Angiogenesis, which is the development of new blood
vessels from existing vasculature, is a major driving force in
numerous types of malignancy by delivering oxygen and nutrients for
the growth of tumors (1), while
facilitating fast metastasis (2).
First introduced by Folkman as a potential target for cancer
treatment (3), angiogenesis was
thereafter considered an essential pathologic feature and
sustaining element of cancer, which has a key role in tumor
dissemination/metastasis (4).
Therefore, it appears reasonable to predict that the extent of
tumor vascularity, measured by the pathological microvessel density
(MVD), may be closely associated with the aggressiveness of a tumor
(5), including its invasive and
metastatic potential. MVD is usually defined by the following
equation:
The endothelial cell or endothelial cell cluster
that was clearly separated from adjacent microvessels, tumor cells
and other connective tissue elements was considered a single,
countable microvessel (6). An
inverse association between MVD and patient survival has been
reported for several malignancies, including breast cancer
(7) and melanoma (8), as well as prostate (9) and bladder (10) cancer. Previous studies have indicated
that the MVD was correlated with vascular endothelial growth factor
(VEGF) expression, which is also a crucial factor in the vascular
biology of multiple tumors as a mediator of angiogenesis. In the
field of metastatic renal cell carcinoma (RCC), which is a highly
vascularized solid tumor type (11),
anti-angiogenic agents targeting VEGF/VEGF receptor, such as
sunitinib, pazopalib and bevacizumab, have been the standard
first-line therapy for years; however, they provide a limited
benefit and metastatic RCC remains a challenge (12), which suggests that there may be an
alternative blood supply besides angiogenesis. Of note,
intra-tumoral MVD has been a controversial prognostic predictor for
RCC. Nativ et al (13) and
Fukata et al (14) reported
that higher MVD is associated with shorter survival in RCC.
Similarly, other studies have demonstrated this association in
patients with ccRCC (15–17). Some of the studies found other
associations. For example, Paradis et al (18) and Zhang et al (19) reported a positive association between
MVD and VEGF expression levels, and Tuna et al (20) reported positive association between
MVD and mast cell infiltration. Notably, Slaton et al
(21) reported no significant
correlation between MVD and VEGF, Mohseni et al (22) reported lack of correlation between
MVD and mast cell infiltration, while others reported a lack of
correlation between MVD and survival (23–26). On
the contrary, numerous studies (27–32) have
reported higher MVD associated with longer survival, and Yoshino
et al (33) and Sabo et
al (34) also reported this
association in patients with low-stage RCC. Delanunt et al
(35) reported this association in
ccRCC, and Sharaml et al (36) reported this tendency yet the P-value
was 0.1. Sandlund et al (37)
reported this trend in 2006, but one year later they switched the
marker from CD105 to CD31 and found the association disappeared
(38). As for the association with
stage or grade, Köhler et al (39) reported a negative association between
MVD and stage, Hemmerlein et al (40) and Baldewijns et al (41) reported a negative association between
MVD and Fuhrman grade and Kavantzas et al (42) reported positive association between
MVD and grade, while Sharma et al (43) reported no association. Therefore,
plethora of literature makes the current understanding of MVD in
the setting of RCC controversial (Table
I).
Microvessel or microvasculature is defined as ‘the
smallest system of blood vessels in a body, including those
responsible for microcirculation, that distribute blood within
tissues’ (44). Besides
angiogenesis, there is an alternative perfusion source termed
‘vasculogenic mimicry’ (VM), also referred to as ‘vascular
mimicry’. The initial study and molecular characterization of VM
was conducted in melanoma (45).
Later, VM was also assessed in breast cancer (46) and hepatic carcinoma (47). Of note, the results of these studies
agreed with those of earlier studies suggesting the perfusion of
tumors via non-endothelial-lined channels. Since VM may also serve
as a supply system of blood including nutrients, the concept of MVD
may require to be modified, as the current understanding of the
complexity of vasculature, either endothelium- or tumor
cell-derived, improves over the years. Therefore, the present study
proposed a modified version of MVD, referred to as total MVD
(TMVD), which incorporates the number of MVD and the status of VM,
and was defined as follows:
In the present study, the capability of MVD, VM and
TMVD in predicting prognosis of patients with RCC was evaluated and
compared, and a bioinformatics analysis of the possible genes
underlying the clinical significance of VM was performed.
A retrospective study was performed involving 183
patients with histopathologically verified RCC who underwent
nephrectomy between January 2006 and December 2016 at Xinhua
Hospital Affiliated to Shanghai Jiao Tong University, School of
Medicine (Shanghai, China). The cohort had a median age of 59.3±7.0
years (range, 44–73 years) and comprised 104 males and 79 females.
The pre-operative radiological evaluation consisted of chest X-ray,
abdominal ultrasonography and contrast-enhanced CT. None of the
patients received irradiation or chemotherapy prior to surgery. The
follow-up comprised of chest X-ray, abdominal ultrasonography or CT
scan. The macroscopic and histological features of RCC were
assessed, including tumor stage and Fuhrman nuclear grade (26). The tumor stage was defined according
to the 2010 TNM classification (48). At presentation, the tumor stage was
pT1 in 73, pT2 in 80 and pT3 in 30 cases, and the Fuhrman grade was
I in 58, II in 90, III in 29 and IV in 6 umors. The follow-up
program included clinical and radiological examinations. The median
follow-up time from diagnosis was 53.9±19.0 months (range, 11–94
months) for surviving patients. The survival time was calculated
from the date of surgery to the date of death or latest follow-up.
The study was approved by the Ethics Committee of Xinhua Hospital
(Shanghai, China; approval no. XHEC-D-2016-061). The requirement
for informed consent was waived by the Ethics Committee due to the
retrospective nature of this study. The overall/disease-free
survival time and gene sequencing data of another 537 patients with
RCC were retrieved from The Cancer Genome Atlas (TCGA) database
(https://cancergenome.nih.gov/), the
Kidney RCC cohort (TCGA, provisional) using cBioPortal (https://www.cbioportal.org/). Survival time was
evaluated based on individual gene expression levels.
IHC was performed on conventional 5-µm-thick
histological paraffin-embedded tissue serial RCC sections on
poly-L-lysine-coated glass slides. After heat-drying, the sections
were deparaffinized in xylene and sequentially rehydrated in
gradients of ethanol, and next incubated overnight at 4°C with
anti-CD34 antibody (cat. no. ab81289; 1:100 dilution; Abcam).
