Introduction
Soft tissue sarcomas can occur in any anatomic
region. Those arising from the retroperitoneal cavity
(retroperitoneal sarcoma; RSar) are rare, aggressive, heterogeneous
malignancies, accounting for approximately 15% of all soft tissue
sarcomas (1). As RSar-induced
symptoms are frequently nonspecific and painless, the median size
at first diagnosis ranges from 15 to 18 cm (2). Enlarged RSars tend to invade the
surrounding vital organs, including the kidneys, liver, pancreas,
duodenum, small intestine, colon, inferior vena cava, and aorta,
making complete surgical resection difficult in clinical practice
(3). The poor prognosis of RSar has
been driving the continual efforts for determining the prognostic
factors (1,3–5),
developing the molecular classification (6,7), and
establishing perioperative interventions (8,9) and
novel antitumor drugs and regimens (10–12).
RSar is characterized by many different cell types, resulting in a
wide variety of histological entities such as well-differentiated
liposarcoma (WDLPS), dedifferentiated liposarcoma (DDLPS),
undifferentiated pleomorphic sarcoma (UPS), and leiomyosarcoma
(LMS). Considering the disease heterogeneity, it is very
complicated to define and manage the treatment strategies.
As some patients with unresectable,
treatment-refractory soft-tissue sarcoma respond to immune
checkpoint inhibitors (ICIs) that target programmed cell death 1
(PD-1), programmed cell death ligand-1 (PD-L1), and cytotoxic
T-lymphocyte antigen, the perioperative administration of ICIs
would be beneficial for treatment-naive patients with less tumor
burden (13). Several prospective
clinical trials are ongoing to verify the efficacy of ICIs and/or
radiotherapy in the neoadjuvant settings for patients with
surgically resectable RSar (8,13). The
immunologic profile, copy number alteration, and tumor mutation
burden are useful predictive factors of the response to ICIs
(14). Although the prognostic
impact and biological role of PD-1 and PD-L1 in sarcomas have been
investigated in previous studies including meta-analyses (15,16),
only a few studies have focused on the immunologic profile of
RSar.
In the current study, we evaluated the clinical
significance and prognostic implications of PD-L1, PD-L2, PD-1, and
Ki-67 expression in patients with RSar. It is well known that PD-L1
binding to PD-1 is a negative regulator of T-cell responses and
contributes to immune escape in the tumor microenvironment
(13). By contrast, the clinical and
biological role of PD-L2 in tumor immunity remains unclear,
especially in RSar (16). Thus, to
better understand the role and prognostication of the
PD-L1/PD-L2/PD-1 axis in RSar, we conducted this multicenter
collaborative study of the Nara Urological Research and Treatment
Group (NURTG) by performing immunohistochemical (IHC) staining
analysis of surgically resected tissue specimens.
Materials and methods
Patient selection and data
collection
This study was approved by the ethics committee of
the Nara Medical University, and informed consent from the
participants was obtained in the form of opt-out in the outpatient
clinic and on the web-site (reference ID: 1256/1966). Between
January 2000 and September 2019, a total of 64 patients were
diagnosed with primary RSar at the Departments of Urology or
Orthopedic Surgery of Nara Medical University Hospital, Urology of
Nara Prefecture General Medical Center, and Urology of Nara City
Hospital. Of the 64 patients, 9 were excluded owing to insufficient
follow-up data. Four patients were excluded because only tissue
biopsy, not surgical tumor resection, was performed; finally, 51
patients with RSar who underwent surgical tumor resection were
included in the analysis. The baseline clinicopathological
information and follow-up data were collected by retrospectively
reviewing the medical charts. The blood parameters included the
levels of hemoglobin (Hb), albumin, C-reactive protein (CRP), and
lactate dehydrogenase (LDH), calcium adjusted by albumin level
(hereafter referred to as adjusted Ca), the
neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte
ratio (PLR), and the monocyte-to-lymphocyte ratio (MLR). All
patients underwent contrast-enhanced computed tomography and/or
magnetic resonance imaging prior to surgery to determine the tumor
location and size.
Surgical resection of RSar
Complete surgical resection is among the principal
treatment modalities for RSar. The resection of contiguous organs,
major blood vessels, and major muscles is associated with a higher
rate of complete resection, thereby resulting in improved
disease-free survival (3,17). A previous study using a nationwide
database revealed that contiguous organ resection was not
associated with increased postoperative morbidity or severe
morbidity (17). The extent of
surgical resection depended on the attending physicians and
surgeons in our cohort. We reviewed the contiguous organs, vessels,
and muscles that were resected concomitantly. The resection of a
tumor with at least one organ, major vessel, or major muscle was
defined as ‘extended resection’.
Pathological evaluation and tumor
staging
All hematoxylin and eosin-stained (H&E)
specimens obtained via surgical resection or biopsy (image-guided
core needle biopsy or open/laparoscopic excision biopsy) were
re-assessed independently by two experienced pathologists (K.H. and
T.F.) considering the tumor histology, tumor-cell-free surgical
margins, and tumor grade according to the French Federation
Nationale des Centres de Lutte le Cancer (FNCLCC) system (18). The resection margins were coded by
using the residual tumor (R) classification on the basis of the
macroscopic and microscopic evaluation (19), and they were categorized into one of
the following three categories: Microscopically negative (R0),
microscopically positive (R1), or grossly positive (R2). TNM
categories were classified and the prognostic stage (from stage IA
to IV) was defined according to the American Joint Committee on
Cancer (AJCC) eighth edition for the retroperitoneum-specific
criteria (20).
Immunohistochemical staining
IHC staining was performed using paraffin-embedded,
formalin-fixed tissue blocks (fixed in 10% buffered formalin at
room temperature for 24–48 h), as previously described (21). The details about the primary and
secondary antibodies and conditions are available in Table SI. Histofine Simple Stain™ MAX PO
(MULTI) kit (catalogue no. 414151; Nichirei Corporation) was used
for peroxidase color development according to the manufacturer
direction. The membranous expression of PD-L1, PD-L2, and PD-1 and
the nuclear expression of Ki-67 were evaluated in at least 3
independent high-power microscopic fields (×400, 0.0625
µm2) of sarcoma cells, which were determined on the
basis of the cell morphology. The percentage of positive sarcoma
cells divided by the total counted sarcoma cells was calculated
(0–100%). The staining intensity of these markers was not taken
into account. The cut-off values for defining low or high
expression on IHC staining were based on the median-positive
scores. The IHC staining results were evaluated by two
investigators (Y.O. and S.H.) who were blinded to any
clinicopathological data.
Postoperative management and
follow-up
Additional chemotherapy and/or radiotherapy were
performed on a case-by-case basis, especially for patients
presenting with R1 or R2 disease. Doxorubicin plus ifosfamide or
doxorubicin alone was administered for most cases (22). A chest/abdomen/pelvis CT scan was
obtained approximately every 3 months for 3 years after surgery,
every 6 months from years 4 to 5, and annually thereafter.
