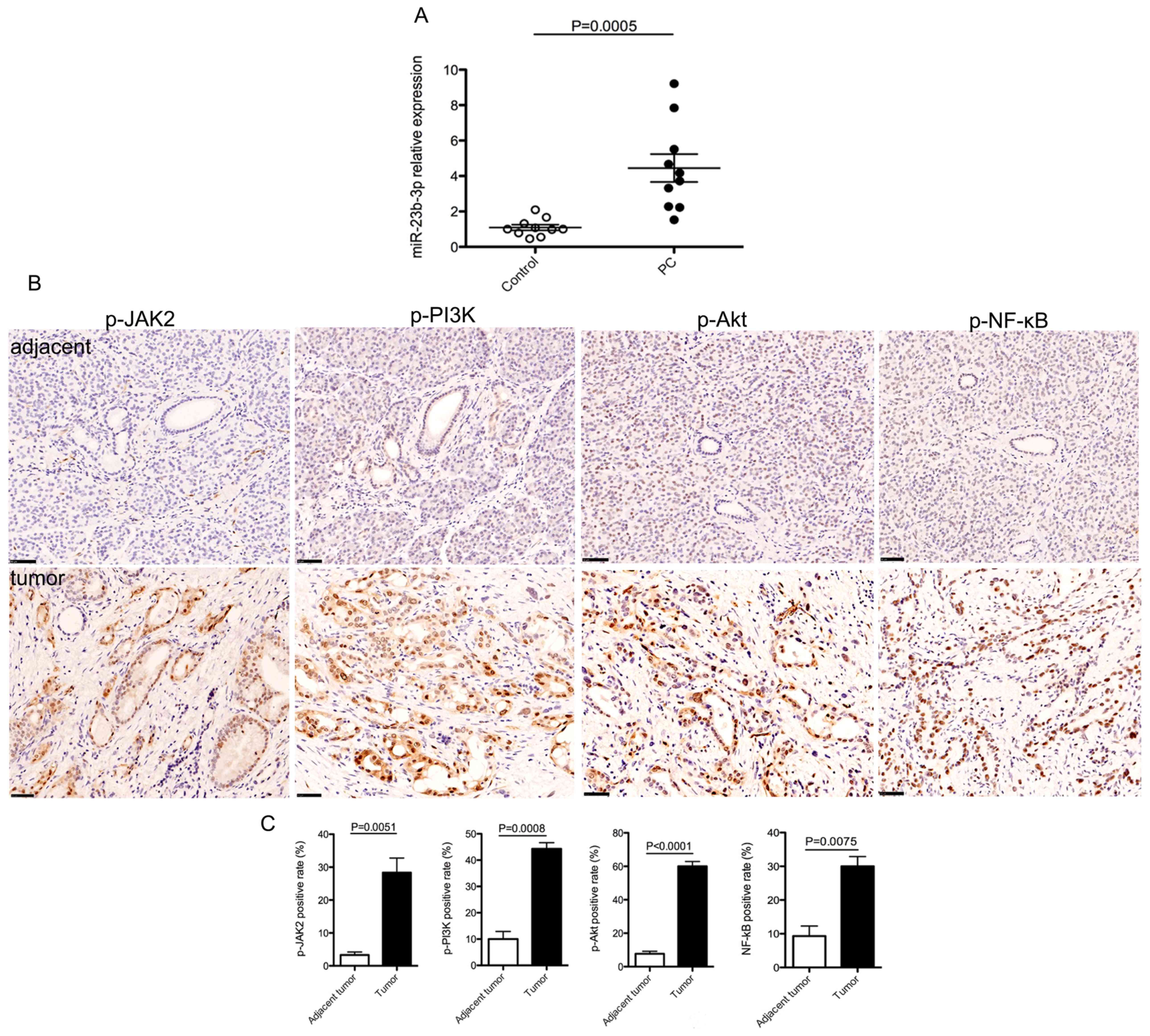
PC, compared with that in healthy controls (Fig. 1A). As JAK/PI3K and Akt/NF-κВ
signaling pathways have well-known roles in the progression of PC
(28,29), the expression patterns of p-JAK2,
p-PI3K, p-Akt and p-NF-κВ were analyzed in PC tissues. The results
showed that the expression of p-proteins examined were markedly
enhanced in PC tissues compared with that in tumor-adjacent tissues
(Fig. 1B and C). These data
suggested a positive association between the levels of serum
miR-23b-3p and JAK/PI3K and Akt/NF-κB signaling activity in PC and
that miR-23b-3p might promote PC development and progression
through the activation of JAK/PI3K and Akt/NF-κB signaling
pathways.
Overexpression of miR-23b-3p activates
JAK/Akt/NF-κВ signaling in PC cells
The possible role of miR-23b-3p in the activation of
the JAK/PI3K and Akt/NF-κB signaling pathways in PC was further
supported by the finding that miR-23b-3p could be induced by IL-6,
a critical inflammatory cytokine involved in the progression of PC
(30) (Fig. 2A). Notably, overexpression of
miR-23b-3p in PANC-1 cells, with the transfection of 23b-3pM
(Fig. 2B), significantly upregulated
the protein expression level of p-JAK2, p-PI3K, p-Akt and p-NF-κВ,
as well as MMP2, which is a downstream target of the PI3K/Akt
signaling pathway (Fig. 2C and D).
In addition, p-JAK2, p-PI3K, p-Akt, p-NF-κВ and MMP2 were
significantly downregulated by miR-23b-3p inhibition with the
transfection of 23b-3pI in PANC-1 cells (Fig. 2B-D). Meanwhile, activation of PTEN,
which inactivates PI3K/Akt signaling, was significantly
downregulated by 23b-3pM and significantly upregulated with 23b-3pI
in PANC-1 cells (Fig. 2C and D). The
expression of the total proteins JAK2, PI3K, Akt, NF-κB and PTEN
had no difference in cells treated with 23b-3pM or 23b-3pI when
compared to their respective controls (data not shown). TargetScan
software was used to identify the putative binding site between
miR-23b-3p and PTEN, which was located between 1,608 and 1,615 bp
in the 3′-UTR of PTEN (Fig. 2E).