Signals were amplified with the VECTASTAIN® ABC kit
(Vector Laboratories, Inc.). At ×200 magnification, most of the
slides had CD34-positive stain and those without any CD34 signal
were considered invalid and restained. Periodic acid Schiff (PAS)
staining was performed using a PAS kit (Sigma-Aldrich; Merck KGaA)
according to the manufacturer's protocol on one of the CD34-stained
slides. Sections were counterstained with Mayer's hematoxylin,
coverslips were mounted with Permount Mounting Medium and samples
were observed using an Olympus IX73 microscope (Olympus, Corp.).
For the negative control, the primary antibody was replaced with
non-immune human serum (cat. no. 31876; Thermo Fisher Scientific,
Inc.).
Values were expressed as the mean ± standard error
of the mean, while in figures MVD were shown in box and whisker
plots as minimum to maximum using GraphPad Prism 6 (GraphPad
Software, Inc.). Statistical analyses involved Student's t-test,
one-way analysis of variance with Bonferroni's post hoc test, the
χ2 test and the log-rank (Mantel-Cox) test. The analyses
were conducted with SPSS 22 (IBM Corp.) or GraphPad Prism 6
(GraphPad Software, Inc.). In the survival analysis, when two
Kaplan-Meier curves crossed, Cox time-dependent covariate analysis
was used for adjustment of the P-value. P<0.05 was considered to
indicate a statistically significant difference.
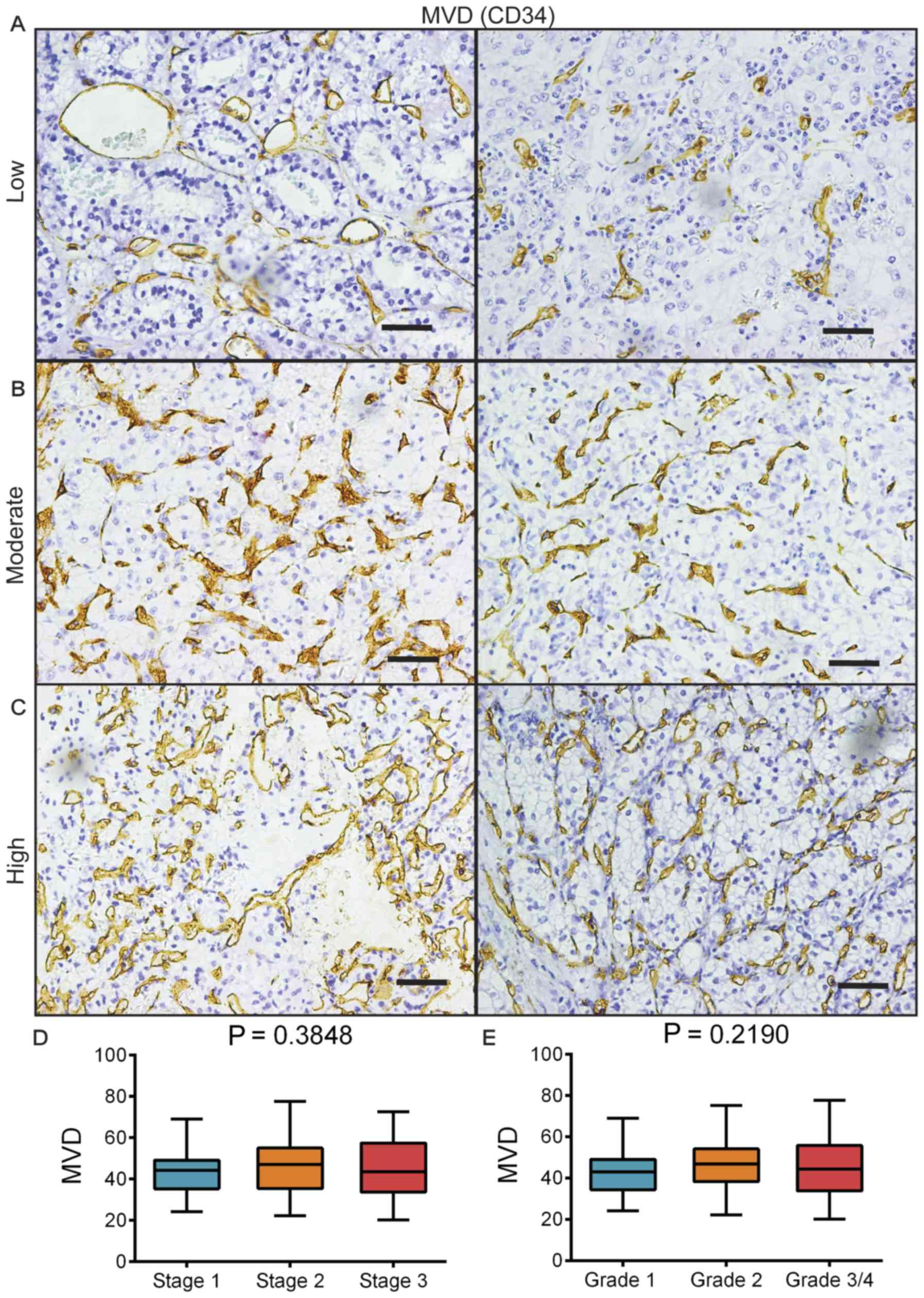
IHC staining for CD34 was performed on the RCC
samples. By microscopic observation under ×200 magnification, MVD
in a hotspot area was able to be classified into low (between 20
and 30; Fig. 1A), moderate (between
40 and 50; Fig. 1B) and high
(between 60 and 80; Fig. 1C). The
mean MVD was calculated to be 44.9±12.4. Regarding different
stages, the mean MVD was 43.5±10.0 for stage 1, 46.3±13.6 for stage
2 and 44.8±14.2 for stage 3 (Fig.
1D). The mean MVD for different grades was 42.6±10.9 for grade
1, 46.2±12.4 for grade 2 and 45.5±14.3 for grades 3/4 (Fig. 1E). There was no significant
difference in MVD between the different stages or grades, and no
increasing or decreasing tendency was observed either. The results
of Fig. 1 suggested a weak
association between MVD and the stage/grade.