Recurrence was defined as radiographically detectable local
recurrence or distant metastases.
Statistical analysis
IBM SPSS version 23 (SPSS Inc.) and PRISM software
version 5.00 (San Diego) were used for statistical analyses and
data plotting, respectively. A P-value <0.05 was considered
statistically significant. Continuous variables were expressed as
the median and interquartile range (IQR) and compared using the
Mann-Whitney U-test or the Kruskal-Wallis test, followed by the
post hoc test (Dunn test). Data are expressed as box plots.
Categorical variables were compared using a Chi-square or Fisher's
exact test, as appropriate. The correlations among the studied
parameters were examined using the Spearman correlation coefficient
(ρ value) and linear regression analysis (Y-slope). The absolute
values of Spearman ρ<0.2–0.4 were considered to indicate weak
correlation; 0.4–0.7, moderate correlation; and >0.7, strong
correlation.
Recurrence-free survival (RFS) and disease-specific
survival (DSS) were the primary outcomes. Survival was estimated
with the Kaplan-Meier method by calculating the RFS and DSS from
the date of surgery to the date of events or the last follow-up,
and were compared using log-rank tests. Multivariate analysis was
used to identify independent prognostic variables by using a
stepwise Cox proportional hazards regression model. Variables that
potentially affected prognosis (P<0.1) on univariate analysis
were included in the multivariate analysis.
Results
Patient characteristics
A total of 51 patients treated at three tertiary
hospitals were included in this study. Table I summarizes the clinicopathological
characteristics. Tumor biopsy prior to surgery was performed in 10
patients (20%) to make the diagnosis of sarcoma and determine its
histologic subtype. The paired pathological results of biopsy
tissues and surgically resected tissues are listed in Table SII. The pathological results of
biopsy tissues and surgically resected tissues were the same or
similar in 8 of the 10 patients (80%), while the pathological
results were changed from LMS or rhabdomyosarcoma to DDLPS and from
inflammatory tissue to DDLPS in 1 patient each. Simple tumor
resection was performed in 20 patients (35%) and extended resection
in 31 patients (65%). The number of resected organs and the
concomitant procedure are shown in Table SIII. Eighteen (58%) of the 31
patients underwent concomitant resection of one organ. Frequently
resected targets included the kidneys (48%) and psoas muscle (35%).
One patient (an 81-year-old man) with a large retroperitoneal DDLPS
underwent multi-visceral resection of the left kidney,
descending/sigmoid colon, psoas muscle, and common iliac artery via
F-F bypass vascular reconstruction. Regarding the tumor
histopathology, liposarcoma (WDLPS, DDLPS, and myxoid LPS) was the
most frequent tumor entity, accounting for 53% of all cases. The
three major types of RSar in our cohort were DDLPS (41%), LMS (20%)
and UPS (16%) (Table I).
 | Table I.Clinicopathologic characteristics of
51 cases with retroperitoneal sarcoma. |
Table I.
Clinicopathologic characteristics of
51 cases with retroperitoneal sarcoma.
| Variables | Value |
|---|
| Total | 51 (100%) |
| Sexa |
|
|
Male | 32 (63) |
|
Female | 19 (37) |
| Age at surgery,
yearsb | 65 (54–71) |
| Tumor size,
cmb | 7.6 (4.9–11.2) |
| Hemoglobin,
g/dlb | 12.8
(11.3–14.3) |
| Albumin,
g/dlb | 4.0 (3.7–4.3) |
| LDH,
IU/lb | 196 (169–226) |
| Adjusted calcium,
mg/dlb | 9.3 (9.1–9.5) |
| CRP,
mg/dlb | 0.2 (0.05–1.7) |
| NLRb | 2.3 (1.7–3.4) |
| PLRb | 147 (97–173) |
| MLRb | 0.30
(0.21–0.49) |
| Biopsy prior to
surgerya,c |
|
| No | 41 (80) |
|
Yes | 10 (20) |
| Extended
resectiona,d |
|
| No | 20 (39) |
|
Yes | 31 (61) |
| Tumor
entitya |
|
|
WDLPS | 5 (10) |
|
DDLPS | 21 (41) |
| Myxoid
LPS | 1 (2) |
|
LMS | 10 (20) |
|
UPS | 8 (16) |
|
Epithelioid sarcoma | 1 (2) |
|
Fibrosarcoma | 1 (2) |
|
Myxofibrosarcoma | 1 (2) |
|
Synovial sarcoma | 1 (2) |
|
Sarcoma, NOS | 2 (4) |
| FNCLCC
gradinga |
|
| G1 | 10 (20) |
| G2 | 14 (27) |
| G3 | 27 (53) |
| Prognostic
staginga,e |
|
| IA | 5 (10) |
| IB | 5 (10) |
| II | 9 (18) |
|
IIIA | 15 (29) |
|
IIIB | 15 (29) |
| NA | 2 (4) |
| Resection
margina,f |
|
| R0 | 23 (45) |
| R1 | 23 (45) |
| R2 | 5 (10) |
| Postoperative
treatmenta |
|
| No | 41 (80) |
|
Chemotherapy | 7 (14) |
|
Chemotherapy +
radiotherapy | 3 (6) |
PD-L1, PD-L2, PD-1, and Ki-67
expression in RSar specimens
As no patients underwent neoadjuvant therapy in the
studied cohort, all the specimens were treatment-naïve RSar.