Therefore, to further confirm the binding of miR-23b-3p to the
3′-UTR of PTEN, the transcriptional regulation of PTEN by
miR-23b-3p in PANC-1 cells was examined. As shown in Fig. 2F, overexpression of miR-23b-3p
significantly repressed the transcriptional activity of the
wild-type PTEN gene; however, the levels seemed to increase
compared to the transcriptional activity of the 3′-UTR-mutated PTEN
gene, however, this change was not significant. This result
indicated that miR-23b-3p downregulated PTEN by directly targeting
the 3′-UTR of PTEN. Taken together, these data show that miR-23b-3p
is a positive regulator of the JAK2/Akt/NF-κВ signaling pathway in
PC cells.
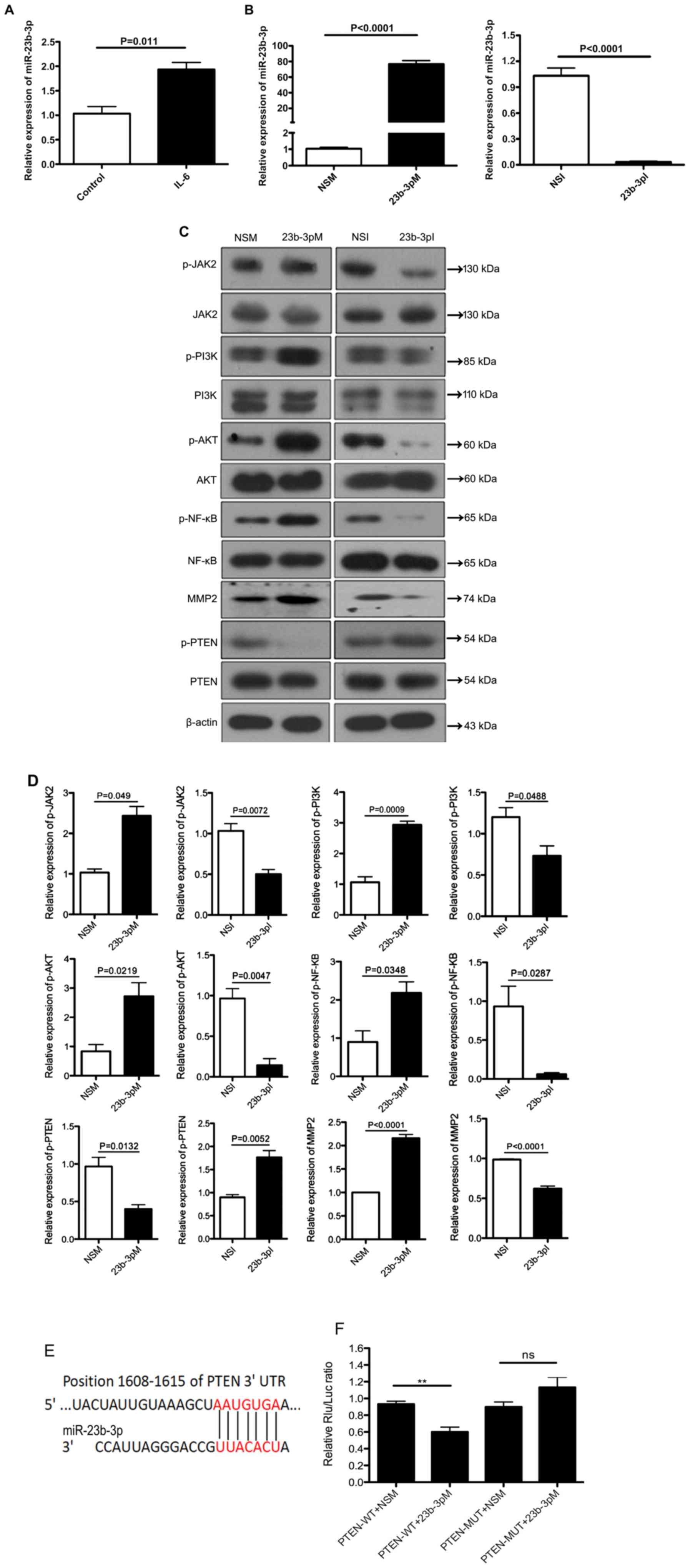 | Figure 2.miR-23b-3p regulates the
JAK/Akt/NF-κВ signaling pathway in pancreatic cancer cells. (A)
RT-qPCR was performed to detect the mRNA levels of miR-23b-3p in
PANC-1 cells in response to IL-6 stimulation. (B) PANC-1 cells were
transfected with 23b-3pM, 23b-3pI and the respective controls, NSM
and NSI for 72 h, and the relative expression of miR-23b-3p was
measured using RT-qPCR. (C) The effect of miR-23b-3p overexpression
and inhibition on the expression levels of proteins in the
JAK/Akt/NF-κВ signaling pathway. Western blot assays were performed
to detect the expression of JAK2, p-JAK2, PI3K, p-PI3K Akt, p-Akt,
NF-κB, p-NF-κВ, MMP2, PTEN and p-PTEN. β-actin was used as the
internal control for total protein, while total protein was used as
the internal control for the phosphorylated protein. (D) The bar
graphs show the quantification of western blot analysis of the
phosphorylated proteins and MMP2. (E) The bioinformatics analysis
using TargetScan shows that miR-23b-3p could bind at positions
1,608 to 1,615 of PTEN 3′UTR (the binding sites were indicated as
red color). (F) The effect of miR-23b-3p overexpression on the
transcriptional activity of the PTEN gene. PANC-1 cells were
transfected with 23b-3pM or NSM for 24 h. The luciferase reporter
assay was performed to determine the transcriptional activity of
the WT PTEN gene and the 3′-UTR-MUT PTEN gene. **P<0.01.