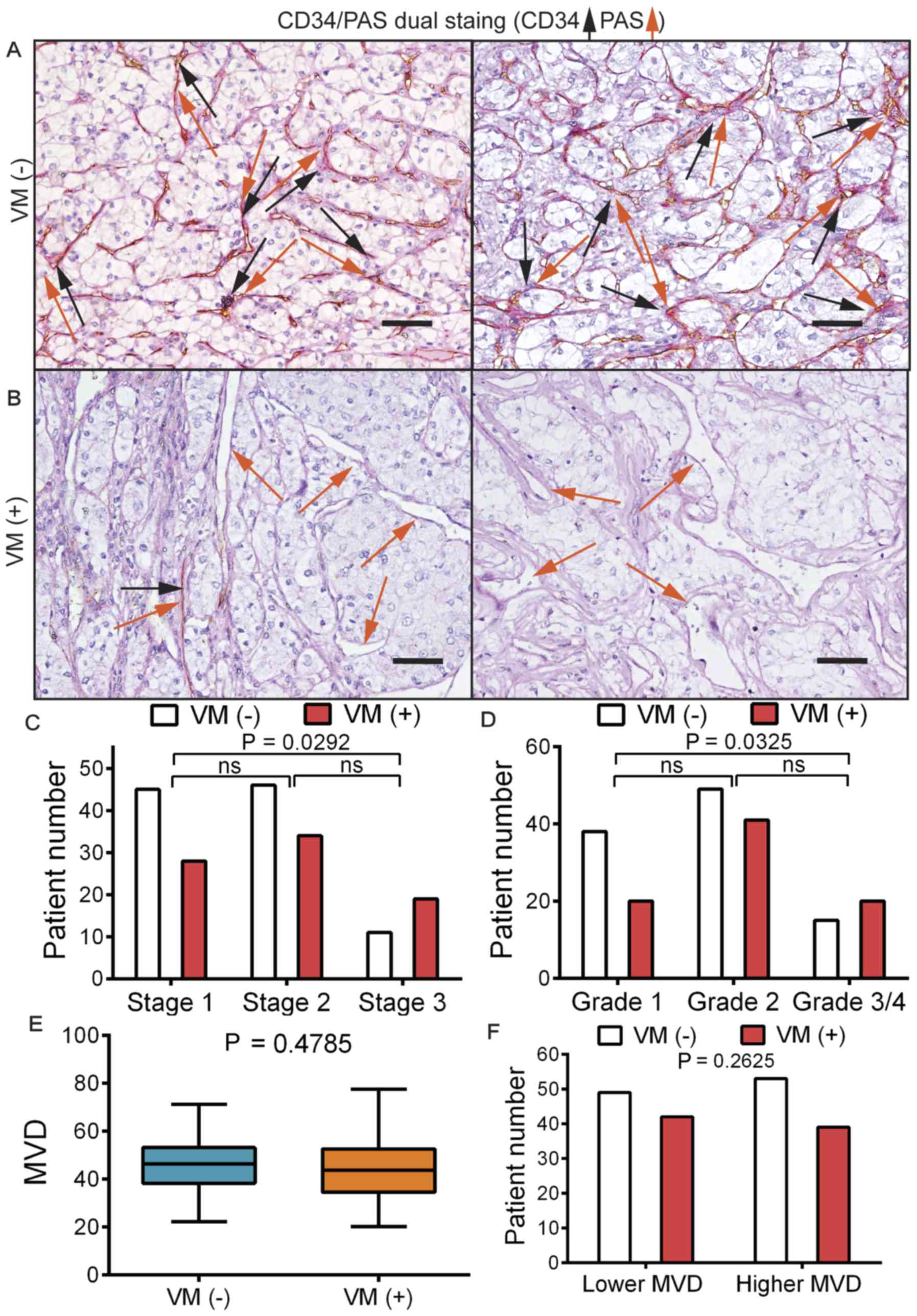
CD34/PAS dual staining was performed on serial RCC
sections in order to identify the VM structure. Based on CD34
expression, the slides were classified into VM(−), which
corresponded to a CD34(+)/PAS(+) status (Fig. 2A), and VM(+), which was defined by
the presence of a CD34(−)/PAS(+) enclosed channel that was lined by
tumor cells rather than endothelial cells (Fig. 2B). Patients were stratified based on
their VM(+) or VM(−) status. By further stratifying the patients
based on their stage/grade information, it was observed that,
although there was a higher proportion of VM(+) patients in stage 3
compared with those in stage 1 (P=0.0292; Fig. 2C), the differences between stage 1
and 2 or stage 2 and 3 were not statistically significant.
Similarly, a higher proportion of VM(+) patients was present in the
grade 3/4 group than in the grade 1 group (P=0.0325; Fig. 2D). There was no difference in MVD
between patients with VM(+) and VM(−) according to Student's t-test
(P=0.4785; Fig. 2E). The patients
were then stratified into high or low MVD groups and it was
observed that there was no difference in the VM(+) ratio between
patients with high or low MVD in their tumor according to the
c2 test (P=0.2625; Fig.
2F).
To clarify why the phenotype of VM was reported to
be closely associated with the survival of patients with RCC
(51,52), the present study attempted to
identify the potentially associated genes using TCGA database via
cBioPortal. Previous studies reported several genes closely