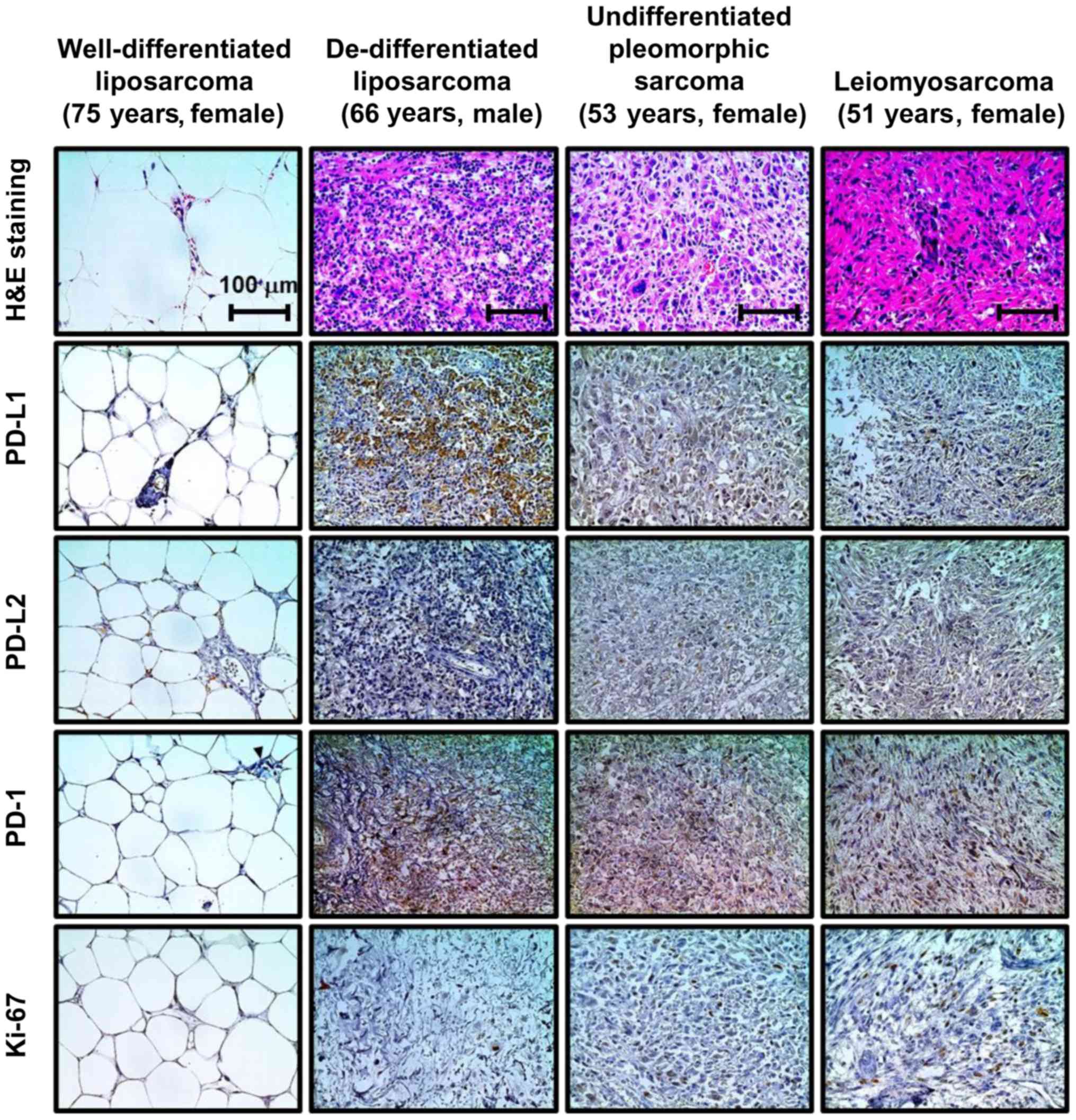
Representative images of IHC staining for PD-L1, PD-L2, PD-1, and
Ki-67 in retroperitoneal sarcoma are shown in Fig. 1. The percentage of positive sarcoma
cells divided by the total counted sarcoma cells was calculated
(0–100%). The median scores and IQRs for PD-L1, PD-L2, PD-1, and
Ki-67 expression in sarcoma cells were 7 (2 to 11), 2 (0 to 8), 15
(5 to 27), and 5 (2 to 19), respectively. These scores were
compared considering the sarcoma subtypes and prognostic stage.
There was a significant difference in the PD-L1-positive score
among the tumor subtypes on the Kruskal-Wallis test, followed by
multiple comparisons showing higher levels of PD-L1 expression in
DDLPS and LMS than in other sarcomas (Fig. 2A). PD-L2, PD-1, and Ki-67 staining
results were not associated with sarcoma subtypes. Although the
prognostic stage was not correlated with the IHC staining results
in our cohort, there seemed to be a tendency that PD-L1 and PD-L2
positive scores decreased from stage II to IIIA in that order
(Fig. 2B).
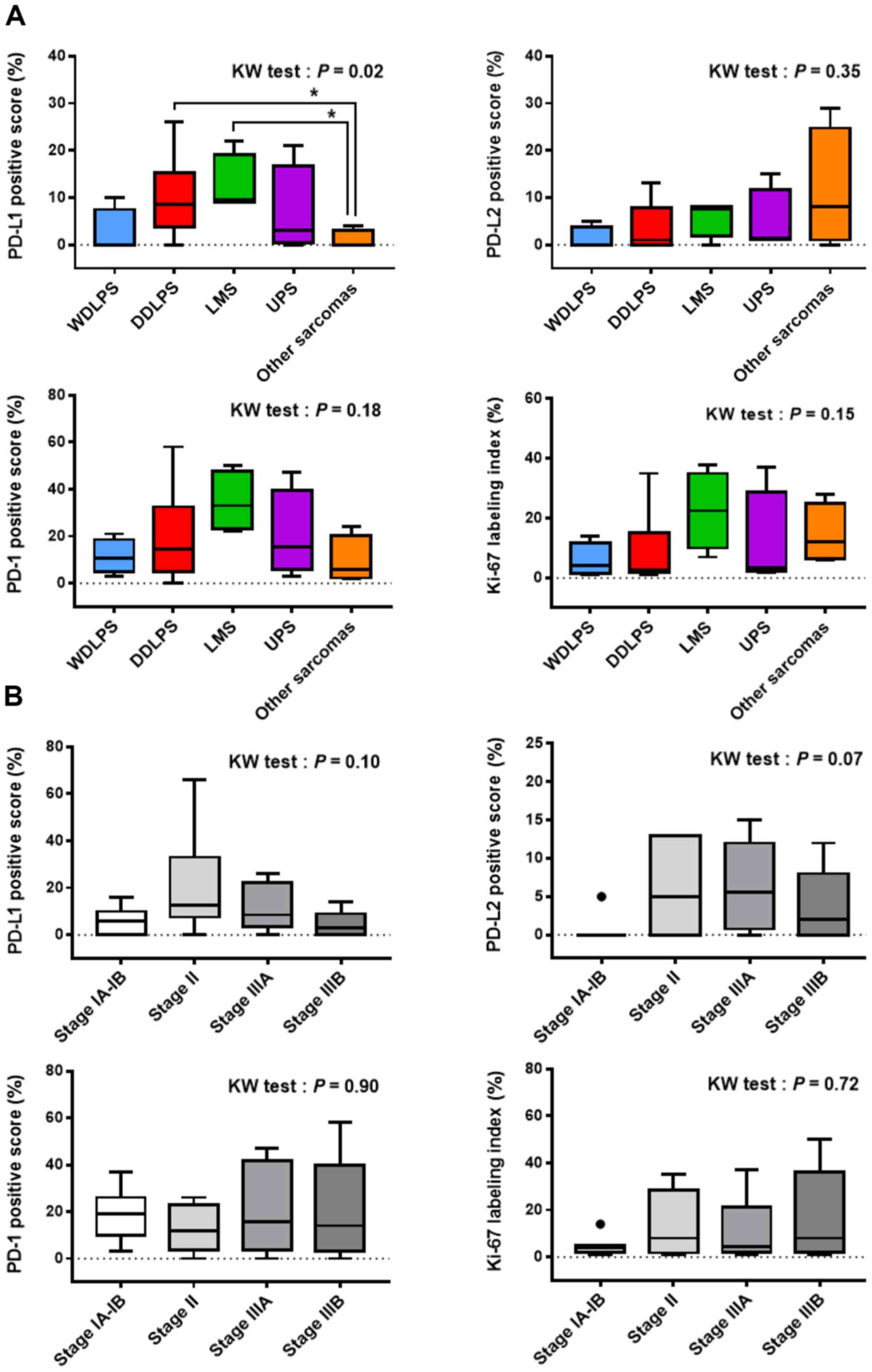 | Figure 2.Association of PD-L1, PD-L2, PD-1,
and Ki-67 expression with the (A) sarcoma subtypes and (B)
prognostic stage. The prognostic stage (from stage IA to IIIB) was
determined according to the TNM categories and tumor grade (the
American Joint Committee on Cancer eighth edition for the
retroperitoneum-specific criteria). All data are expressed in
box-and-whisker plots. The black circles indicate outliers.