23b-3pI, miR-23b-3p inhibitor; 23b-3pM, miR-23b-3p mimic; IL,
interleukin; JAK2, Janus kinase 2; miR, microRNA; MMP, matrix
metalloproteinase; MUT, mutated; NS, not significant; NSI,
non-specific inhibitor; NSM, non-specific mimic; p-,
phosphorylated; RT-qPCR, reverse transcription-quantitative PCR;
UTR, untranslated region; WT, wild-type; Rlu, Renilla
luciferase. |
Overexpression of miR-23b-3p promotes
PC cell-derived tumor growth in vivo and JAK/PI3K and Akt/NF-κВ
signaling activation
To further investigate the in vivo role of
miR-23b-3p in PC cell tumorigenesis, the miR-23b-3p agomir was
injected into a PANC-1 cell-derived subcutaneous tumor, in a
xenograft mouse model. The intratumoral injection of the miR-23b-3p
agomir lead to a significant increase in the tumor size in a
time-dependent manner, whereas injection of the miR-23b-3p
antagomir reduced the tumor size, compared with that in the
negative control agomir group (Fig. 3A
and B), indicating that miR-23b-3p plays a positive role in PC
cell tumorigenesis in vivo. In addition, miR-23b-3p
overexpression could activate JAK/PI3K and Akt/NF-κВ signaling
pathways, as evidenced by miR-23b-3p-induced upregulation of
p-JAK2, p-PI3K, p-Akt, p-NF-κВ and MMP2 in mouse subcutaneous tumor
tissues (Fig. 3C and D), suggesting
that activation of the JAK/PI3K and Akt/NF-κВ signaling pathways
might be mechanism by which miR-23b-3p promotes PC cell
tumorigenesis in vivo.
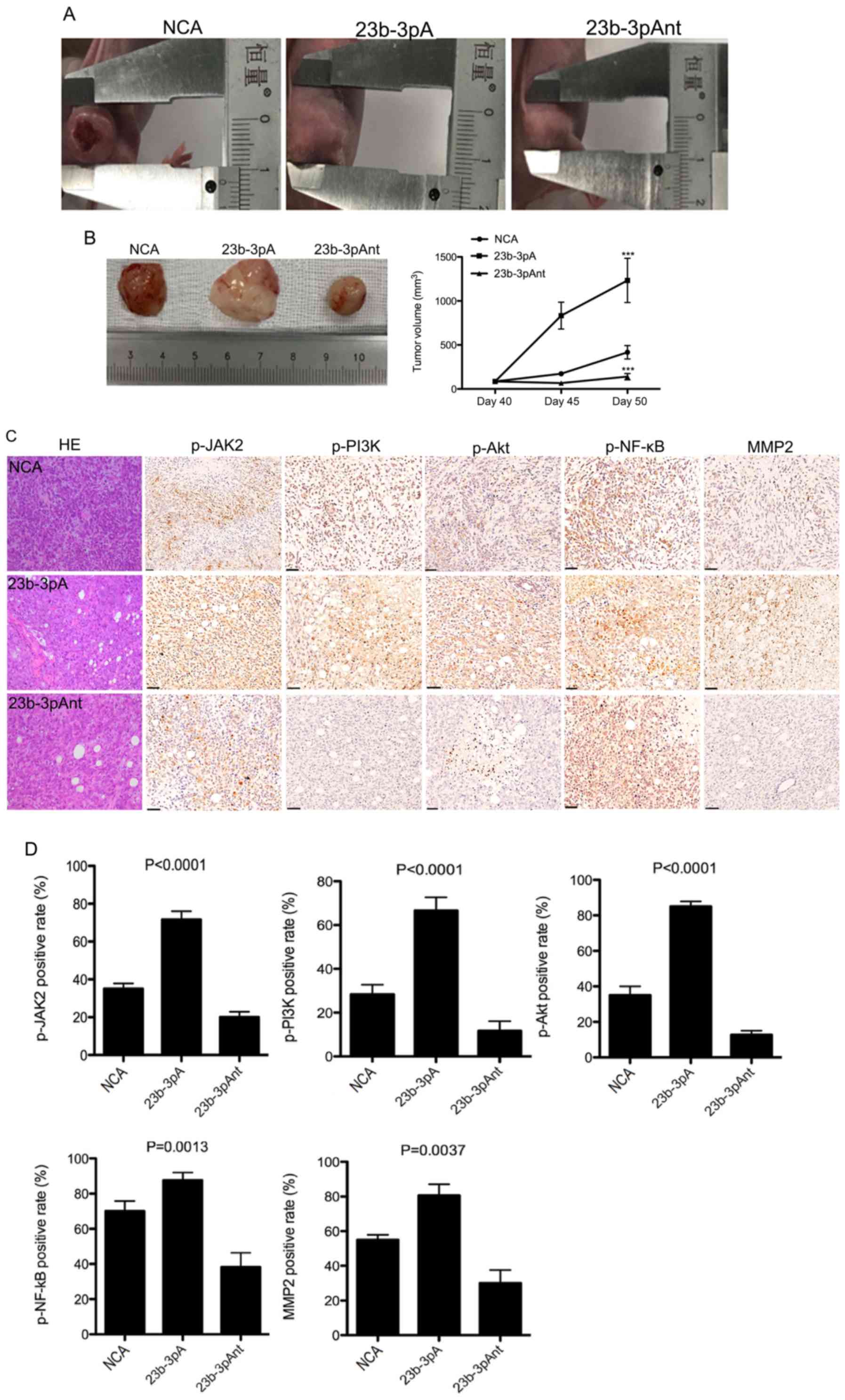 | Figure 3.Effect of miR-23b-3p on cell
tumorigenesis and JAK/Akt/NF-κВ signaling pathway activation in
pancreatic cancer. (A) At day 40, 23b-3pA, 23b-3pAnt or NCA was
injected into PANC-1 cell-derived subcutaneous tumors in a
xenograft mouse model every 3 days. The width and length of tumor
were measured and the volume was calculated in vivo every 5
days. (B) At 50 days, subcutaneous tumors were harvested. The tumor
volume curve was created. ***P<0.001 compared with control. (C)
Hematoxylin and eosin staining of PANC-1 cell-derived tumors and
immunohistochemical staining of p-JAK2, p-PI3K p-Akt, p-NF-κВ and
MMP2 in PANC-1 cell-derived tumor sections. Scale bar, 50 µm. (D)
Quantification of the positive staining rate for p-JAK2, p-PI3K
p-Akt, p-NF-κВ and MMP2 expression. 23b-3pAnt, miR-23b-3p
antagomir; 23b-3pA, miR-23b-3p agomir; NCA, negative control
agomir; JAK2, Janus kinase 2; miR, microRNA; MMP, matrix
metalloproteinase. |
Overexpression of miR-23b-3p enhances
the metastasis of PC in vivo
PC metastasis commonly occurs in the liver (31). To investigate whether miR-23b-3p
plays a role in PC liver metastasis, liver tissues were harvested
from miR-23b-3p agomir-administered nude mice for morphological and
histological examination. Treatment with the miR-23b-3p agomir was
found to increase the number of metastatic lesions and the amount
of inflammatory cell infiltration (black arrows) in liver tissues,
compared with that in the normal and control groups (Fig. 4A). Whereas the treatment with the
miR-23b-3p antagomir had the opposite effect on liver metastasis
(Fig. 4A). These results indicated
that overexpression of miR-23b-3p enhanced liver metastasis from
subcutaneous tumor derived in PANC-1 cells. In addition, the
miR-23b-3p agomir significantly upregulated the expression of the
MMP2 in liver metastasis, while the miR-23b-3p antagomir
downregulated MMP2 expression (Fig.