associated with the formation of VM, including vascular endothelial
(VE)-cadherin (also known as CDH5), vimentin (VIM) and matrix
metalloproteinases (MMPs) (53–55). The
clinical data from a large sample were retrieved from TCGA database
and the survival length of patients with RCC was analyzed based on
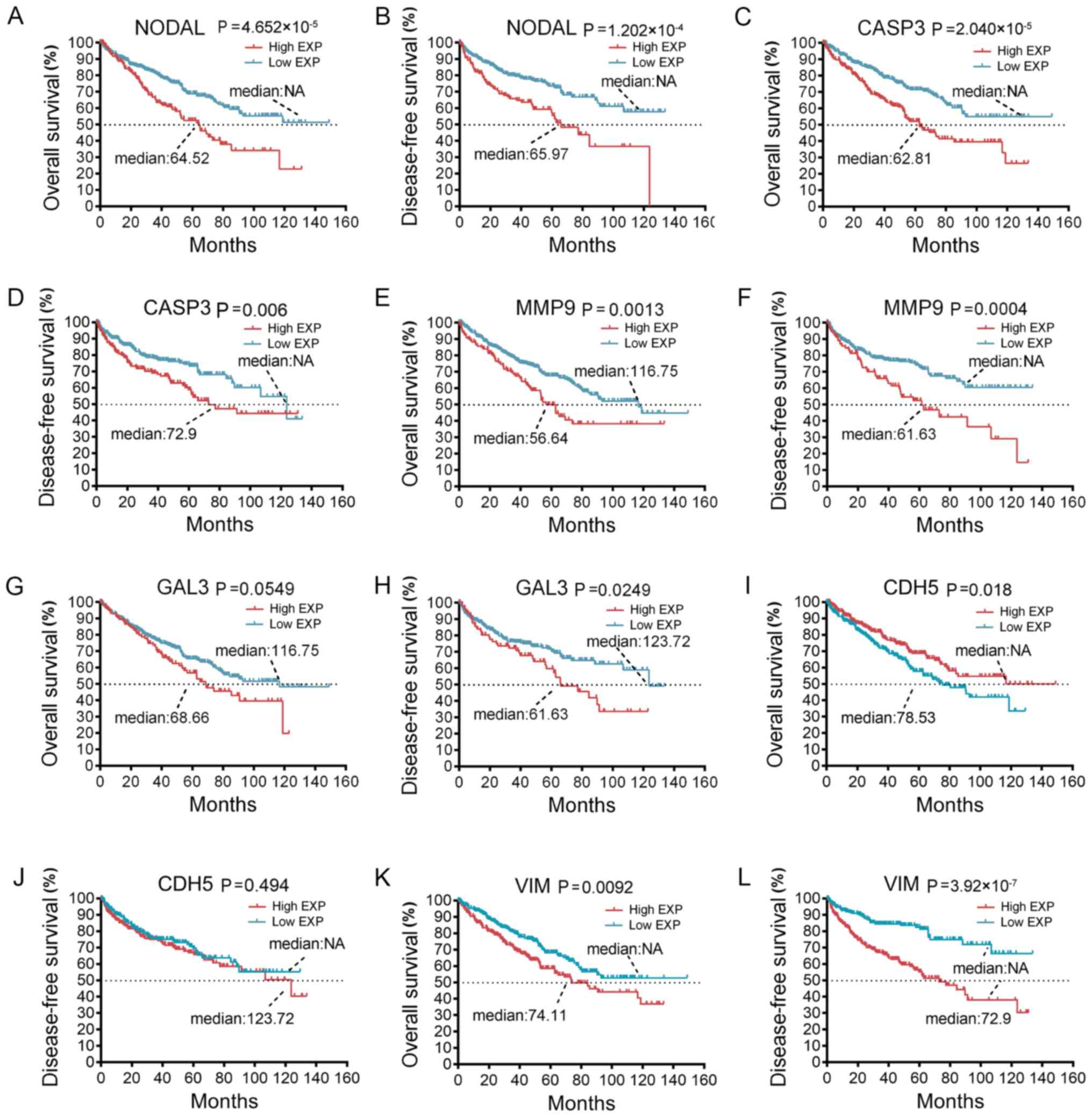
the expression levels of those VM-associated genes. Among them,
certain genes had a significant negative impact on
overall/disease-free survival, including nodal growth
differentiation factor (NODAL), caspase-3 (CASP3), MMP9 and
galectin-3 (GAL3) (Fig. 3A-H,
respectively). Of the two genes that are known to be closely linked
to VM, high VE-cadherin was unexpectedly associated with a longer
overall survival (P=0.018; Fig. 3I),
but not disease-free survival (P=0.494; Fig. 3J). VIM, a well-known oncogene
(56,57), had a significant negative effect on
overall survival (P=0.0092; Fig. 3K)
and disease-free survival (P=3.92×10−7; Fig. 3L).
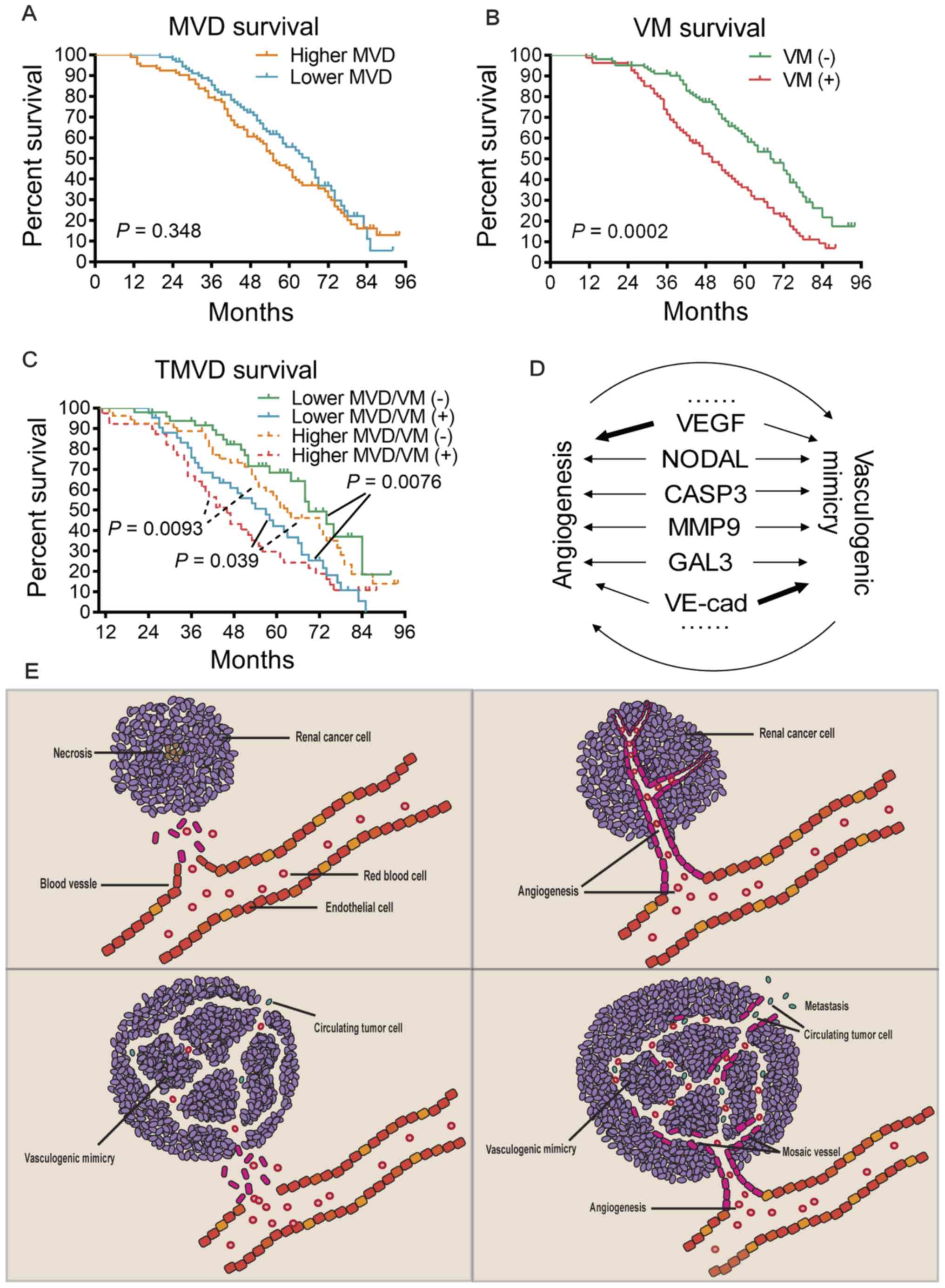
Upon dividing the patients into two groups based on
their MVD levels, there was no significant difference between the
survival time of patients with high or low MVD (P=0.348; Fig. 4A), although the survival time had a
tendency to be shorter in patients with higher MVD. Stratification
of the patients based on their VM status indicated that VM(+)
patients had a significantly shorter survival time (P=0.0002;
Fig. 4B), demonstrating an inverse
association between VM and survival. By applying the TMVD concept,
those patients were further stratified into four subgroups.