Multiple data were compared using the Kruskal-Wallis test.
*P<0.05 (post hoc Dunn test). PD-L1, programmed death ligand-1;
PD-L2, programmed death ligand-2; PD-1, programmed cell death
protein 1; WDLPS, well-differentiated liposarcoma; DDLPS,
dedifferentiated liposarcoma; UPS, undifferentiated pleomorphic
sarcoma; LMS, leiomyosarcoma. |
Correlation analysis
To investigate the correlations among the baseline
parameters and the expression of immune checkpoint proteins in the
RSar specimens, we selected continuous variables for analysis using
the Spearman correlation coefficient. Fig. 3A summarizes the P-values and Spearman
ρ values from the correlation coefficient analyses and the Y-slopes
from the linear regression analyses. The tumor size was inversely
correlated with Hb and albumin levels. Three systemic inflammation
markers, i.e., the NLR, PLR, and MLR, were moderately to strongly
correlated (all P<0.001; NLR vs. PLR ρ=0.56; PLR vs. MLR ρ=0.64:
NLR vs. MLR ρ=0.78, respectively). Serum LDH levels were moderately
and positively correlated with expression of PD-L1 (P=0.018,
ρ=0.41) and PD-L2 (P=0.006, ρ=0.47) (Fig. 3B). A PD-L1 positive score was
positively correlated with a PD-1 positive score (Fig. 3B; P=0.039, ρ=0.41), while the Ki-67
labeling index was positively correlated with PD-L2 (P=0.03,
ρ=0.48) and PD-1 expression (P=0.01, ρ=0.52). Age and adjusted
calcium levels were not significantly correlated with any
parameters (Fig. 3A).
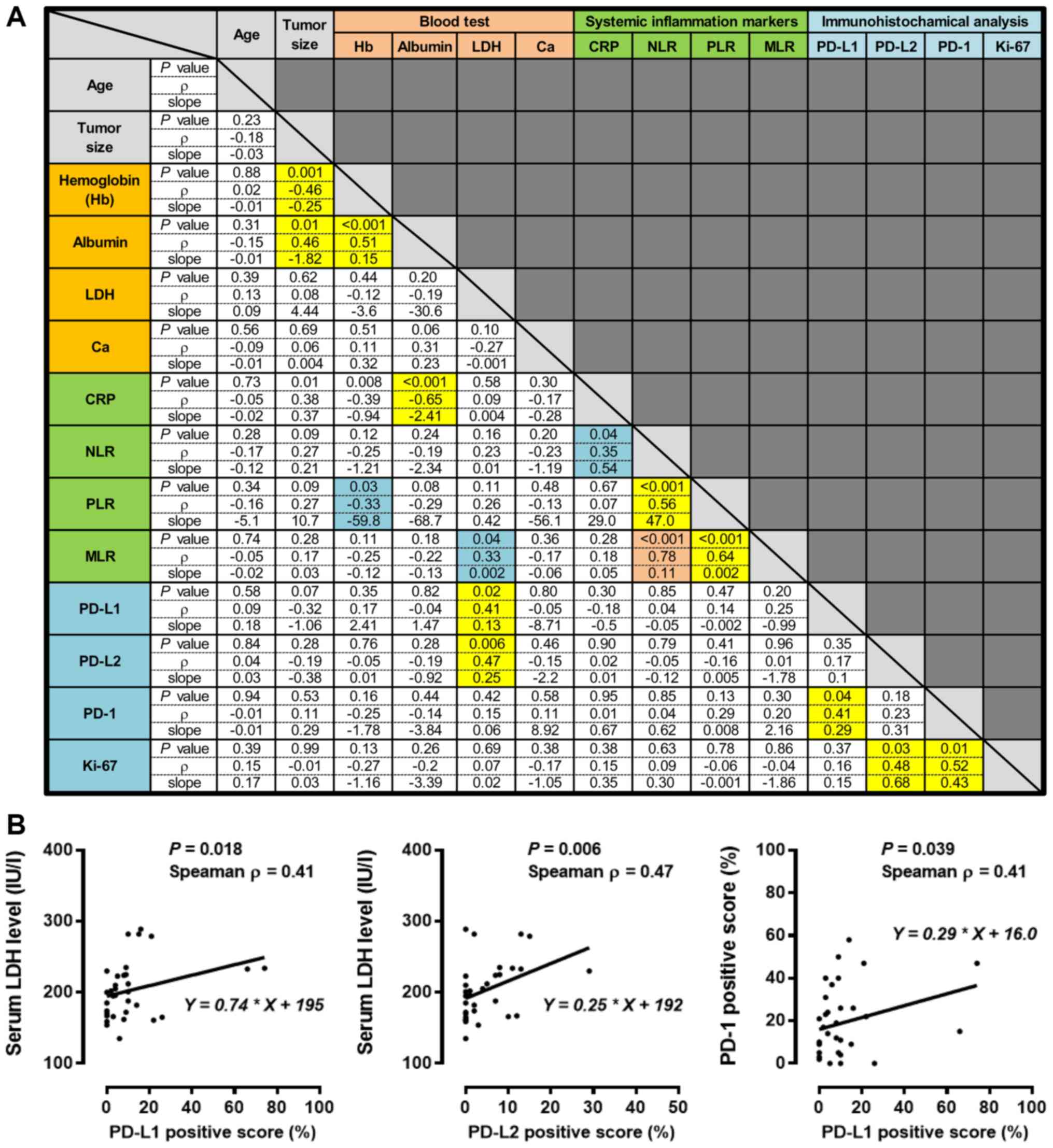 | Figure 3.Correlation analysis for the baseline
clinical parameters and immune checkpoint proteins in 51 patients
with retroperitoneal sarcoma. (A) Summary results from the
correlation analysis for the preoperative clinical parameters and
immunohistochemical staining analysis in the sarcoma specimens. The
P-value and Spearman ρ from the correlation coefficient analysis
and the Y-slope from the linear regression analysis are shown in
each cell. Blue, yellow, and orange cells indicate weak, moderate,
and strong correlations, respectively. (B) The relationships
between the baseline LDH and the PD-L1 and PD-L2 positive scores
were examined using the Spearman correlation coefficient and linear
regression analysis. PD-L1, programmed cell death ligand-1; PD-L2,
programmed death ligand-2; PD-1, programmed cell death protein 1;
Hb, hemoglobin; LDH, lactate dehydrogenase; CRP, C-reactive
protein; NLR, neutrophil-to-lymphocyte ratio; PLR,
platelet-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte
ratio. |
Prognostic values of prognostic
staging and high expression of PD-L1 and Ki-67
The median follow-up period after surgery was 29
months (IQR, 10–50 months). During the follow-up period, 33
patients (65%) experienced tumor recurrence and 19 patients (37%)
succumbed to RSar. As no patients died of other cause during the
follow-up period, overall survival was not evaluated in this study.