4B). MMP2 is one of the key regulators for the metastasis of PC
(32), hence in this experiment,
only MMP2 protein expression in the liver was investigated. To
further confirm the role of miR-23b-3p in PC metastasis, the BXPC-3
cells infected with miR-23b-3p lentivirus (lenti-23b-3p) were
injected into the caudal vein of nude mice, then the whole-body
mice imaging was performed using an animal CT scan. The results
demonstrated that miR-23b-3p overexpression enhanced the total
fluorescence signals from the ROI in mice, and the fluorescence
signals were stronger in the lung and heart when compared with that
in the NCL group (Fig. 4C). These
findings were different from the subcutaneous tumor model as no
fluorescence signals were found in liver, this could be due to
different cell lines or different animal PC models causing
different metastasis modes.
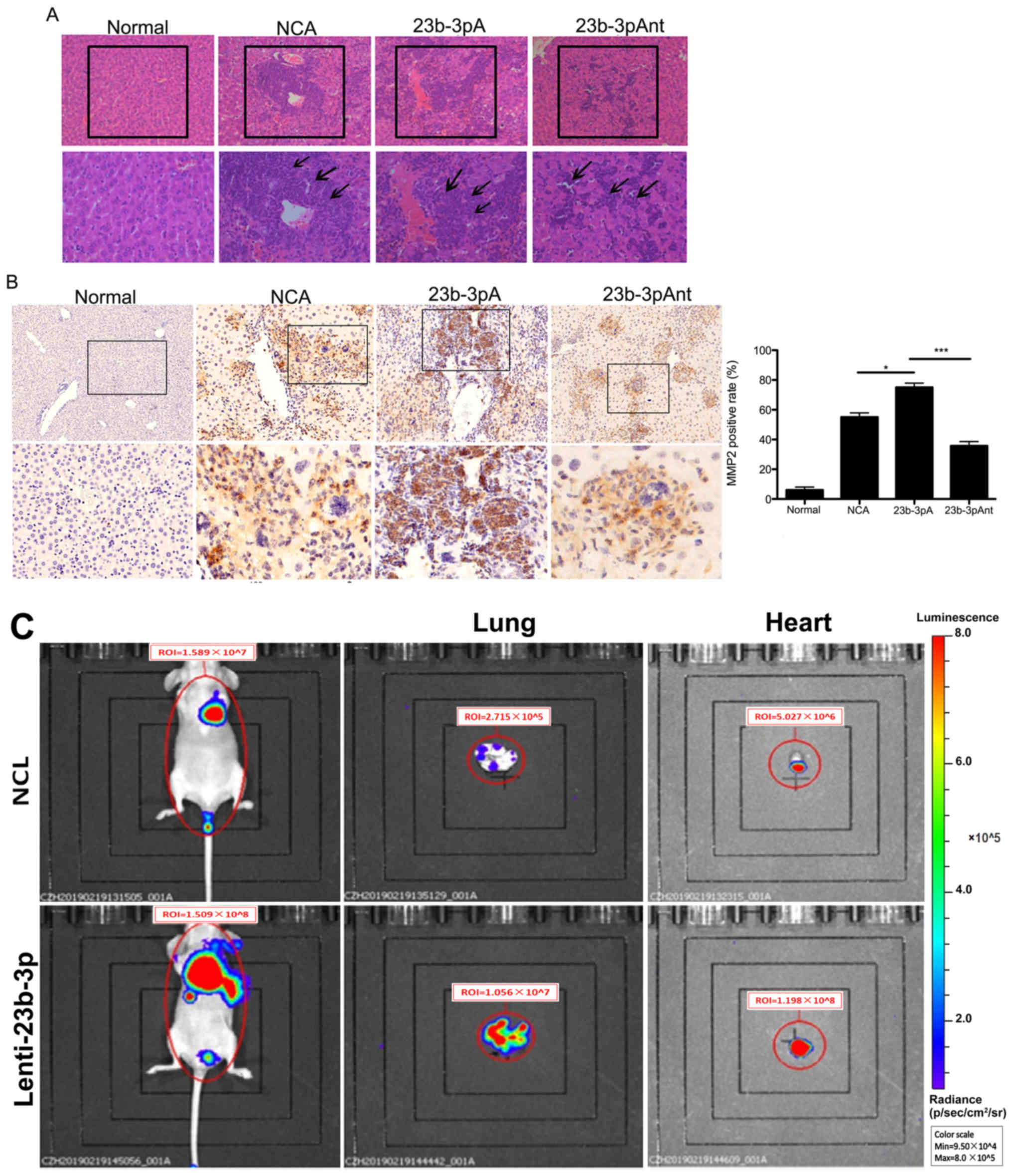 | Figure 4.Effect of miR-23b-3p on pancreatic
cancer metastasis. (A) At 50 days following creation of the
xenograft mouse model, the liver tissues of mice were harvested,
and histological analysis was performed to examine the effect of
miR-23b-3p on liver metastasis. The images in the upper panel were
×100 magnified, and images in the lower panel are a magnification
of the indicated boxes, at ×200 magnification. The metastatic
lesions were indicated with black boxes and the inflammatory cells
were indicated with black arrows. (B) IHC staining of MMP2 in liver
tissues. The images in the upper panel were ×100 magnified, and
images in the lower panel are a magnification of the indicated
boxes, at ×200 magnification. The bar graph shows the
quantification of IHC staining. *P<0.05; ***P<0.001. (C) The
BXPC-3 cells stably expressing miR-23b-3p were injected into the
caudal vein of nude mice, after 52 days, the mice were injected
with D-luciferin for 15 min, then the whole-body imaging was
acquired using Living Image software. The red circle represents the
ROI, and the total luminescence within the ROI was considered as
the signal intensity. The mice injected with saline solution only
were used as Normal group. 23b-3pAnt, miR-23b-3p antagomir;
23b-3pA, miR-23b-3p agomir; control, negative control agomir; NCL,
negative control lentivirus; IHC, immunohistochemical;
lenti-23b-3p, miR-23b-3p lentivirus; miR, microRNA; MMP, matrix
metalloproteinase; ROI, Region of Interest. |
Discussion
Metastasis is a key factor influencing the prognosis
of PC, and it is well-known that miRNAs can regulate the
development, progression and metastasis of breast cancer (33,34). In
PC, miRNAs have been found to affect tumor metastasis and function
as biomarkers, and prognostic and therapeutic modulators of PC
(35–38). For example, miR-141 was associated
with the growth and metastasis of PC, whereas miR203a-3p inhibited
PC cell growth, proliferation, epithelia-mesenchymal transition and
apoptosis (39). However, the exact
mechanism by which miRNAs promote metastasis in PC has not been
fully understood. miR-23b-3p has been shown to promote the
development of a variety of different cancers, such as lung cancer,
gastric cancer and ovarian cancer, and it was also associated with
poor prognosis as well (40–43). A previous study revealed that
miR-23b-3p promotes the proliferation, invasion and migration of