Comparison of the survival curves of these four subgroups indicated
that this stratification was able to distinguish patients with
different survival prognoses (Fig.
4C). Among patients with a lower MVD, VM(−) patients exhibited
significant longer survival than VM(+) patients (P=0.0076); and
among patients with a higher MVD, VM(−) patients also had a
significantly longer survival time than VM(+) patients (P=0.0093).
Of note, patients with a lower MVD combined with a VM(+) status had
an even poorer prognosis than those with a higher MVD combined with
a VM(−) status (P=0.039).
MVD assessment is the most commonly used technique
to quantify intratumoral angiogenesis in cancer. It was first
developed by Weidner et al (58) in 1991, who used panendothelial IHC
staining of blood microvessels. The first step was the
identification of the area with the highest neovessel density (the
so-called ‘hot spot’). Individual microvessels were then counted at
higher power (magnification, ×200) in an adequate area (e.g., 0.74
mm2 per field using a 20× objective lens and a 10×
ocular lens). Any stained endothelial cells or clusters separated
from adjacent vessels were counted as single microvessels. Despite
numerous reports of the clinical prognostic significance of MVD in
various types of tumor, its predictive value regarding outcomes in
RCC remains controversial, as summarized in Table I. Some of them reported negative
correlation between MVD and prognosis (higher MVD correlated with
shorter survival) (13–17), some reported positive correlation
(27–32) and others reported no significance
(21,23–26,38).
This may be associated with several non-mechanistic factors,
including sample size, sampling bias, different blood vessel
markers (such as the more commonly used CD34 or CD31, or the less
frequently used FVIII Rag or CD105), the quality of IHC staining,
the methods of vasculature quantification and the methods of
interpretation. For instance, Sandlund et al (59) reported in 2006 that a higher MVD was
associated with longer survival; however, when CD31 was used as the
vessel marker instead of CD105, no association with survival was
observed (60). Due to the
heterogeneity in methodology among these studies, a forest plot may
be unpractical and unreasonable. Another possible reason is the
different categories of blood vessels. Yao et al (61) proposed that, within clear-cell RCC,
there are at least two major categories of blood vessels with
contrasting prognostic implications, namely undifferentiated
vessels (expressing CD31 but not CD34) and differentiated vessels
(expressing both CD31 and CD34), with a higher undifferentiated
vessel density indicating poorer prognosis and higher
differentiated vessel density correlating with better prognosis.
Qian et al (62) also
discussed the complexity of tumor vasculature in RCC and recent
studies on the concept of vessel co-option (a non-angiogenic
process through which tumor cells utilize pre-existing tissue blood
vessels to support tumor growth, survival and metastasis) have been
published (63–65), thus obscuring whether MVD is a
sufficient prognostic factor.
VM is the formation of fluid-conducting channels by
highly invasive and genetically dysregulated tumor cells and acts
as a complementary source of blood supply. In the present study,
TMVD (i.e., MVD plus VM status) demonstrated a better
prognosis-predicting capability compared with that of the MVD or VM
alone (Fig. 4C), which may be
explained by the fact that endothelium-lined blood vessels as well
as VM are able to transfer blood, nutrients and oxygen, and
theoretically, both may facilitate cancer progression. It is
reasonable to assume that during treatment with an anti-angiogenic
regimen, when neo-angiogenesis is suppressed, tumor growth may be
more dependent on the supply from VM. A comprehensive meta-analysis
review by Yang et al (66)
revealed that VM is associated with unfavorable prognosis in >10
different types of tumor, and with cancer differentiation, lymph
node metastasis and distant metastasis. In other words, VM is not
only functional as a delivering channel, but is in itself is a
hallmark of potent proliferation and metastasizing capability.
Survival analysis of VM-associated genes, including NODAL, CASP3,
MMP9 and GAL3, revealed that these genes had a negative impact on
overall and disease-free survival in the setting of RCC based on
TCGA database. In addition, several studies have been published
demonstrating that the above genes also contribute to angiogenesis
(67–70). The single most important factor in
VM, VE-cadherin, has been indicated to regulate angiogenesis
(71) and the single most important
factor in angiogenesis, VEGF, has also been reported to promote VM
(72). Taken together, angiogenesis
and VM may promote tumor progression independently and probably
interdependently (Fig. 4D and E).
One of the limitations of the present study is that the association
between the above-mentioned genes, VM formation and patient
survival was not assessed in the present cohort, and therefore, it
was not possible to experimentally clarify certain paradoxical
results of the bioinformatics analysis, including higher
VE-cadherin being associated with longer overall survival.
When the concept of TMVD was proposed, it was
expected to be the sum of MVD and VM density, but in reality, the
quantification of VM density, if it is able to be quantitated, is
rather difficult. The identification process relies greatly on
visual observation. If red blood cells (RBCs) are present inside a
CD34(−)/PAS(+) area, it is easier to confirm, while the absence of
RBCs inside such an area complicates the identification, since PAS
staining may not be well demarked. Instead of calculating its
density, the status of VM (positive or negative) was incorporated
into the formula of TMVD in the present study. Generally speaking,
among the four groups classified according to TMVD, the prognosis
of patients with low MVD(≤45)/VM(+) was the best, that of patients
with high MVD(>45)/VM(−) and low MVD(≤45)/VM(+) was intermediate
and that of patients with high MVD(>45)/VM(+) was the worst. The
clinical significance and cost-effectiveness of this novel concept
of TMVD require to be further investigated, not only in the setting
of RCC, but also in other cancer types in which VM may have a
critical role. Recently, novel combinational therapy targeting
other molecules, including programmed cell death 1 (PD1)/programmed
cell death 1 ligand 1 (PDL1) and cytotoxic T-lymphocyte-associated
protein 4 (CTLA-4), has demonstrated promising efficiency (73–75).
With more clinical trials ongoing, it is possible that checkpoint
immunotherapy combined with anti-angiogenesis therapy may be
adopted as the first-line treatment for metastatic RCC, and
PD1/PDL1/CTLA-4 expression levels, and perhaps other gene
expression levels (76–79), combined with TMVD may provide higher
accuracy in predicting patient prognosis.