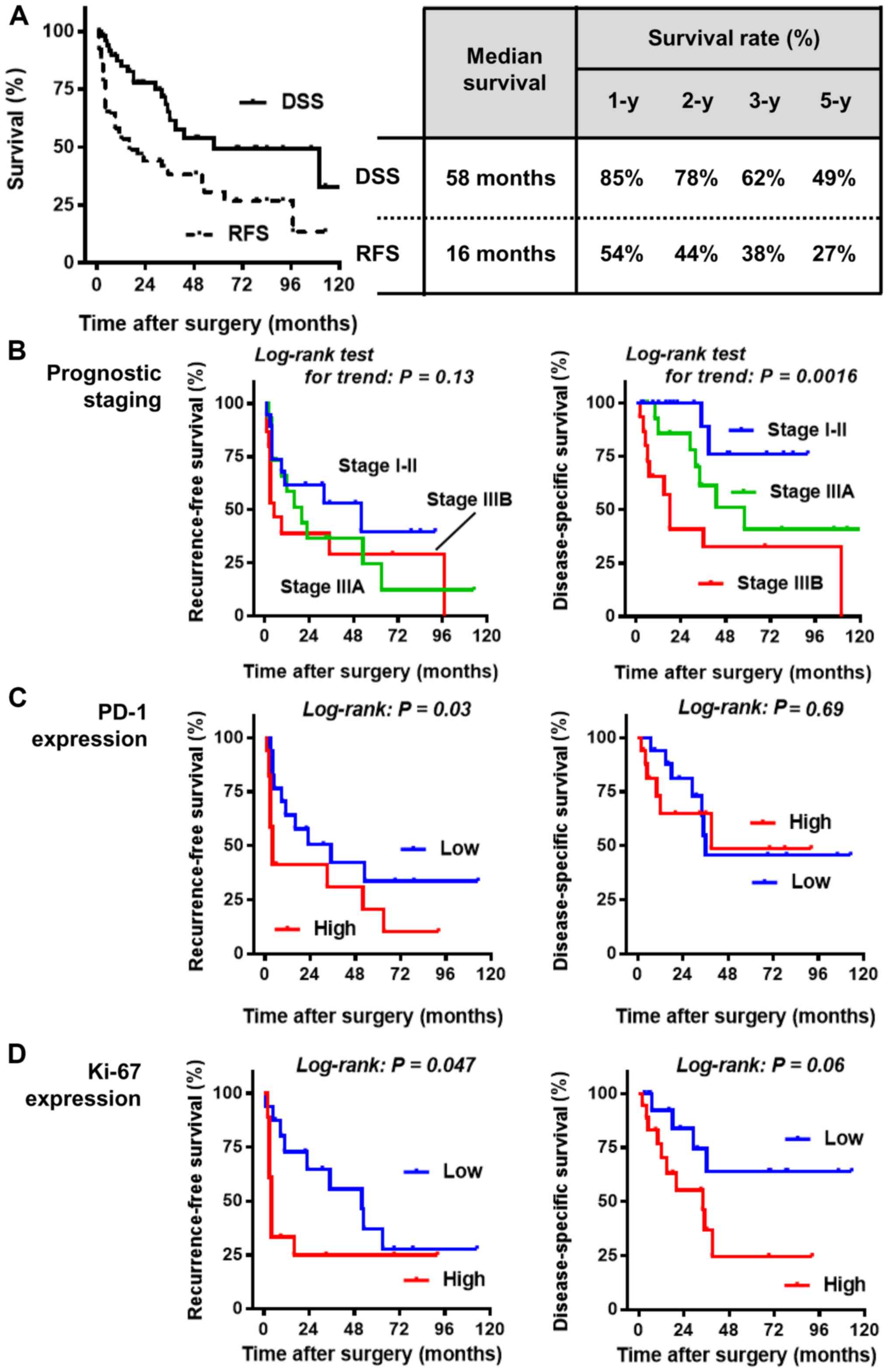
The median DSS and RFS were 58 and 16 months, respectively
(Fig. 4A). The 5-year RFS and DSS
rates were 27 and 49%, respectively. Univariate analyses were
performed to determine the prognostic values of the studied
parameters. The cut-off values on IHC staining were defined on the
basis of the median positive scores as follows: 7% for PD-L1, 2%
for PD-L2, 15% for PD-1, and 5% for Ki-67. On univariate analysis,
a short RFS was associated with high expression levels of PD-1 and
Ki-67, while DSS was significantly associated with prognostic stage
IIIB disease and a high baseline level of CRP (Table II). The Kaplan-Meier curves for RFS
and DSS are shown in Figs. 4B-D and
S1. After controlling for possibly
confounding variables, multivariate analyses revealed that
independent predictors of RFS and DSS were a high expression of
Ki-67 (P=0.03; hazard ratio, 2.29 vs. low expression) and
prognostic stage IIIB (P, 0.04; hazard ratio, 5.11 vs. stage I–II),
respectively (Table II). PD-L1,
PD-L2, or PD-1 expression was not an independent prognostic factor
in our study cohort of RSar.
 | Table II.Prognostic variables for
recurrence-free survival and disease-specific in 51 patients with
retroperitoneal sarcoma undergoing resection surgery. |
Table II.