PANC-1 cells (8). However, the in
vivo effects and the mechanistic role of miR-23b-3p in PC
development remain to be elucidated.
The activation of the PI3K/Akt signaling pathway was
found to promote proliferation and inhibit apoptosis of PC cells
(44,45). In colorectal cancer, miRNAs were
found to regulate numerous genes, such as BRCA1, PIK3CG and IL-6R
involved in this PI3K/Akt signaling pathway (46). In the present study, miR-23b-3p
enhanced PC cell tumorigenesis and metastasis in vivo. In
addition, overexpression of miR-23b-3p significantly increased the
phosphorylation of JAK2, PI3K, Akt, NF-κВ and the total expression
levels of MMP2 in PC cells, as well as in the subcutaneous tumors
of the mouse model, all of which are major components of the
JAK/PI3K/Akt signaling pathway. Notably, increased phosphorylation
of JAK2, PI3K, Akt, NF-κВ and the total expression levels of MMP2
were also observed in the tumor tissues from patients with PC,
suggesting that the activation of the JAK/PI3K/Akt/NF-κВ signaling
pathway mediates the observed effect of miR-23b-3p on the
development, progression and metastasis of PC. The findings of the
present study were consistent with a previous study where the
miR-1224/ELF3 axis was found to regulate the metastasis of PC via
the PI3K/Akt signaling pathway (47).
IL-6 is an important inflammatory cytokine that
plays a critical role in tumor development, progression,
invasiveness and metastasis by activating the JAK2/STAT3, PI3K/Akt,
and MAPK signaling pathways or by mediating all miRNA expression
(48). In addition, it has been
found that IL-6 induces miR-224 expression but inhibits miR-370
expression in cholangiocarcinoma. In addition, miR-224 promoted,
but miR-370 suppressed tumor growth of cholangiocarcinoma (25,49). The
transcriptional expression of IL-6 can be promoted by NF-κВ
(50). In the present study, IL-6
was shown to upregulate miR-23b-3p in PACN-1 cells, suggesting that
IL-6 might regulate PC development by inducing the
miR-23b-3p/PI3K/Akt/NF-κВ cascade, however, further investigation
is required.
In addition, it was also revealed that miR-23b-3p
decreased the protein expression level of PTEN, which is a negative
regulator of PI3K/Akt signaling, by directly targeting PTEN mRNA
(51). Similarly, in renal cancer,
PTEN has been found to also be inhibited by miR-23b-3p (52). Furthermore, in PC, miR-107 inhibits
PC metastasis through the inhibition of the PI3K/Akt signaling
pathway via PTEN (53).
MMP2 is one of the key regulators for the invasion
and metastasis of PC (32), and the
MMP2 protein expression levels have been associated with lymph node
metastasis in PC (54). The present
findings suggested that miR-23b-3p could promote liver metastasis
of PC cell-derived tumors, possibly via the induction of MMP2. This
effect was further confirmed by the administration of a miR-23b-3p
antagomir, which significantly suppressed liver metastasis and was
accompanied by the downregulation of MMP2 protein expression
levels. Notably, MMP2 has been reported to be a downstream target
of PI3K/Akt signaling in tumor cells (55). As a result, regulation of the
PTEN/PI3K/Akt/NF-κВ/MMP2 cascade might be the primary mechanism
underlying the in vivo role of miR-23b-3p in PC cell
tumorigenesis and metastasis.