In conclusion, the present study examined the novel
concept of TMVD, which is a combination of MVD and VM status, and
evaluated its capability in predicting prognosis in patients with
RCC compared to that of MVD or VM alone. TMVD demonstrated superior
predictive capability, and together with the results of the TCGA
data analysis, the present results suggested that angiogenesis and
VM promote tumor progression independently and probably
interdependently.
Not applicable.
This work was supported by the National Natural
Science Foundation (grant nos. 81970657 and 81802522) and the
Shanghai Sailing Program (grant no. 18YF1415200).
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
JQ, ZG, JY and JD designed the study. JY and JD
supervised the whole process. YW, KD, WG, DW, HT and NW performed
the research, among which WG and JY conducted the IHC staining. YW
and KD analyzed the data. YW and JD wrote the manuscript. ZG and JD
revised the statistics and the manuscript. All authors read and
approved the final manuscript.
The study was approved by the Ethics Committee of
Xinhua Hospital (Shanghai, China; approval no. XHEC-D-2016-061).
The requirement of informed consent was waived by the Ethics
Committee due to the retrospective nature of the study.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 (6 Suppl 16):S15–S18. 2002.
View Article : Google Scholar
|
|
3
|
Folkman J: Anti-angiogenesis: New concept
for therapy of solid tumors. Ann Surg. 175:409–416. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hlatky L, Hahnfeldt P and Folkman J:
Clinical application of antiangiogenic therapy: Microvessel
density, what it does and doesn't tell us. J Natl Cancer Inst.
94:883–893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tae K, El-Naggar AK, Yoo E, Feng L, Lee
JJ, Hong WK, Hittelman WN and Shin DM: Expression of vascular
endothelial growth factor and microvessel density in head and neck
tumorigenesis. Clin Cancer Res. 6:2821–2828. 2000.PubMed/NCBI
|
|
7
|
Zhou D, Cheng SQ, Ji HF, Wang JS, Xu HT,
Zhang GQ and Pang D: Evaluation of protein pigment
epithelium-derived factor (PEDF) and microvessel density (MVD) as
prognostic indicators in breast cancer. J Cancer Res Clin Oncol.
136:1719–1727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pastushenko I, Vermeulen PB, Carapeto FJ,
Van den Eynden G, Rutten A, Ara M, Dirix LY and Van Laere S: Blood
microvessel density, lymphatic microvessel density and lymphatic
invasion in predicting melanoma metastases: Systematic review and
meta-analysis. Br J Dermatol. 170:66–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyata Y and Sakai H: Reconsideration of
the clinical and histopathological significance of angiogenesis in
prostate cancer: Usefulness and limitations of microvessel density
measurement. Int J Urol. 22:806–815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang J, Ma X, Chen X, Liu X, Zhang B,
Minmin L, Nie W, Zhang L and Liu L: Microvessel density as a
prognostic factor in bladder cancer: A systematic review of
literature and meta-analysis. Cancer Biomark. 14:505–514. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aziz SA, Sznol J, Adeniran A, Colberg JW,
Camp RL and Kluger HM: Vascularity of primary and metastatic renal
cell carcinoma specimens. J Transl Med. 11:152013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Derosa L, Bayar MA, Albiges L, Le Teuff G
and Escudier B: A new prognostic model for survival in second line
for metastatic renal cell carcinoma: Development and external
validation. Angiogenesis. 22:383–395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nativ O, Sabo E, Reiss A, Wald M, Madjar S
and Moskovitz B: Clinical significance of tumor angiogenesis in
patients with localized renal cell carcinoma. Urology. 51:693–696.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukata S, Inoue K, Kamada M, Kawada C,
Furihata M, Ohtsuki Y and Shuin T: Levels of angiogenesis and
expression of angiogenesis-related genes are prognostic for
organ-specific metastasis of renal cell carcinoma. Cancer.
103:931–942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joo H, Oh D, Kim Y, Lee K and Kim S:
Increased expression of caveolin-1 and microvessel density
correlates with metastasis and poor prognosis in clear cell renal
cell carcinoma. BJU Int. 93:291–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minardi D, Lucarini G, Filosa A, Milanese
G, Zizzi A, Di Primio R, Montironi R and Muzzonigro G: Prognostic
role of tumor necrosis, microvessel density, vascular endothelial
growth factor and hypoxia inducible factor-1alpha in patients with
clear cell renal carcinoma after radical nephrectomy in a long term
follow-up. Int J Immunopathol Pharmacol. 21:447–455. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iakovlev VV, Gabril M, Dubinski W,
Scorilas A, Youssef YM, Faragalla H, Kovacs K, Rotondo F, Metias S,
Arsanious A, et al: Microvascular density as an independent
predictor of clinical outcome in renal cell carcinoma: An automated
image analysis study. Lab Invest. 92:46–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paradis V, Lagha NB, Zeimoura L, Blanchet
P, Eschwege P, Ba N, Benoît G, Jardin A and Bedossa P: Expression
of vascular endothelial growth factor in renal cell carcinomas.
Virchows Arch. 436:351–356. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Yamashita M, Uetsuki H and Kakehi
Y: Angiogenesis in renal cell carcinoma: Evaluation of microvessel
density, vascular endothelial growth factor and matrix
metalloproteinases. Int J Urol. 9:509–514. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuna B, Yorukoglu K, Unlu M, Mungan MU and
Kirkali Z: Association of mast cells with microvessel density in
renal cell carcinomas. Eur Urol. 50:530–534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slaton JW, Inoue K, Perrotte P, El-Naggar
AK, Swanson DA, Fidler IJ and Dinney CP: Expression levels of genes
that regulate metastasis and angiogenesis correlate with advanced
pathological stage of renal cell carcinoma. Am J Pathol.
158:735–743. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mohseni MG, Mohammadi A, Heshmat AS,
Kosari F and Meysamie AP: The lack of correlation between mast
cells and microvessel density with pathologic feature of renal cell
carcinoma. Int Urol Nephrol. 42:109–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
MacLennan GT and Bostwick DG: Microvessel
density in renal cell carcinoma: Lack of prognostic significance.
Urology. 46:27–30. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gelb AB, Sudilovsky D, Wu CD, Weiss LM and
Medeiros LJ: Appraisal of intratumoral microvessel density, MIB-1
score, DNA content, and p53 protein expression as prognostic
indicators in patients with locally confined renal cell carcinoma.
Cancer. 80:1768–1775. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki K, Morita T, Hashimoto S and Tokue
A: Thymidine phosphorylase/platelet-derived endothelial cell growth
factor (PD-ECGF) associated with prognosis in renal cell carcinoma.