Prognostic variables for
recurrence-free survival and disease-specific in 51 patients with
retroperitoneal sarcoma undergoing resection surgery.
|
| Recurrence-free
survival | Disease-specific
survival |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age at surgery,
years |
|
<65 | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
≥65 | 0.73 | 0.37–1.44 | 0.34 |
|
|
| 0.65 | 0.26–1.63 | 0.36 |
|
|
|
| Sex |
|
Male | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
Female | 0.69 | 0.34–1.37 | 0.29 |
|
|
| 0.56 | 0.22–1.35 | 0.21 |
|
|
|
| Prognostic
staginga |
| Stage
I–II | 1 |
|
|
|
|
| 1 |
|
| 1 |
|
|
| Stage
IIIA | 1.54 | 0.64–3.70 | 0.32 |
|
|
| 3.68 | 0.99–13.6 | 0.08 | 2.95 | 0.53–16.5 | 0.22 |
| Stage
IIIB | 1.77 | 0.73–4.31 | 0.16 |
|
|
| 6.70 | 1.96–22.9 | 0.002 | 5.11 | 1.06–24.7 | 0.04 |
| Resection
marginb |
| R0 | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
| R1 | 1.09 | 0.51–2.31 | 0.81 |
|
|
| 0.87 | 0.33–2.31 | 0.89 |
|
|
|
| R2 | 1.79 | 0.53–6.08 | 0.25 |
|
|
| 0.96 | 0.20–4.58 | 0.97 |
|
|
|
| Tumor
entityc |
|
DDLPS | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
LMS | 1.26 | 0.51–3.08 | 0.58 |
|
|
| 0.85 | 0.26–2.74 | 0.79 |
|
|
|
|
UPS | 0.83 | 0.33–2.07 | 0.69 |
|
|
| 1.32 | 0.41–4.22 | 0.62 |
|
|
|
| Other
sarcomas | 0.94 | 0.32–2.78 | 0.91 |
|
|
| 1.09 | 0.22–5.37 | 0.91 |
|
|
|
| LDH |
|
Low | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
High | 1.22 | 0.59–2.55 | 0.58 |
|
|
| 1.12 | 0.44–2.82 | 0.81 |
|
|
|
| CRP |
|
Low | 1 |
|
|
|
|
| 1 |
|
| 1 |
|
|
|
High | 1.83 | 0.87–3.88 | 0.10 |
|
|
| 2.37 | 1.09–5.99 | 0.03 | 1.39 | 0.43–4.47 | 0.58 |
| NLR |
|
Low | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
High | 1.50 | 0.67–3.36 | 0.30 |
|
|
| 1.49 | 0.50–4.46 | 0.47 |
|
|
|
| PLR |
|
Low | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
High | 0.57 | 0.26–1.27 | 0.57 |
|
|
| 0.88 | 0.30–2.62 | 0.82 |
|
|
|
| MLR |
|
Low | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
High | 1.11 | 0.50–2.46 | 0.79 |
|
|
| 1.30 | 0.44–3.86 | 0.64 |
|
|
|
| PD-L1
expression |
|
Low | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
High | 0.94 | 0.41–2.17 | 0.88 |
|
|
| 0.64 | 0.21–1.92 | 0.41 |
|
|
|
| PD-L2
expression |
|
Low | 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
High | 1.37 | 0.59–3.17 | 0.44 |
|
|
| 1.53 | 0.51–4.59 | 0.43 |
|
|
|
| PD-1
expression |
|
Low | 1 |
|
| 1 |
|
| 1 |
|
|
|
|
|
|
High | 2.15 | 1.27–5.32 | 0.03 | 1.61 | 0.66–3.94 | 0.29 | 1.25 | 0.41–3.78 | 0.69 |
|
|
|
| Ki-67
expression |
|
Low | 1 |
|
| 1 |
|
| 1 |
|
| 1 |
|
|
|
High | 2.19 | 1.03–5.13 | 0.047 | 2.29 | 1.16–5.50 | 0.03 | 2.81 | 0.97–8.08 | 0.06 | 2.80 | 0.84–9.29 | 0.09 |
Discussion
The current study investigated the clinical
relevance of the expression of three pivotal immune checkpoint
proteins, PD-L1, PD-L2, and PD-1, and Ki-67 expression in
surgically resected RSar. Although we evaluated the prognostic
value of PD-L1, PD-L2, and PD-1 expression, only high expression of
PD-1 was a possible predictor of postoperative recurrence. By
contrast, a high expression of Ki-67 was associated with a high
risk of recurrence and disease-specific death. Several molecular
markers, such as Ki-67, p27/kip1, α-SMA, and CD133, have been
identified as prognostic factors (3,23–25).
PD-L1 and PD-1 expression in sarcoma cells and tumor-infiltrating
lymphocytes (TILs) as well as TIL density have been well
investigated as potential prognostic biomarkers in various
malignancies, including sarcomas. However, the results were
inconsistent (15). A recent study
demonstrated that preoperative 18F-fluorodeoxyglucose
positron emission tomography/computed tomography was able to
predict preoperatively FNCLCC grade 3 of retroperitoneal LPS and
estimate the short survival after surgery (cutoff: Maximum
standardized uptake value >4.5) (26). However, to date, only a few molecular
and radiographic markers have been used in clinical practice.
Some advanced/metastatic soft tissue sarcomas are
amenable to immunotherapies (13),
although there is a significant lack of evidence regarding the
immunologic profile and immune microenvironment of soft tissue
sarcoma subtypes. Pollack et al compared the expression
levels of genes associated with antigen presentation, T-cell
infiltration, and immune checkpoint proteins among common sarcomas
including WDLPS, DDLPS, UPS, and LMS (16). UPS is a highly mutated sarcoma and
shows high levels of PD-L1 and PD-1 on IHC analysis. By contrast,
LPS was less mutated but highly expressed immunogenic
self-antigens, which may support the need for immunotherapy with
PD-1/PD-L1 blockade in this subset of common sarcomas. The results
of the current study showed significantly higher levels of PD-L1
expression in DDLPS and LMS, but not in UPS, than in other sarcomas
(Fig. 2A), while PD-1 and PD-L2
expression did not show any significant difference among the
sarcoma subtypes. PD-L1 is the most intensively researched immune
checkpoint molecule in all oncological fields, including soft
tissue sarcoma. A meta-analysis of the prognostic value of PD-L1 in
sarcomas showed that the positive rate of PD-L1 expression varied
from 8.5 to 75.0% (15). This
striking variability in the PD-L1 expression rate in sarcomas can
be due to multiple factors, such as differences in the cut-off
values for defining PD-L1 positivity, differences in IHC assays,
antibodies used for PD-L1 expression, and differences in patient
background characteristics, including the sarcoma subtypes
(15). In the current study, we
determined the absolute percentages of positivity in sarcoma cells
and used those cut-off values for prognostic assessment.
Nevertheless, further comprehensive evaluation of multiple
available antibodies (e.g., clones SP263, E1L3N, and 22C3) in
various types of cells including sarcoma cells, tumor-infiltrating
lymphocytes, and macrophages is likely to fill the gaps between the
studies.