However, the results of current study require
further investigation, as the miR-23b-3p serum levels were only
investigated in 10 PC samples, while the mRNA expression of
miR-23b-3p in the tumor and adjacent tumor tissues of PC have not
been investigated. The role of miR-23b-3p in tumor metastasis
should also be studied in different PC models generated using
different PC cell lines in future studies. In future research, the
number of samples should be increased and the levels of miR-23b-3p
should also be measured in the tumor tissues, as well as in the
serum of patients with PC at different stages of the disease and
the expression of other proteins apart from MMP2 should be
investigated in an in vivo model of PC. In addition, the
exact mechanism of how IL-6 can induce the expression of miR-23b-3p
and the role of miR-23b-3p in the JAK/PI3K and Akt/NF-κВ signaling
pathways will be investigated further.
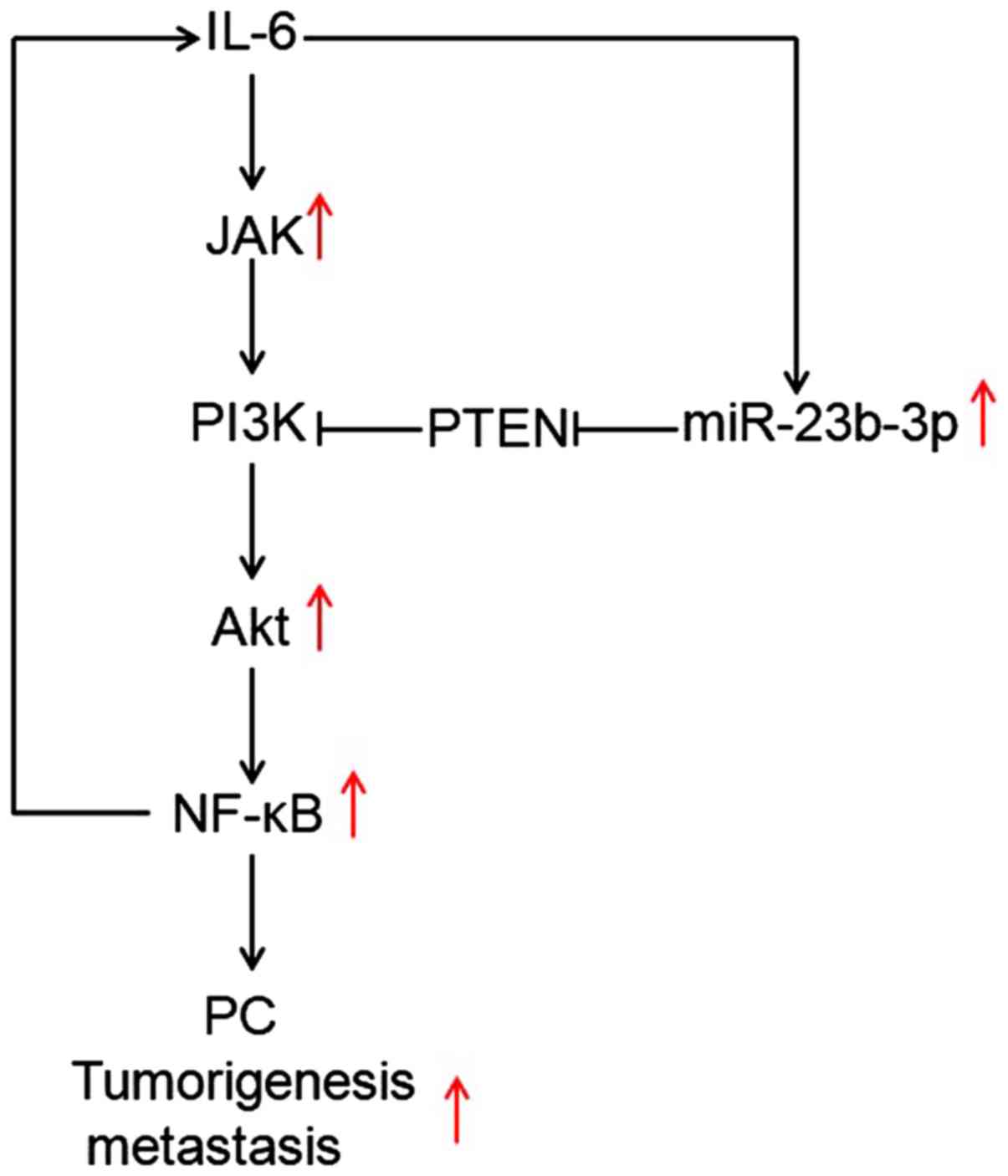
In conclusion, the present study found that IL-6
could regulate miR-23b-3p, which contributed to the activation of
JAK/PI3K and Akt//NF-κВ by targeting PTEN, which in turn promoted
PC cell tumorigenesis and metastasis in vivo (Fig. 5). Together, these findings might
provide potential therapeutic strategies in the future for the
treatment of PC.
Acknowledgements
The abstract has been previously published in two
journals: Journal of Digestive Disease (Yu-Nan ZHANG, Fang-Mei AN:
miR-23b-3p promotes pancreatic cancer cell tumorigenesis and
metastasis through the PTEN/PI3K/Akt signaling pathway. Journal of
Digestive Disease 20(S1): PO-191, 2019) and the United European
Gastroenterology (An F, Zhang Y and Chen D: miR-23b-3p promotes
pancreatic cancer cell tumorigenesis and metastasis through the
PTEN/PI3K/Akt signaling pathway. United European Gastroenterology
7(S): P0119, 2019).
Funding
The present study was partially supported by grants
from The National Natural Science Foundation of China (grant nos.
81502038 and 81773227), The Jiangsu Provincial Medical Youth Talent
(grant no. QNRC2016187), Wuxi Medical Innovation Team (grant no.
CXTD005) and The Major Project in Wuxi (grant no. Z201903).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FA and QZ designed the experiments. YZ and DC
conducted the experiments. GZ, XW and LZ performed the animal
experiments and prepared the figures. YL and JD analyzed the data.
FA and QZ wrote and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Ethics Committee of Nanjing Medical University. Written informed
consent was obtained from all patients included in this study prior
to their enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rawla P, Sunkara T and Gaduputi V:
Epidemiology of pancreatic cancer: Global trends, etiology and risk
factors. World J Oncol. 10:10–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beitzinger M and Meister G: Preview.