Urol Res. 29:7–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Minardi D, Lucarini G, Mazzucchelli R,
Milanese G, Natali D, Galosi AB, Montironi R, Biagini G and
Muzzonigro G: Prognostic role of fuhrman grade and vascular
endothelial growth factor in pT1a clear cell carcinoma in partial
nephrectomy specimens. J Urol. 174:1208–1212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anastassiou G, Duensing S, Steinhoff G,
Zorn U, Grosse J, Dallmann I, Kirchner H, Ganser A and Atzpodien J:
Platelet endothelial cell adhesion molecule-1 (PECAM-1): A
potential prognostic marker involved in leukocyte infiltration of
renal cell carcinoma. Oncology. 53:127–132. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rioux-Leclercq N, Epstein JI, Bansard JY,
Turlin B, Patard JJ, Manunta A, Chan T, Ramee MP, Lobel B and
Moulinoux JP: Clinical significance of cell proliferation,
microvessel density, and CD44 adhesion molecule expression in renal
cell carcinoma. Hum Pathol. 32:1209–1215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yagasaki H, Kawata N, Takimoto Y and
Nemoto N: Histopathological analysis of angiogenic factors in renal
cell carcinoma. Int J Urol. 10:220–227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imao T, Egawa M, Takashima H, Koshida K
and Namiki M: Inverse correlation of microvessel density with
metastasis and prognosis in renal cell carcinoma. Int J Urol.
11:948–953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mertz KD, Demichelis F, Kim R, Schraml P,
Storz M, Diener PA, Moch H and Rubin MA: Automated
immunofluorescence analysis defines microvessel area as a
prognostic parameter in clear cell renal cell cancer. Hum Pathol.
38:1454–1462. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yildiz E, Ayan S, Goze F, Gokce G and
Gultekin EY: Relation of microvessel density with microvascular
invasion, metastasis and prognosis in renal cell carcinoma. BJU
Int. 101:758–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshino S, Kato M and Okada K: Prognostic
significance of microvessel count in low stage renal cell
carcinoma. Int J Urol. 2:156–160. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sabo E, Boltenko A, Sova Y, Stein A,
Kleinhaus S and Resnick MB: Microscopic analysis and significance
of vascular architectural complexity in renal cell carcinoma. Clin
Cancer Res. 7:533–537. 2001.PubMed/NCBI
|
|
35
|
Delahunt B, Bethwaite P and Thornton A:
Prognostic significance of microscopic vascularity for clear cell
renal cell carcinoma. Br J Urol. 80:401–404. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schraml P, Struckmann K, Hatz F, Sonnet S,
Kully C, Gasser T, Sauter G, Mihatsch MJ and Moch H: VHL mutations
and their correlation with tumour cell proliferation, microvessel
density, and patient prognosis in clear cell renal cell carcinoma.
J Pathol. 196:186–193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sandlund J, Hedberg Y, Bergh A, Grankvist
K, Ljungberg B and Rasmuson T: Endoglin (CD105) expression in human
renal cell carcinoma. BJU Int. 97:706–710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sandlund J, Hedberg Y, Bergh A, Grankvist
K, Ljungberg B and Rasmuson T: Evaluation of CD31 (PECAM-1)
expression using tissue microarray in patients with renal cell
carcinoma. Tumor Biol. 28:158–164. 2007. View Article : Google Scholar
|
|
39
|
Köhler HH, Barth PJ, Siebel A, Gerharz EW
and Bittinger A: Quantitative assessment of vascular surface
density in renal cell carcinomas. Br J Urol. 77:650–654. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hemmerlein B, Kugler A, Özisik R, Ringert
RH, Radzun HJ and Thelen P: Vascular endothelial growth factor
expression, angiogenesis, and necrosis in renal cell carcinomas.
Virchows Arch. 439:645–652. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baldewijns MM, Thijssen VL, Van den Eynden
GG, Van Laere SJ, Bluekens AM, Roskams T, van Poppel H, De Bruïne
AP, Griffioen AW and Vermeulen PB: High-grade clear cell renal cell
carcinoma has a higher angiogenic activity than low-grade renal
cell carcinoma based on histomorphological quantification and
qRT-PCR mRNA expression profile. Br J Cancer. 96:1888–1895. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kavantzas N, Paraskevakou H,
Tseleni-Balafouta S, Aroni K, Athanassiades P, Agrogiannis G and
Patsouris E: Association between microvessel density and histologic
grade in renal cell carcinomas. Pathol Oncol Res. 13:145–148. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sharma SG, Aggarwal N, Gupta SD, Singh MK,
Gupta R and Dinda AK: Angiogenesis in renal cell carcinoma:
Correlation of microvessel density and microvessel area with other
prognostic factors. Int Urol Nephrol. 43:125–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:91995.PubMed/NCBI
|
|
45
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shirakawa K, Kobayashi H, Heike Y,
Kawamoto S, Brechbiel MW, Kasumi F, Iwanaga T, Konishi F, Terada M
and Wakasugi H: Hemodynamics in vasculogenic mimicry and
angiogenesis of inflammatory breast cancer xenograft. Cancer Res.
62:560–566. 2002.PubMed/NCBI
|
|
47
|
Sun B, Zhang S, Zhang D, Du J, Guo H, Zhao
X, Zhang W and Hao X: Vasculogenic mimicry is associated with high
tumor grade, invasion and metastasis, and short survival in
patients with hepatocellular carcinoma. Oncol Rep. 16:693–698.
2006.PubMed/NCBI
|
|
48
|
Lee H, Lee M, Lee SE, Byun SS, Kim HH,
Kwak C and Hong SK: Outcomes of pathologic stage T3a renal cell
carcinoma up-staged from small renal tumor: Emphasis on partial
nephrectomy. BMC Cancer. 18:4272018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nowak-Sliwinska P, Alitalo K, Allen E,
Anisimov A, Aplin AC, Auerbach R, Augustin HG, Bates DO, van
Beijnum JR, Bender RHF, et al: Consensus guidelines for the use and
interpretation of angiogenesis assays. Angiogenesis. 21:425–532.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Feng Y, Song K, Shang W, Chen L, Wang C,
Pang B and Wang N: REDD1 overexpression in oral squamous cell
carcinoma may predict poor prognosis and correlates with high
microvessel density. Oncol Lett. 19:431–441. 2020.PubMed/NCBI
|
|
51
|
Vartanian AA, Stepanova EV, Gutorov SL,
Solomko ES, Grigorieva IN, Sokolova IN, Baryshnikov AY and
Lichinitser MR: Prognostic significance of periodic
acid-Schiff-positive patterns in clear cell renal cell carcinoma.