We provided a detailed overview of baseline clinical
parameters and IHC analysis (Fig.
3). We selected the parameters expressed with continuous values
on the basis of the previously reported possible prognostic
factors. A total of 14 parameters were tested, and 15 correlations
were evaluated as follows: 1 Strong, 11 moderate, and 3 weak
correlations. A large tumor size, low Hb level, and low albumin
level were moderately associated with each other. A high NLR, high
PLR, and high MLR were moderately to strongly associated with each
other. High levels of serum LDH were significantly correlated with
high PD-L1 expression (Spearman ρ=0.41) and PD-L2 expression
(ρ=0.47). LDH is an enzyme ubiquitously found in all cell types. In
the last step of aerobic glycolysis, LDH catalyzes the conversion
of pyruvate to lactate, leading to the accumulation of lactate and
the production of an acidic tumor microenvironment (27). Eventually, this condition can cause
immunosuppression in melanoma tumors (27). LDH-A mRNA expression was associated
with an increased number of PD-L1- and PD-1-positive M2 macrophages
in the tumor microenvironment of patients with extramammary Paget
disease (28). A preclinical study
revealed that the stimulation of melanoma cells with lactate
upregulated the expression of PD-L1 and altered immunomodulation in
the tumor microenvironment; also, blockade of LDH-A could improve
the efficacy of anti-PD-1 treatment (27). A clinical study showed that an
integrated algorithm involving baseline serum LDH levels in
patients with metastatic solid tumors was able to provide a higher
performance for response prediction to ICIs (29). A review regarding the biomarkers for
predicting the efficacy of anti-PD-1 antibodies showed that
elevated levels of serum LDH were associated with poor response to
the treatment (30). Therefore, we
believe that more evidence would clarify the potential benefit of
serum and tumor LDH levels as clinically available biomarkers for
RSar.
The present study has several limitations. First,
the sample size was relatively small and there was a possible
selection bias caused by the retrospective nature of the study.
Second, we did not perform subgroup analysis for the different
types of sarcomas considering the prognostic values of the immune
checkpoint molecules because only limited patients with each major
entity (DDLPS, LMS, and UPS) were included. Third, we excluded
patients who were diagnosed with RSar and did not undergo surgical
resection. Fourth, only a single antibody for each molecule was
used in the IHC analysis, which could affect the staining result.
Fifth, we did not evaluate the expression of cytotoxic T
lymphocyte-associated antigen-4 (CTLA-4). Although immune
checkpoint blockade by the combination of anti-CTLA-4 and anti-PD-1
antibodies have successfully been used for several malignancies
(13), this treatment modality
remains understudied in retroperitoneal soft tissue sarcoma. Future
additional assessment of CTLA-4 expression in RSar is needed for
better understanding the immune microenvironment that can affect
tumor initiation and response to therapy.
In conclusion, the findings of the current study
provide novel insights into the prognostic values of PD-L1, PD-L2,
and PD-1 expression in RSar tissues as well as regarding the
correlation between baseline clinical parameters and immune
checkpoint molecules. However, the complex heterogeneity of RSar
hinders the establishment of an ideal therapeutic strategy using
immunotherapy. Nevertheless, further investigations are necessary
to determine the immunologic landscape of RSar and provide a
foundation for therapeutic intervention using ICIs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MM, YM, DG, NT, and KF conceived the study. YO and
SH designed the study. SO performed the immunohistochemical
staining analysis. KH, TF, ST, HF, AK and KH collected and data. NN
performed the statistical analysis. YN, SA, KT, YM and EO
interpreted the data. All authors substantially contributed
drafting the work and revising it critically for important
intellectual content. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
the Nara Medical University (reference ID: 1256 and 1966). Informed
consent to participate in the study was obtained from all
participants. The study was conducted in compliance with the
study's protocol and in accordance with the provisions of the
Declaration of Helsinki (2013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RSar
|
retroperitoneal sarcoma
|
|
WDLPS
|
well-differentiated liposarcoma
|
|
DDLPS
|
de-differentiated liposarcoma
|
|
UPS
|
undifferentiated pleomorphic
sarcoma
|
|
LMS
|
leiomyosarcoma
|
|
ICI
|
immune checkpoint inhibitors
|
|
PD-1
|
programmed cell death 1
|
|
PD-L1
|
program cell death ligand-1
|
|
PD-L
|
program cell death ligand-2
|
|
IHC
|
immunohistochemical
|
|
Hb
|
hemoglobin
|
|
LDH
|
lactate dehydrogenase
|
|
CRP
|
C-reactive protein
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
PLR
|
platelet-to-lymphocyte ratio
|
|
MLR
|
monocyte-to-lymphocyte ratio
|
|
CT
|
computed tomography
|
|
FNCLCC
|
French Federation Nationale des
Centres de Lutte le Cancer
|
|
IQR
|
interquartile range
|
|
RFS
|
recurrence-free survival
|
References
|
1
|
Malinka T, Nebrig M, Klein F, Pratschke J,
Bahra M and Andreou A: Analysis of outcomes and predictors of
long-term survival following resection for retroperitoneal sarcoma.
BMC Surg. 19:612019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hassan I, Park SZ, Donohue JH, Nagorney
DM, Kay PA, Nasciemento AG, Schleck CD and Ilstrup DM: Operative
management of primary retroperitoneal sarcomas: A reappraisal of an
institutional experience. Ann Surg. 239:244–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morizawa Y, Miyake M, Shimada K, Hori S,
Tatsumi Y, Nakai Y, Anai S, Tanaka N, Konishi N and Fujimoto K:
Extended resection including adjacent organs and Ki-67 labeling
index are prognostic factors in patients with retroperitoneal soft
tissue sarcomas. World J Surg Oncol. 14:432016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonvalot S, Gaignard E, Stoeckle E, Meeus
P, Decanter G, Carrere S, Honore C, Delhorme JB, Fau M, Tzanis D,
et al: Survival benefit of the surgical management of
retroperitoneal sarcoma in a reference center: A nationwide study
of the French sarcoma group from the NetSarc database. Ann Surg
Oncol. 26:2286–2293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tseng W, Martinez SR, Tamurian RM, Borys D
and Canter RJ: Histologic type predicts survival in patients with
retroperitoneal soft tissue sarcoma. J Surg Res. 172:123–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan L, Wang Z, Cui C, Guan X, Dong B, Zhao
M, Wu J, Tian X and Hao C: Comprehensive immune characterization
and T-cell receptor repertoire heterogeneity of retroperitoneal
liposarcoma. Cancer Sci. 110:3038–3048. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tseng WW, Malu S, Zhang M, Chen J, Sim GC,
Wei W, Ingram D, Somaiah N, Lev DC, Pollock RE, et al: Analysis of
the intratumoral adaptive immune response in well differentiated
and dedifferentiated retroperitoneal liposarcoma. Sarcoma.