MicroRNAs: From decay to decoy. Cell. 140:612–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gebert LFR and MacRae IJ: Regulation of
microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ortiz-Quintero B: Cell-free microRNAs in
blood and other body fluids, as cancer biomarkers. Cell Prolif.
49:281–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: New trends in the development of miRNA therapeutic
strategies in oncology. Int J Oncol. 49:5–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slotwinski R, Lech G and Slotwinska SM:
MicroRNAs in pancreatic cancer diagnosis and therapy. Cent Eur J
Immunol. 43:314–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen D, Wu X, Xia M, Wu F, Ding J, Jiao Y,
Zhan Q and An F: Upregulated exosomic miR23b-3p plays regulatory
roles in the progression of pancreatic cancer. Oncol Rep.
38:2182–2188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Yang L and Li Y: Neuropilin-1
(NRP-1) upregulated by IL-6/STAT3 signaling contributes to invasion
in pancreatic neuroendocrine neoplasms. Hum Pathol. 81:192–200.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Fu X, Guan Y, Gong M, He K and
Huang B: 1,3-Dicaffeoylquinic acid targeting 14-3-3 tau suppresses
human breast cancer cell proliferation and metastasis through
IL6/JAK2/PI3K pathway. Biochem Pharmacol. 172:1137522020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan K, Yang C, Fan Z, Huang Q, Zhang Y,
Cheng H, Jin K, Lu Y, Wang Z, Luo G, et al: MUC16 C
terminal-induced secretion of tumor-derived IL-6 contributes to
tumor-associated Treg enrichment in pancreatic cancer. Cancer Lett.
418:167–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haddadi N, Lin Y, Travis G, Simpson AM,
Nassif NT and McGowan EM: PTEN/PTENP1: Regulating the regulator of
RTK-dependent PI3K/Akt signalling, new targets for cancer therapy.
Mol Cancer. 17:372018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu W, Yang Z, Xie C, Zhu Y, Shu X, Zhang
Z, Li N, Chai N, Zhang S, Wu K, et al: PTEN lipid phosphatase
inactivation links the hippo and PI3K/Akt pathways to induce
gastric tumorigenesis. J Exp Clin Cancer Res. 37:1982018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki A, de la Pompa JL, Stambolic V,
Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A,
Khoo W, et al: High cancer susceptibility and embryonic lethality
associated with mutation of the PTEN tumor suppressor gene in mice.
Curr Biol. 8:1169–1178. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Cristofano A, Pesce B, Cordon-Cardo C
and Pandolfi PP: Pten is essential for embryonic development and
tumour suppression. Nat Genet. 19:348–355. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luongo F, Colonna F, Calapà F, Vitale S,
Fiori ME and De Maria R: PTEN Tumor-Suppressor: The dam of stemness
in cancer. Cancers (Basel). 11:10762019. View Article : Google Scholar
|
|
19
|
Li G, Robinson GW, Lesche R, Martinez-Diaz
H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, et al:
Conditional loss of PTEN leads to precocious development and
neoplasia in the mammary gland. Development. 129:4159–4170.
2002.PubMed/NCBI
|
|
20
|
Jiang N, Dai Q, Su X, Fu J, Feng X and
Peng J: Role of PI3K/AKT pathway in cancer: The framework of
malignant behavior. Mol Biol Rep. 47:4587–4629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebrahimi S, Hosseini M, Shahidsales S,
Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM and Avan A:
Targeting the Akt/PI3K signaling pathway as a potential therapeutic
strategy for the treatment of pancreatic cancer. Curr Med Chem.
24:1321–1331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wolin EM: PI3K/Akt/mTOR pathway inhibitors
in the therapy of pancreatic neuroendocrine tumors. Cancer Lett.
335:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xueli B, Tao M and Ting-Bo L:
Interpretation of NCCN clinical practice guidelines in oncology:
Pancreatic adenocarcinoma (version 1, 2016). Chin J Pract Surg.
36:870–871. 2016.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
An F, Yamanaka S, Allen S, Roberts LR,
Gores GJ, Pawlik TM, Xie Q, Ishida M, Mezey E, Ferguson-Smith AC,
et al: Silencing of miR-370 in human cholangiocarcinoma by allelic
loss and interleukin-6 induced maternal to paternal epigenotype
switch. PLoS One. 7:e456062012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poling BC, Tsai K, Kang D, Ren L, Kennedy
EM and Cullen BR: A lentiviral vector bearing a reverse intron
demonstrates superior expression of both proteins and microRNAs.
RNA Biol1. 4:1570–1579. 2017. View Article : Google Scholar
|
|
27
|
Song C, Han Y, Luo H, Qin Z, Chen Z, Liu
Y, Lu S, Sun H and Zhou C: HOXA10 induces BCL2 expression, inhibits
apoptosis, and promotes cell proliferation in gastric cancer.
Cancer Med. 8:5651–5661. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conway JR, Herrmann D, Evans TJ, Morton JP
and Timpson P: Combating pancreatic cancer with PI3K pathway
inhibitors in the era of personalised medicine. Gut. 68:742–758.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Wu H, Li W, Yin L, Guo S, Xu X,
Ouyang Y, Zhao Z, Liu S, Tian Y, et al: Downregulated miR-506
expression facilitates pancreatic cancer progression and
chemoresistance via SPHK1/Akt/NF-κB signaling. Oncogene.
35:5501–5514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ying H, Dey P, Yao W, Kimmelman AC,
Draetta GF, Maitra A and DePinho RA: Genetics and biology of
pancreatic ductal adenocarcinoma. Genes Dev. 30:355–385. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao Z, Luo G, Liu C, Wu C, Liu L, Liu Z,
Ni Q, Long J and Yu X: Molecular mechanism underlying lymphatic
metastasis in pancreatic cancer. Biomed Res Int. 2014:9258452014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Shi G, Gao F, Liu P, Wang H and
Tan X: TSPAN1 upregulates MMP2 to promote pancreatic cancer cell
migration and invasion via PLCγ. Oncol Rep. 41:2117–2125.