Can J Urol. 16:4726–4732. 2009.PubMed/NCBI
|
|
52
|
Zhang Y, Sun B, Zhao X, Liu Z, Wang X, Yao
X, Dong X and Chi J: Clinical significances and prognostic value of
cancer stem-like cells markers and vasculogenic mimicry in renal
cell carcinoma. J Surg Oncol. 108:414–419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu
D, Yu X and Tian Y: Advanced research on vasculogenic mimicry in
cancer. J Cell Mol Med. 19:315–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Paulis YW, Soetekouw PM, Verheul HM,
Tjan-Heijnen VC and Griffioen AW: Signalling pathways in
vasculogenic mimicry. Biochim Biophys Acta. 1806:18–28.
2010.PubMed/NCBI
|
|
55
|
Kirschmann DA, Seftor EA, Hardy KM, Seftor
RE and Hendrix MJ: Molecular pathways: Vasculogenic mimicry in
tumor cells: Diagnostic and therapeutic implications. Clin Cancer
Res. 18:2726–2732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bai J, Yeh S, Qiu X, Hu L, Zeng J, Cai Y,
Zuo L, Li G, Yang G and Chang C: TR4 nuclear receptor promotes
clear cell renal cell carcinoma (ccRCC) vasculogenic mimicry (VM)
formation and metastasis via altering the miR490-3p/vimentin
signals. Oncogene. 37:5901–5912. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sabo E, Miselevich I, Bejar J, Segenreich
M, Wald M, Moskovitz B and Nativ O: The role of vimentin expression
in predicting the long-term outcome of patients with localized
renal cell carcinoma. Br J Urol. 80:864–868. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sandlund J, Hedberg Y, Bergh A, Grankvist
K, Ljungberg B and Rasmuson T: Endoglin (CD105) expression in human
renal cell carcinoma. BJU Int. 97:706–710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sandlund J, Hedberg Y, Bergh A, Grankvist
K, Ljungberg B and Rasmuson T: Evaluation of CD31 (PECAM-1)
expression using tissue microarray in patients with renal cell
carcinoma. Tumour Biol. 28:158–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yao X, Qian CN, Zhang ZF, Tan MH, Kort EJ,
Yang XJ, Resau JH and The BT: Two distinct types of blood vessels
in clear cell renal cell carcinoma have contrasting prognostic
implications. Clin Cancer Res. 13:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Qian CN, Huang D, Wondergem B and Teh BT:
Complexity of tumor vasculature in clear cell renal cell carcinoma.
Cancer. 115 (10 Suppl):S2282–S2289. 2009. View Article : Google Scholar
|
|
63
|
Kuczynski EA and Reynolds AR: Vessel
co-option and resistance to anti-angiogenic therapy. Angiogenesis.
23:55–74. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kuczynski EA, Vermeulen PB, Pezzella F,
Kerbel RS and Reynolds AR: Vessel co-option in cancer. Nat Rev Clin
Oncol. 16:469–493. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Latacz E, Caspani E, Barnhill R, Lugassy
C, Verhoef C, Grünhagen D, Van Laere S, Moro CF, Gerling M, Dirix
M, et al: Pathological features of vessel co-option versus
sprouting angiogenesis. Angiogenesis. 23:43–54. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang JP, Liao YD, Mai DM, Xie P, Qiang YY,
Zheng LS, Wang MY, Mei Y, Meng DF, Xu L, et al: Tumor vasculogenic
mimicry predicts poor prognosis in cancer patients: A
meta-analysis. Angiogenesis. 19:191–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hueng DY, Lin GJ, Huang SH, Liu LW, Ju DT,
Chen YW, Sytwu HK, Chang C, Huang SM, Yeh YS, et al: Inhibition of
Nodal suppresses angiogenesis and growth of human gliomas. J
Neurooncol. 104:21–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Feng X, Yu Y, He S, Cheng J, Gong Y, Zhang
Z, Yang X, Xu B, Liu X, Li CY, et al: Dying glioma cells establish
a proangiogenic microenvironment through a caspase 3 dependent
mechanism. Cancer Lett. 385:12–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bekes EM, Schweighofer B, Kupriyanova TA,
Zajac E, Ardi VC, Quigley JP and Deryugina EI: Tumor-recruited
neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately
the levels of tumor angiogenesis and efficiency of malignant cell
intravasation. Am J Pathol. 179:1455–1470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jia W, Kidoya H, Yamakawa D, Naito H and
Takakura N: Galectin-3 accelerates M2 macrophage infiltration and
angiogenesis in tumors. Am J Pathol. 182:1821–1831. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bentley K, Franco CA, Philippides A,
Blanco R, Dierkes M, Gebala V, Stanchi F, Jones M, Aspalter IM,
Cagna G, et al: The role of differential VE-cadherin dynamics in
cell rearrangement during angiogenesis. Nat Cell Biol. 16:309–321.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang JY, Sun T, Zhao XL, Zhang SW, Zhang
DF, Gu Q, Wang XH, Zhao N, Qie S and Sun BC: Functional
significance of VEGF-a in human ovarian carcinoma: Role in
vasculogenic mimicry. Cancer Biol Ther. 7:758–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Powles T, Albiges L, Staehler M, Bensalah
K, Dabestani S, Giles RH, Hofmann F, Hora M, Kuczyk MA, Lam TB, et
al: Updated european association of urology guidelines
recommendations for the treatment of first-line metastatic clear
cell renal cancer. Eur Urol. 73:311–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Azuma T, Sugihara T, Honda S, Yoshizaki U,
Niimi F, Tsuru I and Kume H: Metastatic renal cell carcinoma
regains sensitivity to tyrosine kinase inhibitor after nivolumab
treatment: A case report. Oncol Lett. 17:4011–4015. 2019.PubMed/NCBI
|
|
76
|
Wei W, Lv Y, Gan Z, Zhang Y, Han X and Xu
Z: Identification of key genes involved in the metastasis of clear
cell renal cell carcinoma. Oncol Lett. 17:4321–4328.
2019.PubMed/NCBI
|
|
77
|
Carlsson J, Christiansen J, Davidsson S,
Giunchi F, Fiorentino M and Sundqvist P: The potential role of
miR-126, miR-21 and miR-10b as prognostic biomarkers in renal cell
carcinoma. Oncol Lett. 17:4566–4574. 2019.PubMed/NCBI
|
|
78
|
Gao Y, Qi JC, Li X, Sun JP, Ji H and Li
QH: Decreased expression of TXNIP predicts poor prognosis in
patients with clear cell renal cell carcinoma. Oncol Lett.
19:763–770. 2020.PubMed/NCBI
|
|
79
|
Yan N, Feng X, Jiang S, Sun W, Sun MZ and
Liu S: GRIM-19 deficiency promotes clear cell renal cell carcinoma
progression and is associated with high TNM stage and fuhrman
grade. Oncol Lett. 19:4115–4121. 2020.PubMed/NCBI
|