2015:5474602015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keung EZ, Lazar AJ, Torres KE, Wang WL,
Cormier JN, Ashleigh Guadagnolo B, Bishop AJ, Lin H, Hunt KK, Bird
J, et al: Phase II study of neoadjuvant checkpoint blockade in
patients with surgically resectable undifferentiated pleomorphic
sarcoma and dedifferentiated liposarcoma. BMC Cancer. 18:9132018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diamantis A, Baloyiannis I, Magouliotis
DE, Tolia M, Symeonidis D, Bompou E, Polymeneas G and Tepetes K:
Perioperative radiotherapy versus surgery alone for retroperitoneal
sarcomas: A systematic review and meta-analysis. Radiol Oncol.
54:14–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelly CM, Antonescu CR, Bowler T, Munhoz
R, Chi P, Dickson MA, Gounder MM, Keohan ML, Movva S, Dholakia R,
et al: Objective response rate among patients with locally advanced
or metastatic sarcoma treated with talimogene laherparepvec in
combination with pembrolizumab: A phase 2 clinical trial. JAMA
Oncol. 6:402–408. 2020.(Epub ahead of print). View Article : Google Scholar
|
|
11
|
D'Angelo SP, Mahoney MR, Van Tine BA,
Atkins J, Milhem MM, Jahagirdar BN, Antonescu CR, Horvath E, Tap
WD, Schwartz GK and Streicher H: Nivolumab with or without
ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two
open-label, non-comparative, randomised, phase 2 trials. Lancet
Oncol. 19:416–426. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wisdom AJ, Mowery YM, Riedel RF and Kirsch
DG: Rationale and emerging strategies for immune checkpoint
blockade in soft tissue sarcoma. Cancer. 124:3819–3829. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gibney GT, Weiner LM and Atkins MB:
Predictive biomarkers for checkpoint inhibitor-based immunotherapy.
Lancet Oncol. 17:e542–e551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu Z, Jin Z, Zhang M, Tang Y, Yang G,
Yuan X, Yao J and Sun D: Prognostic value of programmed
death-ligand 1 in sarcoma: A meta-analysis. Oncotarget.
8:59570–59580. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pollack SM, He Q, Yearley JH, Emerson R,
Vignali M, Zhang Y, Redman MW, Baker KK, Cooper S, Donahue B, et
al: T-cell infiltration and clonality correlate with programmed
cell death protein 1 and programmed death-ligand 1 expression in
patients with soft tissue sarcomas. Cancer. 123:3291–3304. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tseng WH, Martinez SR, Tamurian RM, Chen
SL, Bold RJ and Canter RJ: Contiguous organ resection is safe in
patients with retroperitoneal sarcoma: An ACS-NSQIP analysis. J
Surg Oncol. 103:390–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guillou L, Coindre JM, Bonichon F, Nguyen
BB, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V,
Leroux A, et al: Comparative study of the national cancer institute
and French federation of cancer centers sarcoma group grading
systems in a population of 410 adult patients with soft tissue
sarcoma. J Clin Oncol. 15:350–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoang K, Gao Y and Miller BJ: The
variability in surgical margin reporting in limb salvage surgery
for sarcoma. Iowa Orthop J. 35:181–186. 2015.PubMed/NCBI
|
|
20
|
Tanaka K and Ozaki T: New TNM
classification (AJCC eighth edition) of bone and soft tissue
sarcomas: JCOG bone and soft tissue tumor study group. Jpn J Clin
Oncol. 49:103–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyake M, Hori S, Morizawa Y, Tatsumi Y,
Nakai Y, Anai S, Torimoto K, Aoki K, Tanaka N, Shimada K, et al:
CXCL1-mediated interaction of cancer cells with tumor-associated
macrophages and cancer-associated fibroblasts promotes tumor
progression in human bladder cancer. Neoplasia. 18:636–646. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Judson I, Verweij J, Gelderblom H,
Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan
J, Hohenberger P, et al: Doxorubicin alone versus intensified
doxorubicin plus ifosfamide for first-line treatment of advanced or
metastatic soft-tissue sarcoma: A randomised controlled phase 3
trial. Lancet Oncol. 15:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oliveira AM, Nascimento AG, Okuno SH and
Lloyd RV: p27(kip1) protein expression correlates with survival in
myxoid and round-cell liposarcoma. J Clin Oncol. 18:2888–2893.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
She Y, Liang J, Qiu Y, Liu N, Huang X,
Zhao X and Li P: Expression of CD133 in primary retroperitoneal
leiomyosarcoma and its relationship with Ki-67. Zhonghua Yi Xue Za
Zhi. 94:1241–1244. 2014.(In Chinese). PubMed/NCBI
|
|
25
|
Ma C, Li PY, Zhang N, Sun CB, Hou L and
Liu N: Prognostic factors analysis of Ki-67, α-SMA expression in
retroperitoneal leiomyosarcoma. Zhonghua Zhong Liu Za Zhi.
40:258–263. 2018.(In Chinese). PubMed/NCBI
|
|
26
|
Rhu J, Hyun SH, Lee KH, Jo SJ, Lee KW,
Park JB and Kim SJ: Maximum standardized uptake value on
18F-fluorodeoxyglucose positron emission
tomography/computed tomography improves outcome prediction in
retroperitoneal liposarcoma. Sci Rep. 9:66052019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Daneshmandi S, Wegiel B and Seth P:
Blockade of lactate dehydrogenase-A (LDH-A) improves efficacy of
anti-programmed cell death-1 (PD-1) therapy in melanoma. Cancers
(Basel). 11:4502019. View Article : Google Scholar
|
|
28
|
Urata K, Kajihara I, Miyauchi H, Mijiddorj
T, Otsuka-Maeda S, Sakamoto R, Sawamura S, Kanemaru H,
Kanazawa-Yamada S, Makino K, et al: The Warburg effect and tumour
immune microenvironment in extramammary Paget's disease:
Overexpression of lactate dehydrogenase A correlates with immune
resistance. J Eur Acad Dermatol Venereol. 34:1715–1721. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cona MS, Lecchi M, Cresta S, Damian S, Del
Vecchio M, Necchi A, Poggi MM, Raggi D, Randon G, Ratta R, et al:
Combination of baseline LDH, performance status and age as
integrated algorithm to identify solid tumor patients with higher
probability of response to anti PD-1 and PD-L1 monoclonal
antibodies. Cancers (Basel). 11:2232019. View Article : Google Scholar
|
|
30
|
Kambayashi Y, Fujimura T, Hidaka T and
Aiba S: Biomarkers for predicting efficacies of anti-PD1
antibodies. Front Med (Lausanne). 6:1742019. View Article : Google Scholar : PubMed/NCBI
|