2019.PubMed/NCBI
|
|
33
|
Grossi I, Salvi A, Baiocchi G, Portolani N
and De Petro G: Functional role of microRNA-23b-3p in cancer
biology. Microrna. 7:156–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Li J, Lai XN, Jiao XQ, Xiong JP
and Xiong LX: Focus on Cdc42 in breast cancer: New insights, target
therapy development and non-coding RNAs. Cells. 8:462019.
View Article : Google Scholar
|
|
35
|
Weidle UH, Birzele F and Nopora A:
Pancreatic ductal adenocarcinoma: MicroRNAs affecting tumor growth
and metastasis in preclinical in vivo models. Cancer Genomics
Proteomics. 16:451–464. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Daoud AZ, Mulholland EJ, Cole G and
McCarthy HO: MicroRNAs in pancreatic Cancer: Biomarkers,
prognostic, and therapeutic modulators. BMC Cancer. 19:11302019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lv Y and Huang S: Role of non-coding RNA
in pancreatic cancer. Oncol Lett. 18:3963–3973. 2019.PubMed/NCBI
|
|
38
|
Shams R, Saberi S, Zali M, Sadeghi A,
Ghafouri-Fard S and Aghdaei HA: Identification of potential
microRNA panels for pancreatic cancer diagnosis using microarray
datasets and bioinformatics methods. Sci Rep. 10:75592020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
An N and Zheng B: miR-203a-3p inhibits
pancreatic cancer cell proliferation, EMT, and apoptosis by
regulating SLUG. Technol Cancer Res Treat. 19:15330338198987292020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Begum S, Hayashi M, Ogawa T, Jabboure FJ,
Brait M, Izumchenko E, Tabak S, Ahrendt SA, Westra WH, Koch W, et
al: An integrated genome-wide approach to discover deregulated
microRNAs in non-small cell lung cancer: Clinical significance of
miR-23b-3p deregulation. Sci Rep. 5:132362015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lei Y and Li JT: Affinity of
penicillin-binding proteins of Escherichia coli K-12 for
furbenicillin and other beta-lactam antibiotics. Zhongguo Yao Li
Xue Bao. 10:177–180. 1989.(In Chinese). PubMed/NCBI
|
|
42
|
Hu X, Wang Y, Liang H, Fan Q, Zhu R, Cui
J, Zhang W, Zen K, Zhang CY, Hou D, et al: miR-23a/b promote tumor
growth and suppress apoptosis by targeting PDCD4 in gastric cancer.
Cell Death Dis. 8:e30592017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu C, Zhao Y, Liu Y, Yang X, Yan M, Min Y,
Pan Z, Qiu S, Xia S, Yu J, et al: Identifying miRNA-mRNA regulation
network of major depressive disorder in ovarian cancer patients.
Oncol Lett. 16:5375–5382. 2018.PubMed/NCBI
|
|
44
|
Xu X, Yu Y, Zong K, Lv P and Gu Y:
Up-regulation of IGF2BP2 by multiple mechanisms in pancreatic
cancer promotes cancer proliferation by activating the PI3K/Akt
signaling pathway. J Exp Clin Cancer Res. 38:4972019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Falasca M, Selvaggi F, Buus R, Sulpizio S
and Edling CE: Targeting phosphoinositide 3-kinase pathways in
pancreatic cancer-from molecular signalling to clinical trials.
Anticancer Agents Med Chem. 11:455–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Slattery ML, Mullany LE, Sakoda LC, Wolff
RK, Stevens JR, Samowitz WS and Herrick JS: The PI3K/AKT signaling
pathway: Associations of miRNAs with dysregulated gene expression
in colorectal cancer. Mol Carcinog. 57:243–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kong L, Liu P, Zheng M, Wang Z, Gao Y,
Liang K, Wang H and Tan X: The miR-1224-5p/ELF3 Axis regulates
malignant behaviors of pancreatic cancer via PI3K/AKT/Notch
signaling pathways. Onco Targets Ther. 13:3449–3466. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pop VV, Seicean A, Lupan I, Samasca G and
Burz CC: IL-6 roles-molecular pathway and clinical implication in
pancreatic cancer-A systemic review. Immunol Lett. 181:45–50. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang M, Wu X, Cao H, Zhan Q, Xia M, Zhou
Q, Cai X and An F: Regulatory role of serum miR-224 in invasiveness
and metastasis of cholangiocarcinoma. Zhonghua Gan Zang Bing Za
Zhi. 23:748–753. 2015.(In Chinese). PubMed/NCBI
|
|
50
|
Chen X, Lai Y, Song X, Wu J, Wang L, Zhang
H, Liu Z and Wang Y: Polysaccharides from Citrus grandis
associate with luteolin relieves chronic pharyngitis by
anti-inflammatory via suppressing NF-κB pathway and the
polarization of M1 macrophages. Int J Immunopathol Pharmacol.
32:20587384187805932018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F and Ciuffreda L: PTEN: Multiple functions in human
malignant tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zaman MS, Thamminana S, Shahryari V,
Chiyomaru T, Deng G, Saini S, Majid S, Fukuhara S, Chang I, Arora
S, et al: Inhibition of PTEN gene expression by oncogenic
miR-23b-3p in renal cancer. PLoS One. 7:e502032012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xiong J, Wang D, Wei A, Lu H, Tan C, Li A,
Tang J, Wang Y, He S, Liu X and Hu W: Deregulated expression of
miR-107 inhibits metastasis of PDAC through inhibition PI3K/Akt
signaling via caveolin-1 and PTEN. Exp Cell Res. 361:316–323. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li Y, Song T, Chen Z, Wang Y, Zhang J and
Wang X: Pancreatic stellate cells activation and matrix
metallopeptidase 2 expression correlate with lymph node metastasis
in pancreatic carcinoma. Am J Med Sci. 357:16–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu Y, Yan L, Zhu W, Song X, Yang G and
Wang S: MMP2/3 promote the growth and migration of laryngeal
squamous cell carcinoma via PI3K/Akt-NF-kB-mediated
epithelial-mesenchymal transformation. J Cell Physiol. Feb
4–2019.(Epub ahead of print). doi: 10.1002/jcp.28242.
|